Abstract
Objective
The survival benefits of retroperitoneal lymphadenectomy (RLNA) for epithelial ovarian cancer (EOC) remain controversial because clinical behaviors differ among subtypes. The purpose of the present study was to clarify whether RLNA increases the survival rate of advanced high-grade serous carcinoma (HGSC).
Methods
This was a retrospective cohort analysis of 3,227 patients with EOC treated between 1986 and 2017 at 14 institutions. Among them, 335 patients with stage IIB-IV HGSC who underwent optimal cytoreduction (residual tumor of <1 cm) were included. Patients were divided into the RLNA group (n=170) and non-RLNA group (n=165). All pathological slides were assessed based on a central pathological review. Oncologic outcomes were compared between the two groups in the original and weighted cohorts adjusted with the inverse probability of treatment weighting.
Results
The median observation period was 49.8 (0.5–241.5) months. Overall, 219 (65%) out of 335 patients had recurrence or progression, while 146 (44%) died of the disease. In the original cohort, RLNA was a significant prognostic factor for longer progression-free survival (PFS) (hazard ratio [HR]=0.741; 95% confidence interval [CI]=0.558–0.985) and overall survival (OS) (HR=0.652; 95% CI=0.459–0.927). In the weighted cohort in which all variables were well balanced as standardized differences decreased, RLNA was also a significant prognostic factor for more favorable oncologic outcomes (PFS, adjusted HR=0.742; 95% CI=0.613–0.899) and OS, adjusted HR=0.620; 95% CI=0.488–0.787).
Conclusion
The present study demonstrated that RLNA for stage III-IV HGSC with no residual tumor after primary debulking surgery contributed to better oncologic outcomes.
Keywords: Epithelial Ovarian Cancer, Serous Cystadenocarcinoma, Lymphadenectomy, Propensity Score, Recurrence, Survival Rate
Synopsis
Retroperitoneal lymphadenectomy for advanced-stage patients with ovarian high-grade serous carcinoma can improve oncologic outcomes. This multi-centered retrospective study was performed under the same chemotherapeutic protocol and criteria. Inverse probability of treatment weighting method was used to make weighted cohorts.
INTRODUCTION
Epithelial ovarian cancer (EOC) easily metastasizes to the retroperitoneal lymph nodes in more than 50% of patients with advanced disease [1]. It currently remains unclear whether retroperitoneal lymphadenectomy (RLNA) contributes to the complete elimination of occult metastases and subsequent increases in the survival rate. Previous studies reported the potential survival benefits of RLNA for patients with advanced EOC who underwent macroscopically complete tumor resection [2,3,4,5]. However, a randomized controlled trial (the LION study) more recently concluded that patients with advanced EOC and clinically negative lymph nodes who underwent macroscopically complete resection did not achieve any benefits from systematic lymphadenectomy [6].
One of the limitations of these findings is derived from the histological variety of EOC. EOC comprises a number of histological subtypes according to morphological features, such as high- or low-grade serous, clear-cell, mucinous, and endometrioid carcinomas. Biological hallmarks and clinical behaviors markedly vary among the different subtypes [7]. The diversity of EOC subtypes is now the greatest challenge in all relevant research. Therefore, the validity of lymphadenectomy needs to be confirmed for each histological subtype.
The purpose of the present study was to clarify whether RLNA increases the survival rate of advanced high-grade serous carcinoma (HGSC). HGSC is the most frequent subtype of EOC in Western countries as well as in Japan. Most patients with this subtype already have advanced-stage disease at diagnosis. In this multi-institutional cohort study, we retrospectively focused on 335 patients with advanced HGSC who underwent optimal cytoreduction (residual tumor of <1 cm) including complete surgery (no residual tumor) at the initial surgery.
MATERIALS AND METHODS
1. Study design and population
The present study was a retrospective cohort analysis of 3,227 patients with EOC who were treated between 1986 and 2017 by the Tokai Ovarian Tumor Study Group, consisting of Nagoya University Hospital and 13 affiliated institutions in Japan. Patients were included in this analysis if they had all of the following criteria: (i) stage IIB-IV pure-type HGSC based on a central pathological review, (ii) sufficient clinical data, including details on the initial surgery and oncologic outcomes, and (iii) optimal cytoreduction (residual tumor of <1 cm) including complete surgery (no residual tumor) at the initial surgery. Patients were excluded if they received neo-adjuvant chemotherapy. We also excluded patients who did not meet any of these criteria. Patients who met the criteria were divided into two groups according to the surgical procedure. The RLNA group included patients who underwent standard surgery with RLNA. The non-RLNA group comprised patients who underwent incomplete-staging surgery without RLNA. The allocation of patients to each surgical procedure was at the discretion of the attending physician or the institution based on the clinical decision.
Clinical data and follow-up information on the survival status were collected from medical records. Clinical stages were assigned according to the 1988 International Federation of Gynecology and Obstetrics (FIGO) staging system. Histological subtypes were assigned according to the World Health Organization classification criteria [7]. Under a central pathological review system, all histological slides were reviewed by two expert pathologists without any clinical information on patients. The present study was approved by the relevant Review Boards or Ethics Committees of Nagoya University (approval number 2006-0357) and all 13 affiliated institutions. The requirement for written informed consent from patients was waived because of the retrospective nature of this study. All identifiers had been removed before data collection and collected data were confidential. All patients were provided with the opportunity to opt out of this study.
2. Treatments
Peritoneal staging, defined as peritoneal exploration, cytology, and biopsy with or without omentectomy, was conventionally performed for all patients. Standard surgery with RLNA (the RLNA group) included hysterectomy and bilateral salpingo-oophorectomy with complete staging surgery. Complete staging surgery was defined as peritoneal staging and an exploration of the regional lymph nodes. This exploration included the excision of palpable lymph nodes and systematic pelvic and para-aortic lymphadenectomy even in the absence of clinically obvious metastasis to the lymph nodes. Systematic para-aortic lymphadenectomy involved the excision of all lymphatic tissue around the abdominal aorta and inferior vena cava from the origin of the renal vessels to the bifurcation of the abdominal aorta. Systematic pelvic lymphadenectomy involved the excision of all lymphatic tissue around the bilateral common, internal, and external iliac and obturator vessels from the bifurcation of the abdominal aorta to the bilateral femoral rings. On the other hand, incomplete-staging surgery without RLNA (the non-RLNA group) included unilateral or bilateral salpingo-oophorectomy, with or without hysterectomy and omentectomy. The non-RLNA group also included the excision of locally swollen lymph nodes >1 cm in diameter confirmed by preoperative computed tomography (CT). Details on each major first-line chemotherapy regimen were as follows: CAP (cyclophosphamide [300 mg/m2], adriamycin [30 mg/m2], and cisplatin ][70 mg/m2]) (1986–1989); CAP or PVB (cisplatin [70 mg/m2], vinblastine [6 mg/m2], and bleomycin [12 mg/m2]) (1989–1991); PVB or PP (carboplatin [300 mg/m2] and cisplatin [70 mg/m2]) (1992–2000); TC (paclitaxel [180 mg/m2] and carboplatin [area under the curve (AUC 5–6)]) (2000–2002); TC or DC (docetaxel [70 mg/m2] and carboplatin [AUC 5–6]) (2003–2013); TC or DC with or without bevacizumab (15 mg/kg) (2013-) [8].
3. Clinical follow-up
Our protocol for the follow-up of patients was previously described [9]. Briefly, all patients regularly returned for follow-up visits to each institution from the end of treatment. The follow-up interval was every 1–3 months from the first to the second year, every 3–6 months from the third to the fifth year, and annually thereafter. The follow-up procedure included the measurement of serum cancer antigen 125 levels, a pelvic examination, and ultrasonography. CT was repeated every 6 months during the first 2 years, once a year thereafter, and when it was considered necessary. Magnetic resonance imaging or positron emission tomography was also performed where appropriate to detect recurrent tumors. Progression-free survival (PFS) was defined as the time from surgery to recurrence, relapse, or the last date of the follow-up. Overall survival (OS) was defined as the time from surgery to death from any cause or the last date of the follow-up.
4. Statistical analysis
We used propensity score weighting to assess the survival benefits of RLNA in this non-randomized study [10]. We estimated scores by fitting multivariate logistic regression models to the original cohorts of the two groups. We included the following independent variables, which were considered to be clinically relevant: age (≤55 or >55 years old), the FIGO stage (IIB vs. III-IV), residual tumor (none vs. <1 cm), type of first-line chemotherapy (taxane plus platinum [TP] vs. non-TP), and treatment era (<2010 vs. ≥2010). We adjusted cohorts with the inverse probability of treatment weighting (IPTW) approach to balance clinicopathological characteristics between the two surgery groups [11]; each individual was weighted by the inverse probability of receiving RLNA, equal to 1/the propensity score for treated individuals and 1/(1−the propensity score) for control individuals. We compared PFS and OS between the two surgery groups of both the unweighted original and weighted cohorts using Kaplan-Meier estimates and Log-rank tests. We identified each prognostic factor for PFS and OS in both the original and weighted cohorts with Cox proportional hazards regression models. We compared the distributions of clinicopathological characteristics between the two groups by chi-squared tests for categorical variables and the Student’s t-test for continuous variables. We considered p-values <0.05 to be significant. We performed all statistical analyses using SPSS Ver. 26 (IBM Japan, Tokyo, Japan) and JMP Pro Ver. 10.0 (SAS Institute Japan, Tokyo, Japan).
RESULTS
1. Patient characteristics
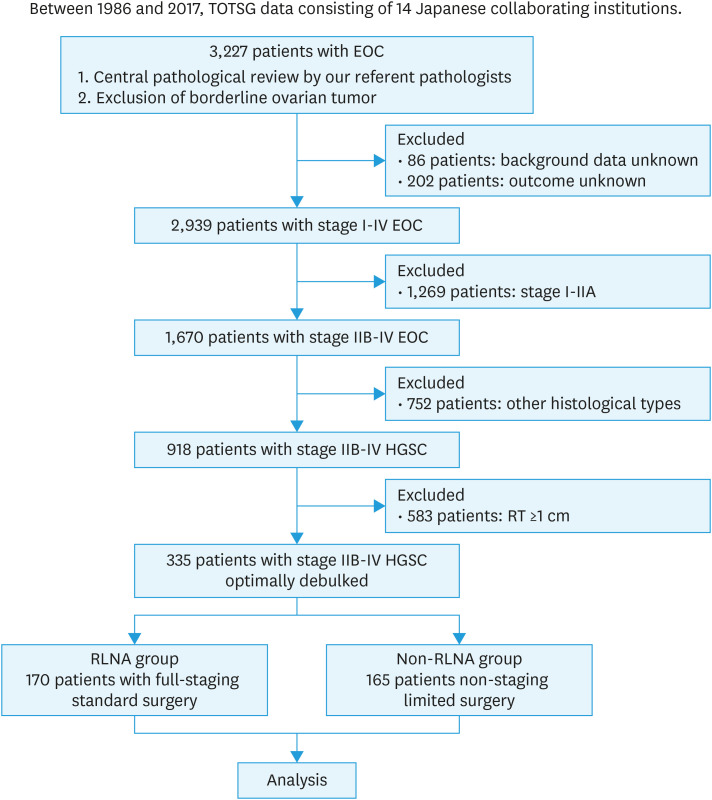
We analyzed 335 patients who met the inclusion criteria. There were 170 patients (51%) in the RLNA group and 165 (49%) in the non-RLNA group (Fig. 1). The median observation period of all patients was 49.8 (range, 0.5–241.5) months. Table 1 shows patient characteristics. Patients were significantly younger in the RLNA group than in the non-RLNA group (p=0.0002). In the RLNA group, 33 (20%) out of 170 patients had stage II disease and 123 (72%) had stage III disease. In the non-RLNA group, 34 (21%) out of 165 patients had stage II disease and 110 (67%) had stage III disease. In both groups, 35 (10%) out of 335 patients had stage IV disease. All patients with limited metastasis to the parenchymal organ underwent complete resection of the metastatic site. More patients were diagnosed with stage IIIC in the RLNA group (p=0.046), while there were more patients with residual tumors <1 cm in diameter in the non-RLNA group (p<0.0001). In addition, more patients were treated before 2010 in the RLNA group than in the non-RLNA group (p=0.0025). The type of first-line chemotherapy did not significantly differ between the groups (p=0.235). Clinicopathological factors did not correlate with the implementation of RLNA in a multivariable analysis (Table S1).
Fig. 1. Outline of enrolled patients.
EOC, epithelial ovarian cancer; HGSC, high-grade serous carcinoma; RLNA, retroperitoneal lymphadenectomy; RT, residual tumor; TOTSG, Tokai Ovarian Tumor Study Group.
Table 1. Patient characteristics (original cohort).
| Characteristics | Total | RLNA* | Non-RLNA† | p-value | |
|---|---|---|---|---|---|
| Total | 335 | 170 | 165 | ||
| Age (yr) | <0.001 | ||||
| ≤55 | 150 | 88 (52) | 62 (38) | ||
| >55 | 185 | 82 (48) | 103 (62) | ||
| Mean (SD) | 55.0 (10) | 59.6 (12) | |||
| FIGO stage | 0.005 | ||||
| IIB | 14 | 5 (3) | 9 (6) | ||
| IIC | 53 | 28 (17) | 25 (15) | ||
| IIIA | 16 | 8 (5) | 8 (5) | ||
| IIIB | 52 | 16 (9) | 36 (22) | ||
| IIIC | 165 | 99 (58) | 66 (40) | ||
| IV | 35 | 14 (8) | 21 (13) | ||
| Uterine preservation | 24 | 3 (2) | 21 (13) | <0.001 | |
| Residual tumor | <0.001 | ||||
| None | 266 | 152 (89) | 114 (69) | ||
| <1 cm | 69 | 18 (11) | 51 (31) | ||
| Chemotherapy | 0.235 | ||||
| Non-TP | 72 | 41 (24) | 31 (19) | ||
| TP | 263 | 129 (76) | 134 (81) | ||
| Treatment era | 0.003 | ||||
| <2010 | 206 | 118 (69) | 88 (53) | ||
| ≥2010 | 129 | 52 (31) | 77 (47) | ||
Values are presented as number (%).
FIGO, International Federation of Gynecology and Obstetrics; RLNA, retroperitoneal lymphadenectomy; SD, standard deviation; TP, taxane plus platinum.
*The RLNA group included patients who underwent full-staging standard surgery with RLNA.
†The non-RLNA group included patients who underwent non-staging limited surgery without RLNA.
2. Survival analyses using the unweighted original cohort
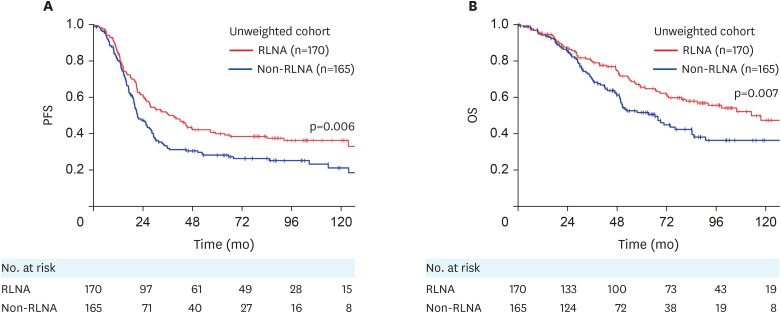
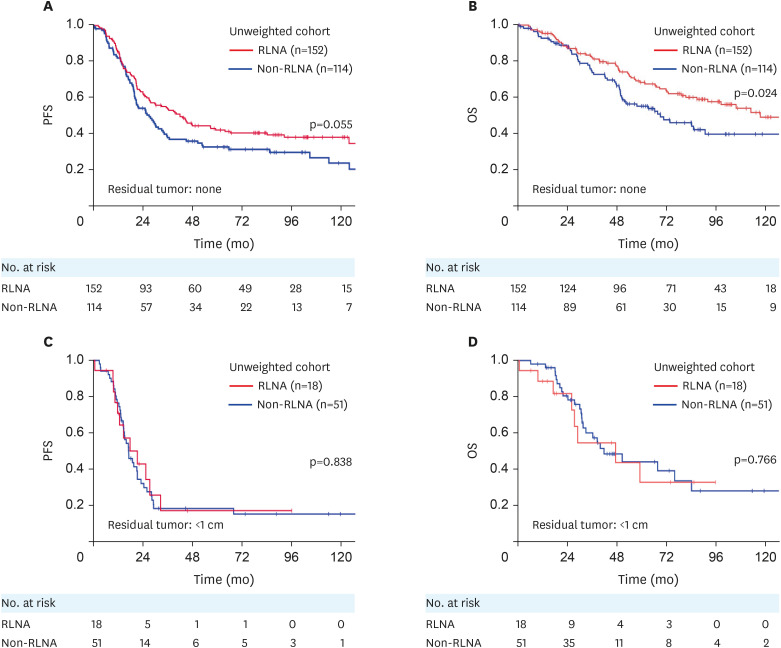
Overall, 219 (65%) out of 335 patients had recurrence or progression, while 146 (44%) died of the disease. Recurrence was detected in 102 (60%) out of 170 patients in the RLNA group and in 117 (71%) out of 165 patients in the non-RLNA group. Sixty-six (39%) out of 170 patients in the RLNA group and 80 (49%) out of 165 patients in the non-RLNA group died of the disease. In the unweighted original cohort, the 5-year PFS and OS rates (95% confidence interval [CI]) of all enrolled patients were 34.1% (29.0–39.6) and 58.8% (52.9–64.5), respectively. Following stratification by patient groups, PFS was significantly longer in the RLNA group than in the non-RLNA group (5-year PFS rate [95% CI], 39.9% [32.5–47.7] vs. 28.1% [21.4–35.8]; p=0.006) (Fig. 2A). OS was also significantly longer in the RLNA group than in the non-RLNA group (5-year OS rate [95% CI], 65.5% [57.4–72.8] vs. 51.6% [43.0–60.1]; p=0.007) (Fig. 2B). We stratified patients according to the residual tumor status. In the absence of residual tumors at the initial surgery (complete surgery), PFS did not significantly differ between the RLNA and non-RLNA groups (p=0.055) (Fig. 3A), whereas OS was significantly longer in the RLNA group than in the non-RLNA group (p=0.024) (Fig. 3B). In patients with a residual tumor <1 cm in diameter at the initial surgery (optimal surgery), PFS and OS did not significantly differ between the RLNA and non-RLNA groups (PFS, p=0.838; OS, p=0.766) (Fig. 3C and D). Cox proportional multivariable analyses identified the type of surgery (RLNA vs. non-RLNA) as a significant prognostic factor for longer PFS and OS (PFS, hazard ratio [HR]=0.741 [95% CI=0.558–0.985]; OS, HR=0.652 [95% CI=0.459–0.927]) (Table 2). We also evaluated the HR of RLNA for PFS and OS in stage IIB-IIIB and IIIC-IV patients as a subgroup analysis. We found that RLNA significantly prolonged both PFS and OS in patients with stage IIIC-IV tumors, while this effect was not significant in those with stage IIB-IIIB tumors (Table S2).
Fig. 2. (A) PFS and (B) OS in the original cohort, and comparisons between surgical types. RLNA group (red): full-staging standard surgery with RLNA; non-RLNA group (blue): incomplete-staging surgery without RLNA.
OS, overall survival; PFS, progression-free survival; RLNA, retroperitoneal lymphadenectomy.
Fig. 3. (A, C) PFS and (B, D) OS in the original cohort, stratified by the residual tumor status. (A, B) Non-residual tumor subgroup; RLNA group (red): full-staging standard surgery with RLNA; non-RLNA group (blue): incomplete-staging surgery without RLNA. (C, D) Optimally debulked subgroup; RLNA group (red); non-RLNA group (blue).
OS, overall survival; PFS, progression-free survival; RLNA, retroperitoneal lymphadenectomy.
Table 2. Multivariate analysis of a Cox’s hazard model in relation to progression-free survival and overall survival (unweighted original cohort).
| Variables | Progression-free survival | Overall survival | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (yr) | |||||
| ≤55 | Reference | 0.513 | Reference | 0.907 | |
| >55 | 1.095 (0.834–1.439) | 0.980 (0.703–1.367) | |||
| FIGO stage | |||||
| II | Reference | <0.001 | Reference | <0.001 | |
| III–IV | 2.866 (1.862–4.411) | 2.565 (1.551–4.241) | |||
| Residual tumor | |||||
| None | Reference | 0.021 | Reference | 0.055 | |
| <1 cm | 1.486 (1.061–2.083) | 1.506 (0.991–2.288) | |||
| Surgery | |||||
| Non-RLNA | Reference | 0.039 | Reference | 0.017 | |
| RLNA | 0.741 (0.558–0.985) | 0.652 (0.459–0.927) | |||
| Chemotherapy | |||||
| Non-TP | Reference | 0.739 | Reference | 0.055 | |
| TP | 0.942 (0.665–1.336) | 0.679 (0.457–1.008) | |||
| Treatment era | |||||
| <2010 | Reference | 0.151 | Reference | 0.757 | |
| ≥2010 | 1.241 (0.924–1.666) | 1.064 (0.720–1.572) | |||
CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; RLNA, retroperitoneal lymphadenectomy; TP, taxane plus platinum.
3. Survival analyses using the weighted cohort
Table S3 summarizes patient characteristics adjusted with the IPTW approach. All variables were well balanced in this weighted cohort as standardized differences decreased (Table S4). PFS was significantly longer in the RLNA group than in the non-RLNA group (median PFS [95% CI], 30.5 [22.6–38.3] vs. 22.1 [19.0–25.2] months; p=0.006). OS was also significantly longer in the RLNA group than in the non-RLNA group (median OS [95% CI], 119.3 [93.4-not applicable] vs. 64.1 [51.3–74.8] months; p<0.0001). Cox multivariable analyses of PFS and OS in the cohort showed that the implementation of RLNA was a significant prognostic factor for more favorable oncologic outcomes (PFS, adjusted HR=0.742 [95% CI=0.613–0.899]; OS, adjusted HR=0.620 [95% CI=0.488–0.787]) (Table 3). We performed forest plot analyses of adjusted HR for death in all groups and subgroups of the unweighted and weighted cohorts (Fig. S1A and B). Regardless of adjustments with IPTW, the implementation of RLNA maintained its significance in subgroups of all ages, no residual tumors, stage III-IV, and all treatment eras.
Table 3. Multivariate analysis of a Cox’s hazard model in relation to progression-free survival and overall survival (weighted cohort).
| Variables | Progression-free survival | Overall survival | |||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||
| Age (yr) | |||||
| ≤55 | Reference | 0.513 | Reference | 0.591 | |
| >55 | 1.044 (0.860–1.266) | 0.937 (0.741–1.187) | |||
| FIGO stage | |||||
| II | Reference | <0.001 | Reference | <0.001 | |
| III–IV | 2.672 (1.977–3.611) | 2.444 (1.716–3.480) | |||
| Residual tumor | |||||
| None | Reference | <0.001 | Reference | <0.001 | |
| <1 cm | 1.567 (1.234–1.990) | 1.562 (1.158–2.107) | |||
| Surgery | |||||
| Non-RLNA | Reference | 0.002 | Reference | <0.001 | |
| RLNA | 0.742 (0.613–0.899) | 0.620 (0.488–0.787) | |||
| Chemotherapy | |||||
| Non-TP | Reference | 0.762 | Reference | 0.021 | |
| TP | 0.961 (0.743–1.243) | 0.709 (0.530–0.949) | |||
| Treatment era | |||||
| <2010 | Reference | 0.034 | Reference | 0.832 | |
| ≥2010 | 1.252 (1.017–1.540) | 0.970 (0.734–1.283) | |||
CI, confidence interval; FIGO, International Federation of Gynecology and Obstetrics; HR, hazard ratio; RLNA, retroperitoneal lymphadenectomy; TP, taxane plus platinum.
DISCUSSION
The results of the present study showed that the complete surgery subgroup of the RLNA group had significantly longer OS and slightly longer PFS than the non-RLNA group in the original cohort. In contrast, the optimal surgery subgroup showed no significant differences in OS or PFS between the RLNA and non-RLNA groups. Furthermore, even after adjustments with IPTW, the implementation of RLNA maintained its significance or was slightly different in all subgroups, except for optimal surgery with residual tumor and stage II tumors.
The complete surgery subgroup of the RLNA group in the present study on advanced HGSC showed significantly longer OS and slightly longer PFS than in the non-RLNA group in the original cohort, which was distinct from the optimal surgery subgroup. Interestingly, RLNA significantly affected survival outcomes, particularly for stage IIIC-IV, which suggested that its effects differed under FIGO IIIB and above IIIC diseases. We previously indicated that the implementation of RLNA did not lead to a significant improvement in the oncologic outcomes of patients with advanced ovarian clear-cell carcinoma [12]. This discrepancy between these two histological types supports our proposal that we need to comprehensively verify the validity of lymphadenectomy for each histological subtype. Regarding endometrioid and mucinous histologies, the effects of RLNA were previously reported to differ in early-stage disease; RLNA significantly affected the prognosis of patients with endometrioid carcinoma, but not those with mucinous carcinoma [13]. Although these findings may not be directly applied to advanced disease, they implied the different impact of RLNA on each histological subtype. In the LION study, systematic retroperitoneum lymphadenectomy was not associated with better oncologic outcomes than no lymphadenectomy [6]. Tumors were completely resected in 99.4% of patients at baseline. More than 70% of patients in the intention to treat population had the HGSC subtype, while the remainder had a wide variety of histological subtypes. However, the LION study excluded patients with any nodes that macroscopically appeared to be involved with tumors. Metastasis to retroperitoneal lymph nodes occurs in more than 50% of patients with advanced EOC [1]. The present study covered the HGSC population that was not included in the LION study. The present results suggest that RLNA significantly improved the oncologic outcomes of advanced HGSC with complete surgery.
In contrast, RLNA is recognized as a basic surgical procedure for the staging of EOC in early-stage patients [14]. It identified patients with the occult metastasis of EOC at the lymph nodes, including the para-aortic region, even among stage I patients [1]. Regarding early-stage disease, RLNA was found to improve survival outcomes compared with patients who did not undergo RLNA [15]. However, previous studies found no significant impact of RLNA on the prognosis of patients [16,17]. This inconsistency may also be attributed to the heterogeneity of cohorts among these studies, including the various proportions of histological subtypes as well as reports of advanced-stage disease. These findings support the validity of the present results and suggest the necessity for further evaluations of RLNA in each histological subtype and clinical stage of EOC.
Even after adjustments with IPTW, the implementation of RLNA maintained its significance or was slightly different in all subgroups, except for optimal surgery and stage II tumors. The present study had various biases because of its retrospective design. It predominantly included patients who underwent complete tumor resection. The present results may reflect the potential selection bias of RLNA being performed on patients whose tumors were expected to be easily resected and whose prognosis was expected to be good. Actually, our current data of optimal surgery rate was low (36%), which might be the feature of our therapeutic approach that gynecologists were likely to choose not radical surgery for optimal cytoreduction but conservative primary surgery to avoid adverse events, relying on the effect of chemotherapy. On the other hand, we used the IPTW method to make weighted cohorts in which patient characteristics had the same distribution [18]. As a result, we minimized the effects of confounding factors as much as possible and obtained well-balanced estimates of average treatment effects. Comparisons between the two weighted surgical cohorts showed that RLNA may have contributed to the better oncologic outcomes observed in the complete surgery subgroup. The results of the present study may facilitate appropriate decisions by physicians and patients for the implementation of RLNA, and may be used as the basis for further studies, such as multi-institutional prospective trials. Additionally, the present study had several limitations because of its retrospective nature. Various clinicopathological factors relevant to decision making were not controlled as strictly as in a randomized clinical trial. Furthermore, the composition of the enrolled patients may have been influenced by a referral bias because the present study was a long-term multi-institutional study. Moreover, crucial data were not provided, such as the completeness of RLNA, the stage IV subclassification, the number of resected lymph nodes, staging classification according to the 2014 FIGO system, any surgical outcomes and complications, including operative times, estimated blood loss, intra-/postoperative complications, lymphoceles, or lymphedema, and detailed surgical procedures, including hepatectomy, lung lobectomy, and intestine resection, which may have affected the reliability of the estimated propensity scores. Moreover, the frequency of the chemotherapeutic regimens used, including VEGF and PARPi, which may affect the survival outcomes of patients, was unknown. Nevertheless, the present study has several strengths. We conducted a central pathological review by expert gynecologic pathologists. In addition, an identical study group had the same chemotherapeutic protocol and criteria. The main clinical utility of the present study may be in the field of preoperative counseling on surgical aggressiveness and the expected prognosis of patients. The present results will not immediately lead to the implementation of RLNA for all patients with advanced HGSC. We intend to reassess and verify the present results in a future trial in order to establish an appropriate treatment strategy for advanced HGSC.
In conclusion, the present study demonstrated that RLNA for stage III-IV HGSC with no residual tumor after primary debulking surgery contributed to better oncologic outcomes. The results provide new insights into the importance of RLNA for advanced HGSC.
ACKNOWLEDGEMENTS
We sincerely thank Drs. Kimio Mizuno (Japanese Red Cross Nagoya Daiichi Hospital), Katsumi Sakakibara (Okazaki City Hospital), Osamu Yamamuro (Japanese Red Cross Nagoya Daini Hospital), Toshiya Misawa (Nagoya Ekisaikai Hospital), Michiyasu Kawai (Toyohashi Municipal Hospital), Hidenori Oguchi (Toyota Memorial Hospital), and Takahiro Suzuki (Anjyo Kosei Hospital) who collaborated for data collection. We also thank Dr. Tetsuro Nagasaka (Nagoya University) who collaborated with the central pathological review.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: I.Y., Y.M., K.H.
- Data curation: I.Y., T.S., Y.A., Y.N.
- Formal analysis: I.Y.
- Investigation: I.Y., T.S., Y.A., Y.N.
- Methodology: T.S., Y.A., Y.N.
- Project administration: K.H.
- Supervision: Y.M., K.H.
- Validation: Y.M., K.H.
- Writing - original draft: I.Y., Y.M., K.H.
- Writing - review & editing: Y.M., K.H.
SUPPLEMENTARY MATERIALS
Independent predictors of retroperitoneal lymphadenectomy for advanced-stage patients with ovarian high-grade serous carcinoma
Multivariate analysis of a Cox’s hazard model in relation to progression-free survival and overall survival in stage subgroups (unweighted original cohort)
Patient characteristics (weighted cohort)
Standardized differences of the variables
Forest plots of the adjusted HR for death in the unweighted original cohort (A) and weighted cohort with the inverse probability of treatment weighting (B).
References
- 1.Morice P, Joulie F, Camatte S, Atallah D, Rouzier R, Pautier P, et al. Lymph node involvement in epithelial ovarian cancer: analysis of 276 pelvic and paraaortic lymphadenectomies and surgical implications. J Am Coll Surg. 2003;197:198–205. doi: 10.1016/S1072-7515(03)00234-5. [DOI] [PubMed] [Google Scholar]
- 2.Chan JK, Urban R, Hu JM, Shin JY, Husain A, Teng NN, et al. The potential therapeutic role of lymph node resection in epithelial ovarian cancer: a study of 13918 patients. Br J Cancer. 2007;96:1817–1822. doi: 10.1038/sj.bjc.6603803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aletti GD, Dowdy S, Podratz KC, Cliby WA. Role of lymphadenectomy in the management of grossly apparent advanced stage epithelial ovarian cancer. Am J Obstet Gynecol. 2006;195:1862–1868. doi: 10.1016/j.ajog.2006.06.068. [DOI] [PubMed] [Google Scholar]
- 4.Scarabelli C, Gallo A, Visentin MC, Canzonieri V, Carbone A, Zarrelli A. Systematic pelvic and para-aortic lymphadenectomy in advanced ovarian cancer patients with no residual intraperitoneal disease. Int J Gynecol Cancer. 1997;7:18–26. doi: 10.1046/j.1525-1438.1997.00418.x. [DOI] [PubMed] [Google Scholar]
- 5.di Re F, Baiocchi G, Fontanelli R, Grosso G, Cobellis L, Raspagliesi F, et al. Systematic pelvic and paraaortic lymphadenectomy for advanced ovarian cancer: prognostic significance of node metastases. Gynecol Oncol. 1996;62:360–365. doi: 10.1006/gyno.1996.0249. [DOI] [PubMed] [Google Scholar]
- 6.Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med. 2019;380:822–832. doi: 10.1056/NEJMoa1808424. [DOI] [PubMed] [Google Scholar]
- 7.Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97(Suppl):2631–2642. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki S, Kajiyama H, Shibata K, Ino K, Nawa A, Sakakibara K, et al. Is there any association between retroperitoneal lymphadenectomy and survival benefit in ovarian clear cell carcinoma patients? Ann Oncol. 2008;19:1284–1287. doi: 10.1093/annonc/mdn059. [DOI] [PubMed] [Google Scholar]
- 9.Kajiyama H, Suzuki S, Utsumi F, Yoshikawa N, Nishino K, Ikeda Y, et al. Comparison of long-term oncologic outcomes between metastatic ovarian carcinoma originating from gastrointestinal organs and advanced mucinous ovarian carcinoma. Int J Clin Oncol. 2019;24:950–956. doi: 10.1007/s10147-019-01438-6. [DOI] [PubMed] [Google Scholar]
- 10.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. Am J Stat Assoc. 1984;79:516–524. [Google Scholar]
- 11.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajiyama H, Suzuki S, Yoshikawa N, Tamauchi S, Shibata K, Kikkawa F. The impact of systematic retroperitoneal lymphadenectomy on long-term oncologic outcome of women with advanced ovarian clear-cell carcinoma. J Gynecol Oncol. 2020;31:e47. doi: 10.3802/jgo.2020.31.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nasioudis D, Chapman-Davis E, Witkin SS, Holcomb K. Prognostic significance of lymphadenectomy and prevalence of lymph node metastasis in clinically-apparent stage I endometrioid and mucinous ovarian carcinoma. Gynecol Oncol. 2017;144:414–419. doi: 10.1016/j.ygyno.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology. Ovarian Cancer including Fallopian Tube Cancer and Primary Peritoneal Cancer, Version 1 [Internet] Plymouth Meeting, PA: National Comprehensive Cancer Network; 2017. [cited 2017 Jun 22]. Available from: https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. [Google Scholar]
- 15.Kleppe M, van der Aa MA, Van Gorp T, Slangen BF, Kruitwagen RF. The impact of lymph node dissection and adjuvant chemotherapy on survival: a nationwide cohort study of patients with clinical early-stage ovarian cancer. Eur J Cancer. 2016;66:83–90. doi: 10.1016/j.ejca.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Maggioni A, Benedetti Panici P, Dell’Anna T, Landoni F, Lissoni A, Pellegrino A, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer. 2006;95:699–704. doi: 10.1038/sj.bjc.6603323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshita T, Itamochi H, Nishimura R, Numa F, Takehara K, Hiura M, et al. Clinical impact of systematic pelvic and para-aortic lymphadenectomy for pT1 and pT2 ovarian cancer: a retrospective survey by the Sankai Gynecology Study Group. Int J Clin Oncol. 2013;18:1107–1113. doi: 10.1007/s10147-012-0483-8. [DOI] [PubMed] [Google Scholar]
- 18.Kajiyama H, Yoshihara M, Tamauchi S, Yoshikawa N, Suzuki S, Kikkawa F. Sparing surgery for young women with ovarian endometrioid carcinoma: a multicenteric comparative study using inverse probability of treatment weighting. Eur J Obstet Gynecol Reprod Biol X. 2019;4:100071. doi: 10.1016/j.eurox.2019.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Independent predictors of retroperitoneal lymphadenectomy for advanced-stage patients with ovarian high-grade serous carcinoma
Multivariate analysis of a Cox’s hazard model in relation to progression-free survival and overall survival in stage subgroups (unweighted original cohort)
Patient characteristics (weighted cohort)
Standardized differences of the variables
Forest plots of the adjusted HR for death in the unweighted original cohort (A) and weighted cohort with the inverse probability of treatment weighting (B).