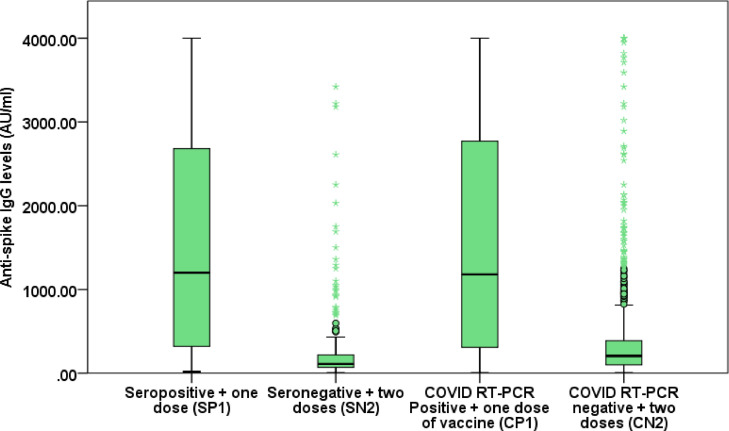
At present, the most effective strategy against the COVID-19 pandemic is to reach a point where the majority of the population is immune, either from natural infection or vaccination. Complete vaccination is a herculean task in a populous country such as India due to limited vaccine availability. Two doses are needed for most vaccines, where the second dose acts as a booster. Alternatively, SARS-CoV-2 infection could act as a natural vaccine (Prime dose) after which, one dose could act as a booster dose and may give adequate immunity. While no large-scale data are available, small series have suggested that this may be true ( Manisty et al., 2021). In a study by Sasikala et al. from India, heightened immune and memory responses to a single dose of ChAdOx1 nCoV-19 were seen in previously infected individuals compared with those with no prior exposure (Sasikala et al., 2021). To explore if this policy works as a part of a larger prospective study on 3258 healthcare workers, the study subjects were divided into four different groups, namely, history of RT-PCR proven COVID-19 (CP), COVID naïve defined by no history of RT-PCR proven COVID-19 (CN), baseline seropositive (SP), and baseline seronegative (SN). Thereafter, a comparison was made on the protective effects of one dose of ChAdOx1 nCoV-19 vaccine (AZD1222) with previous RT-PCR proven COVID-19 (CP1) or seropositive individuals (SP1), and two doses in COVID naïve (CN2) or seronegative (SN2) individuals The study was conducted after approval from the institutional ethics committee. Seropositivity was defined as presence of anti-spike protein IgG antibody (anti-spike IgG) ≥15 AU/ml, produced against SARS-CoV-2 (detected by automated LIAISON® SARS-CoV-2 S1/S2 IgG) and may result from asymptomatic (Nikolai et al., 2020) or symptomatic COVID infections. The mean duration between COVID infection and the vaccination was 140 days, and anti-spike IgG was measured at 14 and 28 days after the first and the second doses, respectively. We found that median anti-spike IgG in group CP1 was significantly higher than in CN2 (1573.2 vs 418.4, P <0.0001) and in SP1 compared with SN2 (1200 vs 111, P <0.0001) (Table 1 ). The mean (geometric) rise in anti-spike IgG after vaccination was also higher in CP1 than CN2 and in SP1 than SN2 (Table 1). Further, the seroconversion rate was similar in CP1 and CN2 (210/214 [98.1%] vs. 822/838 [98.1%], P = 0.969). This suggests that one dose of the AZD1222 vaccine after previous COVID infection or seropositive status mounts a better anti-spike IgG response at 4 weeks than two doses of it in COVID naïve or seronegative individuals. Similar observations have been made in a few other studies with mRNA (Krammer et al., 2021; Bradley et al., 2021) and ChAdOx1 nCoV-19 (Sasikala et al., 2021) vaccine. In conclusion, India and other countries with a low vaccine stocks/population ratio could adopt a strategy of persons with pre-existing anti-spike IgG and/or COVID infection being given only one vaccine dose, thus allowing a quicker and wider coverage of the population to induce immunity against COVID-19. The effectiveness and robustness of immunological responses in these subjects should, however, be confirmed in further prospective studies (Box Plot 1 ).
Table 1.
Anti-spike IgG levels (in terms of median and geometric mean) among seropositive individuals before 1st dose of vaccination (SP), history of COVID-19 RT-PCR positive before 1st dose of vaccination (CP), SP + one dose of vaccine (SP1), seronegative (SN) + two doses (SN2), CP + one dose of vaccine (CP1) and COVID RT-PCR negative (CN) + two doses (CN2)
| Group | n | Anti-spike IgG levels (AU/ml) Median (IQR) | –p-value | Anti-spike IgG levels (AU/ml) Geometric Mean ± SD | –p-value |
|---|---|---|---|---|---|
| Seropositive before 1st dose of vaccination (SP) | 1119 | 39.9 (25.7 – 67.6) | 0.379 | 42.30±1.85 | 0.001a |
| History of COVID-19 RT-PCR positive before 1st dose of vaccination (CP) | 472 | 41.3 (20.5 – 78.6) | 35.68±2.86 | ||
| SP + one dose of vaccine (SP1) | 581 | 1200 (320 – 2680) | < 0.0001a | 906.69±3.40 | < 0.0001a |
| Seronegative (SN) + two doses (SN2) | 420 | 111 (67 – 217) | 117.09±2.89 | ||
| CP + one dose of vaccine (CP1) | 214 | 1573.2 (308.0 – 2770.0) | < 0.0001a | 896.65±4.86 | < 0.0001a |
| COVID RT-PCR negative (CN) + two doses (CN2) | 838 | 418.4 (97.9 – 387.0) | 202.49±3.26 |
P-value < 0.05, statistically significant; IQR , Inter Quartile Range.
Box Plot 1.
Box plot of Anti-spike IgG levels for SP1, SN2, CP1 and CN2
Declaration of Competing Interest
The authors have no competing interests to declare.
Acknowledgments
Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics committee approval
The work has been approved by Institutional Ethics Committee. The approval reference number is 1222/2021 Academic), dated January 11, 2021.
Authors’ contribution
Vikas Deswal: conceptualization, data collection, data analysis and manuscript writing. Rashmi Phogat: Laboratory analysis, data collection. Pooja Sharma: Conceptualization, data analysis and manuscript writing. Sushila kataria Data analysis, manuscript writing, Proof reading Arvinder soin- Conceptualization, data analysis, manuscript writing, proof reading.
References
- Bradley T, Grundberg E, Selvarangan R, LeMaster C, Fraley E, Banerjee D, et al. Antibody responses after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1959–1961. doi: 10.1056/NEJMc2102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Srivastava K, Alshammary H, Amoako AA, Awawda MH, Beach KF, et al. Antibody responses in seropositive persons after a single dose of SARS-CoV-2 mRNA vaccine. N Engl J Med. 2021;384:1372–1374. doi: 10.1056/NEJMc2101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manisty C, Otter AD, Treibel TA, McKnight Á, Altmann DM, Brooks T, et al. Antibody response to first BNT162b2 dose in previously SARS-CoV-2-infected individuals. Lancet. 2021;397:1057–1058. doi: 10.1016/S0140-6736(21)00501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolai LA, Meyer CG, Kremsner PG, Velavan TP. Asymptomatic SARS coronavirus 2 infection: invisible yet invincible. Int J Infect Dis. 2020;100:112–116. doi: 10.1016/j.ijid.2020.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasikala M, Shashidhar J, Deepika G, Ravikanth V, Krishna VV, Sadhana Y, et al. Immunological memory and neutralizing activity to a single dose of COVID-19 vaccine in previously infected individuals. Int J Infect Dis. 2021;108:183–186. doi: 10.1016/j.ijid.2021.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]