Abstract
Background
Current guidelines recommend vaccination against SARS-CoV2 for people with multiple sclerosis (pwMS). The long-term review of the safety and effectiveness of COVID-19 vaccines in pwMS is limited.
Methods
Service re-evaluation. PwMS using the MS service at Barts Health National Health Service Trust were sent questionnaires via email to report symptoms following first and second COVID-19 vaccinations (n = 570). A retrospective review of electronic health records was conducted for clinical and safety data post-vaccination(s); cut-off was end of September 2021. Separate logistic regressions were carried out for symptoms experienced at each vaccination. Two sets of regressions were fitted with covariates: (i) Disease-modifying therapy type and (ii) patient characteristics for symptoms experienced.
Results
193/570 pwMS responded. 184 pwMS had both vaccinations. 144 received the AZD1222 and 49 the BNT162b2 vaccine. 87% and 75% of pwMS experienced any symptoms at first and second vaccinations, respectively. The majority of symptoms resolved within a short timeframe. No severe adverse effects were reported. Two pwMS subsequently died; one due to COVID-19 and one due to aspiration pneumonia. Males were at a reduced risk of reporting symptoms at first vaccination. There was evidence that pwMS in certain treatment groups were at reduced risk of reporting symptoms at second vaccination only.
Conclusions
Findings are consistent with our preliminary data. Symptoms post-vaccination were similar to the non-MS population and were mostly temporary. It is important to inform the MS community of vaccine safety data.
Keywords: SARS-CoV2, COVID-19, Vaccination, Multiple sclerosis
1. Introduction
Vaccination against SARS-CoV2 remains a key mitigation strategy during the COVID-19 pandemic, particularly in vulnerable populations, such as people with multiple sclerosis (pwMS) (Salter et al., 2021; Tallantyre et al., 2021). Adverse effects may be modulated by disease-modifying immunotherapy (DMT) and affect vaccination uptake. Following our preliminary experience (Allen-Philbey et al., 2021), we report here on a significantly larger cohort of pwMS receiving a full course of COVID-19 vaccination.
2. Methods
2.1. Study design
This service re-evaluation is registered with the Clinical Effectiveness Unit (#12274) of Barts Health National Health Service Trust (BHT). COVID-19 vaccination for pwMS was coordinated through the BHT MS service (BartsMS), and patients were issued with a questionnaire via email (Allen-Philbey et al., 2021), which they were asked to complete seven days following immunisations and return. People with MS on high efficacy DMTs were more likely to get an email than pwMS not on DMT or pwMS on platform DMT. Reminder emails and/or text messages were sent to non-responders. Demographic data including age, sex, ethnicity, expanded disability status scale (EDSS) score, disease course and duration, DMT and time from last course of DMT was obtained from the electronic health record. Data cut-off was 30 September 2021.
2.2. Statistical analysis
All symptoms were combined to create a binary “any symptoms” indicator reflecting at least one of the following: sore arm, fever, flu-like symptoms, any other symptoms. Logistic regressions were carried out for each vaccination. Two sets of regressions were fitted to explore associations between symptoms and (i) DMT and (ii) patient-characteristics.
In the analysis of DMT three covariates were included: (i) type of DMT, (ii) time from treatment to vaccination (months) and (iii) vaccine type (BNT162b2 or AZD1222). DMT types were classified as follows: B-cell depleters (ocrelizumab, ofatumumab, rituximab), fingolimod and dimethyl fumarate, immune reconstitution therapies (IRT: alemtuzumab and cladribine), natalizumab, other/none.
In the analysis of patient characteristics, covariates included gender, ethnic group, EDSS, disease course and duration, time from last treatment to vaccination, vaccine type.
Multiple imputation (MI) was used to account for missing data. MI by chained equations with predictive mean matching was applied with each regression. Data was only imputed for those who had received a vaccine at each stage. All analyses were carried out using Team RC et al. (2013) with package ‘mice (Van Buuren and Groothuis-Oudshoorn, 2011).
3. Results
A total of 570 questionnaires were sent. N = 193 pwMS (34%) who received at least one COVID-19 vaccine responded (Table 1 ). All pwMS who had two vaccinations received the same vaccine at both time points; 144 (75%) received AZD1222, and 49 (25%) BNT162b2. Nine pwMS received only one vaccination. The mean time between vaccinations was 2 ± 0.8 (standard deviation.SD) months.
Table 1.
Patient demographics and clinical characteristics.
| Total n | 193 |
| Age at first vaccine [years] mean (SD) | 47.4 (11.6) |
| Sex n (%) | |
| Female | 127 (65.8) |
| Male | 66 (34.2) |
| Ethnicity n (%) | |
| Asian | 12 (6.2) |
| Black | 7 (3.6) |
| Not recorded | 72 (37.3) |
| Other | 6 (3.1) |
| White | 96 (49.7) |
| Disease course n (%) | |
| PPMS | 16 (8.3) |
| RRMS | 147 (76.2) |
| SPMS | 30 (15.5) |
| Disease duration from onset of first symptom [years] (median, IQR) | 12.00 (7.00, 18.00) |
| EDSS (median, IQR) | 3.50 (2.00, 6.00) |
| DMT n (%) | |
| None | 12 (6.2) |
| Alemtuzumab | 14 (7.3) |
| Cladribine | 49 (25.4) |
| Dimethyl fumarate | 16 (8.3) |
| Fingolimod | 21 (10.9) |
| Natalizumab | 14 (7.3) |
| Ocrelizumab | 62 (32.1) |
| Other* | 5 (2.6) |
| Time from last treatment to first vaccine [months] mean (SD) | 13.2 (14.8) |
Other” group for DMTs composed of two pwMS on rituximab, one on ofatumumab, one on glatiramer acetate and one on interferon β−1a (Avonex).
3.1. Adverse events
Any symptoms” were reported by 87% after their first and 75% after their second vaccination. The three main symptoms resolved within 48 h in 65% (first vaccination) and 57% (second vaccination). The most common symptom was a sore arm, followed by flu-like symptoms. Fatigue and headache were the most common “other” symptoms reported (Supplementary Table S1).
Following the first vaccination, 30 pwMS (16%) did not report any symptoms, 126 (65%) had a sore arm, 86 (45%) flu-like symptoms and 53 (28%) fever. Sixty-one pwMS (32%) reported “other symptoms” (Table S1). In 125 (65%) the three key symptoms resolved within 48 h; 37 pwMS (19%) reported that symptoms lasted longer than that and in one pwMS (0.5%) the resolution period was not documented.
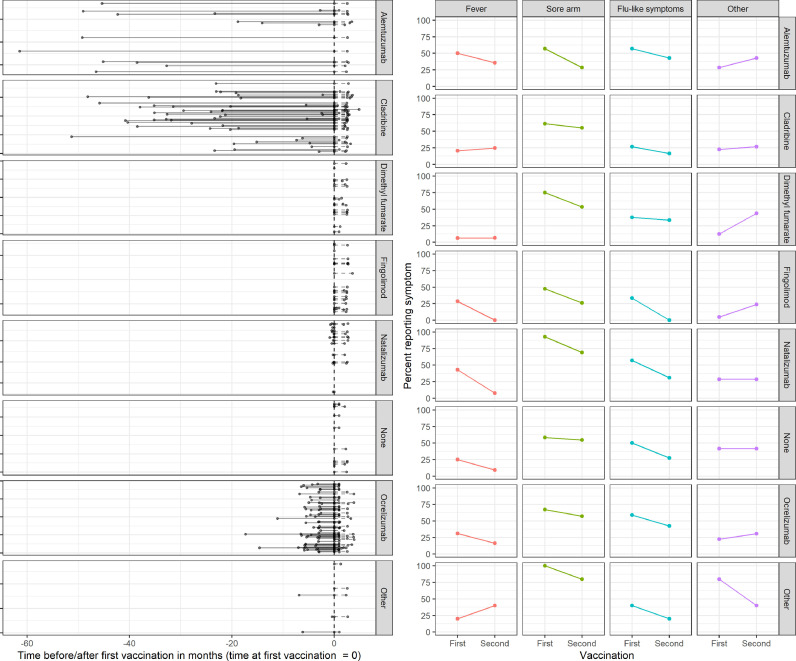
After the second vaccination, 59 pwMS (32%) did not report any symptoms, 98 (53%) had a sore arm, 53 (29%) flu-like symptoms and 32 (17%) fever. Forty-three pwMS (23%) reported “other symptoms” (Table S1). In 104 (57%) the three key symptoms resolved within 48 h; in 17 (9%) it took longer than that; in four (2%) resolution period was not documented. Fig. 1 illustrates the symptoms reported by pwMS at each vaccination, according to their DMT group.
Fig. 1.
Disease modifying treatment and symptoms reported by people with MS following COVID-19 vaccination. People with MS were vaccinated with either BNT162b2 or AZD1222. The left panel indicates the time from last/current disease modifying treatment (DMT) until first vaccination. The first and second dots delineate the time between last treatment and first vaccination; the last dot indicates the time point of second vaccination. The median time from last DMT administration to first vaccination in pwMS was 4 months (3-5.6, n=62) for ocrelizumab, 22 (18.3-32.7, n=49) for cladribine, 40 (20-46.2, n=14) for alemtuzumab, 0.3 (0.2-0.5, n=14) for natalizumab, and 6 (3.3-6.5, n=3) for other DMT. The right panel describes the symptoms reported following first and second vaccination, stratified by DMT.
3.2. Safety
Four females with MS (2%) had a relapse three weeks after their first (n = 1) and seven weeks after their second (n = 3) vaccination. Two pwMS died. The first was a 58-year-old man with relapsing MS and an EDSS=4 who died from COVID-19 four months after his last ocrelizumab infusion and 153 days after his second COVID-19 vaccination. The second was a 60-year-old woman with secondary-progressive MS and an EDSS=8 who died from aspiration pneumonia two weeks following her first vaccination; she was SARS-CoV2-negative. Six pwMS (3%) contracted SARS-CoV2 after second vaccination. Of these, three were on ocrelizumab (one death, see above), one on ofatumumab and two on fingolimod. Mean time from second vaccination to infection was 4 months (range 2.2–6.3). Two pwMS treated with ocrelizumab received AZD1222, all others BNT162b2. One pwMS on ocrelizumab was admitted to hospital, and to intensive care, twice requiring continuous positive airway pressure. They were discharged after 10 weeks, with ongoing home oxygen therapy.
3.3. Regression results
After the first vaccination, there was no evidence of a difference in odds of reporting symptoms between DMT groups (Table S2). After the second vaccination, there was evidence that pwMS who had been treated with fingolimod or dimethyl fumarate had approximately 80% lower odds of reporting any symptoms (odds ratio (OR): 0.18; 95% confidence interval (CI): 0.07, 0.49). There was also some evidence that pwMS who had been treated with either of the IRTs had reduced odds of reporting symptoms (OR: 0.32; 0.1, 1) though there was uncertainty around this estimate (Table S2). All pwMS who had been treated with natalizumab reported at least one symptom so the OR could not be estimated for this subgroup.
There was evidence that men were at lower risk of reporting any symptoms after their first vaccination (OR for men compared to women: 0.17; 0.06, 0.48) (Table S3). There was no evidence that any other factor was associated with any symptoms at first vaccination. There was also no evidence that any patient characteristic was associated with symptoms after the second vaccination, although there was a large degree of uncertainty for some characteristics implied by their wide confidence intervals.
4. Discussion
Immunization through vaccination remains one of the most effective public health strategies to mitigate the COVID-19 pandemic. Particularly vulnerable individuals were encouraged to engage with the vaccination programme, including more recently their priority invitation for third and fourth vaccinations. In the United Kingdom, this subgroup of the population specifically includes pwMS (COVID-19, 2022).
With 144 pwMS receiving AZD1222, our cohort is, to our knowledge, the largest reporting on acute adverse effects of a viral vector SARS-CoV2 vaccination in MS. Our data suggest that both of the COVID-19 vaccines (AZD1222 and BNT162b2) used in our cohort were generally well tolerated (Garjani et al., 2021). The overall number of pwMS experiencing any symptoms was higher than at other centers, though symptoms were mostly transient (Lotan et al., 2021; Briggs et al., 2022). There was evidence that pwMS in certain DMT groups had a lower risk of symptoms after their second vaccination. Men were less likely to report symptoms at first vaccination, as described by another study (Briggs et al., 2022).
Adverse events were generally consistent with those experienced in the general population, and in pwMS receiving other vaccinations. Whilst symptoms were unpleasant, affecting 87% after their first and 75% after their second vaccine, they resolved in 2/3 within a couple of days. Symptoms reported resembled those expected from both the normal population and from experience of pwMS with other vaccinations (Reyes et al., 2020) and our preliminary data (Allen-Philbey et al., 2021).
Six of our patients (3%) contracted SARS-CoV2. This number is rather small to draw any robust conclusion. However, all infections occurred in pwMS treated with DMTs known to impede the immunization response (Tallantyre et al., 2021). The one pwMS who died of COVID was on treatment with ocrelizumab, as were two further pwMS, one of which had to be admitted to intensive care twice.
There have been reports of relapse within a short time frame following first doses of the AZD1222 vaccine (Maniscalco et al., 2021; Nistri et al., 2021). Eight cases were reported after a median of 13 days, with increased disability and lesions on MRI (Fragoso et al., 2021). Whilst reporting of relapses in the literature has thus far been confined to case reports, we detected an overall relapse incidence following either vaccination of 2%, which is consistent with others reporting relapse rates of 2.1% and 1.6% (Achiron et al., 2021). A larger dataset would be required to define the real risk, though the overall incidence appears low, and would, based on current evidence, certainly not support avoiding vaccination (Garjani et al., 2021).
Limitations of this real world study include selection bias given that pwMS at higher risk of COVID-19, particularly those on B cell depleting compounds, were more likely to be contacted and reminded. A degree of responder bias would also be expected given pwMS with an adverse experience are more likely to convey their grievances and return their questionnaire. This is important to bear in mind given the overall modest responder rate (34%). Due to the binary analysis and the brevity of the response window, no grading of symptoms was included. Since mild symptoms may not be reported outside of a survey, a degree of over reporting cannot be excluded.
In conclusion, our data provides supportive evidence that SARS-CoV2 vaccination is generally well tolerated in pwMS. This is important given further vaccinations as a result of the pandemic are likely.
Declaration of Competing Interest
KAP, AS, TB, ACJ, AM and SG have nothing to disclose. RD has received research support from Biogen, Merck, and Celgene, and honoraria/meeting support from Biogen, Merck, Roche, Sanofi-Genzyme, and Teva. GG has received honoraria and meeting support from AbbVie Biotherapeutics, Biogen, Canbex, Ironwood, Novartis, MSD, Merck Serono, Roche, Sanofi Genzyme, Synthon, Teva and Vertex. He also serves as chief editor for Multiple Sclerosis and Related Disorders and is the academic director of the Neurology Academy. SG has received honoraria from Biogen Idec, Sanofi Genzyme, Janssen Cilag, Merck, Neurodiem, Novartis, Roche, and Teva and grant support from ECTRIMS, Genzyme, Merck, National MS Society, Takeda and UK MS Society. MM has received honoraria and travel costs from Genzyme, AbbVie, Roche and Novartis. IS was funded by an ECTRIMS clinical fellowship grant in 2019 and has received honoraria from Biogen Idec, Neurodiem and Merck. BPT has received honoraria, travel grants, and been a member of advisory boards for Biogen, Merck Serono, Novartis, Sanofi Genzyme and Roche. DB has received compensation from InMuneBio, Lundbeck, Merck, Novartis, Rock and Teva. JM has received honoraria and meeting support from Arvelle, Biogen, Novartis, Merck Serono, Roche and Sanofi Genzyme. KS has received research support from Biogen, Merck KGaA, and Novartis, speaking honoraria from, and/or served in an advisory role for, Amgen, Biogen, EMD Serono, Merck KGaA, Novartis, Roche, Sanofi-Genzyme, and Teva; and remuneration for teaching activities from AcadeMe, Medscape and the Neurology Academy.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.msard.2022.104022.
Appendix. Supplementary materials
References
- Salter A., Fox R.J., Newsome S.D., et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol. 2021;78(6):699. doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallantyre E.C., Vickaryous N., Anderson V., et al. COVID-19 vaccine response in people with multiple sclerosis. Ann. Neurol. 2021;22 doi: 10.1002/ana.26251. Published online October. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen-Philbey K., Stennett A., Begum T., et al. Experience with the COVID-19 AstraZeneca vaccination in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2021;52 doi: 10.1016/j.msard.2021.103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team R.C., Others. R: a language and environment for statistical computing. Published online 2013. http://r.meteo.uni.wroc.pl/web/packages/dplR/vignettes/intro-dplR.pdf.
- Van Buuren S., Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J. Stat. Softw. 2011;45:1–67. [Google Scholar]
- COVID-19: Guidance for people whose immune system means they are at higher risk. GOV.UK. Accessed January 17, 2022. https://www.gov.uk/government/publications/covid-19-guidance-for-people-whose-immune-system-means-they-are-at-higher-risk/.
- Garjani A., Middleton R.M., Hunter R., et al. COVID-19 is associated with new symptoms of multiple sclerosis that are prevented by disease modifying therapies. Mult. Scler. Relat. Disord. 2021;52 doi: 10.1016/j.msard.2021.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan I., Wilf-Yarkoni A., Friedman Y., Stiebel-Kalish H., Steiner I., Hellmann M.A. Safety of the BNT162b2 COVID-19 vaccine in multiple sclerosis (MS): early experience from a tertiary MS center in Israel. Eur. J. Neurol. 2021;28(11):3742–3748. doi: 10.1111/ene.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F.B.S., Mateen F.J., Schmidt H., et al. COVID-19 vaccination reactogenicity in persons with multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2022;9(1):e1104. doi: 10.1212/nxi.0000000000001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes S., Ramsay M., Ladhani S., et al. Protecting people with multiple sclerosis through vaccination. Pract. Neurol. 2020;20(6):435–445. doi: 10.1136/practneurol-2020-002527. [DOI] [PubMed] [Google Scholar]
- Maniscalco G.T., Manzo V., Di Battista M.E., et al. Severe multiple sclerosis relapse after COVID-19 Vaccination: a case report. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.721502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistri R., Barbuti E., Rinaldi V., et al. Case report: multiple sclerosis relapses after vaccination against SARS-CoV2: a series of clinical cases. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.765954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso Y.D., Gomes S., Gonçalves M.V.M., et al. New relapse of multiple sclerosis and neuromyelitis optica as a potential adverse event of AstraZeneca AZD1222 vaccination for COVID-19. Mult. Scler. Relat. Disord. 2021 doi: 10.1016/j.msard.2021.103321. Published online October 13. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Achiron A., Dolev M., Menascu S., et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by. Mult. Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. February 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.