Abstract
Objective
Management of heavily pre-treated platinum-resistant ovarian cancer remains a therapeutic challenge. Outcomes are poor with non-platinum, single-agent chemotherapy (CT); however, molecularly targeted anticancer therapies provide new options.
Methods
This open-label, investigator-initiated, phase 2 umbrella trial (NCT03699449) enrolled patients with platinum-resistant ovarian cancer (at least 2 prior lines of CT and Eastern Cooperative Oncology Group 0/1) to receive combination therapy based on homologous recombination deficiency (HRD) and programmed death ligand 1 (PD-L1) status determined by archival tumour sample assessment. HRD-positive patients were randomised to either olaparib 200mg bid tablet + cediranib 30mg qd (arm 1) or olaparib 300mg bid tablet + durvalumab 1,500mg q4w (arm 2). HRD-negative patients were allocated to either durvalumab 1,500 mg q4w + pegylated liposomal doxorubicin (PLD) or topotecan or weekly paclitaxel (6 cycles; arm 3, those with PD-L1 expression) or durvalumab 1,500 mg q4w + tremelimumab 75mg q4w (4 doses) + PLD or topotecan or weekly paclitaxel (4 cycles; arm 4, those without PD-L1 expression). Arm 5 (durvalumab 1,500 mg q4w + tremelimumab 300mg [1 dose] + weekly paclitaxel [60 mg/m2 D1,8,15 q4w for 4 cycles] was initiated after arm 4 completed. The primary endpoint was objective response rate (ORR; Response Evaluation Criteria in Solid Tumours 1.1).
Results
Between Dec 2018 and Oct 2020, 70 patients (median 57 years; median 3 prior treatment lines [range 2–10]) were treated (n=16, 14, 5, 18, and 17, respectively). Overall ORR was 37.1% (26/70, 95% confidence interval=25.9, 49.5); 2 achieved complete response. ORR was 50%, 42.9%, 20%, 33.3%, and 29.4%, respectively. Grade 3/4 treatment-related adverse events (TRAEs) were reported in 37.5%, 35.7%, 20%, 66.7%, and 35.3% of patients, respectively. No TRAEs leading to treatment discontinuation and no grade 5 TRAEs were observed.
Conclusion
This study, the first biomarker-driven umbrella trial in platinum-resistant recurrent ovarian cancer, suggests clinical utility with biomarker-driven targeted therapy. All treatment combinations were manageable, and without unexpected toxicities.
Trial Registration
ClinicalTrials.gov Identifier: NCT03699449
Keywords: Ovarian Cancer, Platinum Resistant, Biomarker, Molecular Targeted Therapy, Immunotherapy
Synopsis
The results of this study support further investigation of durvalumab, tremelimumab, and chemotherapy in a phase 3 trial (Data S1). Furthermore, we support the investigation of the combination therapy of olaparib with an anti-angiogenic agent or immune checkpoint inhibitor.
INTRODUCTION
Ovarian cancer is the most fatal gynaecologic cancer [1]. Most patients present with advanced disease at diagnosis, and despite high responses to initial treatment, the majority will eventually relapse [2]. As platinum resistance continues to be the primary contributor to mortality in ovarian cancer patients, effective treatments for platinum-resistant or platinum-refractory ovarian cancer remain an important unmet need. Standard therapy includes non-platinum single agent chemotherapy (CT) such as pegylated liposomal doxorubicin (PLD), gemcitabine, docetaxel, and paclitaxel, and prognoses are very poor [3,4], with an approximately 10% objective response rate (ORR) and median progression-free survival (PFS) of 3 months with second- or third-line therapy.
Ovarian cancer is a highly heterogeneous disease [5,6], which impacts treatment choice. Anticancer therapy based on molecular biomarkers has enabled improvement in the treatment of ovarian cancer. Applying poly(ADP-ribose) polymerase (PARP) inhibitors for BRCA-mutated (BRCAm) or homologous recombination deficiency (HRD)-positive patients is a prime example. In 2014, the US Food and Drug Administration (FDA) approved olaparib for treatment of patients with germline BRCAm advanced ovarian cancer previously treated with 3 or more lines of CT [7]. Olaparib monotherapy provided ORR of 36% in BRCAm patients but 13% in BRCA wild type (BRCAwt) patients in platinum-resistant disease [8]. The combination of olaparib with various targeted agents has been explored to improve outcomes in the recurrent setting.
Genomic testing has yet to affect the standard treatment of BRCAwt or HRD-negative patients. Several studies have shown moderate activity of immune checkpoint inhibitors in ovarian cancer [9,10,11,12], with patients with high PD-L1 expression achieving greatest benefit [11]. Given these results, attention has turned to the potential role of combination therapies that can improve the efficacy of immune checkpoint inhibitors [13]. Chemoimmunotherapy has been highlighted as some chemotherapeutic agents such as weekly paclitaxel or liposomal doxorubicin induce immunogenic cell death and potentially improve the efficacy of immune checkpoint inhibitors [14].
As ovarian cancer is heterogeneous, it is useful to consider clinical trials using umbrella protocols that test targeted therapies in molecularly defined subsets within a single type of cancer. The present study evaluates biomarker-driven targeted therapies (olaparib; cediranib; durvalumab; tremelimumab) in patients with heavily pre-treated platinum-resistant ovarian cancer.
MATERIALS AND METHODS
1. Study design and participants
A randomised, multicentre, open-label, investigator-initiated phase 2 study was performed in patients with HRD-positive ovarian cancer; and a biomarker-driven, multiple-arm, phase 2 study was performed in HRD-negative patients (Fig. S1). Women ≥20 years of age with Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and histologically confirmed high-grade serous or endometrioid ovarian, primary peritoneal, or fallopian tube cancers were enrolled from 3 cancer centres in Korea through the Korean Gynecologic Oncology Group (KGOG). Patients had received at least 2 prior lines of anticancer therapy and experienced disease progression within 6 months of completing platinum-based CT or had primary platinum-refractory disease. Further details of inclusion/exclusion criteria, randomisation, and study procedure have been published [15], and appear in the Data S2.
This trial was approved by the Institutional Review Board of Yonsei University (approval No. 4-2018-0749). Informed consent was acquired from the participants and the study was conducted in accordance with the principles of the Declaration of Helsinki. The full protocol is available in the Fig. S2.
2. Treatment and randomisation
HRD-positive patients were randomised 1:1 in an open-label manner to either olaparib 200 mg tablet orally (po) twice daily (bid) + cediranib 30 mg po once daily (qd) until disease progression (arm 1) or olaparib 300 mg tablet po bid until disease progression + durvalumab 1,500 mg intravenously (iv) every 4 weeks starting on week 5, day 1 up to 12 months (arm 2). Block randomisation with block size 4 was applied after confirmation of patient eligibility and registration with the KGOG data centre by telephone, fax, or a web-based system.
HRD-negative patients were allocated to a treatment arm based on PD-L1 expression. PD-L1 positivity was defined as PD-L1 expression of 25% or more of the tumour cells as determined by a Ventana SP263 assay [16,17]. Slides were reviewed by a gynaecologic pathologist (EP) blinded to the patient’s characteristics and outcomes. Arm 3 comprised durvalumab 1,500 mg iv every 4 weeks for up to 24 months + PLD or topotecan or weekly paclitaxel every 4 weeks up to 6 cycles in patients with PD-L1 expression (defined as tumour proportion score [TPS] 25% or more). Arm 4 comprised durvalumab 1,500 mg iv every 4 weeks for up to 24 months + tremelimumab 75 mg iv every 4 weeks for up to 4 doses + PLD or topotecan or weekly paclitaxel every 4 weeks up to 4 cycles in patients with low PD-L1 expression. Arm 5, initiated after completion in arm 4, comprised durvalumab 1,500 mg iv every 4 weeks for up to 24 months + tremelimumab 300 mg iv for one dose + weekly paclitaxel 60 mg/m2 iv on days (D) 1, 8, and 15 every 4 weeks for 4 cycles) in patients with low PD-L1 expression. CT regimens were one of the following: weekly paclitaxel 80 mg/m2 (D1, 8, 15, and 22 every 4 weeks), liposomal doxorubicin (40 mg/m2 on D1 every 4 weeks), and topotecan (4 mg/m2 on D1, 8, and 15 every 4 weeks).
3. Homologous recombination repair (HRR) assessment
During screening archival tumour samples were tested for HRR status and PD-L1 status. The HRR gene panel was based on a laboratory-based panel (cancerSCAN version 3.1) including HRR genes [18]. Mutations in 15 HRR genes (BRCA1, BRCA2, ATM, BRIP1, PALB2, RAD51C, BARD1, CDK12, CHEK1, CHEK2, FANCL, PPP2R2A, RAD51B, RAD51D, and RAD54L) were selected based on a biological role in HRR and sensitivity to PARP inhibitors. See more information in the Data S2.
4. Outcomes
Tumour assessment was through computed tomography or magnetic resonance imaging of the chest, abdomen, and pelvis every 12 weeks after initial treatment. Assessments could be done up to 7 days before or after the designated timepoint, and were done by the investigator using Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1.
Safety and adverse events were assessed on day of each cycle and were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. Laboratory assessments and CA-125 was tested on day 1 of each cycle. Patients were followed up for at least 2 years after treatment was completed.
The primary endpoint was ORR according to RECIST version 1.1, which included the best overall response of patients with measurable disease who had a complete or partial response. Secondary endpoints were PFS, overall survival (OS), duration of response, time to response, disease control rate, and safety. PFS was defined as the time from the start of treatment to disease progression or death for any cause (whichever occurred first) or last PFS assessment for patients alive without progression. OS was defined as the time from first treatment until death from any cause. Duration of response was assessed in patients who achieved a response and was defined as the time from the date of the first documented response until the date of documented progression or death from any cause.
5. Statistical analysis
All analyses were based on a modified intent-to-treat (mITT) approach. We analysed efficacy and safety in patients who received at least one treatment dose. No interim analyses were planned.
An exact single-stage phase 2 design was used to calculate sample size. For each arm, an ORR of 5% from conventional monotherapy was assumed (i.e., H0 was 5% of ORR with conventional monotherapy) [8,19,20].Further details of sample size justification have been previously published [15], and appear in the Data S2.
The proportions of patients achieving responses, and 95% confidence intervals [CIs] were assessed using the Clopper-Pearson exact method. Median duration of response and PFS and associated 95% CIs were calculated with the Kaplan-Meier method. A log-rank test compared the PFS between treatment arms. A cut-off date of July 12, 2021 was applied. Statistical analyses were performed in SAS® (version 9.4; SAS Institute, Cary, NC, USA).
An independent data monitoring committee oversaw the study. This study is registered with ClinicalTrials.gov, NCT03699449. The key raw data have been deposited at Yonsei College of Medicine for future reference.
6. Role of funding source
Yonsei College of Medicine and AstraZeneca funded this study. AstraZeneca has no role in study design, data collection, data interpretation, writing of the paper, or the decision to submit for publication, but representatives from the company were given the opportunity to review the manuscript before submission.
All authors participated in critical review and revision of the manuscript and approved the final version for submission. All authors had full access to all of the data and had final responsibility for the decision to submit for publication.
Medical writing support was provided by Helene Wellington, AMICULUM, funded by the corresponding author.
RESULTS
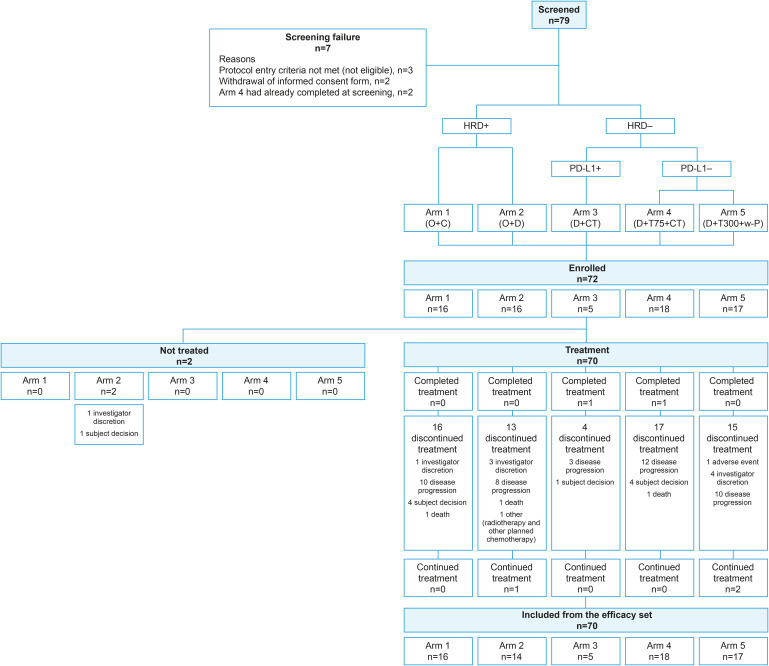
Between Dec 2018 and Oct 2020, 72 patients were enrolled. Two patients were excluded from the mITT analysis because they did not receive study medication based on ineligibility identified after enrolment (n=1 bowel obstruction; n=1 rapid progression). Analyses of efficacy and safety were completed in 70 patients who received at least one dose of study medication. The number of patients in each treatment arm was as follows: 16 patients in arm 1 (olaparib + cediranib), 14 patients in arm 2 (olaparib + durvalumab), 5 patients in arm 3 (durvalumab + CT), 18 patients in arm 4 (durvalumab + tremelimumab 75 mg+ CT), and 17 patients in arm 5 (durvalumab + tremelimumab 300 mg + CT) (Fig. 1). Our previous study shows that PD-L1-positive patient groups defined by on TPS ≥25 with sp263 assay were 10.6% [17], but we found a lower than expected number of patients who were PD-L1-positive during screening. Therefore, due to the difficulty of enrolling patients to arm 3, the data safety monitoring board recommended early closure of arm 3 without completion of preplanned patient recruitment (Nov 11, 2021).
Fig. 1. Study overview and trial profile. Arm 1, O+C (O 200 mg bid + C 30 mg qd); arm 2, O+D (O 300 mg bid + D 1,500 mg q4w); arm 3, D+CT (D 1,500 mg q4w + PLD or topotecan or weekly paclitaxel [6 cycles]) in patients with high PD-L1 expression; arm 4, D+T75+CT (D 1,500 mg q4w + T 75 mg q4w [4 doses] + PLD or topotecan or weekly paclitaxel [4 cycles]) ; arm 5, D+T300+CT (D 1,500 mg q4w + T 300 mg [1 dose] + weekly paclitaxel [60 mg/m2 days 1, 8, and 15 q4w for 4 cycles]).
bid, twice daily; C, cediranib; CT, chemotherapy; D, durvalumab; HRD, homologous recombination deficiency; O, olaparib; PD-L1, programmed death ligand 1; PLD, pegylated liposomal doxorubicin; q4w, every 4 weeks; qd, once daily; T, tremelimumab; T300, tremelimumab 300 mg (1 dose); w-P, weekly paclitaxel.
The median duration of follow-up at the time of data analysis (data cut-off July 12, 2021) was 8.3 months (interquartile range [IQR]=4.6–17.3). At data cut-off, 65 of 70 patients had discontinued the study, 2 had completed treatment cycles, and 3 remained on treatment. The most common primary reason for treatment discontinuation was progressive disease (n=43; 61.4%). Other reasons included adverse events, investigator discretion, withdrawal of consent, death, and receiving other anticancer therapy. The median treatment duration was 4.7 months (IQR=2.8–8.3) and the median number of cycles was 4.5 (IQR=3.0–8.0).
Patient demographics and baseline characteristics were generally balanced across groups (Table 1). Of 70 patients included in the mITT analysis, 31 (44.3%) had received more than 3 prior lines of treatment. Most had high-grade serous carcinoma (HGSC) (90%) and more than half of the patients (54.3%) had progressed on bevacizumab.
Table 1. Baseline patient characteristics.
| Variables | Arm 1 (n=16) | Arm 2 (n=14) | Arm 3 (n=5) | Arm 4 (n=18) | Arm 5 (n=17) | Total (n=70) | |
|---|---|---|---|---|---|---|---|
| Age (yr) | |||||||
| Median | 58.00 | 52.50 | 54.00 | 58.00 | 57.00 | 57.00 | |
| Minimum | 47.00 | 45.00 | 47.00 | 34.00 | 46.00 | 34.00 | |
| Maximum | 76.00 | 72.00 | 58.00 | 70.00 | 77.00 | 77.00 | |
| Histology subtype | |||||||
| HGSC | 14 (87.50) | 13 (92.86) | 5 (100.00) | 16 (88.89) | 15 (88.24) | 63 (90.00) | |
| Endometrioid | 2 (12.50) | 1 (7.14) | 0 (0.00) | 2 (11.11) | 2 (11.76) | 7 (10.00) | |
| Prior treatment for bevacizumab | |||||||
| Yes | 6 (37.50) | 8 (57.14) | 3 (60.00) | 8 (44.44) | 13 (76.47) | 38 (54.29) | |
| No | 10 (62.50) | 6 (42.86) | 2 (40.00) | 10 (55.56) | 4 (23.53) | 32 (45.71) | |
| CA-125 (U/mL) | |||||||
| Mean±SD | 578.90±720.07 | 1,297.31±1,694.89 | 2,691.34±5,381.88 | 1,220.78±1,941.93 | 1,980.34±5,022.95 | 1,378.88±3,079.42 | |
| Minimum | 37.60 | 10.00 | 179.10 | 14.00 | 29.10 | 10.00 | |
| Maximum | 2,772.60 | 4,923.00 | 12,317.90 | 7,657.10 | 20,773.00 | 20,773.00 | |
| ECOG | |||||||
| 0 | 2 (12.50) | 2 (14.29) | 2 (40.00) | 2 (11.11) | 3 (17.65) | 11 (15.71) | |
| 1 | 14 (87.50) | 12 (85.71) | 3 (60.00) | 16 (88.89) | 14 (82.35) | 59 (84.29) | |
| Prior lines of therapy | |||||||
| 2–3 | 10 (62.50) | 6 (42.86) | 4 (80.00) | 9 (50.00) | 10 (58.82) | 39 (55.71) | |
| 4–5 | 3 (18.75) | 5 (35.71) | 1 (20.00) | 7 (38.89) | 5 (29.41) | 21 (30.00) | |
| ≥6 | 3 (18.75) | 3 (21.43) | 0 (0.00) | 2 (11.11) | 2 (11.76) | 10 (14.29) | |
| Regimen of last chemotherapy | |||||||
| Platinum-based | 8 (50.00) | 6 (42.86) | 3 (60.00) | 9 (50.00) | 8 (47.06) | 34 (48.57) | |
| Non-platinum based | 8 (50.00) | 8 (57.14) | 2 (40.00) | 9 (50.00) | 9 (52.94) | 36 (51.43) | |
| Interval between last chemotherapy and date of enrolment | |||||||
| <1 mo | 2 (12.50) | 2 (14.29) | 1 (20.00) | 1 (5.56) | 2 (11.76) | 8 (11.43) | |
| ≥1 and <3 mo | 7 (43.75) | 7 (50.00) | 2 (40.00) | 6 (33.33) | 12 (70.59) | 34 (48.57) | |
| ≥3 mo | 7 (43.75) | 5 (35.71) | 2 (40.00) | 11 (61.11) | 3 (17.65) | 28 (40.00) | |
Data are shown as mean±SD or number (%).
The denominator of percentages is the number of patients in each treatment group.
The interval between last chemotherapy and disease progression was defined as the time from the stop date of last chemotherapy to the date of enrolment.
ECOG, Eastern Cooperative Oncology Group; HGSC, high-grade serous carcinoma.
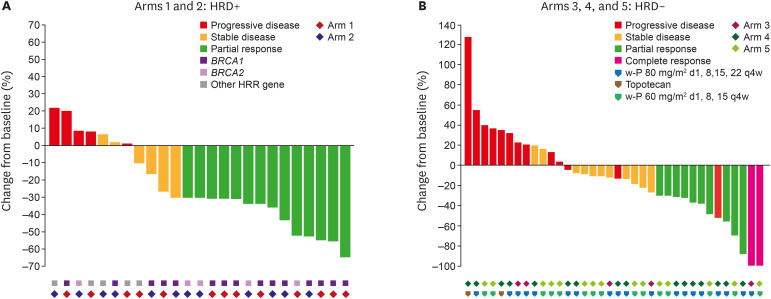
Overall, the confirmed ORR was 37.1% (26/70, 95% CI=25.9, 49.5); complete response was observed in 2 patients (Table 2). The ORRs (95% CI) for each treatment arm were 50% (24.7–75.4), 42.9% (17.7–71.1), 20% (0.5–71.6), 33.3% (13.3–59.0), and 29.4% (10.3–56.0) for arms 1–5, respectively. Median time to response, median duration of response, time to response, and disease control rate are shown in Table 2. Of the 70 patients treated, 61 were evaluable for objective response by RECIST based on imaging availability. Target lesions were reduced in 18 (72%) of 25 from HRD-positive patients and 24 (66.7%) of 36 from HRD-negative patients with at least one post-baseline scan (Fig. 2A). The mean best percentage change of the target lesion size from the baseline was −16.92% (SD=38.22). The durability and timing of objective response are shown in Fig. 2B and Fig. S3.
Table 2. Efficacy endpoints.
| Endpoints | Arm 1 (n=16) | Arm 2 (n=14) | Arm 3 (n=5) | Arm 4 (n=18) | Arm 5 (n=17) | Total (n=70) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (%) | 95% CI | No (%) | 95% CI | No (%) | 95% CI | No (%) | 95% CI | No (%) | 95% CI | No (%) | 95% CI | ||
| Objective response rate* | 8 (50.00) | 24.65, 75.35 | 6 (42.86) | 17.66, 71.14 | 1 (20.00) | 0.51, 71.64 | 6 (33.33) | 13.34, 59.01 | 5 (29.41) | 10.31, 55.96 | 26 (37.14) | 25.89, 49.52 | |
| Best overall response | |||||||||||||
| CR | 0 (0.00) | 0 (0.00) | 1 (20.00) | 0 (0.00) | 1 (5.88) | 2 (2.86) | |||||||
| PR | 8 (50.00) | 6 (42.86) | 0 (0.00) | 6 (33.33) | 4 (23.53) | 24 (34.29) | |||||||
| Stable disease | 2 (12.50) | 4 (28.57) | 2 (40.00) | 3 (16.67) | 6 (35.29) | 17 (24.29) | |||||||
| PD | 3 (18.75) | 2 (14.29) | 2 (40.00) | 7 (38.89) | 4 (23.53) | 18 (25.71) | |||||||
| NE | 3 (18.75) | 2 (14.29) | 0 (0.00) | 2 (11.11) | 2 (11.76) | 9 (12.86) | |||||||
| Disease control rate* | 10 (62.50) | 35.43, 84.80 | 10 (71.43) | 41.90, 91.61 | 3 (60.00) | 14.66, 94.73 | 9 (50.00) | 26.02, 73.98 | 11 (64.71) | 38.33, 85.79 | 43 (61.43) | 49.03, 72.83 | |
| Disease control rate at 24 wk* | 9 (56.25) | 29.88, 80.25 | 9 (64.29) | 35.14, 87.24 | 2 (40.00) | 5.27, 85.34 | 7 (38.89) | 17.30, 64.25 | 10 (58.82) | 32.92, 81.56 | 37 (52.86) | 40.55, 64.91 | |
| Median time to response† (wk) | 11.39 | 11.10, 12.53 | 17.23 | 11.25, 35.59 | 12.81 | 11.10 | 11.10, 23.92 | 11.25 | 11.10, 23.06 | 11.32 | 11.10, 12.53 | ||
| Median duration of response† (wk) | 24.06 | 3.27, 59.08 | 24.49 | 6.69, 36.45 | 23.21 | 3.70, ∞ | 24.20 | 24.20, ∞ | 24.20 | 12.67, 36.45 | |||
| Median PFS† (mo) | 5.62 | 2.60, 10.48 | 5.36 | 2.63, 13.83 | 3.68 | 1.61, ∞ | 3.98 | 2.60, 9.49 | 5.13 | 2.60, 8.12 | 4.76 | 3.38, 8.08 | |
| 6-month PFS rate‡ (%) | 43.27 | 17.98, 66.43 | 47.62 | 20.26, 70.83 | 40.00 | 5.20, 75.28 | 31.67 | 11.60, 54.14 | 45.75 | 21.47, 67.21 | 41.10 | 28.93, 52.86 | |
| Median OS† (mo) | 18.53 | 4.17, ∞ | 15.51 | 7.33, 15.51 | 19.65 | 3.55, ∞ | 15.51 | 12.29, ∞ | |||||
| 6-month OS rate‡ (%) | 77.92 | 45.90, 92.32 | 92.86 | 59.08, 98.96 | 75.00 | 12.79, 96.05 | 83.92 | 49.40, 95.73 | 81.93 | 53.77, 93.80 | 83.31 | 71.09, 90.69 | |
| Median duration of follow-up (mo) | 9.81 | 7.95, 17.62 | 7.41 | 6.76, 16.93 | 4.63 | −2.64, 27.11 | 7.87 | 7.46, 18.82 | 8.08 | 6.14, 10.43 | 8.31 | 9.41, 13.70 | |
The denominator of percentages is the number of patients in each treatment group.
CI, confidence interval; CR, complete response; NE, not evaluable; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response.
*95% CI using Clopper-Pearson exact method; †95% CI using the method known as the log cumulative hazard transformation method; ‡95% CI using the Kaplan-Meier method.
Fig. 2. Tumour responses. (A) Waterfall plot of the maximum percentage change in target lesion size in treated patients who had at least one post-baseline scan. Each bar presents a patient. The colour indicates type of response. (B) Swimmer’s plot for patients with a confirmed response. Each bar presents a patient. From 61 patients who had at least one post-baseline efficacy assessment.
d, day; HRD, homologous recombination deficiency; HRR, homologous recombination repair; q4w, every 4 weeks; w-P, weekly paclitaxel.
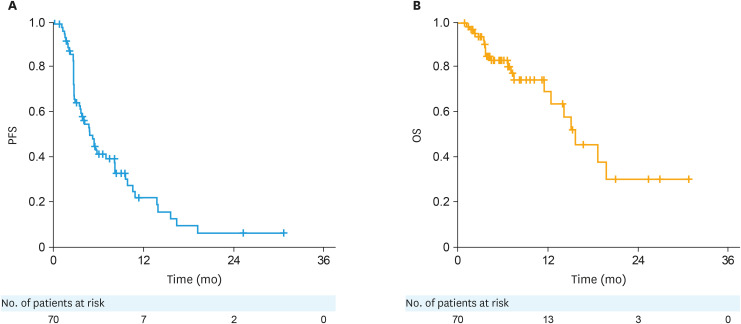
At the data cut-off, 51 PFS events had occurred. Median PFS and the estimated 6-month PFS rate are shown in Table 2 and Fig. 3. Median PFS was 4.76 months (95% CI=3.38, 8.08). 20 deaths had accrued at data cut-off. Median OS and the estimated 6-month OS rate are shown in Table 2 and Fig. 3. No significant differences among the treatment arms were observed in PFS and OS (Fig. S4), and comparison between arms was not intended. The median PFS was 5.62, 5.36, 3.68, 3.98, and 5.13 months in arms 1–5, respectively.
Fig. 3. Kaplan-Meier plots of PFS (A) and OS (B) in patients with modified intent-to-treat population.
OS, overall survival; PFS, progression-free survival.
The safety analysis included 70 patients. Adverse events are summarised in Table S1. Among all participants, treatment-related adverse events (TRAEs) occurred in 61 (87.1%) patients. The incidence of adverse events varied between the treatment arms. Grade 3/4 TRAEs were reported in 37.5%, 35.7%, 20%, 66.7%, and 47.1% of patients in arms 1–5, respectively. No TRAEs leading to discontinuation of treatment and no grade 5 TRAEs were observed. Among all participants, grade 3 or worse TRAEs were reported in 32 (45.7%) patients, the most common being neutropenia (19 [27.1%] patients), anaemia (10 [14.3%] patients), thrombocytopenia (3 [4.3%] patients), and febrile neutropenia (3 [4.3%] patients) (Table 3). The incidence of TRAEs are described according to each arm and immune-related adverse events (irAEs) are described in Tables S2 and S3. Additionally, the use of dose reduction and investigational product delay is summarised in Tables S2 and S3. The median cediranib dose intensity (delivered divided by planned dose based on the dose at cycle 1 day 1) was 50% in the arm 1. The median olaparib dose intensity was 75% in arm 1 and 66.7% in arm 2, respectively. The median paclitaxel dose intensity was 62.5% in arm 3, 50% in arm 4, and 75% in arm 5, respectively.
Table 3. TRAEs* (≥10% of patients).
| Adverse events | Arm 1 (n=16) | Arm 2 (n=14) | Arm 3 (n=5) | Arm 4 (n=18) | Arm 5 (n=17) | Total (n=70) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | |
| Neutrophil count decreased | 8 (50.00) | 3 (18.75) | 5 (35.71) | 3 (21.43) | 1 (20.00) | 1 (20.00) | 13 (72.22) | 9 (50.00) | 3 (17.65) | 3 (17.65) | 30 (42.86) | 19 (27.14) |
| Anaemia | 4 (25.00) | 3 (18.75) | 4 (28.57) | 3 (21.43) | 2 (40.00) | 0 (0.00) | 13 (72.22) | 3 (16.67) | 5 (29.41) | 1 (5.88) | 28 (40.00) | 10 (14.29) |
| Diarrhoea | 6 (37.50) | 0 (0.00) | 3 (21.43) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 6 (33.33) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 16 (22.86) | 1 (1.43) |
| Nausea | 3 (18.75) | 0 (0.00) | 4 (28.57) | 0 (0.00) | 1 (20.00) | 0 (0.00) | 3 (16.67) | 0 (0.00) | 4 (23.53) | 0 (0.00) | 15 (21.43) | 0 (0.00) |
| Platelet count decreased | 3 (18.75) | 1 (6.25) | 2 (14.29) | 1 (7.14) | 0 (0.00) | 0 (0.00) | 6 (33.33) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 12 (17.14) | 3 (4.29) |
| Rash | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (20.00) | 0 (0.00) | 6 (33.33) | 0 (0.00) | 3 (17.65) | 0 (0.00) | 11 (15.71) | 0 (0.00) |
| Hypothyroidism | 3 (18.75) | 0 (0.00) | 2 (14.29) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 7 (10.00) | 0 (0.00) |
| Alanine aminotransferase increased | 0 (0.00) | 1 (6.25) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (11.11) | 0 (0.00) | 2 (11.76) | 0 (0.00) | 5 (7.14) | 2 (2.86) |
| Vomiting | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (16.67) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 5 (7.14) | 0 (0.00) |
| Asthenia | 2 (12.50) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (11.76) | 0 (0.00) | 5 (7.14) | 0 (0.00) |
| Aspartate aminotransferase increased | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (11.11) | 2 (11.11) | 2 (11.76) | 0 (0.00) | 4 (5.71) | 2 (2.86) |
| Lipase increased | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (16.67) | 2 (11.11) | 0 (0.00) | 0 (0.00) | 4 (5.71) | 2 (2.86) |
| Pyrexia | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (11.11) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 4 (5.71) | 0 (0.00) |
| Amylase increased | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (16.67) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (4.29) | 1 (1.43) |
| Abdominal discomfort | 3 (18.75) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (4.29) | 0 (0.00) |
| Colitis | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (16.67) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (4.29) | 0 (0.00) |
| Stomatitis | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (20.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (4.29) | 0 (0.00) |
| Febrile neutropenia | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (16.67) | 3 (16.67) | 0 (0.00) | 0 (0.00) | 3 (4.29) | 3 (4.29) |
| Urticaria | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (20.00) | 0 (0.00) | 2 (11.11) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (4.29) | 0 (0.00) |
| Decreased appetite | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (11.11) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (4.29) | 0 (0.00) |
| Hypertension | 3 (18.75) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 3 (4.29) | 0 (0.00) |
| Blood creatinine increased | 2 (12.50) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (2.86) | 0 (0.00) |
| Abdominal pain | 2 (12.50) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (2.86) | 0 (0.00) |
| Dyspepsia | 2 (12.50) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (2.86) | 0 (0.00) |
| Myalgia | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (20.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (2.86) | 0 (0.00) |
| Alopecia | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (20.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.43) | 0 (0.00) |
| Peripheral sensory neuropathy | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (20.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 1 (1.43) | 0 (0.00) |
Values are presented as number (%).
TRAE, treatment-related adverse event.
*TRAE: all adverse events whose causality is ‘Related’.
PD-L1 immunohistochemistry was completed for the 70 enrolled patients. Of 54 patients who were treated with durvalumab combination, 20 patients had PD-L1 TPS 0%. Survival outcomes were analysed according to PD-L1 expression and the presence of irAE in patients who were treated with durvalumab combination (except arm 1) (Figs. S5 and S6). In a post-hoc exploratory analysis of toxicity and efficacy, we found that patients with irAE had better OS than those without irAE (p=0.05) (Fig. S5).
DISCUSSION
To our knowledge, this is the first biomarker-driven umbrella study conducted in patients with platinum-resistant recurrent ovarian cancer. We observed that 37.1% of patients with platinum-resistant ovarian cancer who were treated with biomarker-driven targeted therapy achieved an objective response. This is comparable with the AURELIA trial showing 30% of ORR in a platinum-resistant setting.3 However, unlike AURELIA, we enrolled a heavily pretreated population with platinum-resistant disease (with no restriction on the number of previous anticancer regimens) and included platinum-refractory cases. More than half of patients in our study had previously received bevacizumab, representing a setting that could be described as a post-AURELIA population.
More specifically, the combination of olaparib with cediranib or durvalumab showed promising efficacy and a manageable toxicity profile to treat HRD-positive platinum-resistant recurrent ovarian cancer. The addition of an immune checkpoint inhibitor to current standard CT showed promising efficacy and a manageable toxicity profile to treat HRD-negative platinum-resistant recurrent ovarian cancer regardless of PD-L1 status.
Recently, the consensus is that ovarian cancer is a highly heterogeneous disease, and this may have implications for ovarian cancer treatment choice. The Cancer Genome Atlas Program suggests 50% of HGSC with homologous recombination defects may benefit from treatment with a PARP inhibitor [5]. A previous study from our group showed genomic profiling of residual tumours from 102 ovarian cancer patients with HGSC and most patients had alterations in at least one targetable pathway [16].
Advancements in genomics has led efforts toward precision medicine targeting cancers on the basis of genetic mutations. Recently, the FDA approved olaparib for first-line maintenance treatment of BRCAm ovarian cancer patients and olaparib plus bevacizumab for first-line maintenance treatment of HRD-positive ovarian cancer patients. However, there is no strong biomarker for immune checkpoint inhibitors in ovarian cancer. KEYNOTE-100 suggested that patients with higher PD-L1 expression (combined positive score ≥10) had increased ORR with pembrolizumab [11]. We hypothesised that PD-L1 inhibitors have a greater likelihood of benefit when high PD-L1 is highly expressed.
Two important biomarkers for ovarian cancer are HRD and PD-L1 status to identify candidates for PARP inhibitor and immune checkpoint inhibitors. Based on the aforementioned biomarker-driven treatment strategy, we designed a molecular screening study for an umbrella trial in patients with platinum-resistant ovarian cancer and allocated patients to treatment arms based on predefined algorithms. For this study, we performed targeted sequencing and immunohistochemistry and assessed HRR gene mutation and PD-L1 status during the screening period.
Olaparib-based combination therapy has a role in patients with HRR gene mutations, in particular BRCAm even in the platinum-resistant setting. Previously, olaparib monotherapy showed an ORR of 30% in platinum-resistant patients with germline BRCA1 and/or BRCA2 mutation (gBRCAm) who had received 3 or more lines of prior therapy [21]. In our study, the combination strategy of olaparib plus cediranib from arm 1 and olaparib plus durvalumab from arm 2 provided ORRs of 50% and 42.9%. These promising results have several implications.
First, it raises questions about whether the combination provides additive or synergistic effects and which combination is best for olaparib in the platinum-resistant setting. According to our study, an anti-angiogenic agent and/or immune checkpoint inhibitor may be synergistic in HRD-positive platinum-resistant ovarian cancer compared with findings from prior studies [21]. The strategy is also being evaluated in the randomised phase 3 trial (NRG-GY005; NCT02502266) comparing olaparib plus cediranib versus cediranib versus standard-of-care CT. Second, it is questionable whether this finding could be applicable in patients with prior PARP inhibitor treatment. While our study was enrolling patients, the standard of care for patients with ovarian cancer changed. With the recent broader FDA approval, more patients have the opportunity to use a PARP inhibitor as maintenance therapy in the front-line setting. In particular, most BRCAm patients are now exposed to PARP inhibitors before platinum resistance occurs. Only a small number of BRCAm patients may not have received a PARP inhibitor prior to the onset of platinum-resistant disease in the future. Lastly, the relatively high proportion of patients requiring dose reduction of cediranib in arm 1 suggests that the regimen could be further optimised.
The results from arms 3, 4, and 5 suggest that the activity of chemo-immunotherapy may be more active than either agent alone, compared with historical trials in platinum-resistant epithelial ovarian cancer. Single-agent PD-1/L1 inhibition had limited activity in platinum-resistant recurrent ovarian cancer [10,11,12,22]. Combination strategies have been suggested to enhance antitumour immune response in ovarian cancer.
First, a combination of PD-1/L1 inhibitor with CT was investigated. Small single-arm phase 2 studies showed preliminary evidence of clinical benefit from PLD and pembrolizumab in a recurrent setting [13]. However, the JAVELIN Ovarian-200 trial failed to show a survival benefit with the addition of avelumab to PLD compared with PLD alone [19]. Although previous studies used PLD as a backbone for combination with an immune checkpoint inhibitor, recent data showed that doxorubicin decreased expression of PD-L1 in cancer cells [23]. Weekly paclitaxel is also suggested as a backbone for an immune checkpoint inhibitor in other ongoing studies as well as in ours. Notably, there were fewer TRAEs of severity grade 3 or higher in arm 5 than in arm 4, which suggests low-dose and -density weekly paclitaxel may be optimal in chemo-immunotherapy.
Second, a signal of efficacy was shown using combination therapy of anti-PD-1 and anti-cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) agents in platinum-resistant ovarian cancer patients [24]. For biomarker-negative patients (HRD negative and PD-L1 negative), we hypothesised that combination therapy with CT, a PD-L1 inhibitor, and a CTLA-4 inhibitor is synergistic even in patients with low PD-L1 expression. To date, there are no clinical data regarding triplet therapy for the treatment of ovarian cancer. This is the first report showing outcomes of durvalumab, tremelimumab, and CT in ovarian cancer. Some studies (NCT03899610, NCT03249142, ACTRN12618000109202) are ongoing to evaluate the efficacy of durvalumab, tremelimumab, paclitaxel, and carboplatin in front-line ovarian cancer treatment [25]. Acknowledging the limitations of cross-arm comparisons, the results of arm 5 (durvalumab + tremelimumab 300 mg + CT) suggest that a single, priming dose of tremelimumab combined with durvalumab may provide a similar ORR and higher PFS when compared with other durvalumab combinations in this study. This finding is consistent with a previous study, which showed that a single, priming dose of tremelimumab may be sufficient to activate the tumour-fighting potential of the immune system [26].
Toxicities attributable to immune checkpoint inhibitors have been reported as predictive biomarkers for treatment efficacy. In a post-hoc exploratory analysis of toxicity and efficacy, we found that patients with irAEs had significantly longer PFS than patients without these events. Our results are in line with previous evidence that treatment-induced toxicities can be independent predictive biomarkers for the efficacy of immune checkpoint inhibitors in other tumours [27].
Our study had several limitations. First, the study had no control group for comparison and selection bias could not be excluded because of the non-randomised. Second, there was a relatively small sample size in each cohort, which affected the statistical power and precluded the ability to distinguish between treatment arms. Lastly, overall ORR is encouraging, and is what would be expected for true synergy for triplet therapy for arms 4 and 5. At present, arm 6 is added to identify the role of tremelimumab in ovarian cancer after completion of arm 5 recruitment (Fig. S2).
Findings from this trial suggest that biomarker-driven targeted therapy may have greater efficacy than other studies published recently in the platinum-resistant setting [19,20]. In conclusion, AMBITION suggests a new concept for platinum-resistant ovarian cancer, demonstrating the clinical utility of molecular subtyping-based targeted therapy. All combinations are worthy of further exploration and phase 3 trials are warranted in the treatment of platinum-resistant ovarian cancer.
ACKNOWLEDGEMENTS
This study was funded by the Yonsei College of Medicine Research Fund for Clinical Excellence (SHRC). This research was an investigator-initiated trial funded by AstraZeneca.
We thank D2S for data management and statistical analysis. We thank the patients and their families and caregivers for participating in this study and all site personnel.
Medical writing support was provided by Helene Wellington, AMICULUM, funded by the corresponding author.
Footnotes
Funding: AstraZeneca, Yonsei College of Medicine Research Fund for Clinical Excellence.
Conflict of Interest: JYL reports grants from AstraZeneca during the conduct of the study; grants and personal fees from Beigene, Bergenbio, Clovis Oncology, Immunogen, Janssen, Merck, MSD, Novartis, Roche, Seagen, Synthon, and Takeda. All other authors have no conflicts of interest to disclose.
Data sharing Statement: The de-identified data that support the findings of this study are available on request to bona fide researchers who provide a methodologically sound proposal. The data will be made available 24 months after study completion. Proposals should be directed to the corresponding author. To gain access, data requesters will need to sign a data access agreement.
- Conceptualization: L.J.Y., K.B.G., K.J.W., L.J.B.
- Data curation: L.J.Y., K.B.G., K.J.W., K.S., C.H.C., K.H.S.
- Formal analysis: L.J.Y., K.B.G., K.J.W., L.J.B., P.E.
- Funding acquisition: L.J.Y.
- Investigation: C.H.C., P.E.
- Methodology: L.J.B.
- Project administration: L.J.Y., K.B.G., K.J.W., K.S.
- Resources: L.J.Y., K.S., C.H.C., K.H.S.
- Software: C.H.C., P.E., L.J.B.
- Supervision: L.J.Y., K.B.G., K.J.W.
- Validation: L.J.Y., K.B.G., K.J.W., L.J.B., P.E., J.J.G., K.S., C.C.H., K.H.S.
- Visualization: L.J.Y., K.B.G., K.J.W., L.J.B., P.E., J.J.G., K.S., C.C.H., K.H.S.
- Writing - original draft: L.J.Y.
- Writing - review & editing: L.J.Y., K.B.G., K.J.W., L.J.B., P.E., J.J.G., K.S., C.C.H., K.H.S.
SUPPLEMENTARY MATERIALS
Research in context
Details of clinical trial
Summary of adverse events
Incidence of TRAEs* according to each arm
Incidence of irAEs*
Revised study protocol. Adapted from Lee et al [1]. with permission of Oxford University Press. Recruitment to arm 6 is initiated after completion in arm 5.
Study schema. Reproduced from Lee et al [1]. with permission of Oxford University Press. Recruitment to arm 5 is initiated after completion in arm 4.
Spider plot showing the change in target lesion size at each time point for 61 patients over the treatment cycle.
Kaplan-Meier plots of PFS (A) and OS (B) in patients with modified intent-to-treat population from each arm.
OS by irAE (arms 2, 3, 4, and 5) (p=0.05).
OS by PD-L1 (arms 2, 3, 4, and 5).
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Lee JY, Kim S, Kim YT, Lim MC, Lee B, Jung KW, et al. Changes in ovarian cancer survival during the 20 years before the era of targeted therapy. BMC Cancer. 2018;18:601. doi: 10.1186/s12885-018-4498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A, Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial. J Clin Oncol. 2014;32:1302–1308. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 4.Lee JY, Park JY, Park SY, Lee JW, Kim JW, Kim YB, et al. Real-world effectiveness of bevacizumab based on AURELIA in platinum-resistant recurrent ovarian cancer (REBECA): a Korean Gynecologic Oncology Group study (KGOG 3041) Gynecol Oncol. 2019;152:61–67. doi: 10.1016/j.ygyno.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee JY, Yoon JK, Kim B, Kim S, Kim MA, Lim H, et al. Tumor evolution and intratumor heterogeneity of an epithelial ovarian cancer investigated using next-generation sequencing. BMC Cancer. 2015;15:85. doi: 10.1186/s12885-015-1077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244–250. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanderstichele A, Nieuwenhuysen EV, Han S, et al. Randomized phase II CLIO study on olaparib monotherapy versus chemotherapy in platinum-resistant ovarian cancer. J Clin Oncol. 2019;37:5507. [Google Scholar]
- 9.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Disis ML, Taylor MH, Kelly K, Beck JT, Gordon M, Moore KM, et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5:393–401. doi: 10.1001/jamaoncol.2018.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. 2019;30:1080–1087. doi: 10.1093/annonc/mdz135. [DOI] [PubMed] [Google Scholar]
- 12.Varga A, Piha-Paul S, Ott PA, Mehnert JM, Berton-Rigaud D, Morosky A, et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecol Oncol. 2019;152:243–250. doi: 10.1016/j.ygyno.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Lee EK, Xiong N, Cheng SC, Barry WT, Penson RT, Konstantinopoulos PA, et al. Combined pembrolizumab and pegylated liposomal doxorubicin in platinum resistant ovarian cancer: a phase 2 clinical trial. Gynecol Oncol. 2020;159:72–78. doi: 10.1016/j.ygyno.2020.07.028. [DOI] [PubMed] [Google Scholar]
- 14.Heinhuis KM, Ros W, Kok M, Steeghs N, Beijnen JH, Schellens JH. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019;30:219–235. doi: 10.1093/annonc/mdy551. [DOI] [PubMed] [Google Scholar]
- 15.Lee JY, Yi JY, Kim HS, Lim J, Kim S, Nam BH, et al. An umbrella study of biomarker-driven targeted therapy in patients with platinum-resistant recurrent ovarian cancer: a Korean Gynecologic Oncology Group study (KGOG 3045), AMBITION. Jpn J Clin Oncol. 2019;49:789–792. doi: 10.1093/jjco/hyz085. [DOI] [PubMed] [Google Scholar]
- 16.Lee YJ, Kim D, Shim JE, Bae SJ, Jung YJ, Kim S, et al. Genomic profiling of the residual disease of advanced high-grade serous ovarian cancer after neoadjuvant chemotherapy. Int J Cancer. 2020;146:1851–1861. doi: 10.1002/ijc.32729. [DOI] [PubMed] [Google Scholar]
- 17.Kim HS, Kim JY, Lee YJ, Kim SH, Lee JY, Nam EJ, et al. Expression of programmed cell death ligand 1 and immune checkpoint markers in residual tumors after neoadjuvant chemotherapy for advanced high-grade serous ovarian cancer. Gynecol Oncol. 2018;151:414–421. doi: 10.1016/j.ygyno.2018.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Shin HT, Choi YL, Yun JW, Kim NK, Kim SY, Jeon HJ, et al. Prevalence and detection of low-allele-fraction variants in clinical cancer samples. Nat Commun. 2017;8:1377. doi: 10.1038/s41467-017-01470-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard IL, et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021;22:1034–1046. doi: 10.1016/S1470-2045(21)00216-3. [DOI] [PubMed] [Google Scholar]
- 20.Lheureux S, Cristea MC, Bruce JP, Garg S, Cabanero M, Mantia-Smaldone G, et al. Adavosertib plus gemcitabine for platinum-resistant or platinum-refractory recurrent ovarian cancer: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet. 2021;397:281–292. doi: 10.1016/S0140-6736(20)32554-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Domchek SM, Aghajanian C, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140:199–203. doi: 10.1016/j.ygyno.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Murayama T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33:4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 23.Kim DJ, Jang JH, Ham SY, Choi SH, Park SS, Jeong SY, et al. Doxorubicin inhibits PD-L1 expression by enhancing TTP-mediated decay of PD-L1 mRNA in cancer cells. Biochem Biophys Res Commun. 2020;522:402–407. doi: 10.1016/j.bbrc.2019.11.106. [DOI] [PubMed] [Google Scholar]
- 24.Zamarin D, Burger RA, Sill MW, Powell DJ, Jr, Lankes HA, Feldman MD, et al. Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an NRG oncology study. J Clin Oncol. 2020;38:1814–1823. doi: 10.1200/JCO.19.02059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JY, Kim JW, Lim MC, Kim S, Kim HS, Choi CH, et al. A phase II study of neoadjuvant chemotherapy plus durvalumab and tremelimumab in advanced-stage ovarian cancer: a Korean Gynecologic Oncology Group Study (KGOG 3046), TRU-D. J Gynecol Oncol. 2019;30:e112. doi: 10.3802/jgo.2019.30.e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley RK, Sangro B, Harris W, Ikeda M, Okusaka T, Kang YK, et al. Safety, efficacy, and pharmacodynamics of tremelimumab plus durvalumab for patients with unresectable hepatocellular carcinoma: randomized expansion of a phase I/II study. J Clin Oncol. 2021;39:2991–3001. doi: 10.1200/JCO.20.03555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou X, Yao Z, Yang H, Liang N, Zhang X, Zhang F. Are immune-related adverse events associated with the efficacy of immune checkpoint inhibitors in patients with cancer? A systematic review and meta-analysis. BMC Med. 2020;18:87. doi: 10.1186/s12916-020-01549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Research in context
Details of clinical trial
Summary of adverse events
Incidence of TRAEs* according to each arm
Incidence of irAEs*
Revised study protocol. Adapted from Lee et al [1]. with permission of Oxford University Press. Recruitment to arm 6 is initiated after completion in arm 5.
Study schema. Reproduced from Lee et al [1]. with permission of Oxford University Press. Recruitment to arm 5 is initiated after completion in arm 4.
Spider plot showing the change in target lesion size at each time point for 61 patients over the treatment cycle.
Kaplan-Meier plots of PFS (A) and OS (B) in patients with modified intent-to-treat population from each arm.
OS by irAE (arms 2, 3, 4, and 5) (p=0.05).
OS by PD-L1 (arms 2, 3, 4, and 5).