Abstract
Advanced wastewater treatment technologies are effective methods and currently attract growing attention, especially in arid and semi-arid areas, for reusing water, reducing water pollution, and explicitly declining, inactivating, or removing SARS-CoV-2. Overall, removing organic matter and micropollutants prior to wastewater reuse is critical, considering that water reclamation can help provide a crop irrigation system and domestic purified water. Advanced wastewater treatment processes are highly recommended for contaminants such as monovalent ions from an abiotic source and SARS-CoV-2 from an abiotic source. This work introduces the fundamental knowledge of various methods in advanced water treatment, including membranes, filtration, Ultraviolet (UV) irradiation, ozonation, chlorination, advanced oxidation processes, activated carbon (AC), and algae. Following that, an analysis of each process for organic matter removal and mitigation or prevention of SARS-CoV-2 contamination is discussed. Next, a comprehensive overview of recent advances and breakthroughs is provided for each technology. Finally, the advantages and disadvantages of each method are discussed.
Keywords: SARS-CoV-2, Micropollutants, Membrane, UV irradiation, Ozonation, Chlorination, Advanced oxidation process, Activated carbon, Algae, Wastewater
Graphical abstract
Introduction
SARS-CoV-2 is a clear danger today, with many anthropogenic micropollutants present in wastewater; therefore, they must be removed before entering the environment (Qi et al., 2015; Sosa-Hernández et al., 2021). Specifically, the SARS-CoV-2 virus has been discovered in stool samples taken from patients with the COVID-19 disease (Cheung et al., 2020), as well as in wastewater (Kitajima et al., 2020) (Schema 1 ). In this regard, some recent publications have focused on SARS-CoV-2 in the whole environment (Núñez-Delgado 2020; Cela-Dablanca et al., 2021), as well as in soils and liquid samples from soil (Anand et al., 2021; Conde-Cid et al., 2021; Conde-Cid et al., 2021). In addition, the drugs used during the pandemic have polluted water bodies and other environmental compartments (Race et al., 2020).
Schema 1.
Monitoring of SARS-CoV-2 RNA has been conducted in municipal wastewater treatment plants (WWTPs).
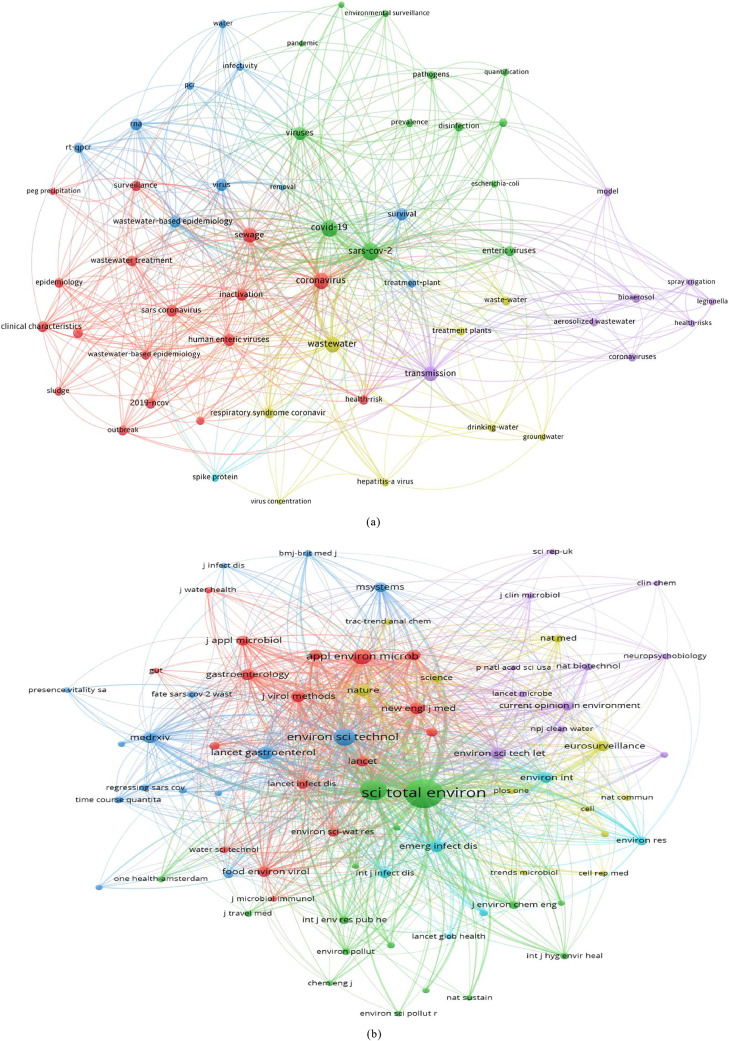
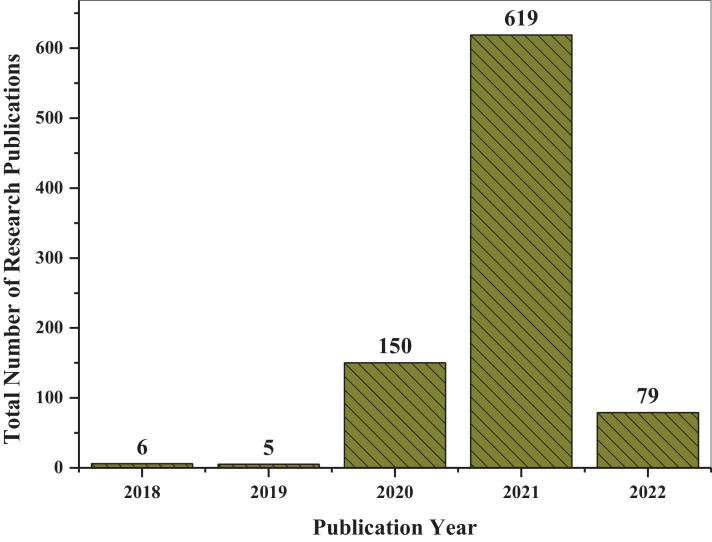
Micropollutants may directly or indirectly impact living organisms due to biomagnification through the food chain (Bonvin et al., 2016; Mackuľak et al., 2021). Wastewater sources contain a variety of pollutants that are determined through different parameters, such as viruses, total organic carbon (TOC), chemical oxygen demand (COD) (Zahmatkesh et al., 2022b), biological oxygen demand (BOD), total suspended solids (TSS), nitrogen (N), and phosphorus (P) (Zahmatkesh et al., 2020). The terms associated with the applications of wastewater treatment and SARS-CoV-2 are summarized in Fig. 1 (a). The report was compiled with the use of the VOSViewer software, and the data was found on the Web of Science using the keywords "wastewater treatment" and "SARS-CoV-2." Fig. 1 (b) shows the co-citation map of journals where recent research associated with wastewater treatment and SARS-CoV-2 has been published. The co-citation map was also composed with the support of VOSViewer. Wastewater treatment and SARS-CoV-2 have been garnering tremendous consideration lately, with a sharp increase in the total number of publications from 5 in 20189 to 619 in 20221 recorded by Sciencedirect (Fig. 2 ).
Fig. 1.
Visualization network map of the keywords (i.e., “SARS-CoV-2,” wastewater, and “transmission”) in the publications surveyed from the Web of Science published from 2018 to 2022: (a) Network visualization of terms related to wastewater treatment and SARS-CoV-2; (b) Co-citation map of journals where recent wastewater treatment and SARS-CoV-2 research was published; the hot topics of SARS-CoV-2 fouling prediction are mined based on the size of nodes in the co-occurrence network mapping, the frequency of keywords, and the distribution of clusters. Each node represents one keyword, and the lines connecting the nodes represent co-occurrence relationships. A larger node indicates a closer relationship between the keywords. The color of an element represents the cluster that it belongs to, and different colors differentiate different clusters.
Fig. 2.
Several research publications related to wastewater treatment and SARS-CoV-2 from 2018 to 2022. Source: Sciencedirect.
It is currently impossible via conventional tertiary treatment methods to remove micropollutants altogether, such as sweeteners, stimulants, medicines, X-ray contrast media, and industrial chemicals (Qi et al., 2015; van Gijn et al., 2021). However, since the outbreak of SARS-CoV-2, micropollutants and SARS-CoV-2 enter surface waters through WWTPs. This virus has been considered a challenge and a significant problem in advanced water treatment decline (Lesimple et al., 2020). Over the past few years, many advanced technologies have been studied, including chlorination (Azuma et al., 2021), ultraviolet (UV) irradiation (Zeng et al., 2020), membrane (Mishra et al., 2022), ozonation (Völker et al., 2019), and advanced oxidation processes (Khan et al., 2020). Although these advanced technologies are being developed, they are unavailable at municipal WWTPs.
Over the past few decades, the technological advancements are: First, membrane technology is considered one of the most advanced wastewater treatment technologies (Tang et al., 2018). Due to its efficiency, membrane technology has gained popularity in the water and wastewater treatment industry since the 1970s (Górecki, 2020). Wastewater treatment using membrane technology provides several advantages, including its small size (Scholz et al., 2013), low energy requirements (Ali et al., 2018), and low capital costs (Bhattacharjee et al., 2017; Judd 2017). Water treatment using membrane technology is currently more effective than other alternatives to promote water reuse. Nevertheless, as regards SARS-CoV-2, it can be detected in the size range of 100 ± 10 nm (Goswami et al., 2020; Wu et al., 2021), making it essential to choose the membrane's pore size that will remove viral particles most effectively when using membrane-based treatments (Lesimple et al., 2020).
Secondly, advanced oxidation processes (AOPs) such as photolysis and photocatalysis are considered attractive technologies for degrading organic pollutants in aquatic environments (Tang et al., 2021) and atmospheric environments (Gültekin et al., 2007; Khan et al., 2020). This process aims to produce hydroxyl radicals (OH•) to clean the water (Wang et al., 2020). Therefore, the hydrogen abstraction process, by which hydroxyl radicals oxidize organic compounds, produces organic radicals, which produce peroxyl radicals upon molecular oxygen. Eventually, these intermediates are degraded oxidatively, resulting in carbon dioxide, water, and salts (Miklos et al., 2018).
Thirdly, the capability of UV radiation to penetrate deep into radiated liquid is extremely important, particularly in opaque environments (Köhler et al., 2012; Karpova et al., 2013). Direct UV radiation can destroy microorganisms (Tran et al., 2021). Some microbes (such as bacteria) are destroyed by UV light depending on the wavelength; ultraviolet A (UVA) generally destroys non-nuclear cellular components, whereas ultraviolet B (UVB) and ultraviolet C (UVC) generally destroy nuclei (Parsa et al., 2021). Furthermore, UVC is effective against SARS-CoV-2 (da Fonseca Filho et al., 2021).
Another method used in advanced wastewater treatment is chlorination; chlorine is among the most common methods used to disinfect wastewater effluents before discharge into streams (Hladik et al., 2014), rivers (Bellanca et al., 1977), or agriculture (Ferro et al., 2015). As a well-established disinfection method, chlorine has a broad germicidal spectrum (Oppenländer, 2007), and its affordability makes it the most popular disinfection procedure (Ao et al., 2021). In addition, a variety of dissolved organic matter compounds attach easily to chlorine to create disinfection by-products (DBPs), which include trihalomethanes (THMs) (Tak et al., 2019) and haloacetic acids (HAAs) (Sillanpää et al., 2018).
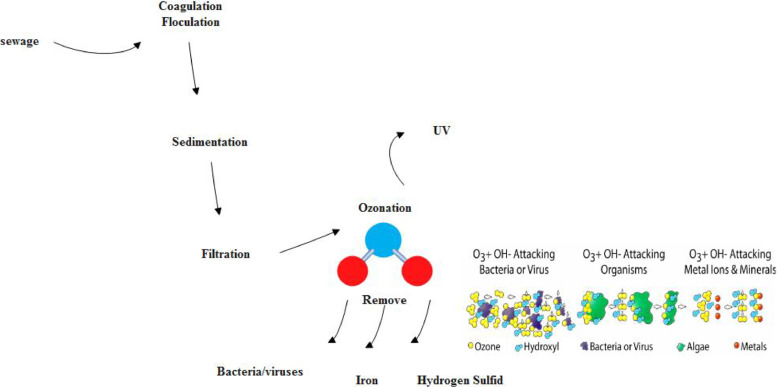
Besides destroying organic pollutants in water, ozone can also degrade organic pollutants (Machulek et al., 2013; An et al., 2020). Ozone is produced using an electric discharge method (Anpilov et al., 2001). In water, two reactions occur between dissolved organic substances and ozone: At low pH levels, molecular ozone attacks the organic molecules in a highly selective manner; ozone-derived free radicals can also attack the organic molecules in an unselective fashion (Pocostales et al., 2010). Furthermore, during the SARS-CoV-2 pandemic, ozonated nanobubbles have been emphasized as an effective method to overcome persistent SARS-CoV-2. Also to remark is that by modifying the pH (Verinda et al., 2021) or introducing hydrogen peroxide (El-Betany et al., 2020) or UV irradiation (Verinda et al., 2021) with a high-pressure mercury lamp, free hydroxyl radicals can be produced in aqueous media using ozone (Du et al., 2019).
Finally, a wide range of micropollutants can be removed from drinking water by means of activated carbon (AC) due to its high specific surface area (Ebie et al., 2001). Due to the organic matter in wastewater effluent, larger quantities of AC are needed to ensure adequate adsorption (Ebie et al., 2001; Nam et al., 2014). Micropollutants can be removed from wastewater by granular AC filtration (Quinlivan et al., 2005; Guillossou et al., 2021), as studied in some WWTPs with varying outcomes depending on the compound and the frequency of granular activated carbon (GAC) regeneration or replacement (Genç et al., 2021; Lu et al., 2021a). Adsorption using powdered activated carbon (PAC) at a dosage of 10-20 mg/L (Ivančev-Tumbas et al., 2020) is more feasible than using GAC (Yu et al., 2021). However, the effectiveness of PAC treatment in removing micropollutants from municipal wastewater has been examined in very few large-scale studies until now (Table 1 ) (Boehler et al., 2012).
Table 1.
Wastewater treatment technologies.a
| Using technology to treat wastewater | Treatment | Description |
|---|---|---|
| AOP (advanced oxidation processes) | Chlorine treatment | It acts as a selective oxidant, reacting in this way with the capsid protein and damaging the Cys, Trp and Tyr, causing the inhibition of replication and injection of the genome as well as UV treatment. |
| Algae systems | Sedimentation, temperature increase, and sunlight degradation are part of these systems' treatment mechanisms. | |
| UV inactivation | 200 to 300 nm is the active UV wavelength range that can damage the bacteria or virus, nonetheless; ∼254 nm is recognized as the best one for microbial disinfection. Its efficiency depends of the contact time, temperature and the presence of organic matter. Usually inactivates the virus by damaging the RNA, so on the replication through oxidation processes, altering the permeability and damaging the capsid proteins. | |
| Nanomaterials | They include the use of photocatalysts and membranes which incorporate nanomaterials. Metal oxide semiconductors utilized are for example TiO2 and ZnO. | |
| Ozone treatment | In order to achieve disinfection, a certain period of contact time is necessary. | |
| Membrane technologies (MTs) | Reverse Osmosis | Usually used as part of pre-treatment systems, in order to remove particles and post-treatment in order to complete the removal of emerging contaminants that may remain after a water treatment process. |
| Nanofiltration | Despite their smaller pore sizes, these membranes are still more resistant to water contaminants such as viruses. | |
| Ultrafiltration | Usually used as a pre-treatment before reverse osmosis, however; several authors have also used it in the removal of bacteria and viruses. | |
| Microfiltration | The purpose of this technology is to remove bacteria and protozoa. | |
| Ceramic membranes | Ozonation and coagulation were used as pre-treatments for filtration to prevent the virus's spread. |
Adapted with permission fromPacheco et al. (2021).
The latest method is that of using microalgae, which is a symbiotic group of microorganisms that feed on light energy (Blanken et al., 2013; Zahmatkesh et al., 2020) and inorganic carbon sources (carbonate and CO2) to produce biomass (Zahmatkesh et al., 2020), releasing oxygen to the atmosphere (Show et al., 2017). Various types of microalgae are used for wastewater treatment (Hussain et al., 2021), and microalgae and cyanobacteria (blue-green algae) are commonly found in microalgal-based systems (Lu et al., 2021b). Although a range of commercial applications is are being developed for microalgal biotechnology (Olaizola 2003; Masojídek et al., 2010; Lu et al., 2021c), algae-based wastewater treatment has become more prevalent in recent years (Larsen et al., 2019). Due to its ability to process wastewater without aeration (Holmes et al., 2020), producing beneficial biofuels such as methane or diesel (Zaimes et al., 2013). Microalgae have also been shown to have several advantages over biological nutrient removal (BNR) processes in wastewater treatment, including nitrification/denitrification without organic carbon (for example, methanol), simultaneous CO2 absorption by photosynthesis, and low cost of installation and operation (Mallick 2002; Molinuevo-Salces et al., 2019).
Advanced wastewater treatment studies have shown the better performance of AC in declining COD or BOD (Zahmatkesh et al., 2020); moreover, AC is more effective than sludge or algae in wastewater reuse (Zahmatkesh et al., 2020). A study using UV radiation in advanced wastewater treatment showed that this radiation is capable of removing pathogens and organic matter in wastewater treatment (Kuzniewski, 2021); especially, UVC is essential for eliminating SARS-CoV-2 (Zahmatkesh et al., 2022a). Ozone catalysis is commonly referred to as an advanced oxidation process; furthermore, ozone can be decomposed using a catalyst to produce more active free radicals, leading to the mineralization of organic pollutants (Schollée et al., 2021). Synthetic polymers are used as membranes in pressure-driven separation processes, to remove organics, bacteria, oil, etc. Using AOP can eliminate nondegradable organic components from industrial or municipal wastewater and avoid removing residual deposits (Kwarciak-Kozłowska et al., 2021). Finally, the chlorination of wastewater caused a substantial reduction in estrogenic activity and an increase in antiestrogenic activity.
In view of all that background, this paper presents a detailed overview of the recent developments and breakthroughs related to advanced wastewater treatment technologies in removing organics and inorganics. It includes a particular additional focus on the removal of SARS-CoV-2. Finally, each method is evaluated for its advantages and disadvantages (Table 2 ).
Table 2.
Advantages and disadvantages of different methods for organic contaminant removal from wastewater a.
| Treatment | Advantages | Disadvantages |
|---|---|---|
| Adsorption |
|
|
| Advanced Oxidation Processes (AOPs) |
|
|
| Biological treatments |
|
|
| Coagulation and flocculation |
|
|
Adapted with permission from (Teymoorian et al., 2021).
Mechanism of SARS-CoV-2 entry into host cells
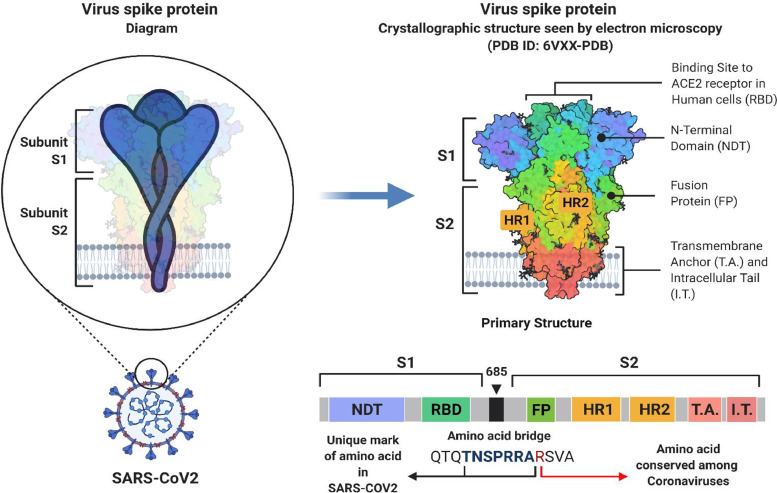
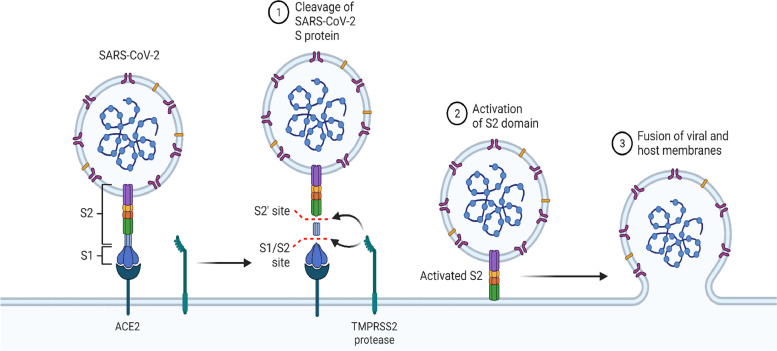
A virus' ability to infect and cause disease depends on gaining entry into host cells. Moreover, infectious diseases pose significant implications for host immune surveillance and human intervention strategies. Coronaviruses go through the complex binding process to a cell surface receptor for viral attachment, entering endosomes and fusing the endosome membranes with the host cell. Spike proteins on the virus surface are responsible for the entry of coronaviruses; thus, a virus with mature spike proteins contains a trimer of three receptor-binding heads atop a stalk of trimeric membrane fusion heads (Kokic et al., 2021). See details in Schema 2 .
Schema 2.
An in-depth look into the structure of the SARS-CoV-2 spike protein.
Furthermore, coronaviruses are within enveloped viruses, Coronaviridae, a family of viruses with a positive-sense RNA genome. Several CoVs associated with high pathogenicity are found in the Betacoronavirus genus, group 2. SARS-CoV-2 is found in this group. In SARS-CoV-2, 80% of the sequences are the same as in SARS-CoV, and 50% are the same as in MERS-CoV. Among the genetic sequences of this virus are 14 open reading frames (ORFs), of which two-thirds encode 16 nonstructural proteins (nsp 1–16), which are used to make the replicase complex. Aside from the nine accessory proteins (ORFs), one-third of the genome contains four structural proteins: spikes (S), envelopes (E), membranes (M), and nucleocapsids (N), the latter of which is required for SARS-CoV entry into host cells (see in Schema 3 ). Even though SARS-CoV-2 shares more than 75% nucleotide identity with SARS-CoV, the S gene is highly variable. Angiotensin-converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) can cleave S1/S2 polybasic cleavage sites in spike proteins' tightly-packed binding domains. Therefore, SARS-CoV-2 Spike entering cells without TMPRSS2 may gain entry through cathepsin L on the plasma membrane surface. However, SARS-CoV-2 Spike is activated by cathepsin L in endosomes, resulting from TMPRSS2 protein helping viruses enter the plasma membrane. A viral genome has been released into the host cell cytosol by ORF1a and ORF1b. These two proteins are translated into viral replicase proteins, which are then cleaved into individual nucleosomal proteins (PSPs); nsp12 belongs to the RNA-dependent RNA polymerase group (derived from ORF1b). Due to these actions, the replication factors rearrange the endoplasmic reticulum (ER) into double-membrane vesicles (DMVs), which facilitate viral replication of genomic and subgenomic RNAs (sgRNAs); the accessory and viral structural proteins that result from sgRNA translation provide virus particle formation (Harrison et al., 2020; Kabinger et al., 2021).
Schema 3.
Mechanism of SARS-CoV-2 Viral Entry.
Various advanced wastewater treatments for removing SARS-CoV-2 from sewage
Membrane
Membranes separate phases by blocking the flow of components through them (Zhao et al., 2020). Phase separation is accomplished through the selective movement of component elements (Ravanchi et al., 2009). Their behaviour makes it possible to classify membranes as anisotropic or isotropic (Obotey Ezugbe et al., 2020). In addition, the composition and physical structure of the anisotropic membrane are uniform, such as in reverse osmosis (RO) (Sagle et al., 2004; Aliyu et al., 2018). They can be either microporous or nonporous (dense); thus, their permeation flux is relatively high, while their low permeation flux severely limits their application (Obotey Ezugbe et al., 2020). Microfiltration membranes frequently use isotropic microporous membranes (Sagle et al., 2004; Obotey Ezugbe et al., 2020). Membranes can be classified based on their material makeup as either organic or inorganic (Moore et al., 2005; Aliyu et al., 2018). Synthetic organic polymers are used for manufacturing organic membranes. Based on their membrane type, membrane treatment processes that eliminate suspended solids and total dissolved compounds can be divided into low-pressure membrane filtration (microfiltration, ultrafiltration) and high-pressure membrane filtration (nanofiltration, reverse osmosis). Polyethylene, polytetrafluoroethylene (PTFE), polypropylene, and cellulose acetate, among others, fall into this category. Among the inorganic membrane, materials are ceramics, metals, zeolites, and silica (Mallada et al., 2008; Baker, 2012; Aliyu et al., 2018; Obotey Ezugbe et al., 2020).
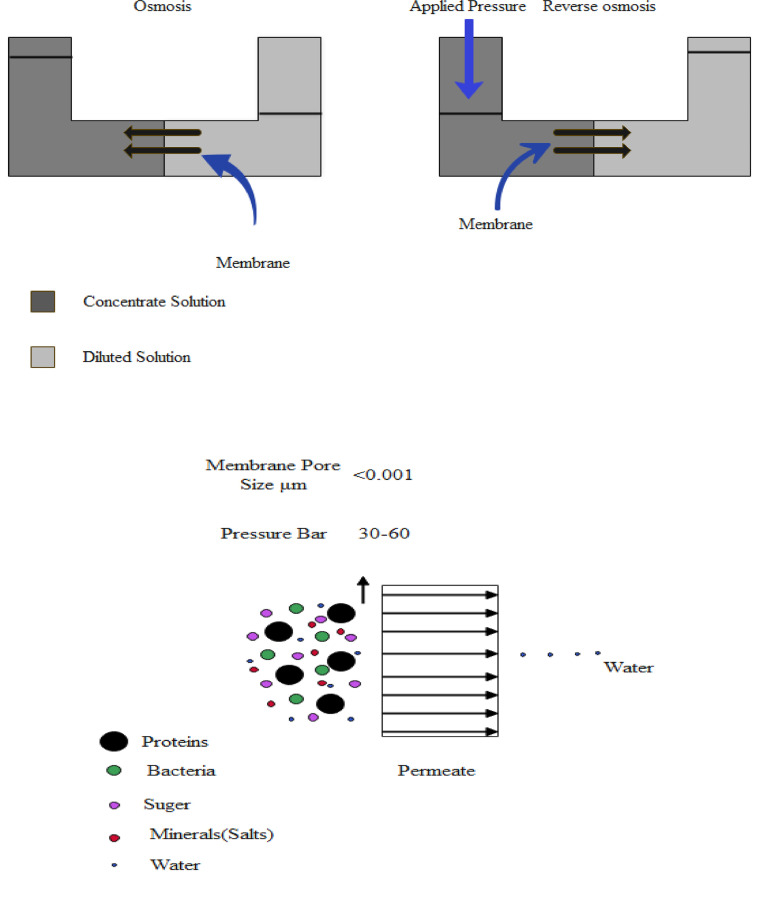
Pressure-driven membranes among wastewater treatment systems are the most commonly used before and after treatment. Hydraulic pressure is used in these processes to achieve separation (Van der Bruggen et al., 2003). The four main types of these processes are microfiltration (MF), ultrafiltration (UF), nanofiltration, and reverse osmosis. The main characteristics of these processes are: The pressure required for the MF membrane is 1-3 bar, and it is porous, asymmetric or symmetric, with a particle size range of 0.15 m to 0.15 m (Anis et al., 2019a), whereas in UF pressure required is 2-5 bar, and the type of this membrane is microporous, asymmetric and with a particle size range of 0.15 μm to 5 × 10−2 μm (Masoudnia et al., 2015); in the case of NF, pressure is 5-15 bar, and the type of membrane use is tight-porous, asymmetric, thin-film composite and a particle size range of 5 × 10−2 μm to 5 × 10−3 μm (Abdel-Fatah, 2018), while for RO pressure required is 15-75 bar, and semi-porous, asymmetric, thin-film composites are used, and the particle size range is of 5 × 10−3 μm to 10−4 μm (Tang et al., 2013). Compared with other membrane processes based on pressure. In addition to separating small particles, such as bacteria, RO is able to separate monovalent ions, such as sodium and chloride ions, up to 99.5% of the time (Wenten, 2016).
In addition, membrane bioreactors (MBRs) are considered an advanced technology for the treatment of wastewater, mainly to produce high-quality effluent suitable for reuse (Sengupta et al., 2021), including for the treatment of emerging contaminants (Nguyen et al., 2022). The technology has matured and has been widely implemented with a growing market share at about a 15% rate, mainly when aiming for effluent reuse (Hoinkis et al., 2012) and sustainability (Holloway et al., 2016). However, membrane fouling is still the major obstacle to boosting the widespread acceptance of MBRs. The need to manage membrane fouling led to inflated operational expenditure, enhanced energy footprint, complicated operation, and eventually reduced confidence in the technology. The complexity of MBRs operation in response to controlling fouling has become the downside compared to other simpler technologies (Hamedi et al., 2019). A high energy input associated with membrane cleaning via coarse bubble aeration is still a critical obstacle (Xiao et al., 2019).
Reverse osmosis
One of the most critical alternatives for water treatment is reverse osmosis membrane technology (Lee et al., 2011), which is currently the leading desalination technology (Lee et al., 2011; Qasim et al., 2019). RO is the most energy-efficient desalination technology, requiring only about 1.8 kWh/m (Jamaly et al., 2014, Park et al., 2020), much lower than other alternatives. Likewise, significant advances have occurred throughout the development of RO technology in materials science, process improvement, membrane synthesis, and modification (Anis et al., 2019b). In addition, RO is mainly used to treat large volumes of brackish groundwater (Afonso et al., 2004) and is an effective method for removing heavy metals without requiring secondary chemical treatment, as shown in Schema 4 (Thaçi et al., 2019). In addition, these techniques remove most of the suspended particles, leading to heavy fouling of lead elements (Pandey et al., 2012). The best example of RO's efficiency in removing ions is dissolved salts (Yoon et al., 2009; Kheriji et al., 2015). It has been widely used in aerospace (Cadotte et al., 1981; Cabrera et al., 2014), food (Hafiz et al., 2020), oil (Bastos et al., 2020), gas (Tang et al., 2020), galvanic (Makisha et al., 2017, Innocenzi et al., 2020), dairy (Balannec et al., 2005), pulp and paper (Mänttäri et al., 2007), and power plants (Emamdoost et al., 2020).
Schema 4.
A practical method for removing organic, inorganic matter, and microorganisms.
The RO process separates ions from water by applying hydrostatic pressure against the osmotic pressure across a semipermeable membrane (Shi et al., 2020). The advantages of this technology include producing high-quality water with low fouling potential (Jiang et al., 2018), ultra or microfiltration pretreatment, regardless of the characteristics of the source water, minimizing the need for frequent chemical cleaning, extending the membrane lifespan, and minimizing overall treatment costs (Khedr 2013; Wenten 2016). However, higher costs and the adverse effects of concentrating on the environment are potential disadvantages (Ghernaout et al., 2017). Further drawbacks for RO membranes include organic fouling caused by dissolved organics and scaling due to an overabundance of marginally soluble salts (Valavala et al., 2011).
In water treatment, RO is a pressure-driven technology that is widely used (Peters et al., 2019). Firstly, RO is controlled by two major factors: membrane characteristics and pore size (Yang et al., 2019; Zhang et al., 2019); moreover, RO systems need pretreatment, including filtering and chemical addition, to avoid membrane biofouling (Anis et al., 2019a). Finally, RO membranes can be classified into aromatic polyamide, thin-film composite (TFC) (Liu et al., 2018; Kasongo et al., 2019), and cellulose (Asempour et al., 2018). Polyamide thin-film composite (PA-TFC) is the most commonly used material in the production of RO membranes because of its excellent water permeability, high salt rejection, and stability, as well as the ability to tolerate low pH or high pH (Liu et al., 2018; Aziz et al., 2021). In RO, cellulose and polyamide derivatives are used as membranes with a 50–100 Da molecular weight cut-off (Hu et al., 2021). According to the USA Environmental Protection Agency, RO is the best technology to remove inorganic contaminants such as radionuclides (alpha and beta emitters) and heavy metals (such as arsenic and antimony) from contaminated groundwater. Thus, bromoform and iodoform in disinfected water can be controlled effectively with RO treatment (Table 3 ) (Wang et al., 2019; Samaei et al., 2020).
Table 3.
Removing micropollutants in various wastewater treatments by RO/NF.
| Technology | Removal micropollutants | Type of micropollutants | Description | Refs. |
|---|---|---|---|---|
| RO | 45-98% | Gemfibrozil, Ketoprofen, Carbamazepine, Diclofenac, Mefenamic acid, Acetaminophen, Sulfamethoxazole, Propyphenazone, Hydrochlorothiazide, Metoprolol, Sotalol, Glibenclamide |
|
(Radjenović et al., 2008) |
| NF/RO | 82-100% | Acetaminophen, Alachlor (Lasso), Atraton, Bisphenol A, Caffeine, Carbadox, Carbamazepine, DEET, Diethylstilbestero, Equilin, 17_-Estradiol, 17_-Estradiol, Estriol, Estrone, 17-Ethynyl Estradiol, Gemfibrozil, Metolachlor, Oxybenzone, Sulfachloropyridazine, Sulfamerazine, Sulfamethizole, Sulfamethoxazole |
|
(Comerton et al., 2008) |
| RO | 25-95% | N-nitrosodimethylamine (NDMA), Nnitrosomethylethylamine (NMEA), Nnitrosopyrrolidine (NPYR), N-nitrosodiethylamine (NDEA), N-nitrosopiperidine (NPIR), Nnitrosomorpholine (NMOR), N-nitrosodipropylamine (NDPA), Caffeine, Simazine, Atrazine, Primidone, Meprobamate, Triamterene, Tris(2- chloroethyl)phosphate (TCEP), Trimethoprim, Nnitrosodi-n-butylamine (NDBA), N,N-Diethyl-metatoluamide (DEET), Bisphenol A, Diuron, Carbamazepine, Linuron, Diazepam, Triclocarban, Clozapine, Omeprazole, Hydroxyzine, Paracetamo, buprofen, Naproxen, Gemfibrozil, Dilantin, Sulfamethoxazole, Ketoprofen. Triclosan, Diclofenac, Enalapril, Simvastatin hydroxy acid, Atenolol, Amitriptyline, Fluoxetine, Verapamil |
|
(Fujioka et al., 2015) |
| NF/RO | 87-99% | Acetaminophen, Bisphenol A, Caffeine, Carbamazepine, Cotinine, Ethinyl Estradiol-17α, Gemfibrozil, Ibuprofen, Progesterone, Sulfamethoxazole, Triclosan, Trimethoprim |
|
(Huang et al., 2011) |
| RO | 57-91% | 2-Naphthol, 4-Phenylphenol, Phenacetine, Caffeine, NAC standard, Primidone, Bisphenol A, Isopropylantipyrine, Carbamazepine, Sulfamethoxazole, 17-Estradiol |
|
(Kimura et al., 2004) |
| RO | 0-85% | 2-Naphthol, 4-Phenylphenol, Phenacetine, Caffeine, NAC standard, Primidone, Bisphenol A, Isopropylantipyrine, Carbamazepine, Sulfamethoxazole, 17-Estradiol |
|
(Kimura et al., 2004) |
| RO | 76.5 | N-nitrosodimethylamine |
|
(Croll et al., 2019) |
Nanofiltration
Since the 1970s, NF has been the newest pressure-driven filtration technology; and the membrane has properties similar to RO and UF (Mohammad et al., 2015). NF membranes are used for various applications, including food (Salehi, 2014; Mallakpour et al., 2021), pharmaceuticals (Zaviska et al., 2013; Mallakpour et al., 2021), wastewater treatment (Bethi et al., 2021; Zhao et al., 2021), and desalination (Mi et al., 2020). Moreover, the separation method removes sparingly soluble salts (Hao et al., 2016) and large organic molecules from fluids (Gao et al., 2020). Calcium and magnesium are two multivalent ions that NF membranes are highly efficient at removing (Cheng et al., 2018). In addition, partially monovalent salts such as sodium and chloride may also be removed depending on the NF membrane cut-off size (Cheng et al., 2018; Li et al., 2021). Note that NF has a 150–1000 Da (Schmidt et al., 2020).
One of the first characteristics to remark is that synthetic polymers, which are thin and spiral, form the membrane elements in the manufacturing of NF membrane separation surfaces. Next, a membrane module filters pressurized source water; finally, sodium ions and organic molecules are rejected from the membrane, and all the water molecules pass through and are reabsorbed in the water that passes through the membrane. This process is accomplished by bypassing pressurized source water along the membrane's surface (Oatley-Radcliffe et al., 2017; Tul Muntha et al., 2017).
The advantages of the NF process include low operation pressure (Li et al., 2019), high flux (Moradi et al., 2020), high retention of multivalent anion salts (Kramer et al., 2019), a molecular weight above 300, relatively low investment, and low operation and maintenance costs. Due to these advantages, NF has become increasingly popular worldwide. Furthermore, NF is widely used for decontaminating and recycling wastewater from diverse industries, including oil and gas, chemicals, and foods and beverages. Finally, water reuse became possible due to the reduction of organic load in the wastewater treated with NF partial desalination. However, Water recovery from wastewater treatment, on the other hand, is an important issue because it should be close to 100%; several researchers studied the feasibility of an integrated membrane system to achieve this goal (Mohammad et al., 2015).
Ultrafiltration
UF, a membrane separation process, is usually applied after conventional treatment as a method for removing particles from solutions. Besides, the separation of macromolecules (103 to 106 Da) from solutions, particularly proteins present in solutions, is usually performed in industry and research (Ahmad et al., 2020). Ultrafiltration membranes are classified based on their molecular weight cut-offs (Kadel et al., 2019). They can be used in cross-flow or dead-end modes in different applications (Ohanessian et al., 2020). Likewise, when the system is in dead-end mode, all source water passes through the membrane and is discharged periodically in batches based on the concentration of solids around the membranes (Roslan et al., 2018). Although a cross-flow system is used, a portion of the source water is discharged or recycled continuously back to the system's inlet (Saeki et al., 2017). Therefore, ultrafiltration membranes can filter only small molecules, such as water, inorganic salts, and micromolecular organics, rather than macromolecules like SS, colloids, proteins, and bacteria. Prior to NF, UF is always used to pretreat leachate with a lower organic concentration (Luo et al., 2013; Oatley-Radcliffe et al., 2017).
Due to its low energy cost and high-water flux compared with NF and RO, UF is widely used to produce drinking water. Moreover, due to a pressure difference between 0.1 and 0.5 MPa, UF uses less energy than traditional pretreatment processes; likewise, pretreatment of effluents and wastewater can be accomplished with UF. In addition, UF recovery processes require far less energy than RO and NF recovery processes. However, they cannot frequently recover DS with lower molecular weight because UF processes have huge pore sizes (Shon et al., 2013; Yang et al., 2019) (Table 4 ).
Table 4.
Removing micropollutants in various wastewater treatments by UF.
| Technology | Removal micropollutants | Type of micropollutants | Description | Refs. |
|---|---|---|---|---|
| UF | 20-80% | Sulfamethoxaz, Carbamazepin, Diclofenac, Ibuprofen |
|
(Burba et al., 2005) |
| NF/UF | 10-75% | Amoxicillin, Naproxen, Metoprolol, Phenacetin |
|
(Javier Benitez et al., 2011) |
| UF | 11-65% | Estrone, Estradiol, Progesterone, Testosterone |
|
(Neale et al., 2012) |
| UF | 25-39% | Perfluorooctanoic acid, Perfluorooctanesulfonic acid |
|
(Pramanik et al., 2017) |
| UF | 17-76% | Bisphenol A, 17α-ethynyl, Estrone 17β-estradiol, Estriol |
|
(Hu et al., 2014) |
| UF | 47-60% | Ibuprofen, Sulfamethoxazole |
|
(Chu et al., 2017) |
| UF | 99.61% | Bisphenol A |
|
(Muhamad et al., 2018) |
| UF | 80-84% | Bisphenol A, 4-Nonylphenol |
|
(Kaminska et al., 2015) |
| UF | 88.97-99.92% | Carbamazepine, Galaxolide, Caffeine, Tonalide, 4-nonylphenol, Bisphenol A |
|
(Wanda et al., 2017) |
| UF | 23-65% | Bisphenol A, Norfloxacin |
|
(Wu et al., 2016) |
Microfiltration
MF is essential in the food (Utoro et al., 2019) and beverage industries (Manni et al., 2020). MF membrane is usually possible following conventional treatment methods, and MF technology is commonly used to remove coarse particles or microbes. In addition, MF technology can remove suspended matter from water, including bacteria, paint, pigment, yeast cells, etc. It is also possible to run such MF either in crossflow mode or dead-end mode (El Rayess et al., 2011).
Hydrophilic polymers are typically used to manufacture MF membranes, such as polyvinylidene fluoride (PVDF), polysulfone, PTFE, polypropylene, and nylon. Teflon membranes have recently been introduced to the market in their newer version. PP is the only membrane that is not resistant to oxidants such as chlorine. During the manufacturing process, special agents are usually added to membranes in order to reduce fouling caused by dissolved organics. As a result, special agents are often applied to the membranes during fabrication to prevent fouling issues. Finally, note that water treatment systems commonly use the MF and UF processes (Anis et al., 2019)
Effect of membrane on SARS-CoV-2
MF and UF, two kinds of low-pressure membrane filtration used in advanced wastewater treatment, have the potential to provide a complete barrier to SARS-CoV-2 transmission. Moreover, a modular membrane system structure can facilitate upgrading existing WWTPs in order to reduce the concentration of SARS-CoV-2 in the effluent. MF > 50 nm and UF 2–50 nm membranes can effectively remove SARS-CoV-2, although this is highly dependent on the pore diameter distribution in relation to the target virus. Thus, UF can effectively remove SARS-CoV-2 with a diameter of 10-100 nm (Kitajima et al., 2020). SARS-CoVs may also be removed depending on membrane surface characteristics based on electrostatic and hydrophobic interactions. UF can be applied in membrane bioreactors (MBRs) to enhance viral removal (not just SARS-CoV) and steric removal, adsorption, and inactivation during biological treatment. Due to this, MBRs have been shown to be more effective at removing enteric viruses (removing up to 6.8 logs) than conventional WWTPs (removing up to 3.6 logs). SARS-CoVs could also be entirely removed by high-pressure membrane systems using tighter and denser membranes (pore size <2 nm) such as NF and RO (Lv et al., 2006; Pendergast et al., 2011; Chaudhry et al., 2015; Bodzek et al., 2019).
UV irradiation
UV irradiation comprises electromagnetic waves invisible to humans but visible to many insects and birds. Also, UV irradiation is a form of electromagnetic energy, having a wavelength shorter than visible light but longer than x-rays (Lu et al., 2019; Milov et al., 2020). Commonly, black lights and mercury lamps are specific lights in UV (Hinds et al., 2019). Frequently, this system is associated with electromagnetic energy with a wavelength of 10 nm to 400 nm. UV is crucial in physical operations for water disinfection (Sommer et al., 2008). In addition, UV light harms and hinders microorganisms' growth. Therefore, UV radiation is capable of removing pathogens and organic matter in wastewater treatment. Advantages of UV include: a) No chemicals were added, b) Cost-effective, c) Fast-acting, d) Effective against a range of organisms broader than chlorine, and g) UV water purifier kills bacteria and viruses. UV disadvantages are: a) incapable of removing dissolved impurities (such as pesticides, rust, arsenic, fluoride, etc.), b) Requires electricity, c) UV light shutoff (Espid et al., 2017; Lei et al., 2021).
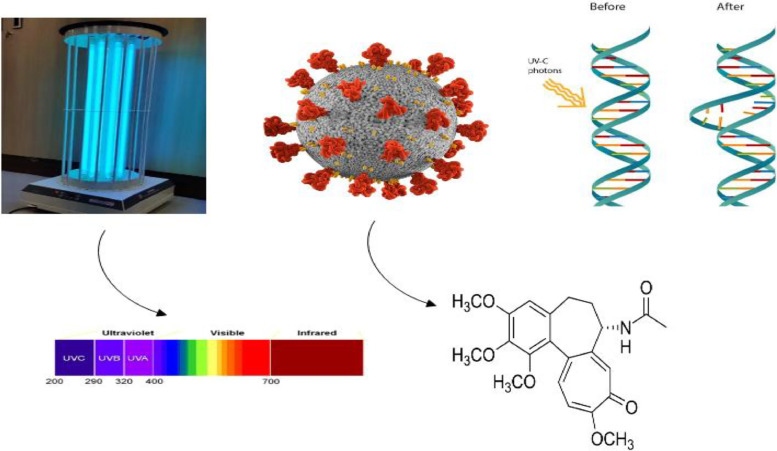
Furthermore, UV is typically used for photoreactions. UV radiation falls into four categories: UVC, UVB, and UVA. It is also common to use far, mid, and near UV instead of VUV (10 - 200)/UVC (100–280 nm), UVB (280-315 nm), and UVA (315–400 nm) (Collivignarelli et al., 2021).
The direct irradiation of UV can destroy microorganisms (Vasilyak, 2021). Using UV radiation as an alternative to chlorination is a standard disinfection method because the usage of Cl2, NaClO, and similar Cl compounds generates substances that promote cancer (Table 5 ) (Tak et al., 2017). Usually, UV disinfection is accomplished by irradiating with 253.7 nm (Sommer et al., 2001; Cervero-Aragó et al., 2014); however, bacterial cells are generally resistant to irradiation under longer wavelengths due to the majority of organic components inside them (Pradhan et al., 2015). Bacteria are removed through photon absorption by DNA (263-275 nm wavelength), resulting in hydrogen bond splitting and thus causing a break in DNA (or other irreversible changes to DNA) that prevents transcription of its genetic material. The shorter wavelengths, which are more energetic, may be absorbed by the cell membrane, preventing osmotic pressure regulation. Depending on the wavelength, bacteria are deactivated differently, with UVA usually destroying non-nuclear components, whereas UVB and UVC usually destroy nuclei (Sharrer et al., 2007).
Table 5.
Removing micropollutants in various wastewater treatments by UV.
| Technology | Removal micropollutants | Type of micropollutants | Refs. |
|---|---|---|---|
| UV | 12-64% | Estrogen, 17α-estradiol, 17β-estradiol, ethinylestradiol, estriol, carbamazepine, naproxen, clarithromycin, diclofenac, ibuprofen, bisphenol A, indomethacin | (Wols et al., 2012; Wang et al., 2016; Yang et al., 2016) |
| UV–chlorine | 50-99.80% | Estrogen, 17α-estradiol, 17β-estradiol, ethinylestradiol, estriol, carbamazepine, naproxen, clarithromycin, diclofenac, ibuprofen, bisphenol A, indomethacin | |
| chlorine | 5-65% | Estrogen, 17α-estradiol, 17β-estradiol, ethinylestradiol, estriol, carbamazepine, naproxen, clarithromycin, diclofenac, ibuprofen, bisphenol A, indomethacin |
In UV irradiation, pathogens' DNA nucleotides are inactivated by UV light (Kumar et al., 2004; Nyangaresi et al., 2019). The National Water Research Institute (NRWI) recommends a fluence of N20 mJ/cm2 to eliminate MS 2 phages successfully and a fluence of 180 mJ/cm2 to successfully inactivate Adenoviruses in drinking water. According to the German water directive, disinfection must be performed using a UV fluence of 40 mJ/cm2 (Hiller et al., 2019). Nevertheless, UV disinfection in water reuse applications is recommended to contain equivalent doses of up to 100 mJ/cm2, though this depends on the pretreatment (e.g., granular media filtration, membrane filtration). Finally, for effluent disinfection at WWTPs, UV fluences are typically applied at 60 to 200 mJ/cm2 (Bourrouet et al., 2001; Hiller et al., 2019).
Specifically regarding COVID-19, since December 2019 the SARS-CoV-2 virus caused the disease, and according to World Health Organization (WHO), by January 30, 2020 this crisis must be considered of public health and international concern. COVID-19 was detected in many countries at the beginning of March 2020, suggesting it could be a pandemic (Lopez Bernal et al., 2021). Coronaviruses and other respiratory viruses are less resistant to sterilization methods; hence, sufficient levels of disinfection can be achieved for the reprocessing of personal protective equipment and supplies, and ultraviolet C (UV-C) irradiation can be effective for this purpose. Many health services have been shown to reduce bacterial and viral contamination by UV-C, which disinfects the air, water, and surfaces (Barnewall et al., 2021; Carleton et al., 2021).
Effect of UV on SARS-CoV-2
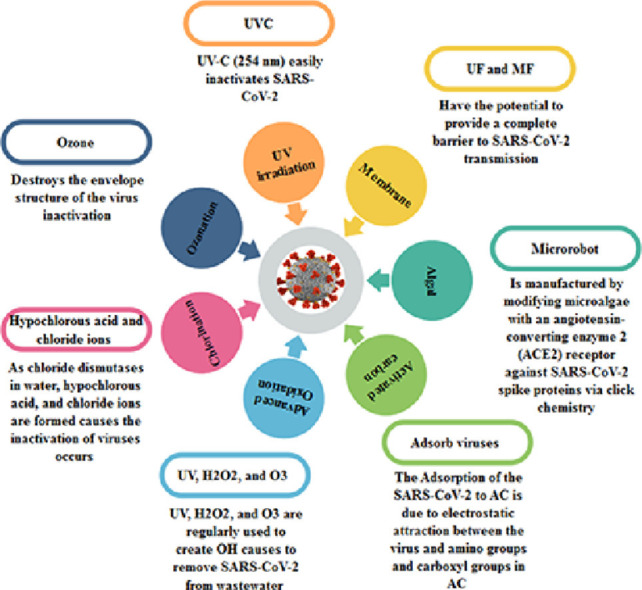
Various viruses, including SARS-CoV, are inactivated by UV radiation, especially UV-C (200-280 nm). Specifically, several studies have shown that UV-C (254 nm) easily inactivates SARS-CoV-2, as indicated in Schema 5 ; UV-C radiation is directly absorbed by nucleic acid bases and capsid proteins (Zahmatkesh et al., 2022c), leading to the production of photoproducts that inactivate the virus. Also, UV light from sunlight effectively inactivates the virus (Parsa et al., 2021).
Schema 5.
Effectiveness of UVC on SARS-CoV-2 inactivation.
Ozonation
France was the first to use ozone for water treatment in 1886 (Kong et al., 2021). As a result of its remarkable properties, such as its strong ability to oxidize, producing fewer by-products of disinfection than other chemical disinfectants, and removing colour and odour from water, ozone has expanded its influence worldwide. Furthermore, some European countries use ozone for taste and color removal. Ozone oxidation and disinfection functions are gaining much attention in water and wastewater treatment. When ozone reacts with water, it produces several ions and free radicals, such as HO⋅, HO2⋅, O, O2, etc. Oxidation is a swift reaction. The residual ozone concentration is typically halved within 30 s and reaches an inactivation level of at least 1-log (Wen et al., 2020). According to the water directives in Germany, 10 mg/L of ozone is the maximum amount that can be used for disinfection (Tyrrell et al., 1995; Shin et al., 2003; Edwards-Brandt et al., 2007; Kong et al., 2021).
Furthermore, the oxidation of trace organic compounds is another potential use of ozone, where hydroxyl radical reactions simultaneously occur (Remucal et al., 2020). In order to remove trace organic compounds, ozone dosages typically range from 0.4 to 1.0 mg O3/mg DOC, depending on the removal objectives and the target compounds (Zhang et al., 2018).
Recent studies indicate that ozone's germicidal effects depend on the type of microbiota (Garcia-Costa et al., 2021). Several water quality parameters can negatively affect ozone stability, including alkalinity, dissolved organic carbon, and particle density (TSS/turbidity), protecting the surface-attached microorganisms (Yan et al., 2007a, 2007b). The effectiveness of ozone disinfection is influenced by exposure to it, specifically by the time-integrated characteristic of ozone concentration applied over contact time. The specific water quality significantly impacts this factor. Wastewater matrices containing DOC, suspended solids, residual nitrite, or particulate matter from activated sludge treatment can deplete ozone, reducing disinfection effectiveness. Increasing ozone doses to improve disinfection is often associated with the formation of carcinogenic bromate (Loeb et al., 2012; Rekhate et al., 2020).
In wastewater treatment, ozone is used in a process called ozonation. The ozonation process involves the dissolution of ozone in wastewater, but this mechanism has resulted in low concentrations of ozone in water, so using ozone to treat wastewater is ineffective. Micro and nanobubble technologies have increased ozone solubility and lifetime (Agustina et al., 2005).
Dry air or pure oxygen generates ozone, a gas with a pungent smell. Because ozone is formed endothermically, it is thermodynamically unstable and rapidly returns to oxygen (3O2 ↔ 2O3) (Prabha et al., 2015; Alimohammadi et al., 2021). In addition, ozone is a powerful oxidizing agent and can be used in water and wastewater treatment, bleaching (Kaur et al., 2019), and synthesis (Mashayekhi et al., 2018). This method is used in water and wastewater treatment for four main reasons: oxidation of bio-recalcitrant pollutants, disinfection, as a way to remove taste and odour, and colour, to reduce the turbidity of water. Ozone has several advantages: (1) it can be produced quickly by electric discharge from air or oxygen on-site; (2) it can react efficiently with organic and inorganic compounds; and (3) it can be used for several purposes, such as disinfection, reducing chemical oxygen demand, and improving colour, and odour. Turbidity of water ozone excess rapidly decomposes into oxygen in water, leaving no residue behind. It should be noted that ozone oxidation generates radicals. This indirect mechanism is highly reactive and unselective. Radicals such as *OH are potent oxidants that play an essential role in the disinfection and oxidation of contaminants (Hussain et al., 2020; Rekhate et al., 2020).
Effect of ozonation on SARS-CoV-2
Virus inactivation can be accomplished by destroying the envelope structure since viruses rely on the specific proteins on their envelopes to invade host cells. According to a study conducted with ozonized water (Martins et al., 2021), the SARS-CoV-2 virus is no longer viable after the envelope is destroyed, although its nucleic acids are still detectable. In some cases, the nucleic acids of non-enveloped viruses become more sensitive due to changes in the capsid proteins, which are very stable, but envelope proteins are much more delicate. Using low doses of ozone, Roy and colleagues found that the capsid protein of poliovirus 1 (PV1) changed; however, the virus's ability to attach to host cells was unaffected (Schema 6 ). Nevertheless, due to RNA destruction, the virus was inactivated (Roy et al., 1981; Jiang et al., 2019; Martins et al., 2021)
Schema 6.
Effect of Ozonation on SARS-CoV-2.
Chlorination
The most common method for disinfecting water and wastewater is chlorination, a critical step in reclaiming water. However, it also produces numerous disinfection by-products (DBPs) when chlorine reacts with dissolved organic matter (Mazhar et al., 2020). Many DBPs have been proven to have carcinogenic, genotoxic, and mutagenic potential, making them toxic to humans and aquatic organisms in chlorinated water (Kali et al., 2021). Likewise, drinking water should be governed by the DBPs Rule (Liu et al., 2020), because of the large production volume and genotoxicity of two influential groups of DBPs: trihalomethanes (THMs) and haloacetic acids (HAAs). However, chemical disinfectants and inactivating agents are two of the many disinfection methods currently used in full-scale WWTPs. Bear in mind that upon chlorine addition to water, a portion of the chlorine reacts immediately with inorganic materials and metals present, and cannot be eliminated; chlorine in water is called total chlorine, while the rest is called chloride demand (Ghernaout, 2017).
Gaseous chlorine is converted to the oxidative species hypochlorous acid (HOCl) as it is injected into wastewater. As part of its oxidation reactions, hypochlorous acid attacks organic molecules at electrophilic sites and adds to unsaturated bonds. Chemical structure plays a crucial role in hypochlorous acid's ability to oxidize organic pollutants since it is highly selective for attacking nucleophilic sites or reducing them (Du et al., 2017).
Following the German water directive, calcium hypochlorite (Ca(OCl)2) and sodium hypochlorite (NaOCl) can be treated with a maximum of 4.7 mg/L free Cl2 and 6 mg/L free Cl2, respectively (Dong et al., 2022). The typical chlorine dosage used to disinfect WWTP effluents is 5–10 mg/L with a 30 min contact time (Mazhar et al., 2020).
Effect of chlorination on SARS-CoV-2
As chloride dismutases in water, hypochlorous acid and chloride ions are formed. Further ionization of hypochlorous acid produces hypochlorite. The primary disinfectant is typically regarded as hypochlorous acid. In Schema 7 , it was shown that the inactivation of viruses occurs when the chlorinated water damages the capsid and destroys the exposed nucleic acids (Fuzawa et al., 2019). It may be because chlorine reacts differently with amino acids (Na et al., 2007) at different sites of viral capsids, which explains these high reactivity sites. However, chlorine is less reactive than ozone, meaning that some microorganisms (and specifically bacteria) are inactivated differently. After chlorination, chlorine reacts more with intracellular components, meaning that the cells show better structural integrity and less plasma leakage because the diffusion of chlorine into cells is less constrained by reactions with cell walls. Chlorination is influenced by factors similar to ozone disinfection (Churn et al., 1983; Cho et al., 2010).
Schema 7.
Inactivation of some microorganisms when using chlorination in water.
Advanced oxidation
AOP (advanced oxidation process) is one of the chemical processes widely used for wastewater, including different mechanisms for organic destruction. Moreover, AOP has been seen as an environmentally friendly, efficient, and cost-effective alternative to traditional disinfection methods for controlling water microbes (Garrido-Cardenas et al., 2020). As a result, chemical oxidants are formed in situ in order to disinfect water and degrade harmful organic contaminants. There is no doubt that AOPs can effectively kill various microorganisms such as viruses, protozoa, spore-forming bacteria, fungi, and yeasts. As part of this process, in order to be able to purify water, an adequate number of hydroxyl radicals (OH•) are produced, and this idea was later extended to sulfate radicals (SO4•*). AOPs have been studied for the inactivation of pathogens, pathogenic indicators, and the degradation of organic and inorganic pollutants. AOPs are the best treatment methods because strong oxidants can rapidly destroy recalcitrant organic pollutants. Several studies using AOPs for textile dye wastewater treatment have been conducted. AOP's advantages include: synthesizing stable inorganic compounds by converting organic materials, preventing pathogens, particularly with the use of UV rapid and robust technology, and removing heavy metals from nearly all organic compounds (Boczkaj et al., 2017; Kanakaraju et al., 2018; Miklos et al., 2018).
The Rodrigues et al. (2008), report demonstrated that heterogeneous photocatalysis could remove more than 90% of COD from textile wastewater treatment effluents. Azbar et al. (2004) showed that AOP results in 60% COD and 50% colour removal when combined with conventional methods using O3/H2O2/UV. Integrating the AOP with the conventional methods results in 96% colour removal and 99% COD removal. Soares et al. (2014) showed that using AOPs in cotton-textile dyeing wastewater resulted in a 60 mg catalyst loading, 85.5% mineralization, and 98.5% decolourization in the solar-photo-Fenton process.
Two decades ago, AOPs gained notable recognition as a technology for enhancing wastewater treatment. Several methods have successfully broken-down organic contaminants at the pilot scale, including Fenton, cavitation, ozonation, and photocatalytic oxidation. In addition, upon activation, AOPs create radicals of the OH type, which react with organic compounds in the presence of dissolved oxygen (Table 6 ) (Abbas et al., 2014).
Table 6.
Utilization of advanced oxidation technologiesa.
| Study conducted on a compound | Applied technologies | Summary |
|---|---|---|
| Hydroxychloroquine | Advanced electrochemical oxidation | As part of oxidation, two boron-doped diamond diodes (BDD) are used to create CO2 and other intermediate compounds that are highly unstable due to a large amount of OH* radicals formed at the surface of the BDD due to electrochemical oxidation of water. |
| Chloroquine | Electro-Fenton oxidation | BDD anodes are used with the addition of FeSO4 in the solution in order to generate a more significant number of free radicals in the AOP and thus improving the cost-effectiveness, even though, the pH has to be controlled. |
| Virus | Photocatalyst | The photocatalyst Ag3PO4/g-C3N4 synergizes the effects of Ag3PO4 with those of g-C3N4, improving the efficiency of the absorption of visible light; consequently, a more significant destruction of the viruses by means of the ROS. |
| Ozone | The powerful oxidant that, by generating ROS, could attack the virus in different places of its structure, especially the S-glycoprotein, inhibiting the infection process. | |
| Flat-sheet PVDF and filters based on PVDF coated with multiwalled carbon nanotubes layer (MWCNTs). | A flat sheet of PVDF is employed, showing a bacteriophage photocatalysis inactivation where the membrane acted as a fence. MWCNTs functionalized with different silver-based filters were demonstrated to remove effectively viral bacteriophages but with a virus retention limitation after the filtration. | |
| Cold plasma (CP) | The plasma acts to facilitate the production of UV radiation with reactive oxygen and/or N species (RONS) that are also the limiting factor and acts to damage the nucleic acids, oxidizing nucleic acids, proteins and lipids |
Adapted with permission from Pacheco et al. (2021).
Ozone based AOPs
Water treatment has long used ozone as an oxidant and disinfectant. Oxidation by ozone is highly selective; ozone attacks primarily electron-rich functional groups such as double bonds, amines, and aromatic rings (e.g., phenol). The fact that its reactions often result in the formation of OH in real aqueous solutions frequently qualifies ozonation as an AOP or similar process. By reacting ozone with hydroxide ions, OH can be formed. Radicals are generated as by-products after reactions involving ozone and organic matter (particularly phenolic and amine functional groups). In particular, these reactions contribute significantly to the radical formation during the ozonation of secondary effluents. The generation of radicals can be actively initiated using ozonation at an elevated pH and combinations of O3/H2O2, O3/UV, and O3/catalysts (Ikehata et al., 2018; Hussain et al., 2020).
UV-based AOPs
AOPs based on UV is typically irradiated with UV-light (mainly UV-C) and use various radical promoters in conjunction with UV light. In advanced oxidation, UV fluences are typically > 200 mJ/cm2 and thus exceed the UV dosage needed to inactivate most pathogens, including those resistant to UV. Typically, UV irradiation is produced by low-pressure or medium-pressure mercury lamps with monochromatic or polychromatic emission spectra. However, in recent research (Miklos et al., 2018), ultraviolet light-emitting diode (LED) sources with specific wavelength distributions have been studied and analyzed for disinfection purposes. In comparison to conventional medium and low-pressure lamps, LEDs have three principal advantages. They eliminate mercury, emit unique peak wavelengths, are compact, have a flexible design, and have a rapid startup time (Deng et al., 2015; Miklos et al., 2018).
Electrochemical AOPs
Various electrodes are typically used in this process, including doped SnO2, PbO2, RuO2, boron-doped diamond (BDD), and sub-stoichiometric and doped TiO2. Despite this, BDD-electrodes are frequently used because their production costs are typically lower than other electrodes, and the diamond layer remains stable under anodic polarization (Oliveira et al., 2021). Using BDD electrodes for electrochemical oxidation of contaminated water can produce OH directly due to the evolution of O2 from water oxidation. In this way, BDD-electrode treatment is considered an eco-friendly and efficient method of removing various pollutants. Due to the fact that the generation of OH occurs directly on the electrode surface and since OH has a reactivity range of about 1 mm, diffusion at the electrode surface limits high oxidation efficiencies. Nevertheless, hydrodynamic parameters for eAOP processes must be considered since the energy used for pumping water might account for the largest share of energy consumption in this process (Ganzenko et al., 2014; Oturan et al., 2014).
Effect of AOP on SARS-CoV-2
Using AOPs widely, emerging contaminants and pathogens will be more efficiently removed from water and wastewater. Different combinations of UV, H2O2, and O3 are regularly used to create OH in sufficient amounts to degrade organic pollutants and some inorganic pollutants. For example, it has been demonstrated that AOP (UV/H2O2) can remove SARS-CoV-2 from wastewater and degrade endocrine-disrupting compounds (Pacheco et al., 2021; Teymoorian et al., 2021).
Activated carbon
The adsorbent properties of AC are primarily due to its highly porous carbon matrix, with a high surface area and a broad range of functional groups. Despite the irregular arrangement, many chemical bonds connect the layers of carbon, creating a highly porous structure that includes lines and cracks between them. In addition, the carbonaceous material AC has a high porosity and a large surface area, making it useful for a wide range of applications. Their characteristics such as pore diameter, hardness, density, iodine number, and ash content make them suitable for different applications. Besides, the pores on ACs have remediable surface chemistry, chemical/thermal stability, and high accessibility (Wong et al., 2018; Völker et al., 2019). Different functional groups of aromatic rings maintain the chemical properties of AC. To create these groups, carbon can be treated chemically, thermally, or hydrothermally. The pores in AC can be divided into macropores (diameter > 50 nm), mesopores (2 ≤ diameter ≤ 50 nm), and micropores (diameter < 2 nm). These filters remove organic substances through a combination of adsorptive and biological processes. A common technique for advanced wastewater treatment is GAC filtration. AC is produced from coconut shells, coals, wood, and lignite in today's wastewater treatment. AC characteristics make them ideal for adsorption: porous surfaces, high surface areas and surfaces containing specific chemical groups that react with molecules. AC adsorption occurs first on the outside of the carbon matrix. A second process is the transfer of materials inside carbon pores. A third is the adsorption of materials on the carbon's internal walls (Perrich, 2018; Zahmatkesh et al., 2020).
Furthermore, in wastewater treatment, AC is one of the most effective adsorbents for removing pollutants like dyes (Moosavi et al., 2020), metals (Karnib et al., 2014), and pesticides (Gupta et al., 2011). Heavy metals in water can also be dangerous to human health; additionally, AC is an economical and straightforward method to remove heavy metals; for this reason, AC is widely used to process water containing these pollutants (Santhy et al., 2004). Meanwhile, three areas in which the AC process is applied include the advanced treatment of drinking water, sewage reclamation, and industrial wastewater treatment. The more efficient AC is a powerful adsorbent, with various activities in wastewater. The COD and BOD removal rate in wastewater by AC is affected by its porous structure and surface area.
Biological activated carbon is based on physicochemical adsorption and biological-oxidation degradation synergistic effects. Different sized organic molecules and dissolved oxygen can enter the pores of AC due to its high adsorption capacity and micropores (Wang et al., 2021), mesopores (Kennedy et al., 2004), and macropores (Dong et al., 2019). In addition, in AC technology, AC microorganisms extend activated carbon's adsorption capacity by biodegrading organic adsorbates to regenerate adsorption sites. However, the pore size of AC has a significant effect on microbial attachment, and the highest biomass retention was measured with a pore size between 2 and 50 nm. Also, AC is more suitable for the control of DBP precursors in systems (Singh et al., 2011; Dos Santos et al., 2020).
Effect of AC on SARS-CoV-2
SARS-CoV-2 can be retained with AC filters, as they adsorb viruses from contaminated deposits. The adsorption of SARS-CoV-2 to AC is due to electrostatic attraction between the virus and amino groups and carboxyl groups in AC (Schema 8 ).
Schema 8.
Images of the use of an activated carbon filter as an absorber for viruses and other microorganisms in the presence of heavy metals in wastewater treatment.
Algae
The comparison between microalgae wastewater treatment systems and conventional biological wastewater treatment indicates that microalgae are a more sustainable, environmentally friendly (Zahmatkesh and Sillanpää, 2022), and economical alternative to conventional biological wastewater treatment (Abdel-Raouf et al., 2012). Algae can produce biofuel and advanced bioremediation by integrating industrial and municipal utilities into holistic urban resources (Wollmann et al., 2019). In general, integrated algal wastewater treatment systems can remove N, P, BOD, and COD from wastewater (Li et al., 2019; Zahmatkesh et al., 2020), capture CO2 from power plants, and produce biofuels by cultivating algae. Organic nitrogen (such as urea) and inorganic nitrogen (such as ammonium and ammonia), nitrites and nitrates, can both be used by micro-algae. During wastewater treatment, NO2 is released due to the environmental conditions under which N is removed (Wollmann et al., 2019).
A recent study highlights different advantages of integrated algae systems, such as sustainable CO2-rich exhausts that can be used as CO2 filters, which stimulate algae growth. Enhanced bio-treatment of wastewater using algae that can remove P and N in wastewater for ecological wastewater removal, manufacturing biofuels using algae that accumulate 20–70% lipid, or using algae for agriculture by producing carbohydrates, proteins, vitamins, etc pigments for fertilizer or pharmaceutical uses. Using high-nutrient resources to cultivate algae is feasible and cost-effective with the abovementioned benefits. Due to this, micro-algae treatment processes produce fewer greenhouse gas emissions; for example, micro-algae can assimilate the majority of nitrogen rather than convert it to oxides of nitrogen (Amenorfenyo et al., 2019; Mohsenpour et al., 2021).
Furthermore, microalgae and bacteria's relationship has been proven to provide a symbiotic environment that protects algae from harmful contaminants while enhancing the removal of hazardous pollutants (Abinandan et al., 2015). Many reports show that microalgae and bacteria have a symbiotic relationship: microalgae utilize CO2 by photosynthesis to produce oxygen, and heterotrophic bacteria can use this oxygen to transform carbon, N, and P in organic matter. As a result of bacteria's aerobic metabolism, CO2, inorganic nitrogen, and P are released that can be used for photosynthesis by microalgae (Li et al., 2019).
Nitrogen can be removed from wastewater using algae-based treatment through nitrogen uptake and ammonia removal caused by a pH increase during algae growth. Moreover, microalgae need good growth to effectively treat wastewater, which means understanding the factors that influence such growth is imperative (Larsdotter, 2006). Physical, chemical, and biological factors influence the growth rate of algae and cyanobacteria. The temperature and light are examples of physical factors. Chemical factors (nutrient availability and carbon dioxide) and biological factors (competition between species, animal grazing, and virus infections) exist.
Additionally, algae cultivation can remove phosphate through bioassimilation, adsorption, and chemical precipitation above pH 8. Despite the different methods of removing N and P, bioassimilation is considered essential, which is affected by various factors, including light conditions and the availability of CO2 purification. As part of wastewater treatment, the initial nutritional concentrations of algae can also play an essential role in determining their growth characteristics and nutrient removal kinetics (Wang et al., 2014). However, microalgae could be particularly important for treating high-strength sewage typical of side streams from WWTPs. Besides, microalgae are used in WWTPs for four main reasons: 1) to absorb or convert contaminants directly or to achieve pathogens and pollutants decline, 2) as a biomass resource for nutrient recovery, 3) to decrease the total energy cost of either direct or indirect oxygen supply, and 4) to reduce CO2 emissions (Ting et al., 2017; Mohsenpour et al., 2021).
Moreover, microalgae's extracellular polymeric substances (EPS) (Cheng et al.,) may also affect their flocculation. EPS production by algae also reflects changes in environmental conditions. For instance, the switch from mixotrophic to heterotrophic growth caused stress for the algae, resulting in EPS production. Furthermore, an alternative method for industrial-scale production of algae biomass and biofuel has been proposed: heterotrophic cultivation. As a result of heterotrophic cultivation, biomass production is more predictable and reliable than photoautotrophic systems since organic carbon uptake is a reliable energy source (Xiao et al., 2016). In addition, algal metabolism will be different depending on growth conditions and the environment, such as nutrient concentrations or light conditions. Finally, depending on the algal metabolism, EPS may have different properties that affect effluent quality, algae harvesting, and digestibility (Mishra et al., 2011; Xiao et al., 2016).
Effect of algae on SARS-CoV-2
A microrobot based on algae has been manufactured by modifying microalgae with an ACE2 receptor against SARS-CoV-2 spike proteins, via click chemistry. As a result, SARS-CoV-2 can be efficiently removed from aquatic media by a biohybrid microrobot. Furthermore, the unique properties of microrobots enable rapid decontamination of a wide range of environmental pollutants by their robust self-propulsion capabilities and facile surface functionalization, which can remove dyes, heavy metals, oil, pathogenic organisms, and chemical and biological warfare agents (Schema 9 ) (Alam et al., 2021; Zhang et al., 2021).
Schema 9.
A microrobot based on algae against SARS-CoV-2.
Trends, challenges, and future research needs
Low-income families across the globe have been hit hardest by this new outbreak, and this is because some of them had little or no access to safe and adequately treated water. Research on pollution levels in rivers and lakes is a possible risk to public health, affecting populations in need of more attention. It is equally important to note the phrases mentioned in the article published by Neal (2020).
“Various countries will emerge from the impacts of COVID-19 at varying times and via different pathways, thus providing an opportunity for those countries that are able to assist and support others humanely and humanly. Due to the fact that water enters into every aspect of human life and every sector of our increasingly globalized and interconnected world, we should be embracing a human rights approach in our immediate, medium-term, and long-term water responses to COVID-19.”
There is an urgent need for an economical alternative to the application of wastewater treatment technologies. Moreover, without the risk of long-term effects, for example, highly toxic chlorine-based disinfectants, the scientific community is apprehensive about the ecological damage they cause in the long run.
The inadequate information about the behaviour of SARS-CoV-2 in wastewater makes proper wastewater treatment difficult and leaves gaps that need to be explored and explored to enhance the proposed technology to eliminate the presence of viruses in water compartments. As COVID-19 raises public awareness of hygiene and disinfection of all types of surfaces that can attach viruses, the impact will be manifested in wastewater treatment chemicals and global water, according to a report made in the UK (Kataki et al., 2021). Natural compounds such as microbial and plant-based surfactants, natural wetting agents, viscosity enhancing agents, essential oils and phenolic compounds are innovative and environmentally friendly solutions to reduce the burden of new contaminants from hygiene products. However, economic aspects must be evaluated with new technologies that increase productivity and reduce costs by optimizing bioprocesses, separation processes or other innovative methods (Daverey and Dutta, 2021). In addition, regions with no regulations on wastewater treatment and inadequate infrastructure need help to solve the problem. Some treatment alternatives are advanced oxidation processes and membrane technologies characterized by environmental friendliness, versatility, high efficiency, and safety.
Conclusion
Using advanced methods such as membrane, UV irradiation, ozonation, chlorination, advanced oxidation process, and AC in advanced water treatment causes the removal of pathogens, a variety of pollutants, microplastics, etc., favouring reuse in irrigation crops and domestic water. This review provides details on different suitable technologies for promoting water reuse. First, to remark that membrane methods can be substituted for conventional wastewater treatment. Thus, for membrane-based treatments, the membrane size determines the maximum pore size of the membrane, which must then be selected in order to remove viral particles. Moreover, in order to inactivate SARS-CoV-2, UV radiation must also be intense enough to penetrate RNA and DNA. In addition, various oxidizing radicals are created during chlorination, which aids in inactivating DNA, thereby inactivating SARS-CoV-2.
Furthermore, a microrobot is fabricated using click chemistry to functionalize algae with an ACE2 receptor against SARS-CoV-2 spike protein. The ACE2-algae-robot demonstrates fast and long-lasting self-propulsion in diverse aquatic media, including drinking water and river water, eliminating the need for external fuel. In addition to removing SARS-CoV-2 spike proteins and SARS-CoV-2 pseudovirus by moving the algae robot, the ACE2-algae robot is also capable of removing SARS-CoV-2 pseudovirus on-the-fly. Finally, the AC with a diameter of almost 1.5 nm, which has a suitable microporous structure, can remove anionic surfactants, and the AC with a smaller pore size between 0.56 and 0.77 nm could adsorb more anionic surfactants and be more effective at removing SARS-CoV-2.
Moreover, it should be stressed that UV is an appropriate technology used in advanced water treatment for achieving the decline and preventing the presence of viable coronavirus. Furthermore, advanced wastewater treatment technologies such as AOPs and membranes can be used to inactivate and remove pathogens and organic contaminants from wastewater. In addition, WHO proposes short-term solutions as UV and solar radiation tend to be less damaging to the environment. Lastly, new low-cost detection and quantification methods in wastewater treatment need to be developed and implemented to inform the assessment of the risk of SARS-CoV-2 to human health.
Funding information
This work did not receive any financial support.
Declaration of Competing Interest
None.
References
- Abbas I., Zaheer S. Advanced oxidation process for wastewater treatment: a review. Am. Int. J. Res. Sci. Technol. Eng. Mater. 2014;7:189–191. [Google Scholar]
- Abdel-Fatah M.A. Nanofiltration systems and applications in wastewater treatment. Ain Shams Eng. J. 2018;9(4):3077–3092. [Google Scholar]
- Abdel-Raouf N., Al-Homaidan A., Ibraheem I. Microalgae and wastewater treatment. Saudi J. Biol. Sci. 2012;19(3):257–275. doi: 10.1016/j.sjbs.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abinandan S., Shanthakumar S. Challenges and opportunities in application of microalgae (Chlorophyta) for wastewater treatment: a review. Renew. Sustain. Energy Rev. 2015;52:123–132. [Google Scholar]
- Afonso M.D., Jaber J.O., Mohsen M.S. Brackish groundwater treatment by reverse osmosis in Jordan. Desalination. 2004;164(2):157–171. [Google Scholar]
- Agustina T.E., Ang H.M., Vareek V.K. A review of synergistic effect of photocatalysis and ozonation on wastewater treatment. J. Photochem. Photobiol. C. 2005;6(4):264–273. [Google Scholar]
- Ahmad T., Guria C., Mandal A. A review of oily wastewater treatment using ultrafiltration membrane: A parametric study to enhance the membrane performance. J. Water Process Eng. 2020;36 [Google Scholar]
- Alam M., Parra-Saldivar R., Bilal M., Afroze C.A., Ahmed M., Iqbal H., Xu J. Algae-derived bioactive molecules for the potential treatment of sars-cov-2. Molecules. 2021;26(8):2134. doi: 10.3390/molecules26082134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A., Tufa R.A., Macedonio F., Curcio E., Drioli E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018;81:1–21. [Google Scholar]
- Alimohammadi M., Naderi M. Effectiveness of ozone gas on airborne virus inactivation in enclosed spaces: a review study. Ozone Sci. Eng. 2021;43(1):21–31. [Google Scholar]
- Aliyu U.M., Rathilal S., Isa Y.M. Membrane desalination technologies in water treatment: a review. Water Pract. Technol. 2018;13(4):738–752. [Google Scholar]
- Amenorfenyo D.K., Huang X., Zhang Y., Zeng Q., Zhang N., Ren J., Huang Q. Microalgae brewery wastewater treatment: potentials, benefits and the challenges. Int. J. Environ. Res. Public Health. 2019;16(11):1910. doi: 10.3390/ijerph16111910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An W., Tian L., Hu J., Liu L., Cui W., Liang Y. Efficient degradation of organic pollutants by catalytic ozonation and photocatalysis synergy system using double-functional MgO/g-C3N4 catalyst. Appl. Surf. Sci. 2020;534 [Google Scholar]
- Anand U., Bianco F., Suresh S., Tripathi V., Núñez-Delgado A., Race M. SARS-CoV-2 and other viruses in soil: an environmental outlook. Environ. Res. 2021;198 doi: 10.1016/j.envres.2021.111297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anis S.F., Hashaikeh R., Hilal N. Microfiltration membrane processes: a review of research trends over the past decade. J. Water Process Eng. 2019;32 [Google Scholar]
- Anis S.F., Hashaikeh R., Hilal N. Reverse osmosis pretreatment technologies and future trends: a comprehensive review. Desalination. 2019;452:159–195. [Google Scholar]
- Anpilov A., Barkhudarov E., Bark Y.B., Zadiraka Y.V., Christofi M., Kozlov Y.N., Taktakishvili M. Electric discharge in water as a source of UV radiation, ozone and hydrogen peroxide. J. Phys. D Appl. Phys. 2001;34(6):993. [Google Scholar]
- Ao X.W., Eloranta J., Huang C.H., Santoro D., Sun W.J., Lu Z.D., Li C. Peracetic acid-based advanced oxidation processes for decontamination and disinfection of water: a review. Water Res. 2021;188 doi: 10.1016/j.watres.2020.116479. [DOI] [PubMed] [Google Scholar]
- Asempour F., Emadzadeh D., Matsuura T., Kruczek B. Synthesis and characterization of novel cellulose nanocrystals-based thin film nanocomposite membranes for reverse osmosis applications. Desalination. 2018;439:179–187. [Google Scholar]
- Azbar N., Yonar T., Kestioglu K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere. 2004;55(1):35–43. doi: 10.1016/j.chemosphere.2003.10.046. [DOI] [PubMed] [Google Scholar]
- Aziz M., Kasongo G. Improving resistance to fouling of aromatic polyamide thin-film composite reverse osmosis membrane by surface grafting of N, N'-dimethyl aminoethyl methacrylate (DMAEMA) J. Water Chem. Technol. 2021;43(4):312–320. [Google Scholar]
- Azuma T., Hayashi T. On-site chlorination responsible for effective disinfection of wastewater from hospital. Sci. Total Environ. 2021;776 doi: 10.1016/j.scitotenv.2021.145951. [DOI] [PubMed] [Google Scholar]
- Baker R.W. John Wiley & Sons; 2012. Membrane Technology and Applications. [Google Scholar]
- Balannec B., Vourch M., Rabiller-Baudry M., Chaufer B. Comparative study of different nanofiltration and reverse osmosis membranes for dairy effluent treatment by dead-end filtration. Sep. Purif. Technol. 2005;42(2):195–200. [Google Scholar]
- Barnewall R.E., Bischoff W.E. Removal of SARS-CoV-2 bioaerosols using ultraviolet air filtration. Infect. Control Hosp. Epidemiol. 2021;1-2 doi: 10.1017/ice.2021.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos P.D., Santos M.A., Carvalho P.J., Crespo J.G. Reverse osmosis performance on stripped phenolic sour water treatment–a study on the effect of oil and grease and osmotic pressure. J. Environ. Manag. 2020;261 doi: 10.1016/j.jenvman.2020.110229. [DOI] [PubMed] [Google Scholar]
- Bellanca M.A., Bailey D.S. Effects of chlorinated effluents on aquatic ecosystem in the lower James River. J. Water Pollut. Control Fed. 1977;49:639–645. [PubMed] [Google Scholar]
- Bethi B., Sonawane S.H., Bhanvase B.A., Sonawane S.S. Textile industry wastewater treatment by cavitation combined with fenton and ceramic nanofiltration membrane. Chem. Eng. Process. Process Intensif. 2021;168 [Google Scholar]
- Bhattacharjee C., Saxena V., Dutta S. Fruit juice processing using membrane technology: a review. Innov. Food Sci. Emerg. Technol. 2017;43:136–153. [Google Scholar]
- Blanken W., Cuaresma M., Wijffels R.H., Janssen M. Cultivation of microalgae on artificial light comes at a cost. Algal Res. 2013;2(4):333–340. [Google Scholar]
- Boczkaj G., Fernandes A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: a review. Chem. Eng. J. 2017;320:608–633. [Google Scholar]
- Bodzek M., Konieczny K., Rajca M. Membranes in water and wastewater disinfection. Arch. Environ. Prot. 2019;45(1) [Google Scholar]
- Boehler M., Zwickenpflug B., Hollender J., Ternes T., Joss A., Siegrist H. Removal of micropollutants in municipal wastewater treatment plants by powder-activated carbon. Water Sci. Technol. 2012;66(10):2115–2121. doi: 10.2166/wst.2012.353. [DOI] [PubMed] [Google Scholar]
- Bonvin F., Jost L., Randin L., Bonvin E., Kohn T. Super-fine powdered activated carbon (SPAC) for efficient removal of micropollutants from wastewater treatment plant effluent. Water Res. 2016;90:90–99. doi: 10.1016/j.watres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Bourrouet A., Garcia J., Mujeriego R., Peñuelas G. Faecal bacteria and bacteriophage inactivation in a full-scale UV disinfection system used for wastewater reclamation. Water Sci. Technol. 2001;43(10):187–194. [PubMed] [Google Scholar]
- Burba P., Geltenpoth H., Nolte J. Ultrafiltration behavior of selected pharmaceuticals on natural and synthetic membranes in the presence of humic-rich hydrocolloids. Anal. Bioanal.Chem. 2005;382(8):1934–1941. doi: 10.1007/s00216-005-3296-z. [DOI] [PubMed] [Google Scholar]
- Cabrera C.R., Miranda F. CRC press; 2014. Advanced Nanomaterials for Aerospace Applications. [Google Scholar]
- Cadotte J.E., Petersen R.J. ACS Publications; 1981. Thin-Film Composite Reverse-Osmosis Membranes: Origin, Development, and Recent Advances. [Google Scholar]
- Carleton T., Cornetet J., Huybers P., Meng K.C., Proctor J. Global evidence for ultraviolet radiation decreasing COVID-19 growth rates. Proc. Natl. Acad. Sci. 2021;118(1) doi: 10.1073/pnas.2012370118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cela-Dablanca R., Santás-Miguel V., Fernández-Calviño D., Arias-Estévez M., Fernández-Sanjurjo M.J., Álvarez-Rodríguez E., Núñez-Delgado A. SARS-CoV-2 and other main pathogenic microorganisms in the environment: Situation in Galicia and Spain. Environ. Res. 2021;197 doi: 10.1016/j.envres.2021.111049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervero-Aragó S., Sommer R., Araujo R.M. Effect of UV irradiation (253.7 nm) on free Legionella and Legionella associated with its amoebae hosts. Water Res. 2014;67:299–309. doi: 10.1016/j.watres.2014.09.023. [DOI] [PubMed] [Google Scholar]
- Chaudhry R.M., Nelson K.L., Drewes J.r.E. Mechanisms of pathogenic virus removal in a full-scale membrane bioreactor. Environ. Sci. Technol. 2015;49(5):2815–2822. doi: 10.1021/es505332n. [DOI] [PubMed] [Google Scholar]
- Cheng W., Liu C., Tong T., Epsztein R., Sun M., Verduzco R., Elimelech M. Selective removal of divalent cations by polyelectrolyte multilayer nanofiltration membrane: Role of polyelectrolyte charge, ion size, and ionic strength. J. Membr. Sci. 2018;559:98–106. [Google Scholar]
- Cheung K.S., Hung I.F., Chan P.P., Lung K., Tso E., Liu R., Tam A.R. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159(1):81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M., Kim J., Kim J.Y., Yoon J., Kim J.H. Mechanisms of Escherichia coli inactivation by several disinfectants. Water Res. 2010;44(11):3410–3418. doi: 10.1016/j.watres.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Chu K.H., Fathizadeh M., Yu M., Flora J.R., Jang A., Jang M., Yoon Y. Evaluation of removal mechanisms in a graphene oxide-coated ceramic ultrafiltration membrane for retention of natural organic matter, pharmaceuticals, and inorganic salts. ACS Appl. Mater. Interfaces. 2017;9(46):40369–40377. doi: 10.1021/acsami.7b14217. [DOI] [PubMed] [Google Scholar]
- Churn C., Bates R., Boardman G. Mechanism of chlorine inactivation of DNA-containing parvovirus H-1. Appl. Environ. Microbiol. 1983;46(6):1394–1402. doi: 10.1128/aem.46.6.1394-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collivignarelli M.C., Abbà A., Miino M.C., Caccamo F.M., Torretta V., Rada E.C., Sorlini S. Disinfection of wastewater by UV-based treatment for reuse in a circular economy perspective. where are we at? Int. J. Environ. Res. Public Health. 2021;18(1):77. doi: 10.3390/ijerph18010077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerton A.M., Andrews R.C., Bagley D.M., Hao C. The rejection of endocrine disrupting and pharmaceutically active compounds by NF and RO membranes as a function of compound and water matrix properties. J. Membr. Sci. 2008;313(1-2):323–335. [Google Scholar]
- Conde-Cid M., Arias-Estévez M., Núñez-Delgado A. How to study SARS-CoV-2 in soils? Environ. Res. 2021;198 doi: 10.1016/j.envres.2020.110464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde-Cid M., Arias-Estévez M., Núñez-Delgado A. SARS-CoV-2 and other pathogens could be determined in liquid samples from soils. Environ. Pollut. 2021;273 doi: 10.1016/j.envpol.2021.116445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croll H., Soroush A., Pillsbury M.E., Castrillón S.R.V. Graphene oxide surface modification of polyamide reverse osmosis membranes for improved N-nitrosodimethylamine (NDMA) removal. Sep. Purif. Technol. 2019;210:973–980. [Google Scholar]
- da Fonseca Filho L.B., Herrera L.E.Y. Proceedings of the 6th Brazilian Technology Symposium (BTSym’20): Emerging Trends and Challenges in Technology. Springer Nature; 2021. Review of Ultraviolet-C Light Against Coronavirus. [Google Scholar]
- Deng Y., Zhao R. Advanced oxidation processes (AOPs) in wastewater treatment. Curr. Pollut. Rep. 2015;1(3):167–176. [Google Scholar]
- Dong L., Liu W., Yu Y., Gu P., Chen G. Preparation, characterization, and application of macroporous activated carbon (MAC) suitable for the BAC water treatment process. Sci. Total Environ. 2019;647:1359–1367. doi: 10.1016/j.scitotenv.2018.07.280. [DOI] [PubMed] [Google Scholar]
- Dong M., Park H.K., Wang Y., Feng H. Control Escherichia coli O157: H7 growth on sprouting brassicacae seeds with high acoustic power density (APD) ultrasound plus mild heat and calcium-oxide antimicrobial spray. Food Control. 2022;132 [Google Scholar]
- Santos Dos, Daniel L. A review: organic matter and ammonia removal by biological activated carbon filtration for water and wastewater treatment. Int. J. Environ. Sci. Technol. 2020;17(1):591–606. [Google Scholar]
- Du T., Adeleye A.S., Zhang T., Yang N., Hao R., Li Y., Chen W. Effects of ozone and produced hydroxyl radicals on the transformation of graphene oxide in aqueous media. Environ. Sci. Nano. 2019;6(8):2484–2494. [Google Scholar]
- Du Y., Lv X.T., Wu Q.Y., Zhang D.Y., Zhou Y.T., Peng L., Hu H.Y. Formation and control of disinfection byproducts and toxicity during reclaimed water chlorination: a review. J. Environ. Sci. 2017;58:51–63. doi: 10.1016/j.jes.2017.01.013. [DOI] [PubMed] [Google Scholar]
- Ebie K., Li F., Azuma Y., Yuasa A., Hagishita T. Pore distribution effect of activated carbon in adsorbing organic micropollutants from natural water. Water Res. 2001;35(1):167–179. doi: 10.1016/s0043-1354(00)00257-8. [DOI] [PubMed] [Google Scholar]
- Edwards-Brandt J., Shorney-Darby H., Neemann J., Hesby J., Tona C. Use of ozone for disinfection and taste and odor control at proposed membrane facility. Ozone Sci. Eng. 2007;29(4):281–286. [Google Scholar]
- El-Betany A.M., Behiry E.M., Gumbleton M., Harding K.G. Humidified warmed CO2 treatment therapy strategies can save lives with mitigation and suppression of SARS-CoV-2 infection: an evidence review. Front. Med. 2020;7:982. doi: 10.3389/fmed.2020.594295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Rayess Y., Albasi C., Bacchin P., Taillandier P., Raynal J., Mietton-Peuchot M., Devatine A. Cross-flow microfiltration applied to oenology: a review. J. Membr. Sci. 2011;382(1-2):1–19. [Google Scholar]
- Emamdoost N., Jafarian A., Kouhikamali R. Implementing multiple-effect distillation and reverse osmosis thermal coupling to improve desalination process performance in combined water and power plants. Energy Convers. Manag. 2020;221 [Google Scholar]
- Espid E., Taghipour F. UV-LED photo-activated chemical gas sensors: a review. Crit. Rev. Solid State Mater. Sci. 2017;42(5):416–432. [Google Scholar]
- Ferro G., Fiorentino A., Alferez M.C., Polo-López M.I., Rizzo L., Fernandez-Ibanez P. Urban wastewater disinfection for agricultural reuse: effect of solar driven AOPs in the inactivation of a multidrug resistant E. coli strain. Appl. Catal. B. 2015;178:65–73. [Google Scholar]
- Fujioka T., Khan S.J., McDonald J.A., Nghiem L.D. Rejection of trace organic chemicals by a hollow fibre cellulose triacetate reverse osmosis membrane. Desalination. 2015;368:69–75. [Google Scholar]
- Fuzawa M., Araud E., Li J., Shisler J.L., Nguyen T.H. Free chlorine disinfection mechanisms of rotaviruses and human norovirus surrogate Tulane virus attached to fresh produce surfaces. Environ. Sci. Technol. 2019;53(20):11999–12006. doi: 10.1021/acs.est.9b03461. [DOI] [PubMed] [Google Scholar]
- Ganzenko O., Huguenot D., Van Hullebusch E.D., Esposito G., Oturan M.A. Electrochemical advanced oxidation and biological processes for wastewater treatment: a review of the combined approaches. Environ. Sci. Pollut. Res. 2014;21(14):8493–8524. doi: 10.1007/s11356-014-2770-6. [DOI] [PubMed] [Google Scholar]
- Gao J., Wang K.Y., Chung T.S. Design of nanofiltration (NF) hollow fiber membranes made from functionalized bore fluids containing polyethyleneimine (PEI) for heavy metal removal. J. Membr. Sci. 2020;603 [Google Scholar]
- Garcia-Costa A.L., Gouveia T.I., Pereira M.F.R., Silva A.M., Alves A., Madeira L.M., Santos M.S. Ozonation of cytostatic drugs in aqueous phase. Sci. Total Environ. 2021;795 doi: 10.1016/j.scitotenv.2021.148855. [DOI] [PubMed] [Google Scholar]
- Garrido-Cardenas J.A., Esteban-García B., Agüera A., Sánchez-Pérez J.A., Manzano-Agugliaro F. Wastewater treatment by advanced oxidation process and their worldwide research trends. Int. J. Environ. Res. Public Health. 2020;17(1):170. doi: 10.3390/ijerph17010170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genç N., Durna E., Kacıra E. The preference of the most appropriate radical-based regeneration process for spent activated carbon by the PROMETHEE approach. Environ. Sci. Pollut. Res. 2021;29:1–16. doi: 10.1007/s11356-021-15833-y. [DOI] [PubMed] [Google Scholar]
- Ghernaout D. Water treatment chlorination: an updated mechanistic insight review. Chem. Res. J. 2017;2:125–138. [Google Scholar]
- Ghernaout D., El-Wakil A. Requiring reverse osmosis membranes modifications-an overview. Am. J. Chem. Eng. 2017;5(4):81–88. [Google Scholar]
- Górecki, R. P. (2020). "From nanoscale assemblies to biomimetic membrane devices."
- Goswami, D., M. Kumar, S. K. Ghosh and A. Das (2020). "Natural product compounds in alpinia officinarum and ginger are potent SARS-CoV-2 papain-like protease inhibitors."
- Guillossou R., Le Roux J., Goffin A., Mailler R., Varrault G., Vulliet E., Rocher V. Fluorescence excitation/emission matrices as a tool to monitor the removal of organic micropollutants from wastewater effluents by adsorption onto activated carbon. Water Res. 2021;190 doi: 10.1016/j.watres.2020.116749. [DOI] [PubMed] [Google Scholar]
- Gültekin I., Ince N.H. Synthetic endocrine disruptors in the environment and water remediation by advanced oxidation processes. J. Environ. Manag. 2007;85(4):816–832. doi: 10.1016/j.jenvman.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Gupta V., Gupta B., Rastogi A., Agarwal S., Nayak A. Pesticides removal from waste water by activated carbon prepared from waste rubber tire. Water Res. 2011;45(13):4047–4055. doi: 10.1016/j.watres.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Hafiz, M., A. H. Hawari and R. Alfahel (2020). "Treatment of wastewater using reverse osmosis for irrigation purposes." [DOI] [PMC free article] [PubMed]
- Hamedi H., Ehteshami M., Mirbagheri S.A., Rasouli S.A., Zendehboudi S. Current status and future prospects of membrane bioreactors (MBRs) and fouling phenomena: a systematic review. Can. J. Chem. Eng. 2019;97(1):32–58. [Google Scholar]
- Hao W., Yang M., Zhao K., Tang J. Dielectric measurements of fouling of nanofiltration membranes by sparingly soluble salts. J. Membr. Sci. 2016;497:339–347. [Google Scholar]
- Harrison A.G., Lin T., Wang P. Mechanisms of SARS-CoV-2 transmission and pathogenesis. Trends Immunol. 2020;41(12):1100–1115. doi: 10.1016/j.it.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller C., Hübner U., Fajnorova S., Schwartz T., Drewes J. Antibiotic microbial resistance (AMR) removal efficiencies by conventional and advanced wastewater treatment processes: a review. Sci. Total Environ. 2019;685:596–608. doi: 10.1016/j.scitotenv.2019.05.315. [DOI] [PubMed] [Google Scholar]
- Hinds L.M., O'Donnell C.P., Akhter M., Tiwari B.K. Principles and mechanisms of ultraviolet light emitting diode technology for food industry applications. Innov. Food Sci. Emerg. Technol. 2019;56 [Google Scholar]
- Hladik M.L., Focazio M.J., Engle M. Discharges of produced waters from oil and gas extraction via wastewater treatment plants are sources of disinfection by-products to receiving streams. Sci. Total Environ. 2014;466:1085–1093. doi: 10.1016/j.scitotenv.2013.08.008. [DOI] [PubMed] [Google Scholar]
- Hoinkis J., Deowan S.A., Panten V., Figoli A., Huang R.R., Drioli E. Membrane bioreactor (MBR) technology–a promising approach for industrial water reuse. Procedia Eng. 2012;33:234–241. [Google Scholar]
- Holloway R.W., Miller-Robbie L., Patel M., Stokes J.R., Munakata-Marr J., Dadakis J., Cath T.Y. Life-cycle assessment of two potable water reuse technologies: MF/RO/UV–AOP treatment and hybrid osmotic membrane bioreactors. J. Membr. Sci. 2016;507:165–178. [Google Scholar]
- Holmes B., Paddock M.B., VanderGheynst J.S., Higgins B.T. Algal photosynthetic aeration increases the capacity of bacteria to degrade organics in wastewater. Biotechnol. Bioeng. 2020;117(1):62–72. doi: 10.1002/bit.27172. [DOI] [PubMed] [Google Scholar]
- Hu Q., Zhou F., Lu H., Li N., Peng B., Yu H., Zhang H. Improved antifouling performance of a polyamide composite reverse osmosis membrane by surface grafting of dialdehyde carboxymethyl cellulose (DACMC) J. Membr. Sci. 2021;620 [Google Scholar]
- Hu Z., Si X., Zhang Z., Wen X. Enhanced EDCs removal by membrane fouling during the UF process. Desalination. 2014;336:18–23. [Google Scholar]
- Huang H., Cho H., Schwab K., Jacangelo J.G. Effects of feedwater pretreatment on the removal of organic microconstituents by a low fouling reverse osmosis membrane. Desalination. 2011;281:446–454. [Google Scholar]
- Hussain F., Shah S.Z., Ahmad H., Abubshait S.A., Abubshait H.A., Laref A., Iqbal M. Microalgae an ecofriendly and sustainable wastewater treatment option: Biomass application in biofuel and bio-fertilizer production. A review. Renew. Sustain. Energy Rev. 2021;137 [Google Scholar]
- Hussain M., Mahtab M.S., Farooqi I.H. The applications of ozone-based advanced oxidation processes for wastewater treatment: a review. Adv. Environ. Res. 2020;9(3):191–214. [Google Scholar]
- Ikehata K., Li Y. Advanced Oxidation Processes for Waste Water Treatment. Elsevier; 2018. Ozone-based processes; pp. 115–134. [Google Scholar]
- Innocenzi V., Cantarini F., Amato A., Morico B., Ippolito N.M., Beolchini F., Vegliò F. Case study on technical feasibility of galvanic wastewater treatment plant based on life cycle assessment and costing approach. J. Environ. Chem. Eng. 2020;8(6) [Google Scholar]
- Ivančev-Tumbas I., Bogunović M., Vasić V., Šćiban M., Tubić A., Leovac Maćerak A., Prodanović J. ‘Green'coagulant application with activated carbon: dosing sequence and removal of selected micropollutants and effluent organic matter from municipal wastewater. Environ. Technol. 2020;43:1–7. doi: 10.1080/09593330.2020.1821788. [DOI] [PubMed] [Google Scholar]
- Jamaly S., Darwish N., Ahmed I., Hasan S. A short review on reverse osmosis pretreatment technologies. Desalination. 2014;354:30–38. [Google Scholar]
- Javier Benitez F., Acero J.L., Real F.J., Roldán G., Rodriguez E. Ultrafiltration and nanofiltration membranes applied to the removal of the pharmaceuticals amoxicillin, naproxen, metoprolol and phenacetin from water. J. Chem. Technol. Biotechnol. 2011;86(6):858–866. [Google Scholar]
- Jiang H.J., Na C., Shen Z.Q., Jing Y., Qiu Z.G., Jing M., Wang X.W. Inactivation of poliovirus by ozone and the impact of ozone on the viral genome. Biomed. Environ. Sci. 2019;32(5):324–333. doi: 10.3967/bes2019.044. [DOI] [PubMed] [Google Scholar]
- Jiang L., Tu Y., Li X., Li H. Proceedings of the E3S Web of Conferences. EDP Sciences; 2018. Application of reverse osmosis in purifying drinking water. [Google Scholar]
- Judd S.J. Membrane technology costs and me. Water Res. 2017;122:1–9. doi: 10.1016/j.watres.2017.05.027. [DOI] [PubMed] [Google Scholar]
- Kabinger F., Stiller C., Schmitzová J., Dienemann C., Kokic G., Hillen H.S., Cramer P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021;28(9):740–746. doi: 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadel S., Pellerin G., Thibodeau J., Perreault V., Lainé C., Bazinet L. How molecular weight cut-offs and physicochemical properties of polyether sulfone membranes affect peptide migration and selectivity during electrodialysis with filtration membranes. Membranes. 2019;9(11):153. doi: 10.3390/membranes9110153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kali S., Khan M., Ghaffar M.S., Rasheed S., Waseem A., Iqbal M.M., Zafar M.I. Occurrence, influencing factors, toxicity, regulations, and abatement approaches for disinfection by-products in chlorinated drinking water: a comprehensive review. Environ. Pollut. 2021;281 doi: 10.1016/j.envpol.2021.116950. [DOI] [PubMed] [Google Scholar]
- Kaminska G., Bohdziewicz J., Calvo J., Prádanos P., Palacio L., Hernández A. Fabrication and characterization of polyethersulfone nanocomposite membranes for the removal of endocrine disrupting micropollutants from wastewater. Mechanisms and performance. J. Membr. Sci. 2015;493:66–79. [Google Scholar]
- Kanakaraju D., Glass B.D., Oelgemöller M. Advanced oxidation process-mediated removal of pharmaceuticals from water: a review. J. Environ. Manag. 2018;219:189–207. doi: 10.1016/j.jenvman.2018.04.103. [DOI] [PubMed] [Google Scholar]
- Karnib M., Kabbani A., Holail H., Olama Z. Heavy metals removal using activated carbon, silica and silica activated carbon composite. Energy Procedia. 2014;50:113–120. [Google Scholar]
- Karpova T., Pekonen P., Gramstad R., Öjstedt U., Laborda S., Heinonen-Tanski H., Jiménez B. Performic acid for advanced wastewater disinfection. Water Sci. Technol. 2013;68(9):2090–2096. doi: 10.2166/wst.2013.468. [DOI] [PubMed] [Google Scholar]
- Kasongo G., Steenberg C., Morris B., Kapenda G., Jacobs N., Aziz M. Surface grafting of polyvinyl alcohol (PVA) cross-linked with glutaraldehyde (GA) to improve resistance to fouling of aromatic polyamide thin film composite reverse osmosis membranes using municipal membrane bioreactor effluent. Water Pract. Technol. 2019;14(3):614–624. [Google Scholar]
- Kaur D., Bhardwaj N.K., Lohchab R.K. Effect of incorporation of ozone prior to ECF bleaching on pulp, paper and effluent quality. J. Environ. Manag. 2019;236:134–145. doi: 10.1016/j.jenvman.2019.01.089. [DOI] [PubMed] [Google Scholar]
- Kennedy L.J., Sekaran G. Integrated biological and catalytic oxidation of organics/inorganics in tannery wastewater by rice husk based mesoporous activated carbon-Bacillus sp. Carbon. 2004;42(12-13):2399–2407. [Google Scholar]
- Khan A.H., Khan N.A., Ahmed S., Dhingra A., Singh C.P., Khan S.U., Alam S. Application of advanced oxidation processes followed by different treatment technologies for hospital wastewater treatment. J. Clean. Prod. 2020;269 [Google Scholar]
- Khedr M.G. Radioactive contamination of groundwater, special aspects and advantages of removal by reverse osmosis and nanofiltration. Desalination. 2013;321:47–54. [Google Scholar]
- Kheriji J., Tabassi D., Hamrouni B. Removal of Cd (II) ions from aqueous solution and industrial effluent using reverse osmosis and nanofiltration membranes. Water Sci. Technol. 2015;72(7):1206–1216. doi: 10.2166/wst.2015.326. [DOI] [PubMed] [Google Scholar]
- Kimura K., Toshima S., Amy G., Watanabe Y. Rejection of neutral endocrine disrupting compounds (EDCs) and pharmaceutical active compounds (PhACs) by RO membranes. J. Membr. Sci. 2004;245(1-2):71–78. [Google Scholar]
- Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler C., Venditti S., Igos E., Klepiszewski K., Benetto E., Cornelissen A. Elimination of pharmaceutical residues in biologically pre-treated hospital wastewater using advanced UV irradiation technology: a comparative assessment. J. Hazard. Mater. 2012;239:70–77. doi: 10.1016/j.jhazmat.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Kokic G., Hillen H.S., Tegunov D., Dienemann C., Seitz F., Schmitzova J., Cramer P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021;12(1):1–7. doi: 10.1038/s41467-020-20542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Lu Y., Ren Y., Chen Z., Chen M. The virus removal in UV irradiation, ozonation and chlorination. Water Cycle. 2021;2:23–31. [Google Scholar]
- Kramer F., Shang R., Rietveld L., Heijman S. Influence of pH, multivalent counter ions, and membrane fouling on phosphate retention during ceramic nanofiltration. Sep. Purif. Technol. 2019;227 [Google Scholar]
- Kumar V., Lockerble O., Kell S.D., Ruane P.H., Platz M.S., Martin C.B., Goodrich R.P. Riboflavin and UV-light based pathogen reduction: extent and consequence of DNA damage at the molecular level. Photochem. Photobiol. 2004;80(1):15–21. doi: 10.1562/2003-12-23-RA-036.1. [DOI] [PubMed] [Google Scholar]
- Kuzniewski S. Prevalence, environmental fate, treatment strategies, and future challenges for wastewater contaminated with SARS-CoV-2. Remediat. J. 2021;31(4):97–110. doi: 10.1002/rem.21691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwarciak-Kozłowska A., Fijałkowski K.L. Efficiency assessment of municipal landfill leachate treatment during advanced oxidation process (AOP) with biochar adsorption (BC) J. Environ. Manag. 2021;287 doi: 10.1016/j.jenvman.2021.112309. [DOI] [PubMed] [Google Scholar]
- Larsdotter K. Wastewater treatment with microalgae-a literature review. Vatten. 2006;62(1):31. [Google Scholar]
- Larsen C., Yu Z.H., Flick R., Passeport E. Mechanisms of pharmaceutical and personal care product removal in algae-based wastewater treatment systems. Sci. Total Environ. 2019;695 doi: 10.1016/j.scitotenv.2019.133772. [DOI] [PubMed] [Google Scholar]
- Lee K.P., Arnot T.C., Mattia D. A review of reverse osmosis membrane materials for desalination—Development to date and future potential. J. Membr. Sci. 2011;370(1-2):1–22. [Google Scholar]
- Lei X., Lei Y., Zhang X., Yang X. Treating disinfection byproducts with UV or solar irradiation and in UV advanced oxidation processes: a review. J. Hazard. Mater. 2021;408 doi: 10.1016/j.jhazmat.2020.124435. [DOI] [PubMed] [Google Scholar]
- Lesimple A., Jasim S.Y., Johnson D.J., Hilal N. The role of wastewater treatment plants as tools for SARS-CoV-2 early detection and removal. J. Water Process Eng. 2020;38 doi: 10.1016/j.jwpe.2020.101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Liu Q., Fang F., Luo R., Lu Q., Zhou W., Addy M. Microalgae-based wastewater treatment for nutrients recovery: a review. Bioresour. Technol. 2019;291 doi: 10.1016/j.biortech.2019.121934. [DOI] [PubMed] [Google Scholar]
- Li S., Wang D., Xiao H., Zhang H., Cao S., Chen L., Huang L. Ultra-low pressure cellulose-based nanofiltration membrane fabricated on layer-by-layer assembly for efficient sodium chloride removal. Carbohydr. Polym. 2021;255 doi: 10.1016/j.carbpol.2020.117352. [DOI] [PubMed] [Google Scholar]
- Li X., Liu C., Yin W., Chong T.H., Wang R. Design and development of layer-by-layer based low-pressure antifouling nanofiltration membrane used for water reclamation. J. Membr. Sci. 2019;584:309–323. [Google Scholar]
- Liu L.F., Gu X.L., Xie X., Li R.H., Yu C.Y., Song X.X., Gao C.J. Modification of PSf/SPSf blended porous support for improving the reverse osmosis performance of aromatic polyamide thin film composite membranes. Polymers. 2018;10(6):686. doi: 10.3390/polym10060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Chen L., Yang M., Tan C., Chu W. The occurrence, characteristics, transformation and control of aromatic disinfection by-products: a review. Water Res. 2020;184 doi: 10.1016/j.watres.2020.116076. [DOI] [PubMed] [Google Scholar]
- Loeb B.L., Thompson C.M., Drago J., Takahara H., Baig S. Worldwide ozone capacity for treatment of drinking water and wastewater: a review. Ozone Sci. Eng. 2012;34(1):64–77. [Google Scholar]
- Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Dabrera G. Effectiveness of Covid-19 vaccines against the B. 1.617. 2 (Delta) variant. N. Engl. J. Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H.C., Lo J.I., Peng Y.C., Chou S.L., Cheng B.M., Chang H.C. Nitrogen-vacancy centers in diamond for high-performance detection of vacuum ultraviolet, extreme ultraviolet, and X-rays. ACS Appl. Mater. Interfaces. 2019;12(3):3847–3853. doi: 10.1021/acsami.9b18372. [DOI] [PubMed] [Google Scholar]
- Lu T., Zhang Q., Zhang Z., Hu B., Chen J., Chen J., Qian H. Pollutant toxicology with respect to microalgae and cyanobacteria. J. Environ. Sci. 2021;99:175–186. doi: 10.1016/j.jes.2020.06.033. [DOI] [PubMed] [Google Scholar]
- Lu X., Cui Y., Chen Y., Xiao Y., Song X., Gao F., Gan Q. Sustainable development of microalgal biotechnology in coastal zone for aquaculture and food. Sci. Total Environ. 2021;780 doi: 10.1016/j.scitotenv.2021.146369. [DOI] [PubMed] [Google Scholar]
- Lu Z., Li C., Jing Z., Ao X., Chen Z., Sun W. Implication on selection and replacement of granular activated carbon used in biologically activated carbon filters through meta-omics analysis. Water Res. 2021;198 doi: 10.1016/j.watres.2021.117152. [DOI] [PubMed] [Google Scholar]
- Luo J., Wan Y. Effects of pH and salt on nanofiltration—a critical review. J. Membr. Sci. 2013;438:18–28. [Google Scholar]
- Lv W., Zheng X., Yang M., Zhang Y., Liu Y., Liu J. Virus removal performance and mechanism of a submerged membrane bioreactor. Process Biochem. 2006;41(2):299–304. doi: 10.1016/j.procbio.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulek A., Oliveira S.C., Osugi M.E., Ferreira V.S., Quina F.H., Dantas R.F., Silva V.O. Vol. 1. InTech; 2013. Application of different advanced oxidation processes for the degradation of organic pollutants; pp. 141–166. (Organic Pollutants-Monitoring, Risk and Treatment). [Google Scholar]
- Mackuľak T., Cverenkárová K., Vojs Staňová A., Fehér M., Tamáš M., Škulcová A.B., Bírošová L. Hospital wastewater-source of specific micropollutants, antibiotic-resistant microorganisms, viruses, and their elimination. Antibiotics. 2021;10(9):1070. doi: 10.3390/antibiotics10091070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makisha N., Yunchina M. Proceedings of the MATEC Web of Conferences. EDP Sciences; 2017. Methods and solutions for galvanic waste water treatment. [Google Scholar]
- Mallada R., Menéndez M. Elsevier; 2008. Inorganic Membranes: Synthesis, Characterization and Applications. [Google Scholar]
- Mallakpour S., Azadi E. Nanofiltration membranes for food and pharmaceutical industries. Emergent Mater. 2021:1–15. [Google Scholar]
- Mallick N. Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals. 2002;15(4):377–390. doi: 10.1023/a:1020238520948. [DOI] [PubMed] [Google Scholar]
- Manni A., Achiou B., Karim A., Harrati A., Sadik C., Ouammou M., El Bouari A. New low-cost ceramic microfiltration membrane made from natural magnesite for industrial wastewater treatment. J. Environ. Chem. Eng. 2020;8(4) [Google Scholar]
- Mänttäri M., Nyström M. Membrane filtration for tertiary treatment of biologically treated effluents from the pulp and paper industry. Water Sci. Technol. 2007;55(6):99–107. doi: 10.2166/wst.2007.217. [DOI] [PubMed] [Google Scholar]
- Martins R.B., Castro I.A., Pontelli M., Souza J.P., Lima T.M., Melo S.R., de Almeida M.T.G. SARS-CoV-2 inactivation by ozonated water: a preliminary alternative for environmental disinfection. Ozone Sci. Eng. 2021;43(2):108–111. [Google Scholar]
- Mashayekhi F., Hazrati H., Shayegan J. Fouling control mechanism by optimum ozone addition in submerged membrane bioreactors treating synthetic wastewater. J. Environ. Chem. Eng. 2018;6(6):7294–7301. [Google Scholar]
- Masojídek J., Prášil O. The development of microalgal biotechnology in the Czech Republic. J. Ind. Microbiol. Biotechnol. 2010;37(12):1307–1317. doi: 10.1007/s10295-010-0802-x. [DOI] [PubMed] [Google Scholar]
- Masoudnia K., Raisi A., Aroujalian A., Fathizadeh M. A hybrid microfiltration/ultrafiltration membrane process for treatment of oily wastewater. Desalin. Water Treat. 2015;55(4):901–912. [Google Scholar]
- Mazhar M.A., Khan N.A., Ahmed S., Khan A.H., Hussain A., Changani F., Vambol V. Chlorination disinfection by-products in Municipal drinking water-a review. J. Clean. Prod. 2020;273 [Google Scholar]
- Mi Y.F., Wang N., Qi Q., Yu B., Peng X.D., Cao Z.H. A loose polyamide nanofiltration membrane prepared by polyether amine interfacial polymerization for dye desalination. Sep. Purif. Technol. 2020;248 [Google Scholar]
- Miklos D.B., Remy C., Jekel M., Linden K.G., Drewes J.E., Hübner U. Evaluation of advanced oxidation processes for water and wastewater treatment-a critical review. Water Res. 2018;139:118–131. doi: 10.1016/j.watres.2018.03.042. [DOI] [PubMed] [Google Scholar]
- Milov I., Lipp V., Ilnitsky D., Medvedev N., Migdal K., Zhakhovsky V., Semin S. Similarity in ruthenium damage induced by photons with different energies: from visible light to hard X-rays. Appl. Surf. Sci. 2020;501 [Google Scholar]
- Mishra A., Kavita K., Jha B. Characterization of extracellular polymeric substances produced by micro-algae Dunaliella salina. Carbohydr. Polym. 2011;83(2):852–857. [Google Scholar]
- Mishra S., Singh R.P., Rout P.K., Das A.P. Development in Wastewater Treatment Research and Processes. 2022. Membrane bioreactor (MBR) as an advanced wastewater treatment technology for removal of synthetic microplastics; pp. 45–60. [Google Scholar]
- Mohammad A.W., Teow Y., Ang W., Chung Y., Oatley-Radcliffe D., Hilal N. Nanofiltration membranes review: recent advances and future prospects. Desalination. 2015;356:226–254. [Google Scholar]
- Mohsenpour S.F., Hennige S., Willoughby N., Adeloye A., Gutierrez T. Integrating micro-algae into wastewater treatment: a review. Sci. Total Environ. 2021;752 doi: 10.1016/j.scitotenv.2020.142168. [DOI] [PubMed] [Google Scholar]
- Molinuevo-Salces B., Riaño B., Hernández D., García-González M.C. Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment. Springer; 2019. Microalgae and wastewater treatment: advantages and disadvantages; pp. 505–533. [Google Scholar]
- Moore T.T., Koros W.J. Non-ideal effects in organic–inorganic materials for gas separation membranes. J. Mol. Struct. 2005;739(1-3):87–98. [Google Scholar]
- Moosavi S., Lai C.W., Gan S., Zamiri G., Akbarzadeh Pivehzhani O., Johan M.R. Application of efficient magnetic particles and activated carbon for dye removal from wastewater. ACS Omega. 2020;5(33):20684–20697. doi: 10.1021/acsomega.0c01905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi G., Zinadini S., Rajabi L. Development of high flux nanofiltration membrane using para-amino benzoate ferroxane nanoparticle for enhanced antifouling behavior and dye removal. Process Saf. Environ. Prot. 2020;144:65–78. [Google Scholar]
- Muhamad M.S., Hamidon N., Salim M.R., Yusop Z., Lau W.J., Hadibarata T. Response surface methodology for modeling bisphenol a removal using ultrafiltration membrane system. Water Air Soil Pollut. 2018;229(7):1–11. [Google Scholar]
- Na C., Olson T.M. Relative reactivity of amino acids with chlorine in mixtures. Environ. Sci. Technol. 2007;41(9):3220–3225. doi: 10.1021/es061999e. [DOI] [PubMed] [Google Scholar]
- Nam S.W., Choi D.J., Kim S.K., Her N., Zoh K.D. Adsorption characteristics of selected hydrophilic and hydrophobic micropollutants in water using activated carbon. J. Hazard. Mater. 2014;270:144–152. doi: 10.1016/j.jhazmat.2014.01.037. [DOI] [PubMed] [Google Scholar]
- Neale P.A., Schäfer A.I. Quantification of solute–solute interactions in steroidal hormone removal by ultrafiltration membranes. Sep. Purif. Technol. 2012;90:31–38. [Google Scholar]
- Nguyen M.L., Nakhjiri A.T., Kamal M., Mohamed A., Algarni M., Yu S.T., Su C.H. State-of-the-Art review on the application of membrane bioreactors for molecular micro-contaminant removal from aquatic environment. Membranes. 2022;12(4):429. doi: 10.3390/membranes12040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez-Delgado A. What do we know about the SARS-CoV-2 coronavirus in the environment? Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyangaresi P.O., Qin Y., Chen G., Zhang B., Lu Y., Shen L. Comparison of the performance of pulsed and continuous UVC-LED irradiation in the inactivation of bacteria. Water Res. 2019;157:218–227. doi: 10.1016/j.watres.2019.03.080. [DOI] [PubMed] [Google Scholar]
- Oatley-Radcliffe D.L., Walters M., Ainscough T.J., Williams P.M., Mohammad A.W., Hilal N. Nanofiltration membranes and processes: a review of research trends over the past decade. J. Water Process Eng. 2017;19:164–171. [Google Scholar]
- Obotey Ezugbe E., Rathilal S. Membrane technologies in wastewater treatment: a review. Membranes. 2020;10(5):89. doi: 10.3390/membranes10050089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanessian K., Monnot M., Moulin P., Ferrasse J.H., Barca C., Soric A., Boutin O. Dead-end and crossflow ultrafiltration process modelling: Application on chemical mechanical polishing wastewaters. Chem. Eng. Res. Des. 2020;158:164–176. [Google Scholar]
- Olaizola M. Commercial development of microalgal biotechnology: from the test tube to the marketplace. Biomol. Eng. 2003;20(4-6):459–466. doi: 10.1016/s1389-0344(03)00076-5. [DOI] [PubMed] [Google Scholar]
- Oliveira T.M., Ribeiro F.W., Morais S., de Lima-Neto P., Correia A.N. Removal and sensing of emerging pollutants released from (micro) plastics degradation: strategies based on boron-doped diamond electrodes. Curr. Opin. Electrochem. 2021 [Google Scholar]
- Oppenländer T. John Wiley & Sons; 2007. Photochemical Purification of Water and Air: Advanced Oxidation Processes (AOPs)-Principles, Reaction Mechanisms, Reactor Concepts. [Google Scholar]
- Oturan M.A., Aaron J.J. Advanced oxidation processes in water/wastewater treatment: principles and applications. A review. Crit. Rev. Environ. Sci. Technol. 2014;44(23):2577–2641. [Google Scholar]
- Pacheco C.A.R., Hilares R.T., d. J. C. Andrade G., Mogrovejo-Valdivia A., Tanaka D.A.P. Emerging contaminants, SARS-COV-2 and wastewater treatment plants, new challenges to confront: a short review. Bioresour. Technol. Rep. 2021;15 doi: 10.1016/j.biteb.2021.100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S.R., Jegatheesan V., Baskaran K., Shu L. Fouling in reverse osmosis (RO) membrane in water recovery from secondary effluent: a review. Rev. Environ. Sci. Bio/Technol. 2012;11(2):125–145. [Google Scholar]
- Park K., Kim J., Yang D.R., Hong S. Towards a low-energy seawater reverse osmosis desalination plant: a review and theoretical analysis for future directions. J. Membr. Sci. 2020;595 [Google Scholar]
- Parsa S.M., Momeni S., Hemmat A., Afrand M. Effectiveness of solar water disinfection in the era of COVID-19 (SARS-CoV-2) pandemic for contaminated water/wastewater treatment considering UV effect and temperature. J. Water Process Eng. 2021;43 doi: 10.1016/j.jwpe.2021.102224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendergast M.M., Hoek E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011;4(6):1946–1971. [Google Scholar]
- Perrich J.R. CRC press; 2018. Activated Carbon Adsorption for Wastewater Treatment. [Google Scholar]
- Peters C.D., Hankins N.P. Osmotically assisted reverse osmosis (OARO): five approaches to dewatering saline brines using pressure-driven membrane processes. Desalination. 2019;458:1–13. [Google Scholar]
- Pocostales J.P., Sein M.M., Knolle W., von Sonntag C., Schmidt T.C. Degradation of ozone-refractory organic phosphates in wastewater by ozone and ozone/hydrogen peroxide (peroxone): the role of ozone consumption by dissolved organic matter. Environ. Sci. Technol. 2010;44(21):8248–8253. doi: 10.1021/es1018288. [DOI] [PubMed] [Google Scholar]
- Prabha, V., R. D. BARMA, R. Singh and A. Madan (2015). "Ozone technology in food processing: a review."
- Pradhan S., Fan L., Roddick F.A. Removing organic and nitrogen content from a highly saline municipal wastewater reverse osmosis concentrate by UV/H2O2-BAC treatment. Chemosphere. 2015;136:198–203. doi: 10.1016/j.chemosphere.2015.05.028. [DOI] [PubMed] [Google Scholar]
- Pramanik B.K., Pramanik S.K., Sarker D.C., Suja F. Removal of emerging perfluorooctanoic acid and perfluorooctane sulfonate contaminants from lake water. Environ. Technol. 2017;38(15):1937–1942. doi: 10.1080/09593330.2016.1240716. [DOI] [PubMed] [Google Scholar]
- Qasim M., Badrelzaman M., Darwish N.N., Darwish N.A., Hilal N. Reverse osmosis desalination: a state-of-the-art review. Desalination. 2019;459:59–104. [Google Scholar]
- Qi W., Singer H., Berg M., Müller B., Pernet-Coudrier B., Liu H., Qu J. Elimination of polar micropollutants and anthropogenic markers by wastewater treatment in Beijing, China. Chemosphere. 2015;119:1054–1061. doi: 10.1016/j.chemosphere.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Quinlivan P.A., Li L., Knappe D.R. Effects of activated carbon characteristics on the simultaneous adsorption of aqueous organic micropollutants and natural organic matter. Water Res. 2005;39(8):1663–1673. doi: 10.1016/j.watres.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Race M., Ferraro A., Galdiero E., Guida M., Núñez-Delgado A., Pirozzi F., Fabbricino M. Current emerging SARS-CoV-2 pandemic: potential direct/indirect negative impacts of virus persistence and related therapeutic drugs on the aquatic compartments. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radjenović J., Petrović M., Ventura F., Barceló D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008;42(14):3601–3610. doi: 10.1016/j.watres.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Ravanchi M.T., Kaghazchi T., Kargari A. Application of membrane separation processes in petrochemical industry: a review. Desalination. 2009;235(1-3):199–244. [Google Scholar]
- Rekhate C.V., Srivastava J. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater-a review. Chem. Eng. J. Adv. 2020;3 [Google Scholar]
- Remucal C.K., Salhi E., Walpen N., von Gunten U. Molecular-level transformation of dissolved organic matter during oxidation by ozone and hydroxyl radical. Environ. Sci. Technol. 2020;54(16):10351–10360. doi: 10.1021/acs.est.0c03052. [DOI] [PubMed] [Google Scholar]
- Rodrigues A.C., Boroski M., Shimada N.S., Garcia J.C., Nozaki J., Hioka N. Treatment of paper pulp and paper mill wastewater by coagulation–flocculation followed by heterogeneous photocatalysis. J. Photochem. Photobiol. A. 2008;194(1):1–10. [Google Scholar]
- Roslan J., Kamal S.M.M., Yunos K.F.M., Abdullah N. Evaluation on performance of dead-end ultrafiltration membrane in fractionating tilapia by-product protein hydrolysate. Sep. Purif. Technol. 2018;195:21–29. [Google Scholar]
- Roy D., Wong P., Engelbrecht R., Chian E. Mechanism of enteroviral inactivation by ozone. Appl. Environ. Microbiol. 1981;41(3):718–723. doi: 10.1128/aem.41.3.718-723.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki D., Minami R., Matsuyama H. Effects of operating conditions on biofouling in crossflow ultrafiltration membrane processes. Sep. Purif. Technol. 2017;189:138–144. [Google Scholar]
- Sagle A., Freeman B. Fundamentals of membranes for water treatment. The future of desalination in Texas. 2004;2(363):137. [Google Scholar]
- Salehi F. Current and future applications for nanofiltration technology in the food processing. Food Bioprod. Process. 2014;92(2):161–177. [Google Scholar]
- Samaei S.M., Gato-Trinidad S., Altaee A. Performance evaluation of reverse osmosis process in the post-treatment of mining wastewaters: case study of Costerfield mining operations, Victoria, Australia. J. Water Process Eng. 2020;34 [Google Scholar]
- Santhy K., Selvapathy P. Removal of heavy metals from wastewater by adsorption on coir pith activated carbon. Sep. Sci. Technol. 2004;39(14):3331–3351. doi: 10.1016/j.biortech.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Schmidt C.M., Sprunk M., Loeffler R., Hinrichs J. Relating nanofiltration membrane morphology to observed rejection of saccharides. Sep. Purif. Technol. 2020;239 [Google Scholar]
- Schollée J.E., Hollender J., McArdell C.S. Characterization of advanced wastewater treatment with ozone and activated carbon using LC-HRMS based non-target screening with automated trend assignment. Water Res. 2021;200 doi: 10.1016/j.watres.2021.117209. [DOI] [PubMed] [Google Scholar]
- Scholz M., Melin T., Wessling M. Transforming biogas into biomethane using membrane technology. Renew. Sustain. Energy Rev. 2013;17:199–212. [Google Scholar]
- Sengupta A., Jebur M., Kamaz M., Wickramasinghe S.R. Removal of emerging contaminants from wastewater streams using membrane bioreactors: a review. Membranes. 2021;12(1):60. doi: 10.3390/membranes12010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V.K., Feng M. Water depollution using metal-organic frameworks-catalyzed advanced oxidation processes: a review. J. Hazard. Mater. 2019;372:3–16. doi: 10.1016/j.jhazmat.2017.09.043. [DOI] [PubMed] [Google Scholar]
- Sharrer M.J., Summerfelt S.T. Ozonation followed by ultraviolet irradiation provides effective bacteria inactivation in a freshwater recirculating system. Aquacult. Eng. 2007;37(2):180–191. [Google Scholar]
- Shi L., Rossi R., Son M., Hall D.M., Hickner M.A., Gorski C.A., Logan B.E. Using reverse osmosis membranes to control ion transport during water electrolysis. Energy Environ. Sci. 2020;13(9):3138–3148. [Google Scholar]
- Shin G.A., Sobsey M.D. Reduction of Norwalk virus, poliovirus 1, and bacteriophage MS2 by ozone disinfection of water. Appl. Environ. Microbiol. 2003;69(7):3975–3978. doi: 10.1128/AEM.69.7.3975-3978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shon H., Phuntsho S., Chaudhary D., Vigneswaran S., Cho J. Nanofiltration for water and wastewater treatment–a mini review. Drink. Water Eng. Sci. 2013;6(1):47–53. [Google Scholar]
- Show P.L., Tang M.S., Nagarajan D., Ling T.C., Ooi C.W., Chang J.S. A holistic approach to managing microalgae for biofuel applications. Int. J. Mol. Sci. 2017;18(1):215. doi: 10.3390/ijms18010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpää M., Ncibi M.C., Matilainen A. Advanced oxidation processes for the removal of natural organic matter from drinking water sources: a comprehensive review. J. Environ. Manag. 2018;208:56–76. doi: 10.1016/j.jenvman.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Singh R.K., Smriti V., Pankaj T. Industrial wastewater treatment by biological activated carbon-a review. Res. J. Pharm. Biol. Chem. Sci. 2011;2(4):1053–1058. [Google Scholar]
- Soares P.A., Silva T.F., Manenti D.R., Souza S.M., Boaventura R.A., Vilar V.J. Insights into real cotton-textile dyeing wastewater treatment using solar advanced oxidation processes. Environ. Sci. Pollut. Res. 2014;21(2):932–945. doi: 10.1007/s11356-013-1934-0. [DOI] [PubMed] [Google Scholar]
- Sommer R., Cabaj A., Hirschmann G., Haider T. Disinfection of drinking water by UV irradiation: Basic principles-specific requirements-international implementations. Ozone Sci. Eng. 2008;30(1):43–48. [Google Scholar]
- Sommer R., Pribil W., Appelt S., Gehringer P., Eschweiler H., Leth H., Haider T. Inactivation of bacteriophages in water by means of non-ionizing (UV-253.7 nm) and ionizing (gamma) radiation: a comparative approach. Water Res. 2001;35(13):3109–3116. doi: 10.1016/s0043-1354(01)00030-6. [DOI] [PubMed] [Google Scholar]
- Sosa-Hernández J.E., Rodas-Zuluaga L.I., López-Pacheco I.Y., Melchor-Martínez E.M., Aghalari Z., Limón D.S., Parra-Saldívar R. Sources of antibiotics pollutants in the aquatic environment under SARS-CoV-2 pandemic situation. Case Stud. Chem. Environ. Eng. 2021;4 doi: 10.1016/j.cscee.2021.100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak S., Kumar A. Chlorination disinfection by-products and comparative cost analysis of chlorination and UV disinfection in sewage treatment plants: Indian scenario. Environ. Sci. Pollut. Res. 2017;24(34):26269–26278. doi: 10.1007/s11356-017-0568-z. [DOI] [PubMed] [Google Scholar]
- Tak S., Vellanki B.P. Applicability of advanced oxidation processes in removing anthropogenically influenced chlorination disinfection byproduct precursors in a developing country. Ecotoxicol. Environ. Saf. 2019;186 doi: 10.1016/j.ecoenv.2019.109768. [DOI] [PubMed] [Google Scholar]
- Tang C.Y., Yang Z., Guo H., Wen J.J., Nghiem L.D., Cornelissen E. ACS Publications; 2018. Potable Water Reuse Through Advanced Membrane Technology. [DOI] [PubMed] [Google Scholar]
- Tang F., Hu H.Y., Wu Q.Y., Tang X., Sun Y.X., Shi X.L., Huang J.J. Effects of chemical agent injections on genotoxicity of wastewater in a microfiltration-reverse osmosis membrane process for wastewater reuse. J. Hazard. Mater. 2013;260:231–237. doi: 10.1016/j.jhazmat.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Tang P., Liu B., Zhang Y., Chang H., Zhou P., Feng M., Sharma V.K. Sustainable reuse of shale gas wastewater by pre-ozonation with ultrafiltration-reverse osmosis. Chem. Eng. J. 2020;392 [Google Scholar]
- Tang S., Xu L., Yu X., Chen S., Li H., Huang Y., Niu J. Degradation of anticancer drug capecitabine in aquatic media by three advanced oxidation processes: Mechanisms, toxicity changes and energy cost evaluation. Chem. Eng. J. 2021;413 [Google Scholar]
- Teymoorian T., Teymourian T., Kowsari E., Ramakrishna S. Direct and indirect effects of SARS-CoV-2 on wastewater treatment. J. Water Process Eng. 2021;42 doi: 10.1016/j.jwpe.2021.102193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaçi B.S., Gashi S.T. Reverse osmosis removal of heavy metals from wastewater effluents using biowaste materials pretreatment. Pol. J. Environ. Stud. 2019;28(1):337–341. [Google Scholar]
- Ting H., Haifeng L., Shanshan M., Zhang Y., Zhidan L., Na D. Progress in microalgae cultivation photobioreactors and applications in wastewater treatment: a review. Int. J. Agric. Biol. Eng. 2017;10(1):1–29. [Google Scholar]
- Tran H.D.M., Boivin S., Kodamatani H., Ikehata K., Fujioka T. Potential of UV-B and UV-C irradiation in disinfecting microorganisms and removing N-nitrosodimethylamine and 1, 4-dioxane for potable water reuse: a review. Chemosphere. 2021 doi: 10.1016/j.chemosphere.2021.131682. [DOI] [PubMed] [Google Scholar]
- Tul Muntha S., Kausar A., Siddiq M. Advances in polymeric nanofiltration membrane: a review. Polym. Plast. Technol. Eng. 2017;56(8):841–856. [Google Scholar]
- Tyrrell S.A., Rippey S.R., Watkins W.D. Inactivation of bacterial and viral indicators in secondary sewage effluents, using chlorine and ozone. Water Res. 1995;29(11):2483–2490. [Google Scholar]
- Utoro P.A.R., Sukoyo A., Sandra S., Izza N., Dewi S.R., Wibisono Y. High-throughput microfiltration membranes with natural biofouling reducer agent for food processing. Processes. 2019;7(1):1. [Google Scholar]
- Valavala R., Sohn J.S., Han J.H., Her N.G., Yoon Y.M. Pretreatment in reverse osmosis seawater desalination: a short review. Environ. Eng. Res. 2011;16(4):205–212. [Google Scholar]
- Van der Bruggen B., Vandecasteele C., Van Gestel T., Doyen W., Leysen R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003;22(1):46–56. [Google Scholar]
- van Gijn K., Chen Y., van Oudheusden B., Gong S., de Wilt H., Rijnaarts H., Langenhoff A. Optimizing biological effluent organic matter removal for subsequent micropollutant removal. J. Environ. Chem. Eng. 2021;9(5) [Google Scholar]
- Vasilyak L. Physical methods of disinfection (a review) Plasma Phys. Rep. 2021;47(3):318–327. [Google Scholar]
- Verinda S.B., Yulianto E., Gunawan M., Nur M. Ozonated nanobubbles-A potential hospital wastewater treatment during the COVID-19 outbreak in Indonesia to eradicate the persistent SARS-CoV-2 in HWWs? Ann. Trop. Med. Public Health. 2021;24:24–197. [Google Scholar]
- Völker J., Stapf M., Miehe U., Wagner M. Systematic review of toxicity removal by advanced wastewater treatment technologies via ozonation and activated carbon. Environ. Sci. Technol. 2019;53(13):7215–7233. doi: 10.1021/acs.est.9b00570. [DOI] [PubMed] [Google Scholar]
- Wanda E.M., Mamba B.B., Msagati T.A. Nitrogen-doped carbon nanotubes/polyethersulfone blend membranes for removing emerging micropollutants. CLEAN Soil Air Water. 2017;45(4) [Google Scholar]
- Wang F., Shi H., Tarabara V.V. Combined precipitative and colloidal fouling of reverse osmosis membranes. J. Environ. Eng. 2019;145(8) [Google Scholar]
- Wang J., Zhuan R. Degradation of antibiotics by advanced oxidation processes: an overview. Sci. Total Environ. 2020;701 doi: 10.1016/j.scitotenv.2019.135023. [DOI] [PubMed] [Google Scholar]
- Wang M., Kuo-Dahab W.C., Dolan S., Park C. Kinetics of nutrient removal and expression of extracellular polymeric substances of the microalgae, Chlorella sp. and Micractinium sp., in wastewater treatment. Bioresour. Technol. 2014;154:131–137. doi: 10.1016/j.biortech.2013.12.047. [DOI] [PubMed] [Google Scholar]
- Wang Q., Lai Z., Luo C., Zhang J., Cao X., Liu J., Mu J. Honeycomb-like activated carbon with microporous nanosheets structure prepared from waste biomass cork for highly efficient dye wastewater treatment. J. Hazard. Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.125896. [DOI] [PubMed] [Google Scholar]
- Wang W.L., Wu Q.Y., Huang N., Wang T., Hu H.Y. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: influence factors and radical species. Water Res. 2016;98:190–198. doi: 10.1016/j.watres.2016.04.015. [DOI] [PubMed] [Google Scholar]
- Wen G., Liang Z., Xu X., Cao R., Wan Q., Ji G., Huang T. Inactivation of fungal spores in water using ozone: kinetics, influencing factors and mechanisms. Water Res. 2020;185 doi: 10.1016/j.watres.2020.116218. [DOI] [PubMed] [Google Scholar]
- Wenten I.G. Reverse osmosis applications: prospect and challenges. Desalination. 2016;391:112–125. [Google Scholar]
- Wollmann F., Dietze S., Ackermann J.U., Bley T., Walther T., Steingroewer J., Krujatz F. Microalgae wastewater treatment: Biological and technological approaches. Eng. Life Sci. 2019;19(12):860–871. doi: 10.1002/elsc.201900071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wols B., Hofman-Caris C. Review of photochemical reaction constants of organic micropollutants required for UV advanced oxidation processes in water. Water Res. 2012;46(9):2815–2827. doi: 10.1016/j.watres.2012.03.036. [DOI] [PubMed] [Google Scholar]
- Wong S., Ngadi N., Inuwa I.M., Hassan O. Recent advances in applications of activated carbon from biowaste for wastewater treatment: a short review. J. Clean. Prod. 2018;175:361–375. [Google Scholar]
- Wu F., Xiao A., Zhang J., Moniz K., Endo N., Armas F., Erickson T.B. Wastewater surveillance of SARS-CoV-2 across 40 US states from February to June 2020. Water Res. 2021;202 doi: 10.1016/j.watres.2021.117400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Niu X., Yang J., Wang C., Lu M. Retentions of bisphenol A and norfloxacin by three different ultrafiltration membranes in regard to drinking water treatment. Chem. Eng. J. 2016;294:410–416. [Google Scholar]
- Xiao K., Liang S., Wang X., Chen C., Huang X. Current state and challenges of full-scale membrane bioreactor applications: a critical review. Bioresour. Technol. 2019;271:473–481. doi: 10.1016/j.biortech.2018.09.061. [DOI] [PubMed] [Google Scholar]
- Xiao R., Zheng Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016;34(7):1225–1244. doi: 10.1016/j.biotechadv.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Yan M.Q., Wang D.S., Shi B.Y., Wei Q.S., Qu J.H., Tang H.X. Transformations of particles, metal elements and natural organic matter in different water treatment processes. J. Environ. Sci. 2007;19(3):271–277. doi: 10.1016/s1001-0742(07)60044-8. [DOI] [PubMed] [Google Scholar]
- Yan M., Wang D., Shi B., Wang M., Yan Y. Effect of pre-ozonation on optimized coagulation of a typical North-China source water. Chemosphere. 2007;69(11):1695–1702. doi: 10.1016/j.chemosphere.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Yang X., Sun J., Fu W., Shang C., Li Y., Chen Y., Fang J. PPCP degradation by UV/chlorine treatment and its impact on DBP formation potential in real waters. Water Res. 2016;98:309–318. doi: 10.1016/j.watres.2016.04.011. [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhou Y., Feng Z., Rui X., Zhang T., Zhang Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers. 2019;11(8):1252. doi: 10.3390/polym11081252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J., Amy G., Chung J., Sohn J., Yoon Y. Removal of toxic ions (chromate, arsenate, and perchlorate) using reverse osmosis, nanofiltration, and ultrafiltration membranes. Chemosphere. 2009;77(2):228–235. doi: 10.1016/j.chemosphere.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Yu S., Wang J., Zhao Z., Cai W. Simultaneous coupling of fluidized granular activated carbon (GAC) and powdered activated carbon (PAC) with ultrafiltration process: A promising synergistic alternative for water treatment. Sep. Purif. Technol. 2021;282 [Google Scholar]
- Zahmatkesh S., Pirouzi A. Effects of the microalgae, sludge and activated carbon on the wastewater treatment with low organics (weak wastewater) Int. J. Environ. Sci. Technol. (IJEST) 2020;17(5) [Google Scholar]
- Zahmatkesh S., Amesho K.T., Sillanpaa M., Wang C. Integration of renewable energy in wastewater treatment during COVID-19 pandemic: challenges, opportunities, and progressive research trends. Clean. Chem. Eng. 2022;3 [Google Scholar]
- Zahmatkesh S., Far S.S., Sillanpää M. RSM-D-optimal modeling approach for COD removal from low strength wastewater by microalgae, sludge, and activated carbon-case study mashhad. J. Hazard. Mater. Adv. 2022;7 [Google Scholar]
- Zahmatkesh S., Klemeš J.J., Bokhari A., Wang C., Sillanpaa M., Hasan M., Amesho K.T. Critical role of Hyssop plant in the possible transmission of SARS-CoV-2 in contaminated human Feces and its implications for the prevention of the virus spread in sewage. Chemosphere. 2022;305 doi: 10.1016/j.chemosphere.2022.135247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahmatkesh S., Sillanpää M. Review of method and a new tool for decline and inactive SARS-CoV-2 in wastewater treatment. Clean. Chem. Eng. 2022;3 [Google Scholar]
- Zaimes G.G., Khanna V. Environmental sustainability of emerging algal biofuels: a comparative life cycle evaluation of algal biodiesel and renewable diesel. Environ. Prog. Sustain. Energy. 2013;32(4):926–936. [Google Scholar]
- Zaviska F., Drogui P., Grasmick A., Azais A., Héran M. Nanofiltration membrane bioreactor for removing pharmaceutical compounds. J. Membr. Sci. 2013;429:121–129. [Google Scholar]
- Zeng F., Cao S., Jin W., Zhou X., Ding W., Tu R., Huang H. Inactivation of chlorine-resistant bacterial spores in drinking water using UV irradiation, UV/hydrogen peroxide and UV/peroxymonosulfate: efficiency and mechanism. J. Clean. Prod. 2020;243 [Google Scholar]
- Zhang F., Li Z., Yin L., Zhang Q., Askarinam N., Mundaca-Uribe R., Zhang L. ACE2 receptor-modified algae-based microrobot for removal of SARS-CoV-2 in wastewater. J. Am. Chem. Soc. 2021;143(31):12194–12201. doi: 10.1021/jacs.1c04933. [DOI] [PubMed] [Google Scholar]
- Zhang S., Wu C., Zhou Y., Wang Y., He X. Effect of wastewater particles on catalytic ozonation in the advanced treatment of petrochemical secondary effluent. Chem. Eng. J. 2018;345:280–289. [Google Scholar]
- Zhang Z., Kang G., Yu H., Jin Y., Cao Y. From reverse osmosis to nanofiltration: Precise control of the pore size and charge of polyamide membranes via interfacial polymerization. Desalination. 2019;466:16–23. [Google Scholar]
- Zhao H., Liu G., Zhang M., Liu H., Zhang M., Zhou L., Jiang Y. Bioinspired modification of molybdenum disulfide nanosheets to prepare a loose nanofiltration membrane for wastewater treatment. J. Water Process Eng. 2021;40 [Google Scholar]
- Zhao Y.G., Zhang H. Phase separation in membrane biology: the interplay between membrane-bound organelles and membraneless condensates. Dev. Cell. 2020;55(1):30–44. doi: 10.1016/j.devcel.2020.06.033. [DOI] [PubMed] [Google Scholar]