Abstract
Objective
Examine the risks of fractures and osteoporosis after risk-reducing bilateral salpingo-oophorectomy (RRBSO) among women with BRCA1/2 mutations.
Methods
In this retrospective population-based study in British Columbia, Canada, between 1996 to 2017, we compared risks of osteoporosis and fractures among women with BRCA1/2 mutations who underwent RRBSO before the age of 50 (n=329) with two age-matched groups without known mutations: 1) women who underwent bilateral oophorectomy (BO) (n=3,290); 2) women with intact ovaries who had hysterectomy or salpingectomy (n=3,290). Secondary outcomes were: having dual-energy X-ray absorptiometry (DEXA) scan, and bisphosphonates use.
Results
The mean age at RRBSO was 42.4 years (range, 26–49) and the median follow-up for women with BRCA1/2 mutations was 6.9 years (range, 1.1–19.9). There was no increased hazard of fractures for women with BRCA1/2 mutations (adjusted hazard ratio [aHR]=0.80; 95% confidence interval [CI]=0.56–1.14 compared to women who had BO; aHR=1.02; 95% CI=0.65–1.61 compared to women with intact ovaries). Among women who had DEXA-scan, those with BRCA1/2 mutations had higher risk of osteoporosis (aHR=1.60; 95% CI=1.00–2.54 compared to women who had BO; aHR=2.49; 95% CI=1.44–4.28 compared to women with intact ovaries). Women with BRCA1/2 mutations were more likely to get DEXA-scan than either control groups, but only 46% of them were screened. Of the women with BRCA1/2 mutations diagnosed with osteoporosis, 36% received bisphosphonates.
Conclusion
Women with BRCA1/2 mutations had higher risk of osteoporosis after RRBSO, but were not at increased risk of fractures during our follow-up. Low rates of DEXA-scan and bisphosphonates use indicate we can improve prevention of bone loss.
Keywords: Hereditary Breast and Ovarian Cancer Syndrome, Osteoporotic Fractures, Osteoporosis, Epidemiology
Synopsis
BRCA mutations carriers had higher osteoporosis risk after risk-reducing bilateral salpingo-oophorectomy (RRBSO) than the groups without mutations. However, they were not at increased risk of fractures during the study period. Only 46% of BRCA mutations carriers were screened for bone loss after RRBSO. The rate of bisphosphonates use was also low.
INTRODUCTION
Women with BRCA1/2 mutations are at an increased lifetime risk for ovarian cancer [1]. A risk-reducing bilateral salpingo-oophorectomy (RRBSO) between 35–45 years, after completion of childbearing, is the gold standard preventive strategy in this population [2,3]. Indeed, it is estimated that RRBSO reduces the risk of ovarian or fallopian cancers by 80% in women with BRCA1/2 mutations [4].
However, RRBSO causes surgical menopause that is known to have a negative impact on long-term health. Studies examining outcomes from surgical menopause among women at general population risk for ovarian cancer report an increased risk of osteoporosis and fractures, cardiovascular diseases, and possible increased risk of cognitive impairment, as well as excess mortality [5,6,7,8,9]. Importantly, a large prospective study observed a 3.64 times increased risk of fractures (95% confidence interval [CI]=1.01–13.04) when bilateral oophorectomy (BO) had been carried out under the age of 45 years [9].
With respect to women with BRCA1/2 mutations, there is evidence of significant bone mineral density loss and increased risk of osteoporosis after RRBSO, but the current literature is limited by small sample sizes, short follow-up periods, and/or lack of control groups [10,11,12,13,14]. Data are even more scarce on the risk of fractures, as evidenced by a 2018 Cochrane review, which could not draw conclusions on the incidence of fractures after RRBSO as none of the studies meeting the inclusion criteria reported this outcome [15]. Moreover, preliminary science suggest that BRCA1/2 mutations may be associated with premature ovarian and systemic aging, including osteoporosis in mice [16,17].
To provide more data on bone health in BRCA1/2 mutation carriers undergoing RRBSO at premenopausal ages, this study investigates the risk of fractures and osteoporosis in such a population-based cohort as compared to 2 groups of women without known BRCA1/2 mutations matched by age at surgery: 1) women who underwent BO for benign gynecologic conditions; and, 2) women who underwent hysterectomy or salpingectomy but retained their ovaries. We also analyzed the use of health services to prevent fractures and maintain bone health (including having bone mineral density scanning and receiving bisphosphonates), and whether the use of hormone replacement therapy (HRT) mitigated the risk of bone diseases in this population.
MATERIALS AND METHODS
In this retrospective study we analyzed population-based administrative data from British Columbia (BC), Canada, between 1996 and 2017. We linked hospitalizations and physicians visits for all BC residents with data from the BC Cancer Registry and the BC PharmaNet, a database including all medications dispensed in an outpatient setting [18,19,20,21,22]. Finally, we linked with data from the Hereditary Cancer Program, (the sole publicly funded site testing for BRCA1/2 mutations in BC) to obtain information on virtually all women tested in the province from 1996 to 2014. Ethics approval was obtained from the University of British Columbia Clinical Research Ethics Board. All inferences, opinions, and conclusions drawn in this paper are those of the authors and do not reflect the opinions or policies of the Data Stewards.
The primary outcomes were fractures and osteoporosis. Secondary outcomes included: 2) having a dual-energy X-ray absorptiometry (DEXA) scan, and 3) use of bisphosphonates initiated after surgery. The outcomes were identified from the hospital and physicians’ visits data using the International Classification of Diseases codes (version-9 and -10) and Medical Services Plan fee item billing codes, and from the BC PharmaNet using the Anatomical Therapeutic Chemical Classification System (Table S1).
1. Study population
Women were included if they: 1) did not have a diagnosis of ovarian, fallopian tube, or peritoneal cancers, and did not have gynecologic cancer listed as an indication for the index surgery; 2) had at least 1 year of follow-up; and 3) were registered in the universal provincial insurance program in the year of their surgery. While we included patients with a history of breast cancer, we excluded those who had a DEXA-scan in the two years before the start of the follow-up, as this suggests their breast cancer treatment was associated with increased risk of bone loss and fractures. Women with a diagnosis of osteoporosis, or hip or vertebral fractures (prototypical osteoporotic fractures) [23] in the 2 years preceding the study entry were also excluded.
We included all women with documented deleterious BRCA1/2 mutations who underwent RRBSO prior to age 50 (before the average age of natural menopause) between January 1st, 1996 and December 31st, 2017. We included 2 control groups, one of women who underwent BO (with or without salpingectomy) for benign gynecologic conditions and a group of women with intact ovaries who underwent hysterectomy or salpingectomy. Both control groups were women who were not tested for BRCA1/2 mutations (without known mutations) matched by age at surgery, randomly selected at a 10:1 rate.
2. Statistical analysis
The beginning of the follow-up was defined as the date of the index surgery. For baseline comparisons we used t-tests for unpaired groups or χ2 tests. All p-values are two-sided and statistical significance was defined as p<0.05. Multivariate Cox proportional hazards models were used to estimate adjusted hazard ratios (aHR) and 95% CI for the outcomes. In this time to event analysis, women were censored at death, date of diagnosis of breast cancer, or if they moved away from the province. The models were adjusted for history of breast cancer, unless otherwise specified.
As osteoporosis is usually asymptomatic, we also estimated the hazard of osteoporosis considering only women who had DEXA-scan, to account for differences in the screening across comparison groups. We performed an analysis stratified by history of breast cancer given that some treatment options (e.g., aromatase inhibitors) are risk factors for osteoporosis and fractures [24]. Finally, we stratified the population by their use of HRT after surgical menopause. Any use of HRT was defined as the dispensation of at least 30 days of systemic estrogen or estrogen plus progesterone preparations. For all stratified analysis, age at baseline was tested as a covariate since the age-matching was no longer valid. Statistical analyses were performed with R software version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
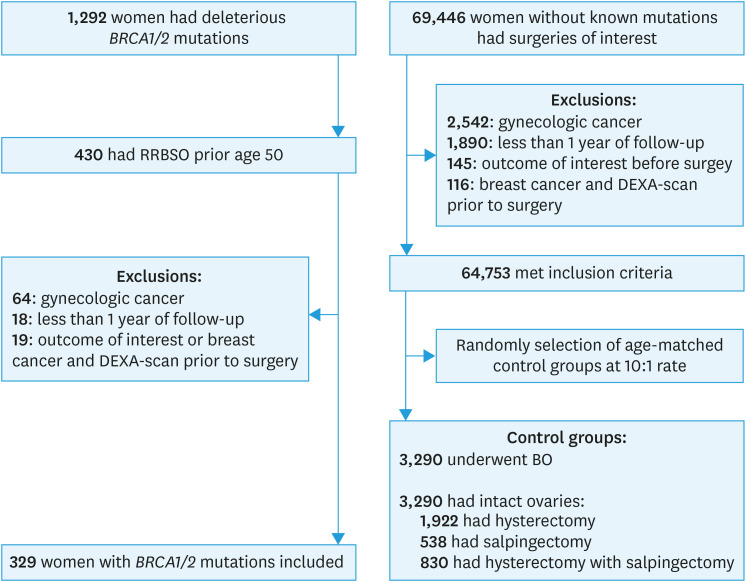
A total of 1,292 women tested positive for deleterious BRCA1/2 mutations, and 430 underwent RRBSO under the age of 50 during the study period. After excluding 64 women due to prior gynecologic cancer, 18 for having less than 1 year of follow-up, and 19 for having an outcome of interest or a history of breast cancer and a DEXA-scan prior to surgery, we included 329 women with BRCA1/2 mutations (Fig. 1). For the control groups, 10,046 women who had BO and 54,707 with intact ovaries who had hysterectomy or salpingectomy filled the same inclusion criteria and did not have known BRCA1/2 mutations. From each group, ten controls matched by age at surgery were randomly selected for each woman with a BRCA1/2 mutation. Of the 3,290 women with intact ovaries, 1,922 underwent hysterectomy, 538 underwent salpingectomy, and 830 underwent hysterectomy with salpingectomy.
Fig. 1. Flow diagram of study population analyzing bone health in women with BRCA1/2 mutations who underwent RRBSO at premenopausal age compared to 2 control groups of age matched women without known mutations, one of women who underwent BO and another group of women with intact ovaries who underwent hysterectomy or salpingectomy.
RRBSO, risk-reducing bilateral salpingo-oophorectomy; BO, bilateral oophorectomy; DEXA, dual-energy X-ray absorptiometry.
The mean age at RRBSO was 42.4 years (range, 26–49) and the median follow-up time for women with BRCA1/2 mutations was 6.9 years (range, 1.1–19.9; Table 1). Follow-up was longer, on average, for the control groups (both p<0.001). Women with BRCA1/2 mutations were more likely to have a history of breast cancer (48.9% compared to 4.6% of women without mutations who had BO; and to 0.3% of women without mutations with intact ovaries, both p<0.001), and to be in the higher income quintiles than both control groups (both p<0.05). Among women without a history of breast cancer (as HRT is not recommended among women with a history of breast cancer) [25,26], there was no significant difference in HRT use after surgical menopause between women with BRCA1/2 mutations and those without a mutation who underwent BO (72.0% vs. 68.5%, p=0.386).
Table 1. Characteristics of the study population matched by age at surgery.
| Characteristics | BRCA mutation with RRBSO (n=329) | BO without BRCA mutation (n=3,290) | p-value* | Intact ovaries without BRCA mutation (n=3,290) | p-value* | |
|---|---|---|---|---|---|---|
| Age (yr) | 0.940 | 0.937 | ||||
| Mean±SD | 42.4±4.8 | 42.5±4.8 | 42.5±4.8 | |||
| Min–Max | 26.7–49.9 | 26.0–49.9 | 26.2–49.9 | |||
| Age category | 1.000 | 1.000 | ||||
| <35 | 23 (7.0%) | 230 (7.0%) | 230 (7.0%) | |||
| 35–39 | 81 (24.6%) | 810 (24.6%) | 810 (24.6%) | |||
| 40–44 | 103 (31.3%) | 1,030 (31.3%) | 1,030 (31.3%) | |||
| 45–49 | 122 (37.1%) | 1,220 (37.1%) | 1,220 (37.1%) | |||
| Follow-up (yr) | <0.001 | <0.001 | ||||
| Median | 6.9 | 10.6 | 8.6 | |||
| Min–Max | 1.1–19.9 | 1.0–22.0 | 1.0–22.0 | |||
| Breast cancer before surgery | 161 (48.9%) | 151 (4.6%) | <0.001 | 9 (0.3%) | <0.001 | |
| No | 168 (51.1%) | 3,139 (95.4%) | 3,281 (99.7%) | |||
| Other cancers | 23 (7.0%) | 310 (9.4%) | 0.175 | 252 (7.7%) | 0.743 | |
| Income quintile | <0.001 | 0.008 | ||||
| 1 | 42 (12.8%) | 670 (20.4%) | 625 (19.0%) | |||
| 2 | 61 (18.5%) | 704 (21.4%) | 650 (19.8%) | |||
| 3 | 58 (17.6%) | 655 (19.9%) | 666 (20.2%) | |||
| 4 | 81 (24.6%) | 598 (18.2%) | 694 (21.1%) | |||
| 5 | 80 (24.3%) | 588 (17.9%) | 592 (18.0%) | |||
| Missing | 7 (2.1%) | 75 (2.3%) | 63 (1.9%) | |||
| HRT after surgery | 153 (46.5%) | 2,159 (65.6%) | <0.001 | - | - | |
| History of breast cancer† | 32 (19.9%) | 8 (5.3%) | <0.001 | - | - | |
| No history of breast cancer‡ | 121 (72.0%) | 2,151 (68.5%) | 0.386 | - | - | |
| Duration HRT (years) | 0.031 | - | - | |||
| Mean±SD | 3.5±2.5 | 4.0±3.7 | ||||
| Min–Max | 0.1–10.7 | 0.1–18.4 | ||||
RRBSO, risk-reducing bilateral salpingo-oophorectomy; BO, bilateral oophorectomy; SD, standard deviation; HRT, hormone replacement therapy.
*Reference group: BRCA mutation with RRBSO; †Denominator is the number of women without prior breast cancer (n=161 and n=151, respectively); ‡Denominator is the number of women with prior breast cancer (n=168 and n=3,139, respectively).
Table 2 shows the frequency of the outcomes by the end of the study period. Of the women with BRCA1/2 mutations, 13.4% (n=44) had fractures, 46.2% (n=152) had DEXA-scan after surgery, and of these 14.5% (n=22) had osteoporosis. The p-values were not provided, as this comparison does not account for differences in the follow-up time.
Table 2. Frequency of the outcomes by study group at the end of the study period.
| Outcomes | BRCA mutation with RRBSO (n=329) | BO without BRCA mutation (n=3,290) | Intact ovaries without BRCA mutation (n=3,290) | |
|---|---|---|---|---|
| Bone fractures | 44 (13.4%) | 616 (18.7%) | 445 (13.5%) | |
| Hip fracture | ≤5 | 21 (0.6%) | 14 (0.4%) | |
| Vertebral fracture | ≤5 | 36 (1.1%) | 31 (0.9%) | |
| Forearm fracture | 10 (3.0%) | 132 (4.0%) | 82 (2.5%) | |
| Humerus fracture | ≤5 | 33 (1.0%) | 31 (0.9%) | |
| Pelvic fracture | ≤5 | 16 (0.5%) | 13 (0.4%) | |
| Other fractures | 27 (8.2%) | 399 (12.1%) | 299 (9.1%) | |
| Osteoporosis | 22 (6.7%) | 127 (3.9%) | 45 (1.4%) | |
| DEXA scan | 152 (46.2%) | 761 (23.1%) | 335 (10.2%) | |
| Bisphosphonates | 27 (8.2%) | 180 (5.5%) | 60 (1.8%) | |
| No. of women who had DEXA scan | 152 | 761 | 335 | |
| Osteoporosis | 22 (14.5%) | 108 (14.2%) | 38 (11.3%) | |
| No. of women with osteoporosis diagnosis | 22 | 127 | 45 | |
| Bisphosphonates | 8 (36.4%) | 65 (51.2%) | 21 (46.7%) | |
RRBSO, risk-reducing bilateral salpingo-oophorectomy; BO, bilateral oophorectomy; DEXA, dual-energy X-ray absorptiometry.
*p-values for the crude incidences were not provided, as this comparison between the groups is not meaningful due to differences in the follow-up time.
1. Primary outcomes
There were no significant differences in the hazard of fractures (Table 3) for women with BRCA1/2 mutations compared to both control groups (aHR=0.80; 95% CI=0.56–1.14 compared to women without mutations who had BO; aHR=1.02; 95% CI=0.65–1.61 compared to women without mutations with intact ovaries). However, fractures occurred earlier after RRBSO, on average, for women with BRCA1/2 mutations (5.8±3.7 years) than for women in both control groups (7.2±4.7 years for women without mutations who had BO p=0.024, and 7.3±4.7 years for women without mutations with intact ovaries, p=0.017).
Table 3. Primary outcomes by surgical and BRCA mutation status.
| Outcomes | BRCA mutation with RRBSO (n=329) | BO without BRCA mutation (n=3,290) | 95% CI | BRCA mutation with RRBSO (n=329) | Intact ovaries without BRCA mutation (n=3,290) | 95% CI | |
|---|---|---|---|---|---|---|---|
| Bone fractures | |||||||
| Persons-years | 2,415.4 | 31,235.9 | - | 2,415.4 | 28,291.0 | - | |
| No. of events | 44 | 616 | - | 44 | 445 | - | |
| Crude HR | 0.96 | 1 | 0.71–1.31 | 1.21 | 1 | 0.89–1.65 | |
| Adjusted HR* | 0.80 | 1 | 0.56–1.14 | 1.02 | 1 | 0.65–1.61 | |
| Osteoporosis | |||||||
| Persons-years | 2,534.0 | 34,307.4 | - | 2,534.0 | 30,780.4 | - | |
| No. of events | 22 | 127 | - | 22 | 45 | - | |
| Crude HR | 2.64 | 1 | 1.67–4.17 | 7.42 | 1 | 4.39–12.55 | |
| Adjusted HR* | 1.83 | 1 | 1.03–3.26 | 6.38 | 1 | 3.11–13.08 | |
| No. of women who had DEXA scan | 152 | 761 | 152 | 335 | |||
| Osteoporosis | |||||||
| Persons-years | 1,304.8 | 9,542.6 | - | 1,304.8 | 4,542.1 | - | |
| No. of events | 22 | 108 | - | 22 | 38 | - | |
| Crude HR | 1.60 | 1 | 1.00–2.54 | 2.54 | 1 | 1.47–4.39 | |
| Adjusted HR† | 1.60 | 1 | 1.00–2.54 | 2.49 | 1 | 1.44–4.28 | |
RRBSO, risk-reducing bilateral salpingo-oophorectomy; BO, bilateral oophorectomy; CI, confidence interval; HR, hazard ratio; DEXA, dual-energy X-ray absorptiometry.
*Adjusted for history breast cancer; †Adjusted for age at surgery.
The likelihood of being diagnosed with osteoporosis was higher among women with BRCA1/2 mutations (aHR=1.83; 95% CI=1.03–3.26 compared to women without mutations who had BO; aHR=6.38; 95% CI=3.11–13.08 compared to women without mutations with intact ovaries). Women with BRCA1/2 mutations were diagnosed with osteoporosis on average 8.0±4.4 years after RRBSO, but the difference in the time to diagnosis compared to the control groups was not significant (8.0±4.5 years for women without mutations who had BO, p=0.923, and 9.4±5.8 years for women without mutations with intact ovaries, p=0.279). The higher hazard of osteoporosis was also observed considering only women who had a DEXA-scan when comparing to women without mutations who had BO (aHR=1.60; 95% CI=1.00–2.54).
The results were similar after excluding women with a history of breast cancer (Table S2). However, the difference in the hazard of osteoporosis was not significant comparing women with BRCA1/2 mutations to women without mutations who had BO, considering only those who had a DEXA-scan (HR=1.52; 95% CI=0.77–3.03). Crude HRs were provided as there were no relevant covariates.
We further compared women without a mutation who underwent BO to women without mutations with intact ovaries who had hysterectomy or salpingectomy (Table S3). We observed a higher hazard of fractures in the group who had BO (aHR=1.22; 95% CI=1.08–1.38).
2. Secondary outcomes
Women with BRCA1/2 mutations were more likely to have DEXA-scan after RRBSO than women in both control groups (aHR=1.58; 95% CI=1.26–1.98, compared to women without mutations who had BO; aHR=6.03; 95% CI=4.64–7.86 compared to women without mutations with intact ovaries, Table 4). Of the 22 women with BRCA1/2 mutations diagnosed with osteoporosis after RRBSO, only 8 (36.4%) filled a prescription for bisphosphonates. Among women with an osteoporosis diagnosis there were no significant differences in the likelihood of receiving bisphosphonates for women with BRCA1/2 mutations compared to both control groups (Table 4).
Table 4. Secondary outcomes by surgical and BRCA mutation status.
| Outcomes | BRCA mutation with RRBSO (n=329) | BO without BRCA mutation (n=3,290) | 95% CI | BRCA mutation with RRBSO (n=329) | Intact ovaries without BRCA mutation (n=3,290) | 95% CI | |
|---|---|---|---|---|---|---|---|
| DEXA-scan | |||||||
| Persons-years | 1,663.82 | 28,518.41 | - | 1,663.82 | 28,441.12 | - | |
| No of events | 152 | 761 | - | 152 | 335 | - | |
| Crude HR | 3.02 | 1 | 2.53–3.60 | 7.54 | 1 | 6.20–9.17 | |
| Adjusted HR* | 1.58 | 1 | 1.26–1.98 | 6.03 | 1 | 4.64–7.86 | |
| Bisphosphonates | |||||||
| Persons-years | 2,489.93 | 33,611.39 | - | 2,489.93 | 30,572.15 | - | |
| No of events | 27 | 180 | - | 27 | 60 | - | |
| Crude HR | 1.93 | 1 | 1.28–2.90 | 5.91 | 1 | 3.73–9.37 | |
| Adjusted HR* | 0.64 | 1 | 0.40–1.04 | 2.41 | 1 | 1.04–5.59 | |
| No. of women with osteoporosis diagnosis | 22 | 127 | 22 | 45 | |||
| Bisphosphonates | |||||||
| Persons-years | 211.10 | 1,217.44 | - | 211.10 | 500.38 | - | |
| No of events | 8 | 65 | - | 8 | 21 | - | |
| Crude HR† | 0.68 | 1 | 0.33–1.43 | 0.97 | 1 | 0.43–2.22 | |
RRBSO, risk-reducing bilateral salpingo-oophorectomy; BO, bilateral oophorectomy; CI, confidence interval; DEXA, dual-energy X-ray absorptiometry; HR, hazard ratio.
*Adjusted for history of breast cancer; †No covariates included due to low number of events.
Higher screening with DEXA was also observed among women with BRCA1/2 after excluding women with prior breast cancer (HR=2.73; 95% CI=2.12–3.52, compared to women without mutations who had BO; HR=6.22; 95% CI=4.76-8.12 compared to women without mutations with intact ovaries, Table S4).
3. HRT
Of the women with BRCA1/2 mutations who underwent RRBSO, 153 (46.5%) received HRT (Table S5), of whom 82 (53.6%) received oral formulations, 32 (20.9%) received transdermal estrogen, and 39 (25.5%) received both HRT formulations. Women who received HRT were younger on average (40.8 vs. 43.9 years, p<0.001) and were less likely to have previous breast cancer than those who did not use HRT (20.9% vs. 73.3%, p<0.001).
We did not observe significant differences in the hazard of fractures between the groups, adjusting for age and history of breast cancer (aHR=0.88; 95% CI=0.43–1.81; Table 5). However, women who received HRT were less likely to be diagnosed with osteoporosis (HR=0.35; 95% CI=0.13–0.95, when examining only women who had DEXA-scan). We could not include covariates for this analysis given the small number of events in the HRT group (n=5).
Table 5. Hazard of outcomes among women with BRCA mutations who received HRT after RRBSO versus the ones who did not.
| Outcomes | HRT (n=153) | No HRT (n=176) | 95% CI | |
|---|---|---|---|---|
| Bone fractures | ||||
| Persons-years | 1,160.63 | 1,254.77 | - | |
| No. of events | 17 | 27 | - | |
| Crude HR | 0.68 | 1 | 0.37–1.25 | |
| Adjusted HR* | 0.88 | 1 | 0.43–1.81 | |
| Osteoporosis | ||||
| Persons-years | 1,208.40 | 1,325.64 | - | |
| No. of events | ≤5 | 17 | - | |
| Crude HR† | 0.29 | 1 | 0.11–0.78 | |
| No. of women who had DEXA scan | 59 | 93 | ||
| Osteoporosis | ||||
| Persons-years | 548.43 | 756.36 | - | |
| No. of events | ≤5 | 17 | - | |
| Crude HR† | 0.35 | 1 | 0.13–0.95 | |
HRT, hormone replacement therapy; RRBSO, risk-reducing bilateral salpingo-oophorectomy; CI, confidence interval; HR, hazard ratio; DEXA, dual-energy X-ray absorptiometry.
*Adjusted for age and breast cancer; †No covariates included due to low number of events.
Considering HRT duration as a continuous variable (Table S6), we did not observe significant differences in the hazard of fractures or osteoporosis for each year of HRT use, adjusting for age and breast cancer history. However, for women without a mutation who underwent BO, each year of HRT use was associated with a small but significant reduction in the hazard of fractures (aHR=0.92; 95% CI=0.90–0.95), adjusting for age and history of breast cancer. Table S7 shows the hazard of the outcomes for women without BRCA1/2 mutations who had BO, comparing those who received HRT and those who did not.
DISCUSSION
In this retrospective population-based study, we observed an increased likelihood of being diagnosed with osteoporosis among women with BRCA1/2 mutations who underwent RRBSO prior to the average age of natural menopause compared to women without mutations with intact ovaries who had hysterectomy or salpingectomy. This was expected as a consequence of the surgical menopause. We also observed a higher likelihood of having a diagnosis of osteoporosis among women with BRCA1/2 mutations compared to women without mutations who had BO considering the entire study population, and also when looking only in those who had received DEXA-scans.
While the risk of fractures was not increased in women with BRCA1/2 mutations, they occurred earlier in this group. There was also an association between surgical menopause and risk of fractures by comparing women who had BO without mutations to women with intact ovaries who had hysterectomy or salpingectomy. In this analysis we had a larger sample size and a longer follow-up period, and thus an older population on average at the end of follow-up.
Women with BRCA1/2 mutations were more likely to be screened for bone loss after the surgery, but less than 50% of that study population had received a DEXA-scan by the end of the follow-up. The rate of bisphosphonates use among women who were diagnosed with osteoporosis was also low. While conclusions should be drawn cautiously with respect to HRT use, as our numbers were small, our findings suggest a decreased risk of receiving an osteoporosis diagnosis among women with BRCA1/2 mutations who were using HRT, and of lower risk of fractures among women without a mutation who had BO and received HRT. These findings are encouraging and suggest that HRT use is likely important to maintain bone health in this population.
By the end of our study, 14.5% of women with BRCA1/2 mutations who underwent RRBSO and had a DEXA-scan had been diagnosed with osteoporosis, a slightly higher rate than reported by Cohen et al. (9%) and Powell et al. (11.6%) for women undergoing premenopausal RRBSO [12,13]. Our observed frequency is also more than double compared to the 6.1% prevalence of osteoporosis for Canadian women between 50–54 years [27]. We observed a similar frequency for the control groups (14.2% and 11.3%), suggesting that the group with intact ovaries may also have been at increased risk of osteoporosis, possibly due to the reduce age of onset of menopause following hysterectomy [28].
Women with BRCA1/2 mutations often have multiple risk factors for bone diseases, including previous chemotherapy and aromatase inhibitors use due to breast cancer, as many women are tested for BRCA1/2 mutations following a breast cancer diagnosis [11]. However, we report an increased risk of osteoporosis among women with BRCA1/2 mutations and no history of breast cancer compared to women without mutations with intact ovaries, indicating that surgical menopause is an important risk factor in this population, and follow-up care should focus on maintaining bone health.
The higher likelihood of having at least one DEXA-scan after surgery among women with BRCA1/2 mutations is also supported by the literature that previously described a more proactive attitude of women undergoing RRBSO towards their health [29]. Nonetheless, under half (46%) of women with BRCA1/2 mutations had a DEXA-scan after RRBSO, similarly to the reports from Chapman et al. and Garcia et al., ranging from 44% to 47% [10,11].
This low rate of DEXA-scan after RRBSO in women with a BRCA1/2 mutation (<50%), and the low proportion of women with diagnosis of osteoporosis who filled prescriptions for bisphosphonates in our study population suggest that improving prevention of bone disease among these women is possible. Although routine screening of bone loss in women with BRCA1/2 mutation undergoing RRBSO has been recommended, there are no formal guidelines on the management of the adverse outcomes of premature surgical menopause in this population, and thus their follow-up care is heterogeneous [10,11]. We recommend further research into the value of regular DEXA scanning in this population, particularly to examine whether DEXA scanning at 3 and 5 years post-surgery could reduce rates of undiagnosed osteoporosis in these patients. Improving our management of the health risks after RRBSO is crucial to prevent non-cancer outcomes and improve long-term survivorship.
This study had a larger sample size and a longer follow-up than most publications on the topic. Unfortunately, as many women were still younger than the average age of osteoporotic fractures by the end of the follow-up period, our study was underpowered to draw firm conclusions on the risk of fractures, despite the inclusion of controls in a 10:1 rate. While there was no increased hazard of bone fractures among women with BRCA1/2 mutations, the observed association between surgical menopause and fractures among women who underwent BO suggests that with longer follow-up and a larger study population, we may see a similar increased risk among women with BRCA1/2 mutations undergoing RRBSO. We were also underpowered to draw firm conclusions on the role of HRT use, and we did not analyze the use of progestogen-only preparations due to small numbers. The duration of HRT varied largely in our population, but the mean use (up to 3.5 years) was well under the recommendation of maintaining HRT treatment until the average age of natural menopause [30]. This further impaired our estimates of the impact of HRT on bone health.
This study was also missing potentially relevant information on other risk factors for osteoporosis and fractures (e.g., parathyroid disease, low body mass index, etc.), and on other medications or preventive strategies to preserve bone health, such as use of vitamin D and calcium. Due to the low rates of DEXA screening (<50%), our overall incidence of osteoporosis was probably underestimated. The high prevalence of breast cancer in our study group is another limitation of this study and our analyses stratified on history of breast cancer had low statistical power as many women were tested for BRCA1/2 mutations due to a breast cancer diagnosis. Breast cancer treatments, especially aromatase inhibitors and tamoxifen, are risk factors for bone loss and osteoporotic fractures [31]. However, a relatively high proportion of BRCA1/2 mutation associated breast cancers are triple negative [32,33]. Thus, many of these women will not receive tamoxifen or aromatase inhibitors. We were also unable to include a control group of women with BRCA1/2 mutations who did not undergo RRBSO, as more than 70% of women with BRCA1/2 mutations who were 40 years of age or older had RRBSO in BC [34]. Our second control group underwent hysterectomy or salpingectomy which may have reduced the age of onset of menopause of these women [28,35]. Thus, we may have underestimated the hazards of the outcomes for women with BRCA1/2 mutations in relation to the general population who have not undergone any gynecologic surgery. The presence of unknown mutation carriers in the control groups may be another source of conservative bias, trending the results towards a null finding.
In conclusion, women with BRCA1/2 mutations who underwent RRBSO had a higher risk of osteoporosis than both control groups but were not at increased risk of bone fractures during our follow-up period. The low rate of DEXA-scan and of bisphosphonates use among women with diagnoses of bone loss indicates that we can better preserve bone health in this population with focused care. Longer follow-up is needed to better investigate the risk of bone fractures and the performance of HRT in this population.
Footnotes
Funding: This study was supported by the Canadian Institutes of Health Research, as well as by donor funds from the Vancouver General Hospital and University of British Columbia Hospital Foundation. Gillian E. Hanley is supported as a CIHR New Investigator and a Michael Smith Foundation for Health Research Scholar. Dr. Hanley is also a Janet D. Cottrelle foundation scholar. The funding sources played no role in study design, collection of data, interpretation of data, writing of the report or decision to submit the article for publication.
Conflict of Interest: Dr. Dawson reports grants from Astra Zeneca Canada, grants from LiquidThermDX, personal fees from Preventum Health, outside the submitted work. Dr. Kwon discloses research funding from Astra Zeneca Canada, and participation in an advisory board for Astra Zeneca Canada. No other potential conflict of interest relevant to this article was reported.
- Conceptualization: A.D.V.H., H.G.E., K.P., K.J.S., C.R., D.L.
- Data curation: A.D.V.H., H.G.E., K.P.
- Formal analysis: A.D.V.H., H.G.E., K.P.
- Investigation: K.J.S., C.R., D.L.
- Methodology: A.D.V.H., H.G.E., K.P., K.J.S., C.R., D.L.
- Project administration: H.G.E.
- Software: A.D.V.H., H.G.E., K.P.
- Supervision: H.G.E.
- Writing - original draft: A.D.V.H., H.G.E.
- Writing - review & editing: K.P., K.J.S., C.R., D.L.
SUPPLEMENTARY MATERIALS
Relevant ICD, ATC codes, and Medical Services Plan fee item billing codes
Primary outcomes by surgical and BRCA mutation status, excluding women with a history of breast cancer
Primary outcomes for women without a BRCA mutation who underwent BO compared to women without a mutation with intact ovaries who had hysterectomy or salpingectomy
Secondary outcomes by surgical and BRCA mutation status, excluding women with a history of breast cancer
Comparison between women with BRCA mutations who received HRT after RRBSO versus the ones who did not
Hazard of primary outcomes considering HRT as a continuous variable in years
Hazard of outcomes among women without BRCA mutations who received HRT after BO versus the ones who did not receive any HRT
References
- 1.Varol U, Kucukzeybek Y, Alacacioglu A, Somali I, Altun Z, Aktas S, et al. BRCA genes: BRCA1 and BRCA2 . J BUON. 2018;23:862–866. [PubMed] [Google Scholar]
- 2.Practice bulletin no 182: Hereditary breast and ovarian cancer syndrome. Obstet Gynecol. 2017;130:e110–e126. doi: 10.1097/AOG.0000000000002296. [DOI] [PubMed] [Google Scholar]
- 3.Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domchek SM, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 4.Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J Natl Cancer Inst. 2009;101:80–87. doi: 10.1093/jnci/djn442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14:567–571. doi: 10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- 6.Shuster LT, Gostout BS, Grossardt BR, Rocca WA. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008;14:111–116. doi: 10.1258/mi.2008.008016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida T, Takahashi K, Yamatani H, Takata K, Kurachi H. Impact of surgical menopause on lipid and bone metabolism. Climacteric. 2011;14:445–452. doi: 10.3109/13697137.2011.562994. [DOI] [PubMed] [Google Scholar]
- 8.Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric. 2015;18:483–491. doi: 10.3109/13697137.2015.1020484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuppurainen M, Kröger H, Honkanen R, Puntila E, Huopio J, Saarikoski S, et al. Risks of perimenopausal fractures--a prospective population-based study. Acta Obstet Gynecol Scand. 1995;74:624–628. doi: 10.3109/00016349509013475. [DOI] [PubMed] [Google Scholar]
- 10.Chapman JS, Powell CB, McLennan J, Crawford B, Mak J, Stewart N, et al. Surveillance of survivors: follow-up after risk-reducing salpingo-oophorectomy in BRCA 1/2 mutation carriers. Gynecol Oncol. 2011;122:339–343. doi: 10.1016/j.ygyno.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Garcia C, Lyon L, Conell C, Littell RD, Powell CB. Osteoporosis risk and management in BRCA1 and BRCA2 carriers who undergo risk-reducing salpingo-oophorectomy. Gynecol Oncol. 2015;138:723–726. doi: 10.1016/j.ygyno.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Cohen JV, Chiel L, Boghossian L, Jones M, Stopfer JE, Powers J, et al. Non-cancer endpoints in BRCA1/2 carriers after risk-reducing salpingo-oophorectomy. Fam Cancer. 2012;11:69–75. doi: 10.1007/s10689-011-9480-8. [DOI] [PubMed] [Google Scholar]
- 13.Powell CB, Alabaster A, Stoller N, Armstrong MA, Salyer C, Hamilton I, et al. Bone loss in women with BRCA1 and BRCA2 mutations. Gynecol Oncol. 2018;148:535–539. doi: 10.1016/j.ygyno.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Kotsopoulos J, Hall E, Finch A, Hu H, Murphy J, Rosen B, et al. Changes in bone mineral density after prophylactic bilateral salpingo-oophorectomy in carriers of a BRCA mutation. JAMA Netw Open. 2019;2:e198420. doi: 10.1001/jamanetworkopen.2019.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eleje GU, Eke AC, Ezebialu IU, Ikechebelu JI, Ugwu EO, Okonkwo OO. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst Rev. 2018;8:CD012464. doi: 10.1002/14651858.CD012464.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben-Aharon I, Levi M, Margel D, Yerushalmi R, Rizel S, Perry S, et al. Premature ovarian aging in BRCA carriers: a prototype of systemic precocious aging? Oncotarget. 2018;9:15931–15941. doi: 10.18632/oncotarget.24638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao L, Li W, Kim S, Brodie SG, Deng CX. Senescence, aging, and malignant transformation mediated by p53 in mice lacking the Brca1 full-length isoform. Genes Dev. 2003;17:201–213. doi: 10.1101/gad.1050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.BC Ministry of Health. Population Data BC (PopData). Discharge abstracts database (hospital separations) data set [Internet] Vancouver: University of British Columbia; 2017. [cited 2021 Jul 20]. Available from: https://www.popdata.bc.ca/data/health/dad. [Google Scholar]
- 19.BC Ministry of Health. Population Data BC (PopData). BC vital events and statistics deaths data set [Internet] Vancouver: University of British Columbia; 2017. [cited 2021 Jul 20]. Available from: https://www.popdata.bc.ca/data/demographic/vs_deaths. [Google Scholar]
- 20.BC Ministry of Health. Population Data BC (PopData). BC cancer registry data set [Internet] Vancouver: University of British Columbia; 2017. [cited 2021 Jul 20]. Available from: https://www.popdata.bc.ca/data/health/bccancer. [Google Scholar]
- 21.BC Ministry of Health. Population Data BC. PharmaNet data set [Internet] Vancouver: University of British Columbia; 2017. [cited 2021 Jul 20]. Available from: https://www.popdata.bc.ca/data/health/pharmanet. [Google Scholar]
- 22.BC Ministry of Health. Population Data BC. Medical Services Plan (MSP) payment information file (PopData) [Internet] Vancouver: University of British Columbia; 2017. [cited 2021 Jul 20]. Available from: https://www.popdata.bc.ca/node/677. [Google Scholar]
- 23.Kaffashian S, Raina P, Oremus M, Pickard L, Adachi J, Papadimitropoulos E, et al. The burden of osteoporotic fractures beyond acute care: the Canadian Multicentre Osteoporosis Study (CaMos) Age Ageing. 2011;40:602–607. doi: 10.1093/ageing/afr085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Yoo JI, Lee YK, Park JW, Won S, Yeom J, et al. Risk of osteoporotic fracture in patients with breast cancer: meta-analysis. J Bone Metab. 2020;27:27–34. doi: 10.11005/jbm.2020.27.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmberg L, Anderson H HABITS steering and data monitoring committees. HABITS (hormonal replacement therapy after breast cancer--is it safe?), a randomised comparison: trial stopped. Lancet. 2004;363:453–455. doi: 10.1016/S0140-6736(04)15493-7. [DOI] [PubMed] [Google Scholar]
- 26.Fahlén M, Fornander T, Johansson H, Johansson U, Rutqvist LE, Wilking N, et al. Hormone replacement therapy after breast cancer: 10 year follow up of the Stockholm randomised trial. Eur J Cancer. 2013;49:52–59. doi: 10.1016/j.ejca.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Public Health Agency of Canada. Osteoporosis and related fractures in Canada: report from the Canadian Chronic Disease Surveillance System 2020 [Internet] Ottawa: Public Health Agency of Canada; 2020. [cited 2021 Aug 5]. Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/osteoporosis-related-fractures-2020.html. [Google Scholar]
- 28.Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956–962. doi: 10.1111/j.1471-0528.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 29.Michelsen TM, Pripp AH, Tonstad S, Tropé CG, Dørum A. Metabolic syndrome after risk-reducing salpingo-oophorectomy in women at high risk for hereditary breast ovarian cancer: a controlled observational study. Eur J Cancer. 2009;45:82–89. doi: 10.1016/j.ejca.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 30.The NAMS 2017 Hormone Therapy Position Statement Advisory Panel. The 2017 hormone therapy position statement of the North American Menopause Society. Menopause. 2017;24:728–753. doi: 10.1097/GME.0000000000000921. [DOI] [PubMed] [Google Scholar]
- 31.Diana A, Carlino F, Giunta EF, Franzese E, Guerrera LP, Di Lauro V, et al. Cancer treatment-induced bone loss (CTIBL): state of the art and proper management in breast cancer patients on endocrine therapy. Curr Treat Options Oncol. 2021;22:45. doi: 10.1007/s11864-021-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atchley DP, Albarracin CT, Lopez A, Valero V, Amos CI, Gonzalez-Angulo AM, et al. Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer. J Clin Oncol. 2008;26:4282–4288. doi: 10.1200/JCO.2008.16.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27:8–16. doi: 10.1097/PPO.0000000000000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanley GE, McAlpine JN, Cheifetz R, Schrader KA, McCullum M, Huntsman D. Selected medical interventions in women with a deleterious BRCA mutation: a population-based study in British Columbia. Curr Oncol. 2019;26:e17–e23. doi: 10.3747/co.26.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanley GE, Kwon JS, McAlpine JN, Huntsman DG, Finlayson SJ, Miller D. Examining indicators of early menopause following opportunistic salpingectomy: a cohort study from British Columbia, Canada. Am J Obstet Gynecol. 2020;223:221.e1–221.11. doi: 10.1016/j.ajog.2020.02.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relevant ICD, ATC codes, and Medical Services Plan fee item billing codes
Primary outcomes by surgical and BRCA mutation status, excluding women with a history of breast cancer
Primary outcomes for women without a BRCA mutation who underwent BO compared to women without a mutation with intact ovaries who had hysterectomy or salpingectomy
Secondary outcomes by surgical and BRCA mutation status, excluding women with a history of breast cancer
Comparison between women with BRCA mutations who received HRT after RRBSO versus the ones who did not
Hazard of primary outcomes considering HRT as a continuous variable in years
Hazard of outcomes among women without BRCA mutations who received HRT after BO versus the ones who did not receive any HRT