Abstract
Background
During the COVID-19 pandemic, the number of hepatitis B virus (HBV) vaccinations among men who have sex with men (MSM) has been considerably lower than before the pandemic. Moreover, less frequent HBV testing and a reduction in numbers of sex partners have been reported. We assessed the impact of these COVID-19-related changes on HBV transmission among MSM in the Netherlands.
Methods
We estimated the changes in sexual activity, HBV testing, and HBV vaccination among MSM during the pandemic from Dutch data. We used a deterministic compartmental model and investigated scenarios with small or large declines in sexual activity, testing, and vaccination for the current phase of the pandemic (without available data). We examined the increase in HBV vaccinations needed to prevent further increase in HBV incidence.
Results
With a decrease in numbers of sex partners of 15–25% during the first lockdown and 5% during the second lockdown, we found a decline of 6.6% in HBV incidence in 2020, despite a >70% reduction in HBV testing and vaccination during the first lockdown. With numbers of sex partners rebounding close to pre-pandemic level in 2021, and a reduction of 15% in testing and 30% in vaccination in 2021, we found an increase of 1.4% in incidence in 2021 and 3.1% in 2026. With these changes, an increase of ≥60% in HBV vaccinations in 2022 would be needed to bring the HBV incidence in 2023 back to the level that it would have had if the COVID-19-related changes had not occurred.
Conclusions
Despite reductions in sexual activity during the COVID-19 pandemic, the decrease in HBV vaccinations may result in a small increase in HBV incidence after 2021, which may persist for years. It is important to restore the vaccination level and limit further increase in HBV transmission among MSM.
Keywords: Hepatitis B virus, Vaccination, Men who have sex with men, Mathematical model, COVID-19, Sexual behaviour
Abbreviations: HBV, hepatitis B virus; MSM, men who have sex with men; CHB, chronic hepatitis B; HCC, hepatocellular carcinoma; HBsAg, hepatitis B surface antigen; anti-HBc, hepatitis B core antibody; STI, sexually transmitted infection; CAI, condomless anal intercourse
1. Introduction
In 2002, the National Hepatitis B Vaccination Programme for Risk Groups was initiated in the Netherlands providing vaccination against hepatitis B virus (HBV) for population subgroups at increased risk of acquiring HBV [1], [2], [3]. Men who have sex with men (MSM) and sex workers are currently included in the programme. Since 2011, there is also a national programme for HBV vaccination among all newborn babies. However, the programme for risk groups continues, since it targets sexually active individuals, who are not yet protected via the vaccination of newborn babies. Major efforts among national and regional public health organisations have resulted in a steadily increasing number of HBV vaccinations among MSM over the years [4]. The COVID-19 pandemic, however, has disrupted this increasing trend and the number of HBV vaccinations administered via the programme since March 2020 has been considerably lower than before [2], [3]. This reduction is a result of a combination of factors, including lockdowns and other restrictive measures that, for example, made on site outreach impossible, the disruptions in healthcare services, and the reluctance of some individuals to visit healthcare facilities. For similar reasons, there has also been a decline in HBV testing [5], [6] that, combined with reduced HBV vaccination, could contribute to increased HBV transmission. On the other hand, MSM have reported lower levels of sexual activity during the pandemic [7], [8], [9] and that could decrease HBV transmission. In this study, we considered changes in the numbers of steady and casual partners during the pandemic as the principal indicator of COVID-19-related changes in sexual activity and investigated how these changes combined with modifications in HBV testing and HBV vaccination could affect HBV transmission among MSM, using a mathematical model.
2. Methods
2.1. Transmission model
We used a deterministic compartmental model that we developed earlier to investigate the impact of risk-group HBV vaccination of MSM [10]. Briefly, the model accounts for HBV transmission among MSM through condomless anal intercourse with steady or casual partners (Supplement Fig. S1) [10]. Parameters relating to sexual activity before the pandemic were estimated based on data from the Amsterdam Cohort Study on HIV among MSM in Amsterdam (Supplement Table S1) [11]. Uninfected MSM in the Netherlands can receive HBV vaccination free of charge from 2003 onwards, and with three vaccine doses they are immune for life [12]. The HBV vaccination rate among MSM was estimated by fitting the model to data on the number of MSM who received their third dose of HBV vaccination in each year (data from the National HBV Vaccination Programme for Risk Groups [1]; see, Supplement Table S2 and Fig. S2). HBV testing rates were derived from the National Database of Sexual Health Centres [13]. Model parameters are shown in Table 1 .
Table 1.
Model parameters.a
| Parameter | Value | Source | |
|---|---|---|---|
| Progression rate active CHB to inactive, per year | 0.1 | [21], [22] | |
| Progression rate inactive to active CHB, per year | 0.02 | [21], [22], [23] | |
| Progression rate active CHB to compensated cirrhosis, per year | 0.05 | [21] | |
| Progression rate compensated to decompensated cirrhosis, per year | 0.02 | [24], [25] | |
| Progression from active CHB to HCC, per year | 0.003 | [21] | |
| Progression from inactive to HCC, per year | 0.0002 | [21] | |
| Progression from compensated cirrhosis to HCC, per year | 0.02 | [21], [26] | |
| Progression from decompensated cirrhosis to HCC, per year | 0.04 | [26] | |
| Death rate from compensated cirrhosis, per year | 0.033 | [24], [27] | |
| Death rate from decompensated cirrhosis, per year | 0.25 | [24], [28] | |
| Death rate from HCC, per year | 0.35 | [29], [30] | |
| Probability acute infection progresses to chronic | 0.07 | [31] | |
| Transition rate out of acute infection, per year | 4 | [32] | |
| HBV clearance rate from chronic infection, per year | 0.01 | [33], [34] | |
| HBV clearance rate from compensated cirrhosis, per year | 0.02 | [33], [34] | |
| % new HBV infections notified during acute phase | 10–30% | [35], [36] | |
| Progression rate treated (T) to HCC (W8), per year | 0.0002 | [37], [38], [39], [40] | |
| HBV-related death rate for those treated, per year | 0.001 | [37], [38], [39], [40] | |
| Treatment rate active CHB, compensated cirrhosis, per year | |||
| % of diagnosed under treatment, until 2011 | 50–70% | Assumption | |
| % of diagnosed under treatment, from 2012 onwards | 70–90% | [41] | |
| % Virally suppressed among treated, until 2011 | 60–80% | [37], [42], [43] | |
| % Virally suppressed among treated, from 2012 onwards | 91–99% | [37], [42], [43] | |
| Rate of entry into and exit out of population, per year | 0.02 | * | |
| Number of MSM | 144,521–263,309 | [44] | |
| Probability HBV transmission per act CAI if infected in state | |||
| Probability HBV transmission per act CAI if infected has inactive chronic HBV | 0.001–0.01 | Assumption | |
| Relative transmissibility acute HBV, compared to inactive chronic HBV | 15–35 | [45] | |
| Relative transmissibility active CHB, compared to inactive chronic HBV | 10–20 | [46] | |
| Relative transmissibility compensated cirrhosis, compared to inactive chronic HBV | 10–20 | [46] | |
| Transmissibility decompensated cirrhosis, HCC | NA | NA | ** |
| Relative transmissibility if virally suppressed, compared to inactive chronic HBV | 0.05–0.15 | [38], [39], [40], [43] | |
| % Reduction probability to acquire HBV due to HBV-related antiretrovirals for HIV | 85–95% | [47], [48] | |
| % Reduction in probability to acquire HBV infection among those receiving PrEP | 88–92% | [49] | |
| Assortative mixing in steady or casual partnerships, respectively | , | 70%, 50% | Assumption |
| Factor increasing CAI frequency before 2002 compared to 2002 and thereafter | 1–30% | Assumption |
Parameters with a range were included in the Latin Hypercube Sampling.
Assuming sexual activity for MSM 15–64 years old.
Assuming that individuals with decompensated cirrhosis or HCC do not engage in sexual practices that could lead to transmission, due to the severity of this phase. Abbreviations: HBV, hepatitis B virus; MSM, men who have sex with men; CHB, chronic hepatitis B; HCC, hepatocellular carcinoma; PrEP, pre-exposure prophylaxis; CAI, condomless anal intercourse.
The model was fitted to data on the number of diagnoses of acute HBV infection among MSM in 2003–19. Using Latin Hypercube Sampling, 5000 combinations of values of uncertain model parameters were sampled and the model calculations were repeated with each of these until 2019, with the levels of sexual activity, testing, and vaccination as of 2019 (before the pandemic). We selected parameter combinations that resulted in number of diagnoses of acute HBV in the range determined by the data (Supplement Fig. S2). For validation, other model results were compared with data (Supplement Figs. S2–S3, Table S3).
2.2. COVID-19 pandemic in the Netherlands
We distinguished five COVID-19-related periods, according to the severity of the pandemic and the stringency of COVID-19 measures [14], [15]:
-
•
First lockdown (mid-March until mid-May 2020), with stringent COVID-19 measures.
-
•
First relaxation (mid-May until mid-October 2020), with less stringent measures.
-
•
Second lockdown (mid-October 2020 until mid-April 2021), with strict measures.
-
•
Second relaxation (mid-April until September 2021), with mild COVID-19 measures that were progressively relaxed.
-
•
Third lockdown (October 2021 until March 2022), starting with mild measures that progressively became more strict, with an “evening lockdown” in November, and a stricter lockdown in December. At the moment of writing (in the middle of this period), there was a strict lockdown in the Netherlands and it was unknown when that would end.
We accounted for COVID-19-related changes in sexual activity, HBV testing, and HBV vaccination during these five periods (see details below and in Table 2 ) and assumed no changes after March 2022. We present results for the two years of the pandemic (2020–2021) and the five years thereafter (2022–2026).
Table 2.
Percentage change* in model parameters due to the COVID-19 pandemic and the related measures.
| 1st lockdown | 1st relaxation | 2nd lockdown | 2nd relaxation | 3rd lockdown | |
|---|---|---|---|---|---|
| Mid Mar–Mid May 2020 | Mid May–Mid Oct 2020 | Mid Oct 2020–Mid Apr 2021 | Mid Apr–Sep 2021 | Oct 2021–Mar 2022 | |
| HBV testinga | −75% | −25% | −30% | −15% | −15%, −5% |
| HBV vaccinationb | −70% | −20% | −35% | −30% | −30%, −5% |
| Casual partners, low activityc | −15% | −15% | −10%, −5% | −10%, −5% | −10%, −5% |
| Casual partners, moderate activityc | −25% | −5% | −15%, −5% | −5%, 0 | −10%, −5% |
| Casual partners, high activityc | −25% | 0 | −15%, −5% | 0 | −5%, 0 |
| Formation main partnerships | −15% | 0 | −10%, −5% | 0 | −5%, 0 |
Percentage change in each parameter calculated compared to its value in 2019 (before the COVID-19 pandemic), as 100*(d-b)/b, where b and d are the values of the parameter before (b) and during (d) the pandemic. Values shown in bold were estimated from data; values not in bold were assumed, as no data were available.
Changes in hepatitis B virus (HBV) testing were based on data from the National Database of Sexual Health Centres in the Netherlands from January 2020 to June 2021 [2], [6], [15]. The reduction in the 2nd relaxation period was based on the reduction in May and June 2021; the reduction in the 3rd lockdown was assumed.
Changes in the number of HBV vaccinations were based on data from the National Hepatitis B Vaccination Programme for Risk Groups [1], [10] until September 2021.
Changes in sexual activity during the 1st lockdown and the 1st relaxation period were based on data from the first round of the “COVID-19, Sex, and Intimacy Survey” [7]. For the changes from October 2020 onwards, two scenarios are shown, assuming a smaller reduction than that until first relaxation period [8], [9].
2.3. COVID-19-related changes in sexual activity
COVID-19-related changes in sexual activity during the first lockdown and the first relaxation period (Table 2) were estimated using data from the first round of the COVID-19, Sex, and Intimacy Survey among MSM [7], [8]. Preliminary results from the second round of this survey indicate that the reduction in sexual activity during the second lockdown was smaller than during the first lockdown, and the level of sexual activity in the second relaxation period was quite similar to the period before the pandemic [8]. Therefore, in these analyses, we examined two levels of reduction in the number of sex partners during the second lockdown and the second relaxation period: one smaller and one much smaller than the respective reductions in the first lockdown and the first relaxation period. We refer to these two levels as the large and small reductions in sex partners in the second phase of the pandemic (Table 2).
2.4. COVID-19-related changes in HBV testing
The change in HBV testing during the first two lockdowns and the subsequent relaxation periods was estimated based on data until June 2021 from the National Database of Sexual Health Centres in the Netherlands [2], [15]. For the period from October 2021 to March 2022, we had no data, and we assumed two levels of reduction in HBV testing: a large reduction of 15% (equal to that in the second relaxation period) and a small reduction of 5% (assuming a trend of returning to the pre-pandemic level continued after the second relaxation period).
2.5. COVID-19-related changes in HBV vaccination
The change in HBV vaccinations during the first and second lockdown and the subsequent relaxation periods was estimated from data from the National HBV Vaccination Programme for Risk Groups until September 2021 [1]. For the period of the third lockdown, we assumed two levels of reduction in HBV vaccination: a large reduction of 30% (equal to that in the second relaxation period); and a small reduction of 5% (assuming a trend of returning to the pre-pandemic level).
2.6. Model scenarios
Model calculations were first carried out for the period until 2026 without COVID-19-related changes in sexual activity (number of sex partners), HBV testing, and HBV vaccination, estimating how the situation in the Netherlands would have been if the COVID-19 pandemic and related changes had not occurred. This is the reference scenario “without COVID-19”. Then, we examined scenarios with changes in individual parameters: two scenarios with changes only in sexual activity (a small or a large reduction in numbers of sex partners after the first relaxation period), two scenarios with changes only in HBV testing (5% or 15% reduction in testing from October 2021 to March 2022), and two scenarios with changes only in HBV vaccinations (5% or 30% reduction in vaccinations from October 2021 to March 2022). Subsequently, we examined the eight combinations of changes in numbers of sex partners, HBV testing, and HBV vaccination. As the reduction in HBV vaccination in September 2021 was still quite high, we examined two additional scenarios in which the 5% or 30% reduction in HBV vaccinations was extended until June 2022 (assuming no changes in number of sex partners or HBV testing from April 2022 onwards). Lastly, we assessed the change in uptake of HBV vaccination needed to prevent an increase in HBV incidence by examining scenarios with different levels of change in HBV vaccinations from January until December 2022 (30% decrease; no change; 40%, 50%, 60%, or 70% increase).
2.7. Presentation of results
We report the percentage change in HBV incidence among MSM due to COVID-19-related changes compared to the reference scenario without COVID-19. This was calculated as , where is the incidence in year for scenario and is the incidence in for the scenario without COVID-19.
3. Results
3.1. Reference scenario without COVID-19-related changes
Without any changes related to the COVID-19 pandemic, the incidence of new HBV infections was calculated at 27.1 (IQR, 24.8–29.8) per 100,000 MSM in 2019, which would have declined to 18.9 (IQR, 17.3–21.6) per 100,000 MSM in 2026 (Supplement Fig. S4).
3.2. Impact of reduced sexual activity
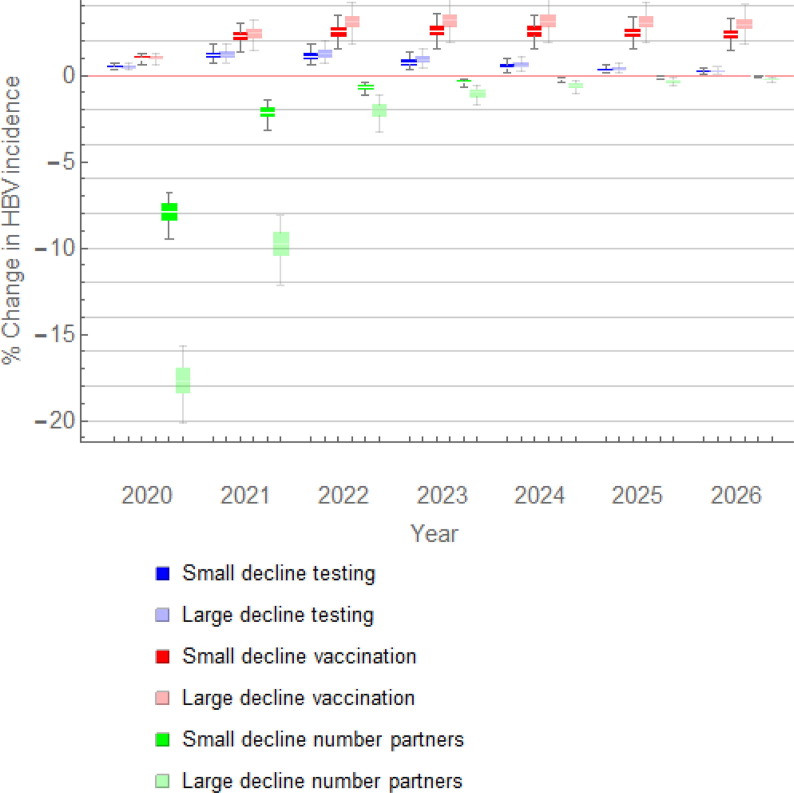
A reduction in the number of sex partners, without changes in HBV testing or vaccination, resulted in a considerable decline in HBV incidence in 2020 (Fig. 1 ). Depending on whether the reduction in the number of sex partners after the first relaxation period was small or large, the decline in incidence in 2020 was 7.9% (IQR, 7.4–8.4%) or 17.7% (IQR, 16.9–18.4%), respectively. The decline was smaller in 2021: 2.1% (IQR, 1.9–2.4%) or 9.8% (IQR, 9.1–10.5%), respectively. The decline in incidence became much smaller in subsequent years and it was less than 0.2% in 2026.
Fig. 1.
Percentage change in HBV incidence among MSM with individual COVID-19-related changes either in sexual activity, or in HBV testing, or in HBV vaccination. The following changes are shown: a small (5%) or a large (15%) decline in HBV testing (dark and light blue box plots, respectively); a small (5%) or a large (30%) decline in HBV vaccination (red and pink box plots, respectively); a small or a large decline in the number of sex partners after the first relaxation period (dark and light green box plots, respectively). HBV, hepatitis B virus; MSM, men who have sex with men.
3.3. Impact of reduced HBV testing
Fig. 1 shows the change in HBV incidence among MSM with a small (5%) or a large (15%) reduction in HBV testing, with the levels of sexual activity and vaccination as before the pandemic. Reduced HBV testing resulted in a small increase in HBV incidence of 0.5% (IQR, 0.4–0.5%) in 2020, 1.2% (IQR, 1.0–1.4%) in 2021, and approximately the same increase in 2022. The increase was smaller after 2022, decreasing to 0.2% (IQR, 0.1–0.3%) in 2026.
3.4. Impact of reduced HBV vaccination
With a reduction in HBV vaccination and no changes in sexual activity or HBV testing, we found an increase of 1.0% (IQR, 0.9–1.1%) in HBV incidence among MSM in 2020 (Fig. 1). The increase grew in the subsequent years, reaching 2.5% (IQR, 2.3–2.8%) or 3.1% (IQR, 2.8–3.5%) in 2022, with a small (5%) or a large (30%) reduction in HBV vaccinations at the end of 2021, respectively. An increase of approximately 2% in HBV incidence remained five years after the level of HBV vaccination was back at the pre-COVID-19 level.
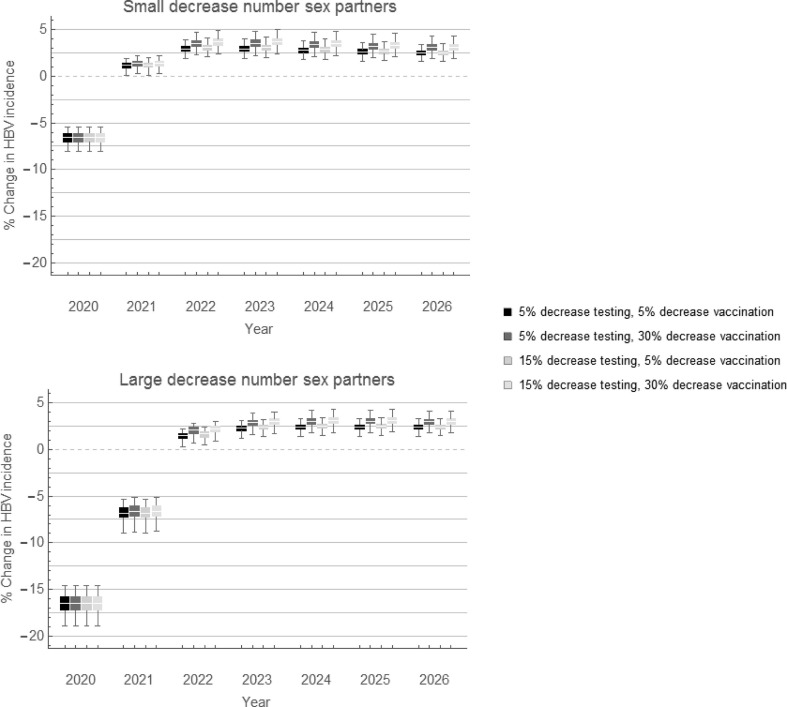
3.5. Impact of combined COVID-19-related changes
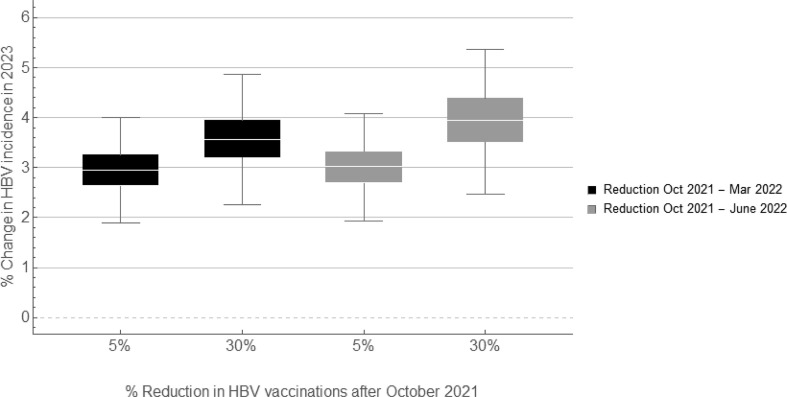
Fig. 2 shows the impact of combinations of COVID-19-related changes on the incidence of HBV infections. We found that a small decline in the number of sex partners after the first relaxation period, combined with reduced HBV testing and HBV vaccination, resulted in a decline of 6.6% (IQR, 6.1–7.1%) in HBV incidence in 2020. A large decline in number of sex partners, combined with reduced testing and vaccination, resulted in a 16.5% (IQR, 15.7–17.3%) decline in incidence. As the impact of reduced sexual activity diminished after 2021 (Fig. 1), the combined impact of reduced sexual activity and reduced testing and vaccination also diminished after 2021, and resulted in a small increase of 2–3% in HBV incidence in 2022 (Fig. 2). The increase in incidence remained five years after the levels of sexual activity, testing, and vaccination were back at their pre-COVID-19 levels (Fig. 2). A longer interval with reduced HBV vaccination resulted in a higher increase in HBV incidence in the subsequent years (Fig. 3, Fig. 4 ). For instance, with a 30% reduction in HBV vaccinations until March (Fig. 2, Fig. 3), June (Fig. 3), or December 2022 (Fig. 4), the increase in incidence in 2023 was 3.6% (IQR, 3.2–4.0%), 4.0% (IQR, 3.5–4.4%), and 4.7% (IQR, 4.2–5.2%), respectively.
Fig. 2.
Percentage change in HBV incidence among MSM with combinations of COVID-19-related changes in sexual activity, HBV testing, and HBV vaccination. Scenarios with (a) small decrease or (b) large decrease in number of sex partners (see Table 2 for the levels of decrease). Black and dark grey box plots: scenarios with 5% decrease in HBV testing; light grey box plots: scenarios with 15% decrease in HBV testing in the last period of the pandemic. Decrease in HBV vaccinations was 5% or 30%, as indicated in the legend. Percentage change was calculated for each scenario of changes compared to the scenario without any COVID-19-related changes. HBV, hepatitis B virus; MSM, men who have sex with men.
Fig. 3.
Impact of a prolonged reduction in HBV vaccination (due to COVID-19) on HBV incidence among MSM. Scenarios with a 5% decrease in HBV testing and a small decrease in number of sex partners until March 2022 (see Table 2 for the levels of decrease). Black box plots: scenarios with 5% or 30% decrease in HBV vaccination from October 2021 to March 2022; grey box plots: scenarios with 5% or 30% decrease in HBV vaccination from October 2021 to June 2022. Percentage change was calculated for each scenario of changes compared to the scenario without any COVID-19 related changes. HBV, hepatitis B virus; MSM, men who have sex with men.
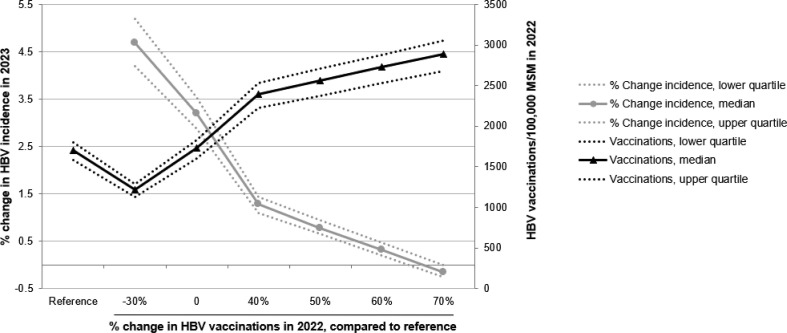
Fig. 4.
Number of HBV vaccinations administered in 2022 and the corresponding change in HBV incidence among MSM the following year. The horizontal axis shows the % change in number of HBV vaccinations until 31 December 2022, compared to the reference scenario (shown first on the horizontal axis) without any COVID-19-related changes. The total number of HBV vaccinations administered in 2022 is shown with black lines (solid line: median; dotted lines: interquartile range) and their values on the right vertical axis. The % change in HBV incidence in 2023, compared to the reference scenario is shown with grey lines (solid line: median; dotted lines: interquartile range) and their values on the left vertical axis. Results are shown for the scenario with small decrease in number of sex partners and 5% decrease in HBV testing until March 2022 (Table 2). HBV, hepatitis B virus; MSM, men who have sex with men.
3.6. Number of HBV vaccinations needed to prevent increase in HBV incidence
Bringing the HBV vaccination rate back to its pre-pandemic level of 1728 (IQR, 1600–1828) HBV vaccinations per 100,000 MSM per year from January 2022 onwards, could limit the increase in HBV incidence in 2023 to 3.2% (IQR, 2.9–3.5%) (Fig. 4). An increase of at least 60% in HBV vaccinations, compared to the number of vaccinations in the reference scenario without COVID-19, may be needed in 2022 to decrease HBV incidence to the level that it would have had if the COVID-19-related changes had not occurred (Fig. 4). Such an expansion of the vaccination programme would result in more than 2700 HBV vaccinations per 100,000 MSM in 2022.
4. Discussion
In this study we found that the reduction in sexual activity due to the COVID-19 pandemic could offset disruptions in HBV testing and HBV vaccination, leading to a decline in HBV incidence in 2020 and possibly also in 2021. However, the long-lasting reduction in HBV vaccination that was documented from the beginning of the pandemic until September 2021, combined with a rebound in sexual activity during 2021, could lead to an increase in HBV incidence from 2022 onwards. The level of HBV incidence may remain slightly higher than what it would have been without COVID-19-related changes, for at least five years after levels of sexual activity, HBV testing, and HBV vaccination have returned to pre-pandemic levels. Expanding the HBV vaccination programme for MSM, such that at least 60% more vaccinations would be administered in 2022, could avert an increase in HBV incidence in MSM in the following years.
The heterogeneity and fluctuations in model outcomes emphasize the importance of monitoring the magnitude and timing of changes in sexual activity during the pandemic. In the short term, HBV incidence can be very sensitive to abrupt changes in sexual activity, but it returns similarly fast to the pre-pandemic level when levels of sexual activity return to those before the pandemic. This is strikingly different from the impact of variations in numbers of HBV vaccinations, which have a more persistent effect. We found that future HBV incidence is more sensitive to disruptions in the vaccination programme than to changes in sexual activity or testing rates. Although the increase may be small, it can be sustained for years after the end of the pandemic.
The long-lasting effect of reduced HBV vaccination highlights the importance of HBV vaccination in limiting transmission among MSM and the need to reach unvaccinated MSM. To this end, social marketing campaigns were conducted during the pandemic, internet fieldwork was intensified when physical outreach was not possible, and vaccination locations were re-organized to be “COVID-19 safe”. Nevertheless, people’s health priorities may have changed during the pandemic, since the risk for COVID-19 infection and the importance of COVID-19 testing and vaccination have emerged. Debates on the benefits and risks of vaccination, prompted by COVID-19 vaccination, may have influenced views about HBV vaccination. Logistics for the HBV vaccination programme are also affected by the pandemic, as the same public health authorities are responsible for both COVID-19 and HBV vaccination programmes. Since booster COVID-19 vaccinations may be repeatedly offered for some time, it is imperative to identify solutions to restore the level of HBV vaccination among MSM, as our results show that longer periods with reduced HBV vaccination result in higher increase in HBV incidence in the subsequent years. This may require exploring options to offer HBV vaccination to those presenting for COVID-19 vaccination and vice-versa. Restoring adequate HBV vaccination levels among MSM will necessitate addressing a complex range of individual, social, and health system barriers.
Our findings have important implications also for sex workers, who are likewise at increased risk of HBV transmission [4]. Reduced HBV testing and HBV vaccination among sex workers have been documented during the pandemic [2]. On the other hand, some sex workers were working during COVID-19 restrictions illegally, since they did not receive government support in the periods that they were not allowed to work due to the restrictions [16]. Therefore, there may have been a small increase in HBV incidence also among sex workers, emphasizing the importance of HBV vaccination.
Our study is the first modelling study to assess the impact of changes related to the COVID-19 pandemic on HBV incidence among MSM. A major strength of our approach is that we based parameters regarding changes in sexual activity on the COVID-19, Sex, and Intimacy Survey that was specifically undertaken to provide data and understanding on COVID-19-related changes in sexual activity and sexual risk behaviours among MSM in the Netherlands. Furthermore, in the model, we used data on levels of HBV testing and HBV vaccination obtained from national databases. This enabled us to include variation in the magnitude of the changes throughout the pandemic.
An important limitation of the study is that the COVID-19 pandemic and related responses continue to evolve. At the time of writing, further COVID-19 restrictive measures were introduced in the Netherlands due to the spread of the Omicron variant of the coronavirus, and the health sector was increasingly engaged in responding to the rising numbers of COVID-19 infections and the roll-out of the third (booster) dose of COVID-19 vaccination. The reduction in HBV vaccinations could be exacerbated (increased and/or prolonged) in 2022, in which case, our results may underestimate the increase in HBV incidence from 2022 onwards. Also, it is uncertain how current and evolving measures will affect sexual activity and it is unknown whether and when levels of sexual activity, HBV testing, and HBV vaccination will return to pre-pandemic levels. Furthermore, we did not account for heterogeneity in COVID-19 related changes according to age or medical indicators of high risk of COVID-19 complications. Moreover, it should be stressed that the increase in HBV incidence, as calculated from the model, was quite small in relative and absolute terms. For instance, we found a 2–3% increase in 2026, that corresponds to one or two more new infections with the COVID-19-related changes compared to the scenario without the COVID-19-related changes. The pandemic has possibly affected many more aspects than the ones considered in our analyses and the magnitude of the COVID-19-related changes is difficult to assess accurately. Projecting the impact of such uncertain changes five years ahead encompasses further uncertainty. Finally, since we used a deterministic rather than a stochastic model, our study does not take into consideration how the variation in results due to stochasticity could influence the impact of changes in testing, vaccination, and sexual activity.
Findings of our study are aligned with earlier modelling studies that assessed the impact of COVID-19-related changes in sexual activity and STI testing on the transmission of HIV, chlamydia, and gonorrhoea [15], [17], [18], [19], [20]. These studies showed that a long-lasting reduction in sexual activity could offset the disruption in STI services, and result in a drop in HIV/STI incidence, while a prolonged disruption of STI services could result in increased STI transmission when sexual activity rebounds to pre-pandemic levels [15], [17], [19].
5. Conclusions
With the COVID-19 pandemic still evolving, it is important to ensure that HBV testing and vaccination programmes continue as much as possible, irrespective of COVID-19 waves, variations in restrictive measures, and additional COVID-19 vaccination rounds. A long-lasting interruption of HBV vaccination in combination with a rebound of sexual activity to pre-pandemic levels can result in a small increase in HBV incidence among MSM, which may take years to return to pre-COVID-19 levels. To prevent a further increase in HBV incidence from 2023 onwards, the number of MSM receiving HBV vaccination in 2022 should be considerably increased, compared to pre-pandemic numbers. This calls for comprehensively and effectively addressing barriers to HBV vaccination during and after the COVID-19 pandemic and implementing innovative ways to provide HBV vaccination to unvaccinated MSM.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
All authors have read and approved the final version of this manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.06.075.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mangen M.J., Stibbe H., Urbanus A., Siedenburg E.C., Waldhober Q., de Wit G.A., et al. Targeted outreach hepatitis B vaccination program in high-risk adults: the fundamental challenge of the last mile. Vaccine. 2017;35(24):3215–3221. doi: 10.1016/j.vaccine.2017.04.068. [DOI] [PubMed] [Google Scholar]
- 2.Staritsky LE, Visser M, van Aar F, Op de Coul E, Heijne J, van Wees D, et al. Sexually transmitted infections in the Netherlands in 2020. Report number 2021-0052. National Institute of Public Health and the Environment, Bilthoven, The Netherlands; 2021. <https://rivm.openrepository.com/bitstream/handle/10029/625007/2021-0052.pdf> [accessed: 14 January 2022].
- 3.National Institute of Public Health and the Environment (RIVM). The National Immunisation Programme in the Netherlands. Surveillance and developments in 2020–2021. Report 2021-0055. Edited by Pluijmaekers AJM, de Melker HE. Bilthoven; 2021. <https://www.rivm.nl/en/national-immunisation-programme/surveillance-and-developments> [accessed: 14 January 2022].
- 4.. Meiberg AE, Urbanus AT. How to reach high behavioral risk groups for HBV-vaccination: combining offline and online strategies. In: Conference of the European public health association. Marseille; 2019. Poster presentation.
- 5.Sonneveld M.J., Veldhuijzen I.K., van de Laar T.J.W., Op de Coul E.L.M., van der Meer A.J. Decrease in viral hepatitis diagnoses during the COVID-19 pandemic in the Netherlands. J Hepatol. 2022 doi: 10.1016/j.jhep.2021.04.015. in print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Institute of Public Health and the Environment (RIVM). Thermometer sexual health November 2021. <https://www.rivm.nl/documenten/thermometer-seksuele-gezondheid-november-2021> [accessed: 14 January 2022].
- 7.Adam P., Op de Coul E., Zuilhof W., Zantkuijl P., den Daas C., de Wit J. Changes in MSM’s sexual activity, PrEP use, and access to HIV/STI testing during and after the first Dutch COVID-19 lockdown. Sex Transmit Infect. 2021;97(Supplement 1):A26. [Google Scholar]
- 8.Adam PCG, Zuilhof W, Den Daas C, Op de Coul E, Zantkuijl P, Paolucci J, et al. Reduction in the magnitude of COVID-19-associated changes in sexual activity and disruption in HIV/STI testing or PrEP use among MSM responding to the 2nd COVID-19, sex and intimacy survey. in: National conference on HIV. Virtual; 2021. Oral presentation.
- 9.de la Court F, Boyd A, Coyer L, van den Elshout MA, de Vries H, Matser A, et al. Three periods of COVID-19 restrictions in 2020 and their impact on sexual healthcare use, PrEP use, and STI incidence among MSM. In: National conference on HIV. Virtual; 2021. Oral presentation.
- 10.Xiridou M., Visser M., Urbanus A., Matser A., van Benthem B., Veldhuijzen I. Ending risk-group HBV vaccination for MSM after the introduction of universal infant HBV vaccination: a mathematical modelling study. Vaccine. 2021;39(21):2867–2875. doi: 10.1016/j.vaccine.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 11.Heijman T., Geskus R.B., Davidovich U., Coutinho R.A., Prins M., Stolte I.G. Less decrease in risk behaviour from pre-HIV to post-HIV seroconversion among MSM in the combination antiretroviral therapy era compared with the pre-combination antiretroviral therapy era. AIDS. 2012;26(4):489–495. doi: 10.1097/QAD.0b013e32834f9d7c. [DOI] [PubMed] [Google Scholar]
- 12.Floreani A., Baldo V., Cristofoletti M., Renzulli G., Valeri A., Zanetti C., et al. Long-term persistence of anti-HBs after vaccination against HBV: an 18 year experience in health care workers. Vaccine. 2004;22(5–6):607–610. doi: 10.1016/j.vaccine.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Visser M., Heijne J.C.M., Hogewoning A.A., van Aar F. Frequency and determinants of consistent STI/HIV testing among men who have sex with men testing at STI outpatient clinics in the Netherlands: a longitudinal study. Sex Transm Infect. 2017;93(6):396–403. doi: 10.1136/sextrans-2016-052918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Government of the Netherlands. Coronavirus COVID-19. <https://www.government.nl/topics/coronavirus-covid-19> [accessed: 14 January 2022].
- 15.Xiridou M., Heijne J., Adam P., Op de Coul E., Matser A., de Wit J., et al. How the disruption in STI care due to the COVID-19 pandemic could lead to increased STI transmission among MSM in the Netherlands: a mathematical modelling study. Sex Transm Dis. 2022;49(2):145–153. doi: 10.1097/OLQ.0000000000001551. [DOI] [PubMed] [Google Scholar]
- 16.Kloek M, Waterman L, Anonymous Sexworker, Spek E, Hendriks S, Luhrs Y, et al. Research on the impact of corona on sexwork in the Netherlands. Report published online 1 December 2021. In Dutch. <https://www.soaaids.nl/files/2021-12/Rapport_Onderzoek_impact_Corona_Sekswerkers.pdf> [accessed: 15 June 2022].
- 17.Jenness S.M., Le Guillou A., Chandra C., Mann L.M., Sanchez T., Westreich D., et al. Projected HIV and bacterial sexually transmitted infection incidence following COVID-19-related sexual distancing and clinical service interruption. J Infect Dis. 2021;223(6):1019–1028. doi: 10.1093/infdis/jiab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jewell B.L., Mudimu E., Stover J., Ten Brink D., Phillips A.N., Smith J.A., et al. Potential effects of disruption to HIV programmes in sub-Saharan Africa caused by COVID-19: results from multiple mathematical models. Lancet HIV. 2020;7(9):e629–e640. doi: 10.1016/S2352-3018(20)30211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitchell K.M., Dimitrov D., Silhol R., Geidelberg L., Moore M., Liu A., et al. The potential effect of COVID-19-related disruptions on HIV incidence and HIV-related mortality among men who have sex with men in the USA: a modelling study. Lancet HIV. 2021;8:e206–e215. doi: 10.1016/S2352-3018(21)00022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booton R.D., Fu G., MacGregor L., Li J., Ong J.J., Tucker J.D., et al. The impact of disruptions due to COVID-19 on HIV transmission and control among men who have sex with men in China. J Int AIDS Soc. 2021;24(4):e25697. doi: 10.1002/jia2.25697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fattovich G., Bortolotti F., Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Fattovich G., Olivari N., Pasino M., D'Onofrio M., Martone E., Donato F. Long-term outcome of chronic hepatitis B in Caucasian patients: mortality after 25 years. Gut. 2008;57(1):84–90. doi: 10.1136/gut.2007.128496. [DOI] [PubMed] [Google Scholar]
- 23.Hsu Y.S., Chien R.N., Yeh C.T., Sheen I.S., Chiou H.Y., Chu C.M., et al. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35(6):1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 24.Fattovich G., Pantalena M., Zagni I., Realdi G., Schalm S.W., Christensen E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97(11):2886–2895. doi: 10.1111/j.1572-0241.2002.07057.x. [DOI] [PubMed] [Google Scholar]
- 25.Liaw Y.F., Sung J.J., Chow W.C., Farrell G., Lee C.Z., Yuen H., et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 26.Raffetti E., Fattovich G., Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: a systematic review and meta-analysis. Liver Int. 2016;36(9):1239–1251. doi: 10.1111/liv.13142. [DOI] [PubMed] [Google Scholar]
- 27.Benvegnù L., Chemello L., Noventa F., Fattovich G., Pontisso P., Alberti A. Retrospective analysis of the effect of interferon therapy on the clinical outcome of patients with viral cirrhosis. Cancer. 1998;83(5):901–909. doi: 10.1002/(sici)1097-0142(19980901)83:5<901::aid-cncr15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 28.Hui A.Y., Chan H.L., Leung N.W., Hung L.C., Chan F.K., Sung J.J. Survival and prognostic indicators in patients with hepatitis B virus-related cirrhosis after onset of hepatic decompensation. J Clin Gastroenterol. 2002;34(5):569–572. doi: 10.1097/00004836-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Kanwal F., Farid M., Martin P., Chen G., Gralnek I.M., Dulai G.S., et al. Treatment alternatives for hepatitis B cirrhosis: a cost-effectiveness analysis. Am J Gastroenterol. 2006;101(9):2076–2089. doi: 10.1111/j.1572-0241.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- 30.Lin X., Robinson N.J., Thursz M., Rosenberg D.M., Weild A., Pimenta J.M., et al. Chronic hepatitis B virus infection in the Asia-Pacific region and Africa: review of disease progression. J Gastroenterol Hepatol. 2005;20(6):833–843. doi: 10.1111/j.1440-1746.2005.03813.x. [DOI] [PubMed] [Google Scholar]
- 31.Edmunds W.J., Medley G.F., Nokes D.J., Hall A.J., Whittle H.C. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253(1337):197–201. doi: 10.1098/rspb.1993.0102. [DOI] [PubMed] [Google Scholar]
- 32.Overby L.R., Ling C.M., Decker R.H., Mushahwar I.K., Chau K. In: Viral hepatitis. Szmuness W., Alter M.J., Maynard J.E., editors. Franklin Institute Press; Philadelphia: 1982. Serodiagnostic profiles of viral hepatitis; pp. 169–182. [Google Scholar]
- 33.Fattovich G. Natural history of hepatitis B. J Hepatol. 2003;39(Suppl 1):S50–S58. doi: 10.1016/s0168-8278(03)00139-9. [DOI] [PubMed] [Google Scholar]
- 34.Lok A.S., McMahon B.J. Chronic hepatitis B. Hepatology. 2007;45(2):507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 35.McMahon B.J., Alward W.L., Hall D.B., Heyward W.L., Bender T.R., Francis D.P., et al. Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. J Infect Dis. 1985;151(4):599–603. doi: 10.1093/infdis/151.4.599. [DOI] [PubMed] [Google Scholar]
- 36.Van Damme P., Tormans G., Beutels P., Van Doorslaer E. Hepatitis B prevention in Europe: a preliminary economic evaluation. Vaccine. 1995;13(Suppl 1):S54–S57. doi: 10.1016/0264-410x(95)93549-o. [DOI] [PubMed] [Google Scholar]
- 37.European Association for the Study of the Liver EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 38.Marcellin P., Gane E., Buti M., Afdhal N., Sievert W., Jacobson I.M., et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381(9865):468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 39.Wong G.L., Chan H.L., Tse Y.K., Yip T.C., Lam K.L., Lui G.C., et al. Normal on-treatment ALT during antiviral treatment is associated with a lower risk of hepatic events in patients with chronic hepatitis B. J Hepatol. 2018;69(4):793–802. doi: 10.1016/j.jhep.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. In. Geneva; 2015. <https://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/>. [accessed: 14 January 2022]. [PubMed]
- 41.Hofman R., Veldhuijzen I.K., van der Lei J., Richardus J.H. Diagnosis, follow-up, and reference of patients with hepatitis B and C. Ned Tijdschr Geneeskd. 2018;162:D2047. In Dutch. [PubMed] [Google Scholar]
- 42.Dutch Association of Gastrointestinal Liver Physicians. Guidelines treatment of chronic hepatitis B virus infection; 2012. <https://www.mdl.nl/sites/www.mdl.nl/files/richtlijnen/Richtlijn_HBV_nieuwe_inzichten_2012.pdf> [accessed: 14 January 2022].
- 43.Heathcote E.J., Marcellin P., Buti M., Gane E., De Man R.A., Krastev Z., et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140(1):132–143. doi: 10.1053/j.gastro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 44.Op de Coul E.L., Schreuder I., Conti S., van Sighem A., Xiridou M., Van Veen M.G., et al. Changing patterns of undiagnosed HIV infection in the Netherlands: who benefits most from intensified HIV test and treat policies? PLoS ONE. 2015;10(7):e0133232. doi: 10.1371/journal.pone.0133232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edmunds W.J., Medley G.F., Nokes D.J., O'Callaghan C.J., Whittle H.C., Hall A.J. Epidemiological patterns of hepatitis B virus (HBV) in highly endemic areas. Epidemiol Infect. 1996;117(2):313–325. doi: 10.1017/s0950268800001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nayagam S., Thursz M., Sicuri E., Conteh L., Wiktor S., Low-Beer D., et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16(12):1399–1408. doi: 10.1016/S1473-3099(16)30204-3. [DOI] [PubMed] [Google Scholar]
- 47.Gatanaga H., Hayashida T., Tanuma J., Oka S. Prophylactic effect of antiretroviral therapy on hepatitis B virus infection. Clin Infect Dis. 2013;56(12):1812–1819. doi: 10.1093/cid/cit145. [DOI] [PubMed] [Google Scholar]
- 48.Heuft M.M., Houba S.M., van den Berk G.E., Smissaert van de Haere T., van Dam A.P., et al. Protective effect of hepatitis B virus-active antiretroviral therapy against primary hepatitis B virus infection. AIDS. 2014;28(7):999–1005. doi: 10.1097/QAD.0000000000000180. [DOI] [PubMed] [Google Scholar]
- 49.Mizushima D, Takano M, Uemura H, Yanagawa Y, Aoki T, Watanabe K, et al. Prophylactic effect of PrEP against HBV infection among MSM. In: Conference on retroviruses and opportunistic infections. Virtual; 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.