Abstract
Background
COVID-19 vaccination reduces SARS-CoV-2 infection and transmission. However, evidence is emerging on the degree of protection across variants and in high-transmission settings. To better understand the protection afforded by vaccination specifically in a high-transmission setting, we examined household transmission of SARS-CoV-2 during a period of high community incidence with predominant SARS-CoV-2 B.1.1.7 (Alpha) variant, among vaccinated and unvaccinated contacts.
Methods
We conducted a household transmission investigation in San Diego County, California, and Denver, Colorado, during January-April 2021. Households were enrolled if they had at least one person with documented SARS-CoV-2 infection. We collected nasopharyngeal swabs, blood, demographic information, and vaccination history from all consenting household members. We compared infection risks (IRs), RT-PCR cycle threshold values, SARS-CoV-2 culture results, and antibody statuses among vaccinated and unvaccinated household contacts.
Results
We enrolled 493 individuals from 138 households. The SARS-CoV-2 variant was identified from 121/138 households (88%). The most common variants were Alpha (75/121, 62%) and Epsilon (19/121, 16%). There were no households with discordant lineages among household members. One fully vaccinated secondary case was symptomatic (13%); the other 5 were asymptomatic (87%). Among unvaccinated secondary cases, 105/108 (97%) were symptomatic. Among 127 households with a single primary case, the IR for household contacts was 45% (146/322; 95% Confidence Interval [CI] 40–51%). The observed IR was higher in unvaccinated (130/257, 49%, 95% CI 45–57%) than fully vaccinated contacts (6/26, 23%, 95% CI 11–42%). A lower proportion of households with a fully vaccinated primary case had secondary cases (1/5, 20%) than households with an unvaccinated primary case (66/108, 62%).
Conclusions
Although SARS-CoV-2 infections in vaccinated household contacts were reported in this high transmission setting, full vaccination protected against SARS-CoV-2 infection. These findings further support the protective effect of COVID-19 vaccination and highlight the need for ongoing vaccination among eligible persons.
Abbreviations: CDC, US Centers for Disease Control and Prevention; CI, Confidence Interval; CSTE, Council of State and Territorial Epidemiologists; FDA, US Food and Drug Administration; IR, infection risk; IQR, interquartile range; NP, nasopharyngeal; RBD, receptor-binding domain; RT-PCR, reverse transcription polymerase chain reaction; WGS, whole genome sequencing
Keywords: SARS-CoV-2, COVID-19, Vaccination, Vaccine effectiveness, Household transmission
1. Introduction
Vaccines currently approved or authorized by the Food and Drug Administration (FDA) for use in the United States are highly effective and prevent severe disease, hospitalization, and death from COVID-19 [1], [2], [3], [4], [5], [6]. However, the risk for SARS-CoV-2 infection in fully vaccinated people cannot be eliminated while there is continued community transmission [7], [8], [9], [10], [11], [12], [13], [14]. Limited data suggest that infections in vaccinated persons may have reduced transmissibility [15], [16]. The effect of vaccination in high transmission settings such as households and prisons is less well understood than in the broader community [17]. Population-based studies have reported high vaccine effectiveness against infection with the B.1.1.7 (Alpha) variant [18], [19], but data suggest infections in fully vaccinated persons may be more common with other variants [11]. Detailed clinical and laboratory data at the individual level remain sparse.
To better understand the effect of vaccination on SARS-CoV-2 infection for household contacts, we examined symptomatic infection, viral dynamics, and antibody titers among vaccinated and unvaccinated persons in infected households. The investigation occurred in San Diego County and the Denver metropolitan area in early 2021, representing areas where COVID-19 incidence was high and Alpha variant circulation predominated; other variants of concern circulating at the time of the investigation included P.1 (Gamma) and B.1.427/B.1.429 (Epsilon) [20], [21], [22]. At the time of this investigation three FDA-authorized COVID-19 vaccines were becoming more widely available [22]. Therefore, this investigation was uniquely timed to describe how vaccination status affected COVID-19 illness, asymptomatic SARS-CoV-2 infection, and viral shedding in the context of multiple circulating variants.
2. Materials and methods
2.1. Household enrollment
To conduct this investigation, the Centers for Disease Control and Prevention (CDC) partnered with state and local public health departments in San Diego County, California (January 18, 2021 to April 14, 2021) and the Tri-County area of Denver, Colorado (March 22, 2021 to April 30, 2021). We recruited individuals with reverse transcription polymerase chain reaction (RT-PCR)–confirmed SARS-CoV-2 infection with onset ≤ 10 days before enrollment (index cases), and we recruited members of their households (some of whom became cases during the investigation) [23].
2.2. Household visits
Investigators visited households at enrollment (Day 0) and closeout (Day 14). At these visits we collected a nasopharyngeal (NP) swab (for RT-PCR testing, whole genome sequencing [WGS], and viral culture) and venous blood samples for serology from all enrolled household members. Household members completed questionnaires assessing demographics, medical history, and COVID-19 vaccination history. One adult from each household completed a household-level questionnaire. Each household member completed a symptom diary daily. A convenience sample of participants volunteered for daily self-antigen testing during days 1–14; a subset volunteered for daily NP swabs during days 1–7.
If a household member developed new symptoms or had a newly positive antigen self-test, then a household visit was conducted to obtain an additional NP swab from all household members.
2.3. Data entry and data management
We collected and managed deidentified questionnaire data using the Research Electronic Data Capture (REDCap) web application [24], [25] hosted at CDC. We collected and managed laboratory data and the final analytic dataset using R version 4.0.2 (R Foundation for Statistical Computing, Vienna, Austria) [26].
2.4. Ethics statement
This activity was reviewed by CDC’s Human Subjects Protection Office and was conducted consistent with applicable federal laws and CDC policy.2
Informed Consent.
We obtained written consent from all participants (or the participant’s guardian) after the nature and possible consequences of the investigation had been fully explained. In addition, participants between the ages of 7 and 17 provided written assent.
2.5. Eligbility criteria
Households were eligible for inclusion in the analysis if the index case had illness onset ≤ 10 days before enrollment, at least one other person resided in the household, the primary case was not currently hospitalized, and the primary case did not live in a congregate setting.3
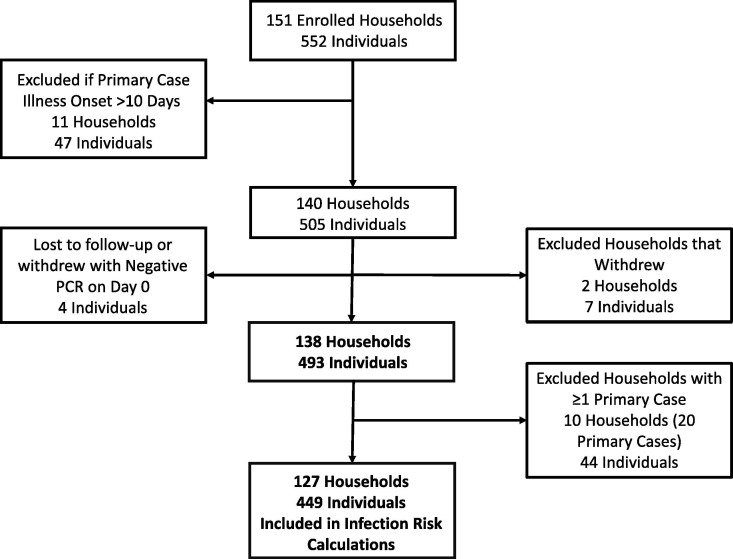
We excluded households from analysis if all household members were lost to follow-up or withdrew, or if all household members had the same date of illness onset. We excluded households from infection risk (IR) calculations if a single primary case could not be determined because multiple persons had the same illness onset date (i.e., co-primary infections) (Fig. 1 ).
Fig. 1.
Investigation flow diagram showing convenience sample enrollment and exclusion criteria, California and Colorado, January-April 2021.
2.6. Definitions
Case – We defined a participant as a case if they had a positive SARS-CoV-2 RT-PCR test during the investigation period.
Illness Onset – We defined each case’s illness onset as the date of symptom onset, or, if asymptomatic, the date of collection of their first positive SARS-CoV-2 RT-PCR test.
Primary case – We defined the primary case as the person within an enrolled household who had the earliest date of illness onset. We defined the infectious period for the primary case as from 2 days before until 10 days after symptom onset, if symptomatic, or from collection date of first positive specimen until 10 days later, if asymptomatic.
Household contact – We defined a household contact as any person who had spent at least 1 night in the same household as the primary case during the primary case’s infectious period.
Secondary case – We defined a secondary case as a household contact who tested positive for SARS-CoV-2 by RT-PCR at least one day after the primary case’s illness onset. The positive test could occur before enrollment in the investigation. We assumed that all secondary cases were due to infection from the primary case and did not investigate possible transmission chains within households.
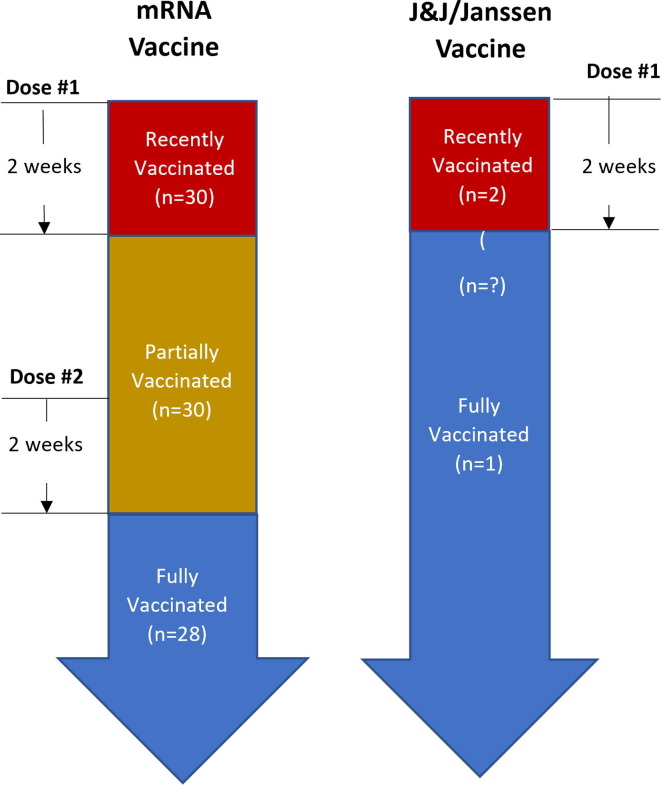
Vaccination status – Persons who had received at least one dose of any FDA-authorized COVID-19 vaccine were classified as recently vaccinated, partially vaccinated, or fully vaccinated (according to the schema shown in Fig. 2 ).
Fig. 2.
Diagram showing classification of persons as recently, partially, or fully vaccinated by vaccine type.
Persons who received an mRNA-based COVID-19 vaccine (BNT162b2 or mRNA-1273) were defined as fully vaccinated if they completed the primary series of an mRNA-based COVID-19 vaccine ≥ 14 days before their illness onset, or, if they were not the primary case, ≥14 days before the illness onset for the primary case in their household.
Persons who had received only one dose of an mRNA-based COVID-19 vaccine were defined as partially vaccinated. They were also defined as partially vaccinated if they had completed the primary series of an mRNA-based COVID-19 vaccine but their second dose was < 14 days before their illness onset, or, if they were not the primary case, <14 days before the illness onset for the primary case in their household. Within the partially vaccinated group, persons were defined as recently vaccinated if they had received a single dose of an mRNA-based COVID-19 vaccine < 14 days before their illness, or, if they were not the primary case, <14 days before the date that the first person in their household became ill.
Persons who received an adenoviral vector-based vaccine (JNJ-78436735) were defined as fully vaccinated if they had received a single dose ≥ 14 days before their illness, or, if they were not the primary case, ≥14 days prior the date that the first person in their household became ill. Persons who had received the vaccine < 14 days before illness onset, or, if they were not the primary case, <14 days prior the date that the first person in their household became ill were classified as recently vaccinated.
Variant Status – For each case, WGS was attempted on at least one NP specimen that met the testing criteria; if successful, a variant status was assigned to each case. For persons with undetermined variant status for whom a specimen was not available or could not be sequenced, their variant was considered to be the variant of the first secondary case within the household; if the variant of the first secondary case was not available or could not be sequenced, then the variant of the primary case was used, if available.
Symptom Status – Persons were classified as symptomatic if they reported at least one of the following symptoms within 14 days of illness onset in their daily symptom diaries, enrollment questionnaire, or final questionnaire: subjective fever, measured fever > 38.0 °C, chills, rigors, myalgia, headache, fatigue, nasal congestion, rhinorrhea, anosmia, ageusia, sore throat, cough, dyspnea, nausea/vomiting, diarrhea, or abdominal pain. Parents or guardians completed the symptom diary for children in the household. This list of symptoms was developed using the interim case definition published by the Council of State and Territorial Epidemiologists (CSTE) and CDC [27].
2.7. Laboratory Methods
RT-PCR testing of NP swabs for SARS-CoV-2 was performed by the Colorado Department of Public Health and Environment (CDPHE) using the TaqPath™ COVID-19 Combo Kit (ThermoFisher Scientific) or by the San Diego County Public Health Laboratories (SD PHL) using the New Coronavirus Nucleic Acid Detection Kit (PerkinElmer). RT-PCR–positive NP specimens with N-gene CT value ≤ 32 underwent viral culture for viral isolation as described previously [28]. Additionally, RT-PCR-positive NP specimen(s) with a cycle threshold (CT) value ≤ 32 underwent WGS at CDC on the MinION platform (Oxford Nanopore Technologies) and the Illumina MiSeq platform (Illumina Inc.), or at CDPHE on the GridION (Oxford Nanopore Technologies) or Illumina (Illumina Inc.) platforms. For households in which no individual had a CT value of ≤ 32, sequencing was performed on the specimen with the lowest CT value. The Phylogenetic Assignment of Named Global Outbreak Lineages (PANGOLIN) web application was used to assign SARS-CoV-2 lineages to sequenced genomes [29].
Serum from blood specimens were tested for SARS-CoV-2-specific antibodies using a multi-spot V-PLEX COVID-19 Serology Kit (Meso Scale Discovery, Rockville, MD) to quantitatively measure antibodies to SARS-CoV-2 receptor binding domain (RBD) and spike antigens, as these antibodies can serve as markers of either vaccination or natural infection. Results were interpolated from a standard curve and reported on a logarithmic scale as assigned Binding Antibody Units (BAU/mL).
2.8. Vaccination rate by location
The state-wide proportion of persons who had received one dose of an mRNA-based vaccine and the proportion of persons who had completed a vaccination series of any of the three FDA-authorized vaccines was determined for California and Colorado using CDC’s COVID-19 Data Tracker [30].
2.9. Statistical Methods
All statistical calculations were performed using SAS software Version 9.4 (SAS Institute, Cary, NC). Persons missing information on the variable of interest were excluded from any analyses of that variable of interest; we did not impute missing data. The Mann-Whitney U test was used to compare age and quantitative serology titers, the χ2 test was used to compare categorical variables, and the Z-test was used to compare proportions. All statistical tests were two-sided and an α-value of 0.05 was considered statistically significant. P-values and confidence intervals (CIs) were not adjusted for multiple comparisons.
We compared the proportion of persons with a positive SARS-CoV-2 viral culture by vaccination status and number of days post-illness onset of specimen collection. Due to limited sample size specimen collection were grouped (0–5, 6–10, or 11–15 days post-illness onset).
To estimate the risk of infection by vaccination status for household contacts, we modeled the relationship between vaccination status and individual odds of infection for household contacts by calculating odds ratios (ORs) using Generalized Estimating Equations (GEE) [31]. The outcome of interest was SARS-CoV-2 infection during the investigation period. The primary predictor variable was vaccination status, categorized as vaccinated and unvaccinated. Persons who were fully or partially vaccinated were considered vaccinated, while persons who were recently vaccinated or unvaccinated were considered unvaccinated. An exchangeable correlation structure was used in GEE models to account for within household correlation. We constructed an additional model to calculate adjusted ORs (aORs). In this adjusted model, the outcome of interest was SARS-CoV-2 infection during the investigation period, and predictor variables were: vaccination status, age (categorized as < 16, 16–64, and ≥ 65 years of age), and sex. Household size was examined as a possible predictor variable in the adjusted model but was not included as it did not change the point estimate by > 10%.
3. Results
A total of 552 persons from 151 households were enrolled in the investigation (Fig. 1). Forty-seven persons from 11 households were excluded as the primary case had illness onset > 10 days before initial enrollment. Additionally, 2 households (7 persons) withdrew from the investigation and 4 persons were lost to follow-up; the final analysis population included 493 persons from 138 households. The median age was 31 years (range 0–86 years) and 258/493 (52%) were female (Table 1 ). Ninety-one (19%) had received at least one dose of any COVID-19 vaccine; 32 (7%) were recently vaccinated, 30 (6%) were partially vaccinated, and 29 (6%) were fully vaccinated. Among 51 persons who received the Pfizer (BNT162b2) vaccine, 17 (33%) were fully vaccinated, 18 (35%) were partially vaccinated, and 16 (31%) were recently vaccinated. Among 36 persons who received the Moderna (mRNA-1273) vaccine, 11 (31%) were fully vaccinated, 12 (33%) were partially vaccinated, and 13 (36%) were recently vaccinated. Three persons received the Janssen (JNJ-78436735) vaccine; 1 was fully vaccinated and 2 were recently vaccinated.
Table 1.
Demographic characteristics by vaccination status for primary cases and household contacts, San Diego, CA, and Denver, CO, January–April 2021.
|
Not Vaccinated (N = 402) |
Recently Vaccinated (N = 32) |
Partially Vaccinated (N = 30) |
Fully Vaccinated (N = 29) |
Overall (N = 493) |
|
|---|---|---|---|---|---|
| Case Status | |||||
| Primary Case | 126 (31%) | 12 (38%) | 6 (20%) | 3 (10%) | 147 (30%) |
| Household Contacts | 276 (69%) | 20 (62%) | 24 (80%) | 26 (90%) | 346 (70%) |
| Age (median years, range) | 23 (0–74) | 39 (15–86) | 50 (17–74) | 48 (32–83) | 31 (0–86) |
| Sex | |||||
| Female | 201 (50%) | 19 (59%) | 17 (57%) | 21 (72%) | 258 (52%) |
| Male | 201 (50%) | 13 (41%) | 13 (43%) | 8 (28%) | 235 (48%) |
| Race/Ethnicity | |||||
| White, NH | 222 (55%) | 25 (78%) | 15 (50%) | 23 (79%) | 285 (58%) |
| Black, NH | 14 (3%) | 1 (3%) | 1 (3%) | 0 (0%) | 16 (3%) |
| AI/AN, NH | 4 (1%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (1%) |
| Asian, NH | 27 (7%) | 0 (0%) | 5 (17%) | 4 (14%) | 36 (7%) |
| NH/PI, NH | 5 (1%) | 0 (%) | 0 (0%) | 0 (0%) | 5 (1%) |
| Multi race, NH | 24 (6%) | 1 (3%) | 1 (3%) | 0 (0%) | 26 (5%) |
| Other race, NH | 2 (<1%) | 0 (0%) | 1 (3%) | 0 (0%) | 3 (1%) |
| Hispanic/Latino | 104 (26%) | 5 (16%) | 7 (23%) | 1 (3%) | 117 (24%) |
| Missing | 0 (0%) | 0 (0%) | 0 (0%) | 1 (3%) | 1 (<1%) |
| Highest Level of Education | |||||
| Child < 18 years | 167 (42%) | 3 (9%) | 1 (3%) | 0 (0%) | 171 (35%) |
| Less than High School | 15 (4%) | 1 (3%) | 3 (10%) | 0 (0%) | 19 (4%) |
| High School/GED | 47 (12%) | 2 (6%) | 5 (17%) | 2 (7%) | 56 (11%) |
| Some college | 58 (14%) | 7 (22%) | 4 (13%) | 1 (3%) | 70 (14%) |
| Technical degree/Associate’s | 18 (4%) | 0 (0%) | 1 (3%) | 0 (0%) | 19 (4%) |
| Bachelor’s degree | 68 (17%) | 14 (44%) | 10 (33%) | 17 (59%) | 109 (22%) |
| Master’s degree | 26 (7%) | 4 (13%) | 5 (17%) | 7 (24%) | 42 (9%) |
| Doctoral/professional degree | 3 (1%) | 1 (3%) | 1 (3%) | 2 (7%) | 7 (1%) |
| Enrollment Location | |||||
| San Diego, CA | 190 (47%) | 9 (28%) | 12 (40%) | 7 (24%) | 218 (44%) |
| Denver, CO | 212 (53%) | 23 (72%) | 18 (60%) | 22 (76%) | 275 (56%) |
| Medical Comorbidities | |||||
| Any Medical Condition | 130 (32%) | 14 (44%) | 23 (77%) | 16 (55%) | 183 (37%) |
| Any Chronic lung disease | 41 (10%) | 5 (16%) | 6 (20%) | 3 (10%) | 55 (11%) |
| Diabetes | 12 (3%) | 3 (9%) | 5 (17%) | 2 (7%) | 22 (4%) |
| Hypertension | 29 (7%) | 6 (19%) | 10 (10%) | 4 (14%) | 49 (10%) |
| Any Cardiovascular disease | 12 (3%) | 3 (9%) | 2 (7%) | 4 (14%) | 21 (4%) |
| Any chronic kidney disease | 4 (1%) | 1 (3%) | 0 (0%) | 0 (0%) | 5 (1%) |
| Any chronic liver disease | 1 (<1%) | 0 (0%) | 0 (%) | 2 (7%) | 3 (1%) |
| Any immunocompromising condition or medication | 12 (3%) | 1 (3%) | 2 (7%) | 1 (3%) | 16 (3%) |
| Any hyperlipidemia | 13 (3%) | 2 (6%) | 3 (10%) | 3 (10%) | 21 (4%) |
| Any hypothyroid | 8 (2%) | 2 (6%) | 3 (10%) | 3 (10%) | 16 (3%) |
| Any neurologic/ neurodevelopmental disorder | 13 (3%) | 1 (3%) | 1 (3%) | 2 (7%) | 17 (3%) |
| Other chronic condition | 47 (12%) | 5 (16%) | 8 (27%) | 5 (17%) | 65 (13%) |
| Vaccine Product | |||||
| BNT162b2 | N/A | 16 (50%) | 18 (60%) | 17 (59%) | 51 (10%) |
| mRNA-1273 | N/A | 13 (41%) | 12 (40%) | 11 (38%) | 36 (7%) |
| JNJ-78436735 | N/A | 2 (6%) | 0 (0%) | 1 (3%) | 3 (1%) |
| Missing | N/A | 1 (3%) | 0 (0%) | 0 (0%) | 1 (<1%) |
Abbreviations: AI/AN – American Indian and Alaska Native, GED – General Educational Development, NH – non-Hispanic, NH/PI – Native Hawaiian and Pacific Islander.
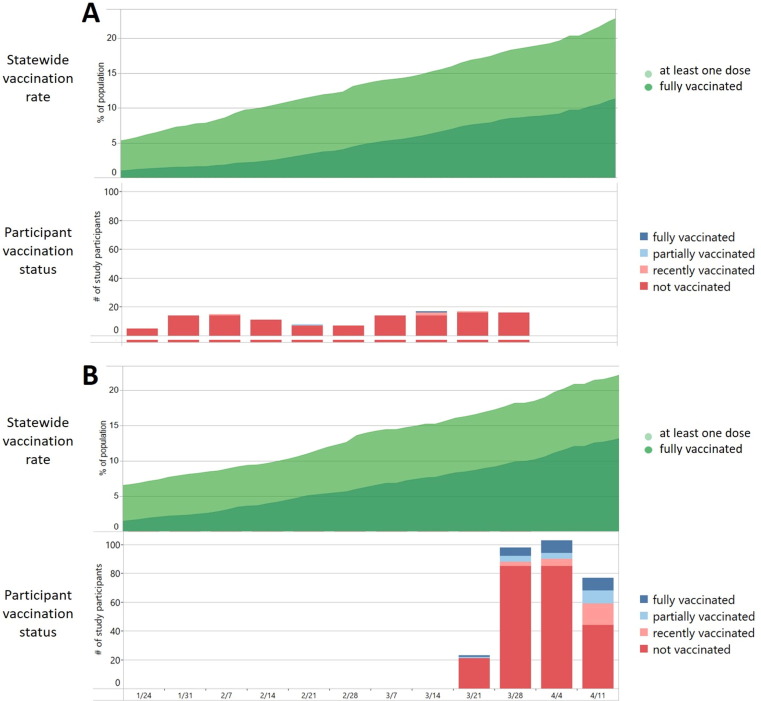
During the investigation period, the proportion of fully vaccinated persons in the state of California increased from 2% to 8%, and the proportion of persons who had received at least one dose of a vaccine ranged from 5% to 17% (Fig. 3 ). For the state of Colorado, the proportion of fully vaccinated persons increased from 8% to 13% over the investigation period, and the proportion of persons who received at least one dose of a vaccine increased from 16% to 23%. A higher proportion of persons enrolled from Colorado were fully, partially, or recently vaccinated compared to persons enrolled from California (Fig. 3; Table 1).
Fig. 3.
Vaccination status for California residents and persons enrolled from California (Panel A) and Colorado (Panel B) as a proportion of the general population (upper chart) and number of persons in the investigation population (bottom chart).
Compared to unvaccinated persons, persons who had received at least one dose of any vaccine were older (median age 46 years, range 15–86 years vs median age 23 years, range 0–74 years, p < 0.0001) and a higher proportion were female (57/91, 63% vs 201/402, 50%, p = 0.02) (Table 1). A higher proportion of persons who received at least one dose of any vaccine were non-Hispanic White compared to persons who had not been vaccinated (63/91, 69% vs 222/402, 55%, p = 0.01). Partially vaccinated persons and fully vaccinated persons had a similar age distribution, and a higher proportion of partially vaccinated persons had at least one medical comorbidity; a lower proportion of partially vaccinated persons were female. Among fully vaccinated persons, the number of days from the final vaccine dose to enrollment was similar in California (median 39 days, range 31–68 days) and Colorado (median 44 days, range 21–97 days; p = 0.71).
Among the 147 primary cases, 21 (14%) had received at least one vaccine dose and 3 (2%) were fully vaccinated; 10 received the BNT162b2 vaccine, 9 received the mRNA-1273 vaccine, and 1 received the J&J-78436735 vaccine (Table 2 ).
Table 2.
Vaccination status for persons enrolled in the investigation, stratified by vaccine product and case status, California and Colorado, January–April 2021.
| N, % | Fully Vaccinated | Partially Vaccinated | Recently Vaccinated | Not Vaccinated | |
|---|---|---|---|---|---|
| All Enrolled Persons (n = 493) | |||||
| BNT162b2 | 51 (10%) | 17 | 18 | 16 | – |
| mRNA-1273 | 36 (7%) | 11 | 12 | 13 | – |
| JNJ-78436735 | 3 (1%) | 1 | – | 2 | – |
| Product not verified | 1 (1%) | – | – | 1 | – |
| Not Vaccinated | 402 (82%) | – | – | – | 402 |
| Primary Cases (n = 147) | |||||
| BNT162b2 | 10 (7%) | 1 | 3 | 6 | – |
| mRNA-1273 | 9 (6%) | 2 | 3 | 4 | – |
| JNJ-78436735 | 1 (1%) | 0 | – | 1 | – |
| Product not verified | 1 (1%) | – | – | 1 | – |
| Not Vaccinated | 126 (86%) | – | – | – | 126 |
| Secondary Cases*(n = 161) | |||||
| BNT162b2 | 15 (9%) | 5 | 3 | 7 | – |
| mRNA-1273 | 2 (1%) | 1 | – | 1 | – |
| JNJ-78436735 | 0 (0%) | 0 | – | 0 | – |
| Product not verified | – | – | – | 0 | – |
| Not Vaccinated | 144 (89%) | – | – | – | 144 |
|
Uninfected household Contacts** (n = 185) |
|||||
| BNT162b2 | 26 (14%) | 11 | 12 | 3 | – |
| mRNA-1273 | 25 (14%) | 8 | 9 | 8 | – |
| JNJ-78436735 | 2 (1%) | 1 | – | 1 | – |
| Product not verified | – | – | – | 0 | – |
| Not Vaccinated | 132 (71%) | – | – | – | 132 |
Any COVID-19 case in the household during the investigation period other than the primary case.
Any member of household who did not test positive for SARS-CoV-2 during the course of the investigation.
Information on symptoms was available for all 308 individuals with confirmed SARS-CoV-2 infection (Table 3 ). A lower proportion of fully vaccinated persons (5/9, 56%) and partially vaccinated persons (6/9, 67%) reported symptoms that met the CSTE case definition [27] than recently vaccinated persons (20/20, 100%) and unvaccinated persons (251/270, 93%) [p < 0.001 comparing fully and partially vaccinated (11/18, 61%) to recently and unvaccinated persons (271/290, 93%)].
Table 3.
Self-reported symptom status for primary and secondary cases, California and Colorado, January–April 2021.
|
Not Vaccinated N (%) |
Recently Vaccinated N (%) |
Partially Vaccinated N (%) |
Fully Vaccinated N (%) |
Overall N (%) |
|
|---|---|---|---|---|---|
| All Cases | (n = 270) | (n = 20) | (n = 9) | (n = 9) | (n = 308) |
| Any symptoms | 258 (96%) | 20 (100%) | 6 (67%) | 6 (67%) | 289 (94%) |
| Symptoms meeting CSTE case definition* |
251 (93%) | 20 (100%) | 6 (67%) | 5 (56%) | 282 (92%) |
| Received medical care† | 23 (9%) | 6 (30%) | 3 (33%) | 1 (11%) | 33 (11%) |
| Primary Cases | (n = 126) | (n = 12) | (n = 6) | (n = 3) | (n = 147) |
| Any symptoms | 123 (98%) | 12 (100%) | 4 (67%) | 1 (33%) | 140 (95%) |
| Symptoms meeting CSTE case definition* |
122 (97%) | 12 (100%) | 4 (67%) | 1 (33%) | 139 (95%) |
| Received medical care† | 16 (13%) | 5 (42%) | 2 (33%) | 0 (0%) | 23 (16%) |
| Secondary Cases | (n = 144) | (n = 8) | (n = 3) | (n = 6) | (n = 161) |
| Any symptoms | 135 (94%) | 8 (100%) | 2 (67%) | 5 (83%) | 149 (93%) |
| Symptoms meeting CSTE case definition* |
129 (90%) | 8 (100%) | 2 (67%) | 4 (67%) | 143 (89%) |
| Received medical care† | 7 (5%) | 1 (13%) | 1 (33%) | 1 (17%) | 10 (11%) |
*Using the interim case definition developed by CSTE and CDC [27].
†Participants self-reported if they sought medical care, including telehealth, primary care provider, urgent care, visited the emergency department, or were hospitalized during the course of their illness as part of the questionnaire administered on day 14 of enrollment. Abbreviations: CSTE – Council of State and Territorial Epidemiologists; CDC – US Centers for Disease Control and Prevention.
The proportion of cases with a positive viral culture during days 0–5 was similar for recently vaccinated (8/16, 50%), and unvaccinated persons (97/160, 61%) (Table 4 ); a lower proportion of fully vaccinated persons had virus recovered in viral culture (2/6, 33%). During days 6–10, fully vaccinated (1/4, 25%) and recently vaccinated persons (2/10, 20%) had a lower proportion of positive viral culture compared to unvaccinated persons (41/103, 40%). No partially vaccinated person had a positive viral culture (n = 5). One fully vaccinated person had a positive viral culture at 12 days after symptom onset; this person continued to have detectable SARS-CoV-2 RNA from NP specimens for at least 29 days after symptom onset. The number of NP specimens from which culturable virus was recovered varied by day post illness onset (Table 4).
Table 4.
Proportion of persons and proportion of positive swabs with a positive viral culture in relation to days after illness onset date (first symptom onset or first positive test for asymptomatic individuals) stratified by vaccination status, California and Colorado, January–April 2021.
|
Time from illness onset date |
||||||
|---|---|---|---|---|---|---|
|
0–5 days |
6–10 days |
>10 days |
||||
| Proportion of persons culture positive (%) | Proportion of RT-PCR-positive swabs culture positive (%) | Proportion of persons culture positive (%) | Proportion of RT-PCR-positive swabs culture positive (%) | Proportion of persons Culture Positive (%) | Proportion of RT-PCR-positive swabs culture positive (%) | |
| Fully Vaccinated | 2/6 (33%) | 2/9 (22%) | 1/4 (25%) | 4/19 (21%) | 1/5 (20%) | 3/14 (21%) |
| Partially Vaccinated | 0/4 (0%) | 0/5 (0%) | 0/2 (0%) | 0/2 (0%) | 0/5 (0%) | 0/5 (0%) |
| Recently Vaccinated | 8/16 (50%) | 9/23 (39%) | 2/10 (20%) | 2/17 (12%) | 0/16 (0%) | 0/18 (0%) |
| Not vaccinated | 97/160 (61%) | 103/181 (57%) | 41/103 (40%) | 49/161 (30%) | 0/168 (0%) | 0/183 (0%) |
| Total | 107/186 (58%) | 114/218 (52%) | 44/119 (37%) | 55/199 (28%) | 1/194 (1%) | 3/220 (1% |
Abbreviation: RT-PCR – reverse transcription polymerase chain reaction.
Among the 127 households included in the IR analysis, the IR for the 322 household contacts of primary cases was 45% (146/322, 95% CI 40–51%) (Table 5 ). The observed IR was highest in unvaccinated household contacts (127/257, 49%, 95% CI 43–55%). Compared to unvaccinated household contacts, the IR was lower for fully vaccinated (23% vs 49%) and partially vaccinated household contacts (14% vs 49%,); the IR for unvaccinated household contacts was similar to that of recently vaccinated household contacts 44% vs 49%. The absolute reduction in the IR was 26% for fully vaccinated compared to unvaccinated contacts. One fully vaccinated secondary case was symptomatic (13%); the other 5 were asymptomatic (87%). Among unvaccinated secondary cases, 105/108 (97%) were symptomatic (Table 3).
Table 5.
Infection risk among household contacts stratified by vaccination status, California and Colorado, January–April 2021.
| SARS-CoV-2 Positive During Investigation | Not SARS-CoV-2 Positive During Investigation | Infection Risk | |
|---|---|---|---|
| Fully Vaccinated | 6 | 20 | 23.1% |
| Partially Vaccinated | 3 | 18 | 14.3% |
| Fully and Partially Vaccinated | 9 | 38 | 19.1% |
| Recently Vaccinated | 8 | 10 | 44.4% |
| Not vaccinated | 127 | 130 | 49.4% |
| Recently and Not Vaccinated | 135 | 140 | 49.1% |
| Total | 144 | 178 | 44.7% |
Among the 127 households included in the IR analysis, there were no secondary cases in the 3 households in which the primary case was fully vaccinated. Among the 5 households in which the primary case was partially vaccinated, secondary cases occurred in 1 household (20%); there were 9 households with secondary cases among the 11 households in which the primary case was recently vaccinated (82%). Among the 108 households in which the primary case was unvaccinated, secondary cases occurred in 66 households (62%).
Using GEE models to account for household clustering, the odds of infection were lower for vaccinated household contacts compared to unvaccinated household contacts (OR = 0.38, 95% CI 0.17–0.85). The odds of infection remained lower for vaccinated persons after adjustment for age and sex (OR = 0.40, 95% CI 0.16–1.03) although this finding did not reach statistical significance.
Among the 161 secondary cases identified during the investigation, 144 (89%) had not been vaccinated, 6 (4%) were fully vaccinated, 3 (2%) was partially vaccinated, and 8 (5%) were recently vaccinated (Table 2). Most of the secondary cases who were vaccinated had received the BNT162b2 vaccine (15/17, 88%). There were 178 household contacts who never had a positive SARS-CoV-2 test during the investigation; of these, 48 (27%) had received at least one dose of any vaccine. Among the 48 vaccinated persons who did not have a positive SARS-CoV-2 test, 10 (21%) were recently vaccinated, 18 (38%) were partially vaccinated, and 20 (42%) were fully vaccinated. Twenty-six persons (49%) received the BNT162b2 vaccine, 25 (47%) received the mRNA-1273 vaccine, and 2 (4%) received the JNJ-78436735 vaccine. None of the fully or partially vaccinated individuals in this investigation with confirmed SARS-CoV-2 infection reported a history of prior infection.
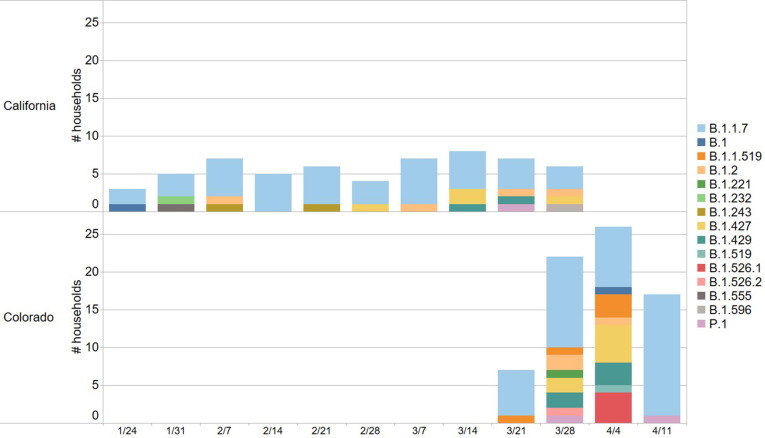
The SARS-CoV-2 lineage was identified for 121/138 households (88%; Fig. 4 ). The most common lineage was Alpha (75/121, 62%), followed by Epsilon (19/121, 16%) and B.1.1.519 (6/121, 5%). Alpha was more common in households enrolled in California compared to Colorado (40/57, 70% vs. 35/64, 56%, p = 0.10). There were no households with discordant lineages among household members.
Fig. 4.
Variant status for enrolled households over time stratified by location. The X-axis shows the week of enrollment.
Among 494 participants, 405 (82%) had blood specimens collected for serology. Adults were more likely to have blood specimens collected (302/324, 93%) compared to children (103/170, 61%) (p < 0.001). After excluding 27 individuals who received a vaccine dose after illness onset, among 281 cases, antibody titers against the spike protein and the receptor-binding domain (RBD) antigen differed by vaccination status and by number of days post illness onset (Table 6 ). Antibody titers against spike and RBD antigens were higher ≥ 14 days post illness onset compared to < 14 days post illness for all persons, and the relative increase in antibody titer was greatest for unvaccinated individuals. Fully and partially vaccinated persons had similar antibody titers against spike and RBD antigens during both time intervals. Unvaccinated persons had lower antibody titers against spike and RBD antigens than partially or fully vaccinated persons during both time intervals.
Table 6.
Quantitative serology results for primary and secondary cases in relation to illness onset date (first symptom onset or first positive test for asymptomatic individuals), California and Colorado, January–April 2021. Individuals who received a vaccine dose after illness onset (n = 27) are excluded from analysis.
|
Geometric mean antibody titer (±geometric standard deviation) log BAU/mL |
||||
|---|---|---|---|---|
|
Anti-Spike |
Anti-Receptor Binding Domain |
|||
| <14 days after illness onset | ≥14 days after illness onset | <14 days after illness onset | ≥14 days after illness onset | |
| Fully Vaccinated (n = 9) | 464.3 (3.9) | 1437.1 (2.1) | 672.5 (4.0) | 2238.6 (2.3) |
| Partially Vaccinated (n = 8) | 609.2 (3.5) | 1854.4 (1.8) | 528.8 (5.5) | 2799.5 (1.9) |
| Recently Vaccinated (n = 11) | 106.8 (25.1) | 1247.3 (4.7) | 83.5 (26.5) | 1266.2 (7.3) |
| Not Vaccinated (n = 253) | 1.8 (10.4) | 123.2 (5.6) | 3.8 (8.1) | 99.1 (6.1) |
Xx Abbreviation: BAU – binding antibody units.
4. Discussion
In a household setting, we found that vaccinated household contacts had a lower risk of SARS-CoV-2 infection compared to persons who were not vaccinated, with fully vaccinated household contacts having an absolute risk reduction of 26% compared to unvaccinated household contacts. Based on this absolute risk reduction, we estimate that 1 SARS-CoV-2 household infection event would be prevented for every 4 vaccinated persons during the period when Alpha was predominant. The protective effect of vaccination for household contacts remained after accounting for clustering by household, although not after adjustment for age and sex. This finding supports previous studies which have demonstrated the efficacy of vaccines in preventing infection [18], [32], [33] and extends these findings to the household setting, which is characterized by intense and repeated exposures, resulting in high risk of transmission [34], [35], [36]. The lower IR observed among household contacts who were fully and partially vaccinated compared to unvaccinated individuals supports the utility of ongoing vaccination efforts.
A lower proportion of secondarily infected persons who were fully or partially vaccinated reported any symptoms, which is consistent with a prior report demonstrating a lower proportion of symptomatic infection in vaccinated persons with SARS-CoV-2 infection [37].
Although the finding did not achieve statistical significance, a lower proportion of fully vaccinated individuals had SARS-CoV-2 recovered in culture from NP specimens than unvaccinated individuals. Two recent reports found that vaccinated persons infected with the Delta variant of SARS-CoV-2 had virus recovered from culture, suggesting that fully vaccinated persons may be able to transmit SARS-CoV-2 [16], [17]. Other studies have found that vaccinated persons with SARS-CoV-2 infection have similar levels of viral RNA detectable by RT-PCR from nasal swab specimens compared to unvaccinated persons [38]. Despite these findings, other studies examining household transmission have found that vaccinated persons do not appear to transmit the virus as effectively as unvaccinated persons within their household [39]. Taken together, the findings from this investigation and those from other studies suggest that vaccinated individuals likely transmit SARS-CoV-2 less efficiently despite the presence of detectable SARS-CoV-2 RNA and recovery of SARS-CoV-2 in viral culture.
One fully vaccinated, immunocompetent individual (BNT162b2) in this investigation had detectable SARS-CoV-2 RNA by NP swab 29 days after illness onset; this person also had virus recovered from cell culture for the first 12 days after illness onset. In a separate laboratory-based study, virus recovery in cell culture was associated with prolonged viral shedding, although the vaccination status of participants was not assessed [40]. Virus recovery in cell culture has been reported to decrease ≥ 8 days of symptom onset [41]; however, increased severity of COVID-19 disease is associated with prolonged viral shedding and virus recovery in cell culture [42]. Similarly, prolonged duration of viral shedding has been reported with the Middle East Respiratory Syndrome coronavirus [43] and the Severe Acute Respiratory Syndrome-associated coronavirus [44]. The finding of prolonged detection of viral RNA and recovery of SARS-CoV-2 in viral culture from this fully vaccinated individual 12 days after symptom onset suggests that fully vaccinated individuals may be capable of transmitting the virus to others. We note this case as an exception, but one that provides important insights for further research regarding factors affecting viral persistence and potential transmissibility following SARS-CoV-2 infection in fully vaccinated individuals.
Although we did observe detectable spike and RBD antibody titers in unvaccinated infected individuals, unvaccinated persons had lower antibody titers to spike and RBD compared to fully vaccinated and partially vaccinated persons who were infected, both early and later after infection. The presence of these antibodies in infected unvaccinated individuals may reflect early antibody response to the current SARS-CoV-2 infection. The antibody titers for recently vaccinated individuals fell between those vaccinated and unvaccinated, as expected. Among persons who acquired SARS-CoV-2 infection during this investigation, spike and RBD antibody titers increased ≥ 14 days after illness onset; spike and RBD antibody titers were higher ≥ 14 days after illness onset for individuals who had received at least one dose of a COVID-19 vaccine compared to unvaccinated individuals, suggesting that natural infection may have provided an immune boosting effect for these individuals. These findings are similar to those reported in a study of antibody titers following SARS-CoV-2 infection, which found substantially higher RBD titers among individuals with a prior history of vaccination compared to unvaccinated individuals [45]. Evidence from other studies suggests that vaccine-induced immune responses may peak 12 weeks after receipt of a second mRNA-based vaccine dose [46].
This investigation had several limitations. First, we do not have information regarding the demographics or household characteristics of persons who declined to participate in this investigation and so cannot determine how representative the participants are compared to the general population. Second, the limited sample size, particularly among vaccinated individuals, limits our ability to perform statistical inference or advanced modeling. Third, fully vaccinated persons may have been more motivated to participate in our investigation, leading to differential enrollment and selection bias. Approximately 20% of all participants were vaccinated in this investigation, which was slightly higher than the percentage of persons vaccinated in their state of residence at the time of enrollment. Fourth, this investigation occurred relatively early after participants had been vaccinated, so we could not assess the long-term protective effects of vaccination on household transmission, nor could we assess the impact of prior infection with SARS-CoV-2 on the risk of household transmission. Fifth, CT values were not standardized across the different testing laboratories and assays; therefore, error in selection of specimens sent for viral culture may have occurred. It is unlikely that this error would be directional due to assay variation within and between testing locations. Sixth, we were not able to analyze the impact of socioeconomic status and household size on household transmission; persons who lived in larger houses may be able to isolate from other family members more easily. Additionally, though we assumed that transmission occurred between the primary case and household contacts, we cannot discern the exact sequence of transmission in households with more than one secondary case. In some instances, community transmission could have accounted for additional infections within the household. Finally, this study was conducted when Alpha was the predominant circulating variant and before booster doses were recommended. These findings may not apply to the evolving situation of COVID-19 transmission in the United States, particularly for variants such as Omicron which have different dynamics in a vaccinated population [47]. Additional studies examining the effect of vaccination and prior infection with SARS-CoV-2 on household transmission with the current predominant variants are recommended.
5. Conclusions
Although COVID-19 infections in vaccinated persons were reported in this high transmission risk setting, both full and partial vaccination were protective against SARS-CoV-2 infection while the Alpha variant was predominant. These findings illustrate the effect of vaccination in reducing transmission within households of persons infected with SARS-CoV-2 and support the need for ongoing vaccination efforts. However, vaccination alone may not be sufficient to control the spread of SARS-CoV-2. Therefore, other interventions such physical distancing, quarantine, and isolation may also be needed. We found no documented transmission from a fully vaccinated case to household contacts, but the recovery of culturable virus from vaccinated persons in this investigation indicates that some infected vaccinated persons may be capable of transmitting SARS-CoV-2. These findings should be interpreted cautiously as the predominant circulating variants continue to change and vaccination recommendations continue to be updated based on age groups, booster doses, and for those with immunocompromising conditions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank the participants in this investigation and the staff at Tri-County Public Health Department, the Colorado Department of Public Health and Environment, County of San Diego Health and Human Services Agency, the California Department of Public Health, and the CDC COVID-19 Response Team.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention, Colorado Department of Public Health and Environment, Tri-County Health Department, or the County of San Diego Health and Human Services Agency.
Footnotes
See e.g., 45C.F.R. part 46.102(l)(2), 21C.F.R. part 56; 42 U.S.C. §241(d); 5 U.S.C. §552a; 44 U.S.C. §3501 et seq.
E.g. dormitory, long-term care facility, group home, or correctional facility.
References
- 1.Tenforde M.W., Patel M.M., Ginde A.A., Douin D.J., Talbot H.K., Casey J.D., et al. Effectiveness of SARS-CoV-2 mRNA Vaccines for Preventing Covid-19 Hospitalizations in the United States. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab687. [DOI] [Google Scholar]
- 2.US Food and Drug Administration. COVID-19 Vaccines 2021. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines (accessed November 2, 2021).
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J., Gray G., Vandebosch A.n., Cárdenas V., Shukarev G., Grinsztejn B., et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N Engl J Med. 2021;384(23):2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Self W.H., Tenforde M.W., Rhoads J.P., Gaglani M., Ginde A.A., Douin D.J., et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions — United States, March–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1337–1343. doi: 10.15585/mmwr.mm7038e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrejko K.L., Pry J., Myers J.F., Jewell N.P., Openshaw J., Watt J., et al. Prevention of COVID-19 by mRNA-based vaccines within the general population of California. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tande AJ, Pollock BD, Shah ND, Farrugia G, Virk A, Swift M, et al. Impact of the Coronavirus Disease 2019 (COVID-19) Vaccine on Asymptomatic Infection Among Patients Undergoing Preprocedural COVID-19 Molecular Screening. Clin Infect Dis 2021;2019:1–7. 10.1093/cid/ciab229. [DOI] [PMC free article] [PubMed]
- 9.Regev-Yochay G., Amit S., Bergwerk M., Lipsitch M., Leshem E., Kahn R., et al. Decreased infectivity following BNT162b2 vaccination: A prospective cohort study in Israel. Lancet Reg Heal Eur. 2021;7:100150. doi: 10.1016/j.lanepe.2021.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fowlkes A., Gaglani M., Groover K., Thiese M.S., Tyner H., Ellingson K. Effectiveness of COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Frontline Workers Before and During B.1.617.2 (Delta) Variant Predominance — Eight U.S. Locations, December 2020–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1167–1169. doi: 10.15585/mmwr.mm7034e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez Bernal J., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., et al. Effectiveness of Covid-19 Vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stowe J, Andrews N, Gower C, Gallagher E, Utsi L, Simmons R, et al. Effectiveness of COVID-19 vaccines against hospital admission with the Delta (B.1.617.2) variant. Public Heal Engl 2021;37. https://khub.net/web/phe-national/public-library.
- 13.Tang P., Hasan M.R., Chemaitelly H., Yassine H.M., Benslimane F.M., Al Khatib H.A., et al. BNT162b2 and mRNA-1273 COVID-19 vaccine effectiveness against the SARS-CoV-2 Delta variant in Qatar. Nat Med. 2021;27(12):2136–2143. doi: 10.1038/s41591-021-01583-4. [DOI] [PubMed] [Google Scholar]
- 14.Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet (London, England) 2021;398(10309):1407–1416. doi: 10.1016/S0140-6736(21)02183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin J.B., Haddix M., Danza P., Fisher R., Koo T.H., Traub E., et al. SARS-CoV-2 Infections and Hospitalizations Among Persons Aged ≥16 Years, by Vaccination Status — Los Angeles County, California, May 1–July 25, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1170–1176. doi: 10.15585/mmwr.mm7034e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ong S.W.X., Chiew C.J., Ang L.W., Mak T.-M., Cui L., Toh M.P.H.S., et al. Clinical and virological features of SARS-CoV-2 variants of concern: a retrospective cohort study comparing B.1.1.7 (Alpha), B.1.315 (Beta), and B.1.617.2 (Delta) Clin Infect Dis. 2021 doi: 10.1093/cid/ciab721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hagan L.M., McCormick D.W., Lee C., Sleweon S., Nicolae L., Dixon T., et al. Outbreak of SARS-CoV-2 B.1.617.2 (Delta) Variant Infections Among Incarcerated Persons in a Federal Prison — Texas, July–August 2021. MMWR Morb Mortal Wkly Rep. 2021;70(38):1349–1354. doi: 10.15585/mmwr.mm7038e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Björk J., Inghammar M., Moghaddassi M., Rasmussen M., Malmqvist U., Kahn F. High level of protection against COVID-19 after two doses of BNT162b2 vaccine in the working age population – first results from a cohort study in Southern Sweden. Infectious Diseases. 2022;54(2):128–133. doi: 10.1080/23744235.2021.1982144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alpert T., Brito A.F., Lasek-Nesselquist E., Rothman J., Valesano A.L., MacKay M.J., et al. Early introductions and transmission of SARS-CoV-2 variant B.1.1.7 in the United States. Cell. 2021;184(10):2595–2604.e13. doi: 10.1016/j.cell.2021.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N Engl J Med. 2021;385(16):1474–1484. doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. COVID Data Tracker: COVID-19 Vaccinations in the United States 2021. https://covid.cdc.gov/covid-data-tracker/#vaccinations_vacc-total-admin-rate-total.
- 23.Donnelly M.A.P., Chuey M.R., Soto R., Schwartz N.G., Chu V.T., Konkle S.L., et al. Household transmission of SARS-CoV-2 Alpha variant - United States, 2021. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. No Title 2020.
- 27.Council of State and Territorial Epidemiologists. Standardized surveillance case definition and national notification for 2019 novel coronavirus disease (COVID-19). 2020.
- 28.Harcourt J., Tamin A., Lu X., Kamili S., Sakthivel S.K., Murray J., et al. Severe Acute Respiratory Syndrome Coronavirus 2 from Patient with Coronavirus Disease. United States Emerg Infect Dis. 2020;26(6):1266–1273. doi: 10.3201/eid2606.200516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rambaut A., Holmes E.C., O’Toole Á., Hill V., McCrone J.T., Ruis C., et al. A dynamic nomenclature proposal for SARS-CoV-2 lineages to assist genomic epidemiology. Nat Microbiol. 2020;5(11):1403–1407. doi: 10.1038/s41564-020-0770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC. Coronavirus disease 2019 (COVID-19): CDC data tracker. United States COVID-19 cases and deaths by states. Atlanta, GA: 2021.
- 31.Zeger S.L., Liang K.Y., Albert P.S. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 32.Thompson MG, Burgess JL, Naleway AL, Tyner HL, Yoon SK, Meece J, et al. Interim Estimates of Vaccine Effectiveness of BNT162b2 and mRNA-1273 COVID-19 Vaccines in Preventing SARS-CoV-2 Infection Among Health Care Personnel, First Responders, and Other Essential and Frontline Workers - Eight U.S. Locations, December 2020-March. MMWR Morb Mortal Wkly Rep 2021;70:495–500. 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed]
- 33.Hall V.J., Foulkes S., Saei A., Andrews N., Oguti B., Charlett A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet (London, England) 2021;397(10286):1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gazit S, Mizrahi B, Kalkstein N, Neuberger A, Peretz A, Mizrahi-Reuveni M, et al. BNT162b2 mRNA Vaccine Effectiveness Given Confirmed Exposure; Analysis of Household Members of COVID-19 Patients. MedRxiv 2021:2021.06.29.21259579. [DOI] [PMC free article] [PubMed]
- 35.Madewell Z.J., Yang Y., Longini I.M., Halloran M.E., Dean N.E. Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3(12):e2031756. doi: 10.1001/jamanetworkopen.2020.31756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grijalva CG, Rolfes MA, Zhu Y, McLean HQ, Hanson KE, Belongia EA, et al. Transmission of SARS-COV-2 Infections in Households - Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep 2020;69:1631–4. 10.15585/mmwr.mm6944e1. [DOI] [PMC free article] [PubMed]
- 37.Thompson M.G., Burgess J.L., Naleway A.L., Tyner H., Yoon S.K., Meece J., et al. Prevention and Attenuation of Covid-19 with the BNT162b2 and mRNA-1273 Vaccines. N Engl J Med. 2021;385(4):320–329. doi: 10.1056/NEJMoa2107058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riemersma KK, Grogan BE, Kita-Yarbro A, Halfmann P, Kocharian A, Florek KR, et al. Shedding of Infectious SARS-CoV-2 Despite Vaccination. MedRxiv 2021:2021.07.31.21261387. [DOI] [PMC free article] [PubMed]
- 39.de Gier B, Andeweg S, Joosten R, Ter Schegget R, Smorenburg N, van de Kassteele J, et al. Vaccine effectiveness against SARS-CoV-2 transmission and infections among household and other close contacts of confirmed cases, the Netherlands, February to May 2021. Euro Surveill 2021;26. 10.2807/1560-7917.ES.2021.26.31.2100640. [DOI] [PMC free article] [PubMed]
- 40.Gniazdowski V, Paul Morris C, Wohl S, Mehoke T, Ramakrishnan S, Thielen P, et al. Repeated Coronavirus Disease 2019 Molecular Testing: Correlation of Severe Acute Respiratory Syndrome Coronavirus 2 Culture With Molecular Assays and Cycle Thresholds. Clin Infect Dis 2021;73:e860–9. 10.1093/cid/ciaa1616. [DOI] [PMC free article] [PubMed]
- 41.Bullard J, Dust K, Funk D, Strong JE, Alexander D, Garnett L, et al. Predicting Infectious Severe Acute Respiratory Syndrome Coronavirus 2 From Diagnostic Samples. Clin Infect Dis 2020;71:2663–6. 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed]
- 42.Folgueira M.D., Luczkowiak J., Lasala F., Pérez-Rivilla A., Delgado R. Persistent SARS-CoV-2 replication in severe COVID-19. MedRxiv. 2020:2–13. doi: 10.1101/2020.06.10.20127837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oh M.-D., Park W.B., Choe P.G., Choi S.-J., Kim J.-I., Chae J., et al. Viral Load Kinetics of MERS Coronavirus Infection. N Engl J Med. 2016;375(13):1303–1305. doi: 10.1056/NEJMc1511695. [DOI] [PubMed] [Google Scholar]
- 44.Peiris J.S.M, Chu C.M., Cheng V.C.C, Chan K.S., Hung I.F.N., Poon L.L.M., et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet (London, England) 2003;361(9371):1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bates T.A., McBride S.K., Winders B., Schoen D., Trautmann L., Curlin M.E., et al. Antibody Response and Variant Cross-Neutralization After SARS-CoV-2 Breakthrough Infection. JAMA. 2022;327(2):179. doi: 10.1001/jama.2021.22898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Naaber P., Tserel L., Kangro K., Sepp E., Jürjenson V., Adamson A., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. The Lancet Regional Health - Europe. 2021;10:100208. doi: 10.1016/j.lanepe.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danza P., Koo T.H., Haddix M., Fisher R., Traub E., OYong K., et al. SARS-CoV-2 Infection and Hospitalization Among Adults Aged ≥18 Years, by Vaccination Status, Before and During SARS-CoV-2 B.1.1.529 (Omicron) Variant Predominance — Los Angeles County, California, November 7, 2021–January 8, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(5):177–181. doi: 10.15585/mmwr.mm7105e1. [DOI] [PMC free article] [PubMed] [Google Scholar]