Abstract
Objective
This study aimed to analyze the trends in cervical cancer screening rates, including organized and opportunistic cancer screening rates, with the Papanicolaou test among Korean women.
Methods
Data were collected from a nationwide, cross-sectional, Korean National Cancer Screening Survey. To evaluate the cervical cancer screening rates, we used the screening approach of “cervical cancer screening rate with recommendation,” defined as the proportion of women who underwent the Papanicolaou test during the previous 2 years according to the Protocol of National Cancer Screening Program for Cervical Cancer in Korea. The joinpoint regression analysis, which describes the annual percent change (APC), was performed to detect significant changes in cervical cancer screening rates in women aged 30-74 years during 2005-2020.
Results
The cervical cancer screening rate was 56.0% in 2020. From 2005 to 2013, there was a rising trend in cervical cancer screening rates (APC=2.70%, 95% confidence interval [CI]:1.05 to 4.38), followed by a falling trend (APC=−2.67%, 95% CI:−4.3 to −1.01). The falling trend was significantly associated with age (≥40 years), education level (below the 15th grade), household income (below the middle-income level), and residence (all residential areas).
Conclusion
The recent falling trend was more common in women with a low socioeconomic status, which suggests that there is a socioeconomic gap in cervical cancer screening. Moreover, young women in their thirties had a low screening rate. Therefore, an active participation strategy for women vulnerable to cervical cancer is required.
Keywords: Early Detection of Cancer, Uterine Cervical Neoplasms, Papanicolaou Test, Socioeconomic Factors, Female, Cross-Sectional Studies
Synopsis
We evaluated trends in cervical cancer screening rates in women aged 30–74 years in 2005–2020. The screening rate rose from 57.0% in 2005 to 67.0% in 2013, and then 56.0% in 2020. The falling trend in the screening rate was significantly associated with low household income, low education level, and all residential areas (especially rural areas).
INTRODUCTION
Globally, approximately 570,000 new cases of cervical cancer were diagnosed and 311,000 women died due to cervical cancer in 2018 [1]. Although the incidence of cervical cancer in Korea is lower than that in other developed countries, new cases and deaths have been consistently reported until recently: 3,500 new cases and 845 deaths in 2018 [2].
The Papanicolaou test (Pap test) is an effective method for screening cervical cancer cases [3,4]. The Korean Guideline for Cervical Cancer Screening and the Protocol of National Cancer Screening Program (NCSP) for cervical cancer have been developed based on the Pap test (Table 1) [5,6,7]. In an organized screening system, the NCSP has offered to conduct a free Pap test every 2 years for all women aged above 30 years since 2002; from 2016, this program also included women in their 20s as target screening recipients [7]. In Korea, cervical cancer screening using the Pap test is also available for the opportunistic screening system. Opportunistic cancer screening entirely depends on individuals’ decisions regarding the test methods and frequency at their own expense. Thus, there is a need for a report on cervical cancer screening rates among all Korean women, which will be a more accurate estimate, as it integrates data from both organized and opportunistic cancer screening systems.
Table 1. The Korean Guideline for Cervical Cancer Screening and the Protocol issued by the NCSP for cervical cancer.
| Year of issue | Korean Guideline for Cervical Cancer Screening | Protocol of NCSP for Cervical Cancer | ||
|---|---|---|---|---|
| 2001 [5] | 2015 [6] | 2002 | 2016 | |
| Target population | Women aged ≥20 yr | Women aged 20–74* yr | Women aged ≥30 yr | Women aged ≥20 yr |
| Test | Pap test | Pap test or liquid-based cytology | Pap test | Pap test |
| Interval | 1 yr | 3 yr | 2 yr | 2 yr |
NCSP, National Cancer Screening Program; Pap, Papanicolaou.
*Women aged >75 years who have undergone ≥3 consecutive Pap tests with negative results within 10 years should stop participating in cervical cancer screening.
The present study aimed to analyze the trends in cervical cancer screening rates, including both the organized and opportunistic cancer screening programs, with the Pap test among Korean women during 2005–2020.
MATERIALS AND METHODS
1. Ethical approval statement
The present study was approved by the National Cancer Center Institutional Review Board of Korea (approval number: NCC2019-0233). Subjects consented to participate in the survey for public purpose, and the requirement for written consent was waived.
2. Study population
We used data from the Korean National Cancer Screening Survey (KNCSS), which is a nationwide cross-sectional survey conducted every year since 2004 and evaluates both organized and opportunistic cancer screening rates among the target screening population of 5 cancers (stomach, liver, colorectal, breast, and cervical cancers). The KNCSS participants were selected through stratified multistage and random sampling according to the geographical area, age, and sex. A detailed description of the target sampling process is provided elsewhere [7]. For data collection, the professional research agency staff visited the selected participants’ houses at least 3 times and conducted face-to-face interviews with them. However, because data collection in 2004 was carried out through computer-assisted telephone interviews, the current analysis excluded that data (Table S1). Women aged 20–29 years were excluded from the trend analysis of cervical cancer screening rates because they were included as participants of the KNCSS since 2014, and thus, only the screening rates for each year were analyzed.
3. Study variables
The KNCSS assesses 2 types of cervical cancer screening rates: lifetime screening rates and screening rates with recommendation. Regarding the lifetime cervical cancer screening rate, study participants were asked the first question, “Have you ever had a cervical cancer screening with the Pap test?” If the answer was “Yes,” then the participants were asked the following question: “When was the last time you had the Pap test?” Based on these questions and answers, we developed an operational definition for “cervical cancer screening rate with recommendation,” defined as the proportion of women who underwent the Pap test during the previous 2 years according to the Protocol of NCSP for Cervical Cancer in Korea. To evaluate the cervical cancer screening rates in our study, we used the “cervical cancer screening rate with recommendation” approach.
To measure the cervical cancer screening rate according to the socioeconomic status, we used the education level and monthly household income variables. These variables have been mainly used to measure the screening rate according to socioeconomic status [8,9]. The education level was categorized into middle school (11 years or less), high school (12–15 years), and university or higher (16 years or more). Monthly household income was classified into tertiles. The first tertile represented the low-income level. The second tertile represented the middle-income level, and the third tertile represented the high-income level.
In addition, the cervical cancer screening rate was assessed in relation to age and residential area (metropolitan, urban, rural).
4. Data analysis
Trends in cervical cancer screening rates were estimated using joinpoint regression with Joinpoint Desktop Software (ver. 4.7.0.0), from the National Cancer Institute (NCI). The joinpoints with the best fitting points were chosen with a significant change in the rate (p<0.05) over the study period [10]. Here, the number of joinpoints from 0 (representing a straight line) to 1 as the best fitting point and the annual percentage change (APC) in each trend segment were estimated. The average APC (AAPC) was calculated as the average trend for the period 2005–2020 [10]. Statistically significant differences in APC and AAPC were estimated at a 95% confidence interval (CI), and the p-value (p<0.05) was determined. We described the trends with estimates of APC based on the cancer trends progress report by the NCI, as follows [11]: 1) STABLE: −0.5≤APC≤0.5, with no statistical significance; 2) NON-SIGNIFICANT CHANGE: APC<−0.5 or APC>0.5, with no statistical significance; 3) RISING: a statically significant APC of >0; 4) FALLING: a statistically significant APC of <0. In addition, we performed descriptive statistics to analyze the annual cervical cancer screening rate and general characteristics of the participants using SAS software (ver. 9.4; SAS Institute, Cary, NC, USA), and the results are presented in Tables S1 and S2.
RESULTS
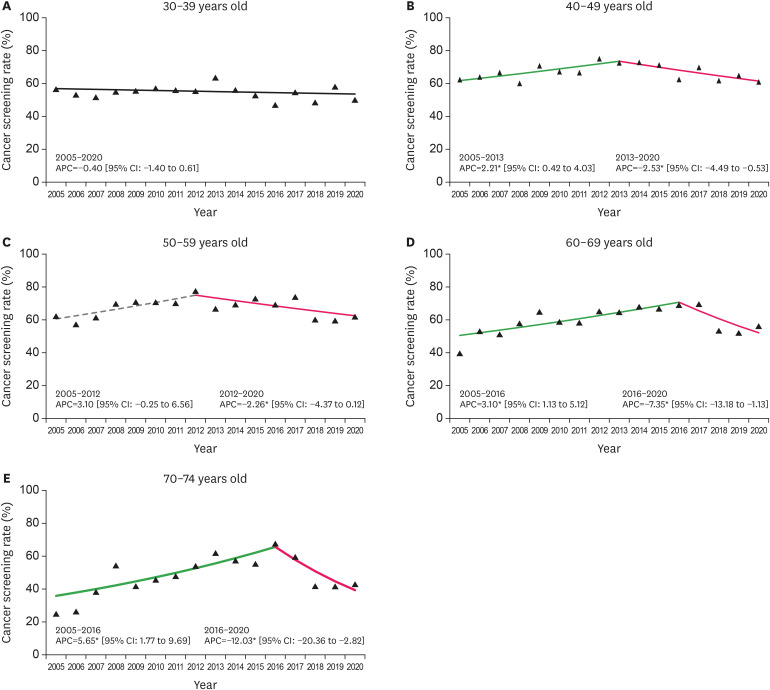
The results of the trend analysis showed that the cervical cancer screening rate started at 57.0% in 2005 and showed a rising trend until 2013 (APC=2.70%, 95% CI:1.05 to 4.38), followed by a falling trend thereafter, reaching 56.0% in 2020 (APC=−2.67%, 95% CI:−4.3 to −1.01) (Fig. 1 and Table S2). As for the cervical cancer screening rate according to age, a falling trend was noted in the recent years in all women aged above 40 years, whereas a stable trend was noted in women aged 30 years old during the entire period (APC=−0.40% during 2005–2020) (Fig. 2). Moreover, the groups with more than the 16th education grade and high-income level showed stable trends; except these groups, we observed falling trends in cervical cancer screening rates in recent years in all subgroups according to education level, monthly household income level, and residential area (Figs. 3, 4, 5). Additionally, 500 women in their 20s have been surveyed each year since 2014. Their screening rates for the period of 2014–2020 were 12.8%, 15.5%, 29.7%, 33.0%, 20.9%, 23.2%, and 26.0% (data not shown).
Fig. 1. Trends in cervical cancer screening rates during 2005–2020. The solid green line denotes a rising trend; the solid pink line denotes a falling trend.
APC, annual percent change; CI, confidence interval.
*p for trends in APCs of <0.05.
Fig. 2. Trends in cervical cancer screening rates by age group during 2005–2020. The solid green line denotes a rising trend; the solid pink line denotes a falling trend; the dashed gray line denotes a non-significant change.
APC, annual percent change; CI, confidence interval.
*p for trends in APCs of <0.05.
Fig. 3. Trends in cervical cancer screening rates by household income during 2005–2020. Monthly household income status classified by tertile. The solid green line denotes a rising trend; the solid pink line denotes a falling trend; the solid black line denotes a stable trend.
APC, annual percent change; CI, confidence interval.
*p for trends in APCs of <0.05.
Fig. 4. Trends in cervical cancer screening rates by education period during 2005–2020. The solid green line denotes a rising trend; the solid pink line denotes a falling trend; the solid black line denotes a stable trend.
APC, annual percent change; CI, confidence interval.
*p for trends in APCs of <0.05.
Fig. 5. Trends in cervical cancer screening rates by residential area during 2005–2020. The solid green line denotes a rising trend; the solid pink line denotes a falling trend; the dashed gray line denotes a non-significant change.
APC, annual percent change; CI, confidence interval.
*p for trends in APCs of <0.05.
DISCUSSION
In the present study, the cervical cancer screening rate showed a rising trend from 2005 to 2013, followed by a falling trend from 2013 to 2020. In particular, the groups with a low socioeconomic status (low income and low education level) had the lowest cervical cancer screening rate, when compared to the other groups; this rate then increased sharply until the early and mid-2010s, and then decreased sharply. The women in their thirties had a stable low screening rate trend.
In the most recent years, the cervical cancer screening rate has decreased from 67.0% in 2013 to 56.0% in 2020 (Table S2). Unfortunately, the latest screening rate was almost the same as the initial screening rate (57.0% in 2005) when the KNCSS was started. This falling trend is in accordance with previously published data from other countries. The National Health System (NHS) in England reported that the cervical cancer screening rate recommended by the United Kingdom National Screening Committee decreased from 75.7% in 2011 to 72.2% in 2020 among women aged 25–64 years [12]. The National Health Interview Survey in the United States reported that the cervical cancer screening rate according to U.S. Preventive Services Task Force (USPSTF) screening recommendations was 82.9% among women aged 21–65 years in 2018, which was seen to be slightly increasing recently, but overall, it was lower than the 85.3% reported in 2005 [13].
A sharp falling trend in the cervical cancer screening rate among groups with a low socioeconomic status was evident since the early and mid-2010s in our study, which could have affected the overall decline of the screening rate in recent years. The low cancer screening rates in the population with a low socioeconomic status have been reported in other countries as well [8,14]. The factors contributing to non- or low-participation in cervical cancer screening in women with a low socioeconomic level were associated to limited accessibility to the healthcare system in terms of the burden of transportation, working schedule, and lack of knowledge and awareness [15,16]. In Korea, according to the KNCSS in 2020, the main reasons for non-participation in cervical cancer screening programs were “because of being healthy” and “no time” (data not shown). In this context, interventions to increase access to the healthcare system (i.e., vaginal self-sampling as an alternative Pap test to reduce logistical barriers about time) or to change the perception of cervical cancer and screening (i.e., educational intervention by community health workers or leaders who are more familiar with non-attendants), especially for persons with a low socioeconomic level [17,18,19], have been conducted, and a positive performance for increasing screening rates has been recorded. So far, in Korea, studies on the feasibility of self-sampling and intervention studies to increase knowledge on cervical cancer prevention have been conducted [20,21,22], but in-depth studies involving women with a low socioeconomic status who do not participate in cervical cancer screening are rare.
The recent falling trend of cervical cancer screening rates can be considered along with government policies. With an increase in the incidence rate of cervical cancer among women in their 20s [23,24], the target age for cervical cancer screening in the NCSP has been extended to the 20s since 2016 [7]. Accordingly, public activities to engage women in cervical cancer screening have focused more on the women in their 20s. In addition, the human papilloma virus (HPV) vaccination program was first introduced in Korea in 2007, and HPV vaccination is provided free of charge to 12-year-old girls since 2016. Although the awareness of HPV vaccination has significantly increased over the past decade, the willingness to take the vaccine has decreased, and the uncertainty of its effectiveness has increased [25]. In a similar context, the vaccination rate among women aged 19–59 years in 2013 was low (12.6%), and was 25.8% among HPV-infected women aged 20-60 years in 2010–2016 [26,27]. Moreover, the cervical cancer screening rate of young women in their 30s was continuously low, along with the recent marked falling in cervical cancer screening rates in our study. From the above, the national policies on cervical cancer prevention are increasing social awareness of cervical cancer prevention, but they are insufficient for triggering health behaviors to prevent cervical cancer in women. Meanwhile, clinics or hospitals designated as national cervical cancer screening units by the National Health Insurance Service is gradually increasing from 1,642 in 2007 to 3,824 in 2019. However, in our study, the screening rate for cervical cancer among rural residents declined from 2013. This suggests that Korean medical institutions are concentrated in large cities [28], and geographic disparities in accessibility to medical institutions may affect the low screening rate.
Our study has several limitations. First, the data were collected by staff members that visited the participants in their homes and conducted face-to-face interviews; therefore, social desirability bias could have been introduced by the participants’ desire to show a positive attitude and desired social behavior. Second, the reasons for not participating in cervical cancer screening could not be identified among the participants’ responses; therefore, we could not discuss the findings more explicitly. Nevertheless, our study used representative samples in Korea, and thus the derived findings are sufficiently meaningful and generalizable.
In conclusion, the cervical cancer screening rate has significantly decreased in recent years among women aged 30–74 years. This trend is more significant in groups with a low socioeconomic status, suggesting some socioeconomic disparities in cervical cancer screening. Further, young women in their thirties continued to have a low screening rate. Therefore, in order to increase participation in cervical cancer screening, active efforts by the government to select a target group with a low cervical cancer screening rate and increase their attitude toward cervical cancer prevention are needed.
ACKNOWLEDGEMENTS
We deeply appreciated all members of Division of Cancer Prevention & Early Detection for data curation and analysis.
Footnotes
Funding: This study was supported by a Grant-in Aid for Cancer Research and Control from the National Cancer Center of Korea (Grant No. 2210771-1). Funding bodies have no role in the study design, study setting, analysis, or writing of the manuscript.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: S.M., C.K.S., J.J.K.
- Formal analysis: Y.L.Y., S.S.Y., P.B.
- Project administration: J.J.K.
- Validation: J.J.K.
- Writing - original draft: S.H.Y.
- Writing - review & editing: S.H.Y., S.M., C.K.S., J.J.K.
SUPPLEMENTARY MATERIALS
Distribution (%) of sociodemographic characteristics of the respondents according to the Korean National Cancer Screening Survey (KNCSS), 2005–2020
Cervical cancer screening rates (%) in Korea during 2005–2020
References
- 1.Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health. 2020;8:e191–e203. doi: 10.1016/S2214-109X(19)30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong S, Won YJ, Lee JJ, Jung KW, Kong HJ, Im JS, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2018. Cancer Res Treat. 2021;53:301–315. doi: 10.4143/crt.2021.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jun JK, Choi KS, Jung KW, Lee HY, Gapstur SM, Park EC, et al. Effectiveness of an organized cervical cancer screening program in Korea: results from a cohort study. Int J Cancer. 2009;124:188–193. doi: 10.1002/ijc.23841. [DOI] [PubMed] [Google Scholar]
- 4.Bui CN, Choi E, Suh M, Jun JK, Jung KW, Lim MC, et al. Trend analysis of process quality indicators for the Korean National Cervical Cancer Screening Program from 2005 to 2013. J Gynecol Oncol. 2021;32:e14. doi: 10.3802/jgo.2021.32.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SY, Khang SK. Guidelines for the screening of uterine cervical cancer. J Korean Med Assoc. 2002;45:1005–1014. [Google Scholar]
- 6.Min KJ, Lee YJ, Suh M, Yoo CW, Lim MC, Choi J, et al. The Korean guideline for cervical cancer screening. J Gynecol Oncol. 2015;26:232–239. doi: 10.3802/jgo.2015.26.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong S, Lee YY, Lee J, Kim Y, Choi KS, Jun JK, et al. Trends in cancer screening rates among Korean men and women: results of the Korean National Cancer Screening Survey, 2004–2018. Cancer Res Treat. 2021;53:330–338. doi: 10.4143/crt.2020.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bao H, Zhang L, Wang L, Zhang M, Zhao Z, Fang L, et al. Significant variations in the cervical cancer screening rate in China by individual-level and geographical measures of socioeconomic status: a multilevel model analysis of a nationally representative survey dataset. Cancer Med. 2018;7:2089–2100. doi: 10.1002/cam4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi E, Lee YY, Suh M, Lee EY, Mai TT, Ki M, et al. Socioeconomic inequalities in cervical and breast cancer screening among women in Korea, 2005–2015. Yonsei Med J. 2018;59:1026–1033. doi: 10.3349/ymj.2018.59.9.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute. Cancer Trends Progress Report. Online Summary of Trends in US Cancer Control Measures [Internet] Bethesda, MD: National Cancer Institute; 2021. [cited 2021 Jul 27]. Available from: https://progressreport.cancer.gov/methodology. [Google Scholar]
- 12.National Health Service. Cervical Screening Programme, England-2017-18 [NS] [Internet] 2018. [cited 2021 Jul 27]. Available from: https://files.digital.nhs.uk/B1/66FF72/nhs-cerv-scre-prog-eng-2017-18-report.pdf.
- 13.Sabatino SA, Thompson TD, White MC, Shapiro JA, de Moor J, Doria-Rose VP, et al. Cancer screening test receipt - United States, 2018. MMWR Morb Mortal Wkly Rep. 2021;70:29–35. doi: 10.15585/mmwr.mm7002a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuche-Berenguer B, Sakellariou D. Socioeconomic determinants of cancer screening utilisation in Latin America: a systematic review. PLoS One. 2019;14:e0225667. doi: 10.1371/journal.pone.0225667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferdous M, Lee S, Goopy S, Yang H, Rumana N, Abedin T, et al. Barriers to cervical cancer screening faced by immigrant women in Canada: a systematic scoping review. BMC Womens Health. 2018;18:165. doi: 10.1186/s12905-018-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akinlotan M, Bolin JN, Helduser J, Ojinnaka C, Lichorad A, McClellan D. Cervical cancer screening barriers and risk factor knowledge among uninsured women. J Community Health. 2017;42:770–778. doi: 10.1007/s10900-017-0316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rees I, Jones D, Chen H, Macleod U. Interventions to improve the uptake of cervical cancer screening among lower socioeconomic groups: a systematic review. Prev Med. 2018;111:323–335. doi: 10.1016/j.ypmed.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 18.O’Donovan J, O’Donovan C, Nagraj S. The role of community health workers in cervical cancer screening in low-income and middle-income countries: a systematic scoping review of the literature. BMJ Glob Health. 2019;4:e001452. doi: 10.1136/bmjgh-2019-001452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savas LS, Atkinson JS, Figueroa-Solis E, Valdes A, Morales P, Castle PE, et al. A lay health worker intervention to improve breast and cervical cancer screening among Latinas in El Paso, Texas: a randomized control trial. Prev Med. 2021;145:106446. doi: 10.1016/j.ypmed.2021.106446. [DOI] [PubMed] [Google Scholar]
- 20.Shin HY, Lee B, Hwang SH, Lee DO, Sung NY, Park JY, et al. Evaluation of satisfaction with three different cervical cancer screening modalities: clinician-collected Pap test vs. HPV test by self-sampling vs. HPV test by urine sampling. J Gynecol Oncol. 2019;30:e76. doi: 10.3802/jgo.2019.30.e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang SH, Shin HY, Lee D, Sung NY, Lee B, Lee DH, et al. A prospective pilot evaluation of vaginal and urine self-sampling for the Roche cobas4800 HPV test for cervical cancer screening. Sci Rep. 2018;8:9015. doi: 10.1038/s41598-018-27390-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mo HS, Choi KB, Kim JS. Effects of a peer cervical cancer prevention education program on Korean female college students’ knowledge, attitude, self-efficacy, and intention. Korean J Adult Nurs. 2013;25:736–746. [Google Scholar]
- 23.Moon EK, Oh CM, Won YJ, Lee JK, Jung KW, Cho H, et al. Trends and age-period-cohort effects on the incidence and mortality rate of cervical cancer in Korea. Cancer Res Treat. 2017;49:526–533. doi: 10.4143/crt.2016.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong S, Won YJ, Park YR, Jung KW, Kong HJ, Lee ES, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2017. Cancer Res Treat. 2020;52:335–350. doi: 10.4143/crt.2020.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh JK, Jeong BY, Yun EH, Lim MK. Awareness of and attitudes toward human papillomavirus vaccination among adults in Korea: 9-year changes in nationwide surveys. Cancer Res Treat. 2018;50:436–444. doi: 10.4143/crt.2017.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim MA, Han GH, Kim JH, Seo K. Current status of human papillomavirus infection and introduction of vaccination to the National Immunization Program in Korea: an Overview. J Korean Med Sci. 2018;33:e331. doi: 10.3346/jkms.2018.33.e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seong J, Ryou S, Yoo M, Lee J, Kim K, Jee Y, et al. Status of HPV vaccination among HPV-infected women aged 20–60 years with abnormal cervical cytology in South Korea: a multicenter, retrospective study. J Gynecol Oncol. 2020;31:e4. doi: 10.3802/jgo.2020.31.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong JH, Shin DB. Analysis of patient disease-oriented medical service areas using the Korea Health Panel Study. J Korean Assoc Geogr Inf Stud. 2020;28:59–67. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution (%) of sociodemographic characteristics of the respondents according to the Korean National Cancer Screening Survey (KNCSS), 2005–2020
Cervical cancer screening rates (%) in Korea during 2005–2020