Abstract
Background
It is unclear whether diabetic retinopathy (DR) can be a predictor of stroke. In this research context, the objective of our study was to investigate whether there is a significant association between DR and stroke in diabetic patients by meta-analysis.
Methods
After a systematic search of studies in electronic databases, we screened all studies reporting the risk of DR status and stroke incidence and calculated their odds ratios (ORs) and hazard ratios (HRs). The effects of type of diabetes and severity of DR were also considered for subgroup analysis.
Results
We included 19 studies involving 45 495 patients. A pooled HR = 1.62 (1.28-2.06) were found for the risk of DR and stroke in diabetic patients. In a subgroup analysis performed on the type of diabetes, the results showed a significant association between stroke incidence and DR status in patients with type 2 diabetes (T2D) (OR: 1.78; 95% CI, 1.53-2.08), but this association was not conclusive in type 1 diabetes (T1D) (OR: 1.77; 95% CI, 0.48-6.61). The results of the subgroup analysis with diabetes severity showed that both mild and moderate nonproliferative diabetic retinopathy (NPDR) status and severe NPDR and worse status significantly increased the risk of stroke with HRs of 2.01 (1.45-2.78) and 2.27 (1.52-3.39), respectively.
Conclusion
DR status in diabetic patients is associated with an increased risk of stroke. This correlation was robust in patients with T2D, but uncertain in T1D. Based on this result, we have perhaps found the new factor for stroke management, so we analyzed the necessity and advantages of considering DR as a factor for stroke screening and risk management in our studies.
Keywords: diabetic retinopathy, stroke, meta-analysis
Diabetic retinopathy (DR), one of the most common microvascular complications of diabetes, is diagnosed in one-third of diabetic patients and is the leading cause of blindness in people of working age [1, 2]. In addition, the incidence and development of DR is associated with numerous systemic diseases, including stroke, which is the second leading cause of death of the world’s population according to epidemiological research [3], especially in low-income and middle-income countries, where the number of stroke patients is increasing by about 2 million per year due to the aging of the population and the growth of the population base [3-5].
To overcome this critical situation, the prediction of risk factors and the related management for stroke is becoming the one of the most important directions of current treatment. Previous studies [6-8] have mostly correlated the risk of stroke with cardiovascular events (CVD); however, as more and more clinical studies and statistical reviews have been conducted, CVD no longer seems to be a strong explanation and predictor of the development of stroke.
For this reason, the studies on the correlation between stroke and diabetes have received a lot of attention in recent years. It is known that diabetes can be an important risk factor for stroke, and the incidence of stroke is 2 to 4 times higher in people with diabetes than in those without diabetes [9]. However, diabetes is also a very important and widespread disease, and the process of microvascular and macrovascular damage that it causes also contributes to the risk of stroke [10, 11]. Therefore, secondary prevention of stroke from a diabetic perspective is now the key to risk management.
DR has now received increasing attention and focus. According to epidemiological and evidence-based surveys [4, 12], the prevalence of DR among diabetic patients worldwide is 34.6%.
Owing to the comprehensive use and development of ophthalmic examinations, combined with its convenient operation and low cost, DR can be included in the routine screening for chronic complications of diabetes, thus providing the potential for early diagnosis of DR and its use as a stroke screening test. On the one hand, it is increasingly recognized that microvascular disease plays an important role in stroke research [13], and DR as a diabetic microvascular complication naturally falls into this range. On the other hand, although clinical studies on DR and stroke have been conducted in recent years [10, 14, 15], the results have been inconsistent and there is a lack of high-level evidence-based studies.
In such a research background, the objective of our study was to investigate whether there is a significant association between DR and stroke in diabetic patients by meta-analysis. We also briefly analyze the progress of those relevant studies, besides the mechanisms of DR-stroke interaction and the prospects of DR screening, concluding that it is necessary and advantageous to include DR as an independent factor in stroke risk management.
Materials and Methods
Data Retrieval and Study Screening
Two researchers (Z.W. and K.Z.) independently searched relevant studies in common databases such as PubMed, Web of Science, Embase, and Cochrane Library. The range was set to studies from all countries during 1976 o 2022, the language of publication was English, and the key words and subject terms (medical subject headings; MeSH) were “stroke,” “cerebrovascular events,” “cerebrovascular accidents,” “cerebrovascular accidents,” “cerebral palsy,” “diabetic retinopathy,” “non-proliferative diabetic retinopathy,” and “proliferative diabetic retinopathy.” Also, to identify potential additional studies, we searched Google Scholar, relevant reviews, and references cited in studies that met the criteria. The eligibility criteria related to study characteristics were population-based retrospective studies, cohort studies, or randomized controlled trials reporting on the association between DR in any state and all types of stroke events. Our supplemental search strategy was consistent the aforementioned criteria, with a last search date of June 1, 2022.
All search results were exported to EndNote X8 (Bld 10063) software for subsequent screening. Two researchers (Z.W. and K.Z.) separated the studies into odds ratio (OR) and hazard ratio (HR) groups according to the type of findings; articles with OR as a outcome were identified with a specific sample size (n) for the DR or stroke group for subsequent analysis. Those articles that did not mention sample size (n) in the full text were treated as follows: (i) the specific sample size was calculated for each group using the given baseline data or outcome event data; and (ii) the corresponding author of the article was contacted to obtain the required data. Studies for which data could not be obtained by the aforementioned methods were excluded.
In addition, the exclusion criteria included (i) studies in which the diagnostic methods for stroke and DR were not described in the text or were considered inappropriate by the researchers; (ii) for different studies of patients in the same region (community, hospital, or medical center), we included only articles with higher-quality assessment results the Newcastle-Ottawa Scale (NOS), and if the quality scores were the same, we included the most recently published one; (iii) studies with a specific coverage and propensity to observe participants. The review of articles and data was performed independently by 2 reviewers (J.Y. and X.Z.), who reviewed and cross-checked the articles before analysis. In case of any disagreement, discussion or consultation with the supervising researcher (D.C.) determined a final decision.
Assessment of Study Quality and Risk of Bias
Two reviewers (Y.C. and R.C.) evaluated study quality and risk of bias for the included studies using the Newcastle-Ottawa Scale. The quality parameters rated include selection (4 points), comparability (2 points), and exposure (3 points) assessment. The two reviewers appropriately adjusted the scoring details before scoring (Table 1 shows the specific entries and scoring details). Studies scoring more than 7 after adding up their quality parameters were considered high-quality studies with a low risk of bias, scores of 5 to 7 indicated moderate-quality studies with a moderate risk of bias, and scores of less than 5 indicated lower-quality studies with a high risk of bias. Any disagreement between the 2 reviewers on this assessment were resolved through discussion with the supervising investigator (D.C.). Publication bias was assessed using funnel plots, completing the symmetry assessment with the Begg test.
Table 1.
Characteristics of inclusion study
| First author | Publication y | Country and region | Findings type | Sample size | Type of diabetes | Mean follow-up y | End points | Diagnosis of DR |
|---|---|---|---|---|---|---|---|---|
| Cheung [19] | 2006 | Australia | OR and HR | 1617 | T2D | 7.8 | Ischemic stroke | Retinal photographs |
| Hitman [20] | 2007 | UK | OR and HR | 2838 | T2D | 3.9 | Stroke | Any retinopathy in DM |
| Bello [26] | 2007 | USA | OR | 4038 | T2D | 2.4 | Stroke | Medical records |
| Fuller [17] | 2001 | UK | OR | 3742 | T1D and T2D | 12 | Fatal or nonfatal stroke | Medical records |
| Hankey [24] | 2012 | Australia | OR and HR | 9795 | T2D | 5 | Ischemic and hemorrhagic stroke | ETDRS |
| Cohen [13] | 2003 | USA | OR | 1689 | T2D | 5.3 | Stroke | ETDRS |
| Hjelmgren [29] | 2018 | Sweden | OR and HR | 445 | T2D | 3.1 | Stroke/TIA | Fundus photography |
| Ono [18] | 2002 | Japan | OR | 223 | T1D | 11.6 | Stroke | Fundus photography |
| Drinkwater [15] | 2020 | Australia | OR and HR | 1521 | T2D | 9.0 | Stroke | ETDRS |
| Hägg [23] | 2013 | Finland | OR and HR | 4083 | T1D | 9.0 | Stroke | Laser treatment |
| Kawasaki [25] | 2013 | Japan | OR | 1620 | T2D | 8 | Stroke | ETDRS |
| Gerstein [22] | 2012 | Canada | OR and HR | 3433 | T2D | 4.7 | Fatal or nonfatal stroke | ETDRS |
| Barlovic [28] | 2018 | Slovenia | OR | 1689 | T1D | 9.5 | Ischemic and hemorrhagic stroke | Laser treatment |
| Wong [14] | 2020 | USA | OR | 2828 | DM (T2D) | 5.4 | Stroke | ETDRS |
| Lip [27] | 2015 | France | OR | 1409 | DM (T2D) | 2.6 | Stroke/TE | Medical records |
| Landers [30] | 2018 | Australia | HR | 737 | DM (T2D) | 8.7 | Stroke | Fundus photography |
| Chou [10] | 2016 | Taiwan | HR | 1311 | DM (T2D) | 15 | Stroke/TIA | Medical records |
| Protopsaltis [21] | 2008 | Greece | HR | 599 | DM (T2D) | 10.1 | Ischemic stroke | NA |
| Klein [16] | 1999 | USA | HR | 1878 | DM (T2D) | 4 | Stroke | Fundus photography |
Abbreviations: DM, diabetes mellitus; DR, diabetic retinopathy; ETDRS, Early Treatment Diabetic Retinopathy Study; HR, hazard ratio; NA, not available; OR, odds ratio; T2D, type 2 diabetes mellitus; TE, thromboembolism; TIA, transient ischemic attack; UK, United Kingdom; USA, United States of America.
Statistical Analysis
After including studies with “no DR” and “with DR” as effects, we examined pooled risk estimates of HRs, ORs, and their 95% CIs calculated from the included studies or from the extracted data to summarize the stroke events associated with DR. In the OR group, statistical heterogeneity was assessed by forest plots and tested using χ 2 and I2 methods. If there was no statistical heterogeneity between outcomes (P > .10 and I2 < 50%), meta-analysis was performed using Mantel-Haenszel statistical methods and fixed-effects models. If there was statistical heterogeneity between the results (P < .10 and I2 > 50%), a meta-analysis was performed using the Mantel-Haenszel statistical method with a random-effects model. For the HR group, we used the statistical method of “inverse variance” to analyze those results with low heterogeneity and “random (I-V heterogeneity)” as an effect model for the meta-analysis. We planned to conduct subgroup analyses using the following factors: type of diabetes (type 1 [T1D] vs type 2 [T2D]), and severity of DR (mild and moderate nonproliferative diabetic retinopathy [NPDR] vs severe NPDR and worse). In addition, we performed sensitivity analyses by excluding studies that were assessed as being of insufficient quality.
All statistical analyses for this study were completed using StataSEWin32 (version 419.12.0.866) and Microsoft Excel for Microsoft 365 MSO (16.0.14026.20202) 64-bit.
Results
Search and Assessment
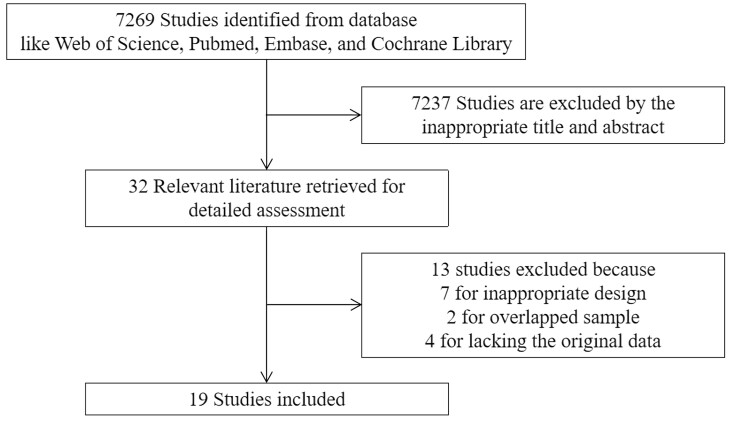
First, we identified 6149 studies in a search of the databases. A supplementary search June 1, 2022, identified an additional 1120 relevant studies that fulfilled the requirements. The study selection process is shown in Fig. 1. We ultimately identified 19 studies [10, 13, 16-28] that included a total of 45 495 patients for analysis (Table 2). Of these studies, 4 involved T1D (sample size n1: 6762; mean follow-up M1: 10.53 years) and 16 involved T2D (n2: 38 733; M2: 6.69 years).
Figure 1.
Screening process for published studies and reasons for exclusion.
Table 2.
The Newcastle-Ottawa Scale
| Study | Selectiona | Comparabilityb | Outcomec | Quality score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representative of treat group | Representative of refer group | Assignment for treatment | Outcome in baseline | For normal baseline | For special baseline | Assessment of outcome | Adequate follow-time | Adequate follow-up | ||
| Cheung [19] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 9 |
| Hitman [20] | No | No | Yes | No | No | Yes | Yes | Yes | No | 5 |
| Bello [26] | Yes | Yes | Yes | No | No | No | Yes | Yes | No | 6 |
| Fuller [17] | Yes | Yes | Yes | No | No | No | Yes | Yes | Yes | 7 |
| Hankey [24] | Yes | Yes | Yes | No | No | No | Yes | Yes | No | 6 |
| Cohen [13] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | 7 |
| Hjelmgren [29] | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | 7 |
| Ono [18] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 9 |
| Drinkwater [15] | Yes | Yes | Yes | No | No | Yes | Yes | Yes | No | 7 |
| Hägg [23] | No | No | Yes | No | Yes | No | Yes | Yes | No | 5 |
| Kawasaki [25] | Yes | Yes | Yes | No | Yes | No | Yes | Yes | No | 7 |
| Gerstein [22] | Yes | Yes | Yes | No | No | No | Yes | Yes | No | 6 |
| Barlovic [28] | Yes | Yes | Yes | No | No | No | Yes | Yes | No | 6 |
| Wong [14] | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 9 |
| Lip [27] | No | No | Yes | No | No | No | Yes | Yes | Yes | 5 |
| Landers [30] | Yes | Yes | Yes | No | NA | NA | Yes | Yes | Yes | 7 |
| Chou [10] | No | No | Yes | No | No | No | Yes | Yes | Yes | 5 |
| Protopsaltis [21] | Yes | Yes | Yes | No | No | No | No | Yes | Yes | 6 |
| Klein [16] | Yes | Yes | Yes | No | Yes | Yes | Yes | No | Yes | 7 |
a Representative if the study is countrywide, not if it is a province/city/continent.
b General baseline: sex/body mass index/vital signs, etc; special baseline: high-density lipoprotein/glycated hemoglobin A1c, etc; comparable if 1 item or less is significant, not if 2 items or more are significant.
c Score if follow-up time has been 5 years or more, and score for missed follow-up rate is less than or equal to 5%.
d If the relevant scoring item is not mentioned, this item will be treated as 0.5 points; and all decimal places will be discarded when the score is counted.
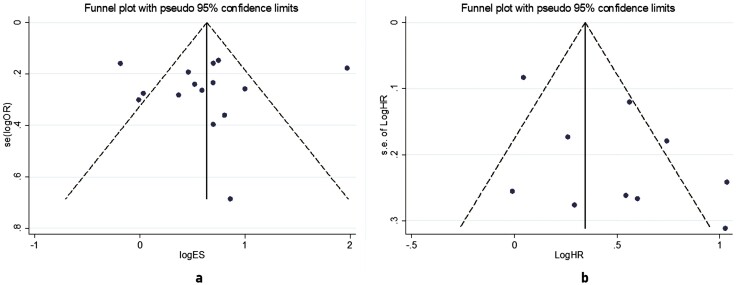
The results of the quality assessment showed that 10 (52.63%) of the included studies were considered to be of high quality, 9 (47.37%) were considered to be of moderate quality, and there were no studies of low quality according to the results of the Newcastle-Ottawa Scale scores (Table 2). This means that all included studies were basically eligible for our study. As for publication bias (Fig. 2), the results of the funnel plot and Begg test showed that there was no significant publication bias or publication bias did not significantly affect the results in the studies of the association between DR and incident stroke in patients with diabetes.
Figure 2.
Funnel plot of included studies, which shows that there was no significant asymmetry in the 15 included studies in the A, odds ratio group, or the 10 studies in the B, hazard ratio group, suggesting no significant publication bias.
Subgroup Analysis
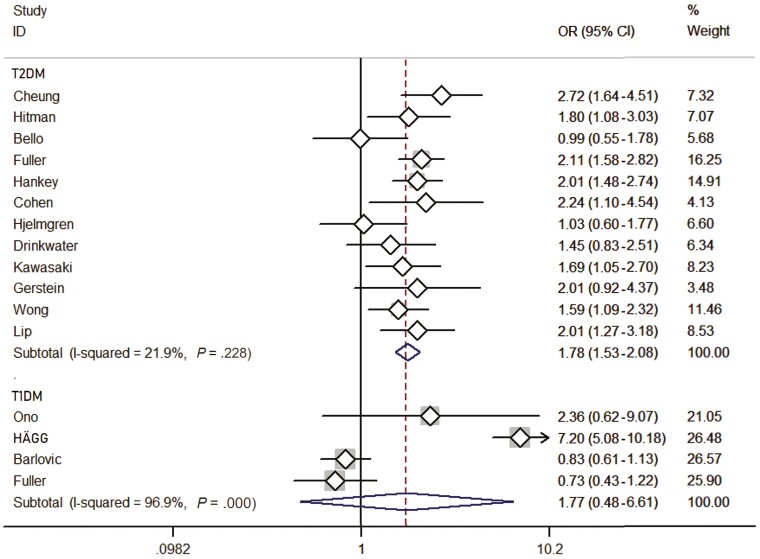
A comprehensive analysis involving patients with T2D included 13 studies (Fig. 3). The presence of DR status was found to be significantly associated with stroke events (OR: 1.78; 95% CI, 1.53-2.08). Thirteen studies included 34 208 patients with a mean follow-up of 6.87 years, which represented 76.33% of all patients, and a fixed-effects model was used because of low heterogeneity (I2: 21.9%; P = .228). Only 4 studies [15, 22, 26, 29] did not have such an association.
Figure 3.
Forest plot of included studies, with 12 studies of type 2 diabetes and 4 studies of type 1 diabetes included, respectively. The P values represent the heterogeneity of the subgroups and the whole studies; and the diamonds in the figure represent the results of the meta-analysis of the different studies that accounted for different weights.
Similarly, the analysis involving patients with T1D included 4 studies (see Fig. 3). The presence of DR was found to be not significantly associated with stroke events (OR: 1.77; 95% CI, 0.48-6.61). A total of 6762 individuals were included in the 4 studies with a mean follow-up of 10.52 years, and a random-effects model was used because of the high heterogeneity (I2: 96.9%; P = .000). No such association was found in any of 3 studies [17, 18, 28].
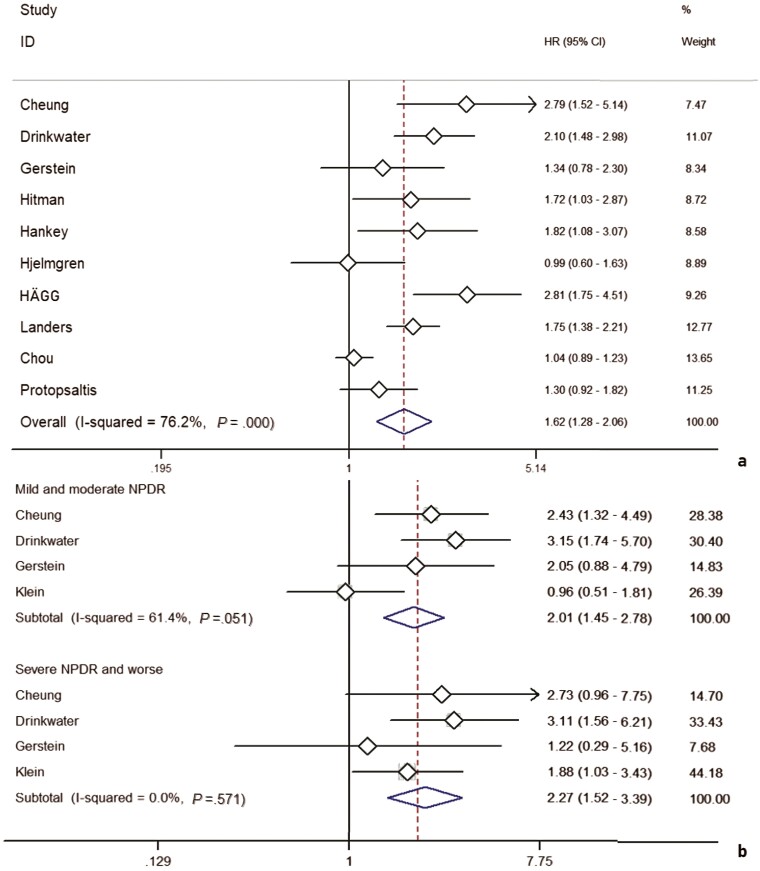
In addition, a comprehensive analysis involving the HR as an effect measure included 10 studies (Fig. 4A). The pooled HR for any DR was found to be significantly associated with HR for stroke events (HR: 1.62; 95% CI, 1.28-2.06). A total of 6762 patients were included in the 10 studies with a mean follow-up of 7.63 years, and a random-effects model was employed because of significant heterogeneity between studies (I2: 76.2%; P = .000).
Figure 4.
Ten studies with hazard ratio as a finding are shown in A, while subgroup analyses of 4 studies with “mild and moderate NPDR” and “severe NPDR and worse” as representative groups of diabetic retinopathy (DR) progression are shown in B.
In a pooled analysis of DR severity as a subgroup, the results included 8 studies (Fig. 4B). HR for mild and moderate NPDR was found to be significantly associated with HR for stroke events (HR: 2.01; 95% CI, 1.45-2.78); DR for severe NPDR and worse states was also significantly associated with HR for stroke events (HR: 2.27; 95% CI, 1.52-3.39).
Sensitivity Analysis
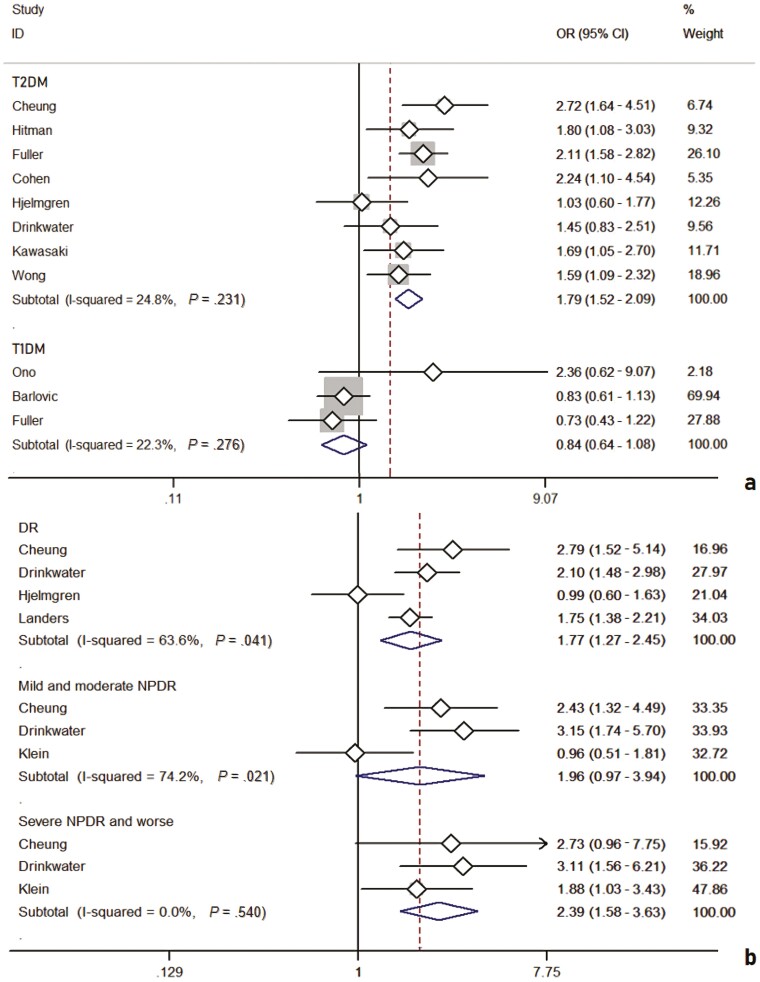
After including high-quality studies only (NOS ≥ 7), the results showed some changes. On the one hand, the analysis involving patients with T2D included 8 studies (Fig. 5A) and found that the presence of DR status remained significantly associated with stroke events (OR: 1.79; 95% CI, 1.52-2.09). The analysis involving patients with T1D included 3 studies and showed that the presence of DR was found to be not significantly associated with stroke events (OR: 0.84; 95% CI, 0.64-1.08) and, moreover, its heterogeneity became insignificant as inappropriate articles were removed (I2: 22.3%; P = .276). On the other hand, the pooled analysis involving HR as an effect measure included 4 studies (Fig. 5B) and the pooled HR for any DR was significantly associated with HR for stroke events (HR: 1.77; 95% CI, 1.49-2.11). The comprehensive analysis with DR severity as a subgroup also included 4 studies (see Fig. 5B) and HR for mild and moderate NPDR was significantly associated with HR for stroke events (HR: 1.96; 95% CI, 0.97-3.94); DR for severe NPDR and worse states was also significantly associated with HR for stroke events (HR: 2.39; 95% CI, 1.58-3.63).
Figure 5.
Eight studies with type 2 diabetes mellitus (T2D) and 3 studies with type 1 diabetes mellitus (T1D) in the odds ratio (OR) group after screening are shown in A, and 4 studies with diabetic retinopathy (DR) and 3 studies with different progression of DR in the hazard ratio group are shown in B.
Discussion
The meta-analysis performed, which included 45 495 patients in 19 studies, showed that the presence of DR was associated with an increased risk of stroke events in patients with diabetes—an association that was robust in patients with T2D but inconclusive in patients with T1D. When the severity of DR was considered, both mild and moderate NPDR and severe NPDR and worse were found to significantly increase the risk of stroke, and in addition, we found a trend toward increased stroke risk with increasing DR stage and DR lesion severity.
The results we have gained are interesting and worthy of further discussion. First, from the perspective of the literature search, most articles were of high quality, not only with some new studies published in the last 5 to 3 years, but also with high representation, large sample size, and long follow-up periods. In addition, the funnel plot and Begg test showed no significantly publication bias, which proved that our analytical work is convincing in terms of data collection.
It has to be mentioned that the results of the subgroup analysis and sensitivity analysis in the T1D were inconsistent and significantly heterogeneous. Although the results still support a significant increase in stroke risk with DR after inclusion of T1D and T2D studies, we were still unsure whether DR is associated with stroke in T1D. Furthermore, this trend seemed to be detectable in the analysis of DR on stroke for different processes—as DR progresses, the risk of stroke also increases. However, this trend could not be supported by high-quality evidence, which may be caused by the different classification criteria for DR progression in different studies, resulting in only 3 studies that could be included.
Some limitations exist in our study, although the relevant analytical work has been refined as much as possible. (i) It is possible that the researchers of the original data used different diagnostic criteria, disease definitions, and statistical methods when conducting their respective clinical studies, leading to changes in the study results. (ii) The inconsistency of the results might be due to the different treatments for the disease. There were different diagnostic criteria and treatments in different regions or at different times in the same region for DR, and even for diabetes itself, and the choice or change of these methods might affect the statistics of clinical results. (iii) Stroke was mostly counted as an outcome of CVD in studies, which often resulted in less comprehensive final data for secondary use. (iv) Few studies included treatment of DR as a baseline variable (like antivascular endothelial growth factor therapy); however, there were many prior studies showing that local treatment of DR as a baseline variable has the potential to have a significant influence on stroke outcome.
Some researches [31] have included 5 studies on DR in stroke risk when exploring the potential relationship between DR and the risk of all-cause mortality, stroke and heart failure, which revealed that DR was significantly associated with an increased risk of stroke compared with patients without DR [RR (Risk Ratio): 1.74; 95% CI, 1.35-2.24]. In a meta-analysis of 18 cohort studies, researchers [32] also concluded that the presence of DR was associated with an increased risk of stroke in patients with diabetes, with an RR of 2.29 (95% CI, 1.77-2.96; P < .0001), while no definitive results were obtained in patients with T1D.
In summary, DR status in diabetic patients is associated with an increased risk of stroke. This correlation was robust in patients with T2D, but uncertain in T1D. Therefore, screening for DR should be considered as a routine procedure in the assessment and management of stroke risk, using optical coherence tomography, optical coherence tomography angiography, scanning laser ophthalmoscopy, and other ophthalmic examinations.
Glossary
Abbreviations
- CVD
cardiovascular events
- DR
diabetic retinopathy
- HR
hazard ratio
- OR
odds ratio
- NPDR
nonproliferative diabetic retinopathy
- T2D
type 2 diabetes mellitus
Contributor Information
Zicheng Wang, School of Medicine, South China University of Technology, Guangzhou, Guangdong, 510000, China; Department of Ophthalmology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510000, Guangdong, China.
Dan Cao, Department of Ophthalmology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510000, Guangdong, China; Guangdong Cardiovascular Institute, Guangzhou, Guangdong, 510000, China.
Xuenan Zhuang, Department of Ophthalmology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510000, Guangdong, China; Shantou University Medical College, Shantou, Guangdong, 515000, China.
Jie Yao, Department of Ophthalmology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510000, Guangdong, China; Shantou University Medical College, Shantou, Guangdong, 515000, China.
Ruoyu Chen, Department of Ophthalmology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510000, Guangdong, China; Southern Medical University, Guangzhou, 510000, China.
Yesheng Chen, Department of Ophthalmology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510000, Guangdong, China; Guangdong Cardiovascular Institute, Guangzhou, Guangdong, 510000, China.
Kangyan Zheng, Department of Ophthalmology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510000, Guangdong, China; Shantou University Medical College, Shantou, Guangdong, 515000, China.
Peiyao Lu, Department of Ophthalmology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510000, Guangdong, China; Guangdong Cardiovascular Institute, Guangzhou, Guangdong, 510000, China.
Liang Zhang, Email: zhangliang5413@163.com, School of Medicine, South China University of Technology, Guangzhou, Guangdong, 510000, China; Department of Ophthalmology, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, 510000, Guangdong, China.
Financial Support
This work was supported by the Natural Science Foundation Project of Guangdong Province (No. 2021A1515010113) and the Guangzhou Science and Technology Program (No. 202102080008).
Conflict of Interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
Clinical Trial Information
PROSPERO registration No. CRD42021268540 (registered at 18/08/2021).
References
- 1. Jeganathan VS, Wang JJ, Wong TY. Ocular associations of diabetes other than diabetic retinopathy. Diabetes Care. 2008;31(9):1905-1912. doi: 10.2337/dc08-0342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Congdon NG, Friedman DS, Lietman T. Important causes of visual impairment in the world today. JAMA. 2003;290(15):2057-2060. doi: 10.1001/jama.290.15.2057 [DOI] [PubMed] [Google Scholar]
- 3. Wu S, Wu B, Liu M, et al. China Stroke Study Collaboration. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394-405. doi: 10.1016/S1474-4422(18)30500-3 [DOI] [PubMed] [Google Scholar]
- 4. Wong TY, Cheung N, Tay WT, et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115(11):1869-1875. doi: 10.1016/j.ophtha.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 5. Stinear CM, Lang CE, Zeiler S, Byblow WD. Advances and challenges in stroke rehabilitation. Lancet Neurol. 2020;19(4):348-360. doi: 10.1016/S1474-4422(19)30415-6 [DOI] [PubMed] [Google Scholar]
- 6. Sun Y, Paul P, Tan N, Rajagopalan R, Lew Y, Heng BH. A risk stratification tool for screening for diabetic retinopathy among type 2 diabetic patients. Value Health. 2014;17(3):A148. doi: 10.1016/j.jval.2014.03.862 [DOI] [Google Scholar]
- 7. Klein BEK, Klein R, McBride PE, et al. Cardiovascular disease, mortality, and retinal microvascular characteristics in type 1 diabetes: Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Intern Med. 2004;164(17):1917-1924. doi: 10.1001/archinte.164.17.1917 [DOI] [PubMed] [Google Scholar]
- 8. Feigin VL, Lawes CMM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8(4):355-369. doi: 10.1016/S1474-4422(09)70025-0 [DOI] [PubMed] [Google Scholar]
- 9. Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775,385 individuals and 12,539 strokes. Lancet. 2014;383(9933):1973-1980. doi: 10.1016/s0140-6736(14)60040-4 [DOI] [PubMed] [Google Scholar]
- 10. Chou AY, Liu CJ, Chao TF, et al. Presence of diabetic microvascular complications does not incrementally increase risk of ischemic stroke in diabetic patients with atrial fibrillation: a nationwide cohort study. Medicine (Baltimore). 2016;95(27):e3992. doi: 10.1097/MD.0000000000003992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alder VA, Su EN, Yu DY, Cringle SJ, Yu PK. Diabetic retinopathy: early functional changes. Clin Exp Pharmacol Physiol. 1997;24(9-10):785-788. doi: 10.1111/j.1440-1681.1997.tb02133.x [DOI] [PubMed] [Google Scholar]
- 12. Baker ML, Hand PJ, Wang JJ, Wong TY. Retinal signs and stroke: revisiting the link between the eye and brain. Stroke. 2008;39(4):1371-1379. doi: 10.1161/STROKEAHA.107.496091 [DOI] [PubMed] [Google Scholar]
- 13. Cohen JA, Estacio RO, Lundgren RA, Esler AL, Schrier RW. Diabetic autonomic neuropathy is associated with an increased incidence of strokes. Auton Neurosci. 2003;108(1-2):73-78. doi: 10.1016/j.autneu.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 14. Wong KH, Hu K, Peterson C, et al. Diabetic retinopathy and risk of stroke: a secondary analysis of the ACCORD Eye Study. Stroke. 2020;51(12):3733-3736. doi: 10.1161/STROKEAHA.120.030350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drinkwater JJ, Davis TME, Hellbusch V, Turner AW, Bruce DG, Davis WA. Retinopathy predicts stroke but not myocardial infarction in type 2 diabetes: the Fremantle Diabetes Study Phase II. Cardiovasc Diabetol. 2020;19(1):43. doi: 10.1186/s12933-020-01018-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Klein R, Klein BE, Moss SE, Cruickshanks KJ. Association of ocular disease and mortality in a diabetic population. Arch Ophthalmol. 1999;117(11):1487-1495. doi: 10.1001/archopht.117.11.1487 [DOI] [PubMed] [Google Scholar]
- 17. Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S54-S64. doi: 10.1007/pl00002940 [DOI] [PubMed] [Google Scholar]
- 18. Ono T, Kobayashi J, Sasako Y, et al. The impact of diabetic retinopathy on long-term outcome following coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40(3):428-436. doi: 10.1016/s0735-1097(02)01983-6 [DOI] [PubMed] [Google Scholar]
- 19. Cheung N, Rogers S, Couper DJ, Klein R, Sharrett AR, Wong TY. Is diabetic retinopathy an independent risk factor for ischemic stroke? Stroke. 2007;38(2):398-401. doi: 10.1161/01.STR.0000254547.91276.50 [DOI] [PubMed] [Google Scholar]
- 20. Hitman GA, Colhoun H, Newman C, et al. CARDS Investigators. Stroke prediction and stroke prevention with atorvastatin in the Collaborative Atorvastatin Diabetes Study (CARDS). Diabet Med. 2007;24(12):1313-1321. doi: 10.1111/j.1464-5491.2007.02268.x [DOI] [PubMed] [Google Scholar]
- 21. Protopsaltis I, Korantzopoulos P, Milionis HJ, et al. Metabolic syndrome and its components as predictors of ischemic stroke in type 2 diabetic patients. Stroke. 2008;39(3):1036-1038. doi: 10.1161/STROKEAHA.107.498311 [DOI] [PubMed] [Google Scholar]
- 22. Gerstein HC, Ambrosius WT, Danis R, et al. ACCORD Study Group. Diabetic retinopathy, its progression, and incident cardiovascular events in the ACCORD trial. Diabetes Care. 2013;36(5):1266-1271. doi: 10.2337/dc12-1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hägg S, Thorn LM, Putaala J, et al. FinnDiane Study Group. Incidence of stroke according to presence of diabetic nephropathy and severe diabetic retinopathy in patients with type 1 diabetes. Diabetes Care. 2013;36(12):4140-4146. doi: 10.2337/dc13-0669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hankey GJ, Anderson NE, Ting RD, et al. Rates and predictors of risk of stroke and its subtypes in diabetes: a prospective observational study. J Neurol Neurosurg Psychiatry. 2013;84(3):281-287. doi: 10.1136/jnnp-2012-303365 [DOI] [PubMed] [Google Scholar]
- 25. Kawasaki R, Tanaka S, Tanaka S, et al. ; Japan Diabetes Complications Study Group. Risk of cardiovascular diseases is increased even with mild diabetic retinopathy: the Japan Diabetes Complications Study. Ophthalmology. 2013;120(3):574-582. doi: 10.1016/j.ophtha.2012.08.029 [DOI] [PubMed] [Google Scholar]
- 26. Bello NA, Pfeffer MA, Skali H, et al. Retinopathy and clinical outcomes in patients with type 2 diabetes mellitus, chronic kidney disease, and anemia. BMJ Open Diabetes Res Care. 2014;2(1):e000011. doi: 10.1136/bmjdrc-2013-000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lip GYH, Clementy N, Pierre B, Boyer M, Fauchier L. The impact of associated diabetic retinopathy on stroke and severe bleeding risk in diabetic patients with atrial fibrillation: the Loire Valley Atrial Fibrillation Project. Chest. 2015;147(4):1103-1110. doi: 10.1378/chest.14-2096 [DOI] [PubMed] [Google Scholar]
- 28. Pongrac Barlovic D, Harjutsalo V, Gordin D, et al. The association of severe diabetic retinopathy with cardiovascular outcomes in long-standing type 1 diabetes: a longitudinal follow-up. Diabetes Care. 2018;41(12):2487-2494. doi: 10.2337/dc18-0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hjelmgren O, Stromberg U, Gellerman K, et al. Does retinopathy predict stroke recurrence in type 2 diabetes patients: a retrospective study?. PLoS One. 2019;14(1):e0210832. doi: 10.1371/journal.pone.0210832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Landers J, Liu E, Estevez J, et al. Presence of diabetic retinopathy is associated with worse 10-year mortality among Indigenous Australians in Central Australia: the Central Australian ocular health study. Clinical & Experimental Ophthalmology. 2019;47(2):226-232. doi: 10.1111/ceo.13375 [DOI] [PubMed] [Google Scholar]
- 31. Zhu XR, Zhang YP, Bai L, et al. Prediction of risk of diabetic retinopathy for all-cause mortality, stroke and heart failure: evidence from epidemiological observational studies. Medicine (Baltimore). 2017;96(3):e5894. doi: 10.1097/MD.0000000000005894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hu K, Jiang M, Zhou Q, et al. Association of diabetic retinopathy with stroke: a systematic review and meta-analysis. Front Neurol. 2021;12:626996. doi: 10.3389/fneur.2021.626996 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”