Abstract
Purpose
Emerging evidence from animal models suggests that intermittent hypoxia due to obstructive sleep apnea (OSA) is a risk factor for breast cancer. Despite their biological plausibility, human epidemiological studies have reported conflicting results. Therefore, we conducted a meta-analysis to delineate this relationship.
Methods
We searched the PubMed, Embase, Scopus, and Cochrane Library databases for eligible studies from inception until June 6, 2021. Two reviewers selected randomized trials or observational studies reporting the association between OSA and breast cancer incidence compared with those without OSA. Two reviewers extracted relevant data and assessed the quality of evidence using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework and Newcastle-Ottawa Scale (NOS). We pooled the maximally covariate-adjusted hazard ratios (HRs) using a random-effects inverse variance-weighted meta-analysis and performed pre-specified subgroup analyses.
Results
We included six studies out of 1,707 records, comprising a combined cohort of 5,165,200 patients. All studies used the International Classification of Diseases codes to classify OSA and breast cancer. OSA patients had a 36% increased breast cancer risk (HR, 1.36; 95% confidence interval [CI], 1.03–1.80; N = 6, I2 = 96%) compared to those without OSA. Most studies adjusted for confounders, such as age, sex, obesity, diabetes mellitus, alcohol use, and hypertension. Subgroup analyses for studies with (1) multivariate adjustment and (2) at least five years of follow-up yielded HRs of 1.35 (95% CI, 0.98–1.87; N = 5, I2 = 96%) and 1.57 (95% CI, 1.14–2.18; N = 4; I2 = 90%), respectively. One Mendelian randomization study suggested a causal relationship, with a two-fold increase in the odds of breast cancer in patients with OSA.
Conclusion
This meta-analysis suggested that OSA is a risk factor for breast cancer. Future studies should explore the dose-response relationship between OSA and breast cancer, and whether treatment may mitigate breast cancer risk or progression.
Keywords: Breast Neoplasms; Hypoxia; Incidence; Mortality; Sleep Apnea, Obstructive
INTRODUCTION
Breast cancer is the most common malignancy in women worldwide [1]. The global incidence of breast cancer has risen steadily at an annual rate of 3.1% over the last 4 decades [2]. Early diagnosis of breast cancer is associated with a significantly better prognosis, making it important to identify risk factors and screen high-risk individuals at an earlier age [3]. Determining the modifiable risks of disease progression may allow intervention and secondary prevention, potentially improving survival.
While hypoxia is a central feature of breast cancer carcinogenesis [4], few studies have explored how hypoxic diseases such as obstructive sleep apnea (OSA) may influence the natural course of breast cancer. OSA, the most prevalent form of sleep-disordered breathing [5], is characterized by recurrent episodes of hypopnea and apnea during sleep [6]. Evidence from in vitro and murine studies suggests that hypoxia caused by sleep apnea plays a significant role in tumor formation and progression [7]. In particular, OSA has been shown to increase the risk of breast cancer metastasis [8]. Although the exact biological mechanism linking OSA and breast cancer remains to be discovered, several explanations for the development of breast cancer may be considered. This includes a variety of harmful mechanisms such as intermittent hypoxia, hypercapnia, increased sympathetic activation, and sleep fragmentation [9]. Murine models have shown that activation of the hypoxia signalling pathway may result in downstream effects that promote angiogenesis and tumor growth [9].
Despite biological plausibility, epidemiological associations have been inconsistent. While some early studies with adjusted hazards showed a null association between OSA and breast cancer [10,11], subsequent studies with a subgroup of breast cancer patients demonstrated that OSA patients were indeed at a higher risk of breast cancer [12,13]. While three meta-analyses previously investigated the association between OSA and overall cancer incidence [14,15,16], evidence has shown a differential association between OSA and various cancer types [10,12,17,18]. Thus, these meta-analyses may not be representative of the specific association between OSA and breast cancer. A recent meta-analysis provided data on the association between OSA and individual cancer types, including breast cancer.[19] However, this study simply reported the descriptive incidence rate of breast cancer in patients with OSA without performing any statistical comparison to those without OSA. In addition, the incidence rates were not adjusted for major confounders, such as age and obesity, which are well-known risk factors for breast cancer. To date, no meta-analysis has investigated the covariate-adjusted association between breast cancer and OSA has been conducted.
Given that breast cancer is the most common malignancy in women, this warrants a specific investigation of its association with OSA. Therefore, we conducted a systematic review and meta-analysis to evaluate the relationship between OSA and breast cancer.
METHODS
This review is composed of an a priori systematic review protocol registered on PROSPERO (CRD42021220836) and is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [20]. The PRISMA checklist is included in Supplementary Table 1.
Search strategy
We searched four databases (PubMed, Embase, Scopus, and Cochrane Library) from inception until June 6, 2021, using the following free text search strategy: (sleep apnea OR nocturnal hypoxia OR nocturnal hypoxemia) AND cancer AND (incidence OR incident OR mortality). “Sleep-disordered breathing” was not used as a search criteria as this is a heterogenous umbrella term that includes primary snoring, OSA, central sleep apnea and sleep-related hypoventilation syndromes [21]. A manual search of the relevant bibliographies was also performed. “Breast cancer” was not used as a search criteria as this would exclude studies which reported overall cancer incidence and only included breast cancer risk as part of the subgroup analysis. The full search strategy is detailed in Supplementary Table 2.
Study selection
Two authors independently selected potential studies, aided by the data management software Rayyan QCRI [22]. The initial screening was based on the title and abstract, while the final inclusion was based on full texts where available. We included randomized controlled trials or longitudinal studies of adults aged at least 18 years, which reported an association between sleep apnea and breast cancer incidence, in comparison to healthy controls without sleep apnea or nocturnal hypoxemia or with less severe forms of these conditions. We accepted the presence or severity of sleep apnea measured by clinical diagnosis, such as International Classification of Diseases (ICD) diagnostic codes, as well as the presence or severity of nocturnal hypoxemia measured by pulse oximetry or any other objective measurements or indices of oxygen saturation, such as sleep duration with arterial oxygen saturation < 90% (T90%). We accepted studies that reported overall cancer incidence and incidence by cancer site, including breast cancer. We accepted conference abstracts, academic dissertations, and other gray literature as per the protocol that fulfilled the above criteria. Case reports, reviews, letters, and non-English publications were excluded.
Data extraction
Data extraction and study quality assessment were independently performed by two authors (DYWT and NTKW). Any disagreements were discussed and resolved by a third reviewer. The following data were from each article into a standardized extraction spreadsheet template: first author, year published, study design, setting, country, sample size, percentage male, mean/median age, body mass index (BMI), intervention/exposure, outcomes, covariates, statistical methods and key findings. All studies that assessed the effect of OSA on the risk of overall cancer had their hazard ratios (HRs) stratified based on cancer type [10,23]. The relevant HRs for the breast cancer subgroups were then extracted.
Quality assessment
As all included studies were observational, we used the Newcastle-Ottawa Scale (NOS) to evaluate the risk of bias at the study level [24,25]. Two authors independently graded studies as having a high (< 5 stars), moderate (5–7 stars), or low (≥ 8 stars) risk of bias according to the NOS grading in past reviews, as shown in Supplementary Tables 3 and 4. The quality of pooled evidence at the outcome level was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, which accounts for statistical heterogeneity, publication bias, risk of bias, indirectness, and statistical imprecision, as shown in Supplementary Table 5.
Statistical analysis
We found sufficient data in our systematic review to meta-analyze the longitudinal associations between baseline OSA measured by ICD implantation and breast cancer incidence. Using the generic inverse variance method, we separately pooled the HRs for breast cancer incidence as measured by the ICD (presence versus absence of diagnosis). Only the study that reported T90% data was excluded from the statistical analysis, as insufficient studies reported T90% to conduct any analysis [26]. We favored maximally covariate-adjusted estimates, where available, to minimize errors introduced by confounders, and included one study that reported standardized incidence ratios (SIRs) in the pooled analysis [13,27], as SIRs sufficiently approximate HRs [28]. We used the random-effects model in all analyses to account for anticipated heterogeneity in the observational estimates and evaluated the extent of between-study heterogeneity using the I2 statistic [29]. For outcomes with significant heterogeneity, we performed pre-specified sensitivity analyses that included only studies that fulfilled the following characteristics: (1) multivariate adjustment and (2) a median follow-up duration of at least five years. There were insufficient studies (< 10 per outcome) to assess publication bias via visual inspection of funnel plot asymmetry, Egger’s bias, or trim-and-fill, as planned. We conducted all analyses using RevMan (version 5.4; Cochrane Collaboration, London) in accordance with statistical approaches from the Cochrane Handbook [25], and considered a two-sided p value <0.05 as statistically significant.
RESULTS
Study selection
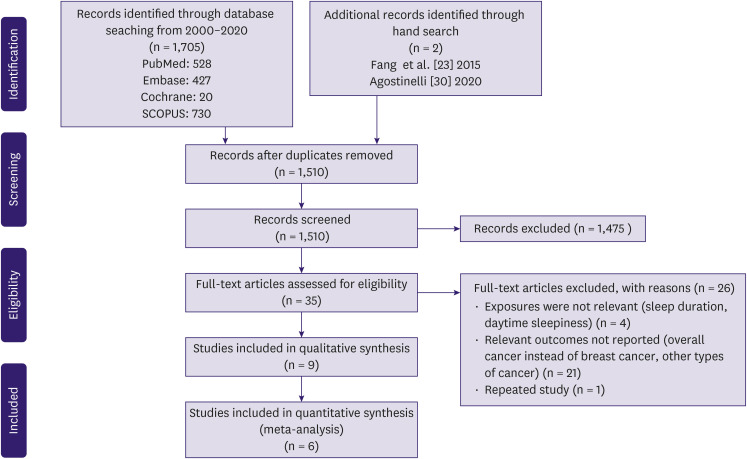
A PRISMA flow diagram is shown in Figure 1. A literature search of four databases (PubMed, Embase, Cochrane, and SCOPUS) retrieved 1705 results, and a manual reference search yielded 2 additional studies [23,30]. In total,197 duplicates were excluded. Title and abstract screening excluded a further 1,475 articles, as they did not report breast cancer incidence, did not report HRs or incidence ratios, were inappropriate study types, or pooled breast cancer with other comorbidities. The full-text screening excluded 26 articles. Nine studies were included in this review [10,11,12,13,23,26,31,32,33].
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram of study selection.
Diagnosis of OSA and breast cancer
Of the nine studies, seven used the ICD to define OSA [10,11,12,13,23,31,32], one used T90% [26], and one used the apnea-hypopnea index (AHI) [33]. All studies reported OSA, except for those by Chang et al. [31] and Sillah et al. [13], which reported sleep apnea. Sillah et al. [13] reported that more than 90% of sleep apnea cases are associated with OSA. One study by Huang et al. [11] relied on self-reported clinically diagnosed OSA and conducted a validation study for a random sample of patients to ascertain the veracity of the self-reported diagnoses, which revealed that all patients had a clinical diagnosis of OSA based on an objective method. Seven studies that used ICD to define OSA also used ICD to define breast cancer cases.
Study characteristics
Among the seven studies using ICD, two by Chang et al. [31] and Fang et al. [23] drew from the same cohort using the National Health Insurance Research Database of Taiwan. We excluded the study by Fang et al. [23] because of the lower quality of evidence (as determined by the NOS score) and adjustment for a smaller number of covariates. Sensitivity analysis, excluding either study, did not alter the meta-analysis results. Hence, the meta-analysis included six independent observational studies using ICD published between 2014 and 2020, comprising a total cohort of 5,165,200 patients and 27,136 breast cancer cases, excluding those that were not reported [10,11,12,13,31,32]. Of the six studies, two were breast cancer-specific, while four reported HRs for overall cancer incidence, as well as individual cancer types, including breast cancer. Four studies were conducted in the United States, one in Taiwan, and one in Korea. One study was prospective [11], while five were retrospective [10,12,13,31,32]. The median follow-up duration ranged from 3.48 to 8 years. All six studies were adjusted for age and sex, and most were adjusted for important confounders such as obesity, type 2 diabetes mellitus, alcohol use, and hypertension. One study using T90% and a Mendelian randomization study were included in the systematic review. The baseline characteristics of the included studies are presented in Tables 1 and 2.
Table 1. Characteristics of studies included in meta-analysis.
| Study | Study design | Sample | Setting/country | Mean/median age | % Male | Covariates | Exposure/case definition | Median follow-up duration (yr) | Hazard ratio (95% CI) | Newcastle-Ottawa Scale score |
|---|---|---|---|---|---|---|---|---|---|---|
| Chang et al. [31] 2014 | Retrospective matched case-control | 5,076 | Administrative database | Not reported | 0 | Age, sex, monthly income, urbanization level, geographic region, hypertension, hyperlipidemia, diabetes, alcohol use disorder, and obesity | ICD-9 codes: 327.23, 780.51, 780.53, 780.57. | 5 | 2.09 (1.06–4.12) | 9 |
| Taiwan | Subjects were required to have received Polysomnography and all ICD codes must have been assigned by pulmonologist, otolaryngologist or neurologist. | |||||||||
| Choi et al. [32] 2019 | Retrospective matched cohort | 274,201 | Administrative database | 48.7 | 0 | Age, sex, income level, diabetes, hypertension, dyslipidemia | At least one claim under ICD-10 code G47.3.* | 3.7 | 1.2 (1.04–1.39) | 7 |
| Korea | ||||||||||
| Gozal et al. [10] 2016 | Retrospective matched cohort | 3,408,906 | Administrative database | Not reported | 50.2 | Age, sex, morbid obesity, hypertension, type 2 diabetes, ischemic heart disease, coronary heart failure, stroke, cardiac arrhythmias, and depression | ICD-9-CM codes: | 3.48 | 0.95 (0.93–0.98) | 8 |
| United States | Obstructive sleep apnea: 327.23, 327.20, 327.29, 780.51, 780.53, 780.57. | |||||||||
| Continuous positive airway pressure: E0601, E0470, E0471 | ||||||||||
| Includes both Obstructive sleep apnea diagnosis and Continuous positive airway pressure | ||||||||||
| Huang et al. [11] 2021 | Prospective cohort | 65,330 | Community-based | 73 | 0 | Age, sex, race/ethnicity, family history of cancer, Body Mass Index, height, pack-years of smoking, alcohol drinking, physical activity, sleep duration, duration of hormonal therapy use by type, history of type 2 diabetes, aspirin use, and recent physical examination | Nurses self-reported clinically diagnosed sleep apnea. | 8 | 1.1 (0.91–1.33) | 5 |
| United States | Additional validation study conducted; all 108 randomly sampled nurses were confirmed to have diagnosis cased on Polysomnography in medical records. | |||||||||
| Jara et al. [12] 2020 | Retrospective matched cohort | 1,377,285 | Administrative database | 55.2 | 94 | Age, sex, year of cohort entry, smoking status, alcohol use, obesity, and Deyo-modified Charlson Comorbidity Index | ICD-9-CM codes: 327.20, 327.23, 327.29, 780.51, 780.53, 780.57, 278.03 | 7.4 | 2.17 (1.83–2.58) | 7 |
| United States | To prevent misclassification, patients were required to have a diagnosis code in at least 1 inpatient or 2 outpatient encounters. Subgroup analysis conducted for Polysomnography ICD-9 codes preceding Obstructive sleep apnea ICD-9 codes. | |||||||||
| Sillah et al. [13] 2018 | Retrospective cohort | 34,402 | Clinic-based | 51.6 | 57.4 | Age and sex | ICD 9 codes: 327.20, 327.21, 327.23, 327.27, 327.29, 780.51, 780.53, and 780.57.* | 5.3 | 1.43 (1.25–1.63) | 6 |
| United States |
CI, confidence interval; ICD, International Classification of Diseases.
*These studies were considered to have not used any ancillary metric to verify ICD codes.
Table 2. Baseline characteristics of studies included in systematic review.
| Study | Study design | Sample | Setting/country | Mean/median age | % Male | Covariates | Median follow-up duration (yr) | Hazard ratio (95% CI) | Newcastle-Ottawa scale score |
|---|---|---|---|---|---|---|---|---|---|
| Fang et al. [23] 2015 | Retrospective nested case-control | 205,266 | Administrative database | NR | Not reported | Age, sex, income, region, urbanization, and CCI | 11 | 2.1 (1.16–3.8) | 8 |
| Taiwan | |||||||||
| Gao et al. [33] 2020 | Mendelian randomization | 4,378 | Clinic-based | NR | 0 | Age, sex, smoking status, family history of cancer and BMI | NR | European population: 2.47 (1.86–3.27) | 6 |
| China | Asian population: 1.33 (1.13–1.56) | ||||||||
| Justeau et al. [26] 2020 | Prospective cohort | 8,748 | Clinic-based | 61 | 64.5 | Age, sex, BMI, smoking status, alcohol intake, diabetes, hypertension, medical history of cardiac disease and Chronic obstructive pulmonary disease, marital status, type of sleep study, and study site | 5.8 | Mild OSA: 2.04 (1.05–3.98) | 9 |
| France | Moderate OSA: 1.40 (0.69–2.87) | ||||||||
| Severe OSA: 1.14 (0.50–2.58) |
CI, confidence interval; CCI, Charlson Comorbidity Index; BMI, body mass index; NR, not reported; OSA, obstructive sleep apnea.
Study quality
The studies scored a range of five to nine using the NOS scale, which corresponds to a low-to-moderate risk of bias.
Meta-analysis of OSA and breast cancer risk based on ICD
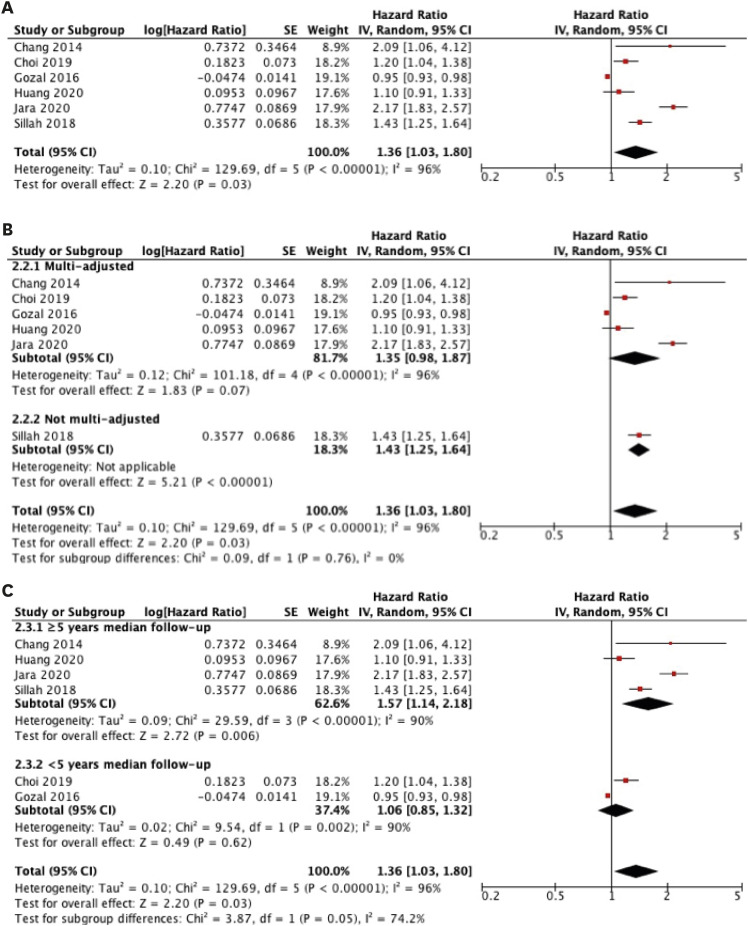
Six studies assessing the relationship between OSA and breast cancer risk were included for overall analysis in our meta-analysis [10,11,12,13,31,32]. The combined effect estimations (HRs) using the random-effects model are presented in Figure 2. The overall results suggest a statistically significant 36% increase in breast cancer risk (combined HR, 1.36; 95% confidence interval [CI], 1.03–1.80). Statistically significant heterogeneity was found across the included studies (I2 = 96%, p < 0.00001).
Figure 2. (A) Random-effects meta-analyses of the association between obstructive sleep apnea diagnosed based on International Classification of Diseases with the incidence of breast cancer and pre-specified subgroup analyses for studies with (B) multi-adjustment and (C) median follow-up duration of at least five years. Black diamonds are the estimated pooled HR for each meta-analysis; red box sizes reflect the relative weight apportioned to studies in the meta-analysis.
SE = standard error; HR = hazard ratio; CI = confidence interval.
Subgroup analysis for studies with (1) multi-variate adjustment and (2) median follow-up duration of at least five years
A subgroup analysis was performed for multi-adjusted studies, as shown in Figure 2. The study by Sillah et al. [13] was excluded because the results were adjusted for age and sex. The multi-adjusted subgroup showed an equivocal association, with an HR (combined HR, 1.35; 95% CI, 0.98–1.87) similar to that of the overall analysis.
Subgroup analysis was performed for studies with a median follow-up duration of at least five years, as shown in Figure 2. Studies with at least five years of follow-up showed a larger significant association (combined HR, 1.57; 95% CI, 1.14–2.18; I2 = 90%, p < 0.00001) compared to the overall analysis. Conversely, the subgroup with less than five years of follow-up showed no association between OSA and breast cancer risk (combined HR, 1.06; 95% CI, 0.85–1.32; I2 = 90%; p < 0.002).
OSA and breast cancer risk based on other measures of OSA
One study that assessed the association between nocturnal hypoxemia measured by T90% and breast cancer incidence was included in our systematic review. Justeau et al. [26] found that patients with mild nocturnal hypoxemia had a significantly elevated risk of breast cancer (adjusted HR, 2.04; 95% CI, 1.05–3.98). However, this association did not hold for those with moderate (adjusted HR, 1.40; 95% CI, 0.69–2.87) and severe nocturnal hypoxemia (adjusted HR, 1.14; 95% CI, 0.50–2.58). No studies have linked other OSA measures, such as AHI and oxygen desaturation index (ODI), to breast cancer risk.
OSA and breast cancer mortality based on ICD
One study using ICD reported no association between OSA and breast cancer mortality (HR, 1.01; 95% CI, 0.80–1.27) [10].
Summary of evidence for a causal relationship between OSA and breast cancer
A Mendelian randomization study was included in our systematic review [33]. Mendelian randomization studies have been suggested as a method to approximate causality and have been widely used in other studies linking risk factors to particular outcomes [34]. Gao et al. [33] investigated the effect of a genetically determined higher risk of OSA on breast cancer risk in two populations. Significant positive associations between genetic risk for OSA and breast cancer risk were found in both the European (OR, 2.47 for BC risk per log-odds increment in OSAS risk; 95% CI, 1.86–3.27) and Asian (OR, 1.33 for BC risk per log-odds increment in OSAS risk; 95% Cl, 1.13–1.56) populations. This study provides a plausible basis for the findings of the meta-analysis.
DISCUSSION
In this meta-analysis of 7 studies comprising a combined cohort of 5,370,466 patients, we demonstrated that patients with a prior OSA diagnosis had a 36% increase in breast cancer risk after multiple adjustments for important confounders. To the best of our knowledge, this is the first meta-analysis to comprehensively summarize the evidence regarding the association between OSA and breast cancer risk.
In our pre-specified subgroup analyses, the subgroup comprised of studies with follow-up duration of more than five years showed increased breast cancer risk (HR, 1.57; 95% CI, 1.14–2.18) compared to the overall analysis (HR, 1.36; 95% CI, 1.03–1.80). Of the two studies excluded from this subgroup, Gozal et al. [10]. showed a null association (HR, 0.95; 95% CI, 0.92–0.97), while Choi et al. [32] showed a positive association (HR: 1.2; 95%CI:1.04-1.39). For Gozal et al. [10], despite the large nationwide cohort (3,408,906 patients), the mean follow-up duration was 3.48 years. In the study by Choi et al. [32], the median follow-up duration was similar at 3.7 years. We suggest that chronicity (for a moderate period such as five years) may be necessary for OSA to play a role in breast cancer carcinogenesis. This may be explained by the need for accumulation of mutations in numerous hypoxia-related breast oncogenes over time [35]. Follow-up duration of approximately three years may not provide sufficient time for OSA to exert a significant impact on breast cancer risk, which may explain the lower number of incident breast cancer cases and reduced HR in the two studies excluded. We chose a median follow-up duration of five years as an arbitrary cutoff for the subgroup analysis, as this was deemed a reasonable period that would also provide for a readily available subgroup analysis. The corollary for future research, especially retrospective studies, is that the follow-up duration after OSA diagnosis should be at least five years.
The studies included in our meta-analysis only used ICD codes to define OSA cases, without stratification based on OSA severity. These OSA cases were likely to have been diagnosed using standard overnight polysomnography, which would have reported the severity of OSA [36]. However, information on severity is lost, since six out of seven included studies are retrospective studies that rely on ICD codes stored in health databases. The ICD codes are binary and only indicate the presence or absence of OSA. Hence, we were unable to determine the dose-response relationship between OSA and breast cancer risk. While recent well-conducted studies using different measures of OSA (AHI, T90%, and ODI) have shown an increased overall cancer risk with greater OSA severity [37,38], one study in our systematic review did not show the same trend. Justeau et al. [26] found that mild OSA, as measured by T90%, was more strongly associated with a higher risk of breast cancer than moderate or severe OSA. However, the low incidence of breast cancer (less than 20 cases per stratum of OSA severity) and large CIs suggest that this study may lack the statistical power to detect any associations in the moderate and severe OSA subgroups. Therefore, more studies are needed to elucidate the dose-response relationship between OSA and breast cancer risk.
Our meta-analysis findings are supported by a recent well-conducted Mendelian randomization study, which we included in our systematic review [33]. The Bradford Hill criteria suggest that one key criterion to prove causation is temporality, where exposure occurs before the outcome [39]. However, it is difficult to establish a relationship between OSA and breast cancer, as both are chronic diseases with varying latencies. Mendelian randomization studies overcome this challenge by using genetic variants as proxies for exposure, such that the variants are associated with the exposure but not the outcome. Because these genetic variants are stable after conception, they always precede other potential confounders [34]. This mitigates the generation of reverse causation and confounding, which is often observed in observational studies [40], and allows any relationship between the genetic variants and the outcome to be interpreted as evidence for causality of the exposure on the outcome [34]. The Mendelian randomization study included in the systematic review strongly suggested a causal relationship between OSA and breast cancer incidence, with two-fold increased odds of breast cancer in patients with OSA [33].
Although the exact biological mechanism linking OSA and breast cancer remains to be discovered, several explanations for the development of breast cancer may be considered. Semenza et al. [41] and Patel et al. [42] showed that hypoxia causes increased expression of hypoxia-inducible factor-1 and -2 (HIF-1 and HIF-2) in cellular and murine models. HIF-1 and HIF-2 are key molecules implicated in the pathogenesis of breast cancer, with over 800 direct target genes [43]. HIF is known to stimulate the upregulation and accumulation of pro-angiogenic factors, such as vascular endothelial growth factor, thus augmenting angiogenesis in OSA patients exposed to intermittent hypoxia and allowing for tumor cell proliferation [44]. In addition, repetitive episodes of hypoxia seen in OSA patients may modify the tissue microenvironment and select for tumor cells that can survive periods of hypoxia. Louie et al. [45] identified a stem-like breast cancer cell subpopulation selected for recurrent hypoxia and reoxygenation cycles. The presence of stem-like breast cancer cells may increase the survival and proliferation of tumor cells through indefinite mitosis.
Several studies have suggested a link between hypoxia and a more aggressive cancer phenotype. Chen et al. [46] showed that intermittent hypoxia induces a metastatic phenotype by increasing the expression of genes that predict lung metastasis. Rausch et al. [47] found that increased HIF-1α gene expression in hypoxic adipocytes is associated with a reduction in estrogen receptor alpha (ERα) gene expression in breast cancer cells, which may result in increased tumor progression and poorer prognosis because ERα-negative tumors tend to be more aggressive and likely to metastasize. However, Campos-Rodriguez et al. [48] found no association between sleep-disordered breathing and breast cancer aggressiveness markers in a Spanish population. Furthermore, a study by Gozal et al. [10] showed no association between OSA and breast cancer mortality (HR, 1.01; 95% CI, 0.80–1.27). Hence, further research is required to fully elucidate the relationship between OSA and breast cancer aggressiveness.
We observed statistically significant heterogeneity in our findings (I2 = 96%, p < 0.00001), for which a random-effects model and subgroup analysis were unable to correct. Sensitivity analysis showed that exclusion of any of the seven studies in the meta-analysis did not significantly change the heterogeneity.
There were several potential sources of heterogeneity. First, some of the heterogeneity may be attributed to the insufficient sensitivity and specificity of ICD codes in defining OSA cases [49], resulting in varying degrees of selection bias across studies. Five of the seven included studies used other ancillary metrics in addition to ICD codes to define OSA patients, such as the use of parallel national registries and endorsement by a clinician (Table 1). Second, the studies were adjusted for different types and number of confounders. Third, the use of ICD codes indicating only the presence or absence of OSA and breast cancer did not comprehensively reflect the heterogeneity present in either disease. OSA is known to have a diverse phenotype and clinical presentation, while breast cancer comprises various histological subgroups with varying latencies and prognoses.
While it is theoretically possible to study the effect of OSA on specific breast cancer subtypes using ICD codes, in practice, it may be difficult to draw an association because the sample size for each breast cancer subtype is likely to be extremely small, given the rarity of some subtypes. Therefore, despite the high heterogeneity based on the GRADE framework, we are moderately confident that the results of this meta-analysis are close to the true effect.
Given the numerous risk factors associated with breast cancer, there is a need to consider the potential confounding variables prevalent in patients with both OSA and breast cancer. The independent risk factors for the development of breast cancer and OSA include obesity, smoking, age, and sex. These risk factors are known to lead to earlier onset and more severe manifestations of OSA. Intriguingly, a recent study on sex differences in OSA revealed that women exhibited stronger associations with diabetes, hypertension, age, BMI, and alcohol use than men, making it important to consider these factors as potential confounders leading to breast cancer, which predominantly affects women [50]. However, most of the included studies adjusted for these confounders as covariates, thus mitigating their effects.
The strengths of our study lie in its systematic methodology, inclusion of studies from a diverse range of ethnicities, and adjustment for several important confounders that make our findings more generalizable. All seven studies in the meta-analysis also achieved a NOS score of at least five, indicating a moderate-to-low risk of bias.
Nonetheless, our findings should be interpreted with consideration of their limitations. First, the present study only showed an association between OSA and breast cancer risk. It is unclear whether the treatment of OSA using current methods, such as continuous positive airway pressure therapy, is sufficient to reduce the subsequent risk of breast cancer. Second, six out of seven studies in the meta-analysis were retrospective in nature, making them prone to misclassification bias. Prospective studies have provided a strong evidence base. Third, due to the limitations of the primary studies, we were unable to explore potential differential associations between OSA and breast cancer based on sex or menopausal status. Thus, this meta-analysis may be updated in the next decade when new prospective studies reporting sex differences and menopause status are available.
In this meta-analysis of seven studies comprising a combined cohort of 5,370,466 patients, we demonstrated that patients with a prior OSA diagnosis had a 36% increase in breast cancer risk after multiple adjustments for important confounders. When limited to studies with longer follow-up periods, studies with at least five years of follow-up showed an even larger significant association. Further studies with long follow-up periods specific to breast cancer are needed to elucidate a possible dose-response relationship and to ascertain whether OSA treatment may reduce the incidence of breast cancer.
Footnotes
Conflict of Interest: The authors declare that they have no competing interests.
Availability of Data: Additional data may reasonably be requested from the corresponding author.
Previous Presentations: Presented at ESMO Breast Cancer Virtual Congress 2021.
- Conceptualization: Tan NKW, Tan BKJ, Teo YH.
- Data curation: Yap DWT, Tan NKW, Tan BKJ, Teo YH.
- Formal analysis: Yap DWT, Tan NKW, Tan BKJ, Teo YH.
- Funding acquisition: Tan BKJ.
- Investigation: Yap DWT, Tan NKW, Tan BKJ, Teo YH.
- Methodology: Yap DWT, Tan NKW, Tan BKJ, Teo YH.
- Project administration: Yap DWT, Tan NKW, Tan BKJ, Teo YH.
- Software: Yap DWT, Tan NKW, Tan BKJ, Teo YH.
- Supervision: Yap DWT, Tan NKW, Tan BKJ, Teo YH, Tan VKM, See A, Toh ST.
- Validation: Yap DWT, Tan NKW, Tan BKJ, Teo YH.
- Visualization: Yap DWT, Tan NKW, Tan BKJ, Teo YH.
- Writing - original draft: Yap DWT, Tan NKW, Tan BKJ, Teo YH.
- Writing - review & editing: Yap DWT, Tan NKW, Tan BKJ, Teo YH, Tan VKM, See A, Toh ST.
SUPPLEMENTARY MATERIALS
PRISMA checklist
Search strategy
Evaluation of risk of bias using the Newcastle-Ottawa Scale: (1) case control
Evaluation of risk of bias using the Newcastle-Ottawa Scale: (2) cohort
GRADE assessment
References
- 1.Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17:43–46. doi: 10.7314/apjcp.2016.17.s3.43. [DOI] [PubMed] [Google Scholar]
- 2.Schneider AP, 2nd, Zainer CM, Kubat CK, Mullen NK, Windisch AK. The breast cancer epidemic: 10 facts. Linacre Q. 2014;81:244–277. doi: 10.1179/2050854914Y.0000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L. Early diagnosis of breast cancer. Sensors (Basel) 2017;17:1572. doi: 10.3390/s17071572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y, Zhang H, Wang M, Schmid T, Xin Z, Kozhuharova L, et al. Hypoxia in breast cancer-scientific translation to therapeutic and diagnostic clinical applications. Front Oncol. 2021;11:652266. doi: 10.3389/fonc.2021.652266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonsignore MR, Saaresranta T, Riha RL. Sex differences in obstructive sleep apnoea. Eur Respir Rev. 2019;28:190030. doi: 10.1183/16000617.0030-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Backer W. Obstructive sleep apnea/hypopnea syndrome. Panminerva Med. 2013;55:191–195. [PubMed] [Google Scholar]
- 7.Almendros I, Montserrat JM, Ramírez J, Torres M, Duran-Cantolla J, Navajas D, et al. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur Respir J. 2012;39:215–217. doi: 10.1183/09031936.00185110. [DOI] [PubMed] [Google Scholar]
- 8.Wang T, Gilkes DM, Takano N, Xiang L, Luo W, Bishop CJ, et al. Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A. 2014;111:E3234–E3242. doi: 10.1073/pnas.1410041111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunyor I, Cook KM. Models of intermittent hypoxia and obstructive sleep apnea: molecular pathways and their contribution to cancer. Am J Physiol Regul Integr Comp Physiol. 2018;315:R669–R687. doi: 10.1152/ajpregu.00036.2018. [DOI] [PubMed] [Google Scholar]
- 10.Gozal D, Ham SA, Mokhlesi B. Sleep apnea and cancer: analysis of a nationwide population sample. Sleep (Basel) 2016;39:1493–1500. doi: 10.5665/sleep.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang T, Lin BM, Stampfer MJ, Schernhammer ES, Saxena R, Tworoger SS, et al. Associations of self-reported obstructive sleep apnea with total and site-specific cancer risk in older women: a prospective study. Sleep (Basel) 2021;44:zsaa198. doi: 10.1093/sleep/zsaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jara SM, Phipps AI, Maynard C, Weaver EM. The association of sleep apnea and cancer in veterans. Otolaryngol Head Neck Surg. 2020;162:581–588. doi: 10.1177/0194599819900487. [DOI] [PubMed] [Google Scholar]
- 13.Sillah A, Watson NF, Schwartz SM, Gozal D, Phipps AI. Sleep apnea and subsequent cancer incidence. Cancer Causes Control. 2018;29:987–994. doi: 10.1007/s10552-018-1073-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palamaner Subash Shantha G, Kumar AA, Cheskin LJ, Pancholy SB. Association between sleep-disordered breathing, obstructive sleep apnea, and cancer incidence: a systematic review and meta-analysis. Sleep Med. 2015;16:1289–1294. doi: 10.1016/j.sleep.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Zhang XB, Peng LH, Lyu Z, Jiang XT, Du YP. Obstructive sleep apnoea and the incidence and mortality of cancer: a meta-analysis. Eur J Cancer Care (Engl) 2017;26:e12427. doi: 10.1111/ecc.12427. [DOI] [PubMed] [Google Scholar]
- 16.Tan BK, Teo YH, Tan NK, Yap DW, Sundar R, Lee CH, et al. Association of obstructive sleep apnea and nocturnal hypoxemia with all-cancer incidence and mortality: a systematic review and meta-analysis. J Clin Sleep Med. doi: 10.5664/jcsm.9772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheong AJ, Tan BK, Teo YH, Tan NK, Yap DW, Sia CH, et al. Obstructive sleep apnea and lung cancer: a systematic review and meta-analysis of 4,885,518 participants. Ann Am Thorac Soc. doi: 10.1513/AnnalsATS.202108-960OC. [DOI] [PubMed] [Google Scholar]
- 18.Tan NK, Yap DW, Tan BK, Teo YH, Tan EK, Chan JY, et al. The association of obstructive sleep apnea with melanoma incidence and mortality: a meta-analysis of 5,276,451 patients. Sleep Med. 2021;88:213–220. doi: 10.1016/j.sleep.2021.10.027. [DOI] [PubMed] [Google Scholar]
- 19.Cheng L, Guo H, Zhang Z, Yao Y, Yao Q. Obstructive sleep apnea and incidence of malignant tumors: a meta-analysis. Sleep Med. 2021;84:195–204. doi: 10.1016/j.sleep.2021.05.029. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carden KA, Chervin RD. Consistency and clarity in sleep medicine terminology. J Clin Sleep Med. 2016;12:157–158. doi: 10.5664/jcsm.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fang HF, Miao NF, Chen CD, Sithole T, Chung MH. Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: a nationwide nested case-control study. J Cancer. 2015;6:1140–1147. doi: 10.7150/jca.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-analyses. 2012. [Accessed April 24th, 2019]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp .
- 25.Higgins JPT, Altman DG, Sterne JAC. In: Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Higgins JPT, Churchill R, Chandler J, Cumpston MS, editors. London: Cochrane Collaboration; 2011. Section 13.5.2.3. Tools for assessing methodological quality or risk of bias in non-randomized studies. [Google Scholar]
- 26.Justeau G, Gervès-Pinquié C, Le Vaillant M, Trzepizur W, Meslier N, Goupil F, et al. Association between nocturnal hypoxemia and cancer incidence in patients investigated for OSA: data from a large multicenter French cohort. Chest. 2020;158:2610–2620. doi: 10.1016/j.chest.2020.06.055. [DOI] [PubMed] [Google Scholar]
- 27.Gislason T, Gumundsson EF, Tryggvadottir L. Obstructive sleep apnea and cancer: a nationwide epidemiological survey. J Sleep Res. 2016;25:53. [Google Scholar]
- 28.Hernán MA. The hazards of hazard ratios. Epidemiology. 2010;21:13–15. doi: 10.1097/EDE.0b013e3181c1ea43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Agostinelli G. Association between obstructive sleep apnea and cancer: a survival analysis [thesis] Pittsburgh: University of Pittsburgh; 2020. p. 50. [Google Scholar]
- 31.Chang WP, Liu ME, Chang WC, Yang AC, Ku YC, Pai JT, et al. Sleep apnea and the subsequent risk of breast cancer in women: a nationwide population-based cohort study. Sleep Med. 2014;15:1016–1020. doi: 10.1016/j.sleep.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 32.Choi JH, Lee JY, Han KD, Lim YC, Cho JH. Association between obstructive sleep apnoea and breast cancer: The Korean National Health Insurance Service Data 2007-2014. Sci Rep. 2019;9:19044. doi: 10.1038/s41598-019-55551-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao XL, Jia ZM, Zhao FF, An DD, Wang B, Cheng EJ, et al. Obstructive sleep apnea syndrome and causal relationship with female breast cancer: a mendelian randomization study. Aging (Albany NY) 2020;12:4082–4092. doi: 10.18632/aging.102725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.König IR, Greco FM. Mendelian randomization: progressing towards understanding causality. Ann Neurol. 2018;84:176–177. doi: 10.1002/ana.25293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jefford CE, Irminger-Finger I. Mechanisms of chromosome instability in cancers. Crit Rev Oncol Hematol. 2006;59:1–14. doi: 10.1016/j.critrevonc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea?: The Rational Clinical Examination systematic review. JAMA. 2013;310:731–741. doi: 10.1001/jama.2013.276185. [DOI] [PubMed] [Google Scholar]
- 37.Gozal D, Farré R, Nieto FJ. Obstructive sleep apnea and cancer: epidemiologic links and theoretical biological constructs. Sleep Med Rev. 2016;27:43–55. doi: 10.1016/j.smrv.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sillah A, Watson NF, Gozal D, Phipps AI. Obstructive sleep apnea severity and subsequent risk for cancer incidence. Prev Med Rep. 2019;15:100886. doi: 10.1016/j.pmedr.2019.100886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 41.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 42.Patel SA, Simon MC. Biology of hypoxia-inducible factor-2alpha in development and disease. Cell Death Differ. 2008;15:628–634. doi: 10.1038/cdd.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schödel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:e207–e217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Louie E, Nik S, Chen JS, Schmidt M, Song B, Pacson C, et al. Identification of a stem-like cell population by exposing metastatic breast cancer cell lines to repetitive cycles of hypoxia and reoxygenation. Breast Cancer Res. 2010;12:R94. doi: 10.1186/bcr2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen A, Sceneay J, Gödde N, Kinwel T, Ham S, Thompson EW, et al. Intermittent hypoxia induces a metastatic phenotype in breast cancer. Oncogene. 2018;37:4214–4225. doi: 10.1038/s41388-018-0259-3. [DOI] [PubMed] [Google Scholar]
- 47.Rausch LK, Netzer NC, Hoegel J, Pramsohler S. The linkage between breast cancer, hypoxia, and adipose tissue. Front Oncol. 2017;7:211. doi: 10.3389/fonc.2017.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Campos-Rodriguez F, Cruz-Medina A, Selma MJ, Rodriguez-de-la-Borbolla-Artacho M, Sanchez-Vega A, Ripoll-Orts F, et al. Association between sleep-disordered breathing and breast cancer aggressiveness. PLoS One. 2018;13:e0207591–e0207591. doi: 10.1371/journal.pone.0207591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jolley RJ, Liang Z, Peng M, Pendharkar SR, Tsai W, Chen G, et al. Identifying cases of sleep disorders through International Classification of Diseases (ICD) codes in administrative data. Int J Popul Data Sci. 2018;3:448. doi: 10.23889/ijpds.v3i1.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fietze I, Laharnar N, Obst A, Ewert R, Felix SB, Garcia C, et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences - results of SHIP-Trend. J Sleep Res. 2019;28:e12770. doi: 10.1111/jsr.12770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist
Search strategy
Evaluation of risk of bias using the Newcastle-Ottawa Scale: (1) case control
Evaluation of risk of bias using the Newcastle-Ottawa Scale: (2) cohort
GRADE assessment