Abstract
Objective
This study investigated the associations between obesity, metabolic syndrome (MetS), the combination of these two components as a metabolic obesity phenotype, and endometrial cancer risk in East Asian women.
Methods
A total of 6,097,686 cancer-free women aged 40–74 years who underwent the National Health Insurance Service health examination between 2009 and 2010 were included. Cancer incidence was identified using the healthcare utilization database. Associations between baseline obesity (body mass index <23 kg/m2, 23–24.9 kg/m2, ≥25 kg/m2), MetS, each component of MetS, MetS stratified by obesity status, combination of obesity and MetS, and endometrial cancer risk were investigated using hazard ratios (HRs).
Results
Obesity, each component of MetS, and MetS increased the endometrial cancer risk. After these factors were mutually adjusted for, the association did not change. When stratified by obesity, MetS and MetS components were not associated with endometrial cancer in normal-weight or overweight women. However, in obese women, MetS and MetS components increased the risk of endometrial cancer (HR=1.29; 95% confidence interval [CI]=1.20–1.39). Compared with normal-weight women without MetS, endometrial cancer risk was not increased in normal-weight women with MetS. Overweight women showed an increased risk of endometrial cancer irrespective of the presence of MetS (HR=1.37 and 1.38, respectively). The HR of obese women with MetS was higher than that of obese women without MetS (HR=2.18 and 1.75).
Conclusion
The association between MetS and endometrial cancer was most prominent in obese women, suggesting that obese women with MetS would be more vulnerable to endometrial cancer.
Keywords: Obesity, Metabolic Syndrome, Endometrial Neoplasms
Synopsis
Obesity, each component of MetS, and MetS increased the endometrial cancer risk. When stratified by obesity, the association between MetS and endometrial cancer was most prominent in obese women, suggesting that obese women with MetS would be more vulnerable to endometrial cancer.
INTRODUCTION
Endometrial cancer is the sixth most common cancer, accounting for 320,000 new cases worldwide in 2012, and the second most common female cancer after breast cancer in developed countries [1]. The incidence rate has largely increased in countries with rapid socioeconomic development, including Asian countries [1]. In South Korea, endometrial cancer is one of the most rapidly increasing gynecological cancers, with an annual incremental increase of 3.5%, despite the general decrement of major gynecological cancers [2].
Risk factors for endometrial cancer include early menarche, late menopause, nulliparity, post-menopausal hormone therapy, and obesity. These risks are mostly related to unopposed estrogen in addition to a family history of endometrial cancer, type 2 diabetes, hypertension, physical activity, and dietary factors [3]. A recent umbrella review suggested that among the various risk factors of endometrial cancer, obesity-related factors such as body mass index (BMI), waist-to-hip ratio, and parity have strong associations without bias [4]. Obesity is associated with 34.0% of the global endometrial cancer incidence, although the rate is higher in Western countries, with a population attributable fraction (PAF) of >40%, than in Asian countries, with a PAF of <20% [5].
Despite the lower obesity rate in Asian countries, the prevalence of obesity has been estimated to increase in Korea [6], followed by an increase in type 2 diabetes and metabolic syndrome (MetS) [7,8]. There is a close relationship between MetS and obesity, with shared mechanisms including insulin resistance, sex hormones, and energy metabolism [9,10]. Thus, MetS has also been suggested to be associated with endometrial cancer, especially the obesity-related component [11,12].
Despite the close relationship, some individuals are obese but do not have any features of MetS. On the other hand, some individuals who are not obese have components of MetS. These individuals are considered metabolically healthy obese (MHO) or metabolically unhealthy normal weight (MUNW). Several review studies have suggested that MHO individuals are at a lower risk of cardiovascular disease and mortality, although MUNW individuals are at an increased risk [13,14]. Moreover, MHO has been associated with increased cancer risks in 5 types of cancer, namely, endometrial, esophageal, renal, pancreatic, and post-menopausal breast cancer [15]. Most of the studies regarding the risk factors for endometrial cancer, including obesity or MetS, have been conducted in European populations, and studies in Asian women have been limited. In this study, we aimed to investigate the associations between obesity, MetS, the combination of these two components as a metabolic obesity phenotype, and endometrial cancer in East Asian women.
MATERIALS AND METHODS
1. Study population
The National Health Information Database (NHID) of the National Health Insurance Service (NHIS) includes information on healthcare utilization, national health screening records, sociodemographic factors, and mortality of the entire Korean population. National health screening records are composed of a self-report questionnaire (lifestyle factors, family history of chronic diseases and cancer, and reproductive factors), results of anthropometric measurements, and laboratory measurements. Each participant provided consent for the transfer of results to the NHID. The NHID data were accessed after the approval of the study proposal by the National Health Insurance Sharing Service. The study protocol was approved by the Institutional Review Board of Hanyang University (approval No. HYI-18-175-1).
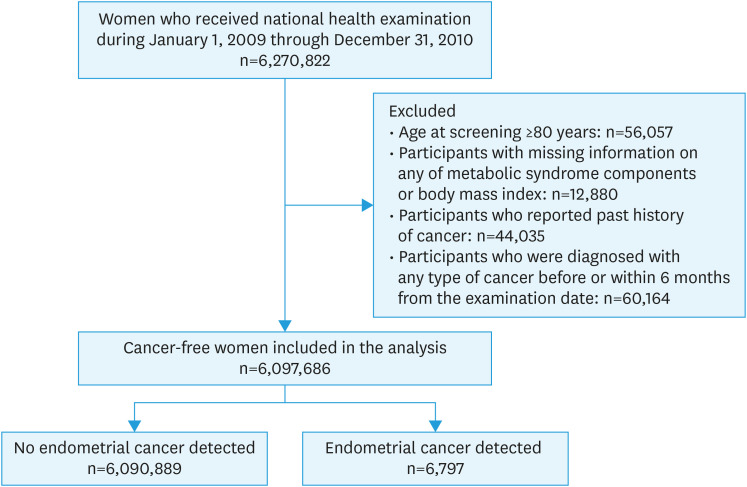
In this study, women aged ≥40 years who underwent health examinations between 2009 and 2010 were included. The biennial cycle of health examination and eligible age of health examination were taken into consideration. Of the 6,270,822 women who underwent health examinations during 2009–2010, women aged ≥80 years (n=56,057), those with missing information on any of the 5 MetS components or BMI (n=12,880), and those who reported that they had a past history of cancer (n=44,035) were excluded. To avoid the possibility of including prevalent cases, women who took healthcare services for any type of cancer and catastrophic illness code before or within 6 months from the date of the health examination (n=60,164) were excluded. In total, 6,097,686 cancer-free women were included in the study (Fig. 1).
Fig. 1. Flow diagram for selection of the eligible population.
2. Definition of baseline obesity and MetS
Obesity status was determined by BMI using anthropometric measurements; BMI <23.0 kg/m2 was defined as having a normal weight, BMI 23.0–24.9 kg/m2 was defined as overweight, and BMI ≥25.0 kg/m2 was defined as being obese according to the criteria for Asians [16,17]. The modified National Cholesterol Rationale Education Program Adult Treatment Program III (NECP-ATP III) was used to define MetS. Based on laboratory investigations and anthropometric measurements, waist circumference (WC) of ≥80 cm was defined as high WC, fasting plasma glucose levels ≥100 mg/dL were defined as elevated fasting glucose, triglyceride (TG) levels ≥150 mg/dL were defined as high TG, high-density lipoprotein (HDL) levels <50 mg/dL were defined as low HDL, and systolic blood pressure (BP) ≥130 mmHg or diastolic BP ≥85 mmHg was defined as elevated BP [18]. The presence of three or more of the above 5 components was defined as the presence of MetS. Participants were classified into six groups: metabolically healthy normal weight (MHNW, BMI <23.0 kg/m2 and no MetS), metabolically healthy overweight (MHOW, BMI 23.0–24.9 kg/m2 and no MetS), MHO (BMI ≥25.0 kg/m2 and no MetS), MUNW (BMI <23.0 kg/m2 and MetS present), metabolically unhealthy overweight (MUOW, BMI 23.0–24.9 kg/m2 and MetS present), and metabolically unhealthy obese (MUO, BMI ≥25.0 kg/m2 and MetS present).
3. Follow-up and identification of breast cancer incidence
Endometrial cancer incidence was identified through a linkage to the NHID healthcare utilization database. A combination of codes for endometrial cancer (C54.1) of the International Classification of Disease (ICD) 10th version and catastrophic illness codes that provide reduction in the coinsurance rate for patients with diseases based on relevant clinical information was applied to define the endometrial cancer incidence. Studies have suggested that the estimation of cancer incidence based on the ICD and catastrophic illness codes is reliable [19]. Participants were considered censored if they had not developed endometrial cancer by December 31, 2018, or until death. If a woman had cancer other than endometrial cancer or 2 or more types of cancer, she was censored at the first cancer. The follow-up period was defined as the period from the date of the health examination to the first date of any of these 3: December 31, 2018, date of death, or date of first cancer.
Although we excluded cancer cases identified through catastrophic illness code within 6 months from the date of screening, additional sensitivity analysis was performed after excluding endometrial cancer cases identified within the first 2 years of follow-up to minimize possible reverse causation.
4. Covariate assessment
Other variables measured through self-administered questionnaires were included as adjusted variables. Age based on the birth year; the year of health examination; and lifestyle factors such as smoking, drinking, and physical activity were measured through questionnaires of the general health examination. Reproductive histories such as age at menarche, menopausal status and age, number of children, breastfeeding history, and oral contraceptive use were evaluated through cancer screening questionnaires.
5. Statistical analysis
The association between obesity, MetS, and endometrial cancer risk was estimated using a Cox proportional hazards regression model adjusted for the variables described above, after testing the proportional hazard assumption based on Kaplan–Meier curves, and parallels of the survival distribution function. In addition, the associations of the total number of MetS components and each of the five components of MetS on endometrial cancer risk were assessed, with or without adjusting for the other components of MetS. To identify the associations between MetS, obesity, and endometrial cancer, excluding other effects, we additionally adjusted for each of them mutually. Subsequently, the associations of MetS, each component of MetS, and endometrial cancer were assessed, stratified by the obesity status (normal weight, overweight, and obesity) with or without additionally adjusted for BMI in each stratum. The hazard ratios (HRs) and 95% confidence intervals (CIs) of the MHOW, MHO, MUNW, MUOW, and MUO groups were estimated using MHNW as a reference group to investigate the combined effect of metabolic health and obesity. In addition, the combined associations of each component of MetS and obesity were analyzed using normal-weight women without each component of MetS as a reference group. All statistical analyses were performed using SAS (version 9.4; SAS Institute, Cary, NC, USA).
RESULTS
Table 1 shows the baseline characteristics of the study participants. Of the 6,097,686 women included in the analysis, 1,497,812 (24.6%) had MetS, 2,649,564 (43.5%) had normal weight, 1,507,663 (24.7%) were overweight, and 1,940,459 (31.8%) were obese. The factors included in Table 1 showed significantly different distributions between the normal, overweight, and obese groups (p<0.05). The number of incident endometrial cancer cases was 6,797, with an incidence rate per 100,000 person-years of 12.6.
Table 1. Baseline characteristics of study participants.
| Characteristics | BMI | |||
|---|---|---|---|---|
| Normal (<23 kg/m2) | Overweight (23–25 kg/m2) | Obese (≥25 kg/m2) | ||
| Age (yr) | 51.5±10.6 | 54.7±10.3 | 56.5±10.4 | |
| Age at menarche | ||||
| <15 years | 649,555 (24.5) | 311,311 (20.6) | 376,918 (19.4) | |
| 15–16 years | 1,000,585 (37.8) | 568,461 (37.7) | 709,674 (36.6) | |
| ≥17 years | 703,627 (26.6) | 491,009 (32.6) | 687,917 (35.5) | |
| Missing | 295,797 (11.2) | 136,882 (9.1) | 165,950 (8.6) | |
| Age at menopause | ||||
| Premenopause | 1,285,464 (48.5) | 584,477 (38.8) | 655,987 (33.8) | |
| <45 years | 77,549 (2.9) | 51,923 (3.4) | 81,942 (4.2) | |
| 45–52 years | 736,844 (27.8) | 515,903 (34.2) | 714,443 (36.8) | |
| ≥ 53 years | 217,939 (8.2) | 191,292 (12.7) | 287,751 (14.8) | |
| Missing | 331,768 (12.5) | 164,068 (10.9) | 200,336 (10.3) | |
| Delivery | ||||
| Nullipara | 291,726 (11.0) | 124,236 (8.2) | 139,635 (7.2) | |
| Para | 2,104,768 (79.4) | 1,272,189 (84.4) | 1,668,946 (86.0) | |
| Missing | 253,070 (9.6) | 111,238 (7.4) | 131,878 (6.8) | |
| Breast feeding | ||||
| Never | 461,092 (17.4) | 181,093 (12.0) | 175,361 (9.0) | |
| <1 year | 536,149 (20.2) | 295,555 (19.6) | 336,132 (17.3) | |
| ≥ 1 year | 1,388,895 (52.4) | 914,445 (60.7) | 1,289,924 (66.5) | |
| Missing | 263,428 (9.9) | 116,570 (7.7) | 139,042 (7.2) | |
| Oral contraceptive use | ||||
| Never | 1,969,875 (74.3) | 1,123,003 (74.5) | 1,434,555 (73.9) | |
| Ever | 423,389 (16.0) | 270,968 (18.0) | 370,700 (19.1) | |
| Missing | 256,300 (9.7) | 113,692 (7.5) | 135,204 (7.0) | |
| Family history of cancer | ||||
| No | 1,939,504 (73.2) | 1,142,825 (75.8) | 1,504,057 (77.5) | |
| Yes | 501,464 (18.9) | 280,098 (18.6) | 338,520 (17.4) | |
| Missing | 208,596 (7.9) | 84,740 (5.6) | 97,882 (5.0) | |
| Drinking frequency during the last 1 year | ||||
| No | 2,044,220 (77.2) | 1,196,744 (79.4) | 1,577,633 (81.3) | |
| 1 day/week | 365,867 (13.8) | 182,501 (12.1) | 208,724 (10.8) | |
| ≥2 day/week | 214,931 (8.1) | 113,871 (7.6) | 135,583 (7.0) | |
| Missing | 24,546 (0.9) | 14,547 (1.0) | 18,519 (1.0) | |
| Smoking | ||||
| Never | 2,501,378 (94.4) | 1,441,995 (95.6) | 1,853,413 (95.5) | |
| Ever | 134,321 (5.1) | 57,521 (3.8) | 76,654 (4.0) | |
| Missing | 13,865 (0.5) | 8,147 (0.5) | 10,392 (0.5) | |
| Vigorous physical activity | ||||
| No | 1,841,554 (69.5) | 1,034,814 (68.6) | 1,389,025 (71.6) | |
| 1–2 day/week | 435,974 (16.5) | 239,121 (15.9) | 277,335 (14.3) | |
| ≥3 day/week | 352,755 (13.3) | 223,056 (14.8) | 261,032 (13.5) | |
| Missing | 19,281 (0.7) | 10,672 (0.7) | 13,067 (0.7) | |
| Moderate physical activity | ||||
| No | 1,614,403 (60.9) | 925,499 (61.4) | 1,245,696 (64.2) | |
| 1–2 day/week | 514,755 (19.4) | 268,274 (17.8) | 318,461 (16.4) | |
| ≥3 day/week | 497,679 (18.8) | 300,644 (19.9) | 358,942 (18.5) | |
| Missing | 22,727 (0.9) | 13,246 (0.9) | 17,360 (0.9) | |
| Walking | ||||
| No | 869,356 (32.8) | 511,071 (33.9) | 707,277 (36.4) | |
| 1–3 days/week | 913,497 (34.5) | 488,657 (32.4) | 600,652 (31.0) | |
| 4–6 days/week | 543,164 (20.5) | 308,035 (20.4) | 369,369 (19.0) | |
| 7 days/week | 305,866 (11.5) | 189,568 (12.6) | 249,517 (12.9) | |
| Missing | 17,681 (0.7) | 10,332 (0.7) | 13,644 (0.7) | |
Values are presented as number (%) not otherwise specified.
BMI, body mass index.
Overweight and obesity were associated with increased endometrial cancer risk, with an HR of 1.36 (95% CI=1.28–1.45) and 1.92 (95% CI=1.82–2.04), respectively (Table 2), after adjusting for age, smoking, drinking, vigorous physical activity, moderate physical activity, walking, age at menarche, age at menopause, number of children, breast feeding, oral contraceptive use, and family history of cancer. MetS and all components of MetS at baseline significantly increased endometrial cancer risk by 1.21- to 1.49-fold. After adjusting for BMI or other components of MetS, the associations remained significant. In particular, MetS defined as 3 or more MetS components and high WC increased the endometrial cancer risk (HR=1.42; 95% CI=1.34–1.50 for MetS; HR=1.49; 95% CI=1.42–1.57 for high WC).
Table 2. Associations between obesity, MetS, and the risk of endometrial cancer.
| BMI, metabolic phenotype | No. participants | Person-years | No. cases | HR (95% CI)* | p-value | HR (95% CI)† | p-value | HR (95% CI)‡ | p-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | |||||||||||
| <23 | 2,649,564 | 23,964,689.4 | 2,253 | 1 | |||||||
| 23–25 | 1,507,663 | 13,124,206.3 | 1,650 | 1.36 (1.28–1.45) | <0.001 | ||||||
| ≥25 | 1,940,459 | 16,863,092.8 | 2,894 | 1.92 (1.82–2.04) | <0.001 | ||||||
| MetS | |||||||||||
| No | 4,599,874 | 40,029,489.5 | 4,810 | 1 | 1 | ||||||
| Yes | 1,497,812 | 13,022,499.0 | 1,987 | 1.44 (1.36–1.52) | <0.001 | 1.42 (1.34–1.50) | <0.001 | ||||
| Per 1 increment component of MetS | - | - | 1.17 (1.15–1.20) | <0.001 | 1.17 (1.15–1.19) | <0.001 | |||||
| Component of MetS | |||||||||||
| Elevated BP | |||||||||||
| No | 3,930,473 | 34,198,779.3 | 4,220 | 1 | 1 | 1 | |||||
| Yes | 2,167,213 | 18,853,209.2 | 2,577 | 1.22 (1.16–1.82) | <0.001 | 1.21 (1.15–1.27) | <0.001 | 1.13 (1.07–1.19) | <0.001 | ||
| Elevated FBG | |||||||||||
| No | 4,324,091 | 37,644,549.6 | 4,650 | 1 | 1 | 1 | |||||
| Yes | 1,773,595 | 15,407,438.8 | 2,147 | 1.19 (1.13–1.26) | <0.001 | 1.18 (1.12–1.25) | <0.001 | 1.10 (1.05–1.16) | <0.001 | ||
| High WC | |||||||||||
| No | 3,636,723 | 31,656,013.0 | 3,554 | 1 | 1 | 1 | |||||
| Yes | 2,460,963 | 21,395,975.5 | 3,243 | 1.52 (1.45–1.60) | <0.001 | 1.49 (1.42–1.57) | <0.001 | 1.43 (1.36–1.51) | <0.001 | ||
| Elevated TG | |||||||||||
| No | 4,679,785 | 40,706,563.9 | 5,042 | 1 | 1 | 1 | |||||
| Yes | 1,417,901 | 12,345,424.6 | 1,755 | 1.23 (1.17–1.30) | <0.001 | 1.22 (1.16–1.29) | <0.001 | 1.10 (1.03–1.16) | 0.002 | ||
| Reduced HDL | |||||||||||
| No | 4,257,796 | 37,025,479.4 | 4,549 | 1 | 1 | 1 | |||||
| Yes | 1,839,890 | 16,026,509.1 | 2,248 | 1.17 (1.12–1.24) | <0.001 | 1.18 (1.12–1.24) | <0.001 | 1.09 (1.04–1.15) | 0.001 | ||
BMI, body mass index; BP, blood pressure; CI, confidence interval; FBG, fasting blood glucose; HDL, high-density lipoprotein; HR, hazard ratio; MetS, metabolic syndrome; TG, triglyceride; WC, waist circumference.
*Adjusted for age, smoking, drinking, vigorous physical activity, moderate physical activity, walking, age at menarche, age at menopause, number of children, breast feeding, oral contraceptive use, and family history of cancer; †Adjusted for the variables mentioned above and BMI; ‡Adjusted for the other components of MetS.
Table 3 shows the association between MetS and endometrial cancer risk stratified by obesity status. In women who were of normal weight or overweight, MetS and all MetS components were not associated with increased endometrial cancer risk. Only reduced HDL increased the likelihood of endometrial cancer in overweight women (HR=1.12; 95% CI=1.01–1.24). However, MUO women with MetS were more likely to develop endometrial cancer (HR=1.29; 95% CI=1.20–1.39) than MHO women. Furthermore, all MetS components were significantly more likely to develop endometrial cancer (HR range: 1.13–1.26). After additionally adjusting for BMI in each stratum, the results did not change (Table S1).
Table 3. Associations between MetS and the risk of endometrial cancer according to obesity status.
| Metabolic phenotype | BMI | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal (<23 kg/m2) | Overweight (23–25 kg/m2) | Obese (≥25 kg/m2) | ||||||
| HR (95% CI)* | p-value | HR (95% CI)* | p-value | HR (95% CI)* | p-value | |||
| MetS | ||||||||
| No | 1 | 1 | 1 | |||||
| Yes | 1.02 (0.87–1.19) | 0.790 | 1.00 (0.89–1.13) | 0.998 | 1.29 (1.20–1.39) | <0.001 | ||
| Component of MetS | ||||||||
| Elevated BP | ||||||||
| No | 1 | 1 | 1 | |||||
| Yes | 0.98 (0.88–1.08) | 0.653 | 0.93 (0.84–1.03) | 0.177 | 1.26 (1.17–1.36) | <0.001 | ||
| Elevated FBG | ||||||||
| No | 1 | 1 | 1 | |||||
| Yes | 0.97 (0.87–1.07) | 0.542 | 1.10 (0.99–1.23) | 0.069 | 1.17 (1.09–1.26) | <0.001 | ||
| High WC | ||||||||
| No | 1 | 1 | 1 | |||||
| Yes | 1.15 (0.99–1.32) | 0.058 | 0.92 (0.83–1.02) | 0.113 | 1.26 (1.14–1.39) | <0.001 | ||
| Elevated TG | ||||||||
| No | 1 | 1 | 1 | |||||
| Yes | 1.00 (0.89–1.13) | 0.980 | 1.00 (0.90–1.13) | 0.942 | 1.19 (1.10–1.28) | <0.001 | ||
| Reduced HDL | ||||||||
| No | 1 | 1 | 1 | |||||
| Yes | 1.03 (0.93–1.13) | 0.599 | 1.12 (1.01–1.24) | 0.028 | 1.13 (1.05–1.22) | 0.001 | ||
BP, blood pressure; CI, confidence interval; FBG, fasting blood glucose; HDL, high-density lipoprotein; HR, hazard ratio; MetS, metabolic syndrome; TG, triglyceride; WC, waist circumference.
*Adjusted for age, smoking, drinking, vigorous physical activity, moderate physical activity, walking, age at menarche, age at menopause, number of children, breast feeding, oral contraceptive use, and family history of cancer.
The combined association of MetS and obesity with endometrial cancer risk is presented in Table 4. MUNW women did not have increased endometrial cancer risk compared to MHNW women. MHOW women were had an increased risk (HR=1.37; 95% CI=1.27–1.46) compared to MHNW women, and the HR was comparable to that of MUOW women (HR=1.38; 95% CI=1.23–1.55). The HR of MHO women was 1.75 (95% CI=1.64–1.87) and it was more prominent in MUO women (HR=2.18; 95% CI=2.03–2.34). The four components of MetS showed a similar trend: no increased risk in normal-weight women with each component of MetS, increased risk in overweight women with or without each component, increased risk in obese women without each component, and highest risk in obese women with the components. Interestingly, normal-weight women with high WC showed significantly increased endometrial cancer risk than women with normal weight and normal WC (HR=1.23; 95% CI=1.07–1.41).
Table 4. HR and 95% CI of the combined association of MetS, obesity and the risk of endometrial cancer.
| Variables | BMI (kg/m2) | No. participants | Person-years | No. cases | HR* (95% CI) | p-value | |
|---|---|---|---|---|---|---|---|
| MeS | |||||||
| No | <23 | 2,415,963 | 21,028,271.6 | 2,067 | 1 | ||
| 23–25 | 1,143,111 | 9,949,836.8 | 1,287 | 1.37 (1.27–1.46) | <0.001 | ||
| ≥25 | 1,040,800 | 9,051,381.1 | 1,456 | 1.75 (1.64–1.87) | <0.001 | ||
| Yes | <23 | 233,601 | 2,036,417.8 | 186 | 1.13 (0.97–1.32) | 0.111 | |
| 23–25 | 364,552 | 3,174,369.5 | 363 | 1.38 (1.23–1.55) | <0.001 | ||
| ≥25 | 899,659 | 7,811,711.7 | 1,438 | 2.18 (2.03–2.34) | <0.001 | ||
| Elevated BP | |||||||
| No | <23 | 1,988,840 | 17,306,485.3 | 1,725 | 1 | ||
| 23–25 | 957,264 | 8,332,062.0 | 1,108 | 1.40 (1.29–1.51) | <0.001 | ||
| ≥25 | 984,369 | 8,560,231.9 | 1,387 | 1.76 (1.64–1.89) | <0.001 | ||
| Yes | <23 | 660,724 | 5,758,204.1 | 528 | 1.05 (0.95–1.16) | 0.319 | |
| 23–25 | 550,399 | 4,792,144.3 | 542 | 1.32 (1.19–1.45) | <0.001 | ||
| ≥25 | 956,090 | 8,302,860.9 | 1,507 | 2.13 (1.98–2.29) | <0.001 | ||
| Elevated FBG | |||||||
| No | <23 | 2,073,820 | 18,054,351.6 | 1,788 | 1 | ||
| 23–25 | 1,062,974 | 9,257,278.9 | 1,150 | 1.32 (1.22–1.42) | <0.001 | ||
| ≥25 | 1,877,297 | 10,332,919.1 | 1,712 | 1.81 (1.69–1.94) | <0.001 | ||
| Yes | <23 | 575,744 | 5,010,337.8 | 465 | 1.01 (0.91–1.12) | 0.825 | |
| 23–25 | 444,689 | 3,866,927.4 | 500 | 1.45 (1.32–1.61) | <0.001 | ||
| ≥25 | 753,162 | 6,530,173.6 | 1,182 | 2.08 (1.93–2.24) | <0.001 | ||
| High WC | |||||||
| No | <23 | 2,392,369 | 20,824,185.1 | 2,024 | 1 | ||
| 23–25 | 896,336 | 7,803,106.0 | 1,051 | 1.42 (1.31–1.53) | <0.001 | ||
| ≥25 | 348,018 | 3,028,721.9 | 479 | 1.66 (1.50–1.84) | <0.001 | ||
| Yes | <23 | 257,195 | 2,240,504.3 | 229 | 1.23 (1.07–1.41) | 0.003 | |
| 23–25 | 611,327 | 5,321,100.3 | 599 | 1.32 (1.20–1.45) | <0.001 | ||
| ≥25 | 1,592,441 | 13,834,370.9 | 2,415 | 2.02 (1.90–2.15) | <0.001 | ||
| Elevated TG | |||||||
| No | <23 | 2,263,454 | 19,698,543.4 | 1,941 | 1 | ||
| 23–25 | 1,132,443 | 9,854,374.7 | 1,260 | 1.36 (1.27–1.46) | <0.001 | ||
| ≥25 | 1,283,888 | 11,153,645.8 | 1,841 | 1.82 (1.70–1.94) | <0.001 | ||
| Yes | <23 | 386,110 | 3,366,146.0 | 312 | 1.06 (0.94–1.19) | 0.361 | |
| 23–25 | 375,220 | 3,269,831.6 | 390 | 1.37 (1.26–1.53) | <0.001 | ||
| ≥25 | 656,571 | 5,709,447.0 | 1,053 | 2.14 (1.98–2.31) | <0.001 | ||
| Reduced HDL | |||||||
| No | <23 | 2,020,563 | 17,575,636.9 | 1,717 | 1 | ||
| 23–25 | 1,023,987 | 8,908,487.7 | 1,093 | 1.32 (1.22–1.42) | <0.001 | ||
| ≥25 | 1,213,246 | 10,541,354.7 | 1,739 | 1.84 (1.72–1.97) | <0.001 | ||
| Yes | <23 | 629,001 | 5,489,052.5 | 536 | 1.05 (0.96–1.16) | 0.303 | |
| 23–25 | 483,676 | 4,215,718.6 | 557 | 1.47 (1.34–1.62) | <0.001 | ||
| ≥25 | 727,213 | 6,321,738.1 | 1,155 | 2.08 (1.93–2.25) | <0.001 | ||
BP, blood pressure; CI, confidence interval; FBG, fasting blood glucose; HDL, high-density lipoprotein; HR, hazard ratio; MetS, metabolic syndrome; TG, triglyceride; WC, waist circumference.
*Adjusted for age, smoking, drinking, vigorous physical activity, moderate physical activity, walking, age at menarche, age at menopause, number of children, breast feeding, oral contraceptive use, and family history of cancer.
Sensitivity analysis excluded any identified cancers within the first 2 years of the screening date and showed comparable results, minimizing the possibility of reverse causation (data not shown).
DISCUSSION
This large, nationally representative study identified that both obesity and MetS increased the risk of endometrial cancer in women, although the association between MetS and endometrial cancer risk was especially significant in obese women. Compared to MHNW women, MHOW, MUOW, MHO, and MUO women had an increased endometrial cancer risk, whereas in MUNW women, the risk did not increase, except for high WC. The increased risk was comparable between MHNW and MUNW women and MHOW and MUOW women. However, a higher risk was found in MUO women than in MHO women. This finding suggests an increased association between MetS and endometrial cancer in obese women. For obese women, endometrial cancer risk increases not only because of obesity but also because of their metabolic health status.
Internal and external estrogen exposure plays an important role in endometrial cancer risk. The role of obesity in endometrial cancer risk has been established as per the “unopposed estrogen” theory, which describes the increase of circulating estrogen levels from sex steroid-metabolizing enzymes expressed by adipose tissue and the influence of low levels of sex hormone-binding globulin on the increased endometrial cancer risk. A positive linear association between increased BMI and serum estrogen concentration supports this theory [20,21]. Meta-analysis and intervention studies have also shown that obesity measured through both BMI, WC, waist-hip ratio, or hip circumference increases the endometrial cancer risk, whereas weight loss interventions and management may reduce the risk [22,23,24]. The results of this study concurred with those of previous studies [22,23,24], and the association of obesity measured by BMI and high WC was found to be the most prominent [5]. Based on the “unopposed estrogen” theory, it is expected that the effect of obesity would be higher in post-menopausal women, as is the case in breast cancer [20,21]. However, a case-control study showed a stronger association between MetS and endometrial cancer in premenopausal women, compared with the association in post-menopausal women [25]. In this study, we did not observe a similar association between obesity, MetS, and endometrial cancer risk according to menopausal status (data not shown). A previous meta-analysis also found a positive association between BMI and endometrial cancer risk in both pre- and post-menopausal women [22], suggesting that the influence of MetS remains constant throughout all stages of menopause.
Other suggested mechanisms include insulin resistance, abnormal fat metabolism, and chronic inflammation, which are the common mechanisms that drive the negative effects of obesity and MetS [12,23]. Comparable results before and after adjustment for BMI in this study confirmed the independent effect of MetS and its components on endometrial cancer through the mechanism of each other. Obesity, type 2 diabetes, and hypertension are referred to as the triplets of endometrial cancer based on their frequent coexistence in patients with endometrial cancer [12]. Recently updated meta-analysis results also confirmed the association of type 2 diabetes and hypertension with increased endometrial cancer risk [4,11,26,27]. Regarding dyslipidemia, despite inconsistent results among available studies [28,29,30], a meta-analysis showed significantly increased endometrial cancer risk in women with high TG levels, but no association was observed with low HDL [11]. In this study, after adjusting for BMI, all components of MetS, including even high TG and low HDL, were significantly associated with increased endometrial cancer risk. The higher influence of blood cholesterol on the bioavailability of estrogen from adipose tissue after menopause might explain these results [31].
Despite strong evidence of the association between obesity, type 2 diabetes, hypertension, and endometrial cancer, studies investigating the effect of a combination of these factors have been less studied compared with those investigating other diseases, such as cardiovascular diseases or breast cancer. A few studies have identified that type 2 diabetes or hypertension is a significant risk factor for endometrial cancer in obese women [25,31,32,33,34]. This study identified that hypertension and type 2 diabetes, as well as other components of MetS and MetS itself, increased the endometrial cancer risk, especially in obese women. Cao et al. [15] also identified that the combination of MetS and obesity, MUO, MHO, MHOW, and MUOW were all associated with increased endometrial cancer risk, although there was no association between MUNW and endometrial cancer risk. These findings are consistent with those of this study. In this study, the association between the presence of each of the five components of MetS and obesity showed a consistent pattern, although women with normal weight and high WC were also likely to have increased endometrial cancer risk, which is consistent with previous results [15,33], suggesting a possible role of body shape. The lower levels of circulating insulin in non-obese women with diabetes than in obese women could explain the greater effect of MetS in obese women [32]. These results suggest that despite the independent association, the impact of obesity might outweigh metabolic health in general. However, obese women with impaired metabolic health had increased endometrial cancer risk compared to MHO women, suggesting that obese women are more vulnerable to metabolic health issues. Despite a high percentage of body fat, MHO women showed higher insulin sensitivity and adiponectin concentration, lower inflammation biomarkers such as C-reactive protein [35], and better fitness [36]. These characteristics may reduce their endometrial cancer risk compared to MUO women. Considering that obese women have a higher endometrial cancer risk, the additional risk due to metabolic health issues should be carefully considered. In addition, a previous study showed that endometrial cancer risk in MUO women was higher (HR=4.03) than the risk of other types of cancer (HR<2) [22], suggesting a higher vulnerability of MUO women to endometrial cancer. Considering the association of insulin resistance with the more advanced stages and higher invasion at diagnosis [37], the management of MetS is important not only for the prevention of endometrial cancer, especially in obese women, but also for a better prognosis of the endometrial cancer.
There are several limitations to be considered. First, although the NECP-ATP III guideline for MetS considers pharmacologic treatment of BP, fasting glucose, TG, and HDL, we did not include the information because we did not obtain the medication data. Thus, women who took medications to control their BP, fasting glucose, TG, or HDL were classified into the normal group. Second, only baseline MetS and obesity status were considered, and changes over the follow-up period were not analyzed in this study. However, we expected non-differential changes in the MetS status and BMI to be related to baseline estimates based on the results from another longitudinal study [38] and that the effect would yield conservative results. Considering that weight loss management through bariatric surgery or medication reduces endometrial cancer risk and hyperplastic changes in the endometrium [23], further studies considering the short- and long-term changes in weight and the components of MetS need to be conducted. Third, women who did not participate in the national health examinations were excluded. The participation rate of the national health examination increased from 43% in 2002 to 75% in 2017 [39]; thus, considering the high participation rate (>70%) in the NHIS health examination and inclusion of all women who underwent a national health examination, the selection bias of this study would not be significant. Fourth, unconsidered confounders may have caused residual confounding effects.
In conclusion, obesity, each component of MetS, and MetS itself, increased the endometrial cancer risk. The associations of MetS, components of MetS, and endometrial cancer risk were more prominent in obese women. The observed associations of obesity, MetS, and endometrial cancer in South Korean women were comparable with the results in Western populations [15,33]. As a preventive measure for endometrial cancer, weight control should take on a scientifically rigorous approach. Moreover, for obese women, controlling MetS may also significantly reduce their endometrial cancer risk. Thus, efforts for lifestyle management and healthy behaviors need to be implemented to increase the likelihood of women transitioning from MUO to MHO, and in so doing, reduce the endometrial cancer risk.
Footnotes
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (grant number 2021R1A2C1011958).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
SUPPLEMENTARY MATERIAL
Associations between metabolic syndrome and the risk of endometrial cancer according to obesity status, adjusted for the BMI as a continuous variable within the obesity strata
References
- 1.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst. 2018;110:354–361. doi: 10.1093/jnci/djx214. [DOI] [PubMed] [Google Scholar]
- 2.Lim MC, Won YJ, Ko MJ, Kim M, Shim SH, Suh DH, et al. Incidence of cervical, endometrial, and ovarian cancer in Korea during 1999-2015. J Gynecol Oncol. 2019;30:e38. doi: 10.3802/jgo.2019.30.e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leslie KK, Thiel KW, Goodheart MJ, De Geest K, Jia Y, Yang S. Endometrial cancer. Obstet Gynecol Clin North Am. 2012;39:255–268. doi: 10.1016/j.ogc.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, et al. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019;145:1719–1730. doi: 10.1002/ijc.31961. [DOI] [PubMed] [Google Scholar]
- 5.Arnold M, Pandeya N, Byrnes G, Renehan PA, Stevens GA, Ezzati PM, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baik I. Forecasting obesity prevalence in Korean adults for the years 2020 and 2030 by the analysis of contributing factors. Nutr Res Pract. 2018;12:251–257. doi: 10.4162/nrp.2018.12.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baik I. Projection of diabetes prevalence in Korean adults for the year 2030 using risk factors identified from national data. Diabetes Metab J. 2019;43:90–96. doi: 10.4093/dmj.2018.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim S, Shin H, Song JH, Kwak SH, Kang SM, Won Yoon J, et al. Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998-2007. Diabetes Care. 2011;34:1323–1328. doi: 10.2337/dc10-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saltiel AR, Olefsky JM. Inflammatory mechanisms linking obesity and metabolic disease. J Clin Invest. 2017;127:1–4. doi: 10.1172/JCI92035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellastella G, Scappaticcio L, Esposito K, Giugliano D, Maiorino MI. Metabolic syndrome and cancer: “The common soil hypothesis”. Diabetes Res Clin Pract. 2018;143:389–397. doi: 10.1016/j.diabres.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 11.Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Giugliano D. Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine. 2014;45:28–36. doi: 10.1007/s12020-013-9973-3. [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Wang J. The role of metabolic syndrome in endometrial cancer: a review. Front Oncol. 2019;9:744. doi: 10.3389/fonc.2019.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stefan N, Häring HU, Hu FB, Schulze MB. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol. 2013;1:152–162. doi: 10.1016/S2213-8587(13)70062-7. [DOI] [PubMed] [Google Scholar]
- 14.Stefan N. Metabolically healthy and unhealthy normal weight and obesity. Endocrinol Metab (Seoul) 2020;35:487–493. doi: 10.3803/EnM.2020.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao Z, Zheng X, Yang H, Li S, Xu F, Yang X, et al. Association of obesity status and metabolic syndrome with site-specific cancers: a population-based cohort study. Br J Cancer. 2020;123:1336–1344. doi: 10.1038/s41416-020-1012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization, Regional Office for the Western Pacific. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 17.Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac J Clin Nutr. 2008;17:370–374. [PubMed] [Google Scholar]
- 18.American Heart Association; National Heart, Lung, and Blood Institue. Grundy SM, Cleeman JI, Daniels SR, Donato KA, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322–327. [PubMed] [Google Scholar]
- 19.Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev. 2012;13:6163–6168. doi: 10.7314/apjcp.2012.13.12.6163. [DOI] [PubMed] [Google Scholar]
- 20.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11:1531–1543. [PubMed] [Google Scholar]
- 21.Key TJ, Appleby PN, Reeves GK, Roddam A, Dorgan JF, Longcope C, et al. Body mass index, serum sex hormones, and breast cancer risk in postmenopausal women. J Natl Cancer Inst. 2003;95:1218–1226. doi: 10.1093/jnci/djg022. [DOI] [PubMed] [Google Scholar]
- 22.Aune D, Navarro Rosenblatt DA, Chan DS, Vingeliene S, Abar L, Vieira AR, et al. Anthropometric factors and endometrial cancer risk: a systematic review and dose-response meta-analysis of prospective studies. Ann Oncol. 2015;26:1635–1648. doi: 10.1093/annonc/mdv142. [DOI] [PubMed] [Google Scholar]
- 23.Mackintosh ML, Crosbie EJ. Obesity-driven endometrial cancer: is weight loss the answer? BJOG. 2013;120:791–794. doi: 10.1111/1471-0528.12106. [DOI] [PubMed] [Google Scholar]
- 24.Ward KK, Roncancio AM, Shah NR, Davis MA, Saenz CC, McHale MT, et al. Bariatric surgery decreases the risk of uterine malignancy. Gynecol Oncol. 2014;133:63–66. doi: 10.1016/j.ygyno.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Friedenreich CM, Biel RK, Lau DC, Csizmadi I, Courneya KS, Magliocco AM, et al. Case-control study of the metabolic syndrome and metabolic risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:2384–2395. doi: 10.1158/1055-9965.EPI-11-0715. [DOI] [PubMed] [Google Scholar]
- 26.Aune D, Sen A, Vatten LJ. Hypertension and the risk of endometrial cancer: a systematic review and meta-analysis of case-control and cohort studies. Sci Rep. 2017;7:44808. doi: 10.1038/srep44808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saed L, Varse F, Baradaran HR, Moradi Y, Khateri S, Friberg E, et al. The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta-analysis. BMC Cancer. 2019;19:527. doi: 10.1186/s12885-019-5748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosato V, Zucchetto A, Bosetti C, Dal Maso L, Montella M, Pelucchi C, et al. Metabolic syndrome and endometrial cancer risk. Ann Oncol. 2011;22:884–889. doi: 10.1093/annonc/mdq464. [DOI] [PubMed] [Google Scholar]
- 29.Bjørge T, Stocks T, Lukanova A, Tretli S, Selmer R, Manjer J, et al. Metabolic syndrome and endometrial carcinoma. Am J Epidemiol. 2010;171:892–902. doi: 10.1093/aje/kwq006. [DOI] [PubMed] [Google Scholar]
- 30.Arthur RS, Kabat GC, Kim MY, Wild RA, Shadyab AH, Wactawski-Wende J, et al. Metabolic syndrome and risk of endometrial cancer in postmenopausal women: a prospective study. Cancer Causes Control. 2019;30:355–363. doi: 10.1007/s10552-019-01139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parazzini F, La Vecchia C, Negri E, Riboldi GL, Surace M, Benzi G, et al. Diabetes and endometrial cancer: an Italian case-control study. Int J Cancer. 1999;81:539–542. doi: 10.1002/(sici)1097-0215(19990517)81:4<539::aid-ijc6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Shoff SM, Newcomb PA. Diabetes, body size, and risk of endometrial cancer. Am J Epidemiol. 1998;148:234–240. doi: 10.1093/oxfordjournals.aje.a009630. [DOI] [PubMed] [Google Scholar]
- 33.Moore LL, Chadid S, Singer MR, Kreger BE, Denis GV. Metabolic health reduces risk of obesity-related cancer in Framingham study adults. Cancer Epidemiol Biomarkers Prev. 2014;23:2057–2065. doi: 10.1158/1055-9965.EPI-14-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Furberg AS, Thune I. Metabolic abnormalities (hypertension, hyperglycemia and overweight), lifestyle (high energy intake and physical inactivity) and endometrial cancer risk in a Norwegian cohort. Int J Cancer. 2003;104:669–676. doi: 10.1002/ijc.10974. [DOI] [PubMed] [Google Scholar]
- 35.Aguilar-Salinas CA, García EG, Robles L, Riaño D, Ruiz-Gomez DG, García-Ulloa AC, et al. High adiponectin concentrations are associated with the metabolically healthy obese phenotype. J Clin Endocrinol Metab. 2008;93:4075–4079. doi: 10.1210/jc.2007-2724. [DOI] [PubMed] [Google Scholar]
- 36.Ortega FB, Lee DC, Katzmarzyk PT, Ruiz JR, Sui X, Church TS, et al. The intriguing metabolically healthy but obese phenotype: cardiovascular prognosis and role of fitness. Eur Heart J. 2013;34:389–397. doi: 10.1093/eurheartj/ehs174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berstein LM, Kvatchevskaya JO, Poroshina TE, Kovalenko IG, Tsyrlina EV, Zimarina TS, et al. Insulin resistance, its consequences for the clinical course of the disease, and possibilities of correction in endometrial cancer. J Cancer Res Clin Oncol. 2004;130:687–693. doi: 10.1007/s00432-004-0587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akinyemiju T, Moore JX, Pisu M, Judd SE, Goodman M, Shikany JM, et al. A prospective study of obesity, metabolic health, and cancer mortality. Obesity (Silver Spring) 2018;26:193–201. doi: 10.1002/oby.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheol Seong S, Kim YY, Khang YH, Heon Park J, Kang HJ, Lee H, et al. Data resource profile: the national health information database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Associations between metabolic syndrome and the risk of endometrial cancer according to obesity status, adjusted for the BMI as a continuous variable within the obesity strata