Abstract
Postoperative fever is mostly transient and inconsequential but may portend a serious postoperative infection requiring a thorough evaluation, especially during the recent COVID-19 pandemic. We aimed to determine the incidence, causes and outcomes of postoperative fever in neurosurgical patients, as well as to evaluate a protocol for management of postoperative fever. We conducted a prospective study over 12 months, recruiting 425 adult patients operated for non-traumatic neurosurgical indications. We followed a standard protocol for the evaluation and management of postoperative fever collecting data regarding operative details, daily maximal temperature, clinical features, as well as use of surgical drains, urinary catheters, and other invasive adjuncts. Elevated body temperature of > 99.9°F or 37.7 °C for over 48 h or associated with clinical deterioration or localising features was considered as “fever” and was evaluated according to our protocol. We classified elevated temperature not meeting this criterion as a transient elevation in temperature (TET). Sixty-five patients (13.5%) had postoperative fever. Transient elevation of temperature, occurring in 40 patients (8.8%) was most common in the first 48 h after surgery. The most common causes of fever were urinary tract infections (13.7%), followed by aseptic meningitis (10.8%), wound infections and pneumonia. Various aetiologies of fever followed distinct patterns, with COVID-19 and meningitis causing high-grade, prolonged fever. Multivariate analysis revealed cranial surgery, prolonged duration of surgery, urinary catheters and wound drains retained beyond POD 3 to predict fever. Postoperative fever was associated with significantly longer duration of hospital admission. COVID-19 had a high mortality rate in the early postoperative period.
Keywords: Postoperative, Fever, Infections, Neurosurgery, COVID-19
1. Introduction
Fever, as a natural response of the body to infection and other insults, leading to tissue injury and inflammation, is mediated by pyrogenic cytokines. Early postoperative fever is reported to occur in 50–70% of patients [1], [2], [3] but prolonged fever lasting >5 days occurs less frequently in about 16% [4]. While surgery per se triggers pro-inflammatory stimuli eliciting a temporary febrile response, postoperative fever may indicate serious surgical site infections, urinary tract infections, pneumonia, post-operative meningitis or other non-infectious aetiologies, such as drug fever, deep venous thrombosis (DVT), phlebitis, transfusion-associated fever and haemorrhage [5]. The recent SARS-COV-2 pandemic has added an extra dimension to the management of fever in postoperative patients. The development of routine “fever protocols” for investigation of postoperative fever, although useful, cannot substitute for real-time bedside clinical examination [2]. As high-quality prospective data on the causes of postoperative fever in neurosurgical patients is sparse, we conducted this prospective study aimed at determining the incidence, causes and outcomes of postoperative fever in neurosurgical patients. We also evaluated a protocol for management of postoperative fever during the COVID-19 pandemic. Our secondary objective was determining the incidence and pattern of COVID-19 among patients with postoperative fever.
2. Materials and methods
2.1. Study design, setting and participants
The study was approved by the Institutional Review Board (IRB Min. No. 12,914 dated 24.06.2020). We recruited all adult patients, >18 years of age, operated electively and as emergencies at our neurosurgical service between 31st March 2020 and 31st March 2021. “Emergency cases” were those who presented to the emergency room requiring urgent intervention and cases operated after-hours due to our long elective surgical waitlist. As trauma is an independent factor triggering the release of a host of pro-inflammatory factors, with up to 40% of patients admitted with trauma manifesting fever in the first 48 h [6], we chose to exclude patients operated for traumatic brain injuries. Informed consent was obtained from all patients prior to recruitment. Assuming a prevalence of post-operative fever of 20%, based on prior data [7] we calculated a sample size of 246 patients.
2.2. Preoperative COVID testing protocol
All patients were advised to isolate themselves in their residences for 1 week before admission, after which we performed two COVID-19 reverse transcription-polymerase chain reaction (RT-PCR) tests, five days apart. Patients who tested positive for COVID-19 were admitted in isolation wards or advised home quarantine based on their clinical condition. Unless emergent intervention was indicated, they were operated a minimum of 21 days from the date of their positive test. In cases operated by the endonasal route, we employed a specially developed negative-pressure face-mounted system and additional personal protective equipment (PPE) [8].
2.3. Data collection and protocol for evaluation of postoperative fever
All data was collected prospectively, with documentation of daily maximum temperature, number of fever spikes, as well as other factors such as preoperative fever, presence of central lines, drains, urinary catheters, and use of invasive ventilation. Details of surgery such as the procedure performed, time taken for surgery and intra-operative blood transfusions were documented from surgical and anaesthetic records on the day of surgery. Temperature measurements were taken every 4 h for patients in the wards and every hour when in the intensive care unit (ICU).
2.4. Definitions
Postoperative “Day 0” was defined as the day of surgery. The “early postoperative period” encompassed the first 48 h following surgery (POD 0 and 1), the “intermediate postoperative period” referred to postoperative Days 2–7 and the “late postoperative period” Day 8 and beyond. We defined temperature > 99.9°F or 37.7 °C for <48 h as “transient elevated temperature (TET)” when not associated with any localising features or clinical deterioration. These cases were not further evaluated for the cause of elevated temperature.
Elevated temperature lasting for >48 h or with clinical deterioration or localising features was considered as “fever” and was evaluated according to our protocol. We obtained history of localizing value, thoroughly examined the patient, and performed focused blood, urine and/or radiological investigations as demonstrated in Fig. 1 . Urinary tract infections (UTI) were diagnosed in the presence of pyuria (>10 WBC/mm3 per HPF) and bacteriuria (≥105 cfu/mL) [9]. Postoperative meningitis was diagnosed in patients with CSF pleocytosis (>10 cells/µl) and low glucose. Empiric antibiotics were initiated pending CSF culture reports and changed according to the drug sensitivity when an organism was cultured. Those with sterile cultures were treated as aseptic meningitis. Pneumonia was diagnosed based on clinical manifestations and chest X-ray showing infiltrates, or consolidation with or without effusion. Pneumonias were classified as ventilator-associated pneumonia (VAP) if associated with > 48 h of invasive ventilation [10]. Thrombophlebitis was diagnosed when a discoloured, swollen, or tender vein with cord-like structure was noted. Sepsis was diagnosed if two or more quick Sequential Organ Failure Assessment (qSOFA) criteria were met, and blood culture grew an organism [11].
Fig. 1.
Protocol for evaluation and management of postoperative fever in our cohort.
If fever persisted and no cause was identified on initial evaluation, we searched for other infectious diseases common to our setting, including dengue, malaria, and typhoid. Our threshold for COVID-19 testing varied through the study period. From April 2020 through June 2020, the incidence of COVID-19 cases in our region was low and community transmission had not commenced. Hence, we tested patients with postoperative fever for COVID-19 only when all other investigations were normal. However, as the pandemic progressed, we began testing patients for COVID-19 when postoperative fever occurred without clinical features suggestive of any other focus. Fever was labelled as “unclassified” if no aetiology was identified on the clinical picture and investigations.
2.5. Routine antibiotic policy and infection control
Our preoperative antibiotic protocol included Inj. Ceftriaxone 1.5 gm intravenously 30 min prior to surgery in all cases, with the addition of Inj. Cloxacillin 2gm and Inj. Gentamicin 160 mg continued up to the 3rd postoperative day in cases where surgical implants were used. In endoscopic anterior skull base surgeries lumbar subarachnoid drains were typically removed on postoperative Day 5 if there was a CSF leak, and the patient was administered Inj. Ceftriaxone 1 gm daily. Patients were catheterised following induction of anaesthesia, except in cases of documented bladder dysfunction, wherein a silicon catheter was inserted preoperatively in the ward and removed after recovery of bladder function, usually on outpatient basis. We routinely removed urinary catheters on the first postoperative day, except in cases where strict monitoring of urine output was indicated such as in diabetes insipidus. Central venous catheters were removed by the third postoperative day, and peripheral intravenous catheters were removed when intravenous medications were discontinued. All patients were encouraged to use incentive spirometers to prevent postoperative atelectasis, and knee-length thromboembolism deterrent stockings were employed in all patients confined to bed.
2.6. Statistical methods
Data was entered into an electronic database via Microsoft Access, and analysed with SPSS software (version 24, Chicago, IL). Descriptive statistics were calculated for all variables of interest, for the entire cohort as well as stratified by TET or postoperative fever. Categorical variables were analysed with the chi square, or Fishers exact test and continuous variables were analysed using Student’s t-test. Mann-Whitney test was used to compare continuous variables with non-standard distributions. Receiver Operating Characteristic (ROC) analysis was performed to assess the cut-off for maximal elevation in temperature that may predict an infective cause of postoperative fever. A significance level of 0.05 was considered significant for all statistical tests.
3. Results
Between March 31st, 2020, and March 31st, 2021, we operated on 613 patients. We excluded 188 patients with acute trauma, hence, this paper assimilates the data of 425 patients. The mean age of our cohort was 43.63 years (range 19 to 81 years). The majority were male (247; 58.1%). Nearly one-fifth (19.5%) were diagnosed to have diabetes mellitus preoperatively.
3.1. Management of patients testing positive for COVID-19 prior to surgery
Twenty-three patients tested positive for COVID-19 preoperatively; all were asymptomatic or mildly symptomatic for COVID-19, except for one who had moderate illness requiring oxygen therapy but no ventilatory support. These patients underwent surgery following a mean duration of 40.2 days (between 21 and 71 days) after testing positive. 1 patient was positive for COVID-19 four months prior to surgery. One patient who was COVID-19 positive at the time of surgery underwent emergency CSF shunting for acute hydrocephalus secondary to a 4th ventricular tumor.
3.2. Surgical interventions performed
We performed 457 surgeries; 393 (86%) were electively performed, while 64 (14%) were emergency procedures. Three hundred and ten (71.2%) were cranial surgeries − 234 supratentorial and 76 infratentorial. Among 147 spine surgeries, the most common levels operated were cervical (64 cases), followed by lumbar (59 cases), thoracic (21 cases) and sacral (3 cases). Two cases of cervico-medullary tumors required simultaneous excision via cranial and spinal approaches. Implants such as pedicle screws and cervical cages were used in 81 cases (52.6% of all spine surgeries).
The dura was opened in 295 surgeries (64.6%). The remaining 162 surgeries (35.4%) were extradural; these were mainly spinal (110/162 surgeries). Fifty-four patients (11.8%) required blood transfusion intraoperatively.
3.3. Incidence and causes of postoperative fever
(Table 1 )Overall, 102 patients (22.3%) had elevated temperature postoperatively, 40 (39.2%) of which were transient (TET) while the remaining 62 (60.7%) had persistent fever requiring evaluation. - TET occurred most frequently in the early postoperative period (20 of 40 cases), while postoperative fever was most frequent in the intermediate postoperative period (29 of 62 cases) (Fig. 2 ). Overall, the most frequent causes of postoperative fever were UTIs (13.7%), followed by aseptic meningitis (10.8%), wound infections (7.8%), pneumonia (5.8%), bacterial meningitis (3.9%), thrombophlebitis (2.9%), COVID-19 infection (1.9%), multiple foci (1.9%), and catheter-associated bloodstream infection (1%). The most common cause of early postoperative fever was bacterial meningitis (3 cases), while in the intermediate and late postoperative period, UTI (8 cases) and wound infections (6 cases) predominated, respectively. In 5 cases, we were unable to classify the cause of fever, however, in all cases, fever subsided spontaneously without antibiotics and was not associated with clinical deterioration. Miscellaneous causes included 3 confirmed acute febrile illnesses (1 each due to Dengue fever, melioidosis, and enteric fever due to Salmonella typhi), 1 shunt-associated abdominal collection, 1 drug fever and 1 intestinal obstruction that led to E.coli septicaemia.
Table 1.
Causes of postoperative fever classified by type of surgery and day of fever onset.
| Cause of fever | Overall (N = 102) | Type of surgery |
Day of fever onset |
|||
|---|---|---|---|---|---|---|
| Cranial (N = 76) | Spinal (N = 26) | Early postoperative (Days 0–1) | Intermediate postoperative (Days 2–7) | Late postoperative (Day 8 and later) | ||
| UTI | 14 (13.7%) | 10 | 4 | 3 (9.1%) | 8 (16.7%) | 3 (19%) |
| Aseptic meningitis | 11 (10.8%) | 11 | – | 2 (3%) | 5 (10.4%) | 4 (14.3%) |
| Wound infection | 8 (7.8%) | 5 | 3 | – | 2 (4.2%) | 6 (28.5%) |
| Pneumonia | 6 (5.8%) | 5 | 1 | 2 (6.1%) | 4 (8.3%) | – |
| Unclassified | 5 (4.9%) | 5 | – | 4 (8.3%) | 1 (4.8%) | |
| Bacterial meningitis | 4 (3.9%) | 4 | – | 3 (9.1%) | – | 1 (4.8%) |
| Thrombophlebitis | 3 (2.9%) | 3 | – | – | 2 (4.2%) | 1 (4.8%) |
| COVID-19 | 2 (1.9%) | 0* | 2 | – | 2 (4.2%) | 0* |
| Multiple foci | 2 (1.9%) | 2 | – | – | 2 (4.2%) | – |
| Catheter-associated blood stream infection (CABSI) | 1 (1%) | 1 | – | – | – | 1 (4.8%) |
| Miscellaneous | 6 (5.8%) | 6 | – | 3 (9.1%) | – | 3 (14.3%) |
| Transient elevated temperature (TET) | 40 (39.2%) | 25 | 15 | 20 (60.6%) | 19 (39.5%) | 1 (4.8%) |
| Total | 102 | 76 | 26 | 33 | 48 | 21 |
*One patient had postoperative COVID infection 31 days after surgery incidentally detected on preoperative testing for a VP shunt.
Fig. 2.
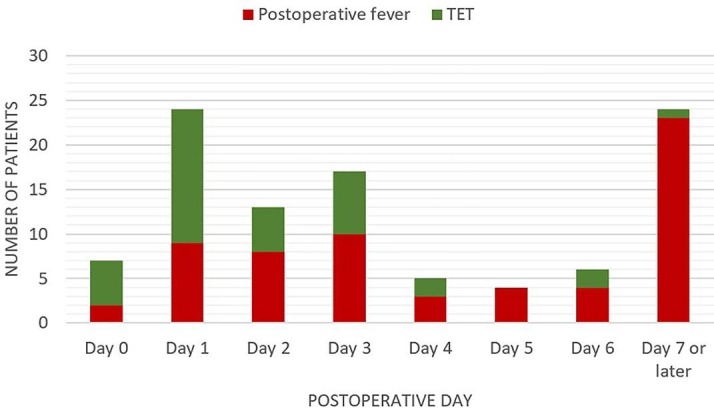
Incidence of TET and postoperative fever across the postoperative period.
Characteristics and outcomes of patients with postoperative fever (Table 2 )Patients who had postoperative fever did not differ in age, gender or prevalence of diabetes mellitus as compared to those who did not, however, their duration of surgery was longer (210 min vs. 180 min, p = 0.016) and they more frequently required blood transfusions (33.8% vs. 8.3%, p < 0.001). The proportion of emergency surgeries was similar between the groups. The cohort of patients that developed postoperative fever more frequently underwent cranial surgery (83.8 vs. 65.3%, p = 0.005) and surgery on the posterior fossa (40.3% vs. 21.3%, p = 0.012). There was no difference in the rates of dural, air sinus or ventricular opening at surgery. Reoperation at the current admission was more frequent among patients with significant fever (14.5% vs. 6.1%, p = 0.011), as was readmission after discharge (24.2% vs. 2.8%, p < 0.001). The duration of hospital stay was also significantly higher among those who had fever (13 days vs. 4 days, p < 0.001). On the other hand, patients with TET had a median duration of hospital stay of 5 days as compared to 4 days among those that did not have any rise in temperature (p = 0.576).
Table 2.
Comparison of clinical characteristics and outcomes among patients that had significant fever and those that did not.
| Characteristic | Entire cohort (N = 457) | Had fever (N = 62) | Did not have fever (N = 395) | p value (Had fever vs. Did not have fever) |
|---|---|---|---|---|
| Preoperative characteristics | ||||
| Mean age (years) | 43.6 ± 14.1 | 43.7 ± 15.2 | 43.6 ± 13.9 | 0.300 |
| Male gender | 263 (57.5%) | 36 (58.1%) | 227 (57.4%) | 0.872 |
| Diabetes | 88 (19.3%) | 14 (22.6%) | 74 (18.7%) | 0.422 |
| Preoperative fever | 20 (4.4%) | 8 (12.9%) | 12 (3%) | <0.001 |
| Preoperative lower cranial nerve dysfunction | 9 (1.9%) | 3 (4.8%) | 6 (1.5%) | 0.114 |
| Operative features | ||||
| Emergency surgery | 64 (14%) | 10 (16.1%) | 54 (13.9%) | 0.221 |
| Duration of surgery (min) | 180 (150, 240) | 210 (180, 300) | 180 (150, 240) | 0.016 |
| Intraoperative blood transfusion | 54 (11.8%) | 21 (33.8%) | 33 (8.3%) | <0.001 |
| Cranial vs. spinal surgery | 310 (67.8%) | 52/62 (83.8%) | 258/395 (65.3%) | 0.005 |
| Cranial surgery (N = 310) | ||||
| Dura opened | 258 (83.2%) | 48/52 (92.3%) | 210/258 (85.2%) | 0.565 |
| Ventricle entered | 43 (13.9%) | 9/52 (17.3%) | 34/258 (13.2%) | 0.514 |
| Ventricular shunt inserted | 26 (5.7%) | 6/52 | 20/258 | |
| Air sinuses opened | 55 (17.7%) | 8/52 (15.3%) | 47/258 (18.2%) | 0.913 |
| Posterior fossa surgery | 76 (24.5%) | 21/52 (40.3%) | 55/258 (21.3%) | 0.012 |
| Endonasal surgery | 46 (14.8%) | 8/52 (15.3%) | 38/258 (14.7%) | 0.677 |
| Burrhole surgery | 18 (3.9%) | 1/52 | 17/258 | |
| Spine surgery (N = 147) | ||||
| No. of levels operated | 2 (1, 4) | 4 (2.25, 4.75) | 2 (1, 3) | 0.031 |
| Dura opened | 37 (11.9%) | 4/10 (40%) | 33/137 (22.6%) | 0.406 |
| Instrumentation | 81 (17.7%) | 7/10 | 74/137 | 0.665 |
| Postoperative events and outcomes | ||||
| Reoperation at current admission | 33 (7.2%) | 9 (14.5%) | 24 (6.1%) | 0.011 |
| Readmission within 1 month | 26 (5.7%) | 15 (24.2%) | 11 (2.8%) | <0.001 |
| Median days of hospital admission | 4 (3, 7) | 13 (7.5, 19.5) | 4 (3, 5) | <0.001 |
Three patients with postoperative fever died − 1 each due to COVID-19 acute respiratory distress syndrome (ARDS), postoperative meningitis and intratumoral haemorrhage. One patient who did not have postoperative fever died due to a postoperative venous infarct.
3.4. Distinct patterns and clinical features distinguishing various causes of elevated temperature
TET and bacterial meningitis occurred most frequently in the early postoperative period, while COVID-19 infection, pneumonia, thrombophlebitis, UTI and aseptic meningitis occurred in the intermediate postoperative period and wound infection in the late postoperative period (Table 3 ). COVID-19 infection, bacterial meningitis, aseptic meningitis and UTI produced fever in excess of 101°F or 38.3 °C. Bacterial meningitis, COVID-19 infection, aseptic meningitis and pneumonia manifested fever for >3 days, while thrombophlebitis and wound infections produced 1 day of fever. This data is represented in Fig. 3 .
Table 3.
A comparison of the characteristics of fever occurring due to various aetiologies in the cohort.
| Cause of fever | Median postoperative day of onset of fever | Median no. of days of fever | Median peak temperature (°C) | Localising features present (%) |
|---|---|---|---|---|
| UTI | 5 (3, 7) | 3 (2, 3.25) | 38.7 (±0.5) | 6 (42.8%) |
| Pneumonia | 2 (1, 3.25) | 4 (2, 6) | 38.3 (±0.4) | 5 (83.3%) |
| Aseptic meningitis | 6 (2, 17) | 4 (3, 5) | 38.8 (±0.3) | 6 (54.5%) |
| Bacterial meningitis | 1 (1, 6.25) | 6.5 (3.75, 7.75) | 39.1 (±0.2) | 3 (75%) |
| Postoperative COVID-19 infection | 2 (2, 2) | 4 (2, 4) | 39.7 (±0.4) | 2 (100%) |
| Thrombophlebitis | 3 (3, 3.5) | 1 (1, 1) | 38.2 (±0.3) | 3 (100%) |
| Wound infection | 12 (6, 32) | 1.5 (1, 3) | 37.9 (±0.4) | 8 (100%) |
| Unclassified | 5 (3.5, 33) | 3 (1.5, 6.5) | 38,3 (±0.7) | – |
| Transient elevated temperature (TET) | 1 (1, 3) | 1 (1, 2) | 38.1 (±0.2) | – |
Fig. 3.
Patterns of postoperative fever among various aetiologies.
ROC analysis demonstrated that a temperature in excess of 100.7°F or 38.2 °C predicted an infective cause of fever with a sensitivity of 83% and specificity of 54.5%. (AUC- 0.674, Fig. 4 ).
Fig. 4.
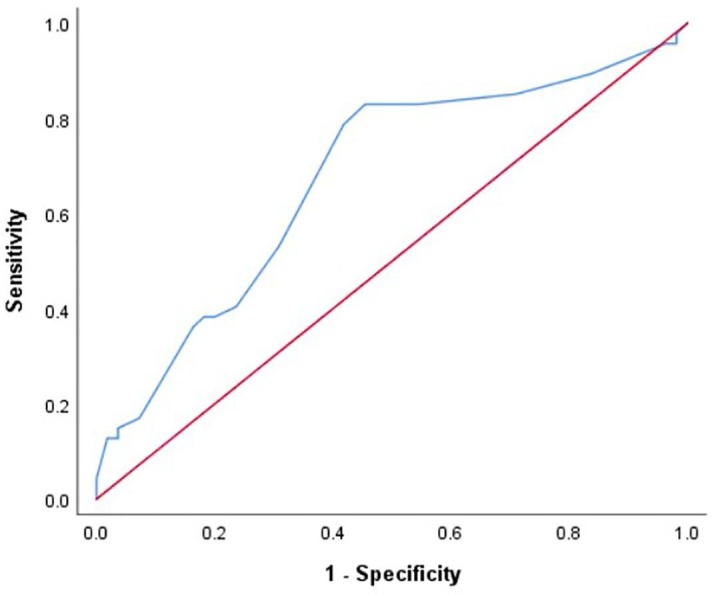
Receiver Operating Characteristics analysis of the threshold of maximum elevation of temperature predicting an infective cause of fever.
Localising clinical features at the onset of fever were present in all cases of thrombophlebitis, wound infection, and postoperative COVID-19 infection, 83.3% of pneumonias, 75% of bacterial meningitis, 54.5% of aseptic meningitis and less than half (42.8%) of UTIs.
3.5. Postoperative fever in infectious diseases
The cohort included ten infective cases, including 7 cases of spondylodiscitis, 2 intracranial abscesses, and 1 frontal osteomyelitis. Three of these cases manifested fever preoperatively, while 5 others had fever preoperatively for other reasons. We found that the presence of fever preoperatively significantly predicted fever postoperatively (12.9% of patients with postoperative fever had fever preoperatively vs. 3% of patients that did not, p < 0.001).
3.6. Urinary catheters and postoperative fever
Most of our patients were on a urinary catheter postoperatively (97.5%) for a median duration of 1 day (IQR: 1,1). UTI accounted for fever in 46.2% (12/26) of patients who were catheterised for 3 days or longer, compared to 6.7% of patients catheterised for ≤ 2 days (2 of 30) (p = 0.002). Thirty-five patients (7.8%) were discharged on an indwelling catheter and followed up on an outpatient basis. Longer duration on a catheter (Median 2 days: IQR 1, 14 vs. 1 day: IQR 1, 1, p < 0.001) and discharge from hospital on an indwelling catheter (15 patients among those with fever-24.1% and 20 patients among those without fever- 5.1%, p < 0.001) were significantly associated with postoperative fever.
3.7. Central venous catheters, wound drains, and intraventricular/subarachnoid drains
A central venous catheter (CVC) was in-situ postoperatively in 396 patients (86.7%) for a median duration of 2 days (IQR: 2, 2), that was significantly longer among patients with postoperative fever (3 days: IQR 2, 4.75 vs. 2 days: IQR 2, 2, p < 0.001). An isolated case of CVC-associated bloodstream infection was noted.
Wound drains were inserted in 279 patients (61.1%) and retained for a median duration of 1 day postoperatively (IQR: 1, 2 days). Among patients who had fever, wound drains were retained for longer compared to those who did not have fever (2 days: IQR 1, 3 vs. 1 day: IQR 1, 2, p = 0.005). Wound infections accounted for fever among 55.4% (6/11) of patients with a wound drain in-situ for 3 days or longer, compared to 9.5% (2/21) of patients that had drains removed earlier (p = 0.01).
An intraventricular or lumbar CSF drain was placed in 27 patients (5.9%), for a median duration of 4 days (IQR: 3, 5). The presence of these drains was associated with postoperative fever (9 patients among 27 in whom a CSF drain was placed developed fever vs. 53 of 430 patients in whom they were not employed, p = 0.003). Meningitis accounted for 55.6% (5/9) of fever in patients with a lumbar drain, compared to 17.9% (10/56) in patients without a lumbar drain (p = 0.02).
One of 4 cases of bacterial meningitis was a shunt-related infection caused by Klebsiella pneumoniae.
3.8. Postoperative invasive ventilation
Seventeen patients required invasive ventilation postoperatively, for a median duration of 48 h (IQR:15, 72). Use of invasive ventilation postoperatively was significantly associated with fever (9 patients- 13.8% vs. 8 patients- 2.04%, p < 0.001). However, there was no significant difference in the duration of ventilation between both groups (48 h: IQR 23, 72 vs. 21 h: IQR 13.5, 66, p = 0.521). Pneumonia accounted for fever in 50% (5/10) patients that were ventilated and 1.7% (1/56) in those that were not (p < 0.001).
3.9. Multivariate analysis of factors predicting postoperative fever
Multivariate analysis revealed that cranial over spinal surgery (HR: 1.82, 95%CI- 1.07, 17.14, p = 0.042), duration of surgery (HR:1.08, 95%CI- 1.02, 1.14, p = 0.013), indwelling urinary catheter beyond POD 3 (HR: 8.40, 95%CI- 1.82, 38.46, p = 0.006) and wound drain retained beyond POD 3 (HR: 6.94, 95%CI- 1.53, 31.25, p = 0.012) were significant predictors of postoperative fever. (Table 4 ).
Table 4.
Multivariate regression analysis of factors predicting postoperative fever.
| Variable | HR | P value | 95% CI |
|
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Cranial surgery | 1.824 | 0.042 | 1.076 | 17.142 |
| Duration of surgery | 1.080 | 0.013 | 1.020 | 1.141 |
| Urinary catheter beyondDay 3 | 8.403 | 0.006 | 1.824 | 38.461 |
| Wound drain beyondDay 3 | 6.944 | 0.012 | 1.538 | 31.25 |
| Preoperative fever | 0.548 | 0.622 | 0.050 | 5.975 |
| Blood transfusion | 1.973 | 0.429 | 0.366 | 10.622 |
| Postoperative invasive ventilation | 0.205 | 0.125 | 0.027 | 1.554 |
| EVD | 0.634 | 0.681 | 0.073 | 5.545 |
| Steroids beyond day 3 | 0.595 | 0.672 | 0.054 | 6.585 |
3.10. Organisms causing postoperative infections
Overall, 47 bacterial organisms were isolated in 36 cases. We noted a wide array of organisms responsible for postoperative infections (Table 5 ). In 11 cases, significant growth of more than one organism was noted. The most frequently encountered organism was E. coli, accounting for 14 of 47 positive cultures. The incidence of antibiotic resistance was highest among cultures of Acinetobacter baumanii (75%), followed by Klebsiella pneumoniae (50%).
Table 5.
Spectrum of organisms isolated in postoperative infections.
| Organism/Infection | Meningitis | UTI | Pneumonia | Wound infection | Diarrhoea | Sepsis | MRSA/VRE/CRO/ESBL-producing |
|---|---|---|---|---|---|---|---|
| Staphylococcus aureus | 1 | 2 | 1/3 | ||||
| Pseudomonas aeruginosa | 1 | 3 | 1 | 2 | 0 | ||
| Enterococcus | 2 | 1 | 1/3 | ||||
| Escherichia coli | 1 | 11 | 1 | 1 | 2/14 | ||
| Klebsiella pneumoniae | 1 | 4 | 3 | 2 | 5/10 | ||
| Stenotrophomonas | 1 | 0 | |||||
| Citrobacter | 1 | 0 | |||||
| Enterobacter | 2 | 1 | 0 | ||||
| Salmonella typhi | 1 | 0 | |||||
| Acinetobacter baumanii | 1 | 2 | 1 | 3/4 |
MRSA-Methicillin-resistant staphylococcus aureus.
VRE-Vancomycin-resistant enterococci.
CRO-Colistin-resistant organism.
ESBL-Extended-spectrum beta lactamase.
3.11. COVID-19 and postoperative fever
We report 3 cases of postoperative COVID-19 infection. The first was a 72-year-old hypertensive male with chronic obstructive pulmonary disease, operated for cervical spondylotic myelopathy. He developed fever on the 2nd postoperative day, and rapidly progressed to acute respiratory distress syndrome (ARDS), with increasing oxygen requirements. He was transferred to a COVID-19 ward, for non-invasive positive pressure ventilation, however he rapidly deteriorated and passed away on POD 9. Our next case was an 81-year-old lady operated for a L2-3 central intervertebral disc prolapse. She developed fever on the 2nd postoperative day with ARDS by the 6th postoperative day requiring invasive mechanical ventilation for a period of 2 weeks in the ICU. She was gradually weaned off her ventilatory supports and discharged on a tracheostomy on the 41st postoperative day. The third case was a 24-year-old male operated for a cerebellar haemangioblastoma. This patient developed postoperative fever on the 1st postoperative day and was found to have E.coli meningitis. During his treatment, he developed hydrocephalus requiring CSF shunting. A COVID-19 RT-PCR done prior to CSF shunting was positive on the 31st day after initial surgery. He remained asymptomatic for COVID-19 and was discharged in stable condition.
4. Discussion
4.1. Incidence of postoperative fever and its significance
Fever presents a common dilemma in the postoperative period contributing to increased investigations, complications and cost [12]. While several physiological factors may contribute to fever in a postoperative patient, including the accelerated production of pyrogenic cytokines, fever due to infective causes need to be identified and treated early. In this prospective study of 457 adult neurosurgical procedures, we noted the incidence of postoperative elevation of temperature to be 22.3% of which 4.4% were transient elevations on POD 0 and 1 and 13.5% had fever that required investigation. This is lower than observed in some prior reports [3], [13], [14], [15], despite a similar definition (Temperature>100°F or 37.7 °C) [16], while being on par with others [4], [17]. This wide variation of reported incidence of fever is explained by the fact that several studies include or are focused solely on children, who appear to have a higher incidence of fever than do adults [13], [14] or on specific neurosurgical procedures, such as hemispherectomy [14] or posterior fossa surgery [18].
4.2. Implications of “early” postoperative fever
More than a third of elevated temperature in the postoperative period was classified as transient elevated temperature, and, as per our protocol, not investigated further. The “<102F one-time spike rule” in the ICU setting, assumes that a single spike of fever measuring <102°F or 38.8 °C is unlikely to be due to an infection, but more likely to be due to drug reactions, transfusion-associated fever, or transient bacteraemia from manipulation of indwelling urinary or venous catheters [5], [19]. It is not surprising that 50% of TET in our cohort occurred in the first 48 h following surgery, when CVCs, urinary catheters and endotracheal tubes were usually removed. These patients had outcomes comparable to those without any elevation of temperature at all. Nevertheless, bacterial meningitis, UTI and postoperative COVID infection also manifested fever in the same time frame. Thus, if fever exceeds 102°F or 38.8 °C, is associated with clinical deterioration, has distinguishing clinical features or extends beyond the first 48 h after surgery a thorough investigation for an infectious aetiology must be carried out. We support the view that patients may be discharged when afebrile for > 24 h, however, in view of the ongoing threat of COVID-19 infection, we recommend early testing of patients with fever>102°F or 38.8 °C, thus minimising the risk of exposure to others. Among the 3 cases of postoperative COVID-19 infection in our cohort, 2 cases that manifested the infection in the early postoperative period developed severe ARDS- both required prolonged invasive ventilation and 1 of them succumbed to the virus. The other patient acquired the infection >31 days after primary surgery and had an asymptomatic course. While both patients who developed severe ARDS had a recognised risk factor for severe COVID-19 complications, namely advanced age, current data on the outcomes of postoperative COVID-19 infection in neurosurgical patients, though sparse, suggests a similarly high mortality [20]. Our low number of postoperative COVID-19 infections, likely a factor of our rigorous preoperative testing strategy prevents us from drawing more definitive conclusions, however it seems prudent to employ strict measures in the postoperative period to avoid COVID-19 transmission.
4.3. Factors associated with postoperative fever
Fever in the postoperative period significantly prolongs the duration of hospital stay (13 days compared to 4 days), and therefore the expenditure incurred by a patient [2], [21]. We found that the factors predisposing to postoperative fever were cranial surgery, prolonged duration of surgery, urinary catheters and wound drains retained beyond POD 3. Cranial surgery may contribute to the risk of fever due to its longer duration than spinal surgery, more frequent opening of the dura, blood in the subarachnoid space and access to the ventricles [22], [23]. Reports from general surgical cohorts suggest that the use of wound drains and prolonged operative time are associated with postoperative infections, although some also suggest that contaminated types of surgery, emergency cases and those operated by residents rather than attending surgeons may also be predisposed to developing postoperative surgical site infections [24], [25]. Thus, a consistent policy regarding the early removal of urinary catheters and wound drains will help mitigate the risk of postoperative fever.
Such measures must take precedence over the use of broad-spectrum antibiotic prophylaxis, as a conservative prophylactic antibiotic policy is sufficient to provide a low rate of postoperative infection with a low incidence of multi-drug resistant organisms [26]. In the present report, we noted that only a quarter of organisms cultured (25.5%) were resistant to first-line antibiotics.
It is tempting in cases of postoperative fever to attribute pyrexia to spurious “infections”, such as positive urine cultures in the absence of pyuria. It has been demonstrated in various institutional settings that bacteriuria is a common incidental finding, and initiation of antibiotic therapy in such patients is not warranted [27], [28]. Indeed, in our series, 6 cases evaluated for fever demonstrated organisms on urine culture in the absence of pyuria, and, in consultation with our infectious disease experts, we deferred antibiotics targeted toward those organisms. In 2 cases, further evaluation revealed other foci of infection (1 aseptic meningitis and 1 pneumonia), while the other 4 resolved spontaneously without clinical deterioration and were categorised as “unclassified” fever.
4.4. Developing a protocol for the investigation of postoperative fever
Our protocol for the evaluation and management of postoperative fever is outlined in Fig. 1. Patients were systematically examined for features suggestive of meningitis, wound infections, urinary tract infections, respiratory infections, thrombophlebitis, drug-related eruptions, and gastroenteritis. We also took into consideration risk factors of postoperative infections, such as invasive ventilation, CSF catheters, and prolonged urinary catheterization. Investigations for the source of fever were tailored to the results of our examination. For example, clinical features of meningitis prompted imaging of the brain followed by a lumbar puncture for CSF analysis, while features suggestive of a UTI prompted a urine examination. While awaiting culture and sensitivity reports, we started empirical antibiotics - meropenem in cases where meningitis was suspected and piperacillin-tazobactam in other infections. As shown in Table 2, however, localizing clinical features may be absent in up to 25% of cases of meningitis and 58% of UTIs. While we agree that clinical judgement must be used to reduce unnecessary testing [15], it should be emphasized that undifferentiated fever in the absence of any accompanying clinical features must be investigated systematically. This is particularly true in cases where risk factors for postoperative infection are present, such as prolonged cranial surgery and the prolonged use of urinary catheters or wound drains. We also recommend analysis of the day of onset of fever and the degree of elevation in temperature produced, that, in many cases, may hint at the underlying cause.
5. Conclusions
Transient elevation of temperature is common following neurosurgery in the early postoperative period but does not portend serious infection. It is important to distinguish these insignificant temperature elevations from true postoperative fever, while cautiously evaluating patients who have risk factors for postoperative infections. As localising features may be absent in several infections, the patterns of fever appear to be distinct and may guide surgeons as to the possible aetiology. The incidence of postoperative infections may be reduced by early removal of urinary catheters and wound drains.
6. Limitations
In our series of 457 surgeries, we report only 3 cases of COVID-19 infection postoperatively. While this demonstrated the efficacy of our preoperative testing and isolation policy, it limited our ability to achieve one of our objectives, which was to assess the outcomes of postoperative COVID-19 infections among neurosurgical patients. Our threshold for COVID-19 testing was also higher during the period of April-June 2020, during which period the incidence of postoperative COVID-19 infection may have been underestimated.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kinoshita Y., Tominaga A., Saitoh T., et al. Postoperative fever specific to neuroendoscopic procedures. Neurosurg Rev. 2014;37:99–104. doi: 10.1007/s10143-013-0505-7. [DOI] [PubMed] [Google Scholar]
- 2.Lesperance R., Lehman R., Lesperance K., et al. Early postoperative fever and the “routine” fever work-up: results of a prospective study. J Surg Res. 2011;171:245–250. doi: 10.1016/j.jss.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Raviv N., Field N., Adamo M.A. Postoperative fever workup in pediatric neurosurgery patients. J Neurosurg Pediatr. 2020;26:691–695. doi: 10.3171/2020.5.PEDS2019. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z., Shen M., Qiao M., et al. Clinical factors and incidence of prolonged fever in neurosurgical patients. J Clin Nurs. 2017;26:411–417. doi: 10.1111/jocn.13409. [DOI] [PubMed] [Google Scholar]
- 5.Cunha B. Clinical approach to fever in the neurosurgical intensive care unit: Focus on drug fever. Surg Neurol Int. 2013;4:318. doi: 10.4103/2152-7806.111432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bengualid V., Talari G., Rubin D., et al. Fever in trauma patients: evaluation of risk factors, including traumatic brain injury. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2015;24:e1–e5. doi: 10.4037/ajcc2015856. [DOI] [PubMed] [Google Scholar]
- 7.Orsi G.B., Scorzolini L., Franchi C., et al. Hospital-acquired infection surveillance in a neurosurgical intensive care unit. J Hosp Infect. 2006;64:23–29. doi: 10.1016/j.jhin.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Gupta A., Goyal-Honavar A., Jonathan G.E., et al. Adapting management strategies for sellar-suprasellar lesions during the COVID-19 pandemic: a pragmatic approach from the frontline. Br J Neurosurg. 2021:1–8. doi: 10.1080/02688697.2021.1940852. [DOI] [PubMed] [Google Scholar]
- 9.Rowe T.A., Juthani-Mehta M. Diagnosis and Management of Urinary Tract Infection in Older Adults. Infect Dis Clin North Am. 2014;28:75–89. doi: 10.1016/j.idc.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plachouras D., Lepape A., Suetens C. ECDC definitions and methods for the surveillance of healthcare-associated infections in intensive care units. Intensive Care Med. 2018;44:2216–2218. doi: 10.1007/s00134-018-5113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer M., Deutschman C.S., Seymour C.W., et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward D.T., Hansen E.N., Takemoto S.K., Bozic K.J. Cost and effectiveness of postoperative fever diagnostic evaluation in total joint arthroplasty patients. J Arthroplasty. 2010;25:43–48. doi: 10.1016/j.arth.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 13.de Kunder SL, ter Laak - Poort MP, Nicolai J, et al (2016) Fever after intraventricular neuroendoscopic procedures in children. Childs Nerv Syst 32:1049–1055. 10.1007/s00381-016-3085-3. [DOI] [PMC free article] [PubMed]
- 14.Kamath A.A., Limbrick D.L., Smyth M.D. Characterization of postoperative fevers after hemispherotomy. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2015;31:291–296. doi: 10.1007/s00381-014-2572-7. [DOI] [PubMed] [Google Scholar]
- 15.Stricsek G.P., Montenegro T.S., Gonzalez G.A., et al. Association between postoperative fever and readmission rates in lumbar fusion patients. Clin Spine Surg. 2021;34 doi: 10.1097/BSD.0000000000001131. E349 E353. [DOI] [PubMed] [Google Scholar]
- 16.Walid M.S., Sahiner G., Robinson C., et al. Postoperative fever discharge guidelines increase hospital charges associated with spine surgery. Neurosurgery. 2011;68:945–949. doi: 10.1227/NEU.0b013e318209c80a. [DOI] [PubMed] [Google Scholar]
- 17.Walid M.S., Woodall M.N., Nutter J.P., et al. Causes and risk factors for postoperative fever in spine surgery patients. South Med J. 2009;102:283–286. doi: 10.1097/SMJ.0b013e31819676a4. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Li Z., Cao X., et al. Postoperative antibiotic management strategy for febrile patient with posterior cranial fossa tumor resection: Retrospective clinical study. J Clin Neurosci Off J Neurosurg Soc Australas. 2020;80:80–86. doi: 10.1016/j.jocn.2020.07.066. [DOI] [PubMed] [Google Scholar]
- 19.Cunha B.A. Fever in the critical care unit. Crit Care Clin. 1998;14:1–14. doi: 10.1016/s0749-0704(05)70378-x. [DOI] [PubMed] [Google Scholar]
- 20.Marenco-Hillembrand L., Erben Y., Suarez-Meade P., et al. Outcomes and surgical considerations for neurosurgical patients hospitalized with COVID-19–A multicenter case series. World Neurosurg. 2021;154 doi: 10.1016/j.wneu.2021.06.147. e118–e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uçkay I., Agostinho A., Stern R., et al. Occurrence of fever in the first postoperative week does not help to diagnose infection in clean orthopaedic surgery. Int Orthop. 2011;35:1257–1260. doi: 10.1007/s00264-010-1128-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carmel P.W., Greif L.K. The aseptic meningitis syndrome: a complication of posterior fossa surgery. Pediatr Neurosurg. 1993;19:276–280. doi: 10.1159/000120744. [DOI] [PubMed] [Google Scholar]
- 23.Zarrouk V., Vassor I., Bert F., et al. Evaluation of the management of postoperative aseptic meningitis. Clin Infect Dis. 2007;44:1555–1559. doi: 10.1086/518169. [DOI] [PubMed] [Google Scholar]
- 24.Isik O., Kaya E., Dundar H.Z. Sarkut P (2015) surgical site infection: re-assessment of the risk factors. Chir Buchar Rom. 1990;110:457–461. [PubMed] [Google Scholar]
- 25.Barie PS, Eachempati SR (2005) Surgical site infections. Surg Clin North Am 85:1115–1135, viii–ix. 10.1016/j.suc.2005.09.006. [DOI] [PubMed]
- 26.Moorthy R.K., Sarkar H., Rajshekhar V. Conservative antibiotic policy in patients undergoing non-trauma cranial surgery does not result in higher rates of postoperative meningitis: An audit of nine years of narrow-spectrum prophylaxis. Br J Neurosurg. 2013;27:497–502. doi: 10.3109/02688697.2013.771138. [DOI] [PubMed] [Google Scholar]
- 27.Terpenning M.S., Bradley S.F., Wan J.Y., et al. Colonization and infection with antibiotic-resistant bacteria in a long-term care facility. J Am Geriatr Soc. 1994;42:1062–1069. doi: 10.1111/j.1532-5415.1994.tb06210.x. [DOI] [PubMed] [Google Scholar]
- 28.Gallegos Salazar J., O’Brien W., Strymish J.M., et al. Association of screening and treatment for preoperative asymptomatic bacteriuria with postoperative outcomes among US veterans. JAMA Surg. 2019;154:241–248. doi: 10.1001/jamasurg.2018.4759. [DOI] [PMC free article] [PubMed] [Google Scholar]




