Abstract
The number of minimally invasive surgeries, such as video‐assisted thoracoscopic surgery and robot‐assisted thoracoscopic surgery, has increased enormously in recent years. More and more relevant studies report that anatomic pulmonary segmentectomy has the same effect as traditional lobectomy in the surgical treatment of early stage non‐small cell lung cancer (diameter less than 2.0 cm). Segmentectomy requires sufficient knowledge of the location of the pulmonary nodules, as well as the anatomy of the target segments, blood vessels, and bronchi. With the rapid development of imaging technology and three‐dimensional technology, three‐dimensional reconstruction has been widely used in the medical field. It can effectively assess the vascular branching patterns, discover the anatomic variations of the blood vessels and bronchi, determine the location of the lesion, and clarify the division of the segments. Therefore, it is helpful for preoperative positioning, surgical planning, preoperative simulation and intraoperative navigation, and provides a reference for formulating an individualized surgical plan. It therefore plays a positive role in anatomic pulmonary segmentectomy. This study reviews the progress made in three‐dimensional computed tomography reconstruction in anatomic pulmonary segmentectomy.
Keywords: lung cancer, pulmonary segmentectomy, three‐dimensional computed tomography, three‐dimensional reconstruction
Three‐dimensional reconstruction can effectively assess the vascular branching patterns, discover the anatomic variations of the blood vessels and bronchi, determine the location of the lesion, and clarify the division of the segments. It is helpful for preoperative positioning, surgical planning, preoperative simulation and intraoperative navigation, which provides a reference for formulating an individualized surgical plan. It plays a positive role in anatomic pulmonary segmentectomy.

INTRODUCTION
Lung cancer remains the leading cause of cancer and mortality in China and worldwide. 1 , 2 Although the treatment of lung cancer is increasingly diverse, surgical resection is still the mainstay option. 3 , 4 Minimally invasive surgeries such as video‐assisted thoracoscopic surgery (VATS) and robot‐assisted thoracoscopic surgery (RATS) have achieved remarkable results in the treatment of early stage non‐small cell lung cancer (NSCLC), 5 , 6 , 7 and have been gradually promoted.
Segmentectomy for early stage lung cancer has achieved a good long‐term prognosis while maximizing removal of the lesion and protecting lung function. 8 , 9 According to the meta‐analyses results of Zhang et al. 10 and Bao et al. 11 anatomic pulmonary segmentectomy combined with systemic hilar and mediastinal lymph node dissection can achieve a satisfactory result for stage I NSCLC patients with tumors less than 2 cm. Segmentectomy requires the surgeon to have a clear understanding of the location of the target tumors, as well as the anatomy of the target lobes, segments, bronchi, arteries and veins.
With the development of imaging technology, such as multidetector computed tomography (MDCT) and three‐dimensional computed tomography bronchography and angiography (3D‐CTBA), two dimensional (2D) images can be converted into three dimensional (3D) images. 12 , 13 In order to reduce the intraoperative risks, accurately locate the lesions, improve the safety of surgery and achieve a precise resection, 3D reconstruction came into being and developed rapidly, especially in thoracic surgery. In this study, we review the progress made in 3D computed tomography (CT) reconstruction in anatomic pulmonary segmentectomy.
ANATOMIC PULMONARY SEGMENTECTOMY
Segmentectomy is defined as resection of one segment or one segment and its additional adjacent segment. From the technical aspect, segmentectomy is further categorized into simple or complex: resection of the right or left superior segment of the lower lobe (segment 6), the left superior, and the lingular segment is defined as simple. Complex segmentectomy is defined as resection of a segment that has more than one intersegmental plane. Even for certified thoracic surgeons, two or more intersegmental planes make segmentectomy technically more difficult. 4 Churchill and Belsey first reported the application of segmentectomy for the treatment of pulmonary tuberculosis and atelectasis in 1939. 14 Le Roux first reported the use of segmentectomy for the surgical treatment of primary lung cancer in 1972. 15 Now segmentectomy has been gradually popularized and has been shown to achieve good results. Dai et al. 16 reported that in patients with peripheral NSCLC of less than 2 cm, segmentectomy and lobectomy were comparable in the short‐term clinical outcomes, and produced comparable symptom burden and functional impairment during the early postoperative period. Chan et al. 17 showed that in the setting of clinical T1cN0M0 NSCLC, anatomic segmentectomy was not associated with significant differences in recurrence‐free or overall survival at 5 years. Wen et al. 18 reported that segmentectomy achieved similar recurrence‐free and overall survival compared with lobectomy for patients with clinical N0 invasive lung adenocarcinomas of no more than 2 cm. In addition, segmentectomy was found to help preserve more pulmonary function than lobectomy. 19 It is worth mentioning that the ongoing JCOG0802/WJOG4607L, JCOG1211, and CALGB140503 trials will disclose the influence of segmentectomy for patients with early‐stage NSCLCs that are small peripheral tumors based on preoperative high‐resolution CT findings about preserved pulmonary function and long‐term prognosis.
THREE‐DIMENSIONAL RECONSTRUCTION TECHNOLOGY
Three‐dimensional technology has previously been widely used in the medical field. 20 , 21 , 22 , 23 , 24 , 25 , 26 Three‐dimensional reconstruction means that the 2D image information such as CT value is extracted and segmented by the software and with the help of the supplementary judgment of the bronchus and blood vessels by the doctor, so as to build a 3D image of the lung. Three‐dimensional reconstruction technology began in the 1970s. Tomasi and Kanade completed the first image‐based 3D reconstruction system in 1992. 27 Scholars then began to apply 3D reconstruction to determine the relationship between tumor and blood vessels, and identify pulmonary vessels and bronchus. 13 , 28 , 29 Now, 3D reconstruction is used in thoracic surgery.
Take Materialise's interactive medical image control system (Mimics) (developed by Materialise Nv Co., Kingdom of Belgium) as an example. First, after manually confirming the starting point of each segmental pulmonary artery, the computer automatically divides the pulmonary segments according to the distribution of the pulmonary artery system, and divides the pulmonary subsegments according to the starting point of the subsegmental pulmonary artery. Then, the following seed line method is the main method, while the manual extraction is the auxiliary method. The lung parenchyma is extracted to the greatest extent, which combines with the division of pulmonary segments and subsegments to generate the corresponding modules and define the corresponding colors. Next, adjust the CT image to the pulmonary artery window, and divide the whole arterial tree by the seed point method. The venous vascular tree can be divided in the same way. Then, adjust the threshold to the required range of the bronchus, and complete the bronchial reconstruction by the threshold division and the seed point method. Finally, the pulmonary nodules can be divided and reconstructed. Larger nodules can be extracted by the threshold division and the seed point method, while smaller nodules can be extracted manually. The 3D images of the pulmonary nodules and the 3D images of the lung are combined to clearly show the specific location of the nodules and the pulmonary segment or subsegment to which they belong by adjusting the transparency of the 3D images of the lung. The reconstructions of trachea and bronchi, pulmonary lesion, pulmonary vessels and lung are shown in Figure 1, 2, 3, 4. The 3D reconstruction model is shown in Figure FIGURE 5.
FIGURE 1.

Trachea and bronchi reconstruction
FIGURE 2.
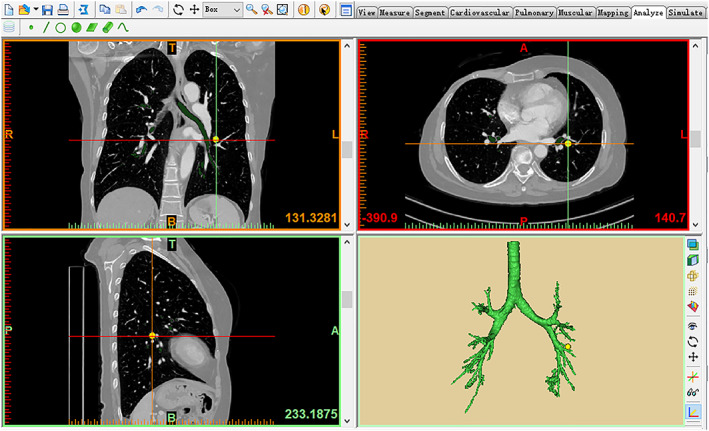
Pulmonary lesion reconstruction
FIGURE 3.

Pulmonary artery and vein reconstruction
FIGURE 4.

Lung reconstruction
FIGURE 5.

3D reconstruction model
ADVANTAGES OF THREE‐DIMENSIONAL RECONSTRUCTION
Three‐dimensional reconstruction can convert 2D images of the pulmonary arteries, pulmonary veins, and bronchi into 3D images of the vascular tree and bronchial tree, clearly showing the structure of the blood vessels and bronchi, effectively assessing vascular branching patterns and discovering the anatomic variations of the blood vessels and bronchi. 30 Three‐dimensional reconstruction can show the morphological characteristics of the lesion three‐dimensionally, and also clearly display the blood supply inside and around the lesion, helping physicians to make more accurate judgments of the nature of the lesion. Three‐dimensional reconstruction can accurately identify the normal anatomic structure, clarify the division of the pulmonary segments, determine the location of the lesion and the positional relationship with the target segment, 31 , 32 which is helpful for the complete resection of the target segment and the guarantee of the surgical margins.
Studies have indicated that the 3D images can be rotated freely and visualized interactively from any angle, which can directly measure the distance and positional relationship between the blood vessels, bronchus and the lesion, perform intraoperative navigation with the assistance of related software, 33 avoid the unnecessary dissection of lung tissue, and save intraoperative time to find and discern the segmental pulmonary venous vessels, thus shortening the operation time, 34 reducing the amount of bleeding during the operation, and improving the success rate of the operation. Three‐dimensional reconstruction is helpful for preoperative positioning, surgical planning, preoperative simulation and intraoperative navigation, and provides a reference for formulating an individualized surgical plan. 35 , 36 , 37
THREE‐DIMENSIONAL RECONSTRUCTION IN ANATOMIC PULMONARY SEGMENTECTOMY
Scholars have reported the use of thin‐layer CT data for processing 3D‐CT models for lobectomy and segmentectomy since 2001. 38 , 39 Three‐dimensional reconstruction has been gradually applied to anatomic pulmonary segmentectomy and achieved satisfactory results.
She et al. 40 reported that in comparison with the 2D group, operative duration shortened significantly, the extent of intraoperative bleeding and postoperative drainage lowered significantly, and chest tube duration shortened significantly in the 3D group. Hemoptysis and pulmonary air leakage (more than 3 days) occurred significantly less frequently in the 3D than in the 2D group. Xue et al. 41 showed that median operation time for the 3D group was shorter than in the non‐3D group. The study indicated that 3D images can be used to reduce the risk of insufficient surgical margins. The guidance of 3D images may enable accurate locate of the lesions and illustrate the variation pattern of the segmental vessels and bronchi. Preoperative 3D simulation can be helpful in precise surgical planning. Liu et al. 42 reported that compared with the general CT group, intraoperative blood loss significantly decreased operation time and postoperative hospital stay was significantly shortened in the 3D‐CT group. The incidence of postoperative hemoptysis in the 3D‐CT group occurred lower in the general group, but the differences were not statistically significant. This article shows that 3D‐CT and 3D printing for making a preoperative plan have an equivalent effect in thoracoscopic pulmonary segmentectomy for experienced surgeons. Qiu et al. 43 showed that intraoperative bleeding in 3D‐reconstruction was significantly lower than in the non‐3D group. With regard to complex segmentectomy, the 3D‐reconstruction group spent a shorter operation time when compared with the non‐3D group. Three‐dimensional reconstruction imaging and 3D printed model were both found to have significant advantages in locating nodules and identifying vascular variations. Moreover, this study proposed a preliminary preoperative rating scale to select appropriate patients for 3D reconstruction or 3D printing, which would develop detailed guidelines for the application of this technique in thoracic surgery. Lin et al. 44 reported that patients with preoperative 3D image simulations had fewer relapses than patients without preoperative 3D image simulations, which confirmed the relationship between the tumor and surrounding blood vessels and bronchus and ensured an oncological safety margin. In addition, preoperative 3D simulation may alter the oncological outcomes for patients with clinical stage IA2 NSCLC. Wu et al. 45 reported that 57 patients received segmentectomy assisted by 3D‐CTBA, and the surgical results were as follows: duration of surgery, 129.8 ± 16.1 min; blood loss, 48.8 ± 26.2 ml; length of postoperative hospital stay, 6.4 ± 1.3 days. The study concluded that preoperative 3D‐CTBA images could clearly and vividly display the targeted structure and the variations of vessels and bronchi. Meanwhile, the application of 3D‐CTBA with a virtual 3D surgical margin assisted the surgeon to determine accurate distances and the positional relationship among the tumor, bronchial trees, and the intersegmental vessels. The advantages of the 3D group compared with the non‐3D group are shown in Table TABLE 1.
TABLE 1.
The advantages of the 3D group compared with the non‐3D group
| 3D group | Non‐3D group | |
|---|---|---|
| Operation time (minute) | ||
| Liu et al. 41 | 115.5 ± 37.2 | 133.0 ± 35.7 |
| Qiu et al. 42 | 116.1 ± 30.7 | 125.1 ± 23.6 |
| She et al. 43 | 141.9 ± 29.1 | 160.9 ± 31.5 |
| Xue et al. 44 | 111 | 139 |
| Intraoperative bleeding (ml) | ||
| Liu et al. 41 | 75.1 ± 57.4 | 106.3 ± 70.8 |
| Qiu et al. 42 | 20.9 ± 12.2 | 18.2 ± 12.2 |
| She et al. 43 | 96.4 ± 47.5 | 131.7 ± 48.5 |
| Postoperative hospital stay (days) | ||
| Liu et al. 41 | 4.5 ± 1.7 | 5.1 ± 1.8 |
| Complication | ||
| Postoperative hemoptysis | ||
| Liu et al. 41 | 2.6% (1/39) | 13.2% (7/53) |
| Hemoptysis | ||
| She et al. 43 | 1.9% (1/51) | 15.6% (8/51) |
| Pulmonary air leakage | ||
| She et al. 43 | 3.9% (2/51) | 19.6% (10/51) |
| Postoperative drainage (ml) | ||
| She et al. 43 | 425.4 ± 163.5 | 664.7 ± 245.6 |
| Chest tube duration (days) | ||
| She et al. 43 | 2.7 ± 1.0 | 4.2 ± 1.6 |
| Recurrence | ||
| Lin et al. 45 | 2% (2/99) | 11.5% (21/182) |
Oizumi et al. 46 indicated that 3D‐CT could effectively guide thoracoscopic pulmonary segmentectomy and ensure the safety of the operation, especially when difficult pulmonary segmentectomies were performed. Hagiwara et al. 47 concluded that preoperative simulations using 3D‐CT for the assessment of pulmonary vessel branching patterns appear to be beneficial for the safe and efficient performance of thoracoscopic pulmonary segmentectomy and for further understanding of the surgical anatomy related to general thoracic surgery. LeMoal et al. 48 indicated that all 3D reconstructions met their expectations: anatomical accuracy (bronchi, arteries, veins, tumor, and the thoracic wall with intercostal spaces), accurate delimitation of each segment in the lobe of interest, margin resection, free space rotation, portability (smartphone, tablet) and time saving technique. The operative planning by 3D CT reconstruction is useful in robot‐assisted segmentectomy.
INSUFFICIENCIES OF THREE‐DIMENSIONAL RECONSTRUCTION
The application of the 3D reconstruction in pulmonary segmentectomy has been increasing, but it still has some shortcomings. (a) Three‐dimensional reconstruction has strict requirements for the 2D images of CT. If the distribution of pixels in the source images is affected by the poor cooperation of patients, artifact, insufficient breathholding, insufficient air in the distal small airways, or inappropriate injection phase of contrast agent into the human body, there will be errors in the reconstruction of the pulmonary blood vessels and bronchi, which will affect the quality of 3D reconstruction. Therefore, patients are required to cooperate as best as possible during the CT examination. (b) Because the branches of the pulmonary blood vessels and bronchi are more variable, there are still errors in the imaging of the blood vessels and bronchi in 3D reconstruction. Therefore, surgeons should combine the 3D images with the CT images before the operation, and carefully identify and analyze the branches during the operation; at the same time, it also requires further optimization of the 3D reconstruction software. (c) In most cases, adjacent pulmonary segments are interlaced with each other instead of being bounded by a straight line. During the operation, a broken line boundary may appear when searching for the intersegmental plane. It means that the results of 3D reconstruction do not completely reflect the division of the intersegmental plane, and the surgeons need to combine the results of 3D images with the actual intersegmental boundary. (d) During 3D reconstruction, the lung is in a state of expansion and normal anatomic position. However, after intraoperative one‐lung ventilation, the affected lung is in a state of collapse and is pulled in multiple directions, which may deviate from the normal anatomic position. There will therefore be a certain degree of differences in the running directions of the pulmonary blood vessels and bronchi in the two different situations. It requires surgeons to place the lung in a relatively normal anatomic position, and combine the 3D images to accurately identify the blood vessels and bronchi. (e) The 3D images still need to be displayed in a 2D window, which lacks a sense of entity. As a result, 3D reconstruction can be combined with 3D printing, virtual reality (VR) or other technologies to make it more convenient for surgeons to view a 3D model and realize the establishment and understanding of the overall space, 41 (f) Three‐dimensional reconstruction software are difficult to operate, some are expensive or not open to individual users.
PROSPECT OF THREE‐DIMENSIONAL RECONSTRUCTION
In order to break through the limitation that traditional 3D visualization software can only form the static simulation, Tokuno et al. 49 developed research progress map (RPM) software, which can semi‐automatically generate virtual dynamic images based on the specific CT data of patients, which can quickly and accurately reflect the dynamic simulation of lung traction, resection and other deformation during the operation, and guide the operation process in real time.
In future, the application of contrast agents with higher contrast, the innovation of image acquisition technology, and the advancement of computer processing software will play a positive role in the development of 3D reconstruction. The combination of artificial intelligence technologies such as 3D printing and virtual reality with 3D reconstruction technology can enhance the surgeon's overall perception of the target area. Three‐dimensional reconstruction technology has a broad application prospect, which can further promote the development of anatomic pulmonary segmentectomy and even thoracic surgery.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Wu Z, Huang Z, Qin Y, Jiao W. Progress in three‐dimensional computed tomography reconstruction in anatomic pulmonary segmentectomy. Thorac Cancer. 2022;13(13):1881–1887. 10.1111/1759-7714.14443
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. [DOI] [PubMed] [Google Scholar]
- 2. Allemani C, Matsuda T, di Carlo V, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD‐3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population‐based registries in 71 countries. Lancet 2018;391:1023–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhao ZR, Situ DR, Lau RW, et al. Comparison of Segmentectomy and lobectomy in stage IA adenocarcinomas. J Thorac Oncol. 2017;12:890–6. [DOI] [PubMed] [Google Scholar]
- 4. Suzuki K, Saji H, Aokage K, Watanabe SI, Okada M, Mizusawa J, et al. Comparison of pulmonary segmentectomy and lobectomy: safety results of a randomized trial. J Thorac Cardiovasc Surg. 2019;158:895–907. [DOI] [PubMed] [Google Scholar]
- 5. Hung MH, Cheng YJ, Chan KC, Han SC, Chen KC, Hsu HH, et al. Nonintubated uniportal thoracoscopic surgery for peripheral lung nodules. Ann Thorac Surg. 2014;98:1998–2003. [DOI] [PubMed] [Google Scholar]
- 6. Wu H, Jin R, Yang S, Park BJ, Li H. Long‐term and short‐term outcomes of robot‐ versus video‐assisted anatomic lung resection in lung cancer: a systematic review and meta‐analysis. Eur J Cardiothorac Surg. 2020;59:732–40. [DOI] [PubMed] [Google Scholar]
- 7. Gharagozloo F, Margolis M, Tempesta B, Strother E, Najam F. Robot‐assisted lobectomy for early‐stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg. 2009;88:380–4. [DOI] [PubMed] [Google Scholar]
- 8. Nomori H, Mori T, Shiraishi A, Fujino K, Sato Y, Ito T, et al. Long‐term prognosis after Segmentectomy for cT1 N0 M0 non‐small cell lung cancer. Ann Thorac Surg. 2019;107:1500–6. [DOI] [PubMed] [Google Scholar]
- 9. Stiles BM, Mao J, Harrison S, Lee B, Port JL, Altorki NK, et al. Sublobar resection for nodenegative lung cancer 2‐5cm in size. Eur J Cardiothorac Surg. 2019;56:858–66. [DOI] [PubMed] [Google Scholar]
- 10. Zhang L, Li M, Yin R, Zhang Q, Xu L. Comparison of the oncologic outcomes of anatomic segmentectomy and lobectomy for early‐stage nonsmall cell lung cancer. Ann Thorac Surg. 2015;99:728–37. [DOI] [PubMed] [Google Scholar]
- 11. Bao F, Ye P, Yang Y, Wang L, Zhang C, Lv X, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta‐analysis. Eur J Cardiothorac Surg. 2014;46:1–7. [DOI] [PubMed] [Google Scholar]
- 12. Akiba T. Utility of three‐dimensional computed tomography in general thoracic surgery. Gen Thorac Cardiovasc Surg. 2013;61:676–84. [DOI] [PubMed] [Google Scholar]
- 13. Akiba T, Marushima H, Harada J, Kobayashi S, Morikawa T. Importance of preoperative imaging with 64‐row three‐dimensional multidetector computed tomography for safer video‐assisted thoracic surgery in lung cancer. Surg Today. 2009;39:844–7. [DOI] [PubMed] [Google Scholar]
- 14. Churchill ED, Belsey R. Segmental pneumonectomy in bronchiectasis: the lingula segment of the left upper lobe. Ann Surg. 1939;109:481–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Le Roux BT. Management of bronchial carcinoma by segmental resection. Thorax. 1972;27:70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dai W, Chang S, Pompili C, Qiu B, Wei X, Mu Y, et al. Early postoperative patient‐reported outcomes after Thoracoscopic Segmentectomy versus lobectomy for small‐sized peripheral non‐small‐cell lung cancer. Ann Surg Oncol. 2022;29:547–56. [DOI] [PubMed] [Google Scholar]
- 17. Chan EG, Chan PG, Mazur SN, Normolle DP, Luketich JD, Landreneau RJ, et al. Outcomes with segmentectomy versus lobectomy in patients with clinical T1cN0M0 non‐small cell lung cancer. J Thorac Cardiovasc Surg. 2021;161:1639–1648.e2. [DOI] [PubMed] [Google Scholar]
- 18. Wen Z, Zhao Y, Fu F, Hu H, Sun Y, Zhang Y, et al. Comparison of outcomes following segmentectomy or lobectomy for patients with clinical N0 invasive lung adenocarcinoma of 2 cm or less in diameter. J Cancer Res Clin Oncol. 2020;146:1603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen L, Gu Z, Lin B, Wang W, Xu N, Liu Y, et al. Pulmonary function changes after thoracoscopic lobectomy versus intentional thoracoscopic segmentectomy for early‐stage non‐small cell lung cancer. Transl Lung Cancer Res. 2021;10:4141–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fiz N, Delgado D, Sanchez X, et al. Application of 3D technology and printing for femoral derotation osteotomy: case and technical report. Ann Transl Med. 2017;5:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flores RL, Liss H, Raffaelli S, Humayun A, Khouri KS, Coelho PG, et al. The technique for 3D printing patient‐specific models for auricular reconstruction. J Craniomaxillofac Surg. 2017;45:937–43. [DOI] [PubMed] [Google Scholar]
- 22. Dupret‐Bories A, Vergez S, Meresse T, Brouillet F, Bertrand G. Contribution of 3D printing to mandibular reconstruction after cancer. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135:133–6. [DOI] [PubMed] [Google Scholar]
- 23. Hadeed K, Acar P, Dulac Y, Cuttone F, Alacoque X, Karsenty C. Cardiac 3D printing for better understanding of congenital heart disease. Arch Cardiovasc Dis. 2018;111:1–4. [DOI] [PubMed] [Google Scholar]
- 24. Kuroda S, Kobayashi T, Ohdan H. 3D printing model of the intrahepatic vessels for navigation during anatomic resection of hepatocellular carcinoma. Int J Surg Case Rep. 2017;41:219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Igami T, Nakamura Y, Hirose T, Ebata T, Yokoyama Y, Sugawara G, et al. Application of a threedimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience. World J Surg. 2014;38:3163–6. [DOI] [PubMed] [Google Scholar]
- 26. Silberstein JL, Maddox MM, Dorsey P, Feibus A, Thomas R, Lee BR. Physical models of renal malignancies using standard cross‐sectional imaging and 3‐dimensional printers: a pilot study. Urology. 2014;84:268–72. [DOI] [PubMed] [Google Scholar]
- 27. Tomasi C, Kanade T. Shape and motion from image streams: a factorization method. Proc Natl Acad Sci USA. 1993;90:9795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strunk H, Schweden F, Schild H, Thelen M. Spiral CT with three‐dimensional (3D) surface reconstruction in assessing solitary pulmonary foci. Rofo. 1993;158:26–30. [DOI] [PubMed] [Google Scholar]
- 29. Watanabe S, Arai K, Watanabe T, Koda W, Urayama H Use of three‐dimensional computed tomographic angiography of pulmonary vessels for lung resections. Ann Thorac Surg 2003;75:388–392; discussion 392. [DOI] [PubMed] [Google Scholar]
- 30. Shimizu K, Nagashima T, Ohtaki Y, Obayashi K, Nakazawa S, Kamiyoshihara M, et al. Analysis of the variation pattern in right upper pulmonary veins and establishment of simplified vein models for anatomical segmentectomy. Gen Thorac Cardiovasc Surg. 2016;64:604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kato H, Oizumi H, Suzuki J, Hamada A, Watarai H, Sadahiro M. Thoracoscopic anatomical lung segmentectomy using 3D computed tomography simulation without tumour markings for nonpalpable and non‐visualized small lung nodules. Interact Cardiovasc Thorac Surg. 2017;25:434–41. [DOI] [PubMed] [Google Scholar]
- 32. Ji Y, Zhang T, Yang L, Wang X, Qi L, Tan F, et al. The effectiveness of three‐dimensional reconstruction in the localization of multiple nodules in lung specimens: a prospective cohort study. Transl Lung Cancer Res. 2021;10:1474–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ikeda N, Yoshimura A, Hagiwara M, Akata S, Saji H. Three dimensional computed tomography lung modeling is useful in simulation and navigation of lung cancer surgery. Ann Thorac Cardiovasc Surg. 2013;19:1–5. [DOI] [PubMed] [Google Scholar]
- 34. Wang B, Guo Y, Tang J, Yu F. Three‐dimensional custom‐made carbon‐fiber prosthesis for sternal reconstruction after sarcoma resection. Thorac Cancer. 2019;10:1500–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yao F, Wang J, Yao J, Hang F, Lei X, Cao Y. Three‐dimensional image reconstruction with free open‐source OsiriX software in video‐assisted thoracoscopic lobectomy and segmentectomy. Int J Surg. 2017;39:16–22. [DOI] [PubMed] [Google Scholar]
- 36. Ma Q, Bao T, Zhang H, Liang C, Liu D. Anatomic video‐assisted thoracoscopic surgery segmentectomies based on the three‐dimensional reformation images. J Vis Surg. 2017;3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Heuts S, Sardari Nia P, Maessen JG. Preoperative planning of thoracic surgery with use of three‐dimensional reconstruction, rapid prototyping, simulation and virtual navigation. J Vis Surg. 2016;2:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kanzaki M, Wachi N, Onuki T. Simulating video‐assisted thoracoscopic lung resection using a virtual 3‐dimensional pulmonary model on a personal computer. J Thorac Cardiovasc Surg. 2011;142:243–4. [DOI] [PubMed] [Google Scholar]
- 39. Onuki T. Virtual reality in video‐assisted thoracoscopic lung segmentectomy. Kyobu Geka. 2009;62:733–8. [PubMed] [Google Scholar]
- 40. She XW, Gu YB, Xu C, Li C, Ding C, Chen J, et al. Three‐dimensional (3D)‐ computed tomography bronchography and angiography combined with 3Dvideo‐assisted thoracic surgery (VATS) versus conventional 2DVATS anatomic pulmonary segmentectomy for the treatment of non‐small cell lung cancer. Thorac Cancer. 2018;9:305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xue L, Fan H, Shi W, Ge D, Zhang Y, Wang Q, et al. Preoperative 3‐dimensional computed tomography lung simulation before video‐assisted thoracoscopic anatomic segmentectomy for ground glass opacity in lung. J Thorac Dis. 2018;10:6598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu X, Zhao Y, Xuan Y, Lan X, Zhao J, Lan X, et al. Three‐dimensional printing in the preoperative planning of thoracoscopic pulmonary segmentectomy. Transl Lung Cancer Res. 2019;8:929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Qiu B, Ji Y, He H, Zhao J, Xue Q, Gao S. Three‐dimensional reconstruction/personalized three‐dimensional printed model for thoracoscopic anatomical partial‐lobectomy in stage I lung cancer: a retrospective study. Transl Lung Cancer Res. 2020;9:1235–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lin KH, Huang YJ, Lee SC, Huang HK, Chen YY, Chang H, et al. Benefit of three‐dimensional image simulation in surgical resection of early stage lung cancer. Ann Thorac Surg. 2021:S0003‐4975(21)01392‐8. 10.1016/j.athoracsur.2021.06.091 [DOI] [PubMed] [Google Scholar]
- 45. Wu YJ, Shi QT, Zhang Y, Wang YL. Thoracoscopic segmentectomy and lobectomy assisted by three‐dimensional computed‐tomography bronchography and angiography for the treatment of primary lung cancer. World J Clin Cases. 2021;9:10494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oizumi H, Kanauchi N, Kato H, Endoh M, Suzuki J, Fukaya K, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3‐dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg. 2011;141:678–82. [DOI] [PubMed] [Google Scholar]
- 47. Hagiwara M, Shimada Y, Kato Y, Nawa K, Makino Y, Furumoto H, et al. High‐quality 3‐dimensional image simulation for pulmonary lobectomy and segmentectomy: results of preoperative assessment of pulmonary vessels and short‐term surgical outcomes in consecutive patients undergoing video‐assisted thoracic surgery. Eur J Cardiothorac Surg. 2014;46:e120–6. [DOI] [PubMed] [Google Scholar]
- 48. LeMoal J, Peillon C, Dacher JN, et al. Three‐dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: a pilot study. J Thorac Dis. 2018;10:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tokuno J, Chen‐Yoshikawa TF, Nakao M, Matsuda T, Date H. Resection process map: a novel dynamic simulation system for pulmonary resection. J Thorac Cardiovasc Surg. 2020;159:1130–8. [DOI] [PubMed] [Google Scholar]


