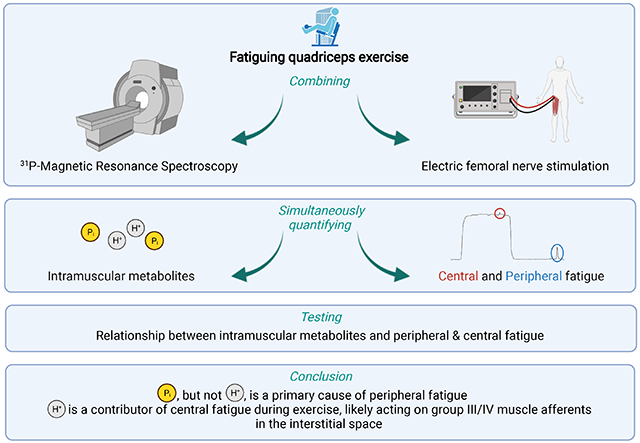
Abstract
Intramuscular hydrogen ion (H+) and inorganic phosphate (Pi) concentrations were dissociated during exercise to challenge their relationships with peripheral and central fatigue in vivo. Ten recreationally active, healthy men (27±5 years; 180±4 cm; 76±10 kg) performed two consecutive intermittent isometric single-leg knee-extensor trials (60 maximal voluntary contractions; 3s-contraction, 2s-relaxation) interspersed with 5 min of rest. Phosphorus magnetic resonance spectroscopy (31P-MRS) was used to continuously quantify intramuscular [H+] and [Pi] during both trials. Using electrical femoral nerve stimulation, quadriceps twitch force (Qtw) and voluntary activation (VA) were quantified at rest and throughout both trials. Decreases in Qtw and VA from baseline were used to determine peripheral and central fatigue, respectively. Qtw was strongly related to both [H+] (β Coefficient: −0.9, P < 0.0001) and [Pi] (−1.1, P < 0.0001) across trials. There was an effect of trial on the relationship between Qtw and [H+] (−0.5, P < 0.0001), but not Qtw and [Pi] (0.0, P = 0.976). This suggests that, unlike the unaltered association with [Pi], a given level of peripheral fatigue was associated with a different [H+] in trial 1 vs trial 2. VA was related to [H+] (−0.3, P < 0.0001), but not [Pi] (−0.2, P = 0.243), across trials and there was no effect of trial (−0.1, P = 0.483). Taken together, these results support intramuscular Pi as a primary cause of peripheral fatigue, and muscle acidosis, likely acting on group III/IV muscle afferents in the interstitial space, as a contributor to central fatigue during exercise.
Keywords: intramuscular metabolic by-product, magnetic resonance spectroscopy, neuromuscular fatigue, exercise
Graphical Abstract
INTRODUCTION
Exercise-induced neuromuscular fatigue is characterized by a temporary reduction in the force or power generating capacity of a muscle. This impairment stems from intramuscular biochemical changes that cause an attenuated contractile response for a given neural input (i.e. peripheral fatigue) and a central nervous system (CNS)-mediated decrease in muscle activation (i.e. central fatigue) (Taylor et al., 2016). In vitro and animal studies have highlighted several intramuscular metabolites as potential causes of peripheral fatigue, with hydrogen ion (H+) and inorganic phosphate (Pi) at the forefront (Nosek et al., 1987; Cooke et al., 1988; Allen et al., 2008; Nelson et al., 2014). Elegant arguments for and against muscle acidosis, or Pi, as the primary cause of peripheral fatigue have been made (Fitts, 2016; Westerblad, 2016). Furthermore, the intramuscular accumulation of metabolites previously suggested to cause peripheral fatigue, including H+ and Pi, have also been suggested to facilitate the development of central fatigue (Blain et al., 2016; Amann et al., 2020). Specifically, when the concentration of these metabolites increases during exercise, the central projection of metabosensitive group III/IV muscle afferents rises, resulting in a CNS-mediated restriction of muscle activation (i.e. an increase in central fatigue) (Amann et al., 2009; Kennedy et al., 2014; Hureau et al., 2019).
Fundamentally, if indeed a cause of neuromuscular fatigue, the accumulation of H+, or Pi, must be related to its development during exercise. Evidence for this postulate was offered by earlier human studies using phosphorus magnetic resonance spectroscopy (31P-MRS) to reveal general associations between the exercise-induced accumulation of both intramuscular H+ and Pi and decreases in voluntary contraction strength (Dawson et al., 1978; Newham & Cady, 1990; Sundberg et al., 2019). Wilson et al. (Wilson et al., 1988) further discriminated between the role of muscle acidosis and Pi and found that elevating intramuscular [H+] and [Pi] immediately prior to maximal wrist flexion exercise causes the relationship between contraction force and [H+] to shift. Interestingly, the relationship between contraction force and [Pi] was not affected in this scenario and the authors suggested [Pi], but not [H+], as the primary cause of muscle fatigue. However, these previous studies were all based on exercise-induced decreases in maximal voluntary contraction force (MVC), a ‘non-specific’ index of fatigue that cannot discriminate between peripheral and central sites (Gandevia, 2001). Furthermore, it is now recognized that Pi primarily affects skeletal muscle force production by altering calcium (Ca2+) handling and that force does not decrease until the cytosolic [Pi] exceeds a ‘critical’ concentration. Specifically, when [Pi] exceeds this threshold, Pi enters the sarcoplasmic reticulum and precipitates with Ca2+, which reduces Ca2+ release during excitation-contraction coupling and, ultimately, decreases force production (Fryer et al., 1997; Posterino & Fryer, 1998; Allen et al., 2008; Korzeniewski & Rossiter, 2020).
Based on this background and our previous observation that intramuscular [Pi] returns to baseline, while [H+] remains elevated, 5 min after a 60 quadriceps contraction protocol (Broxterman et al., 2017), participants in the current study repeated this exercise protocol twice (with a 5 min break in between), allowing us to dissociate [H+] and [Pi] during the second bout and thus challenge their independent relationships with peripheral and central fatigue. Moreover, concomitant quantification of quadriceps twitch force (Qtw) and [Pi] throughout these exercise trials allowed us to assess the critical [Pi] (i.e., PiBP) in vivo and incorporate this into the determination of the relationship between Pi and peripheral fatigue. To this end, intramuscular [H+] and [Pi] and Qtw and voluntary activation (VA) were simultaneously quantified during exercise using 31P-MRS and electrical femoral nerve stimulation, respectively. We hypothesized that: 1) Qtw would be related to both intramuscular [H+] and [Pi], 2) the relationship between Qtw and [H+], but not [Pi], would be altered during the second trial, and 3) VA would be related with [H+] and [Pi] during exercise.
METHODS
Ethical approval
Written informed consent was obtained from each participant prior to the beginning of the study. All experimental procedures were approved by the Institutional Review Boards of the University of Utah and the Salt Lake City Veterans Affairs Medical Center, and conducted according to the Declaration of Helsinki for human experimentation, except for registration in a database.
Subjects
Ten recreationally active, healthy men participated in this study (age: 27 ± 5 years; height: 180 ± 4 cm; body mass: 76 ± 10 kg). Given the previously documented age (Christie et al., 2011) and sex (Hunter, 2014) differences in the development of neuromuscular fatigue during exercise, this investigation was limited to young, male participants. The subjects were non-smokers, not medicated and asymptomatic for cardiovascular or respiratory disease, and refrained from vigorous exercise, caffeine and alcohol consumption for 24 h prior to each study visit.
Experimental protocol
A single-leg intermittent isometric knee-extensor exercise protocol (60 MVCs) was performed during a familiarization visit and repeated twice in a subsequent experimental visit. The 60 MVCs were conducted over 5 min using a 3 s contraction and 2 s relaxation duty cycle, maintained by audio cues. Subjects performed the exercise in a recumbent position with the knee supported (~ 45° knee joint angle) and the ankle fixed to an immovable strain gauge (SSM-AJ-250, Interface Inc., Scottsdale, AZ, USA). Non-elastic straps were positioned over the hips to minimize extraneous movements. For the experimental visit, participants performed the 60 MVCs protocol (Trial 1) followed by 5 min of rest followed by another 60 MVCs protocol (Trial 2) inside a whole-body magnetic resonance imaging (MRI) system. This protocol was designed to initiate Trial 2 with complete recovery of intramuscular [Pi], but incomplete recovery of intramuscular pH (Broxterman et al., 2017). The peak force was determined for each of the 60 MVCs. To quantify exercise-induced neuromuscular fatigue, quadriceps function was assessed before, during, and immediately after the exercise.
31P-MRS
A clinical 2.9 Tesla MRI system (Tim-Trio, Siemens Medical Systems, Malvern, PA, USA) operating at 49.9 MHz for 31P resonance was utilized for MRS. 31P-MRS data acquisition was obtained using a dual 31P-1H surface coil (110 mm 1H coil loop surrounded by the 31P single-loop coil with a diameter of 125 mm) with linear polarization (Rapid Biomedical GmbH, Rimpar, Germany). Given the median mid-thigh circumference of 55 cm for 20-39 years old physically active males (McDowell et al., 2008), the 6.25 cm semi-sphere projection (depth) of the 12.5 cm coil (Gadian, 1982) limited the quantification of the signals to the exercising quadriceps. The surface coil was secured with elastic straps at mid-thigh level and advanced localized volume shimming was performed after a three-plane scout proton image was acquired to ensure sampling of all major quadriceps muscles. Before starting exercise Trial 1, two fully relaxed spectra (3 averages per spectrum; repetition time of 30 s) and two partially relaxed spectra (3 averages per spectrum; repetition time of 2.5 s) were acquired at rest. MRS data were then acquired throughout the exercise Trials using a using a free-induction decay pulse sequence with a 2.56 ms adiabatic-half-passage excitation RF pulse, a repetition time of 2.5 s, a receiver bandwidth of 5 kHz, 1024 data points, and two averages per spectrum equaling 5 s to match the contraction duty cycle. Saturation factors were quantified by the comparison between the fully relaxed and partially relaxed spectra acquired at rest. Relative concentrations of metabolites were determined by a time-domain fitting routine using the AMARES algorithm (Vanhamme et al., 1997) incorporated into CSAIPO software (Layec et al., 2008; Le Fur et al., 2010), while absolute concentrations were estimated assuming a resting [ATP] of 8.2 mM (Harris et al., 1974). Resting concentrations were calculated from the average peak areas of the two fully relaxed spectra. Muscle intracellular pH was calculated from the chemical shift difference (δ) of the Pi peak relation to the phosphocreatine peak as: pH = 6.75 + log[(δ – 3.27) / (5.69 – δ)]. When Pi splitting was evident, the pH corresponding to each Pi pool was calculated separately as pH1 and pH2 on the bases of the chemical shift of each Pi peak relative to the phosphocreatine peak. The overall muscle pH was then calculated as: pH = pH1 · (areaPi1/total Pi area) + pH2 · (areaPi2/total Pi area). The pH values were transformed to H+ concentration by: H+ = 10−pH. The concentration of the H2PO4− was calculated as (Lanza et al., 2006): H2PO4− = [Pi]/(1+10pH-6.75).
Neuromuscular function
Self-adhesive, MRI-safe electrodes were placed at the stimulation site over the femoral nerve that resulted in the maximal torque response (cathode) and on the greater trochanter (anode). A constant current stimulator (model DS7AH; Digitimer, Welwyn Garden City, UK), located outside the MRI room, delivered a square-wave stimulus (200 μs). A supramaximal stimulation intensity (205 ± 66 mA) of 120% of the intensity that elicited maximal force was used to ensure maximal spatial recruitment of the quadriceps. Single potentiated twitches (Qtw) were delivered 1 s after MVCs 1, 3, 9, 15, 21, 27, 33, 39, 45, 51, 57 and 60 (i.e., 12 Qtw during exercise). To maximize force output during every MVC, uniform and strong verbal encouragements were given from the same investigator. We measured peak force for MVC and Qtw assessments. For each MVC preceding a Qtw, a superimposed twitch was delivered during the peak force of the MVC to calculate voluntary activation (VA) of the quadriceps (Merton, 1954).
Statistical analysis
Normality of every dependent variable and sphericity of the variance of the distributions (equal variance) were confirmed with the Kolmogorov–Smirnov test and the Mauchly test, respectively. A Greenhouse–Geisser correction was used when sphericity was violated. A student’s paired t test was used to determine potential difference between the two trials in mean force output (exercise performance). Two-way ANOVA with repeated measures (condition × time) was used to test differences during exercise for MVC, Qtw, VA, H+, and Pi. When a significant difference was found, multiple-comparison analysis was performed with Tukey’s honestly significant difference test. These statistical analyses were conducted with Statistica 8.0 (StatSoft, Tulsa, OK) and the data presented as means ± SD. Statistical significance was set at P < 0.05.
For each Trial, Stata mixed (Stata 17, StataCorp LLC, College Station, TX, USA) was used to conduct linear mixed-effects analyses of the relationship between neuromuscular fatigue and intramuscular metabolites. For Pi or H+, the linear mixed effects model consisted of a dependent variable (Qtw, VA, or MVC), independent variables (metabolite × Trial interaction) as fixed effects, and random effects for subject (intercept) to account for repeated measures within subjects. The acidic diprotonated form of Pi (H2PO4−), which is included in the total Pi measurement, has been suggested as an inhibitor of force production (i.e., peripheral fatigue) (Nosek et al., 1987). Therefore, a linear mixed effects model was conducted with Qtw as the dependent variable, H2PO4− × Trial interaction as fixed effects dependent variables, and random effects for subject (intercept). To assure a similar level of muscle potentiation for all fatigue related variables included in the analysis, the first, or first two, neuromuscular function measurements were omitted (due to incomplete potentiation; (Kufel et al., 2002; Tillin & Bishop, 2009). The data from this maximal Qtw to the initial measurement in which Qtw reached a nadir (< 3% point-to-point change relative to maximal Qtw; range 5th – 11th measurement) were used for the linear mixed effects models to focus on when fatigue is occurring and to minimize the data along the plateau from leveraging the regression analyses. We tested for a critical [Pi] (i.e., PiBP), below which Qtw decreases relatively little, by fitting each individual Qtw vs Pi with a two-segment piecewise linear function: 1st segment) Qtw = a1 + b1Pi, for Pi < PiBP ; 2nd segment) Qtw = (a1 + b1PiBP) + b2(Pi – PiBP), for Pi ≥ PiBP; where a1 and b1 are the respective y-intercept and slope of the first segment, PiBP is the Pi concentration at the breakpoint between the two segments, and b2 is the slope of the second segment (OriginPro, OriginLab, Northampton, Massachusetts, USA). A PiBP was detectable for each trial and Pi concentrations less than PiBP were not included in the linear mixed effects model, based on evidence that Pi induces peripheral fatigue by entering the sarcoplasmic reticulum only when PiBP is exceeded (Fryer et al., 1997; Posterino & Fryer, 1998; Allen et al., 2008; Korzeniewski & Rossiter, 2020). We did not perform these analyses on the Qtw vs H+ data because this relationship is consistently linear (after the initial alkalosis is omitted) (Adams et al., 1991) and we are not aware of any evidence supporting a “break point” in the mechanisms by which H+ is thought to induce peripheral fatigue. The standardized (Z-score) dependent (Qtw, VA, and MVC) and independent (Pi and H+) variables were used for the linear mixed effects models to determine the standardized regression coefficients and to allow more appropriate comparison of the fixed effects. To clarify, in models based on the original scales, the interpretation of the main effects is not possible, or at least made difficult, if an interaction term is in the model. If the model is mean centered (part of Z-score calculation), however, where the total sample means are subtracted from the measurements, the main effects from a model that includes an interaction term can be interpreted. The results from the linear mixed effects analyses are presented as β Coefficient, p-value, 95% confidence interval.
RESULTS
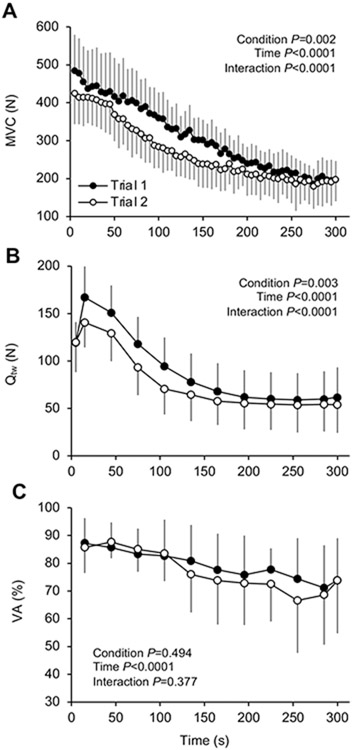
The profiles of quadriceps force output during the 60 MVCs Trials are shown in Figure 1A. There was an effect of Trial for the force output across Trials (P = 0.002). Overall, MVCs were 13 ± 9% (269 ± 62 N) lower in Trial 2 compared to Trial 1 (309 ± 64 N). Qtw decreased throughout both Trials (P < 0.0001). More specifically, Qtw was lower from the second to the fifth assessment (i.e. 6th – 20th MVC) in Trial 2 compared to Trial 1, and Qtw was not different between Trials thereafter (Figure 1B). VA decreased throughout exercise (P < 0.0001) with no difference between Trials (Figure 1D). As displayed in Figure 2, intramuscular Pi, H+ and H2PO4− increased over time during both Trials (P < 0.0001), and we also identified an interaction effect with time (P < 0.0001).
Figure 1. Exercise-induced changes in neuromuscular function.
A: maximal voluntary contraction force (MVC). B: Quadriceps twitch force (Qtw). D: Voluntary quadriceps activation (VA) during 60 knee-extensor MVCs. Trial 1 and 2 were separated by 5 min of rest. Data are presented as mean ± SD. Significant time, trial, and time × trial interaction effects are indicated on the graphs. N = 10.
Figure 2. Exercise-induced changes in intramuscular [H+], [Pi] and [H2PO4−].
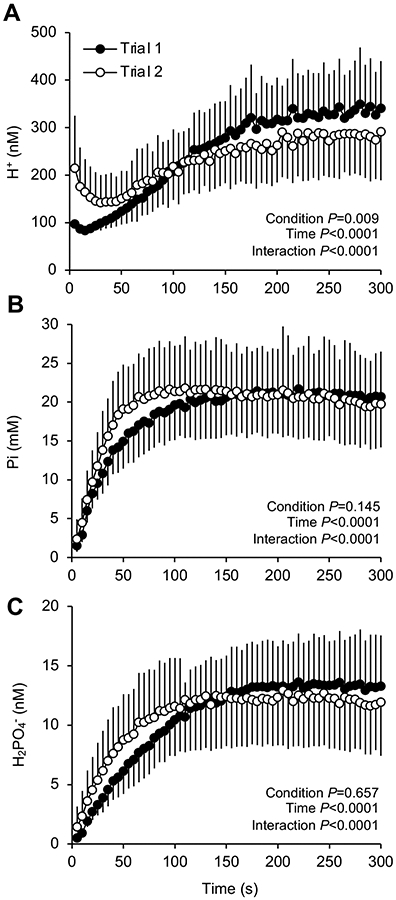
Intramuscular hydrogen ion (H+), inorganic phosphate (Pi) and the acidic diprotonated form of Pi (H2PO4−) were measured using phosphorus magnetic resonance spectroscopy (31P-MRS) during 60 knee-extensor MVCs. Trial 1 and 2 were separated by 5 min of rest. Data are presented as mean ± SD. Significant time, trial, and time × trial interaction effects are indicated on the graphs. N = 10.
Relationship between peripheral fatigue and intramuscular [H+] and [Pi] during exercise
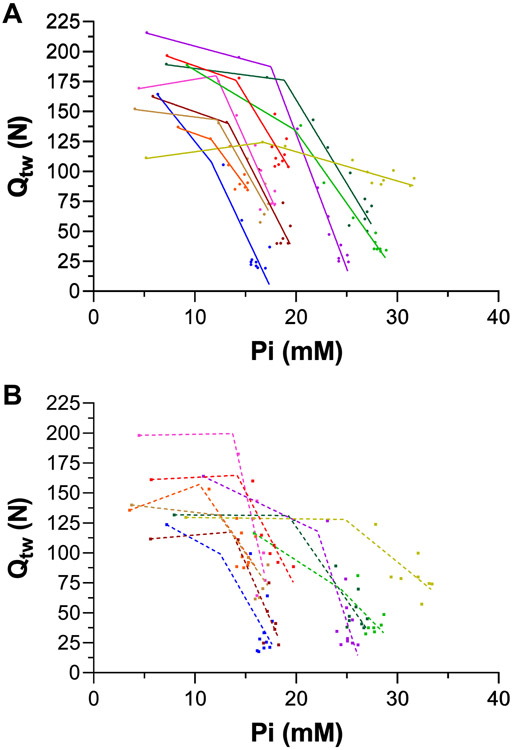
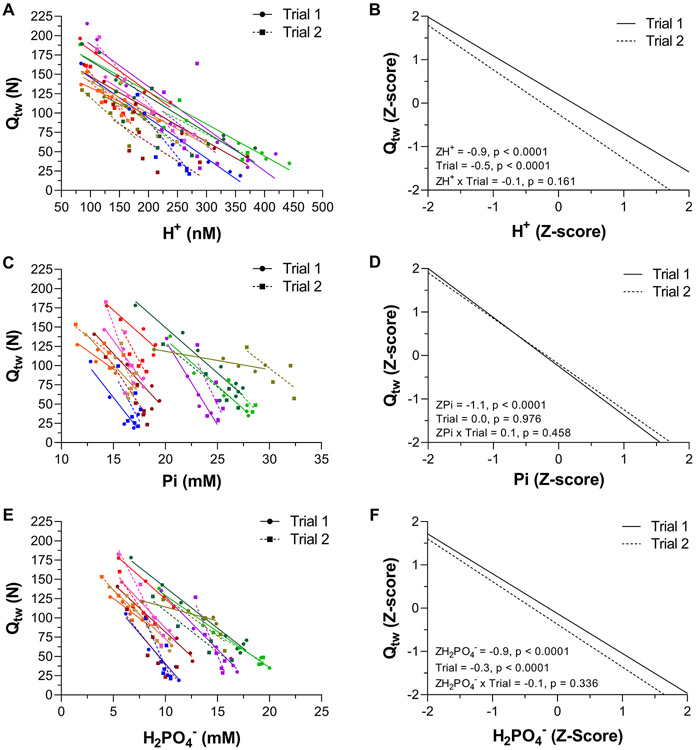
There was no difference between the PiBP between Trial 1 (14.8 ± 3.2 mM; r2 = 0.90 ± 0.06) and Trial 2 (16.8 ± 5.4 mM; r2 = 0.72 ± 0.12). Thus, the two-segment linear regression was fit to the data collapsed from both Trials to improve accuracy in the estimation of the PiBP by increasing the number of data points on both linear segments. The PiBP from the collapsed data was 15.1 ± 3.3 mM, r2 = 0.72 ± 0.13, which was used for the linear mixed models. The individual data for Qtw vs Pi are shown in Figure 3. The individual data and linear mixed effects regression analyses for Qtw vs H+ and Qtw vs Pi are shown in Figure 4. Qtw was strongly related to H+ across both Trials (−0.9, P < 0.0001, −1.0 to −0.8). There was an effect of Trial on the relationship between Qtw and H+ (−0.5, P < 0.0001, −0.6 to −0.3), but no H+ × Trial interaction (−0.1, −0.1 to 0.4). Qtw was strongly related to Pi across both Trials (−1.0, P < 0.0001, −1.2 to −0.8). There was no effect of Trial on the relationship between Qtw and Pi (0.0, −0.2 to 0.2) and no Pi × Trial interaction (0.1, −0.1 to 0.3). The data for H2PO4−, which is part of the Pi measurement, are presented in Figure 4 (Panels E & F). Qtw was strongly related to H2PO4− across both Trials (−0.9, P < 0.001, −1.0 to −0.8). There was an effect of Trial on the relationship between Qtw and H2PO4− (−0.3, P < 0.0001, −0.4 to −0.1), but no H2PO4− × Trial interaction (−0.1, P = 0.336, −0.6 to 0.3).
Figure 3. Relationship between quadriceps twitch force (Qtw) and intramuscular [Pi] during exercise.
Qtw and Pi were measured using electric femoral nerve stimulation and phosphorus magnetic resonance spectroscopy (31P-MRS), respectively, during two 60 knee-extensor MVCs trials. The data were fit with a two-segment piecewise linear function to test for a break point between two linear segments (i.e., critical [Pi]). The Pi break point was 15.1 ± 3.3 mM, r2 = 0.72 ± 0.13. N = 10.
Figure 4. Relationship between quadriceps twitch force (Qtw) and intramuscular [H+], [Pi], and [H2PO4−] during exercise.
Qtw, H+, Pi, and H2PO4− were measured using electric femoral nerve stimulation and phosphorus magnetic resonance spectroscopy (31P-MRS) during 60 knee-extensor MVCs. Trial 1 and 2 were separated by 5 min of rest. Individual participant data are presented in panels A,C, & E (these data are fit with linear regressions for illustrative purposes only). The linear mixed effects model results on the standardized (Z-score) Qtw, H+, Pi, and H2PO4− are presented in panels B, D, & F. N = 10.
Relationship between central fatigue and intramuscular [H+] and [Pi] during exercise
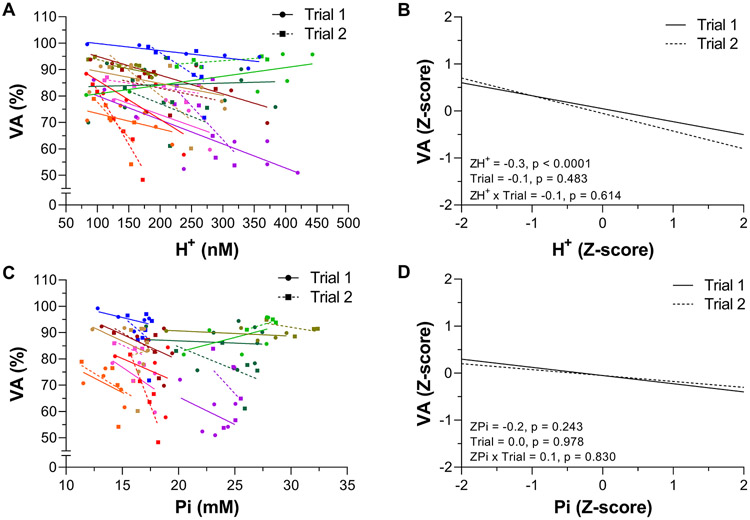
The individual data and linear mixed effects regression analyses for VA vs H+ and VA vs Pi are shown in Figure 5. VA was related to H+ across both Trials (−0.3, P < 0.0001, −0.4 to −0.1). There was no effect of Trial (−0.1, −0.3 to 0.2) or H+ × Trial interaction (−0.1, −0.4 to 0.2). VA was not related to Pi across Trials (−0.2, −0.5 to 0.1). There was no effect of Trial (0.0, −0.3 to 0.3) or Pi × Trial interaction (0.1, −0.2 to 0.5).
Figure 5. Relationship between quadriceps voluntary activation (VA) and intramuscular [H+] and [Pi] during exercise.
VA, H+, and Pi were measured using electric femoral nerve stimulation and phosphorus magnetic resonance spectroscopy (31P-MRS) during 60 knee-extensor MVCs. Trial 1 and 2 were separated by 5 min of rest. Individual participant data are presented in panels A & C (these data are fit with linear regressions only to illustrate trends). The linear mixed effects model results on the standardized (Z-score) VA, H+, and Pi are presented in panels B & D. N = 10.
Relationship between global fatigue and intramuscular [H+] and [Pi] during exercise
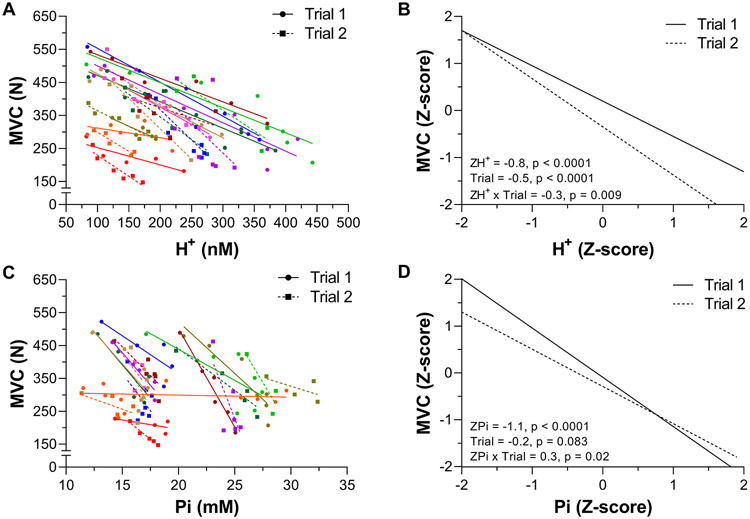
The individual data and linear mixed effects regression analyses for MVC vs H+ and MVC vs Pi are shown in Figure 6. MVC was strongly related to H+ across both Trials (−0.8, P < 0.0001, −0.9 to −0.7). There was an effect of Trial (−0.5, P < 0.0001, −0.7 to −0.4) and H+ × Trial interaction (−0.3, P = 0.009, −0.5 to −0.1). MVC was strongly related to Pi across Trials (−0.9, P < 0.0001, −1.2 to −0.6). There was no effect of Trial (−0.2, −0.5 to 0.1), but there was a Pi × Trial interaction (0.3, P = 0.020, 0.1 to 0.5).
Figure 6. Relationship between maximal voluntary quadriceps force (MVC) intramuscular [H+] and [Pi] during exercise.
H+ and Pi were measured using phosphorus magnetic resonance spectroscopy (31P-MRS) during 60 knee-extensor MVCs. Trial 1 and 2 were separated by 5 min of rest. Individual participant data are presented in panels A & C (these data are fit with linear regressions only to illustrate trends). The linear mixed effects model results on the standardized (Z-score) MVC, H+, and Pi are presented in panels B & D. N = 10.
DISCUSSION
This study was designed to dissociate intramuscular [H+] and [Pi] during exercise to challenge their relationships with peripheral and central fatigue. To this end, we concomitantly applied 31P-MRS (to quantify intramuscular metabolic changes) and electric nerve stimulation (to quantify neuromuscular function) throughout two consecutive all-out exercise bouts separated by 5 minutes of rest. Qtw was, as predicted, related to both [H+] and [Pi] across trials. However, the relationship between Qtw and [H+] was leftward-shifted in trial 2 compared to trial 1, while the relationship between Qtw and [Pi] was not different between the two trials. This suggests that, unlike the stable association with [Pi], a given level of peripheral fatigue was associated with a different [H+] in trial 1 vs trial 2. In contrast, VA was related to [H+], but not [Pi], and this association was not different between trials. The current findings therefore support intramuscular Pi as a primary, but not sole, cause of peripheral fatigue and muscle acidosis, likely acting on group III/IV muscle afferents interstitially, as a contributor to central fatigue.
Intramuscular H+, Pi, and peripheral fatigue
The exact mechanisms by which [Pi] and [H+] mediate the development of peripheral fatigue have previously been described in detail (Allen et al., 2008; Nelson et al., 2014; Fitts, 2016; Westerblad, 2016). Based on the particular relevance for the current investigation, it should be mentioned that Pi causes peripheral fatigue by entering the sarcoplasmic reticulum, precipitating with Ca2+, and, subsequently, reducing Ca2+ release during excitation-contraction coupling. Importantly, however, this only occurs after cytosolic [Pi] exceeds a ‘critical’ value, below which Pi has little effect on the contractile function of a muscle (Fryer et al., 1997; Posterino & Fryer, 1998; Allen et al., 2008; Korzeniewski & Rossiter, 2020).
The Qtw vs [Pi] data in the current study were well fit by a two-segmented linear model with a discernable breakpoint (i.e., the critical [Pi]) (Figure 3). Interestingly, the average critical [Pi] of ~15 mM in the current study is relatively close to the ~18 mM previously proposed by (Korzeniewski & Rossiter, 2020). Although accuracy in detecting the Pi breakpoint in the current study was bolstered by using the data collapsed from both trials, overall sensitivity was limited. It is, therefore, important to note that future studies with greater temporal resolution surrounding the breakpoint are needed to increase the degree of accuracy in the PiBP estimation. Importantly, this is, to our knowledge, the first direct identification and quantification of PiBP in exercising humans.
The present study revealed a strong linear relationship between Qtw and both intramuscular [H+] and [Pi] during exercise (Figure 4). While the linear nature of this relationship is consistent with earlier studies utilizing MVC as the sole index of neuromuscular function / fatigue (Massie et al., 1987; Wilson et al., 1988; Weiner et al., 1990), it deviates from the curvilinear relationship documented by others (Dawson et al., 1978; Degroot et al., 1993). Although speculative, this discrepancy may be due to differences in the temporal resolution of intramuscular metabolites and fatigue measurements and/or the lack of considering the now recognized critical [Pi]. Regardless, as an advantage over earlier studies based on changes in MVC, a non-specific marker of fatigue, we used Qtw and VA allowing us to offer more specific insights on the relationship between H+ and Pi and neuromuscular fatigue. Finally, the current linear relationships, based on multiple measurements obtained during exercise (Figure 4 A&C), are also consistent with findings from earlier studies based on pre/post exercise measurements of Qtw and [H+] and [Pi] (Blain et al., 2016; Broxterman et al., 2017).
In agreement with a previous investigation based only on MVCs (Wilson et al., 1988), without affecting its association with [Pi], the relationship between Qtw and [H+] was left shifted in trial 2 compared to trial 1 (Figure 4 B). While this suggests that a given [Pi] is associated with the same Qtw (i.e. causes the same amount of peripheral fatigue) in both trials, it also reveals that a given [H+] is associated with a lower Qtw (i.e., more peripheral fatigue) in trial 2 compared to trial 1. We interpret these findings as evidence for intramuscular [Pi] as the primary, but not sole, cause of peripheral fatigue during exercise, but also caution that a contribution of [H+], which might vary depending on other factors (Knuth et al., 2006; Debold et al., 2013), cannot be excluded. The intramuscular Pi measurement in the current study includes H2PO4−, which has been demonstrated to be strongly related to Qtw (Blain et al., 2016; Broxterman et al., 2017) and suggested as the only form of Pi that inhibits muscle force production (Nosek et al., 1987). Consistent with the former, Qtw was strongly related to [H2PO4−] across both trials (Figure 4). This relationship, however, was left shifted during trial 2 compared to trial 1. In light of the unaltered relationship between Qtw and [Pi] between trials, this finding suggests that H2PO4− is not the only form of Pi causing peripheral fatigue during exercise and emphasizes the importance of total [Pi]. Overall, the findings of the current study are consistent with the growing body of literature suggesting intramuscular Pi as the primary cause, or ‘maker’, and H+ as a ‘marker’ of peripheral fatigue during exercise (Westerblad, 2021).
Intramuscular H+, Pi, and central fatigue
In contrast to our findings related to peripheral fatigue, intramuscular [H+], but not [Pi], was related to VA and thus the development of central fatigue (Figure 5). Given the etiology of central fatigue and the kinetics of both metabolites within the exercising muscle, the relationship between VA and H+ (and presumably the lack of a relationship with Pi) is likely mediated by feedback from metabosensitive group III/IV muscle afferents (Hureau et al., 2018; Amann et al., 2020). Specifically, in addition to mechanical stimuli, group III/IV muscle afferents are activated by metabolic perturbations, such as increases in H+, within their receptive field (i.e., the interstitial space of skeletal muscle). These sensory neurons project to the CNS and limit motoneuronal output and VA (i.e., facilitate central fatigue) by inhibiting voluntary descending drive ‘upstream’ of the motor cortex and/or by compromising the responsiveness of motor cortical cells (Sidhu et al., 2018; Amann et al., 2020). Importantly, while protons are transported out of the muscle fiber and into the interstitial space (Steinhagen et al., 1976; Victor et al., 1988; Juel, 1996) where they trigger group III/IV muscle afferents, Pi remains, with few exceptions (Meyer & Adams, 1990; Lewis et al., 2019b, a), confined within the muscle fiber and may therefore only have little effect on raising central fatigue via muscle afferent feedback.
It should be mentioned that in addition to H+, other exercise-induced metabolic byproducts, which have previously been linked with peripheral fatigue (e.g., K+ or reactive oxygen species) (Allen et al., 2008), also accumulate within the interstitial space and stimulate metabosensitive group III/IV muscle afferents (Rotto & Kaufman, 1988; Light et al., 2008; Delliaux et al., 2009). Taken together, as intramuscular [Pi] and [H+] typically change in tandem, but Pi is confined to the muscle while protons also rise within the interstitial space, these findings suggest muscle acidosis as an important contributor to the development of central fatigue during exercise.
It is important to emphasize that the generalizability of the current findings is limited to healthy, young males. Previous studies documenting significant differences in the exercise-induced development of neuromuscular fatigue between healthy young and old individuals (Christie et al., 2011) and between females and males (Hunter, 2014) bound this initial investigation to be limited to a single population. Future studies are needed to evaluate the potential for significant sex- and age-related differences.
Conclusion
Based on the use of 31P-MRS and the simultaneous quantification of neuromuscular function throughout two fatiguing exercise trials, this study aimed to assess the relationship between intramuscular metabolites and peripheral and central fatigue in exercising humans. The findings further support intramuscular Pi, but not H+, as a primary cause of peripheral fatigue and suggest muscle contraction-induced increases in H+ as a contributor to central fatigue during exercise.
Supplementary Material
KEY POINTS.
We investigated the relationship between intramuscular metabolites and neuromuscular function in humans performing 2 maximal, intermittent, knee-extension trials interspersed with 5min of rest.
Concomitant measurements of intramuscular hydrogen (H+) and inorganic phosphate (Pi) concentrations, and quadriceps twitch-force (Qtw) and voluntary activation (VA), were made throughout each trial using 31P-MRS and electrical femoral nerve stimulations.
While [Pi] fully recovered prior to the onset of the second trial, [H+] did not.
Qtw was strongly related to both [H+] and [Pi] across both trials. However, the relationship between Qtw and [H+] shifted leftward from the first to the second trial, while the relationship between Qtw and [Pi] remained unaltered. VA was related to [H+], but not [Pi], across both trials.
These in-vivo findings support the hypotheses of intramuscular Pi as a primary cause of peripheral fatigue, and muscle acidosis, likely acting on group III/IV muscle afferents, as a contributor to central fatigue.
Funding
This study was supported by the National Heart, Lung, and Blood Institute (HL-139451, and HL-125756) and the Veterans Affairs Rehabilitation Research and Development (E3343-R) and Clinical Science Research and Development (IK2CX002114).
Biographies

Thomas J. Hureau, Ph.D., is currently an Assistant Professor in the Faculty of Sport Sciences at the University of Strasbourg (France) after completing a postdoctoral fellowship at the University of Utah Vascular Research Laboratory (USA). Using an integrative physiology approach, his research is focused on the etiology of neuromuscular fatigue during exercise in health and disease.

Ryan M. Broxterman, Ph.D., is currently an Assistant Professor in the Division of Geriatrics at the University of Utah and the Geriatric Research, Education, and Clinical Center (GRECC) at the Salt Lake City VA Medical Center. Dr. Broxterman’s research focuses on the integration of vascular, metabolic, and neuromuscular function as determinants of exercise capacity and tolerance in health and disease.
Footnotes
Competing interest
The authors declare no conflict of interest.
Data availability statement
All data supporting the results are presented in the manuscript.
References
- Adams GR, Fisher MJ & Meyer RA. (1991). Hypercapnic acidosis and increased H2PO4- concentration do not decrease force in cat skeletal muscle. Am J Physiol 260, C805–812. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD & Westerblad H. (2008). Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88, 287–332. [DOI] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF & Dempsey JA. (2009). Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. The Journal of physiology 587, 271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Wan HY, Thurston TS, Georgescu VP & Weavil JC. (2020). On the Influence of Group III/IV Muscle Afferent Feedback on Endurance Exercise Performance. Exerc Sport Sci Rev 48, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blain GM, Mangum TS, Sidhu SK, Weavil JC, Hureau TJ, Jessop JE, Bledsoe AD, Richardson RS & Amann M. (2016). Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J Physiol 594, 5303–5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxterman RM, Layec G, Hureau TJ, Amann M & Richardson RS. (2017). Skeletal muscle bioenergetics during all-out exercise: mechanistic insight into the oxygen uptake slow component and neuromuscular fatigue. J Appl Physiol (1985) 122, 1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie A, Snook EM & Kent-Braun JA. (2011). Systematic review and meta-analysis of skeletal muscle fatigue in old age. Med Sci Sports Exerc 43, 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R, Franks K, Luciani GB & Pate E. (1988). The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. The Journal of physiology 395, 77–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG & Wilkie DR. (1978). Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature 274, 861–866. [DOI] [PubMed] [Google Scholar]
- Debold EP, Walcott S, Woodward M & Turner MA. (2013). Direct observation of phosphate inhibiting the force-generating capacity of a miniensemble of Myosin molecules. Biophys J 105, 2374–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degroot M, Massie BM, Boska M, Gober J, Miller RG & Weiner MW. (1993). Dissociation of [H+] from fatigue in human muscle detected by high time resolution 31P-NMR. Muscle & nerve 16, 91–98. [DOI] [PubMed] [Google Scholar]
- Delliaux S, Brerro-Saby C, Steinberg JG & Jammes Y. (2009). Reactive oxygen species activate the group IV muscle afferents in resting and exercising muscle in rats. Pflugers Arch 459, 143–150. [DOI] [PubMed] [Google Scholar]
- Fitts RH. (2016). The Role of Acidosis in Fatigue: Pro Perspective. Med Sci Sports Exerc 48, 2335–2338. [DOI] [PubMed] [Google Scholar]
- Fryer MW, West JM & Stephenson DG. (1997). Phosphate transport into the sarcoplasmic reticulum of skinned fibres from rat skeletal muscle. J Muscle Res Cell Motil 18, 161–167. [DOI] [PubMed] [Google Scholar]
- Gadian DG. (1982). Nuclear magnetic resonance and its applications to living systems. Oxford University Press. [Google Scholar]
- Gandevia SC. (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81, 1725–1789. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E & Nordesjö LO. (1974). Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scandinavian journal of clinical and laboratory investigation 33, 109–120. [PubMed] [Google Scholar]
- Hunter SK. (2014). Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol (Oxf) 210, 768–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hureau TJ, Romer LM & Amann M. (2018). The 'sensory tolerance limit': A hypothetical construct determining exercise performance? Eur J Sport Sci 18, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hureau TJ, Weavil JC, Thurston TS, Wan HY, Gifford JR, Jessop JE, Buys MJ, Richardson RS & Amann M. (2019). Pharmacological attenuation of group III/IV muscle afferents improves endurance performance when oxygen delivery to locomotor muscles is preserved. Journal of applied physiology (Bethesda, Md : 1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel C (1996). Lactate/proton co-transport in skeletal muscle: regulation and importance for pH homeostasis. Acta Physiol Scand 156, 369–374. [DOI] [PubMed] [Google Scholar]
- Kennedy DS, McNeil CJ, Gandevia SC & Taylor JL. (2014). Fatigue-related firing of distal muscle nociceptors reduces voluntary activation of proximal muscles of the same limb. Journal of applied physiology (Bethesda, Md : 1985) 116, 385–394. [DOI] [PubMed] [Google Scholar]
- Knuth ST, Dave H, Peters JR & Fitts RH. (2006). Low cell pH depresses peak power in rat skeletal muscle fibres at both 30 degrees C and 15 degrees C: implications for muscle fatigue. J Physiol 575, 887–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewski B & Rossiter HB. (2020). Exceeding a "critical" muscle Pi: implications for [Formula: see text] and metabolite slow components, muscle fatigue and the power-duration relationship. Eur J Appl Physiol 120, 1609–1619. [DOI] [PubMed] [Google Scholar]
- Kufel TJ, Pineda LA & Mador MJ. (2002). Comparison of potentiated and unpotentiated twitches as an index of muscle fatigue. Muscle Nerve 25, 438–444. [DOI] [PubMed] [Google Scholar]
- Lanza IR, Wigmore DM, Befroy DE & Kent-Braun JA. (2006). In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol 577, 353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layec G, Bringard A, Vilmen C, Micallef JP, Fur YL, Perrey S, Cozzone PJ & Bendahan D. (2008). Accurate work-rate measurements during in vivo MRS studies of exercising human quadriceps. Magma (New York, NY) 21, 227–235. [DOI] [PubMed] [Google Scholar]
- Le Fur Y, Nicoli F, Guye M, Confort-Gouny S, Cozzone PJ & Kober F. (2010). Grid-free interactive and automated data processing for MR chemical shift imaging data. Magma (New York, NY) 23, 23–30. [DOI] [PubMed] [Google Scholar]
- Lewis MT, Kasper JD, Bazil JN, Frisbee JC & Wiseman RW. (2019a). Quantification of Mitochondrial Oxidative Phosphorylation in Metabolic Disease: Application to Type 2 Diabetes. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MT, Kasper JD, Bazil JN, Frisbee JC & Wiseman RW. (2019b). Skeletal muscle energetics are compromised only during high-intensity contractions in the Goto-Kakizaki rat model of type 2 diabetes. Am J Physiol Regul Integr Comp Physiol 317, R356–R368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z & Lee J. (2008). Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100, 1184–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massie BM, Conway M, Yonge R, Frostick S, Sleight P, Ledingham J, Radda G & Rajagopalan B. (1987). 31P nuclear magnetic resonance evidence of abnormal skeletal muscle metabolism in patients with congestive heart failure. The American journal of cardiology 60, 309–315. [DOI] [PubMed] [Google Scholar]
- McDowell MA, Fryar CD, Ogden CL & Flegal KM. (2008). Anthropometric reference data for children and adults: United States, 2003–2006. Natl Health Stat Report, 1–48. [PubMed] [Google Scholar]
- Merton PA. (1954). Voluntary strength and fatigue. J Physiol 123, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer RA & Adams GR. (1990). Stoichiometry of phosphocreatine and inorganic phosphate changes in rat skeletal muscle. NMR Biomed 3, 206–210. [DOI] [PubMed] [Google Scholar]
- Nelson CR, Debold EP & Fitts RH. (2014). Phosphate and acidosis act synergistically to depress peak power in rat muscle fibers. American journal of physiology Cell physiology 307, C939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newham DJ & Cady EB. (1990). A 31P study of fatigue and metabolism in human skeletal muscle with voluntary, intermittent contractions at different forces. NMR Biomed 3, 211–219. [DOI] [PubMed] [Google Scholar]
- Nosek TM, Fender KY & Godt RE. (1987). It is diprotonated inorganic phosphate that depresses force in skinned skeletal muscle fibers. Science (New York, NY) 236, 191–193. [DOI] [PubMed] [Google Scholar]
- Posterino GS & Fryer MW. (1998). Mechanisms underlying phosphate-induced failure of Ca2+ release in single skinned skeletal muscle fibres of the rat. J Physiol 512 (Pt 1), 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotto DM & Kaufman MP. (1988). Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol (1985) 64, 2306–2313. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Weavil JC, Thurston TS, Rosenberger D, Jessop JE, Wang E, Richardson RS, McNeil CJ & Amann M. (2018). Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. J Physiol 596, 4789–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen C, Hirche HJ, Nestle HW, Bovenkamp U & Hosselmann I. (1976). The interstitial pH of the working gastrocnemius muscle of the dog. Pflugers Archiv : European journal of physiology 367, 151–156. [DOI] [PubMed] [Google Scholar]
- Sundberg CW, Prost RW, Fitts RH & Hunter SK. (2019). Bioenergetic basis for the increased fatigability with ageing. J Physiol 597, 4943–4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Amann M, Duchateau J, Meeusen R & Rice CL. (2016). Neural Contributions to Muscle Fatigue: From the Brain to the Muscle and Back Again. Med Sci Sports Exerc 48, 2294–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillin NA & Bishop D. (2009). Factors modulating post-activation potentiation and its effect on performance of subsequent explosive activities. Sports Med 39, 147–166. [DOI] [PubMed] [Google Scholar]
- Vanhamme L, van den Boogaart A & Van Huffel S. (1997). Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. Journal of magnetic resonance (San Diego, Calif : 1997) 129, 35–43. [DOI] [PubMed] [Google Scholar]
- Victor RG, Bertocci LA, Pryor SL & Nunnally RL. (1988). Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. The Journal of clinical investigation 82, 1301–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner MW, Moussavi RS, Baker AJ, Boska MD & Miller RG. (1990). Constant relationships between force, phosphate concentration, and pH in muscles with differential fatigability. Neurology 40, 1888–1893. [DOI] [PubMed] [Google Scholar]
- Westerblad H (2016). Acidosis Is Not a Significant Cause of Skeletal Muscle Fatigue. Med Sci Sports Exerc 48, 2339–2342. [DOI] [PubMed] [Google Scholar]
- Westerblad H (2021). Biochemical mechanisms of muscle fatigue. In Fatigue as a limitation to performance symposium. Madrid, Spain. [Google Scholar]
- Wilson JR, McCully KK, Mancini DM, Boden B & Chance B. (1988). Relationship of muscular fatigue to pH and diprotonated Pi in humans: a 31P-NMR study. J Appl Physiol (1985) 64, 2333–2339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the results are presented in the manuscript.