Abstract
Wound healing differs significantly between men and women in a tissue-dependent manner. Dermal wounds heal faster in women whereas mucosal wounds heal faster in men. However, the effect of sex as a variable in corneal wound healing is largely unknown. The primary objective of this study was to test whether sex is a biological variable in corneal wound healing activated by the trauma or injury using an established in vivo rabbit model with male and female New Zealand White rabbits. Corneal wounds in rabbits were produced by a single topical alkali (0.5N Sodium hydroxide) application. Serial slit-lamp, stereo biomicroscopy, and applanation tonometry evaluated corneal opacity, anterior segment ocular health, and intraocular pressure (IOP), respectively, at various times during the study. Fourteen days after alkali-wound, corneal tissues were collected after humane euthanasia to examine cellular and molecular wound healing parameters. Quantitative PCR (qPCR) and immunofluorescence were used to quantify changes in the extracellular modeling protein levels of alpha-smooth muscle actin (α-SMA), Fibronectin (FN), Collagen-I (Col-I), and Transforming growth factor beta 1 (TGFβ1) involved in corneal healing. Hematoxylin and Eosin (H&E) staining was used to study histopathological changes in morphology and TUNEL assay to evaluate levels of apoptotic cell death. Male and female rabbits showed no significant differences in corneal opacity (Fantes score) or intraocular pressure (IOP) values (9.5 ± 0.5 mm Hg) in live animals. Likewise, no statistically significant sex-based differences in the mRNA levels of α-SMA (male = 5.95 ± 0.21 fold vs. female = 5.32 ± 0.043), FN (male = 3.02 ± 0.24 fold vs. female = 3.23 ± 0.27), Col-I (male = 3.12 ± 0.37 fold vs. female = 3.31 ± 0.24), TGFβ1 (male = 1.65 ± 0.06 fold vs. female = 1.59 ± 0.053); and protein levels of α-SMA (male = 74.16 ± 4.6 vs. female = 71.58 ± 7.1), FN (male = 60.11 ± 4.6 vs. female = 57.41 ± 8.3), Col-I (male = 84.11 ± 2.8 vs. female = 84.55 ± 3.6), TGFβ1 (male = 11.61 ± 2.8 vs. female = 9.5 ± 3.04) were observed. Furthermore, H&E and TUNEL analyses found no statistically significant differences in cellular structures and apoptosis, respectively, in male vs. female corneas. Consistent with earlier reports, wounded corneas showed significantly increased levels of these parameters compared to the unwounded corneas. Our data suggest that sex is not a major biological variable during active early stages of corneal wound healing in rabbits in vivo.
Keywords: Cornea, Fibrosis, Wound healing, Sex variable, Male vs. female
1. Introduction
Corneal wound healing is a complex process. It involves cell death, migration, proliferation, differentiation, and extracellular matrix (ECM) remodeling (Mohan et al., 1997; Gosain and DiPietro, 2004; Chaurasia et al., 2009; Guo and DiPietro, 2010; Bukowiecki et al., 2017). Successful wound healing is contingent upon all phases occurring in a proper, finely tuned sequence, and continuing for a specific duration, each with optimal intensities (Gosain and DiPietro, 2004; Mathieu et al., 2006; Guo and DiPietro, 2010; Tandon et al., 2010). Several cytokines including IL-1α, IL-1β, Fas ligand, and TNFα (Mohan et al., 1997, 2000; Wilson et al., 2001; Ambrósio et al., 2009) and chemokines regulate this process, as well as ECM deposition (Li et al., 2007; Velnar et al., 2009; Guo and DiPietro, 2010; Pavletic, 2010). Factors affecting wound healing may be categorized as either “local” or “systemic” (Guo and DiPietro, 2010). Important local factors that influence wound healing include wound size and depth, the presence of a foreign body and/or infection, and health of the wound bed. Systemic factors that can affect normal wound healing processes include age, stress, central hypoxia, hematologic disorders, obesity, and sex.
Sex has been considered to be a critical factor in cutaneous wound healing (Ashcroft et al., 1997; Jorgensen et al., 2002; Ashcroft and Ashworth, 2003) and showed a female predilection towards improved healing rates compared to males. By contrast, women healed significantly more slowly (Conrad et al., 1999; Phillips et al., 2003) and required additional post-surgical interventions (Phillips et al., 2003; Benediktsdottir et al., 2004) compared to men during healing of mucosal tissues after third-molar surgery. Similarly, wound healing studies in ovariectomized female mice demonstrated that estrogen supplementation accelerated cutaneous wound healing (Ashcroft et al., 2003; Hardman et al., 2008; Emmerson et al., 2010). Additionally, estrogen and dehydroepiandrosterone (DHEA) accelerated skin repair whereas androgens appeared to retard the healing process. When young male mice were castrated, the healing of full-thickness skin incisions was markedly accelerated compared to age-matched controls (Ashcroft and Mills, 2002). Also, androgen receptor blockade or castration improves the cutaneous wound healing process in rodents (Gilliver et al., 2006).
Several mechanisms have been suggested for sex-based differences in wound healing. One study noted that increased levels of estrogen in females, which affects wound healing gene regulation via dermal endoplasmic reticula, contributes to more efficient dermal wound healing by reducing inflammation and increasing matrix deposition (Strudwick et al., 2006). Estrogen also accelerates cutaneous wound healing by promoting epidermal keratinocyte proliferation. (Ashcroft et al., 1997, 1999). By contrast, endogenous testosterone inhibits the cutaneous wound healing response in males due to hormone-dependent enhanced inflammatory response (Ashcroft et al., 1997; Ashcroft and Mills, 2002; Gilliver et al., 2006, 2008); therefore, male sex hormones are inversely related to the wound healing process in a tissue-specific manner.
The role of sex as a biological variable in corneal wound healing is poorly understood at present. Corneal epithelial wound healing is regulated by estrogen. Estrogen negatively regulates corneal epithelial wound healing by delaying wound closure in female mice compared with their male counterparts (Wang et al., 2012). Corneal structure, stiffness, and function have been found to differ between men and women, partially due to the effect of sex hormones (Ashcroft et al., 1999). Receptors for female and male sex hormones are present in the human and rabbit cornea and likely contribute to the regulation of corneal structure and function (Wickham et al., 2000; Suzuki et al., 2001). Sex plays a role in nerve innervation in injured mouse corneas. Female mice demonstrate more nerve innervation after corneal injury than male mice in a strain-specific manner (Pham et al., 2019).
Therefore, the present study sought to determine whether a biological difference exists in the corneal wound healing response of males and females using a well-established in vivo rabbit alkali wound healing model. Understanding the influence of sex on corneal wound healing is of paramount importance in order to develop effective corneal therapies for men and women. This investigation will improve our knowledge-base and may afford new insights into the sex-based molecular and physiological regulation of corneal wound healing.
2. Materials and methods
2.1. Animals
Animal protocols were approved by the Harry S. Truman Memorial Veterans’ Hospital and the University of Missouri Institutional Animal Care and Use Committee and were conducted in accordance with the principles of the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research. Six female (n = 6) and six male (n = 6) New Zealand White rabbits (Charles River, Wilmington, MA, USA) each weighing 2–3 kg were used in the study. The animals were anesthetized by intramuscular injection of combination ketamine hydrochloride (JHP Pharmaceuticals, LLC, Rochester, MI, USA) (50 mg/kg) and xylazine hydrochloride (XylaMed, Bimeda Inc., IL, USA) (10 mg/kg), prior to induction of corneal alkali wounds. Time-dependent clinical examinations were regularly performed using slit-lamp biomicroscopy (SL-15; Kowa Optimed, Torrance, CA, USA), ocular stereo biomicroscopy (Leica DM 4000B) equipped with digital camera (SpotCamRT KE; Diagnostic Instruments, Sterling Heights, MI, USA), and applanation tonometry (Tono-Pen AVIA; Reichert Technologies, Depew, NY, USA) for IOP measurements. Topical ophthalmic proparacaine hydrochloride (0.5%; Alcon, Fort Worth, TX, USA) was administered for local anesthesia prior to all procedures and IOP measurements.
2.2. Alkali-induced corneal wounding in male and female rabbits
Corneal wounds with fibrotic changes were induced in the left eye while the right eye served as the unwounded control for each rabbit using a well-established protocol (Gupta et al., 2017, 2018). Briefly, rabbits were anesthetized, topical anesthesia was applied, and topical 0.5N sodium hydroxide solution was placed onto the central cornea for 30 s using an 8 mm soaked filter paper under a surgical microscope (Leica Wild Microscope MEL53; Leica, Wetzlar, Germany). The wounded corneas were rinsed immediately with sterile balanced salt solution to remove residual alkali solution after removal of the filter paper. Wound healing was consequently initiated, and the progression of corneal fibrosis (scarring) was monitored throughout the experimental duration (14 days).
2.3. Intraocular pressure measurements
Intraocular pressure (IOP) variability due to sex differences after corneal wounding are largely unknown. IOP measurements will evaluate the ocular abnormalities such as glaucoma (intraocular pressures above normal reference ranges) and uveitis (intraocular pressures below normal reference ranges). Adverse sequela such as uveitis and glaucoma are essential to monitor as both are vision-robbing diseases and alkali-induced corneal wounds may affect the globe by causing one or both secondary problems. IOP measurements in rabbit eyes were therefore recorded using an applanation tonometer (Tonopen AVIA; Reichert Technologies, Depew, NY, USA) before wounding and on days 0, 3, 7, and 14 after wounding.
2.4. Ocular stereo biomicroscopy, slit-lamp examination, and haze analysis
Clinical examination of corneas was conducted before and after wounding on days 0, 3, 7, and 14 to assess anterior segment ocular health. A digital imaging system incorporated into the surgical microscope and a hand-held slit-lamp biomicroscope with a similar imaging system (SL-15 equipped with VK image filing quantitative software, Kowa Optimed, Torrance, CA, USA) were used to capture stages of corneal wound healing. The Fantes scale was used to grade the degree of corneal haze at each time point. Independent observers (RT, NPH, JTR, SG, LMM, MKF, and/or HBG) masked to the healing stage did Fantes scale grading. The grading system used was as follows: Grade 0 = completely transparent cornea; Grade 1 = trace haze with careful oblique illumination; Grade 2 = more obvious haze but not interfering with the visualization of fine iris details; Grade 3 = mild obscuration of iris details; Grade 4 = moderate obscuration of the iris and lens; and Grade 5 = complete opacification of the stroma in the wounded area.
2.5. Corneal tissue collection
Rabbits underwent humane euthanasia on day 14 after all final clinical examinations were complete. Intravenous Beuthanasia-D (150 mg/kg; Schering-Plough Animal Heath, Union, NJ) was administered while under general anesthesia. The wounded corneas were collected using sharp dissection and placed in 24 × 24 × 5 mm molds (Fischer Scientific, Pittsburgh, PA, USA) containing optical cutting temperature (OCT) (Sakura FineTek, Torrance, CA, USA) compound. The molds were immediately snap frozen by immersion in a cryo-cup containing 2-methylbutane in liquid nitrogen container. Frozen tissue blocks were stored at −80 °C. The corneas were cut in two halves: one half was used for histology studies while the other half was used for molecular studies. For histology, serial corneal sections (8 μm) were prepared using a cryostat (HM525 NX UV; Microm GmbH, Walldorf, Germany), placed on labeled glass microscope slides (Superfrost Plus; Fisher Scientific, Pittsburgh, PA, USA), and stored at −80 °C until analysis. For molecular studies, the corneas were cut into small pieces, immersed in a cryo-cup placed in liquid nitrogen, and subsequently ground and processed for RNA isolation and cDNA synthesis following manufacturer’s protocols (Qiagen, Germantown, MD, USA).
2.6. RNA extraction, cDNA synthesis, and quantitative PCR
Total RNA was isolated from the corneal tissue using the RNeasy kit (Qiagen, Germantown, MD, USA) following the manufacturer’s protocol. First-strand cDNA was synthesized by reverse transcriptase enzyme (Promega, Madison, WI, USA). Quantitative PCR (qPCR) was performed using the One Step Plus Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA). A 20 μl reaction mixture containing 2 μl cDNA, 2 μl forward and reverse primers (200 nM each), and 10 μl of 2X All-in-One qPCR mix (GeneCopoeia, Rockville, MD, USA) was run at a universal cycle (95 °C for 10 min, 40 cycles at 95 °C for 15 s, and 60 °C for 60 s) as previously reported (Mohan et al., 2011; Tandon et al., 2013). Gene-specific forward and reverse primer sequences used in PCR analyses are summarized in Table 1. The GAPDH was used for the normalization of qPCR data and showed no detectable relative fold change at various tested points or groups. The relative gene expression was calculated by the 2−ΔΔCt method and reported as relative fold-change over the respective control values. A minimum of three independent experiments were conducted, qPCR was performed in triplicate for each sample, and the average fold changes in mRNA levels were reported.
Table 1.
Quantitative PCR primer sequences.
| Gene | Primers (5′−3′) | Tm (°C) | |
|---|---|---|---|
| α-SMA | Forward | TGG GTG ACG AAG CAC AGA GC | 60 |
| Reverse | CTT CAG GGG CAA CAC GAA GC | 60 | |
| FN | Forward | CGC AGC TTC GAG ATC GTG C | 60 |
| Reverse | TCG ACG GGA TCA CAC TTC CA | 60 | |
| Col-I | Forward | TGT GGC CCA GAA CTG GTA CAT | 60 |
| Reverse | ACT GGA ATC CAT CGG TCA TGC TCT | 60 | |
| TGFβ1 | Forward | TGG ACA CCA ACT ACT GCT TCA GCT C | 60 |
| Reverse | CAG GTC CTT GCG GAA GTC AAT GTA | 60 | |
| GAPDH | Forward | GCC TCA AGA TCA TCA GCA ATG CCT | 60 |
| Reverse | TGT GGT CAT GAG TCC TTC CAC GAT | 60 |
2.7. Hematoxylin and Eosin, immunofluorescence staining, and TUNEL assay
Corneal cellular structure before and after wounding was evaluated by H&E staining. This was done using the standard procedure for visualizing morphologic details, as previously reported (Gupta et al., 2018). Immunofluorescence staining was performed to measure the following: α-SMA, a marker for myofibroblast; TGFβ1, a pro-fibrotic cytokines involved in fibroblast recruitment and fibrosis; FN, a protein secreted by fibroblasts involved in extracellular matrix formation; Col-I, secreted by fibroblasts to strengthen the tissue; and 4′,6-diamidine-2’phenylindole dihydrochloride (DAPI), a blue stain of nuclei to indicate the presence of fibroblasts and myofibroblasts. For immunofluorescence, corneal sections were first blocked with 2% bovine serum albumin at room temperature for 30 min. Protein-specific primary antibodies were probed and incubated for 90 min as follows: mouse monoclonal anti-α-SMA (1:200 dilution, M0851; Dako, Carpentaria, CA, USA), goat polyclonal anti-FN (1:200 dilution, sc-6952; Santa Cruz Biotechnology, Dallas, TX, USA), anti-Col-I (1:200 dilution, ab19811; Abcam, Cambridge, MA, USA), and goat polyclonal anti-TGFβ1 (1:200 dilution, AF-246-NA; R&D System, Inc., Minneapolis, MN, USA) and then incubated with Alexa-Fluor 488 goat anti-mouse IgG secondary antibody (1:1000 dilution, A11001; Invitrogen, Carlsbad, CA, USA) or Alexa-Fluor 594 donkey anti-goat IgG secondary antibody (1:1000 dilution, A11058; Invitrogen, Carlsbad, CA, USA) for 1 h at room temperature. Antifade Mounting Medium containing DAPI (H1200, Vector Laboratories, Inc. Burlingame, CA, USA) was used to mount corneal sections. A digital imaging software (SpotCamRT KE; Diagnostic Instruments, Sterling Heights, MI, USA) associated with the fluorescence microscope (Leica) was used to image-capture stained corneas. Distribution of antibody stained cells was quantified by selecting six randomly, non-overlapping, full-thickness central corneal columns, extending from the anterior stromal surface to the posterior stromal surface at 200x and 400x magnification fields using the computer software program ImageJ.
Cell death was determined by performing a TUNEL assay (S7165 ApopTag; Millipore, Temecula, CA, USA). Corneal sections were fixed for 10 min in acetone at −20 °C, and the assay was performed following the manufacturer’s instructions. Briefly, sections were washed with equilibration buffer and incubated with TdT enzyme diluted with reaction buffer containing digoxigenin composed nucleotide for 1 h at 37 °C. Enzyme activity was stopped by stop buffer followed by PBS rinse and then incubated with anti-digoxigenin rhodamine antibody for 30 min at room temperature followed by PBS wash and mounting with Vectashield antifade DAPI containing medium (H1200, Vector Laboratories, Inc. Burlingame, CA, USA). Rhodamine-conjugated apoptotic cells (red) and DAPI-stained nuclei (blue) were viewed and photographed with the fluorescence microscope (Leica) fitted with a digital camera system (SpotCamRT KE). The DAPI-stained nuclei and TUNEL positive (+ve) cells in unwounded and wounded tissues were quantified at 200x and 400x magnification in six randomly selected non-overlapping areas.
2.8. Statistical analysis
Statistical analyses were conducted with Prism Version 6 (GraphPad Software Inc., San Diego, CA, USA). Quantification studies were performed using Student’s t-test, two-way ANOVA followed by Bonferroni multiple comparisons test. The data are represented as the mean ± standard error of the mean (SEM). P < 0.05 was considered statistically significant.
3. Results
3.1. Corneal fibrosis after wounding in male and female rabbits
All male (Fig. 1A) and female (Fig. 1F) rabbit corneas were healthy prior to alkali-wound. Immediately after wounding (day 0), corneas in both male (Fig. 1B) and female (Fig. 1G) rabbits were opaque and no iris was visible due to dense corneal inflammation and edema. On day 3, corneas remained opaque, though to a slightly lesser extent, but iris details were still not visible through the male (Fig. 1C) and female (Fig. 1H) corneas. In addition, the peripheral corneal tissue adjacent to the area of alkali wound demonstrated increased corneal edema on day 0 compared to day 3 in both male (Fig. 1C) and female (Fig. 1H) rabbits. On day 7 after wounding, the corneas remained opaque with evidence of peripheral neovascularization emanating from the limbus and encroaching axially in the both males (Fig. 1D) and females (Fig. 1I) At study endpoint on day 14 after wounding, corneas were deemed opaque with hazy visualization of the underlying lens and iris, a more significant neovascularized perilimbal corneal response in both males (Fig. 1E) and females (Fig. 1J). On the basis of these observations, a Fantes score was assigned to male and female rabbit corneas at all study time points. As evident from Table 2, Fantes scores were consistent and no significant differences in haze levels between male and female rabbit corneas were observed at any examined time points.
Fig. 1.
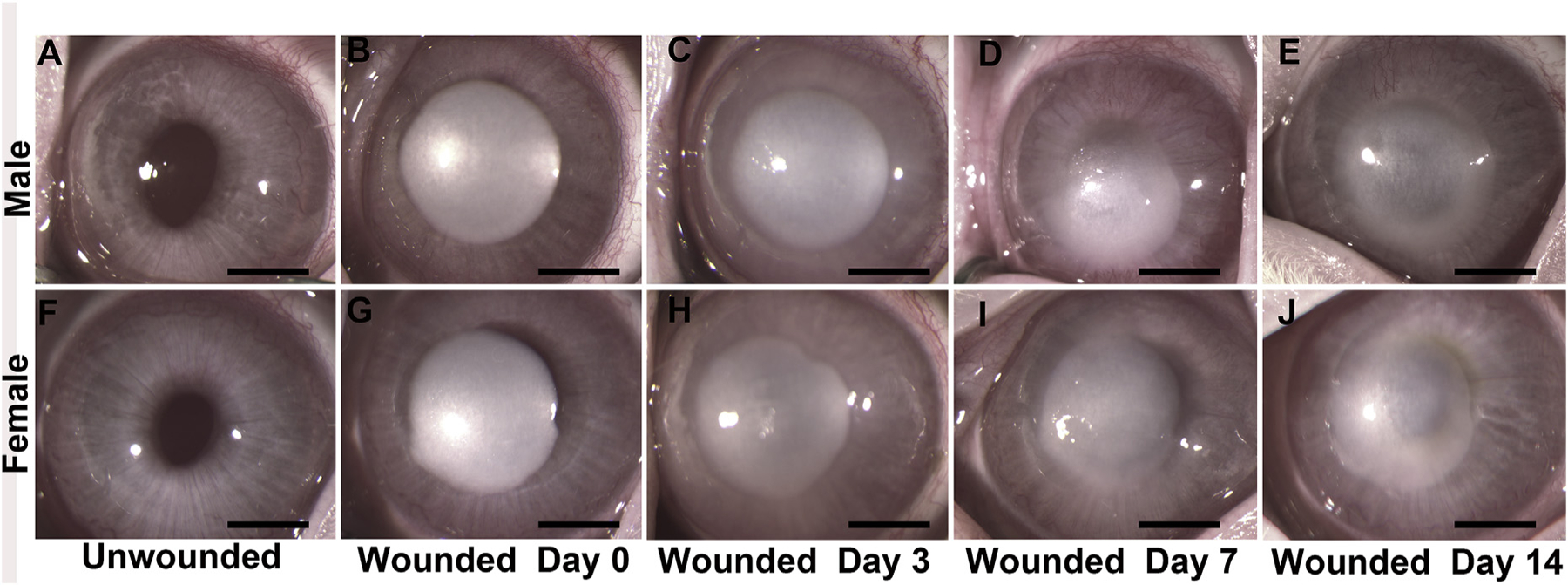
Stereo biomicroscopy images of the rabbit corneas before wounding (unwounded) and 0, 3, 7, and 14 days after wounding in male (A–E) and female rabbits (F–J). Corneal opacification is very dense after wounding (0 day) in males (B) and females (G) with a gradual decrease in opacification over time but with no clinical differences observed between male (E) and female (J) rabbits by day 14. n = 6. Scale bar = 2 mm.
Table 2.
Fantes scores for the unwounded and wounded corneas. All corneas were graded by two independent observers using the Fantes scale. The scores were averaged and compared between male and female rabbits. No significant difference in corneal haze was found between male and female rabbits at any observed time points (0, 3, 7, and 14 days). Data are presented as Mean ± SEM. n= 6.
| Group | Male | Female | P Value |
|---|---|---|---|
| Unwounded | 0.00 | 0.00 | > 0.999 |
| Wounded Day 0 | 4.4 ± 0.20 | 4.7 ± 0.20 | > 0.999 |
| Wounded Day 3 | 4.1 ± 0.10 | 4.1 ± 0.10 | > 0.999 |
| Wounded Day 7 | 3.4 ± 0.10 | 3.3 ± 0.10 | > 0.999 |
| Wounded Day 14 | 3.1 ± 0.10 | 3.1 ± 0.10 | > 0.999 |
Corneal transparency was evaluated with slit-lamp biomicroscopy (Fig. 2). Slit beams (corneal parallelepipeds) traversing the stroma were found to be homogeneously continuous in thickness and shape prior to wound (unwounded) in both male (Fig. 2A) and female (Fig. 2C) corneas, thus defining similar levels of baseline corneal health. On day 14 after alkali wounding, the corneal parallelepiped was thicker and demonstrated less translucency in both male (Fig. 2B) and female (Fig. 2D) corneas consistent with the presence of corneal haze and edema. Likewise, no significant differences in slit-lamp biomicroscopy were noted at other tested time points (data not shown).
Fig. 2.

Representative slit-lamp images of rabbit corneas before wounding (unwounded) in male (A) and female (C) rabbits and 14 days after wounding in male (B) and female (D) rabbits. Corneas were clear prior to wounding, and similar haze was present in both male (B) and female (D) rabbit corneas after wounding. n = 6, scale bar = 2 mm.
3.2. Intraocular pressure in male and female rabbits
At day 0, the average IOP measurement (mmHg) of the six male rabbits before alkali wounding (unwounded) was 8.3 ± 0.21 and after wounding was 9.50 ± 0.56; day 3 was 8.16 ± 0.31; day 7 was 8.83 ± 0.31; and day 14 was 9.0 ± 0.26. The average IOP measurement of the six female rabbits before alkali wounding (unwounded) was 8.5 ± 0.34 and after wounding at day 0 was 9.83 ± 0.49; day 3 was 8.75 ± 0.28; day 7 was 8.75 ± 0.42; and day 14 was 9.17 ± 0.25. While measurements varied slightly between male and female rabbits, the differences observed were not statistically significant within the same sex or between the sexes at any time points studied. The alkali-wounding did not significantly alter IOP from normal values between the sexes (Fig. 3).
Fig. 3.

Intraocular pressure (IOP) measurements taken before wounding (unwounded) and on days 0, 3, 7, and 14 after wound. No significant changes were observed in IOP in male and female rabbits at any time points studied. Error bars represent Mean ± SEM. n = 6.
3.3. Corneal morphology in male and female rabbits
H&E staining of unwounded male (Fig. 4A) and female (Fig. 4B) corneal tissues exhibited normal epithelial, stromal, and endothelial layers. The tissue thickness appeared normal in both groups. H&E staining of male (Fig. 4C) and female (Fig. 4D) corneal tissues on day 14 after alkali wounding exhibited normal-appearing recovered epithelium and endothelium, however, the stroma was thickened and irregular as a result of edema and fibrotic processes.
Fig. 4.

Representative H&E stained unwounded male (A) and female (B) rabbit corneas demonstrated normal cellular structures with no differences between the sexes. At day 14 after wounding, both male (C) and female (D) corneal stroma showed inter-lamellar disorganization, consistent with corneal edema secondary to wounding. n = 6 for each group. Scale bar = 100 μM.
3.4. Fibrotic gene profiling during corneal wound healing in male and female rabbits
To explore the progression of the wound healing process, principal genes involved in corneal stromal wound healing were tested (Fig. 5). α-SMA showed a 5.95 ± 0.21-fold increase in males and 5.32 ± 0.043-fold increase in females; FN showed 3.02 ± 0.24-fold increase in males and 3.23 ± 0.27-fold increase in females; Col-I showed 3.12 ± 0.37-fold increase in males and 3.31 ± 0.24-fold increase in females; TGFβ1 showed 1.65 ± 0.06-fold increase in males and 1.59 ± 0.053-fold increase in females compared to the unwounded corneas (Fig. 5). There was no significant change in the expression of all tested genes between male and female rabbits. As expected, there were significantly increased levels of all tested fibrotic genes in wounded corneas when compared to unwounded corneas (p < 0.001 or p < 0.01), except TGFβ1.
Fig. 5.

Bar graph showing mRNA expression of α-SMA, FN, Col-I, and TGFβ1 in unwounded and wounded rabbit corneas. No significant changes were observed in the expression of α-SMA, FN, Col-I, or TGFβ1 in wounded corneas between males and females after 14 days. Error bars represent Mean ± SEM. n = 6 in each group. ns = no significance between wounded male vs. wounded female corneas.
3.5. Effect of corneal wounding on profibrotic proteins in male and female rabbits
Next, the protein expression in unwounded and wounded male and female rabbit corneas were explored (Fig. 6). Immunofluorescence showed a similar number and distribution of α-SMA (green) +ve cells in both male (Fig. 6C) and female (Fig. 6D) corneas on day 14 after alkali wounding. In unwounded male (Fig. 6A) and female (Fig. 6B) corneas, α-SMA +ve cells were not observed as reported earlier (Gupta et al., 2017). As seen in Fig. 6, wounded male corneas stained 74.16 ± 4.6 and female corneas stained 71.58 ± 7.5 cells +ve for α-SMA (Fig. 6E). There was no significant difference in α-SMA protein expression observed between male and female rabbits.
Fig. 6.

Immunofluorescence staining of α-SMA in male (C) and female (D) rabbit corneas 14 days after wounding. Arrows indicate α-SMA +ve cells in anterior corneal stroma. No α-SMA +ve cells were observed in unwounded male (A) or female (B) rabbit corneas. Quantification data (E) represents no significant changes in the distribution of α-SMA +ve cells in male and female rabbits. Error bars represent Mean ± SEM. n = 6 in each group. ns = no significance between wounded male vs. wounded female corneas. ***p < 0.001 unwounded vs. wounded. Scale bar = 100 μM.
Similarly, the number and distribution of FN +ve cells (Fig. 7) was similar in both male (Fig. 7C) and female (Fig. 7D) corneas on day 14 after alkali wounding. As demonstrated in Fig. 7, alkali wounded male corneas showed 60.11 ± 4.6 FN +ve cells, and female corneas showed 57.41 ± 8.3 FN stained cells, however, the differences in the number of FN +ve cells between male and female corneas was not statistically significant (Fig. 7E). The unwounded male (Fig. 7A) and female (Fig. 7B) corneas showed 0–2 FN +ve cells. On the other hand, the differences between unwounded and wounded rabbit corneas of both sexes were significant (Fig. 7E), which is consistent to our previous report (Gupta et al., 2017).
Fig. 7.

Immunofluorescence staining of FN in male (C) and female (D) rabbit corneas 14 days after wounding. Arrows show FN +ve cells in corneal stroma. A similar distribution of FN +ve cells were observed in unwounded male (A) and female (B) rabbit corneas. Quantification data (E) indicate no significant differences in the distribution of FN +ve cells between male and female rabbits. Error bars represent Mean ± SEM. n = 6 in each group. ns = no significance between wounded male vs. wounded female corneas. ***p < 0.001 unwounded vs. wounded. Scale bar = 100 μM.
The distribution of Col-I stained cells in both male (Fig. 8C) and female (Fig. 8D) corneas on day 14 after alkali wounding was found to be similar. As demonstrated in Fig. 8, wounded male corneas exhibited 84.11 ± 2.8 and female corneas showed 84.55 ± 3.6 +ve stained cells for Col-I (Fig. 8E) whereas unwounded male rabbit corneas showed 30.02 ± 4.5 (Fig. 8A) and female corneas 30.11 ± 3.7 (Fig. 8B) +ve stained cells for Col-I. The difference in the Col-I levels between male and female wounded corneas was not statistically significant, but it was significant between the unwounded vs. wounded (p < 0.001) rabbit corneas (Fig. 8E).
Fig. 8.

Immunofluorescence staining of Col-I in male (C) and female (D) rabbit corneas 14 days after wounding. Arrows show Col-I +ve cells in corneal stroma. A similar distribution of Col-I +ve cells were observed in unwounded male (A) and female (B) rabbit corneas. Quantification data (E) demonstrate no significant changes in the distribution of Col-I +ve cells between male and female rabbits after wounding. Error bars represent Mean ± SEM. n = 6 in each group. ns = no significance between wounded male vs. wounded female corneas. ***p < 0.001 unwounded vs. wounded. Scale bar = 100 μM.
Fig. 9 shows the distribution of TGFβ1 +ve cells in both male (Fig. 9C) and female (Fig. 9D) corneas on day 14 after alkali wounding, which was similar in distribution. In unwounded male (Fig. 9A) and female (Fig. 9B) corneas, TGFβ1 +ve cells were not observed. Conversely, alkali wounded male corneas showed 11.61 ± 2.8 and female corneas 9.5 ± 3.04 +ve stained cells for TGFβ, but there was no significant difference observed between male and female rabbit corneas (Fig. 9E).
Fig. 9.

Immunofluorescence staining of TGFβ1 in male (C) and female (D) rabbit corneas 14 days after wounding. Arrows show TGFβ1 +ve cells in corneal stroma. No TGFβ1 +ve cells were observed in unwounded male (A) and female (B) rabbit corneas. Quantification graph (E) shows no significant differences in the distribution of TGFβ1 +ve cells between male and female rabbits. Error bars represent Mean ± SEM. n = 6 in each group. ns = no significance between wounded male vs. wounded female corneas. ***p < 0.001 unwounded vs. wounded. Scale bar = 100 μM.
3.6. Apoptosis in the cornea of male and female rabbits after corneal wounding
TUNEL assay was performed to compare apoptotic cell death in male and female rabbit corneas after wounding. As shown in Fig. 10 and 44.30 ± 1.21 cells stained +ve for TUNEL in male corneas (Fig. 10C), while 42.75 ± 2.15 of cells stained +ve for TUNEL in female corneas (Fig. 10D) after alkali wounding. Unwounded corneas also showed 14.80 ± 1.7 TUNEL +ve cells in males (Figs. 10A) and 16.16 ± 1.6 +ve cells in females mostly in the epithelium due to cell renewal (Fig. 10B). These results, taken together with the results of unwounded male and female data, suggest that there were no significant differences in TUNEL +ve cells between male and female rabbit corneas during corneal wound healing (Fig. 10E). As expected, a significantly increased TUNEL +ve cells were detected in the wounded rabbit corneal stroma compared to the unwounded corneas (p < 0.001).
Fig. 10.

TUNEL +ve cells indicating apoptosis in male (C) and female (D) rabbit corneas 14 days after wounding. Arrows show TUNEL +ve cells in the corneal stroma. TUNEL +ve cells were also observed in unwounded male (A) and female (B) rabbit corneas. Quantification data (E) indicate no significant changes in the distribution of TUNEL +ve cells between male and female rabbits. Error bars represent Mean ± SEM. n = 6 in each group. ns = no significance between wounded male vs. wounded female corneas. ***p < 0.001 unwounded vs. wounded. Scale bar = 100 μM.
4. Discussion
Wound healing is a complex process influenced by multiple factors including age, weight, and stress levels (Guo and DiPietro, 2010). The role of sex and sex hormones was shown to be significantly involved in the dermal and mucosal wound healing (Engeland et al., 2009; Ashcroft et al., 1997; Ashcroft and Mills, 2002). To date, information about sex as a biological variable in corneal wound healing is extremely limited (Wang et al., 2012; Pham et al., 2019; Krishnan et al., 2012). Differences in wound healing capabilities between males and females are essential to ensure that any therapeutic recommendations are appropriately tailored, if deemed necessary, based on sex. Therefore, in this study, we compared the corneal wound healing process in male and female rabbits using an established in vivo alkali wound model.
Clinical data from slit-lamp biomicroscopy, stereo imaging, and IOP measurements suggest sex plays a limited role in corneal wound healing in rabbits. There were no significant differences found in corneal wound healing between male and female rabbits when examining gross corneal haze and morphology. Microscopic data derived from corneal histopathologic examinations, immunofluorescence, H&E, and TUNEL staining procedures supported the clinical observations. The clinical and pathological data, slit-lamp biomicroscopy, stereo imaging, H&E, and IOP measurements demonstrate that male rabbits exhibit virtually identical responses to female rabbits after the same corneal wound protocol. The cellular and molecular data, qPCR, and immunoflurorescence related to master regulatory genes such as α-SMA, FN, and Col-I showed no differences in expression between male and female rabbit corneas, supporting previous results predominantly performed in female rabbits (Sharma et al., 2011; Mohan et al., 2011; Gupta et al., 2017, 2018). It was interesting that TGFβ1 levels were not significantly elevated in contrast to RNA and protein levels on day 14 in wounded male and female rabbit corneas, which most likely is due to closure of the surface epithelium 3–5 days after wound. Our in vivo measurement of TGFβ1 in rabbit corneas is in contrast to the previous ex vivo rabbit studies (Sriram et al., 2017) and our ongoing in vitro human studies (Mohan et al., unpublished observation) in which recombinant TGFβ1 was added and maintained in culture medium for an extended period of time to promote transdifferentiation of stromal fibroblasts to myofibroblasts, and hence may have increased TGFβ1 transcript level. Nevertheless, this interesting observation regarding TGFβ1 requires further investigation.
The epithelial expression of TGFβ1 in wounded rabbit corneas (Fig. 9) in both sexes was detected higher as reported previously (Tandon et al., 2010; Nishida et al., 1994; Imanishi et al., 2000; Pasquale et al., 1993). Tandon et al. (2010) reported that TGFβ1 expression was observed at early time points of wound healing but later (e.g. on day 14 post-wounding), its expression was diminished and mainly remained in the corneal endothelial cell layer. Tandon et al., (2010) results strongly suggest that TGFβ1 is paramount for activating wound healing processes and that once healing is activated, TGFβ1 expression is self-diminishing.
The sex-based corneal wound healing findings in the present study are different from previous investigations, which reference sex as a contributing factor in several models of tissue wound healing. Previous studies suggest that the differences between male and female tissue healing were related to hormone levels between the sexes. For example, dermal thickness and collagen levels were a function of age and sex, mostly associated with estrogen levels. On the other hand, mucosal proliferation including re-epithelialization and angiogenesis are suggested to be related to testosterone levels. In ocular studies, mRNA of estrogen, androgen, and progesterone receptors are present (Wickham et al., 2000). In humans, the thickness of the central cornea varies with the stage of menstrual cycle (Goldich et al., 2011). However, female rabbits have a different estrous cycle compared to humans (Lebas et al., 1997), and this might contribute to the lack of difference found in corneal wound healing between males and females in our rabbit model. Female rabbits do not have an estrous cycle with regular periods of heat during which ovulation occurs. Female rabbits are considered to be in estrus cycle more or less continuously but ovulation occurs only after mating. Therefore, in the present study, both sexes of rabbits likely had a relatively stable level of male and female hormones over time because no breeding occurred.
Collagen is a critical component in the corneal stroma, and matrix metalloproteinases (MMPs) are zinc-dependent proteases that participate in wound healing and tissue remodeling (Mauch et al., 1994; Zhao et al., 1997; Benaud et al., 1998; Folgueras et al., 2004). Collagen is the main component of the cornea. The corneal stroma consists of dense collagen fibrils (Collagen-I, Collagen V) (Fujikawa et al., 1984; John and David, 2010). Surface epithelium is anchored to the underlying corneal stroma due to constituent collagen components and proteoglycans (Fujikawa et al., 1984). Col-I expression in the unwounded corneal epithelium (Fig. 8) is normal, and it serves to provide both mechanical and tensile strength, which further ensures surface epithelium attachment to corneal stroma (Long et al., 2015; John and David, 2010).
A previous in vitro study of rabbit corneal fibroblasts demonstrated that treatment with female sex hormones (17β-estradiol and progesterone) inhibited the activation and expression of MMP1–3 and MMP9, and inhibited IL-1β-induced collagen degradation (Zhou et al., 2011). In another study, women affected by fungal corneal ulcers took longer to re-epithelialize their corneas compared to men (Krishnan et al., 2012). Similarly, female mice exhibit slower re-epithelialization compared to male mice after corneal wounds (Gronert et al., 2009). These findings indicate that sex hormones do modulate wound healing processes nevertheless likely in a species-dependent manner. Previous studies have shown that human corneas possess sex hormone receptors, which might have clinical implications for the treatment of corneal diseases in people (Suzuki et al., 2001).
Recently Sharif et al., (2019) measured the levels of Prolactin-Induced Protein (PIP) whose expression might be regulated by hormones such as androgens, estrogens, and prolactin. PIP was found in tears, plasma, and saliva collected from 147 patients affected with keratoconus (KC) (105 men and 42 women) and 67 healthy (27 men and 33 women) subjects. Similar to the trend detected in the present study, no statistically significant difference in any tested protein or hormone levels were observed between men and women within groups (e.g. those patients affected by KC or healthy controls) at any time point. By contrast, differences in PIP, estrone, estriol, 17β-estradiol and DHEA were statistically significant in diseased KC vs. normal patients, but those differences demonstrated no evidence of sex predilection between men and women within each cohort. Interestingly in the skin, topically applied estrogen accelerates wound healing (Ashcroft et al., 1997) through the upregulation of hyaluronic acid biosynthesis (Uzuka et al., 1980, 1981). In fact, during corneal wound healing, hyaluronic acid is produced endogenously (Weber et al., 1997; Podskochy and Fagerholm, 1998) and topical application of hyaluronic acid accelerates corneal wound healing (Nakamura et al., 1997; Stiebel-Kalish et al., 1998).
In conclusion, we found that sex does not influence in vivo corneal wound healing in rabbits. However, additional research is warranted, mainly using animal models with estrous cycles more comparable to humans in order to validate the definitive role of sex as a variable in corneal wound healing.
Acknowledgments
This work was primarily supported by the University of Missouri Ruth M. Kraeuchi Missouri Endowed Chair Ophthalmology Fund (RRM), and partially by grants from the National Eye Institute, NIH, R01 EY017294 (RRM), and the Veterans Health Affairs Merit 1I01BX00357 (RRM).
References
- Ambrósio R, Kara-José N, Wilson SE, 2009. Early keratocyte apoptosis after epithelial scrape injury in the human cornea. Exp. Eye Res 89, 597–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Dodsworth J, van Boxtel E, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MW, 1997. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med 3, 1209–1215. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW, 1999. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am. J. Pathol 155, 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Mills SJ, 2002. Androgen receptor-mediated inhibition of cutaneous wound healing. J. Clin. Investig 110, 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Ashworth JJ, 2003. Potential role of estrogens in wound healing. Am. J. Clin. Dermatol 4, 737–743. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, et al. , 2003. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Investig 111, 1309–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benaud C, Dickson RB, Thompson EW, 1998. Roles of the matrix metalloproteinases in mammary gland development and cancer. Breast Canc. Res. Treat 50, 97–116. [DOI] [PubMed] [Google Scholar]
- Benediktsdottir IS, Wenzel A, Petersen JK, Hintze H, 2004. Mandibular third molar removal: risk indicators for extended operation time, postoperative pain, and complications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod 97, 438–446. [DOI] [PubMed] [Google Scholar]
- Bukowiecki A, Hos D, Cursiefen C, Eming SA, 2017. Wound healing studies in cornea and skin: parallels, differences and opportunities. Int. J. Mol. Sci 18 pii:E1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaurasia SS, Kaur H, de Medeiros FW, Smith SD, Wilson SE, 2009. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp. Eye Res 89, 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad SM, Blakey GH, Shugars DA, Marciani RD, Phillips C, White RP Jr., 1999. Patients’ perception of recovery after third molar surgery. J. Oral Maxillofac. Surg 57, 1288–1294. [DOI] [PubMed] [Google Scholar]
- Emmerson E, Campbell L, Ashcroft GS, Hardman MJ, 2010. The phytoestrogen genistein promotes wound healing by multiple independent mechanisms. Mol. Cell. Endocrinol 321, 184–193. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Sabzehei B, Marucha PT, 2009. Sex hormones and mucosal wound healing. Brain Behav. Immun 23, 629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folgueras AR, Pendas AM, Sanchez LM, Lopez-Otin C, 2004. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int. J. Dev. Biol 48, 411–424. [DOI] [PubMed] [Google Scholar]
- Fujikawa LS, Foster CS, Gipson IK, Colvin RB, 1984. Basement membrane components in healing rabbit corneal epithelial wounds: immunofluorescence and ultrastructural studies. J. Cell Biol 98, 128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliver SC, Ashworth JJ, Mills SJ, Hardman MJ, Ashcroft GS, 2006. Androgens modulate the inflammatory response during acute wound healing. J. Cell Sci 119, 722–732. [DOI] [PubMed] [Google Scholar]
- Gilliver SC, Ruckshanthi JP, Hardman MJ, Nakayama T, Ashcroft GS, 2008. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology 149, 5747–5757. [DOI] [PubMed] [Google Scholar]
- Goldich Y, Barkana Y, Pras E, Fish A, Mandel Y, Hirsh A, Tsur N, Morad Y, Avni I, Zadok DJ, 2011. Cataract Refractive Surgery Variations in Corneal Biomechanical Parameters and Central Corneal Thickness during the Menstrual Cycle, vol 37. pp. 1507–1511. [DOI] [PubMed] [Google Scholar]
- Gosain A, DiPietro LA, 2004. Aging and wound healing. World J. Surg 28, 321–326. [DOI] [PubMed] [Google Scholar]
- Gronert K, Sullivan A, Lam K, 2009. Expression of protective lipid circuit and levels of inflammatory lipid mediators demonstrate marked gender differences in a mouse model of acute epithelial injury. Investig. Ophthalmol. Vis. Sci 50, 5526. [Google Scholar]
- Guo S, DiPietro LA, 2010. Factors affecting wound healing. J. Dent. Res 89, 219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Rodier JT, Sharma A, Giuliano EA, Sinha PR, Hesemann NP, Ghosh A, Mohan RR, 2017. Targeted AAV5-Smad7 gene therapy inhibits corneal scarring in vivo. PLoS One 24 (12), e0172928 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Fink MK, Ghosh A, Tripathi R, Sinha PR, Sharma A, Hesemann NP, Chaurasia SS, Giuliano EA, Mohan RR, 2018. Novel combination BMP7 and HGF gene therapy instigates selective myofibroblast apoptosis and reduces corneal haze in vivo. Investig. Ophthalmol. Vis. Sci 59, 1045–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman MJ, Emmerson E, Campbell L, Ashcroft GS, 2008. Selective estrogen receptor modulators accelerate cutaneous wound healing in ovariectomized female mice. Endocrinology 149, 551–557. [DOI] [PubMed] [Google Scholar]
- Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S, 2000. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog. Retin. Eye Res 9, 113–129. [DOI] [PubMed] [Google Scholar]
- John RH, David EB, 2010. The molecular basis of corneal transparency. Exp. Eye Res 91, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen LN, Sorensen LT, Kallehave F, Vange J, Gottrup F, 2002. Premenopausal women deposit more collagen than men during healing of an experimental wound. Surgery 131, 338–343. [DOI] [PubMed] [Google Scholar]
- Krishnan T, Prajna NV, Gronert K, Oldenburg CE, Ray KJ, Keenan JD, Lietman TM, Acharya NR, 2012. Gender differences in re-pithelialisation time in fungal corneal ulcers. Br. J. Ophthalmol 96, 137–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebas F, Coudert P, de Rochambeau H, Thebault RG, 1997. The Rabbit: Husbandry, Health and Production Food and Agriculture Organization of the United Nations, pp. 45–60. [Google Scholar]
- Li J, Chen J, Kirsner R, 2007. Pathophysiology of acute wound healing. Clin. Dermatol 25, 9–18. [DOI] [PubMed] [Google Scholar]
- Long K, Wang L, Liu S, Wang Y, Ren L, 2015. Collagen based film with well epithelial and stromal regeneration as corneal repair materials: improving mechanical property by crosslinking with citric acid. Mater. Sci. Eng. C 55, 201–208. [DOI] [PubMed] [Google Scholar]
- Mathieu D, Linke JC, Wattel F, 2006. Non-healing wounds. In: Mathieu DE (Ed.), Handbook on Hyperbaric Medicine Springer, Netherlands, pp. 401–427. [Google Scholar]
- Mauch C, Krieg T, Bauer EA, 1994. Role of the extracellular matrix in the degradation of connective tissue. Arch. Dermatol. Res 287, 107–114. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Liang Q, Kim WJ, Helena MC, Baerveldt F, Wilson SE, 1997. Apoptosis in the cornea: further characterization of Fas/Fas ligand system. Exp. Eye Res 65, 575–589. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Mohan RR, Kim WJ, Wilson SE, 2000. Modulation of TNF-α-induced apoptosis in corneal fibroblasts by transcription factor NF-κB. Investig. Ophthalmol. Vis. Sci 41, 1327–1336. [PubMed] [Google Scholar]
- Mohan RR, Tandon A, Sharma A, Cowden JW, Tovey JC, 2011. Significant inhibition of corneal scarring in vivo with tissue selective, targeted AAV5 decorin gene therapy. Investig. Ophthalmol. Vis. Sci 52, 4833–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Sato N, Chikama TI, 1997. Hasegawa Y, Nishida T. Hyaluronan facilitates corneal epithelial wound healing in diabetic rats. Exp. Eye Res 64, 1043–1050. [DOI] [PubMed] [Google Scholar]
- Nishida K, Kinoshita S, Yokoi N, Kaneda M, Hashimoto K, Yamamoto S, 1994. Immunohistochemical localization of transforming growth factor-beta 1, -beta 2, and -beta 3 latency-associated peptide in human cornea. Investig. Ophthalmol. Vis. Sci 35, 3289–3294. [PubMed] [Google Scholar]
- Pasquale LR, Dorman-Pease ME, Lutty GA, Quigley HA, Jampel HD, 1993. Immunolocalization of TGF-beta 1, TGF-beta 2, and TGF-beta 3 in the anterior segment of the human eye. Investig. Ophthalmol. Vis. Sci 34, 23–30. [PubMed] [Google Scholar]
- Pavletic MM, 2010. Basic principles of wound healing. In: Atlas of Small Animal Wound Management and Reconstructive Surgery, third ed. Wiley-Blackwell, pp. 17–29. [Google Scholar]
- Pham TL, Kakazu A, He J, Bazan HEP, 2019. Mouse strains and sexual divergence in corneal innervation and nerve regeneration. FASEB J 33, 4598–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips C, White RP Jr., Shugars DA, Zhou X, 2003. Risk factors associated with prolonged recovery and delayed healing after third molar surgery. J. Oral Maxillofac. Surg 61, 1436–1448. [DOI] [PubMed] [Google Scholar]
- Podskochy A, Fagerholm P, 1998. Cellular response and reactive hyaluronan production in UV-exposed rabbit corneas. Cornea 17, 640–645. [DOI] [PubMed] [Google Scholar]
- Sharma A, Tandon A, Tovey JC, Gupta R, Robertson JD, Fortune JA, Klibanov AM, Cowden JW, Rieger FG, Mohan RR, 2011. Polyethylenimine-conjugated gold nanoparticles: gene transfer potential and low toxicity in the cornea. Nanomedicine 7, 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif R, Bak-Nielsen S, Sejersen H, Ding K, Hjortdal J, Karamichos D, 2019. Prolactin-induced protein is a novel biomarker for keratoconus. Exp. Eye Res 179, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram S, Tran JA, Guo X, Hutcheon AEK, Kazlauskas A, Zieske JD, 2017. Development of wound healing models to study TGFβ3’s effect on SMA. Exp. Eye Res 161, 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiebel-Kalish H, Gaton DD, Weinberger D, Loya N, Schwartz-Ventik M, Solomon A, 1998. A comparison of the effect of hyaluronic acid versus gentamicin on corneal epithelial healing. Eye 12, 829–833. [DOI] [PubMed] [Google Scholar]
- Strudwick X, Powell BC, Cowin AJ, 2006. Role of sex hormones in acute and chronic wound healing. Prim. Intention Aust. J. Wound Manag 14, 35–38. [Google Scholar]
- Suzuki T, Kinoshita Y, Tachibana M, Matsushima Y, Kobayashi Y, Adachi W, Sotozono C, Kinoshita S, 2001. Expression of sex steroid hormone receptors in human cornea. Curr. Eye Res 22, 28–33. [DOI] [PubMed] [Google Scholar]
- Tandon A, Tovey JC, Sharma A, Gupta R, Mohan RR, 2010. Role of transforming growth factor Beta in corneal function, biology and pathology. Curr. Mol. Med 10, 565–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon A, Sharma A, Rodier JT, Klibanov AM, Rieger FG, Mohan RR, 2013. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PLoS One 8, e66434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzuka M, Nakajima K, Ohta S, Mori Y, 1980. The mechanism of estrogen-induced increase in hyaluronic acid biosynthesis, with special reference to estrogen receptor in the mouse skin. Biochem. Biophys. Acta 627, 199–206. [DOI] [PubMed] [Google Scholar]
- Uzuka M, Nakajima K, Ohta S, Mori Y, 1981. Induction of hyaluronic acid synthetase by estrogen in the mouse skins. Biochim. Biophys. Acta 673, 387–393. [DOI] [PubMed] [Google Scholar]
- Velnar T, Bailey T, Smrkoj V, 2009. The wound healing process: an overview of the cellular and molecular mechanism. J. Int. Med. Res 37, 1528–1542. [DOI] [PubMed] [Google Scholar]
- Wang SB, Hu KM, Seamon KJ, Mani V, Chen Y, Gronert K, 2012. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J 26, 1506–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber BA, Fagerholm P, Johansson B, 1997. Colonization of hyaluronan and water in rabbit corneas after photorefractive keratectomy by specific staining for hyaluronan and by quantitative microradiography. Cornea 16, 560–563. [PubMed] [Google Scholar]
- Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA, 2000. Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol. Scand 78, 146–153. [DOI] [PubMed] [Google Scholar]
- Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Hong J, Lee J, 2001. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog. Retin. Eye Res 20, 625–637. [DOI] [PubMed] [Google Scholar]
- Zhao H, Cai G, Du J, Xia Z, Wang L, Zhu T, 1997. Expression of matrix metalloproteinase-9 mRNA in osteoporotic bone tissues. J. Tongji Med. Univ 17, 28–31. [DOI] [PubMed] [Google Scholar]
- Zhou H, Kimura K, Orita T, Nishida T, Sonoda KH, 2011. Inhibition by female sex hormones of collagen degradation by corneal fibroblasts. Mol. Vis 17, 3415–3422. [PMC free article] [PubMed] [Google Scholar]


