Abstract
Objective
Ozone adjuvant in COVID-19 management showed conflicting results in prior studies. Here, we aimed to comprehensively evaluate benefits and side effects of ozone as adjuvant therapy in COVID-19 patients.
Methods
Systematic searches were conducted in MEDLINE, ScienceDirect, Cochrane Library, Springer, medRxiv, and ProQuest for articles investigating ozone as adjuvant therapy in COVID-19. Clinical and laboratory outcomes, mortality, length of hospital stay, intensive care unit (ICU) admission, and adverse events were assessed.
Results
Thirteen studies were included in this review. Case-control studies, but not randomized controlled trials (RCTs), showed a decrease in mortality following ozone therapy (OR = 0.24 (95% CI [0.07–0.76]), p = 0.02, I2 = 0%, fixed-effect). However, ozone therapy did not improve the length of hospital stay (SMD = -0.99 (95 %CI −2.44 to 0.45), p = 0.18, I2 = 84%, random-effects) and ICU admission (RR = 0.57 (95 %CI [0.05–6.71]), I2 = 73%, p = 0.65, random-effects). Consecutive case control studies suggested that ozone therapy significantly improved levels of D-dimer (p = 0.0060), lactate dehydrogenase (LDH; p = 0.0209), C-reactive protein (CRP; p = 0.0040) and interleukin (IL)-6 (p = 0.0048) as compared to standard therapy alone.
Conclusions
The beneficial effect of ozone in COVID-19 management seems to be limited to the improvements of laboratory parameters among severe patients, including the reduction of IL-6, LDH, CRP, and D-dimer levels. Meanwhile, other study endpoints, such as mortality, length of stay and ICU admission, were not improved following ozone therapy, although it may partly be due to a shorter duration of viral clearance. Furthermore, no serious adverse event was reported following ozone therapy, suggesting its high safety profile. (PROSPERO ID: CRD42021278018)
Keywords: COVID-19, SARS-CoV-2, Ozone, Adjuvant therapy, Integrative medicine
1. Introduction
At the end of December 2019, a novel coronavirus began to spread in Wuhan, China. Since the outbreak, the 2019 Coronavirus Disease (COVID-19) has brought major harm and challenges to>200 countries and regions [1]. To date (i.e., 30 May 2022), there have been 526,182,662 confirmed cases of COVID-19, including 6,286,057 deaths, reported to WHO. As of 17 March 2022, a total of 10,925,055,390 vaccine doses have been administered [2]. A wide range of interventions, including antiviral medications, immunomodulators, convalescent plasma, and herbal medicinal therapy is being used to treat the disease [3], [4], [5]. Due to many uncertainties around the management of COVID-19, there has been lots of interest in the potential role of adjuvant therapies that can complement the standard COVID-19 therapy. One of those previously explored adjuvant therapies for COVID-19 is ozone therapy [6].
Ozone (O3) is a gas composed of three atoms of oxygen, including a stable pair (O2) and a third, unstable, atom, which gives ozone its beneficial effects on humans’ health. Common routes of ozone administration include ozonated autohemotherapy and rectal ozone insufflation [7]. Several studies showed that ozone could improve blood circulation and oxygen delivery to ischemic tissue [8]. Thus, it may contribute to overcoming hypercoagulation, a frequently observed pathology in COVID-19 patients. Additionally, a hyperinflammatory response is a hallmark of severe SARS-CoV-2 infection and cytokine modulation is a key to avoid patients’ deterioration [9]. Remarkably, ozone was able to modulate the release of anti-inflammatory cytokines, reduce the activity of pro-inflammatory cytokines and it has a direct antiviral effect [10], [11], [12], suggesting its potential benefits in COVID-19 management.
Several randomized controlled trials (RCTs) and observational studies on ozone therapy in COVID-19 patients have recently been published. However, at present, the application of ozone as adjuvant therapy in COVID-19 remains debatable due to conflicting results in prior studies, demanding further investigations. Therefore, in this systematic review and meta-analysis, we aim to comprehensively evaluate the benefit and side effects of ozone as adjuvant therapy in COVID-19.
2. Methods
This systematic review and meta-analysis was conducted in compliance with the 2020 Preferred Reporting Items for Systematic Review and meta-analysis (PRISMA) guideline [13] and has been registered in the PROSPERO database (https://www.crd.york.ac.uk/prospero/) with a registration number of CRD42021278018.
2.1. Eligibility criteria
The following study types were included in this review: randomized controlled trials (RCTs), clinical trials, cohort studies, case-control studies, and observational reports (case series and case reports). The authors screened the title and abstract independently based on the following criteria: (1) COVID-19 patients, regardless of their severity; (2) the study involved ozone as adjuvant therapy; (3) eligible studies should have reported at least one of our outcomes of interest; (4) studies written in English. Our primary outcomes included mortality, clinical outcome, length of hospital stay, and ICU admission. Our secondary outcomes included laboratory outcomes and adverse events. We excluded review articles, non-human studies, irrelevant articles, and duplications.
2.2. Search strategy and selection of studies
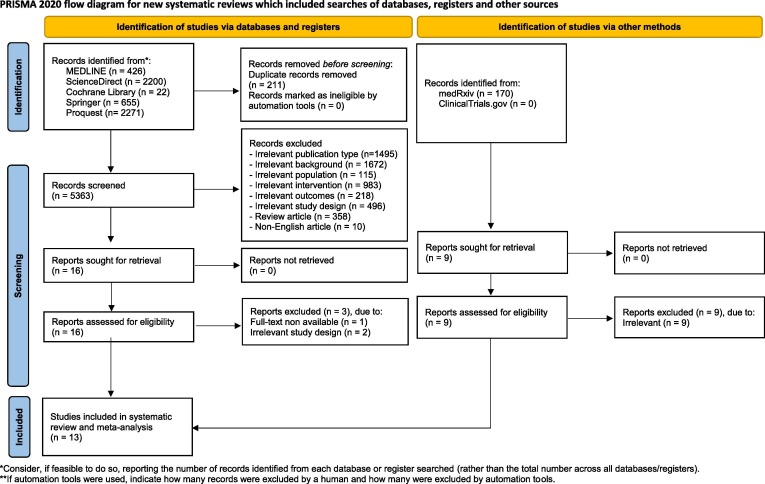
Two authors (A.S.H. and S.R.D.) conducted a keyword search for articles published in trial registries (ClinicalTrials.gov) and databases (MEDLINE, ScienceDirect, Springer, ProQuest, and Cochrane Library) on May 21st, 2022. Our search is limited to studies involving humans. Extended manual search (e.g., in medRxiv) and bibliographical search were also conducted from May 22nd, 2022, to May 25th, 2022, to obtain additional data. The following keywords were used: “((COVID-19) OR (Covid) OR (SARS-COV-2)) AND ((Ozone) OR (Autohemotherapy))”. Detailed search strategies are available in Supplementary Materials. We exported all studies retrieved from the electronic searches into the Mendeley reference manager for duplication removal and screening independently. Any disagreements between these two authors were resolved by discussion with all authors until consensus was reached. Excluded studies were described in the PRISMA flow diagram alongside their reasons for exclusion (Fig. 1 ).
Fig. 1.
PRISMA 2020 flow diagram for included studies *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
2.3. Data extraction
Two review authors (D.S.B. and I.F.R.) independently extracted relevant data from each selected study using a structured and standardized form. The following information was extracted: first authors’ names and publication year, study design, country of origin, sample size, patient age, disease severity, administration technique of ozone therapy, concurrent therapy, and outcome (clinical outcome, mortality, length of hospital stay, intensive care unit (ICU) admission, laboratory outcome, and adverse event).
2.4. Quality assessment
Two review authors (D.S.B and A.S.H) independently assessed the risks of bias from each included studies using the Cochrane risk of bias tool for randomized trials (RoB ver.2) [14], ROBINS-I for non-randomized trials [15], Newcastle-Ottawa Scale (NOS) assessment tool for case-control studies [16], and Joanna Briggs Institute (JBI) critical appraisal checklist for case report and case series studies [17]. Any discrepancies were resolved by discussion until consensus was reached.
2.5. Statistical analysis
Primary analyses were carried out using the Review Manager version 5.4 (The Cochrane Collaboration). Pooled risk ratios (RRs) for dichotomous outcomes were evaluated using the Mantel-Haenszel method. Standardized mean differences (SMDs) of continuous outcomes were pooled using the inverse variance method. The I2 test was used to quantify heterogeneity between studies, with values I2 > 50% representing moderate-to-high heterogeneity. If the value of I2 statistics was < 50% or the p-value was > 0.1, the fixed-effects model could be applied; otherwise, the random-effects model would be used. Begg's funnel plots were performed for publication bias analysis, and if present, the trim-and-fill method was used. All statistical analysis with a p-value < 0.05 was considered statistically significant. Leave-one-out sensitivity analysis was conducted to find the source of statistical heterogeneity and demonstrate how each study affected the overall result.
3. Result
3.1. Study selection
From both the databases and manual research, 5574 and 170 records were retrieved, respectively. Following the screening of titles and abstracts, 25 potential articles were selected for review. After a full-text review, 13 studies were included for narrative synthesis and meta-analysis. The study selection process is summarized in the PRISMA flow chart (Fig. 1 ). Three RCTs [23], [25], [26] were considered to be in high-quality studies and an RCT [24] had some concerns according to Cochrane’s Risk of Bias 2 (RoB2) assessment (Table S2). Robins-I assessment determined that a clinical trial had a moderate risk of bias [22] (Table S3). The quality assessment of case-control studies utilizing the NOS critical appraisal checklist resulted in three high-quality [18], [19], [20] and one medium-quality study [21] (Table S4). Moreover, the quality of the case series was assessed using JBI and summarized in Supplementary Material s [27], [28], [29], [30] (Table S5-S6). Overall, none of the included studies had a low quality.
3.2. Study characteristics
Thirteen studies were finally included, with a total of 241 COVID-19 patients belonging to the ozone as an adjuvant therapy group and 157 patients belonging to the standard therapy only as of the control group, respectively. In this review, the majority were observational studies (4 case-control studies, and 4 case-series) conducted in Spain [19], [20], [29], Italy [18], [28], Turkey [21], Egypt [27], and China [30]. In addition, there is one clinical trial from India [22], and four RCTs conducted in Italy [23], [24], India [25] and Turkey [26]. Based on the administration route, eight studies were conducted using major autohemotherapy [18], [19], [21], [22], [23], [25], [28], [29], two studies using rectal insufflation [19], [27], and one study using intravenous ozonated saline [22], one study using ozone nebulization [26] and one study using the combination of minor autohemotherapy and rectal insufflation [25]. Standard therapy consists of corticosteroids, antivirals (lopinavir, ritonavir, remdesivir), antibiotics (such as azithromycin), and vitamin supplements (vitamin E, vitamin C, vitamin D, and zinc) [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]. Clinical outcomes assessed including the Italian Society of Emergency Medicine (SIMEU) phenotype class, Taylor’s radiology scale, arterial oxygen saturation (SpO2), and time to PCR COVID-19 negative. SIMEU phenotype class includes phenotype 1 = fever and without respiratory failure; phenotype 2 = fever but with ABG and/or Chest XR indicative of modest respiratory insufficiency (PO2 > 60 mmHg in ambient air) and/or pulmonary consolidation area; phenotype 3 = fever associated with moderate-severe respiratory insufficiency (at triage PO2 < 60 mmHg in ambient air) and/or bilateral pulmonary consolidation area at Chest XR; phenotype 4 = respiratory failure with suspected ARDS (Adult Respiratory Distress Syndrome); phenotype 5 = subjects suffering from ARDS initially [18], [23]. The mean value of the patient's phenotype at baseline will be compared with the average after receiving the intervention to determine clinical improvement. Meanwhile, chest radiography improvement was assessed based on the patient's average score between baseline compared to the average grade after receiving the intervention. Taylor has proposed a Severity Scale for (SARS) Severe Acute Respiratory Infection, ranging from 1 to 5 degrees. Grade 1 = normal; Grade 2 = shows patchy atelectasis or hyper inflammation or thickening of the bronchial wall; Grade 3 = focal alveolar consolidation but without involving more than one segment or lobe; Grade 4 = multifocal consolidation; Grade 5 = diffuse alveolar consolidation [19]. The characteristics and outcomes summary for each study are presented in Table 1 and Table 2 .
Table 1.
A. Characteristics of the included studies B. Outcomes of the individual studies.
| Reference | Study design | Country | Sample size |
Age, years Mean ± SD |
Disease severity | Dosage and administration |
|||
|---|---|---|---|---|---|---|---|---|---|
| Intervention (n) |
Control (n) |
Intervention | Control | Intervention | Control | ||||
| Tascini et al. [18] | Case-control study |
Italy | 9 | 9 | 57 ± 12 | 65 ± 13 | Hospitalized, moderate to critical illness | major autohemotherapy + standard therapy | standard therapy |
| Fernández-Cuadros et al. [19] | Case-control study |
Spain | 14 | 14 | 84.35 ± 9.5 | 83 ± 12.55 | Hospitalized, severe illness | ozonized rectal insufflation + standard therapy | standard therapy |
| Hernández et al.[20] | Case-control study |
Spain | 9 | 9 | 64 ± 11 | 71 ± 18 | Hospitalized, severe illness | major autohemotherapy + standard therapy | standard therapy |
| Çolak et al.[21] | Case-control study |
Turkey | 37 | 18 | 58.03 ± 16.3 | 64.7 ± 10.4 | Hospitalized, mild to severe illness | major autohemotherapy + standard therapy | standard therapy |
| Sharma et al.[22] | Clinical trials | India | 10 | NA | 36.2 | NA | Hospitalized, moderate illness | IV ozonide saline + standard therapy | NA |
| Sozio et al.[23] | Randomized controlled trial | Italy | 44 | 48 | 64.2 ± 14.1 | 63.5 ± 12.5 | Hospitalized, mild to moderate illness | major autohemotherapy + standard therapy | standard therapy |
| Araimo et al.[24] | Randomized controlled trial | Italy | 14 | 14 | 63.3 ± 12.1 | 60.1 ± 14.4 | Hospitalized, mild to severe illness | major autohemotherapy + standard therapy | standard therapy |
| Shah et al.[25] | Randomized controlled trial | India | 30 | 30 | 44 ± 8.6 | 43.6 ± 9.7 | Hospitalized, mild to moderate illness | minor autohemotherapy and rectal insufflation + standard therapy | standard therapy |
| Dengiz et al.[26] |
Randomized controlled trial | Turkey | 15 | 15 | 51 ± 16 | 51 ± 16 | Hospitalized, any illness severity | ozone nebulization + standard therapy | standard therapy |
| Reference | Clinical improvement |
All-cause mortalityN (%) |
Length of Hospital Stay (Mean ± SD / Median) |
ICU Admission N (%) |
Laboratory outcome (Mean ± SD / Median) |
Any adverse eventsN (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | Intervention | Control | |
| Tascini et al.[18] |
Baseline SIMEU Class: 2.87 ± 0.78 After therapy SIMEU Class: 2.27 ± 0.83*** |
Baseline SIMEU Class: 2.53 ± 0.51 After therapy SIMEU Class: 2.43 ± 0.94 |
0 (0%) | 2 (7%) | 9.37 ± 3.84 | 9.37 ± 5.38 | NA | NA |
|
|
No adverse events (0) | No adverse events (0) |
| Fernández-Cuadros et al.[19] |
|
|
1 (8.3%) | 2 (16.6%) | 28.58± 16.97 | 35.67 ± 21.04 | NA | NA |
|
|
slight meteorism and a feeling of bloating | |
| Hernández et al.[20] | a.Time to clinical improvement, median [IQR], days: 7 [6], [7], [8], [9], [10]*b.Clinical improvement at day 14, n (%): 8 (89%)*c.Time to PCR COVID-19 negative, mean ± SD, days: 13.1 ± 5.7 |
|
|
|
8 [7], [8], [9], [10], [11], [12] | 28 [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31] | 0 (0%) | 0 (0%) |
|
|
No adverse events (0) | No adverse events (0) |
| Çolak et al.[21] | NA | NA | 2 (5.4%) | 5 (27.8%) | NA | NA | 6 (16.2%) | 4 (22.2%) | NA | NA | No adverse events (0) | No adverse events (0) |
| Sharma et al.[22] | Average time to recovery on 8-point ordinal scale (1-3) = 5.7 days. Moreover, at day 8, all patients improved from moderate to mild category. | NA | 0 | NA | 9.7 days | NA | NA | NA | IL6before:113.17±80after:17.12±21**CRPbefore:65.24±48.28after:7.02±3.82**D-dimerbefore:695±704after:232±158*LDHbefore:624.40±309after:422.62±108*SPO2/FiO2before:299.45±113after:467.61±2* | NA | Pain at the site of injection = 4/10 (40%)Transiently raised LFT =8/10 (80%)Headache=1/10 (10%)Dilutional Hyponatremia= 1/10 (10%) | NA |
| Sozio et al.[23] |
|
|
2 (4.2%) | 2 (4.7%) | 10[6.75–15] | 9[6.5–13] | 6 (12.5%) | 3 (6.8%) | Baseline:
|
Baseline:
|
NR | NR |
| Araimo et al.[24] | NA | NA | 1 (7.1%) | 1 (7.1%) | NA | NA | 1 (7.1%) | 1 (7.1%) | Baseline:
|
Baseline:
|
No adverse events | diarrhea (30%) |
| Shah et al.[25] |
|
|
0 (0%) | 2 (15.4%) | 8 days | 9 days | 0 (0%) | 3 (10%) | Baseline:
|
Baseline:
|
No adverse events | No adverse events |
| Dengiz et al.[26] |
|
|
NA | NA | 18.73±2.91** | 21.2±1.97 | NA | NA | Baseline:
|
Baseline:
|
NR | NR |
NR, not reported; SD, standard deviation; NA, Not Available; CRP, c-reactive protein; LDH, lactate dehydrogenase; WBC, white blood cells; IL, Interleukin; AST, aspartate aminotransferase; ALT, alanine transaminase ; LFT, liver function test ; ICU, intensive care unit; NEWS, national early warning score; SIMEU, Italian society of emergency medicine; RT-PCR, reverse transcription polymerase chain reaction * p<0.05 ** p<0.001
SD, standard deviation; IV, intravenous ; NA, Not Available
Table 2.
A. Characteristics of the included studies B. Outcomes of the individual studies
| Reference | Study design | Country | Sample size | Age, yMean ± SD or Median (IQR) | Disease severity | Intervention |
|---|---|---|---|---|---|---|
| Hendawy et al.[27] | case series | Egypt | 2 | 50 (min-max: 40-60) | Hospitalized, severe illness | Ozonized rectal insufflation +standard therapy |
| Franzini et al.[28] | case series | Italy | 50 | 75 ± 11.4 | Hospitalized, severe illness | Major autohemotherapy + standard therapy |
| Hernandez et al.[29] | case series | Spain | 3 | 61 (min-max: 49-64) | Hospitalized, severe illness | Major autohemotherapy + standard therapy |
| Wu et al.[30] | case series | China | 4 | 60 (54.5-70.5) | Hospitalized, mild to severe illness | Major autohemotherapy + standard therapy |
| Reference | Clinical improvement | All-cause mortalityN (%) | Length of Hospital Stay(Mean ± SD) | Laboratory outcome(Mean ± SD / Median) | Any adverse eventsN (%) | ||
|---|---|---|---|---|---|---|---|
| Baseline | Outcome | Baseline | Outcome | ||||
| Hendawy et al.[27] |
|
|
0 (0%) | N/A | N/A | N/A | 0 (0%) |
|
|
||||||
| Franzini et al.[28] |
|
|
2 (4%) | 22.13 ± 3.44 |
|
|
NR |
| |||||||
| Hernandez et al.[29] |
|
|
0 (0%) | 3.67 ± 0.58 |
|
|
0 (0%) |
|
|
|
|
||||
|
|
|
|
||||
| Wu et al.[30] | P/F ratio: 80 | P/F ratio: 362 | 0 (0%) | 22 ± 7.87 | NA | NA | 0% |
| P/F ratio: 423 | P/F ratio: 405 | NA | NA | ||||
| P/F ratio: 410 | P/F ratio: 306 | NA | NA | ||||
| P/F ratio: 575 | P/F ratio: 412 | NA | NA | ||||
N/A: not available; SD, standard deviation; RR, respiratory rate; HR, Heart rate; CRP, c-reactive protein; LDH, lactate dehydrogenase; WBC, white blood cells; IL, Interleukin; PCT, pro-calcitonin; ALT, alanine transaminase; LFT, liver function test; * p<0.05; ** p<0.001
SD, standard deviation; IQR: inter-quartile range
3.3. Mortality
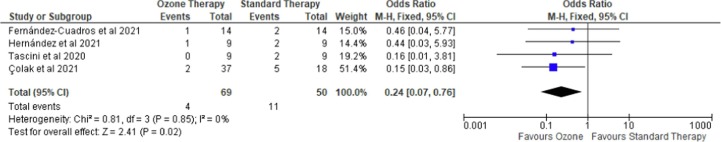
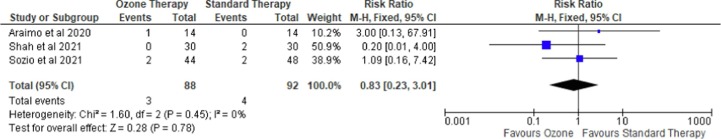
Mortality risk was examined in seven studies [18], [19], [20], [21], [23], [24], [25]: three RCTs and four case-control studies with a total of 180 and 119 patients, respectively. As shown in Fig. 2 , four case-control studies consistently showed that ozone therapy was associated with lower odds of mortality than the conventional strategy (OR = 0.24 (95 %CI 0.07 to 0.76), p = 0.02, I2 = 0%, fixed-effect). Interestingly, leave-one-out sensitivity analyses showed that the statistical significance was revoked if Colak et al. [23] was omitted. A further subgroup analysis of evaluating only case-control studies [19], [20] with hospitalized, severe illness, patients also showed an insignificant result (OR = 0.45 (95 %CI 0.07 to 2.76), p = 0.39, I2 = 0%, fixed-effect). Meanwhile, as shown in Fig. 3 , in three RCTs, ozone therapy was not associated with a reduction in mortality risk (RR = 0.83 (95 %CI 0.23 to 3.01), p = 0.78, I2 = 0%, fixed-effect). Neither statistical significance nor heterogeneity was revoked when leave-one-out sensitivity analyses were performed. A subgroup analysis to evaluate mortality among mild to moderate illness subgroups [23], [25] was performed but it also showed an insignificant result (RR = 0.59 (95 %CI 0.13 to 2.64), p = 0.49, I2 = 0%, fixed-effect). At last, the funnel plot did not indicate any publication bias (Figure S1).
Fig. 2.
Forest plot on risk mortality comparing ozone as an adjuvant therapy with standard therapy in case-control studies.
Fig. 3.
Forest plot on risk mortality comparing ozone as an adjuvant therapy with standard therapy in randomized controlled trials.
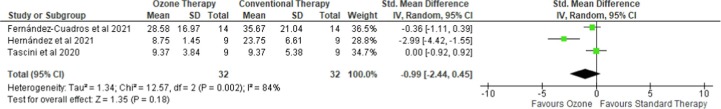
3.4. Length of hospital stay
Three case-control studies reported on the length of hospital stay [18], [19], [20]. The meta-analysis of those studies (Fig. 4 ) concluded that administering ozone as an adjuvant therapy did not have any significant effect on the length of hospitalization as compared to the standard therapy only (SMD = -0.99 (95 %CI −2.44 to 0.45), p = 0.18, I2 = 84%, random-effects). Consistently, the statistical insignificance and heterogeneity remained when leave-one-out sensitivity analyses were performed. A subgroup analysis showed that in hospitalized, severe illness patients [19], [20], ozone improved the length of hospitalization (SMD = -1.62 (95 %CI −4.17 to 0.97), p = 0.22, I2 = 90%, random-effects). No publication bias was detected from the funnel plot (Figure S2).
Fig. 4.
Forest plot on length of hospital stay comparing ozone as an adjuvant therapy with standard therapy.
Three RCT studies reported the length of hospital stay outcome [23], [25], [26]. However, among these studies, only Dengiz et al. [26] demonstrated a significant improvement of the length of hospital stay among patients who received ozone therapy. This study reported the length of stay (mean ± SD) of the ozone group vs. standard therapy as 18.73 ± 2.9 days vs 21.2 ± 1.97 days (p = 0.001). Meanwhile, Sozio et al. [23] reported that the length of stay (median [IQR]) of the ozone group vs. standard therapy was 10 [IQR: 6.75–15] days vs. 9 [IQR: 6.5–13] days (p = 0.182) and in that of Shah et al. [25], the mean length of stay duration was 8 days vs. 9 days (p > 0.05).
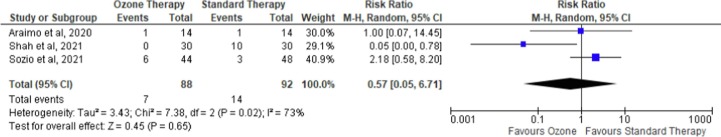
3.5. ICU admission
Three RCTs were reported upon the ICU admission [23], [24], [25]. As shown in Fig. 5 , the overall effect of the intervention (i.e., ozone therapy) on ICU admission was insignificant (RR = 0.57 (95 %CI [0.05–6.71]), I2 = 73%, p = 0.65, random-effects). The statistical insignificance and heterogeneity remained when leave-one-out sensitivity analyses were performed. A subgroup analysis of mild to moderate hospitalized patients [23], [25] showed that ozone did not favor either group in reducing the risk of ICU admission (RR = 0.32 (95 %CI 0.00 to 30.88), p = 0.63, I2 = 87%, random-effects). The funnel plot did not indicate any publication bias (Figure S3).
Fig. 5.
Forest plot on ICU admission comparing ozone as an adjuvant therapy with standard therapy.
3.6. Clinical outcomes
Eight studies reported clinical outcomes, and different parameters were used, such as SIMEU class, and chest radiography scoring using Taylor’s score [18], [19], [20], [22], [23], [25], [27], [28] ( Table 1 B). Based on the case-control studies, there was a significant decrease in SIMEU Class phenotypes after the intervention in ozone therapy (2.87 ± 0.78 vs. 2.27 ± 0.83, p < 0.001), but not in the standard therapy group (2.53 ± 0.51 vs. 2.43 ± 0.93, p = 0.522). In addition, 53% of patients in the ozone therapy arm showed clinical improvements, compared to 33% of patients in the standard therapy arm [18]. In addition, the ozone-treated patients showed a shorter time to clinical improvement compared to the patients treated with standard therapy only (median [IQR]),7 days [IQR: 6–10] vs 28 days [IQR: 8–31], p = 0.04). Moreover, there was a trend of reduction of time required to be negative for COVID-19 by PCR test [mean ± SD; 13.1 ± 5.7 days vs. 21.4 ± 7.4 days, p = 0.05) [20].
There was also a significant improvement in Taylor’s score, from a 4.78 to a 3 grade (p = 0.0001), meanwhile, patients in the standard therapy also experienced an improvement but it was an insignificant 4.25 to a 3.75 (p = 0.3145) [19]. In line with the results of the case-control studies, the RCTs also displayed a significant reduction in time required to be negative for COVID-19 by PCR test in the ozone adjuvant group as compared to standard therapy. On the tenth day, 100% of patients in the ozone adjuvant group were negative, as compared to 70% of patients in the standard therapy group (p = 0.01) [25]. However, an RCT conducted by Sozio et al. [23] reported that after three days of ozone administration, there was no significant difference in chest radiography improvement compared to the standard therapy group (10 (38.5%) vs 2 (11.8%), (p = 0.119)).
In a case series study conducted by Hendawy et al. [27], two patients with severe illness’ oxygen saturation improved after administration of high dose rectal insufflation (SpO2: 85% on room air and 95% with O2 face mask 5 L/min) to (SpO2 became 94–97% on room air) and (SaO2 60% room air, 90% (FiO2 90%) to SaO2 94% (FiO2 60% after 24 h), while another case series in severe COVID-19 reported SaO2 improvement after administration of ozone therapy (p < 0.05) [28] ( Table 2 B).
3.7. Laboratory outcomes
Seven studies reported the laboratory outcomes following ozone therapy [19], [20], [22], [23], [24], [25], [28] (Table 1 B). One case-control study [19] reported that after the ozone adjuvant therapy, there was a significant reduction in D-Dimer (3240 ± 2484 to 1343 ± 1320 ng/mL; p = 0.0060), LDH (329 ± 111 to 241 ± 89 U/L; p = 0.0209), CRP (8.9 ± 6.14 to 2.46 ± 3.78 mg/mL; p = 0.0040) and IL-6 (85.07 ± 50.5 to 30.48 ± 38.1 pg/mL; p = 0.0048). Meanwhile, improvements in the laboratory parameters, including inflammatory markers, were also seen after the standard therapy, although they did not reach statistical significance, except for D-Dimer. Following the standard COVID-19 treatment, D-Dimer lowered significantly from 1153 ± 595 to 853 ± 330 ng/mL (p = 0.0251) and there was a declining trend of LDH, CRP and IL-6, from 262 ± 128 to 242 ± 84 U/L (p = 0.0570), 6.5 ± 6.6 to 1.91 ± 2.3 mg/mL (p = 0.0525) and 44.2 ± 23.2 to 23.3 ± 17.3 pg/mL (p = 0.2365), respectively [19]. In addition, compared to the standard therapy, ozone adjuvant was also associated with a shorter time for reaching a two-times reduction of CRP (3.5 days [IQR: 3–28] vs. 13 days [IQR: 8–25]; p = 0.008), D-dimer (4 days [IQR: 1–10] vs. 19.5 days [IQR: 10–28]; p = 0.009), and LDH (9 days [IQR: 7–9] vs. 25 days [IQR: 12–26]; p = 0.01) [20]. Another study reported a decrease in some inflammatory markers between baseline compared to after receiving ozone intervention, such as D-Dimer (695 ± 704 vs 232 ± 158, p = 0.002), and LDH (624.40 ± 309 vs 422.62 ± 108, p = 0.049) [22]. As reported in RCTs [23], ozone adjuvant administration resulted in a significantly lower LDH than the baseline (531 ± 162 vs. 478 ± 180; p = 0.012), while the LDH reduction in the standard therapy group failed to reach a statistical significance (525 ± 216 vs. 489 ± 168; p = 0.891). Similarly, CRP level was also reduced at the end of both the ozone therapy (48.0 [IQR: 18.2–87.8] vs. 9.2 [IQR: 1.9–24.9]; p < 0.001) and the standard therapy (42.0 [IQR: 13.1–71.2] vs. 3.2 [IQR: 1.6–11.5]; p < 0.001). On the contrary, neither ozone adjuvant nor standard therapy showed a significant reduction of D-Dimer (p = 0.446 and p = 0.211, respectively) and IL-6 (p = 0.107 and p = 0.341, respectively) [23]. Moreover, the RCT conducted by Shah et al. [25] reported no significant difference between the beginning and the end of the study for both CRP and LDH in the ozone therapy group and standard therapy group (p > 0.05 and p > 0.05, respectively), although numerical reduction of 21.29% for CRP and 30% for LDH were noted [25]. Meanwhile, AST levels did not increase significantly from baseline after intervention in the ozone adjuvant group (41.93 ± 23.83 vs. 39.45 ± 25.48, p = 0.81) and standard therapy group (29.71 ± 11.33 vs 65.29 ± 68.38, p = 0.08). However, there was a significantly increased ALT level from baseline in the standard therapy group after intervention (36.64 ± 33.18 vs. 130.7 ± 142.9, p = 0.03), which did not occur in the ozone adjuvant group (56.07 ± 46.04 vs. 101.9 ± 88.7, p = 0.14) [24]. Additionally, Dengiz et al. [26] reported no significant difference between the beginning and the end of the study for LDH, and D-Dimer in the ozone therapy group and standard therapy group (p = 0.419 and p = 0.710), respectively. One case series [28] of severely ill patients showed a significant decrease of inflammatory markers after an intervention with ozone adjuvant therapy, in which the CRP decreased 48.15% (covariance = 9576.177, p = 0.0167), and IL-6 decreased 86.17% (covariance = 9113.337, p = 0.0275) ( Table 2 B).
3.8. Adverse events
Two studies reported minor adverse events following ozone therapy as an adjuvant support treatment [19], [22]. The administration of intravenous ozonized saline in clinical trials conducted by Sharma et al. [22] showed several adverse events, such as pain at the site of injection (4/10 [40%]), an increase in AST/ALT (8/10 [80%]), headache (1/10 [10%]) and dilutional mild hyponatremia (1/10 [10%]) ( Table 1 B). In rectal insufflation administration, three studies reported no major adverse events (e.g., respiratory failure, severe transaminitis, or death related to adverse events) [19], [25], [27]. A case-control study by Fernandez-Cuadros et al. [19] reported that following a mean of 7.83 ± 2.4 sessions of rectal ozone treatment (150 ml of ozone at 35 g/mL, total dose 5.25 mg), symptoms of bloating and slight meteorism complained, although they resolved spontaneously [19]. All studies using ozonated autohemotherapy did not report any adverse events [18], [20], [21], [23], [24], [25], [28], [29], [30]. Additionally, in the three case-series, all participants did not experience any adverse events (0%) [27], [29], [30] ( Table 2 B).
4. Discussion
Studies on adjuvant therapy for COVID-19 are interesting because ozone, due to its biological plausibility, is considered one of the candidate agents to improve clinical outcomes [7]. Recently, ozone was shown to have a therapeutic potential in viral diseases, including COVID-19 [30]. In our meta-analysis, case-control studies reported that ozone therapy was associated with lower odds of mortality than the standard therapy [18], [19], [20], [21]. Meanwhile, RCTs showed a trend towards a decrease in mortality associated to ozone therapy, although not statistically significant [23], [24], [25]. Therefore, such different results require prudent interpretation. Additionally, the meta-analysis of case-control studies revealed that the length of stay was not significantly different between ozone and control groups. However, an RCT by Dengiz et al. [26] reported that ozone significantly improved the length of stay. Noteworthily, the participants included in this RCT were any hospitalized patients regardless of the disease severity and no stratification was performed, in contrast to the other two RCTs [23], [25] which only included mild to moderate hospitalized patients. Additionally, Dengiz et al. [26] administered ozone through nebulization, whereas in Sozio et al. [23] and Shah et al. [25], ozone was administered through major autohemotherapy and minor autohemotherapy in combination with rectal insufflation, respectively. Regarding the ICU admission risk, only RCTs were employed for the analysis and the result showed no association between ozone and ICU admission risk improvement.
Observational (real-world) studies tend to have confounders that are difficult to control. For instance, patients may receive different standard therapies which may influence the outcomes. To the best of our knowledge, no (completed or ongoing) study utilizes ozone as monotherapy for COVID-19. Thus, all patients in this meta-analysis reportedly received standard COVID-19 therapies, including corticosteroids and antivirals [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]. Additionally, there was a notable difference in the disease severity between the included case-control studies and RCTs. In the case-control studies, most patients were severely ill, whereas the majority of RCT patients were in mild and moderate conditions. Corticosteroid was effective in reducing mortality in severe COVID-19 infection, but not in mild and moderate infection [32]. Thus, the administration of corticosteroids on top of the ozone therapy could potentially obscure the effects of ozone against COVID-19, especially in mortality endpoint. It is also important to note that the small sample size could affect the findings of included studies.
Some studies reported clinical improvements after ozone intervention. The observed clinical improvement seems to be associated with the disease severity and is correlated with the mechanism of action of ozone in COVID-19 [33]. In mild and moderate conditions, the ozone adjuvant group showed a reduction in time required to be negative for COVID-19 by PCR test as compared to standard therapy [25]. In addition, there was a significant improvement in symptoms, such as cough and breathlessness, in the ozone group as compared to the standard therapy [25]. However, there was no difference in the chest radiography of the ozone group as compared to the standard therapy group [23]. Meanwhile, in severe COVID-19, ozone adjuvant therapy also improved the chest radiograph [19] and lowered inflammatory markers [19], [20].
With respect to the mechanism of action, ozone has the potential to be a virucidal agent, as ozone could oxidize cysteine and tryptophan residues in the spike glycoprotein of COVID-19 viral particle, and induce nuclear factor erythroid 2–related factor 2 (Nrf2), blocking the fusion of SARS-CoV2 S protein with the ACE2 receptor [33]. Ozone-mediated generation of Nitric Oxide (NO) also inhibits fusion by inhibiting the palmitoylation of the spike protein [28]. The presence of oxidants produced by ozone also oxidizes viral capsid, leading to damage of viral particles [22]. Meanwhile, in severe COVID-19, the presence of chest radiograph improvement could be related to the immune-modulating property of ozone, for example through the reduction of inflammation and the modulation of the antioxidant system. Ozone can increase O2 delivery to tissues by increasing the erythrocyte 2,3-diphosphoglycerate (2,3-DPG) level and inducing the production of vasodilators such as NO and prostacyclin [33]. Moreover, ozone can inhibit the inflammasome pathway (NF-κβ pathway), which plays a role in cytokine storm by inducing the production of pro-inflammatory cytokines (TNFα, IFNγ, IL1β, IL6, and IL8) and induction of release and modulation of interferon and anti-inflammatory cytokines (IL-4, IL-6, IL-10, TNFα) [22], [33].
The SARS‐CoV‐2 infection could induce a cytokine storm, in which the expression of inflammatory cytokines, mainly IL‐6 and tumor necrosis factor‐α (TNF‐α), results in an uncontrolled inflammatory response [34], [35]. Thus, it is important to analyze the effect of COVID-19 therapy on the level of inflammatory markers. Nevertheless, because the available data were limited, we were not able to conduct a meta-analysis on the laboratory outcomes. Qualitatively, there were conflicting results on inflammatory markers which also seem to be influenced by differences in the disease severity of each study. All studies investigating severely ill patients reported that there was a significant decrease in inflammatory markers, such as IL-6, CRP, and D-Dimer [19], [20], whereas in mild to moderate illness, there was no difference in the level of inflammatory markers following the administration of ozone therapy as compared to the standard therapy [23], [25]. These results indicate that the effect of ozone on inflammatory markers is more prominent in severely ill patients than in mildly to moderately ill patients [33]. Differences in laboratory outcomes could also arise due to differences in concomitant therapies given to the patients. Sozio et al. [23] revealed that steroids were given more frequently to patients treated with standard therapy than to ozone-treated patients, although this was not allowed in the study protocol [23]. Steroids (i.e., glucocorticoids) are non-specific cytokine inhibitors, inhibiting immune cells such as T-cells and neutrophils. Glucocorticoids also work as an inhibitor of the production of major inflammatory molecules [36].
There are five routes of administering ozone discussed in this review: major autohemotherapy, intravenous ozonated saline, ozone nebulization, rectal insufflation, and a combination of rectal insufflation with minor autohemotherapy. Major autohemotherapy consists of extracorporeal mixing of 50–225 ml of the patient's venous blood with an O2/O3 mixture at concentrations of 15–70 g mL [27], whereas minor autohemotherapy, uses 2–3 ml of venous blood together with 5 ml of Ozone at 25 Aμg/ml [25]. In the saline injection method, the ozone-oxygen mixture was bubbled in the saline at 5mcg/ml concentration at 500 ml/min flow of oxygen for 20 min this achieved 2 μgN/ mL dose of ozone at intravenous administration of the solution, then 200 ml ozonated saline was then immediately administered intravenously [22]. Dengiz et al. [26] used a self-designed prototype nebulization device for the ozone administration which consisted of three sessions applied for 10 min at intervals of 5 min daily for 5 days. In each session; 0.2 ppm ozone gas was given as cold vapor using lung disinfection technique inhalation devices. Meanwhile, rectal O3 insufflation is done by introducing O2/O3 gas mixture into the colon. O3 therapy was used safely with a dose range from 0.2 to 79 mg kg − 1 body weight, a concentration between 10 and 50 g, and a volume up to 300 ml [37]. Major autohemotherapy and intravenous ozonated saline can deliver 100% ozone, which is more effective than rectal insufflation administration which delivers 95–96% ozone [33]. Nevertheless, rectal insufflation is the choice in pediatrics and the elderly because it is considered easier to do and relatively non-invasive without going through involvement in venous extraction, venous oxygenation, and reinfusion. In addition, rectal insufflation is well tolerated and allows scaling doses [38]. A study by Hendawy et al. [27] found that high-volume insufflation with the target of filling the whole colon with gas (but as tolerated by the patients and X-ray guided) improves the patient's oxygenation faster than small volume insufflations. This is probably due to the larger surface area used for O3 absorption [27]. This opens up opportunities for dose adjustments and the optimal number of times to administer rectal ozone therapy to COVID-19 patients because improvements begin to appear when ozone is given at high doses after several repetitions with X-ray monitoring.
In general, although ozone therapy did not cause major adverse events, some literature reported the presence of minor adverse events [19], [22]. Most of the reported adverse events were related to the administration route, technique, and concentration of ozone administered. On rectal O3 insufflation, it was reported that the patient only had bloating and slight meteorism, which improved spontaneously [19]. Other studies have also revealed the possibility of rectal insufflation causing changes in the normal flora of the gut, which could be corrected by probiotic supplementation [27]. Interestingly, there was no adverse event reported in autohemotherapy. This is in line with a previous systematic review by Wen et al. which utilized ozone autohemotherapy for chronic wounds, in which they found no reports of adverse effects associated with ozone autohemotherapy [39]. In intravenous ozonated saline administration, there were several reported adverse events, including pain at the site of injection, temporary increase in AST/ALT, headache, and mild dilutional hyponatremia. An increase in AST/ALT could be due to antiviral, ozone, or their combination [22]. Nonetheless, the use of ozone as adjuvant therapy for COVID-19 needs careful consideration, especially regarding the dosage, route selection, and monitoring. In addition, patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency are not advised to receive ozone therapy due to acute hemolysis on ozone exposure [40] and the lack of studies on ozone in pregnancy makes the therapy in this special population inadvisable [40].
Finally, this meta-analysis has several limitations. First, the included studies were predominantly observational studies and only three out of twelve studies were RCTs. Second, the included RCTs had a low number of participants and one of three RCTs was open-label [24]. The small sample size makes the trial to be underpower to detect small, but clinically significant, differences in mortality. Moreover, the open-label design could lead to a higher selection bias. Third, we could not provide any recommendation regarding the application method of ozone due to differences in dosage and route of administration across included studies. At last, further well-powered studies with more extensive adjustment of confounders, as well as larger double-blind RCTs, are warranted to address some limitations of our current meta-analysis.
5. Conclusion
Overall, our systematic review and meta-analysis concluded that the observed benefit of ozone as COVID-19 adjuvant therapy was limited to the improvements of laboratory parameters. In severe illness, ozone improved inflammatory markers, such as IL-6, LDH, D-Dimer, and CRP. Although the meta-analysis of case-control studies revealed a significant mortality risk reduction, especially in those with severe illness, the meta-analysis of (small-sampled) RCTs did not show statistically significant associations between ozone and mortality risk reduction, as well as ICU admission and length of hospital stay. However, ozone therapy could potentially be associated with a shorter time for being COVID-19 PCR-negative. Regarding the side effects following ozone adjuvant therapy, no major safety concern was reported regarding the use of ozone as COVID-19 adjuvant therapy, but some minor adverse reactions (e.g., the elevation of transaminase enzymes, gastrointestinal disturbance, and pain at the injection site) were documented.
Funding
There was no funding source for this study.
CRediT authorship contribution statement
David Setyo Budi: Conceptualization, Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Ihsan Fahmi Rofananda: Conceptualization, Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Nando Reza Pratama: Conceptualization, Data curation, Methodology, Visualization, Writing – original draft, Writing – review & editing. Henry Sutanto: Conceptualization, Methodology, Writing – original draft. Arisvia Sukma Hariftyani: Methodology, Visualization, Data curation. Saskia Ratna Desita: Methodology, Visualization, Data curation. Aulia Zinedinita Rahmasari: Methodology, Visualization, Data curation. Tri Pudy Asmarawati: Writing – review & editing, Validation. Langgeng Agung Waskito: Writing – review & editing, Validation. Citrawati Dyah Kencono Wungu: Conceptualization, Validation, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.intimp.2022.109014.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., Xing X., Xiang N., Wu Y., Li C., Chen Q.i., Li D., Liu T., Zhao J., Liu M., Tu W., Chen C., Jin L., Yang R., Wang Q.i., Zhou S., Wang R., Liu H., Luo Y., Liu Y., Shao G.e., Li H., Tao Z., Yang Y., Deng Z., Liu B., Ma Z., Zhang Y., Shi G., Lam T.T.Y., Wu J.T., Gao G.F., Cowling B.J., Yang B.o., Leung G.M., Feng Z. Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N. Engl. J. Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. 2022. Coronavirus disease 2019 (COVID-19) https://www.who.int/emergencies/diseases/novel-coronavirus-2022/situation reportsSituation Report-100.
- 3.Hernández A., Papadakos P.J., Torres A., González D.A., Vives M., Ferrando C., Baeza J. Two known therapies could be useful as adjuvant therapy in critical patients infected by COVID-19. Rev. Española Anestesiol. y Reanim. (English Ed. 2020;67(5):245–252. doi: 10.1016/j.redar.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang M., Hu Z., Yue R. Efficacy and safety of Chinese herbal medicine for Coronavirus disease 2019: A protocol for systematic review and meta-analysis. Medicine (Baltimore). 2020;99(22):e20157. doi: 10.1097/MD.0000000000020157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simonovich V.A., Burgos Pratx L.D., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., Savoy N., Giunta D.H., Pérez L.G., Sánchez M.D.L., Gamarnik A.V., Ojeda D.S., Santoro D.M., Camino P.J., Antelo S., Rainero K., Vidiella G.P., Miyazaki E.A., Cornistein W., Trabadelo O.A., Ross F.M., Spotti M., Funtowicz G., Scordo W.E., Losso M.H., Ferniot I., Pardo P.E., Rodriguez E., Rucci P., Pasquali J., Fuentes N.A., Esperatti M., Speroni G.A., Nannini E.C., Matteaccio A., Michelangelo H.G., Follmann D., Lane H.C., Belloso W.H. A Randomized Trial of Convalescent Plasma in COVID-19 Severe Pneumonia. N Engl J Med. 2021;384(7):619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cattel F., Giordano S., Bertiond C., Lupia T., Corcione S., Scaldaferri M., et al. Ozone therapy in COVID-19: A narrative review. Virus Res. 2021;291:198207. doi: 10.1016/j.virusres.2020.198207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yousefi B., Banihashemian S.Z., Feyzabadi Z.K., Hasanpour S., Kokhaei P., et al. Potential therapeutic effect of oxygen-ozone in controlling of COVID-19 disease. Med Gas Res. 2022;12(2):33–40. doi: 10.4103/2045-9912.325989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bocci V.A., Zanardi I., Travagli V. Ozone acting on human blood yields a hormetic dose-response relationship. J Transl Med. 2011;9:66. doi: 10.1186/1479-5876-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hertanto D.M., Wiratama B.S., Sutanto H., Wungu C.D.K. Immunomodulation as a Potent COVID-19 Pharmacotherapy: Past. Present and Future. J Inflamm Res. 2021;14:3419–3428. doi: 10.2147/JIR.S322831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chirumbolo S., Valdenassi L., Simonetti V., Bertossi D., Ricevuti G., et al. Insights on the mechanisms of action of ozone in the medical therapy against COVID-19. Int Immunopharmacol. 2021;96:107777. doi: 10.1016/j.intimp.2021.107777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Şahin M., Eryilmaz F., Keser Şahin H.H. Ozone therapy may be an option for COVID-19 patients. Eur Rev Med Pharmacol Sci. 2021 Mar;25(6):2470–2472. doi: 10.26355/eurrev_202103_25407. [DOI] [PubMed] [Google Scholar]
- 12.Bayarri B., Cruz-Alcalde A., López-Vinent N., Micó M.M., Sans C. Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J Hazard Mater. 2021 Aug;5(415) doi: 10.1016/j.jhazmat.2021.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne J.A.C., Savovic J., Page M.J., Elbers R., Blencowe N., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 15.Sterne J.A.C., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., et al. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions. BMJ. 2016;355 doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.G. Wells, B. Shea, D. O’Connell, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed Jun 27, 2021.
- 17.J. Briggs, Institute. Checklist for systematic reviews and research syntheses. Available at: https://jbi.global/critical-appraisal-tools. Accessed Jun 26, 2021.
- 18.Tascini C., Sermann G., Pagotto A., Sozio E., De Carlo C., Giacinta A., Sbrana F., Ripoli A., Castaldo N., Merelli M., Cadeo B., Macor C., De Monte A. Blood ozonization in patients with mild to moderate COVID-19 pneumonia: a single centre experience. Intern Emerg Med. 2021;16(3):669–675. doi: 10.1007/s11739-020-02542-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-Cuadros M.E., Albaladejo-Florín M.J., Álava-Rabasa S., Gallego-Galiana J., Pérez-Cruz G.F., Usandizaga-Elio I., Pacios E., Torres-García D.E., Peña-Lora D., Casique-Bocanegra L., López-Muñoz M.J., Rodríguez-de-Cía J., Pérez-Moro O.S. Compassionate Use of Rectal Ozone (O3) in Severe COVID-19 Pneumonia: a Case-Control Study. SN Compr Clin Med. 2021;3(5):1185–1199. doi: 10.1007/s42399-021-00849-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernández A., Viñals M., Pablos A., Vilás F., Papadakos P.J., et al. Ozone therapy for patients with COVID-19 pneumonia: Preliminary report of a prospective case-control study. Int Immunopharmacol. 2021;90:107261. doi: 10.1016/j.intimp.2020.107261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Çolak Ş., Genç Yavuz B., Yavuz M., Özçelik B., Öner M., Özgültekin A., Şenbayrak S. Effectiveness of ozone therapy in addition to conventional treatment on mortality in patients with COVID-19. Int J Clin Pract. 2021;75(8) doi: 10.1111/ijcp.14321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sharma A., Shah M., Lakshmi S., Sane H., Captain J., et al. A pilot study for treatment of COVID-19 patients in moderate stage using intravenous administration of ozonized saline as an adjuvant treatment-registered clinical trial. Int Immunopharmacol. 2021;96:107743. doi: 10.1016/j.intimp.2021.107743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sozio E., De Monte A., Sermann G., et al. CORonavirus-19 mild to moderate pneumonia Management with blood Ozonization in patients with Respiratory failure (CORMOR) multicentric prospective randomized clinical trial. Int Immunopharmacol. 2021;98:107874. doi: 10.1016/j.intimp.2021.107874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araimo F., Imperiale C., Tordiglione P., Ceccarelli G., Borrazzo C., Alessandri F., Santinelli L., Innocenti G.P., Pinacchio C., Mauro V., Recchia G.E., Zancla S., Calò A., Poscia R., Ruberto F., d'Ettorre G., Bilotta F., Mastroianni C., Pugliese F. Ozone as adjuvant support in the treatment of COVID-19: A preliminary report of probiozovid trial. J Med Virol. 2021;93(4):2210–2220. doi: 10.1002/jmv.26636. [DOI] [PubMed] [Google Scholar]
- 25.Shah M., Captain J., Vaidya V., et al. Safety and efficacy of ozone therapy in mild to moderate COVID-19 patients: A phase 1/11 randomized control trial (SEOT study) Int Immunopharmacol. 2021;91:107301. doi: 10.1016/j.intimp.2020.107301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendawy H.A., Mosallam W., Abuelnaga M.E., Sabry A.M. Old Treatment for a New Disease: Can Rectal Ozone Insufflation Be Used for COVID-19 Management? A Case Report [published online ahead of print, 2021 Apr 14]. SN Compr. Clin Med. 2021;3(6):1424–1427. doi: 10.1007/s42399-021-00895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franzini M., Valdenassi L., Ricevuti G., Chirumbolo S., Depfenhart M., Bertossi D., Tirelli U. Oxygen-ozone (O2–O3) immunoceutical therapy for patients with COVID-19. Preliminary evidence reported. Int Immunopharmacol. 2020 Nov;88 doi: 10.1016/j.intimp.2020.106879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernández A., Viñals M., Isidoro T., Vilás F. Potential Role of Oxygen-Ozone Therapy in Treatment of COVID-19 Pneumonia. The American journal of case reports. 2020;21 doi: 10.12659/AJCR.925849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J., Tan C.S., Yu H., Wang Y., Tian Y., et al. Recovery of Four COVID-19 Patients via Ozonated Autohemotherapy. Innovation (N Y). 2020;1(3):100060. doi: 10.1016/j.xinn.2020.100060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valdenassi L., Franzini M., Ricevuti G., Rinaldi L., Galoforo A.C., Tirelli U. Potential mechanisms by which the oxygen-ozone (O2–O3) therapy could contribute to the treatment against the coronavirus COVID-19. Eur Rev Med Pharmacol Sci. 2020 Apr;24(8):4059–4061. doi: 10.26355/eurrev_202004_20976. [DOI] [PubMed] [Google Scholar]
- 31.Sarma P., Bhattacharyya A., Kaur H., Prajapat M., Prakash A., Kumar S., Bansal S., Kirubakaran R., Reddy DibbantiHarikrishna, Muktesh G., Kaushal K., Sharma S., Shekhar N., Avti P., Thota P., Medhi B. Efficacy and safety of steroid therapy in COVID-19: A rapid systematic review and Meta-analysis. Indian J Pharmacol. 2020;52(6):535. doi: 10.4103/ijp.ijp_1146_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernández-Cuadros M.E., Albaladejo-Florín M.J., Peña-Lora D., Álava-Rabasa S., Pérez-Moro O.S. Ozone (O3) and SARS-CoV-2: Physiological Bases and Their Therapeutic Possibilities According to COVID-19 Evolutionary Stage. SN Compr. Clin. Med. 2020;2(8):1094–1102. doi: 10.1007/s42399-020-00328-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirano T., Murakami M. COVID-19: a new virus, but a familiar receptor and cytokine release syndrome. Immunity. 2020;52(5):731–733. doi: 10.1016/j.immuni.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.S., Lee J.Y., Yang J.W., Lee K.H., Effenberger M., Szpirt W., Kronbichler A., Shin J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–329. doi: 10.7150/thno.49713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinezs G., Re L. Rectal administration and its application in ozone therapy. Int J Ozone Ther. 2012;11:41–49. [Google Scholar]
- 37.A. Schwartz, G.M. Sanchez, F. Sabah, et al., Madrid Declaration on the ozone therapy. 3rd edition. 2020.
- 38.Wen Q., Liu D., Wang X., Zhang Y., Fang S., et al. Effects of ozone for treating chronically refractory wounds and ulcers: A protocol for systematic review and meta-analysis of randomized clinical trials. Medicine (Baltimore). 2020;99(22):e20457. doi: 10.1097/MD.0000000000020457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chatterjee K., Wu C.P., Bhardwaj A., Siuba M. Steroids in COVID-19: An overview. Cleve Clin J Med. 2020 doi: 10.3949/ccjm.87a.ccc059. [DOI] [PubMed] [Google Scholar]
- 40.Bocci V. The Potential Toxicity of Ozone: Side Effects and Contraindications of Ozonetherapy. OZONE. 2010 Sep;24:75–84. doi: 10.1007/978-90-481-9234-2_7. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.