Abstract
Group 3 innate lymphoid cells (ILC3s) produce interleukin (IL)-22 and orchestrate with other cells in the gut, to mount productive host immunity against bacterial infection. However, the role of ILC3s in Salmonella enterica serovar Typhimurium (S. Typhimurium) infection, which causes foodborne enteritis in humans, remains elusive. Here, we show that S. Typhimurium exploits ILC3-produced IL-22 to promote its infection in mice. Specifically, S. Typhimurium secretes flagellin through activation of the TLR5-Myd88-IL-23 signaling pathway in antigen presenting cells (APCs) to selectively enhance IL-22 production by ILC3s, but not T cells. Deletion of ILC3s but not T cells in mice leads to better control of S. Typhimurium infection. We also show that S. Typhimurium can invade ILC3s directly and cause caspase-1-mediated ILC3 pyroptosis independently of flagellin. Genetic ablation of Casp1 in mice leads to increased ILC3 survival and IL-22 production, and enhanced S. Typhimurium infection. Collectively, our data suggest a key host defense mechanism against S. Typhimurium infection via induction of ILC3 death to limit intracellular bacteria and reduce IL-22 production.
Introduction
Salmonella enterica serovar Typhimurium (hereafter referred to as S. Typhimurium) is a facultative intracellular pathogen that actively invades and replicates within host cells, which can cause both gastrointestinal and systemic diseases1,2. S. Typhimurium hijacks the host signaling pathways via its secreted virulence effectors during infection1,2, which are mainly delivered by the two Type III secretion systems (T3SSs) encoded by the Salmonella pathogenicity islands (SPI), SPI-1 and SPI-22,3. In addition to the SPI-1 and SPI-2, the flagella system is also considered as a T3SS and responsible for flagellin delivery and assembly on the exterior surface4. S. Typhimurium flagellin includes the conserved N- and C-terminal D0 and D1 domains, and a central hypervariable region (D2 and D3 domains)5. It can trigger either extracellular immune signaling via Toll-like receptor 5 (TLR5) by binding of the D1 domain6, or cytoplasmic NLRC4 (NLR family caspase recruitment domain-containing 4) inflammasome activation and assembly via NAIP 5/6 (NLR family apoptosis-inhibitory protein) by binding of the D0 domain5,7.
During S. Typhimurium infection, the host immune system mounts a quick response through producing specific cytokines, e.g., interferon gamma (IFN-γ), to defend against these invaders8. However, IL-22, one of the most upregulated cytokines in the gut 9, enhances S. Typhimurium colonization at mucosal surfaces by mechanisms involving induction of antimicrobial proteins to inhibit the growth of commensal microbes that otherwise compete against S. Typhimurium 9,10.
Innate lymphoid cells (ILCs) include group 1 ILCs expressing transcription factor (TF) T-bet (ILC1s, including natural killer (NK) cells and ILC1 with or without expression of TF Eomes, respectively) and producing IFN-γ; group 2 ILCs (ILC2s) expressing TF GATA3 and producing IL-5 and IL-13; and group 3 ILCs (ILC3s) expressing TF RORγt and producing IL-22 and/or IL-17A (in short, IL-17 hereafter)11. ILCs play important roles in tissue homeostasis and host defense during infection, particularly at barrier surfaces like the intestine11. It is known that IFN-γ produced by ILC1s is important for controlling Salmonella during early infection8. Given that ILC3s can also produce IFN-γ, it was suggested that ILC3s could control Salmonella infection as well12. However, this notion requires experimental validation.
Pyroptosis is a major form of programmed host cell death triggered by infection, and is primarily mediated by the activation of caspase-1, a canonical inflammasome-dependent pro-inflammatory caspase13. Pyroptosis process is best studied in myeloid cells during infection13,14. Recently, caspase-1-induced pyroptosis was also shown to be the major cell death mechanism of CD4+ T cells during HIV infection15. Nevertheless, pyroptosis in ILCs during infection has not been reported.
In this study, we investigate the role of ILC3s during S. Typhimurium infection. S. Typhimurium promotes IL-22 production in ILC3s activated by antigen presenting cells (APCs) through the flagellin-TLR5-MyD88 pathway, and IL-22 facilitates S. Typhimurium colonization, thus uncovering a Salmonella hijacking mechanism of host innate immune system. Notably, S. Typhimurium invasion also elicits a host defense mechanism involving activation of the caspase-1-mediated pyroptosis in ILC3s. Genetic depletion of ILC3s leads to decreased S. Typhimurium infection while knockout of Casp1 in ILC3s enhances infection severity. Thus, our data suggest a co-evolving interaction between Salmonella and ILC3s in establishing infection and immunity.
Results
Distinct ILC responses during early S. Typhimurium infection
IFN-γ produced by ILC1s is critical for controlling early Salmonella infection8. However, the role of other ILCs, including ILC3s during this process is unclear. To this end, we orally challenged the cohoused, age-matched, and streptomycin-pretreated C57BL/6 (WT), Rag1−/− (lack of adaptive immune system), and Rag2 and Il2 receptor common gamma chain double-knockout (Rag2−/−γc−/−) (lack of both innate and adaptive lymphocytes) mice with S. Typhimurium wild-type SL1344 experimental strain that was engineered to be streptomycin-resistant16 (Extended Data Fig. 1a). Although Rag1−/− mice exhibited similar body weight loss and survival (Fig. 1a,1b) to WT mice, Rag2−/−γc−/− mice were more susceptible to S. Typhimurium infection, evidenced by more body weight loss and mortality (Fig. 1a,1b), suggesting a protective role of common gamma chain-dependent ILCs during S. Typhimurium infection.
Fig. 1. Distinct ILC responses during early S. Typhimurium infection.
a and b, C57BL/6 WT, Rag1−/−, and Rag2−/−γc−/− streptomycin pretreated mice were orally inoculated with 2 × 107 CFUs of S. Typhimurium SL1344 and assessed for survival following infection. Body weight change (a) and survival curve (b) are compiled data of two independent experiments (C57BL/6 WT, n = 10 mice; Rag1−/−, n = 7 mice; Rag2−/−γc−/−, n = 10 mice). The log-rank test for statistical analysis of survival curve in (b). c–h, FACS analysis of Eomes (c) and IFN-γ (f) expression after gating on group 1 ILCs (Lin−RORγt−NKp46+NK1.1+) in the large intestine (LI) of uninfected and S. Typhimurium-infected WT mice on day 2 post-infection. c and f are representative of four independent experiments. Shown are percentages (d) and absolute cell numbers (e) of Eomes− (ILC1) and Eomes+ (NK) cells in group 1 ILCs, and percentages (g) and absolute cell numbers (h) of IFN-γ+ cells in ILC1 and NK cells. (d, e, g and h) are compiled data from four independent experiments (n = 6 mice for uninfected group; n = 7 mice for infected group). i, Ifng mRNA determined by qRT-PCR in LI LPLs of uninfected and S. Typhimurium-infected C57BL/6 WT mice on day 2 post-infection. Data are representative of two independent experiments (n = 3 mice for uninfected group; n = 4 mice for infected group). j, FACS analysis of GATA3 expression after gating on Lin− in the LI of uninfected and S. Typhimurium-infected WT mice. Data are representative of three independent experiments. See also Extended Data Fig. 1.
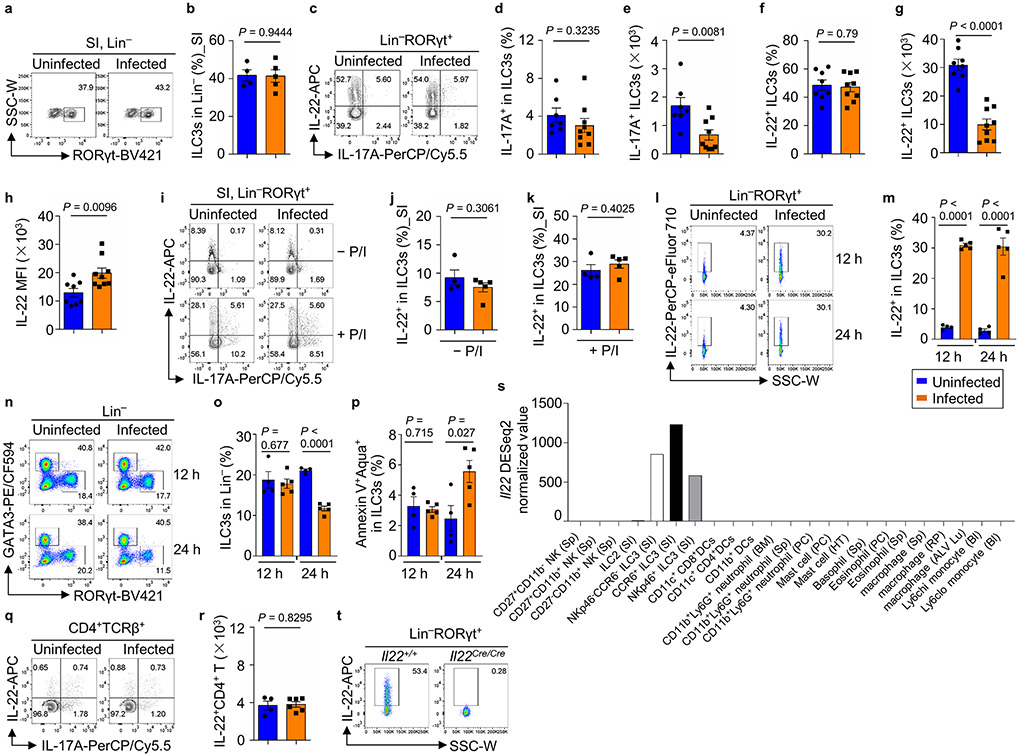
We examined different ILC responses in the large intestine (LI) lamina propria of WT mice at day 2 post-infection. ILC1 (Lin−RORγt−NKp46+NK1.1+Eomes−) percentage and cell number decreased, while NK (Lin−RORγt−NKp46+NK1.1+Eomes+) cell percentage and number increased upon infection, suggesting a preferential expansion of NK cells at the expense of ILC1 (Fig. 1c-1e). IFN-γ+ ILC1s (ILC1 and NK cells) were markedly enhanced in the LI after infection (Fig. 1f-1h). Consistently, the mRNA of Ifng in the LI was also upregulated following infection (Fig. 1i). Notably, the frequency, absolute cell number, and proliferation (revealed by Ki-67 staining) of ILC2s did not have significant changes (Fig. 1j, and Extended Data Fig. 1b-1d). However, ILC2-produced cytokines (i.e., IL-5 and IL-13) were reduced in mice after S. Typhimurium infection (Extended Data Fig. 1d), consistent with the fact that S. Typhimurium infection predominantly induces a type 1 immune response (Fig. 1f-1i). Moreover, the phenotypes mentioned above were only observed in the LI but not the small intestine (SI) (Extended Data Fig. 1e-1j), in agreement with the fact that severe inflammation was mostly observed in the LI instead of the SI during S. Typhimurium infection in the streptomycin-pretreated mouse model of colitis17.
Various subsets of ILC3s, including NKp46+, CD4+, and CD4− ILC3s, were all reduced in the LI but not in the SI (Fig. 2a-2i, and Extended Data Fig. 2a,2b). In contrast to abundant production by ILC1s, limited amounts of IFN-γ were produced by ILC3s with or without infection (Fig. 2j-2l). Furthermore, while IL-17 has been reported to play a protective role in S. Typhimurium infection18, there was no induction of IL-17 in ILC3s during early infection (Extended Data Fig. 2c-2e). Together, these data suggest that S. Typhimurium infection induces distinct ILC1 and ILC3 responses characterized by production of IFN-γ and IL-22, respectively.
Fig. 2. IL-22+ ILC3s are specifically induced in early S. Typhimurium infection.
a–i, FACS analysis of RORγt, NKp46 and CD4 expression after gating on Lin− or Lin−NKp46− cells in LI-LPLs of uninfected or S. Typhimurium-infected WT mice on day 2 post-infection. Representative data of four independent experiments (a). Percentages and absolute numbers of ILC3s (b and c), NKp46+ ILC3s (d and e), CD4+ ILC3s (f and g), and CD4− ILC3s (h and i). Compiled data from four independent experiments (b, d, f, h, n = 8 and 9 mice for uninfected and infected groups, respectively; c, e, g, i, n = 6 and 8 mice for uninfected and infected groups, respectively) (b–i). j–l, IFN-γ and RORγt expression in LI ILC3s of uninfected or S. Typhimurium-infected WT mice on day 2 post-infection. Representative data of three independent experiments (j). Percentages (k) and absolute numbers (l) of IFN-γ+ ILC3s are shown. Compiled data from three independent experiments (n = 6 and 7 mice for uninfected and infected groups, respectively) (k and l). m–p, IL-22 and IL-17 expression in the LI ILC3s of uninfected or S. Typhimurium-infected WT mice on day 2 post-infection. Representative data of two independent experiments (m). Percentages (n), absolute numbers (o) and MFI (p) of IL-22+ ILC3s (no P/I stimulation) are shown. Compiled data from two independent experiments (n = 4 and 5 mice for uninfected and infected groups, respectively) (n–p). q, Il22 mRNA in LI-LPLs of uninfected or S. Typhimurium-infected WT mice on day 2 post-infection. Complied data of three independent experiments (n = 3 and 4 mice for uninfected and infected groups, respectively). r–t, mRNA in LI tissue of uninfected or S. Typhimurium-infected WT mice on day 2 post-infection. Il22 (r, per group: WT: n = 5 mice; Rag1−/−: n = 3 mice; Rag2−/−γc−/−: n = 3 mice), Reg3g (s, per group: WT: n = 3 mice) and Reg3b (t, per group: WT: n = 3 mice). Compiled data from two independent experiments. u, S. Typhimurium CFUs in feces of Il22+/+Rag1−/− (n = 7) and Il22Cre/CreRag1−/− (n = 6) mice on day 4 post-infection. Compiled data from two independent experiments. See also Extended Data Fig. 2-3.
IL-22+ ILC3s are induced in early S. Typhimurium infection
Despite of total ILC3 reduction, IL-22-producing LI ILC3s were markedly increased after S. Typhimurium infection (Fig. 2m-2o). Of note, in vitro PMA and ionomycin stimulation (+ P/I) significantly enhanced IL-22 expression by ILC3s from uninfected mice, and no further increase was observed after S. Typhimurium infection (Extended Data Fig. 2c,2f and 2g). IL-22 protein expression in ILC3s as indicated by mean fluorescence intensity (MFI) of flow cytometry (FACS) and mRNA expression as determined by qRT-PCR, were also increased in the LI lamina propria lymphocytes (LPLs) (Fig. 2p,2q, and Extended Data Fig. 2h-2k).
Remarkably, the increase of IL-22+ ILC3s was observed as early as 12 h post-infection while the ILC3 cell number and survival were not reduced until 24 h post-infection (Extended Data Fig. 2l-2p), suggesting a prompt induction of IL-22 in ILC3s followed by the reduction of ILC3 cell number after infection. Together, these data demonstrate that while other populations of ILC3s were reduced after S. Typhimurium infection, there was a selective induction of IL-22+ ILC3s.
S. Typhimurium infection does not induce IL-22+ CD4+ T cells
There was no significant induction of IL-22 in CD4+ T cells at day 2 post-infection (Extended Data Fig. 2q,2r), in accordance with a dispensable role of adaptive immunity for disease development (Fig. 1a,1b). In the LI tissue of Rag1−/− and WT mice, S. Typhimurium significantly induced the expression of Il22, while its expression was ablated in Rag2−/−γc−/− mice. In contrast to ILC3s, ILC1s, ILC2s, and myeloid cells had little or no expression of Il22 at least at the steady state (Extended Data Fig. 2s). Together, these data suggest that ILC3s are the predominant source of IL-22 during early S. Typhimurium infection (Fig. 2r).
Total lethality of mice at day 8 with 2–3 × 107 colony-forming units (CFUs) of S. Typhimurium challenge prevented us from carefully assessing adaptive immunity. Thus, we determined the host immune responses with a lower infection dosage (2–3 × 103 CFUs) at day 8 in mice that were still alive but with notable development of colitis and loss of body weight (Extended Data Fig. 3a,3p). The percentage and number of ILC1s and ILC3s declined after S. Typhimurium infection (Extended Data Fig. 3b-3h). In contrast to day 2, IFN-γ+ ILC1s were markedly down-regulated (Extended Data Fig. 3i,3j), while IFN-γ+ CD4+ T cells were increased but without statistical significance at day 8 (Extended Data Fig. 3i,3k). IL-22+ ILC3s were markedly reduced at day 8 after infection (Extended Data Fig. 3l,3m). IL-22+ CD4+ T cells were also low at day 8 (Extended Data Fig. 3n,3o). Together, these data suggest a state of host immune suppression, presumably due to uncontrolled infection. Consistent with the dispensable role of adaptive immunity (Fig. 1a,1b), Rag1−/− mice showed similar disease severity to WT mice after low dosage of S. Typhimurium infection, as evidenced by weight loss and fatality (Extended Data Fig. 3p,3q).
Innate IL-22 facilitates S. Typhimurium infection
IL-22 is important for host defense against bacterial infection via inducing gut epithelial cell production of antimicrobial proteins19,20. Indeed, the expression of antimicrobial genes Reg3g and Reg3b in the LI tissues were upregulated upon S. Typhimurium infection (Fig. 2s,2t), consistent with the enhancement of IL-22 produced by ILC3s. Paradoxically, IL-22 was also shown to be deployed by S. Typhimurium to cause host dysbiosis and thus facilitates its colonization through competition with the gut commensal microbiota9,10,21. Thus, we determined the bacterial CFUs in the feces and various organs (i.e., LI, liver, spleen and mesenteric lymph nodes (mLNs)) of WT and Il22Cre/Cre (22) (a.k.a Il22−/−, Extended Data Fig. 2t) mice infected with S. Typhimurium at different time points. Bacterial CFUs were significantly lower in the feces, as well as the tissues of LI, liver, and spleen of S. Typhimurium-infected Il22Cre/Cre mice compared with those in controls, especially at day 4 post-infection (Extended Data Fig. 3r-3v), supporting a pro-Salmonella role for IL-22. Further ablation of IL-22 expression in Rag1-deficient mice (Il22Cre/CreRag1−/−) led to less bacterial counts in feces after S. Typhimurium infection, compared with the Il22+/+Rag1−/− littermate control mice (Fig. 2u), demonstrating that innate IL-22 benefits S. Typhimurium infection. Collectively, our data support a model that ILC3s were specifically induced to produce IL-22 that is pro-Salmonella infection in the LI.
Salmonella-secreted proteins enhance IL-22+ ILC3s in vitro
We hypothesized that Salmonella-released components might be responsible for induction of ILC3-produced IL-22. To this end, LI-LPLs were isolated from WT mice and incubated for 5 hours in vitro in the presence of wild-type S. Typhimurium SL1344 cell-free culture supernatant (Extended Data Fig. 4a). Remarkably, the S. Typhimurium supernatant in a dose-dependent manner significantly enhanced IL-22 but not IL-17 production by ILC3s in LPLs (Extended Data Fig. 4b-4d). In contrast, IL-22 production by CD4+ T cells, and IFN-γ production by CD4+ T cells and ILC1s (ILC1 and NK cells), were unchanged upon the bacterial supernatant treatment (Extended Data Fig. 4e-4i). The mRNA amounts of Il22, but not Il17α, detected by qRT-PCR, were also significantly augmented in LPLs co-incubated with the bacterial supernatant (Extended Data Fig. 4j,4k). Furthermore, treatment of bacterial culture supernatant by proteinase K (PK) markedly diminished its ability to enhance ILC3-produced IL-22 in LPLs, as did heat treatment (Extended Data Fig. 4l-4q). Together, these data suggest that the protein (s) in S. Typhimurium SL1344 supernatant are responsible for IL-22 enhancement in ILC3s.
Salmonella flagellin protein promotes IL-22+ ILC3s
SPI-1 and SPI-2, are known to inject virulence factors (effectors) into eukaryotic cells to interfere with host signaling transduction pathways, thereby affecting host cell survival and proliferation1,2. Notably, the bacterial supernatants from various pathogenic S. Typhimurium strains with different virulence (SL1344 and UK-1)23 exhibited similar capability to enhance IL-22 production by ILC3s (Extended Data Fig. 4c,5a,5b compare –P/I condition). To determine whether the protein components in S. Typhimurium supernatant were the virulence effectors secreted by T3SSs, we employed the single or double knockout of SPI-1 and SPI-2 S. Typhimurium mutants in the UK-1 background24. We collected culture supernatants of these mutants and determined their activity on ILC3-produced IL-22 in LPL-bacterial supernatant culture system. Deletion one or both of T3SSs still promoted IL-22 production by ILC3s (Extended Data Fig. 5a,5b). However, size exclusion data showed that small molecules from the supernatants less than 3 kDa failed to enhance IL-22 (Extended Data Fig. 5b), suggesting that the protein component (s) secreted or released by secretion systems independent of T3SSs in S. Typhimurium are responsible for induction of IL-22+ ILC3s.
The flagella of Salmonella are another primary secretion machinery associated with the bacterial virulence and sensed by the host extracellular TLR54,5. S. Typhimurium possesses two genes (fliC and fljB) to alternate expression of flagellin between two antigenically different proteins, FliC and FljB, in a process known as flagellar phase variation. They elicit similar host immune responses and usually only one of the flagellins is produced at a time in a given cell25,26. Notably, in contrast to the single fliC or fljB gene-deficient mutant, or the deletion of O-antigen of lipopolysaccharide (LPS) ΔwaaL46 mutant (Fig. 3a-3c), bacterial supernatant from S. Typhimurium UK-1 lacking both flagellin genes (ΔfliC/fljB) failed to upregulate IL-22 production by ILC3s. Furthermore, purified flagellin protein (FliC) encoded by fliC from S. Typhimurium boosted IL-22 production by ILC3s (Fig. 3d-3f), and similar enhancement was also observed using flagellin protein (FljB) encoded by fljB (Extended Data Fig. 5c,5d). Together, these data indicate that S. Typhimurium flagellin promotes ILC3 production of IL-22.
Fig. 3. S. Typhimurium flagellin protein promotes IL-22+ ILC3s.
a–c, FACS analysis of IL-22 and IL-17 expression after gating on ILC3s (Lin−RORγt+) in LI-LPLs co-incubated with bacterial supernatants of different S. Typhimurium mutants (no P/I stimulation). Representative data of three experiments (a). Percentages of IL-22+ cells (b) and IL-22 MFI (c) in ILC3s are shown. Compiled data from three independent experiments (n = 5 repeats with independent mouse LPLs) (b). Compiled data from two independent experiments (n = 3 repeats with independent mouse LPLs) (c). d–f, FACS analysis of IL-22 and IL-17 expression after gating on ILC3s from LI-LPLs co-incubated with purified S. Typhimurium flagellin protein (no P/I stimulation). Representative data of two independent experiments (d). Percentages of IL-22+ cells (e) and IL-22 MFI (f) in ILC3s are shown. Compiled data from two independent experiments (n = 3 repeats with independent mouse LPLs) (e and f). g, Schematic depiction of experimental design. h–j, FACS analysis of IL-22 and IL-17 expression in sorted ILC3s (no P/I stimulation) co-incubated with S. Typhimurium culture supernatant, with or without the addition of CD11c+ APCs from Tlr5+/+ or Tlr5−/− mice. Representative data of two independent experiments (h). Percentages of IL-22+ cells (i) and IL-22 MFI (j) in ILC3s are shown. Compiled data of two independent experiments (n = 3 repeats with independently sorted LI cells from mice with indicated genotypes) (i and j). k and l, Il23a p19 and Il12a p35 mRNA determined by qRT-PCR in LI-LPLs of uninfected or infected WT mice on day 2 post-infection (k) and in LI-LPLs treated with empty medium (MgM) or with 15 μl or 45 μl of S. Typhimurium culture supernatant (l). Data are representative of three independent experiments (k, n = 3 mice for uninfected group, n = 4 mice for infected group; l, n = 5 repeats with independent mouse LPLs). See also Extended Data Fig. 4-6.
CD11c+ APCs induce IL-22+ ILC3s by engagement of TLR5-MyD88
Notably, the bacterial supernatant induced minimal amounts of IL-22 production by FACS-purified ILC3s from LPLs (Fig. 3g-3j), suggesting that S. Typhimurium culture supernatant does not directly activate ILC3s. Indeed, co-culture of ILC3s with WT CD11c+ APCs but not Tlr5−/− CD11c+ APCs markedly enhanced IL-22 production (Fig. 3h-3j). Tlr5−/− CD11c+ APCs can be activated by other TLR ligands except flagellin27, and thus our data further support the notion that flagellin is at least one of the key components in S. Typhimurium culture supernatant to activate IL-22 production from ILC3s. Consistently, purified flagellin (FliC) promoted IL-22 production by sorted ILC3s co-cultured with CD11c+ APCs from WT mice but not from Tlr5−/− mice (Extended Data Fig.5e,5f).
Genome-wide transcriptome profiling of sorted ILC3s from the LI of uninfected and S. Typhimurium-infected mice by RNA-sequencing (RNA-seq) showed that ILC3s had various expression of TLRs but minimal expression of Tlr5 mRNA compared with CD11c+ APCs (Extended Data Fig. 5g,5h), consistent with the involvement of APCs in upregulation of IL-2228. We observed increased Il23a (p19) but not Il12a (p35) mRNA in LPLs of the mice after S. Typhimurium infection (Fig, 3k) and in LPLs that were stimulated with S. Typhimurium culture supernatant in vitro (Fig. 3l), indicating the activation of IL-23-IL-22 axis during S. Typhimurium infection. Furthermore, the enhancement of Il23a (p19) mRNA expressed by CD11c+ APCs upon bacterial culture supernatant stimulation was abolished by Tlr5 deletion (Extended Data Fig. 5i), demonstrating that IL-23 production by APCs is TLR5-dependent.
We further determined the domain (s) of flagellin required for IL-22 upregulation by generating various flagellin mutants (Extended Data Fig. 6a and Supplementary Table 1). In contrast to the individual ND0/ND1 or CD1/CD0 domain of flagellin that failed to enhance IL-22, ΔD2/D3 mutant that consisted of the amino acids (aa) 1-180 fused with aa 361-495 promoted IL-22 expression by ILC3s (Extended Data Fig. 6b-6d), in line with their abilities to activate TLR5 as measured by the HEK-blue mTLR5 reporter cell lines (InvivoGen, USA) (Extended Data Fig. 6e). MyD88 plays a critical role in APCs for signaling downstream of the TLR and interleukin-1 (IL-1) receptor families29. ILC3s in Myd88-deficient LPLs had a marked reduction of IL-22 with or without bacterial supernatant stimulation (Extended Data Fig. 6f-6h), indicating an essential role of MyD88. Furthermore, we purified CD11c+ APCs from the LI of Myd88+/+ or Myd88−/− littermate mice and performed the co-culture experiment with LI ILC3s sorted from WT mice. Our data showed that flagellin in a dose-dependent manner markedly increased IL-22 production by ILC3s when co-cultured with Myd88+/+ but not with Myd88−/− CD11c+ APCs (Extended Data Fig. 6i,6j). Together, our data provide evidence that S. Typhimurium indirectly enhances IL-22 production from ILC3s by engagement of TLR5-MyD88 signaling axis in CD11c+ APCs.
ILC3s promotes S. Typhimurium infection in mice
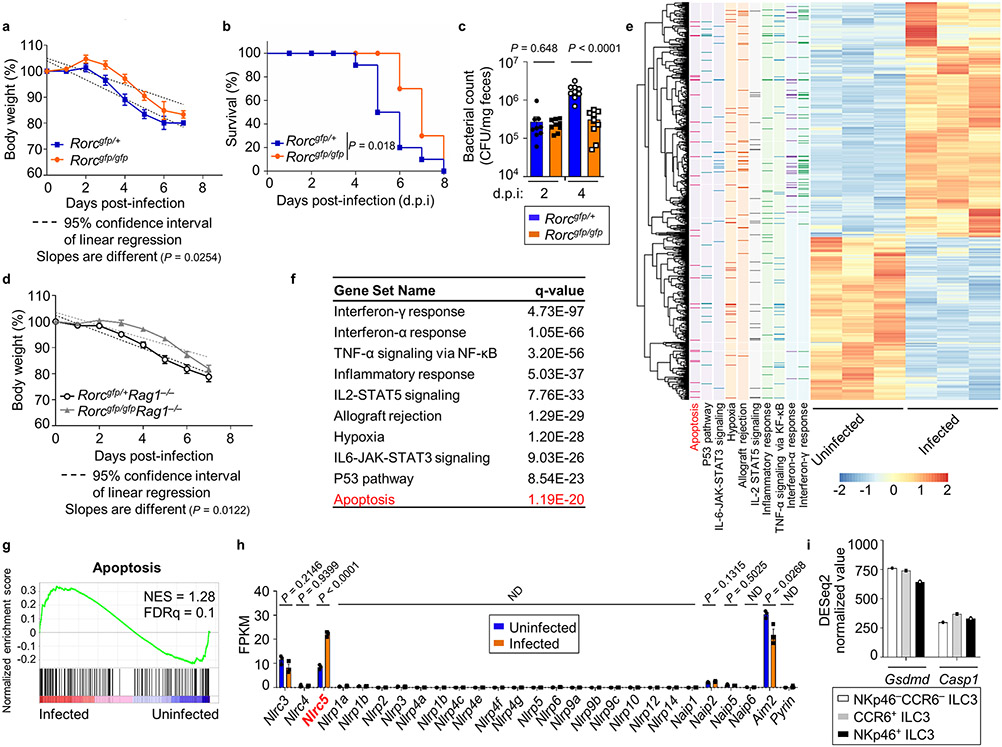
To further determine the role of ILC3s during S. Typhimurium infection, we firstly employed RORγt-deficient mice (Rorcgfp/gfp) mice to deplete ILC3s, which are known for host immunity against enteric bacterial infection by producing IL-2230,31. Notably, the body weight loss and survival were significantly improved, and lower fecal bacterial CFUs were detected in Rorcgfp/gfp mice compared with heterozygous littermate controls (Extended Data Fig. 7a-7c), indicating an unexpected role of ILC3s in promoting S. Typhimurium infection. We further generated Rorcgfp/gfpRag1−/− mice, which lacked ILC3s and adaptive immune system. Rorcgfp/+Rag1−/− mice exhibited similar percentage of weight loss and survival as WT mice after S. Typhimurium infection, consistent with the dispensable role of adaptive immunity during this process (compare Fig. 1a,1b with Fig. 4a and Extended Data Fig. 7d). However, in sharp contrast to Rag2−/−γc−/− mice that lack all ILCs and had exacerbated infection, ablation of ILC3s in Rorcgfp/gfpRag1−/− mice alleviated S. Typhimurium infection severity as evidenced by less weight loss, better survival, and less bacterial loads in feces, especially at day 4 post-infection (Fig. 4a,4b and Extended Data Fig. 7d). As expected, hydrodynamic injection of a plasmid expressing IL-22 to Rorcgfp/gfp mice increased IL-22 target gene Reg3g expression in the gut (Fig. 4c). Importantly, forced expression IL-22 in Rorcgfp/gfp mice increased S. Typhimurium counts in their feces compared with the mice receiving the control plasmid (Fig. 4d). Together, these data suggest that ILC3s, presumably by production of IL-22 facilitate S. Typhimurium infection, representing a bacterial hijacking mechanism of host immunity.
Fig. 4. Presence of ILC3s increases the susceptibility to S. Typhimurium infection in mice.
a and b, Rorcgfp/+Rag1−/− and Rorcgfp/gfpRag1−/− mice were orally inoculated with 2 × 107 CFUs of S. Typhimurium. Survival was assessed following infection, and the data are compiled from two independent experiments (Rorcgfp/+Rag1−/−: n = 10 mice; Rorcgfp/gfpRag1−/−: n = 10 mice) with the log-rank test for statistical analysis (a). Bacterial CFUs in feces were measured on day 2 and day 4 post-infection. Representative of two independent experiments and presented as CFU per mg of feces (Rorcgfp/+Rag1−/−: n = 7 mice; Rorcgfp/gfpRag1−/−: n = 6 mice) (b). c, Reg3g mRNA determined by qRT-PCR in LI tissue of Rorcgfp/gfp mice hydrodynamically injected with IL-22-expressing or control plasmids followed by S. Typhimurium infection on day 4-7 post-infection. Compiled data from two independent experiments (Rorcgfp/gfp + vector: n = 5 mice; Rorcgfp/gfp + IL-22: n = 6 mice). d, CFUs in feces of the mice treated as in (c) on day 2 and day 4 post-infection were determined. Compiled data from two independent experiments, and presented as CFU per mg of feces (Rorcgfp/gfp + vector: n = 5 mice; Rorcgfp/gfp + IL-22: n = 6 mice). e, Confocal analysis of RORγt+ ILC3s in the LI of uninfected or S. Typhimurium-infected Rag1−/− mice. Red, RORγt; Green: S. Typhimurium; Blue: DAPI. Scale bar, 50 μm. Representative data of three independent experiments. f–i, RNA-seq analysis of sorted ILC3s from the LI of uninfected or S. Typhimurium-infected WT mice (n = 3 mice per group). f, Principal component analysis (PCA) plot of the RNA-seq. g, Scatterplot of log2 (FPKM) gene expression of the RNA-seq generated with Excel. Differentially expressed genes (fold change ≥ 1.5, q value ≤ 0.05) are highlighted in red (upregulated) and blue (downregulated), respectively. h, FPKM of Caspase genes in sorted ILC3s from uninfected or S. Typhimurium-infected WT mice (n = 3 mice per group). i, FPKM of Gasdermin genes in sorted ILC3s from uninfected or S. Typhimurium-infected WT mice (n = 3 mice per group). See also Extended Data Fig. 7.
S. Typhimurium invasion activates ILC3 cell death pathways
Next, we determined the bacterial localization in the LI of mice after S. Typhimurium infection by confocal microscopy. While the cryptopatches composed of RORγt+ ILC3 clusters in the uninfected mice were readily detectable, these distinctive lymphoid structures had largely disappeared in the LI of mice after S. Typhimurium infection (Fig. 4e), consistent with the marked reduction of ILC3 cell number after S. Typhimurium infection (Fig. 2). Of note, multiple bacteria were present within and/or adherent to ILC3s (Fig. 4e), suggesting that S. Typhimurium can invade gut ILC3s directly in vivo.
To determine transcriptional changes of ILC3s upon infection, we performed the RNA-seq analysis using the sorted ILC3s from LI of uninfected and S. Typhimurium-infected mice, and identified 1261 significantly changed genes (q-value ≤ 0.05, fold change ≥ 1.5) out of 21,940 expressed genes (fragments per kilobase of transcript per million mapped reads [FPKM] ≥ 1); among these genes, 746 were upregulated and 515 were downregulated (Fig. 4f,4g, and Extended Data Fig. 7e). Genes upregulated by S. Typhimurium challenge showed the enrichment in the pathway of general apoptosis (Extended Data Fig. 7e-7g). Careful examination of the apoptosis pathway genes revealed that S. Typhimurium induced the genes related to pyroptosis, including Casp1, Il1b, Nlrc5 and Il18 (Fig. 4g,4h). Of note, Casp1, encoding the canonical inflammasome-dependent inflammatory caspase32, was the only caspase gene in ILC3s that had significant changes after infection. mRNA expression of other caspases, including Casp11 that is involved in the non-canonical pyroptosis activation33, had modest or no changes between infected and uninfected ILC3s (Fig. 4h). Additionally, a few of inflammasome-encoding genes, Nlrc3, Nlrc5, Naip2, and Aim 2, were expressed in ILC3s, but only Nlrc5 mRNA was significantly enhanced after S. Typhimurium infection (Extended Data Fig. 7h). On note, NLRC5 regulates MHC class I gene transcription and is also suggested to interact with NLRP3 to activate the inflammasome34,35. The Gasdermin family proteins are the main effectors downstream of pro-inflammatory caspases36, among which Gsdmd was the only one highly expressed in ILC3s and enhanced by S. Typhimurium infection (Fig. 4i). Comparable expression of Casp1 and Gsdmd were observed in various subsets of ILC3s (Extended Data Fig. 7i). Together, these data suggest that S. Typhimurium may induce caspase-1-GSDMD-mediated canonical pathway of pyroptosis in ILC3s.
S. Typhimurium induces ILC3 pyroptosis
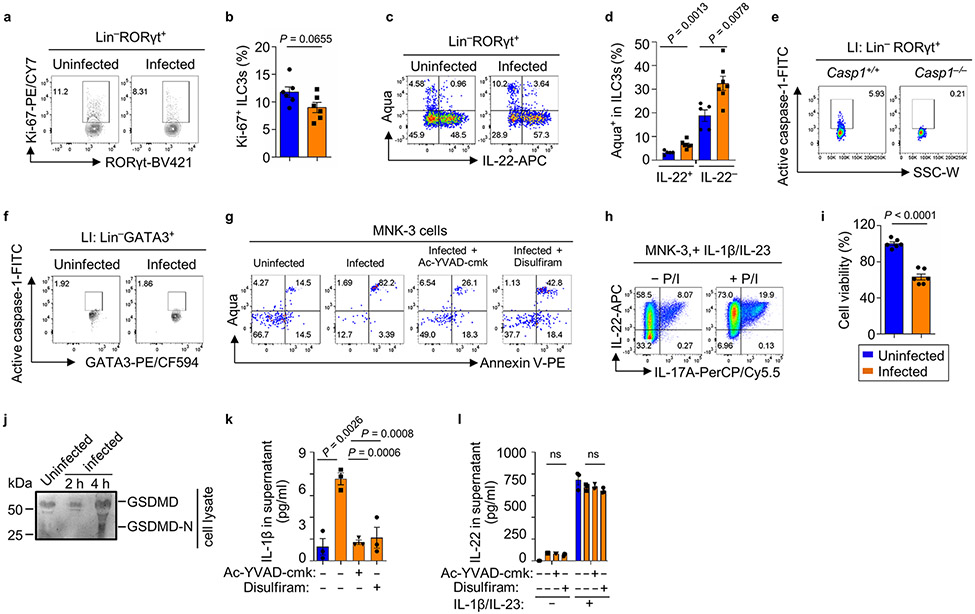
Consistent with the transcriptional alteration in ILC3s, FACS analysis showed the increase of annexin V+ and/or Aqua+ intestinal ILC3s after S. Typhimurium infection (Fig. 5a-5c) without apparent changes in ILC3 proliferation as revealed by Ki-67 staining (Extended Data Fig. 8a,8b), indicating that S. Typhimurium infection induces ILC3 cell death and consequently cell reduction in the gut. Of note, the cell death of IL-22+ ILC3s and IL-22− ILC3s were both significantly enhanced after S. Typhimurium infection (Extended Data Fig. 8c,8d), suggesting that the increase of IL-22+ ILC3s (Fig. 2) is not due to their preferential survival after infection.
Fig. 5. Pyroptosis in gut ILC3s was induced by S. Typhimurium independent of flagellin.
a–c, FACS analysis of Aqua and Annexin V expression in ILC3s from LI-LPLs of uninfected or S. Typhimurium-infected WT mice on day 2 post-infection. Representative data of two independent experiments (a). Percentages of Annexin V+ (b) and Annexin V+ Aqua+ (c) cells are shown. Compiled data from two independent experiments (n = 6 mice per group). d and e, FACS analysis of active caspase-1 expression in LI ILC3s of uninfected or S. Typhimurium-infected WT mice on day 2 post-infection. Representative data of two independent experiments. Percentages of active caspase-1+Aqua− cells in LI ILC3s (n = 3 mice per group) (e). Percentages of Annexin V+ (f) and Annexin V+ Aqua+ (g) uninfected or infected ILC3s, without or with pretreatment of Ac-YVAD-cmk or Disulfiram in vitro. Representative data of two independent experiments (n = 3 independent wells). h, LDH assay in uninfected or infected MNK-3 cells, without or with the pretreatment of Ac-YVAD-cmk. Representative data of three independent experiments (n = 3 independent wells). i–k, FACS analysis of Aqua and Annexin V expression in uninfected or infected MNK-3 cells, without or with the pretreatment of Ac-YVAD-cmk. Representative data of two independent experiments (i). Percentages of Annexin V+ (j) and Annexin V+ Aqua+ (k) MNK-3 cells (n = 3 independent wells). l, Western blot to determine GSDMD activation in the supernatant of MNK-3 cells uninfected or infected with S. Typhimurium. Representative data of two independent experiments. m, LDH assay in uninfected or infected MNK-3 cells, with or without pretreatment of Disulfiram. Representative data of two independent experiments (n = 3 independent wells). n and o, FACS analysis of Annexin V and Aqua staining of MNK-3 cells treated as in (m). Percentages of Annexin V+ (n) and Annexin V+ Aqua+ (o) cells. Representative data of two independent experiments (n = 3 independent wells). p, Confocal analysis of intracellular GFP-S. Typhimurium in MNK-3 cells. Green, S. Typhimurium. Blue, DAPI nuclear staining. Scale bar, 10 μm. Representative data of two independent experiments. q, LDH assay in MNK-3 cells uninfected or infected with indicated S. Typhimurium. Representative data of two independent experiments (n = 4 independent wells). See also Extended Data Fig. 8.
We further assessed the activation of caspase-1 in live gut ILC3s (Aqua−) during infection, using the fluorochrome-labeled caspase-1 inhibitor (YVAD) that allows detection of the active caspase-1 by FACS (Extended Data Fig. 8e). Intestinal ILC3s but not ILC2s of S. Typhimurium-infected mice had significantly more active caspase-1 than those of uninfected mice (Fig. 5d, 5e and Extended Data Fig. 8f). Disulfiram has recently been reported to inhibit pyroptosis by blocking GSDMD pore formation37. In vitro infection of sorted ILC3s by S. Typhimurium induced cell death as revealed by increased percentages of annexin V+ and/or Aqua+ ILC3s, which were prevented by pretreatment of ILC3s with the caspase-1 inhibitor Ac-YVAD-cmk or the GSDMD inhibitor Disulfiram (Fig. 5f, 5g, and Extended Data Fig. 8g). Together, these data indicate the pyroptotic cell death of ILC3s during S. Typhimurium infection.
To study the ILC3 cell death at the molecular level, we employed the ILC3-like MNK-3 cell line (Extended Data Fig. 8h, and ref.38 ). S. Typhimurium infection induced MNK-3 cell death as shown by lactate dehydrogenase (LDH) release and increased live/dead viability dye staining (Fig. 5h-5k, and Extended Data Fig. 8i), both of which could be inhibited by the caspase-1 inhibitor Ac-YVAD-cmk (Fig. 5h-5k). In addition, activation of GSDMD in MNK-3 cells upon infection was evidenced by the detection of cleaved GSDMD-C in the supernatant (Fig. 5l and Extended Data Fig. 8j). Similar to Ac-YVAD-cmk, the GSDMD inhibitor Disulfiram pretreatment significantly inhibited the cell death induced by S. Typhimurium infection (Fig. 5m-5o), indicating that S. Typhimurium-induced MNK-3 cell death is mediated by caspase-1 and GSDMD. Although the biological relevance of IL-1β produced by MNK-3 cells during S. Typhimurium infection remains to be determined, limited amounts of IL-1β were detected in the S. Typhimurium-infected MNK-3 culture supernatant. The secretion of IL-1β was further abolished by pretreatment of MNK-3 cells with Ac-YVAD-cmk or Disulfiram before infection, suggesting GSDMD-mediated pore formation after S. Typhimurium infection (Extended Data Fig. 8k). Of note, the inhibitors’ treatment had no significant effect on IL-22 amounts in the culture supernatant (Extended Data Fig. 8l), suggesting that IL-22 was not released through GSDMD-mediated membrane pore. Together, these data suggested that the caspase-1-mediated pyroptosis is responsible for MNK-3 cell death during S. Typhimurium infection. Notably, S. Typhimurium UK-1 flagellin-deficient mutant (ΔfliC/fljB) showed similar bacterial entry and induced the comparable cell death to that of WT strain (Fig. 5p,5q), indicating that MNK-3 cell death is flagellin-independent. Intriguingly, S. Typhimurium UK-1 ΔSPI-1/2 mutant could not invade the cells, consistent with the requirement of SPI-1 for cell entry3, also did not induce MNK-3 cell death (Fig. 5q). Together, these data suggest that S. Typhimurium-induced pyroptosis in MNK-3 cells is a bacterial cell entry-dependent but flagellin-independent event.
Caspase-1 controls ILC3 death in a cell-intrinsic manner
To determine the role of caspase-1 in S. Typhimurium infection, we challenged Casp1−/− and littermate Casp1+/+ control mice with S. Typhimurium. Compared with the control mice, the Casp1−/− mice had more ILC3s after infection with less cell death (Fig. 6a,6b, and Extended Data Fig. 9a,9b). Furthermore, Casp1−/− mice had increased IL-22+ ILC3s and accordingly accelerated mortality with higher bacterial counts in the feces (Fig. 6c-6e, and ref.39), supporting the notion that the increase of IL-22 in caspase-1-deficient mice promotes S. Typhimurium infection. To further determine the cell-intrinsic role of caspase-1 in ILC3s during S. Typhimurium infection, we performed a mixed bone-marrow (BM) transfer experiment. Specifically, BM cells from Casp1−/− mice and Rorc+/+ or Rorcgfp/gfp mice were mixed at a 1:1 ratio and transferred into lethally irradiated Rag2−/−γc−/− littermate recipient mice, in which the Casp1+/+ ILC3s (BM: Casp1−/− + Rorc+/+) (Casp1WT) or Casp1−/− ILC3s (BM: Casp1−/− + Rorcgfp/gfp) (Casp1ΔILC3) were reconstituted with comparable expression of caspase-1 in other cell types (e.g., ILC2s) (Fig. 6f,6g). After S. Typhimurium infection, the recipients reconstituted with Casp1-deficient ILC3s (Casp1ΔILC3) had significantly higher bacterial counts, more body weight loss and less survival, compared with the mice with Casp1-sufficient ILC3s (Casp1WT). These data phenocopied the results of Casp1−/− mice during S. Typhimurium infection (Fig. 6f,6h, and Extended Data Fig. 9c,9d), suggesting that caspase-1-mediated pyroptosis of ILC3s is critical for bacterial control during S. Typhimurium infection. Together, our data support a model that induction of pyroptotic death in intestinal ILC3s thereby reducing their cell number and function (i.e., IL-22) during S. Typhimurium infection may represent a host defensive mechanism to control the infection (Extended Data Fig. 9e).
Fig. 6. Caspase-1 controls ILC3 cell death in a cell-intrinsic manner during S. Typhimurium infection.
a, Absolute numbers of ILC3s in LI-LPLs of uninfected or S. Typhimurium-infected Casp1+/+ and Casp1−/− mice on day 2 post-infection. Complied data from two independent experiments (Casp1+/+: uninfected group, n = 4 mice and infected group, n = 6 mice; Casp1−/−: uninfected group, n = 4 mice and infected group, n = 4 mice). b, Percentages of Aqua+ proportion (cell death) in total ILC3s from Casp1+/+ and Casp1−/− mice infected with S. Typhimurium. Complied data from two independent experiments (Casp1+/+: n = 6 mice; Casp1−/−: n = 4 mice). c, Absolute numbers of IL-22+ ILC3s in uninfected or S. Typhimurium-infected Casp1+/+ and Casp1−/− mice (no P/I stimulation). Compiled data from two independent experiments (Casp1+/+: uninfected group, n = 4 mice and infected group, n = 6 mice; Casp1−/−: uninfected group, n = 4 mice and infected group, n = 4 mice). d, Survival curve of S. Typhimurium-infected Casp1+/+ and Casp1−/− mice (Casp1+/+: n = 6 mice; Casp1−/−: n = 4 mice). Compiled data from two independent experiments with the log-rank test for statistical analysis. e, S. Typhimurium CFUs in feces of Casp1+/+ and Casp1−/− mice on day 2 or day 4 post-infection. Complied data from two independent experiments, and presented as CFU per mg of feces (Casp1+/+: n = 6 mice; Casp1−/−: n = 4 mice). f, Schematic depiction of experimental design of bone marrow (BM) reconstitution followed by S. Typhimurium infection. g, Casp1 mRNA determined by qRT-PCR in sorted ILC3s and ILC2s from LI-LPLs of BM reconstituted recipients (Casp1ΔILC3 and Casp1WT mice). Representative data of two independent experiments (n = 3 technical repeats). h, S. Typhimurium CFUs in the feces of BM chimeric mice on day 2 and day 4 post-infection. Compiled data from two independent experiments and presented as CFU per mg of the feces (Casp1WT: n = 5 mice; Casp1ΔILC3: n = 6 mice). See also Extended Data Fig. 9.
Discussion
ILC3s are important to control enteric infections such as C. rodentium by their effector cytokine IL-2231. However, the contribution of ILC3s to the early infection of S. Typhimurium remains unknown. In this study, we demonstrate that S. Typhimurium infection induces ILC3 production of IL-22 and ILC3 pyroptosis through flagellin-dependent and -independent mechanisms. In contrast to the protective function against other enteropathogenic pathogens, ILC3s facilitate S. Typhimurium infection. Genetic depletion of ILC3s alleviates the mortality and disease severity in S. Typhimurium-infected mice.
The paradoxical role of ILC3s is presumably mediated by IL-22, a cytokine that is most abundantly produced by ILC3s and selectively enhanced by the infection, but facilitates S. Typhimurium colonization. Consistent with this notion, IL-22 has been reported to be employed by S. Typhimurium to benefit its competition against commensals, which otherwise suppress S. Typhimurium in the gut9. Of note, IL-22 is an important regulator of epithelial homeostasis via multiple mechanisms, including promoting epithelial cell survival upon radiation or chemotherapeutic agent-induced epithelial damage40-42. Thus, whether S. Typhimurium upregulates IL-22 to promote intestinal epithelial cell (IEC) survival and thus to prolong its infection needs to be further determined. Intriguingly, ILC3 death is triggered by S. Typhimurium infection through caspase-1- mediated pyroptosis. Ablation of Casp1 enhances ILC3 survival and number after infection, but exacerbates S. Typhimurium infection. Thus, our data reveal that ILC3s, an otherwise well-known anti-bacterial innate lymphocyte subset, are pro-Salmonella, and ILC3 pyroptosis represents a host defense mechanism to limit intracellular S. Typhimurium and reduce pro-Salmonella cytokine IL-22. Our data showing IL-22 secretion by MNK-3 cells was not affected by pretreatment of the caspase-1-inhibitor or the GSDMD inhibitor suggest that pyroptosis and IL-22 production of MNK-3 cells are two distinct events after S. Typhimurium infection.
ILC3s were previously speculated to control S. Typhimurium infection because of their ability to produce IFN-γ12. However, the former study did not determine whether ILC3s indeed can produce IFN-γ during S. Typhimurium infection and the impact of ILC3 depletion on infection. Strikingly, our data show that in contrast to ILC1s, limited amounts of IFN-γ can be produced by ILC3s without further upregulation after S. Typhimurium infection. Since ILC3s are not major producers of anti-Salmonella cytokine IFN-γ, ILC3 pyroptosis induced S. Typhimurium would presumably not compromise the protective IFN-γ-mediated host immunity.
The selective upregulation of IL-22 but not IL-17 in ILC3s during S. Typhimurium infection remains to be determined. However, our findings are consistent with a report showing that flagellin is not required for the induction of IL-17 in the gut43. We further provide evidence that flagellin works on ILC3s indirectly through the ND0/ND1 and CD1/CD0 domains by engaging APCs in a TLR5-MyD88 dependent manner, consistent with the substantial binding of the flagellin D1 domain to TLR5 from a structural study6. Notably, we have attributed the role of ILC3s in IL-22 production observed in vivo to the flagellin-TLR5-IL-23 signaling pathway established through in vitro and ex vivo experiments. This notion requires further validation by in vivo experiments, for example, infection with bacteria lacking synthesis of flagellin. Flagella contribute to S. Typhimurium invasion44, inflammasome activation45 and TLR5 signaling25. As a result, a comparison of inflammatory responses elicited by WT S. Typhimurium with a ΔfliC/fljB mutant in vivo would be difficult to interpret, because multiple pathways are affected at once. A ΔflgK mutant, which is defective in flagella assembly (i.e., non-motile) but secretes flagellin into the supernatant (thus activating caspase-1 and TLR5), was generated previously43. Although IL-22 expression was not examined in the study, inflammatory responses elicited by a ΔflgK mutant in the LI of streptomycin pretreated mice are intriguingly similar to those elicited by a ΔflgK/fliC/fljB mutant (non-motile and lacking flagellin)43. These data highlight the complexity of in vivo experiments in mice, which are very much dependent on means for growth and preparation of bacterial strains for their delivery to mice and the treatment practices for the hosts46-48.
The activation of caspase-1-dependent pyroptosis in ILC3s provides a mechanism underlying the marked reduction of ILC3 number in the gut during S. Typhimurium infection. Pyroptosis is primarily recognized as a host defense strategy to release pro-inflammatory cytokines and clear infected cells during infection49. It is well studied in myeloid cells like macrophages, in which pyroptosis is initiated by forming inflammasome complexes (canonical) through the signals from distinct pathogen-associated molecular patterns (PAMPs) of intracellular pathogens, including bacterial flagellin, or non-canonical inflammatory pathway, e.g., by binding to caspase-11 directly through LPS13,14,33,49. It is still unclear how the pyroptosis signal is activated in ILC3s by S. Typhimurium to induce the cell death during infection and merits further study on the molecular mechanisms.
Our study showed that the activation of caspase-1-induced ILC3 pyroptosis probably is a host defense mechanism during S. Typhimurium infection by limiting intracellular bacteria and reducing IL-22 production at the expense of ILC3s. Interestingly, it has been shown that pyroptosis in CD4+ T cells during HIV infection in the gut enhances inflammation15. Moreover, intestinal ILC3s have been shown to decrease in HIV/simian immunodeficiency virus (SIV) infected Macaca mulatta50,51. Although the molecular mechanisms remain to be determined, the apoptosis of ILC3s in viral infection has been shown to contribute to the HIV-induced damage of gut-associated lymphoid tissue structure and function, indicating that ILC3 cell death could have distinct impact on tissue inflammation and infection. Thus, better understanding of ILC3 cell death may provide new therapeutic opportunity to control S. Typhimurium and other pathogen infections.
Methods
Mice
All the mice in this study were maintained in specific-pathogen-free (SPF) facilities at the University of Florida, with 14:10 hour light cycle, temperature between 68-79°F and humidity between 30-70%. Mice (both sexes) were littermates and were 6–8 weeks old unless otherwise indicated in the text. No statistical methods were used to pre-determine sample sizes but our sample sizes are similar to those reported in previous publications52,53. Rorcgfp/gfp (54) and Myd88−/− (55) mice were previously described. Tlr5−/−, Casp1−/−, Il22Cre/Cre (22) (Il22 knockout mice, Extended Data Fig. 2t) and Rag1−/− mice were purchased from Jackson Laboratory. Rag2−/−γc−/− mice were purchased from Taconic Farms. All studies with mice were approved by the Animal Care and Use Committee of the University of Florida. For experiments related to mice and cells sorted from mice, age- and sex-matched littermate mice were randomized and used for experiments.
Bacterial strains and growth conditions
Wild-type S. Typhimurium strain SL1344 is from ATCC and UK-1 is from Roy Curtiss’ lab. S. Typhimurium mutant strains used in this study were derived from UK-1 and listed in Supplementary Table 1. All strains were grown aerobically at 37°C by shaking at 220 RPM in Luria–Bertani (LB) broth or low magnesium-containing minimal medium MgM56. For S. Typhimurium used for infection of the mice or cells, overnight cultures (14–16 hours) in LB medium were subcultured in fresh LB medium containing 0.3 M sodium chloride to OD600 of 0.7–0.9 under low-aeration conditions (20–30 rpm)39,57.
S. Typhimurium culture supernatant preparation
Wild-type or mutant S. Typhimurium SL1344 or UK-1 strains were cultured in MgM medium aerobically for 14–16 hours to OD600 of 2.0 at 37°C at 220 RPM, and then at 1:50 dilution inoculated into the fresh MgM medium and grown for 4–5 hours aerobically at 37°C at 220 RPM to the log phase (OD600 of 0.4–0.5). The culture was centrifuged at 3,000 g for 15 mins and the supernatant was filtered (0.2 μm) for downstream use. 15 μl or 45 μl of 5 ml of bacterial culture supernatants were then used for culture experiment with indicated cells.
Size exclusion filtration, proteinase K and heat treatment of bacterial supernatant
Bacterial supernatant prepared as above was subjected to various treatments when necessary, including: (1) size exclusion filtration: log phase S. Typhimurium culture supernatant was subjected to size filtration by centrifugation (3000 g) using 3 kDa cutoff filter (Ultrafree filter membrane, Millipore) per manufacture instruction; (2) Proteinase K digestion: log phase S. Typhimurium culture supernatant was digested with 50 μg/ml proteinase K for 12 hours; (3) Heat treatment: log phase S. Typhimurium culture supernatant was inactivated at 100°C for 2 hours. 15 μl or 45 μl of the treated supernatant was used for culture experiment with indicated cells.
Antibiotic pretreatment and mouse infection
Mice used for S. Typhimurium infection were cohoused for 3 weeks prior to experiments. After that, mice were pretreated with 20 mg of streptomycin 24 hour before infection with S. Typhimurium strain, to induce a Serovar Typhimurium colitis in the LI17. Mice were orally gavaged 2–3 × 107 or 2–3 × 103 CFUs of S. Typhimurium or PBS, and body weight change and morbidity were monitored.
Isolation of gut LPLs and flow cytometry (FACS)
Isolation of intestinal LPLs and flow cytometry were done as described previously52. Antibodies were purchased from Invitrogen/eBioscience, BD Pharmingen, BioLegend, TONBO, Abcam, Cell Signaling Tech or Sigma-Aldrich. CD16/32 antibody was used to block nonspecific binding to Fc receptors before all surface staining. For transcription factor staining, cells were fixed and permeabilized with a Foxp3 staining buffer kit (eBioscience). For cytokine staining used for FACS analysis, cells were stimulated with 50 ng/ml phorbol-12-myristate-13-acetate (PMA) and 500 ng/ml ionomycin for 4 hours unless indicated otherwise, and Brefeldin A (2 μg/ml) was added 2 hours before cells harvest. The live and dead cells were discriminated by Live and Dead Violet Viability Kit (Invitrogen) or Zombie Aqua Fixable Viability Kit (BioLegend). Sample acquisition was performed on FACSCantoII (BD Biosciences) and Aurora (Cytek), and analyzed with FlowJo (version 10.6, Tree Star). FACS gating strategies for different immune cell populations are shown in Extended Data Fig. 1k.
RNA-seq analyses
ILC3s (Lin−CD90hiCD45lo)58 were sorted from the LI of uninfected and S. Typhimurium-infected C57BL/6 WT mice, and RNA was extracted using RNAeasy Micro Kit, followed by cDNA generation with SMART-Seq® HT Kit (TaKaRa). Sequencing libraries were generated with Nextera® XT DNA Library Preparation Kit (Illumina), and sequenced on an Illumina HiSeq 2500 instrument. FastQC (v0.11.5) was used to ensure high per-base sequence quality of reads. Sequenced reads were mapped and raw count values quantified with STAR (v2.7.4a)59 to the Mus musculus genome (GRCm38/mm10 assembly) and filtered for uniquely mapped reads. Quantitated mRNA expression levels, FPKM aligned reads, were calculated based on exon regions using RSEM (v1.2.25)60. Differentially expressed genes (max FPKM ≥ 1, fold change ≥ 1.5, q-value ≤ 0.05) were identified by DESeq2 (v1.26.0) analysis61. Gene Set Enrichment Analysis (GSEA) was performed for GO analysis. Log2 transformed FPKM values were used for principal component analysis in R (v3.6.2) with the prcomp function and then visualized using the factoextra package (v1.0.7). Heatmaps were created using the R package pheatmap (v1.0.12).
Detection of caspase-1 activation in ILC3s (FLICA assay)
Active caspase-1 was detected by flow cytometry with the carboxyfluorescein FLICA kit (FAM-YVAD-FMK, Immunochemistry Techniques). Mice were orally gavaged with S. Typhimurium or PBS as indicated above. The intestinal LPLs were freshly prepared from these mice two days post-infection and incubated with the kit at 37°C for one hour according to the manufacturer’s instructions, followed by flow cytometry analysis.
In vitro culture of LPLs
Freshly isolated LI LPLs (2–3 × 106) were cultured in RPMI with 5% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mM L-glutamine (Gibco, Life Technologies), with S. Typhimurium supernatant for 5 hours in total 500 μl culture volume. For the in vitro co-culture experiment using purified cells, ILC3s were sorted from LI LPLs as described above, and CD11c+ APCs were isolated from LI LPLs using the Mouse CD11c Positive Selection Kit II (STEMCELL, 18780A) after depletion of adaptive immune cells using PE positive Selection Kit II (STEMCELL, 17684). Cells were cultured for 5 hours and subjected to cytokine production examination for FACS analysis were stimulated with 50 ng/ml PMA and 500 ng/ml ionomycin for 4 hours unless indicated otherwise, and Brefeldin A (2 μg/ml) was added 2 hours before cells harvest. Cells used for qRT-PCR were directly collected after co-incubation with bacterial supernatant without PMA/ionomycin stimulation.
Silver staining
Silver staining was performed as described previously with slight modification62. Briefly, the SDS-PAGE gel after electrophoresis was fixed in fixing solution (50% MeOH, 10% HOAc), followed by washing, treating with silver nitrate and development with sodium carbonate sodium until achieve the desired intensity of staining.
Real-time RT-PCR
RNA of large intestine tissue or cells was isolated with Trizol (Invitrogen), without PMA/ionomycin stimulation. cDNA was synthesized using the GoScript reverse transcription kit (Promega). Real-time RT-PCR was performed using SYBR Green (Bio-Rad) and various primer sets (Supplementary Table 2). Reactions were run using CFX Connect Real-Time PCR Detection System (v 3.1) (Bio-Rad). The mRNA levels of target genes were calculated by the comparative CT (2-ΔΔCt) method and normalized to β-actin.
Plasmid construction and histidine-tagged protein purification
Full length or truncated fragments of S. Typhimurium flagellin (FliC and FljB) were cloned into plasmid pET30a. The resulting plasmids were electro-transformed into E. coli BL21. IPTG-induced recombinant proteins were purified by Ni2+-nitrilotriacetic acid affinity chromatography (Ni-NTA agarose; Qiagen), as described by the manufacturer.
TLR5 activation assay
The human embryonic kidney (HEK)-Blue-mTLR5 reporter cells (Invivogen) were used to determine the TLR5 activation by full-length or truncated flagellin proteins. Briefly, cells were washed with PBS, re-suspended in the HEK-Blue™ detection medium (Invivogen) and seeded in 96-well plates at a density of 5 × 104 cells per well in 100 μl volume, and stimulated with flagellin proteins for 16 hours. TLR5 activation was indicated by SEAP (secreted embryonic alkaline phosphatase) activities according to the manufacturer's instructions.
MNK-3 cell line culture and bacterial infection
The MNK-3 cells were maintained in the RPMI complete media with 10% FBS, 2 mM GlutaMAX, 1 mM sodium pyruvate, 55 mM 2-mercaptoethanol, 10 mM HEPES, 50 μg/ml gentamicin, 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco, Life Technologies), as previously described38.
For S. Typhimurium infection, MNK-3 cells were cultured in the RPMI infection media with 2 mM GlutaMAX, 1 mM sodium pyruvate, 55 mM 2-mercaptoethanol, 10 mM HEPES but without serum and antibiotics. WT or mutant S. Typhimurium strains were added into the cells at a multiplicity of infection (MOI) of 100, and centrifuged for 10 mins at 500 g to facilitate infection. The infection mixture was further incubated at 37°C for 1 hour before pre-warmed PBS (1 ml per well) wash, followed by exchanging with the fresh RPMI infection media with the addition of gentamicin (final concentration: 100 μg/ml) to stop extracellular bacterial growth. Infection-induced cell death was measured 4 hours after infection by the lactate dehydrogenase (LDH) assay from the supernatant using CytoTox 96 Non-Radioactive Cytotoxicity Assay kit (Promega). The caspase-1 specific inhibitor (Ac-YVAD-cmk, 50 μM, Sigma-Aldrich) or the GSDMD inhibitor Disulfiram (20 μM, Sigma-Aldrich) was added 30 mins or one hour before infection as specified.
ELISA assay
IL-1β and IL-22 secretion in culture supernatants of MNK-3 cells with or without S. Typhimurium infection were measured using the ELISA kits purchased from R&D Systems, following the manufacturer’s instructions.
Western blot
After infection with S. Typhimurium, the culture supernatants were collected and precipitated with trichloroacetic acid (TCA). The whole-cell extracts were made in lysis buffer (50 mM Tris-HCl (pH 8.0), 120 mM NaCl, 4 mM EDTA, 1% NP40, 1 mM DTT) with protease inhibitor cocktails (Roche) and the insoluble materials were removed by centrifugation at 12,000 g for 10 mins. The precipitated supernatants or cell lysates were subjected to SDS-PAGE and immunoblotting with the indicated antibodies.
Immunofluorescence staining and confocal microscopy analysis
Large intestine of S. Typhimurium-infected and uninfected Rag1−/− mice were dissected, washed with ice-cold PBS, followed by fixation in 4% paraformaldehyde (PFA) at 4 °C overnight. The fixed tissues were washed with ice-cold PBS three times, made in swiss rolls, and then saturated in a 30% sucrose gradient in Phosphate Buffer (PB) at 4 °C overnight. After that, the samples were embedded in optimal-cutting-temperature (OCT) compound (Tissue-Tek). Tissue blocks were snap frozen in a bath of dry ice and ethanol, and cut into 6 μm sections. The tissue section slides were stained with anti-human/mouse RORγt antibody (eBioscience 14-6988-82, 1:100 dilution) and anti-GFP antibody (abcam ab290, 1:100 dilution) at 4 °C overnight, followed by secondary antibody staining of donkey anti-rat IgG-Alexa Fluor 555 (1:400) and goat anti-rabbit IgG Alexa Fluor 488 (1:400) (Invitrogen) at room temperature for one hour, and then 4′,6-diamidino-2-phenylindole (DAPI) staining. Data were collected by a confocal microscope (Zeiss 710 confocal microscope). ZEN 2011 (v14.0.10.201) was used to collect confocal images.
Hydrodynamic gene delivery of IL-22
Plasmid DNA was introduced into the mice using a hydrodynamic tail vein injection-based gene transfer technique as previously described53. Briefly, plasmid DNA was diluted in TransIT-EE Hydrodynamic Delivery Solution (Mirus) (10 μg DNA/mouse) in a volume of 10% body weight (0.1 ml/g). The DNA solution was injected into mice through the tail vein using a 27-gauge needle within a time period of 5 to 10 seconds. To ensure the success of delivery, the large intestinal tissue RNA was prepared and evaluated for IL-22 target gene (i.e., Reg3g) expression (Fig. 4c). Mice were rested for 24 hours before pretreated with streptomycin and orally infected with S. Typhimurium.
Bone marrow transfer
Bone marrow from Casp1−/− and Rorc+/+ or Rorcgfp/gfp mice was mixed at a 1:1 ratio, and a total of 4 × 106 cells were intravenously injected into lethally irradiated Rag2−/−γc−/− recipient mice. Recipient mice were treated with antibiotics cocktails for 2 weeks after bone marrow transfer. At the time of 8 weeks after transfer, reconstituted mice were challenged with S. Typhimurium as indicated above.
Statistical methods
Unless otherwise noted, statistical analysis was performed with unpaired two-tailed Student’s t test on individual biological samples with GraphPad Prism 9. All the data with error bars were expressed as mean ± standard error of the mean (SEM).
Extended Data
Extended Data Fig. 1. Distinct ILC response during early S. Typhimurium infection, (Related to Fig. 1).
a, Schematic depiction of experimental design of S. Typhimurium infection in mice. b and c, Percentages in the Lin− (b) and absolute numbers (c) of the ILC2s in LI-LPLs of uninfected and infected WT mice. Compiled data from two independent experiments (n = 5 and 7 mice for uninfected and infected groups, respectively). d, FACS analysis of Ki-67, IL-5 and IL-13 expression in LI ILC2s of uninfected and infected WT mice on day 2 post-infection (d.p.i 2). Representative data of two independent experiments. e–h, FACS analysis of Eomes (e), IFN-γ (g) expression in SI ILC1s of uninfected and infected WT mice on d.p.i 2. Representative data of two independent experiments (e and g). Percentages of Eomes− (ILC1) and Eomes+ (NK) cells in group 1 ILCs (f), and percentages of IFN-γ+ cells in NK and ILC1 cells (h). Compiled data from two independent experiments (n = 4 and 5 mice for uninfected and infected groups, respectively) (f and h). i and j, FACS analysis of GATA3 expression after gating on Lin− in the SI of uninfected and infected WT mice on d.p.i 2. Representative data of two independent experiments (i). Percentages of ILC2s in Lin− cells in SI LPLs. Compiled data from two independent experiments (n = 4 and 5 mice for uninfected and infected groups, respectively) (j). k, Representative FACS gating/sorting strategies used for identification of different immune cell populations in this study. Gating strategies indicated in this panel were applied to the following FACS data panels in the figures: group 1 ILCs (Lin−RORγt−NKp46+NK1.1+): Fig. 1c, 1f; ILC3s for intracellular staining and gating (Lin−RORγt+): Fig. 2a, 2j, 2m, 3a, 3d, 5a, 5d, 6a; ILC3s for surface staining and sorting (Lin−CD90hiCD45lo): Fig. 3h, 4f-4g, 6g; ILC2 (Lin−GATA3+): Fig. 1j.
Extended Data Fig. 2. IL-22+ ILC3s are specifically induced in early S. Typhimurium infection, (Related to Fig. 2).
a and b, FACS analysis of RORγt expression in SI Lin− of uninfected and S. Typhimurium-infected WT mice on day 2 post-infection (d.p.i 2). Representative data of two independent experiments (a). Percentages of SI ILC3s in Lin−. Compiled data from two independent experiments (n = 4 and 5 mice for uninfected and infected groups, respectively) (b). c–h, IL-22 and IL-17 expression in LI ILC3s of uninfected and infected WT mice on d.p.i 2. Representative FACS data of three independent experiments (a). Percentages (d) and absolute numbers (e) of IL-17+ LI ILC3s of uninfected and infected WT mice on d.p.i 2. Compiled data from three independent experiments (n = 7 and 9 mice for uninfected and infected groups, respectively) (d and e). Percentages (f), absolute numbers (g) and MFI (h) of IL-22+ LI ILC3s of uninfected and infected WT mice on d.p.i 2. Compiled data from three independent experiments (n = 8 and 9 mice for uninfected and infected groups, respectively) (f–h). i–k, FACS analysis of IL-22 and IL-17 expression in SI ILC3s of uninfected and infected WT mice on d.p.i 2, with or without P/I stimulation. Representative data of two independent experiments (i). Percentages of IL-22+ ILC3s, without (j) or with (k) P/I stimulation. Compiled data from two independent experiments (n = 4 and 5 mice for uninfected and infected groups, respectively) (j and k). l–m, FACS analysis of IL-22 expression in LI ILC3s of uninfected and infected WT mice at 12 h and 24 h post-infection. Percentages of IL-22 in LI ILC3s (l). Compiled data from two independent experiments (n = 4 and 5 mice for uninfected and infected groups, respectively) (m). n and o, FACS analysis of RORγt and GATA3 expression after gating on Lin− cells in LI-LPLs of uninfected and infected WT mice at 12 h and 24 h post-infection. Percentages of ILC3s in Lin− cells in LI-LPLs. Representative data of two independent experiments (n). Compiled data from two independent experiments (n = 4 and 5 mice for uninfected and infected groups, respectively) (o). p, Percentages of Annexin V+ Aqua+ in total ILC3s from LI-LPLs of uninfected and infected WT mice at 12 h and 24 h post-infection. Compiled data from two independent experiments (n = 4 and 5 mice for uninfected and infected groups, respectively). q and r, FACS analysis of IL-22 and IL-17 expression in LI CD4+ T cells of uninfected and infected WT mice on d.p.i 2. Representative data of two independent experiments (q). r, Absolute numbers of LI IL-22+ CD4+ T cells. Compiled data from two independent experiments (n = 4 and 6 mice for uninfected and infected groups, respectively). s, Expression value of Il22 normalized by DESeq2 in ILCs and myeloid cells from ImmGen database. t, FACS analysis of IL-22 expression in LI ILC3s of Il22+/+ and Il22Cre/Cre mice. Representative data of two independent experiments.
Extended Data Fig. 3. IL-22 was repressed by S. Typhimurium infection in late stage, (Related to Fig. 2).
a, LI of uninfected and S. Typhimurium-infected WT mice on d.p.i 8. b–f, Analysis of RORγt, NKp46 and CD4 expression in LI Lin− or Lin−NKp46− cells of uninfected and infected WT mice on d.p.i 8. Representative FACS data of two independent experiments (b). Percentages of ILC3s in LI Lin− (c), NKp46+ ILC3s (NKp46+RORγt+ among Lin−) (d), CD4+ ILC3s (CD4+RORγt+ among Lin−NKp46−) (e), CD4− ILC3s (CD4−RORγt+ among Lin−NKp46−) (f) of uninfected and infected WT mice. Compiled data from two independent experiments (n = 5 and 6 mice for uninfected and infected groups, respectively). g and h, Analysis of T-bet and RORγt expression in LI Lin− of uninfected and infected WT mice on d.p.i 8. Representative FACS data of two independent experiments (g). Percentages of ILC1s in Lin− (h). Compiled data from two independent experiments (n = 5 and 6 mice for uninfected and infected groups, respectively). i–k, Analysis of IFN-γ expression in LI CD4+ T cells or ILC1s of uninfected and infected WT mice on d.p.i 8. Representative FACS data of two independent experiments (i). Percentages of IFN-γ+ ILC1s (j) and CD4+ T cells (k). Compiled data from two independent experiments (n = 5 and 6 mice for uninfected and infected groups, respectively). l–o, Analysis of IL-22 and IL-17 expression in LI ILC3s (l and m) or CD4+ T cells (n and o) of uninfected and infected WT mice on d.p.i 8. Representative FACS data of two independent experiments (l and n). Percentages of IL-22+ ILC3s (m) and IL-22+ and IL-17+ CD4+ T cells (o). Complied data from two independent experiments (n = 5 and 6 mice for uninfected and infected groups, respectively). p and q, WT and Rag1−/− mice were orally inoculated with 2 × 103 CFUs of S. Typhimurium. Body weight analysis using linear regression to compare slope difference (p) and survival curve with the log-rank test for statistical analysis (q). Complied data from two independent experiments (WT, n = 4 mice; Rag1−/−, n = 5 mice). r–v, S. Typhimurium CFUs in feces (r), LI (s), liver (t), spleen (u) and mLNs (v) of Il22+/+ and Il22Cre/Cre mice on d.p.i 2 and 4. Compiled data from two independent experiments (n = 4 and 5 mice for d.p.i 2 and 4, respectively).
Extended Data Fig. 4. S. Typhimurium-secreted proteins enhance IL-22+ ILC3s in vitro, (Related to Fig. 3).
a, Schematic depiction of experimental design of in vitro LPL cultured with S. Typhimurium supernatant. b–d, Analysis of IL-22 and IL-17 expression in WT LI-LPLs cultured with bacterial supernatant, with or without P/I stimulation. Representative FACS data of four independent experiments (b). Percentages of IL-22+ (c) and IL-17+ (d) ILC3s, with or without PMA and ionomycin (P/I) stimulation. Compiled data from four independent experiments (n = 9 repeats with independent mouse LPLs) (c and d). e–g, Analysis of IL-22 and IL-17 expression in LI CD4+ T cells cultured with S. Typhimurium supernatant. Representative data of three independent experiments (e). Percentages of IL-17+ (f) and IL-22+ (g) CD4+ T cells. Compiled data from three independent experiments (n = 8 repeats with independent mouse LPLs) (f and g). h and i, Percentages of IFN-γ+ ILC1 and NK (h) and IFN-γ+ CD4+ T (i) cells. Compiled data from two independent experiments (n = 3 repeats with independent mouse LPLs). j and k, Il22 (j, n = 9 repeats with independent mouse LPLs) and Il17 (k, n = 3 repeats with independent mouse LPLs) mRNA determined by qRT-PCR in LI-LPLs of WT mice co-incubated with bacterial supernatant. Compiled data from two independent experiments. l, Silver staining of S. Typhimurium culture supernatant with or without proteinase K (PK) treatment. Representative data of two independent experiments. m and n, Analysis of IL-22 and IL-17 expression in ILC3s of LPLs cultured with S. Typhimurium supernatant with or without PK treatment (no P/I stimulation). Representative FACS data of two independent experiments (m). n, Percentages of IL-22+ ILC3s. Compiled data from two independent experiments (n = 3 repeats with independent mouse LPLs). o, Il22 mRNA determined by qRT-PCR in LI-LPLs co-incubated with S. Typhimurium culture supernatant with or without PK treatment. Representative data of two independent experiments (o) (n = 3 repeats with independent mouse LPLs). p and q, Analysis of IL-22 and IL-17 expression in ILC3s from LI-LPLs cultured with S. Typhimurium supernatant treated with or without heat (no P/I stimulation). Representative FACS data of two independent experiments (p). Percentages of IL-22+ ILC3s (q). Compiled data from two independent experiments (n = 3 repeats with independent mouse LPLs).
Extended Data Fig. 5. S. Typhimurium flagellin protein promotes IL-22+ ILC3s but not IL-22+ CD4+ T cells, (Related to Fig. 3).
a and b, Analysis of IL-22 and IL-17 expression in ILC3s of LPLs cultured with bacterial supernatants of WT strain UK-1 or indicated S. Typhimurium mutants (no P/I stimulation). Representative FACS data of three independent experiments (a). b, Percentages of IL-22+ cells in ILC3s. Compiled data from three independent experiments (n = 3 repeats with independent mouse LPLs). 15 μl or 45 μl of S. Typhimurium culture supernatant was added, respectively. Empty medium (MgM) was included as a negative control. Fractionated supernatant fractions (< 3 kDa) were used in LPL culture as well. c and d, Analysis of IL-22 and IL-17 expression in ILC3s of LI-LPLs cultured with purified S. Typhimurium flagellin protein encoded by fljB (no P/I stimulation). Representative FACS data of two independent experiments (c). Percentages of IL-22+ cells in ILC3s (d). Compiled data from two independent experiments (n = 3 repeats with independent mouse LPLs). e and f, Analysis of IL-22 and IL-17 expression in sorted WT ILC3s (no P/I stimulation) co-cultured with purified S. Typhimurium flagellin proteins, with or without the addition of CD11c+ APCs from Tlr5+/+ or Tlr5−/− mice. Representative FACS data of two independent experiments (e). Percentages of IL-22+ cells in ILC3s (f). Compiled data from two independent experiments (n = 3 repeats with independently sorted LI cells from mice with indicated genotypes). g, FPKM of Tlr genes in sorted ILC3s from uninfected and infected WT mice (n = 3 mice per group). h, Expression value of Tlr5 normalized by DESeq2 in ILC3s and CD11c+ DCs from ImmGen database. i, Il23 (p19) mRNA determined by qRT-PCR in cell culture containing CD11c+ APCs sorted from the LI of Tlr5+/+ or Tlr5−/− mice, and sorted LI WT ILC3s (no P/I stimulation), stimulated with S. Typhimurium culture supernatant for 5 hours. Representative data of two independent experiments (n = 3 repeats with independently sorted LI cells from mice with indicated genotypes). 15 μl or 45 μl of S. Typhimurium culture supernatant was added, respectively. Empty medium (MgM) was included as a negative control.
Extended Data Fig. 6. Stimulation of IL-22+ ILC3s by S. Typhimurium infection is mediated by CD11c+ APCs via TLR5-flagellin signaling, (Related to Fig. 3).
a, Schematic depiction of flagellin and its truncated mutant proteins. b–d, Analysis of IL-22 and IL-17 expression in ILC3s of LI-LPLs cultured with S. Typhimurium flagellin proteins (no P/I stimulation). Representative FACS data of two independent experiments (b). Percentages of IL-22+ cells (c) and IL-22 MFI (d) in ILC3s. Compiled data from two independent experiments (n = 3 repeats with independently sorted LI cells from mice with indicated genotypes) (c and d). e, TLR5 activation by S. Typhimurium flagellin proteins as indicated by SEAP activities (OD650nm) in HEK-Blue-mTLR5 reporter cells. Representative data of two independent experiments (n = 3 independent wells per group). f–h, Analysis of IL-22 and IL-17 expression in ILC3s from LI-LPLs of Myd88+/+ and Myd88−/− littermates, cultured with S. Typhimurium supernatant (no P/I stimulation). Representative FACS data of two independent experiments (f). Percentages of IL-22+ cells (g) and IL-22 MFI (h) in ILC3s. Compiled data from two independent experiments (n = 3 repeats with independently sorted LI cells from mice with indicated genotypes) (g and h). i and j, Analysis of IL-22 and IL-17 expression in sorted WT ILC3s (no P/I stimulation) co-cultured with S. Typhimurium flagellin, with or without the addition of CD11c+ APCs from Myd88+/+ or Myd88−/− mice. Representative FACS data of two independent experiments (i). Percentages of IL-22+ cells (j) in ILC3s. Compiled data from two independent experiments (n = 3 repeats with independently sorted LI cells from mice with indicated genotypes).
Extended Data Fig. 7. Presence of ILC3s increases the susceptibility to S. Typhimurium infection in mice, (Related to Fig. 4).
a and b, Rorcgfp/+ and Rorcgfp/gfp mice were orally gavaged with 2 × 107 CFUs of S. Typhimurium. Body weight change (a) and survival curve (b) are compiled from two independent experiments (Rorcgfp/+, n = 10 mice; Rorcgfp/gfp, n = 10 mice). Linear regression for comparing slope difference in (a) and the log-rank test for statistical analysis of survival curve in (b). c, Bacterial CFUs in the feces of Rorcgfp/+ and Rorcgfp/gfp were measured on d.p.i 2 and 4. Compiled data from two independent experiments and presented as CFU per mg of the feces (n = 9 mice per group). d, Rorcgfp/+Rag1−/− and Rorcgfp/gfpRag1−/− mice were orally inoculated with 2 × 107 CFUs of S. Typhimurium. Compiled data from two independent experiments (Rorcgfp/+Rag1−/−, n = 10 mice; Rorcgfp/gfpRag1−/−, n = 10 mice). Linear regression for comparing slope difference. e, Heatmap of all of the differentially expressed genes (fold change ≥ 1.5, q value ≤ 0.05) and pathway analysis in the RNA-seq of sorted ILC3s (Lin−CD90hiCD45lo) from LI of uninfected and infected WT mice (n = 3 mice per group). f, Top 10 pathways of the differentially expressed genes identified by RNA-seq in ILC3s (fold change ≥ 1.5, q value ≤ 0.05) from LI of uninfected and infected WT mice (n = 3 mice per group). g, GSEA showing enrichment of apoptosis-related genes in LI ILC3s from infected mice, compared with ILC3s from uninfected mice. h, FPKM of inflammasome genes in sorted ILC3s from uninfected or infected mice (n = 3 mice per group). i, Expression value of Gsdmd and Casp1 normalized by DESeq2 in ILC3s from ImmGen database.
Extended Data Fig. 8. Pyroptosis in gut ILC3s was induced by S. Typhimurium, (Related to Fig. 5).
a and b, Analysis of Ki-67 and RORγt expression in ILC3s from LI-LPLs of uninfected and S. Typhimurium-infected WT mice on d.p.i 2. Representative FACS data of two independent experiments. Percentages of Ki-67+ cells in ILC3s (b). Compiled data from two independent experiments (n = 6 mice per group). c and d, Analysis of Aqua and IL-22 expression in LI ILC3s of uninfected and infected WT mice on d.p.i 2. Representative FACS data of two independent experiments (c). Percentages of Aqua+ cells in IL-22+ vs IL-22− ILC3s (d). Compiled data from two independent experiments (n = 5 and 7 mice for uninfected and infected groups, respectively). e, FACS analysis of active caspase-1 expression (Ac-YVAD-cmk, FLICA assay) in LI ILC3s of Casp1+/+ and Casp1−/− mice. Representative data of two independent experiments f, FACS analysis of active caspase-1 expression in LI ILC2s of uninfected and infected WT mice on d.p.i 2. Representative data of two independent experiments. g, FACS analysis of Aqua and Annexin V expression in LI ILC3s uninfected and infected with S. Typhimurium in vitro. Cells were pretreated with Ac-YVAD-cmk or Disulfiram as indicated. Representative data of two independent experiments. h, FACS analysis of IL-22 and IL-17 expression in MNK-3 cells, with or without P/I stimulation. Representative data of two independent experiments. i, Cell viability of MNK-3 cells infected with S. Typhimurium counted by FACS. Compiled data from three independent experiments (n = 6 independent wells). j, Western blot analysis to determine GSDMD in cell lysates of MNK-3 cells uninfected or infected with S. Typhimurium. Representative data of two independent experiments. k, Quantitative evaluation of mature IL-1β secreted in the culture supernatant of S. Typhimurium-infected MNK-3 cells using ELISA, with or without pretreatment of Ac-YVAD-cmk or Disulfiram. l, Quantitative evaluation of IL-22 secreted in the culture supernatant of S. Typhimurium-infected MNK-3 cells using ELISA, with or without pretreatment of Ac-YVAD-cmk or Disulfiram.
Extended Data Fig. 9. Caspase-1 controls ILC3 cell death in a cell-intrinsic manner during S. Typhimurium infection, (Related to Fig. 6).
a and b, FACS analysis of RORγt and Lin expression (a), and Aqua and Annexin V expression (b) in LI-LPLs of S. Typhimurium-infected Casp1+/+ and Casp1−/− mice on d.p.i 2. Representative data of two independent experiments. c and d, Bone-marrow chimeric mice were orally gavaged with 2 × 107 CFUs of S. Typhimurium and assessed for survival following infection. Survival curve with the log-rank test for statistical analysis (c) and body weight change with linear regression to compare slope difference (d) are compiled from two independent experiments (Casp1WT: n = 5 mice; Casp1ΔILC3: n = 6 mice). e, Working model of ILC1 and ILC3 responses during S. Typhimurium infection, and pyroptotic cell death of ILC3s to defend the host against S. Typhimurium infection.
Supplementary Material
Acknowledgments
We thank the entire Zhou lab for help and suggestions. We thank Ms. Lesa N. Howell at Animal Care Services of the University of Florida for the hydrodynamic injection, and Dr. Yuping Sun from Molecular Pathology Core, Dept. of Pathology of the University of Florida for tissue section and immunofluorescence staining. We thank Dr. Marina Cella at Washington University to kindly provide us with the MNK-3 cell line, and the Genomics Facility (University of Chicago) for sequencing services and assistance. We acknowledge Dr. Francesco Vacca at the University of Edinburgh for sharing the protocol of proteinase K treatment of bacterial culture supernatant. L.Z. is an Investigator in the Pathogenesis of Infectious Disease supported by the Burroughs Wellcome Fund. The work was supported by the National Institutes of Health grants AI132391 and DK105562 (to L.Z.), AI60557 (to R.C.), and AI126172 (to S.W.).
Footnotes
Competing interests
Roy Curtiss III is a founder and part owner of Curtiss Healthcare, Inc., which is involved in developing vaccines against infectious diseases of farm animals. The authors declare no competing interests.
Data Availability
All data supporting the findings of this study are available within the article and its supplementary materials. The Mus musculus reference genome (GRCm38/mm10 assembly) https://www.ncbi.nlm.nih.gov/assembly/GCF_000001635.20/ was used for RNA-seq read alignment. The accession GEO number of the RNA-seq data in this paper is GSE201292.
References
- 1.Chong A, Starr T, Finn CE & Steele-Mortimer O A role for the Salmonella Type III Secretion System 1 in bacterial adaptation to the cytosol of epithelial cells. Mol Microbiol 112, 1270–1283, doi: 10.1111/mmi.14361 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennings E, Thurston TLM & Holden DW Salmonella SPI-2 Type III Secretion System Effectors: Molecular Mechanisms And Physiological Consequences. Cell Host Microbe 22, 217–231, doi: 10.1016/j.chom.2017.07.009 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Patel JC & Galán JE Manipulation of the host actin cytoskeleton by Salmonella--all in the name of entry. Curr Opin Microbiol 8, 10–15, doi: 10.1016/j.mib.2004.09.001 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Thomson NM et al. Bacterial Flagellins: Does Size Matter? Trends Microbiol 26, 575–581, doi: 10.1016/j.tim.2017.11.010 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Vijayan A, Rumbo M, Carnoy C & Sirard JC Compartmentalized Antimicrobial Defenses in Response to Flagellin. Trends Microbiol 26, 423–435, doi: 10.1016/j.tim.2017.10.008 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Yoon SI et al. Structural basis of TLR5-flagellin recognition and signaling. Science 335, 859–864, doi: 10.1126/science.1215584 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duncan JA & Canna SW The NLRC4 Inflammasome. Immunol Rev 281, 115–123, doi: 10.1111/imr.12607 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McSorley SJ Immunity to intestinal pathogens: lessons learned from Salmonella. Immunol Rev 260, 168–182, doi: 10.1111/imr.12184 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behnsen J et al. The cytokine IL-22 promotes pathogen colonization by suppressing related commensal bacteria. Immunity 40, 262–273, doi: 10.1016/j.immuni.2014.01.003 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grizotte-Lake M et al. Commensals Suppress Intestinal Epithelial Cell Retinoic Acid Synthesis to Regulate Interleukin-22 Activity and Prevent Microbial Dysbiosis. Immunity 49, 1103–1115 e1106, doi: 10.1016/j.immuni.2018.11.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artis D & Spits H The biology of innate lymphoid cells. Nature 517, 293–301, doi: 10.1038/nature14189 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Klose CS et al. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature 494, 261–265, doi: 10.1038/nature11813 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Kesavardhana S, Malireddi RKS & Kanneganti TD Caspases in Cell Death, Inflammation, and Pyroptosis. Annu Rev Immunol 38, 567–595, doi: 10.1146/annurev-immunol-073119-095439 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergsbaken T, Fink SL & Cookson BT Pyroptosis: host cell death and inflammation. Nat Rev Microbiol 7, 99–109, doi: 10.1038/nrmicro2070 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doitsh G et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature 505, 509–514, doi: 10.1038/nature12940 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoiseth SK & Stocker BA Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291, 238–239, doi: 10.1038/291238a0 (1981). [DOI] [PubMed] [Google Scholar]
- 17.Barthel M et al. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect Immun 71, 2839–2858, doi: 10.1128/iai.71.5.2839-2858.2003 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayuzumi H, Inagaki-Ohara K, Uyttenhove C, Okamoto Y & Matsuzaki G Interleukin-17A is required to suppress invasion of Salmonella enterica serovar Typhimurium to enteric mucosa. Immunology 131, 377–385, doi: 10.1111/j.1365-2567.2010.03310.x (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Y et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 14, 282–289, doi: 10.1038/nm1720 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Perez-Lopez A, Behnsen J, Nuccio SP & Raffatellu M Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol 16, 135–148, doi: 10.1038/nri.2015.17 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Miki T, Goto R, Fujimoto M, Okada N & Hardt WD The Bactericidal Lectin RegIIIβ Prolongs Gut Colonization and Enteropathy in the Streptomycin Mouse Model for Salmonella Diarrhea. Cell Host Microbe 21, 195–207, doi: 10.1016/j.chom.2016.12.008 (2017). [DOI] [PubMed] [Google Scholar]
- 22.Ahlfors H et al. IL-22 fate reporter reveals origin and control of IL-22 production in homeostasis and infection. J Immunol 193, 4602–4613, doi: 10.4049/jimmunol.1401244 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Kelly SM, Bollen WS & Curtiss R 3rd. Characterization and immunogenicity of Salmonella typhimurium SL1344 and UK-1 delta crp and delta cdt deletion mutants. Infect Immun 65, 5381–5387, doi: 10.1128/iai.65.12.5381-5387.1997 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dieye Y, Ameiss K, Mellata M & Curtiss R 3rd. The Salmonella Pathogenicity Island (SPI) 1 contributes more than SPI2 to the colonization of the chicken by Salmonella enterica serovar Typhimurium. BMC Microbiol 9, 3, doi: 10.1186/1471-2180-9-3 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayashi F et al. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410, 1099–1103, doi: 10.1038/35074106 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Fujita H, Yamaguchi S & Iino T Studies on H-O variants in Salmonella in relation to phase variation. J Gen Microbiol 76, 127–134, doi: 10.1099/00221287-76-1-127 (1973). [DOI] [PubMed] [Google Scholar]
- 27.Uematsu S et al. Detection of pathogenic intestinal bacteria by Toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol 7, 868–874, doi: 10.1038/ni1362 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Kinnebrew MA et al. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity 36, 276–287, doi: 10.1016/j.immuni.2011.12.011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akira S & Takeda K Toll-like receptor signalling. Nat Rev Immunol 4, 499–511, doi: 10.1038/nri1391 (2004). [DOI] [PubMed] [Google Scholar]
- 30.Satoh-Takayama N et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970, doi: 10.1016/j.immuni.2008.11.001 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Collins JW et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol 12, 612–623, doi: 10.1038/nrmicro3315 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Man SM & Kanneganti TD Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol 16, 7–21, doi: 10.1038/nri.2015.7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi J et al. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514, 187–192, doi: 10.1038/nature13683 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Davis BK et al. Cutting edge: NLRC5-dependent activation of the inflammasome. J Immunol 186, 1333–1337, doi: 10.4049/jimmunol.1003111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobayashi KS & van den Elsen PJ NLRC5: a key regulator of MHC class I-dependent immune responses. Nat Rev Immunol 12, 813–820, doi: 10.1038/nri3339 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Broz P, Pelegrín P & Shao F The gasdermins, a protein family executing cell death and inflammation. Nat Rev Immunol 20, 143–157, doi: 10.1038/s41577-019-0228-2 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Hu JJ et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol 21, 736–745, doi: 10.1038/s41590-020-0669-6 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Allan DS et al. An in vitro model of innate lymphoid cell function and differentiation. Mucosal Immunol 8, 340–351, doi: 10.1038/mi.2014.71 (2015). [DOI] [PubMed] [Google Scholar]
- 39.Lara-Tejero M et al. Role of the caspase-1 inflammasome in Salmonella typhimurium pathogenesis. J Exp Med 203, 1407–1412, doi: 10.1084/jem.20060206 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keir M, Yi Y, Lu T & Ghilardi N The role of IL-22 in intestinal health and disease. J Exp Med 217, e20192195, doi: 10.1084/jem.20192195 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gronke K et al. Interleukin-22 protects intestinal stem cells against genotoxic stress. Nature 566, 249–253, doi: 10.1038/s41586-019-0899-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aparicio-Domingo P et al. Type 3 innate lymphoid cells maintain intestinal epithelial stem cells after tissue damage. J Exp Med 212, 1783–1791, doi: 10.1084/jem.20150318 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winter SE et al. Contribution of flagellin pattern recognition to intestinal inflammation during Salmonella enterica serotype typhimurium infection. Infect Immun 77, 1904–1916, doi: 10.1128/iai.01341-08 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones BD, Lee CA & Falkow S Invasion by Salmonella typhimurium is affected by the direction of flagellar rotation. Infect Immun 60, 2475–2480, doi: 10.1128/iai.60.6.2475-2480.1992 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miao EA et al. Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 7, 569–575, doi: 10.1038/ni1344 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Galán JE & Curtiss R 3rd. Expression of Salmonella typhimurium genes required for invasion is regulated by changes in DNA supercoiling. Infect Immun 58, 1879–1885, doi: 10.1128/iai.58.6.1879-1885.1990 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Galán JE & Curtiss R 3rd. Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A 86, 6383–6387, doi: 10.1073/pnas.86.16.6383 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lockman HA & Curtiss R 3rd. Salmonella typhimurium mutants lacking flagella or motility remain virulent in BALB/c mice. Infect Immun 58, 137–143, doi: 10.1128/iai.58.1.137-143.1990 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vande Walle L & Lamkanfi M Pyroptosis. Curr Biol 26, R568–R572, doi: 10.1016/j.cub.2016.02.019 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Xu H, Wang X, Lackner AA & Veazey RS Type 3 innate lymphoid cell depletion is mediated by TLRs in lymphoid tissues of simian immunodeficiency virus-infected macaques. FASEB J 29, 5072–5080, doi: 10.1096/fj.15-276477 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Z et al. Plasmacytoid dendritic cells promote HIV-1-induced group 3 innate lymphoid cell depletion. J Clin Invest 125, 3692–3703, doi: 10.1172/jci82124 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xiong L et al. Ahr-Foxp3-RORγt axis controls gut homing of CD4(+) T cells by regulating GPR15. Sci Immunol 5, doi: 10.1126/sciimmunol.aaz7277 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu J et al. The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104, doi: 10.1016/j.immuni.2011.11.011 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eberl G et al. An essential function for the nuclear receptor RORgamma(t) in the generation of fetal lymphoid tissue inducer cells. Nat Immunol 5, 64–73, doi: 10.1038/ni1022 (2004). [DOI] [PubMed] [Google Scholar]
- 55.Mühlbauer M, Perez-Chanona E & Jobin C Epithelial cell-specific MyD88 signaling mediates ischemia/reperfusion-induced intestinal injury independent of microbial status. Inflamm Bowel Dis 19, 2857–2866, doi: 10.1097/01.mib.0000435445.96933.37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beuzón CR, Banks G, Deiwick J, Hensel M & Holden DW pH-dependent secretion of SseB, a product of the SPI-2 type III secretion system of Salmonella typhimurium. Mol Microbiol 33, 806–816, doi: 10.1046/j.1365-2958.1999.01527.x (1999). [DOI] [PubMed] [Google Scholar]
- 57.Chen LM, Kaniga K & Galán JE Salmonella spp. are cytotoxic for cultured macrophages. Mol Microbiol 21, 1101–1115, doi: 10.1046/j.1365-2958.1996.471410.x (1996). [DOI] [PubMed] [Google Scholar]
- 58.Guo X et al. Induction of innate lymphoid cell-derived interleukin-22 by the transcription factor STAT3 mediates protection against intestinal infection. Immunity 40, 25–39, doi: 10.1016/j.immuni.2013.10.021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dobin A et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21, doi: 10.1093/bioinformatics/bts635 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li B & Dewey CN RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 12, 323, doi: 10.1186/1471-2105-12-323 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Love MI, Huber W & Anders S Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550, doi: 10.1186/s13059-014-0550-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shevchenko A, Wilm M, Vorm O & Mann M Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68, 850–858, doi: 10.1021/ac950914h (1996). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the article and its supplementary materials. The Mus musculus reference genome (GRCm38/mm10 assembly) https://www.ncbi.nlm.nih.gov/assembly/GCF_000001635.20/ was used for RNA-seq read alignment. The accession GEO number of the RNA-seq data in this paper is GSE201292.