Abstract
PURPOSE:
To investigate the effects of mitomycin-C (MMC) and 5-fluorouracil (5-FU) on the viability, proliferation, and migratory capacity of cultured ocular adnexal sebaceous carcinoma (SC) cells.
DESIGN:
Laboratory investigation.
METHODS:
Human SC cell lines (Bascom Palmer 50 and 52 [BP50 and BP52]) and human limbal stem cells (LSCs) were treated with various concentrations of MMC and 5-FU. Cytotoxicity was assessed with the tetrazolium MTT colorimetric viability assay on normal corneal versus tumor cells. Growth curves and scratch assays were performed to characterize the effects of these chemotherapeutic agents on SC proliferation and migration, respectively.
RESULTS:
MMC decreased BP52 cell viability in a dose-dependent manner with a half-maximal effective dose (EC50) of 11.8 μM after 72 hours. SC viability decreased >50% at 80 mM 5-FU after 72 hours. MMC reduced LSC viability in a dose-dependent manner with an EC50 value of 3.24 μM, and 5-FU decreased LSC viability >50% at 160 μM. MMC decreased SC cell proliferation and migration in a dose-dependent manner. 5-FU displayed antiproliferative effects but did not affect cell migration at concentrations below 1000 μM.
CONCLUSIONS:
Our in vitro data corroborate clinical observations that MMC is efficacious for treating ocular adnexal SC, albeit at the expense of LSC viability. Our findings also demonstrate that topical 5-FU exhibits antiproliferative effects that supersede its cancer-killing and anti-migratory effects on cultured SC cells.
Graphical Abstract
Despite limited biological data, topical mitomycin-c and 5-fluorouracil are used with variable success for cases of locally advanced ocular adnexal sebaceous carcinoma. In this laboratory investigation, the effects of these agents on the viability, proliferation, and migratory capacity of cultured ocular adnexal sebaceous carcinoma cells were evaluated. Mitomycin-c effectively kills sebaceous carcinoma cells, albeit at the expense of limbal stem cells. 5-fluoruracil exhibits antiproliferative effects that supersede its cancer-killing and anti-migratory effects.
INTRODUCTION
Sebaceous carcinoma (SC) is a malignant neoplasm that most commonly arises from the sebaceous glands of the ocular adnexa.1–3 It often masquerades as benign lesions, inflammatory conditions, or other malignancies, leading to delayed diagnosis, misdirected therapy, and higher morbidity and mortality.4–7 SC frequently exhibits diffuse pagetoid (intraepithelial) spread and multicentric origins, which are difficult to treat and generally require orbital exenteration to control.8 No standardized treatment exists, but surgical excision is the mainstay of therapy.9
Various medical therapies have been attempted to treat the affected ocular surface and preserve the globe in cases of locally advanced SC. Topical chemotherapeutic agents like mitomycin-C (MMC) and 5-fluorouracil (5-FU) are used for conjunctival melanoma and ocular surface squamous carcinoma.10–14 This has translated to their empirical use for advanced SC as primary or adjuvant treatments.15–19 Yet, there is a dearth of scientific evidence on the efficacy of these topical agents for SC due in part to the lack of appropriate experimental models.
Recently, we reported the establishment and characterization of an ocular adnexal SC cell line named Bascom Palmer 50 (BP50) and demonstrated the effectiveness of MMC on its viability.20 In this present study, we further characterize the effects of MMC and 5-FU on three clinically salient cancer hallmarks: resistance to cell death, sustained proliferation, and cell migration.21 We also investigated the effects of these medications on the viability of cultured limbal stem cells (LSCs) to gauge their toxicity on normal corneal structures. Together, these findings may help inform clinical decision-making about topical chemotherapy for advanced ocular adnexal SC.
METHODS
Ethics Statement
The University of Miami Institutional Review Board approved this study. Written informed consent for the establishment of the cell lines was obtained from the patients.
Sebaceous carcinoma (SC) cell culture
BP50 and BP52 SC cell lines were used for this study.20 They were maintained in Dulbecco’s Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12; Gibco; Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (Thomas Scientific, Swedesboro, NJ, USA; Premium U.S. Source Heat Inactivated) supplemented with 1% antibiotic/antimycotic at 37°C in humidified 5% CO2. The cells were validated by short tandem repeat profiling.
Limbal stem cell (LSC) isolation and culture
Sclerocorneal rims from two deceased donors were obtained from the Florida Lions Eye Bank (Miami, FL, USA) as excess tissue from cornea transplantations. Sclerocorneal rims were processed (no later than 12 hours post-collection) to remove undesired tissue components, including conjunctiva, iris, and Tenon’s capsule. The cleansed tissues were cut into equal size (2mm x 2mm) explants and subjected to overnight digestion (12-16 hours) in 1 mg/mL of collagenase A in DMEM/F-12 GlutaMAX™ medium (Thermo Fisher Scientific) with 50 μg/ml gentamicin at 37°C. The tissue was then gently disaggregated, and cells were passed through a 100 μm cell strainer and centrifuged at 2000 rpm for 5 min. LSCs were cultured in StemPro® hESC serum-free medium (Thermo Fisher Scientific) at 37 °C in humidified 5% CO2.
Drugs
Mitomycin-C (MMC) prepared for topical administration (0.4 mg/mL) and 5-fluorouracil (5-FU; Roche, Nutley, NJ, USA) prepared for intravenous administration (50 mg/mL) were diluted in fresh complete media before each assay.
Cell viability assay
SC and LSC viabilities after 72-hour treatment with MMC or 5-FU were assessed with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Biotium, Fremont, CA, USA) according to the manufacturer’s protocol, as previously described.20 Optical density was quantified with a SpectraMax MiniMax 300 Imaging Cytometer (Molecular Devices, San Jose, CA, USA) at 570 nm, and background absorbance was measured at 630 nm. Quadruplicate readings were recorded. Values were normalized to the untreated control. The half-maximal effective doses (EC50) on cell viability were determined with Combenefit software.22
Cell proliferation assay
Proliferation was measured with the CellTiter-Glo Luminescent Cell Viability Assay (Promega, Madison, WI, USA) following the manufacturer’s protocol. SC cells were seeded with complete medium then treated with MMC or 5-FU the next day. Media with drug were replaced every 48 hours. Luminescence measurements were taken every 24 hours on the SpectraMax MiniMax 300 Imaging Cytometer (Molecular Devices). The mean percent luminance change compared to the baseline was graphed. Each treatment was performed in triplicate. Growth curves were analyzed by calculating the area under the curves (AUCs), which provide estimates of percent inhibition throughout the entire experiment. Ordinary one-way ANOVAs with Dunnett’s post-hoc comparison tests were performed to determine differences between the control and treated groups.
Migration assay
SC cells were grown to confluence and then pre-treated with MMC or 5-FU for 24 hours. A uniform scratch was made in the center of each well with the 0.50 mm width SPLScar Scratcher (Kisker Biotech, Steinfurt, Germany). Baseline phase-contrast images were taken with the EVOS FL Auto Imaging System (Thermo Fisher Scientific). After 24 hours with treatment, cells were fixed, stained with crystal violet (Sigma-Aldrich, St. Louis, MO, USA), and re-imaged. To measure SC cell migration without the confounding influence of proliferation, the experiment was limited to 24 hours after the scratch. The surface areas of the gap at baseline and after 24 hours were determined via manual tracing on software Fiji, and the percent closure was calculated.23 Each treatment was performed in triplicate. The mean percent wound closure was graphed. Ordinary one-way ANOVAs with Dunnett’s post-hoc comparison tests were performed.
Statistics
GraphPad Prism 6 version 9.0.2 (San Diego, CA, USA) was used for statistical data analysis. Values are presented as the mean ± standard deviation.
RESULTS
Effects of MMC and 5-FU on SC and LSC viability
The effects of MMC and 5-FU on SC and LSC viability were evaluated quantitatively with the colorimetric MTT assay, which measures cellular metabolic activity as an indicator of cell viability.
MMC decreased BP52 viability in a dose-dependent manner with a half-maximal effective dose (EC50) of 11.8 μM after 72 hours of treatment (Figure 1A), similar to what was reported in BP50 previously (EC50 = 10.4 μM).20 5-FU did not display potent acute toxic effects on cultured SC cells. SC viability decreased >50% with approximately 80 mM 5-FU (3 orders of magnitude greater concentration than MMC) after 72 hours (Figure 1B).
Figure 1. Effects of MMC and 5-FU on SC cell viability.
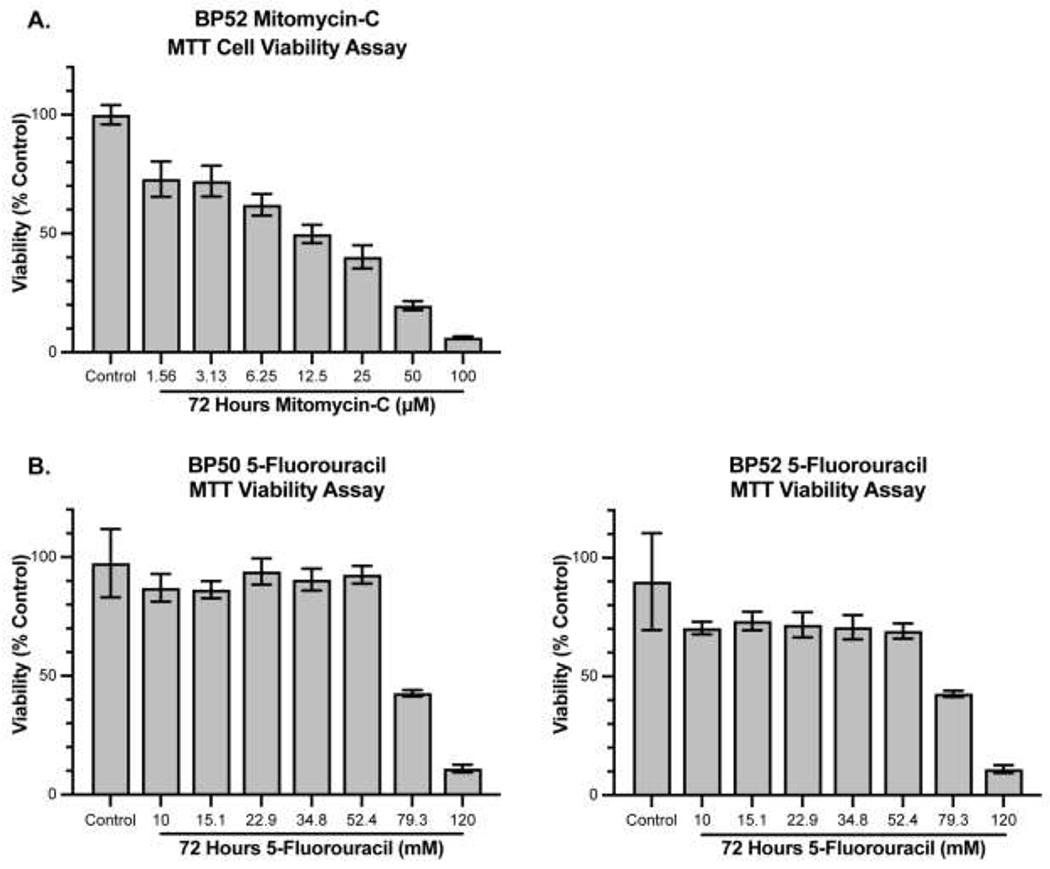
A) 72-hour treatment of MMC decreases cell viability in a dose-dependent manner with an EC50 of 11.8 μM in BP52. In a previous study, we reported that MMC decreases BP50 cell viability in a dose-dependent manner with an EC50 of 10.4 μM.20 B) Cell viability of BP50 and BP52 decreased after 72-hour treatment with 5-FU only at concentrations greater than about 50 mM. Values are expressed as mean ± standard deviation.
MMC decreased LSC viability in a dose-dependent manner with an EC50 of 3.24 μM (Figure 2A). LSC viability decreased >50% with 160 μM 5-FU after 72 hours (Figure 2B).
Figure 2. Effects of MMC and 5-FU on the viability of cultured LSCs.

A) 72-hour treatment with MMC decreased LSC viability in a dose-dependent manner with an EC50 of 3.4 μM. B) LSC viability decreased >50% with 160 μM 5-FU after 72 hours. Error bars are standard deviations. Values are expressed as mean ± standard deviation.
SC cell proliferation after MMC or 5-FU treatment
We measured the effects of MMC and 5-FU on SC cell proliferation by quantifying adenosine triphosphate (ATP) levels over time. The amount of cellular ATP is directly proportional to the number of viable cells.
MMC decreased SC cell proliferation in a dose-dependent manner according to the area under the curves (AUCs) quantification. Concentrations less than or equal to the MMC EC50 were tested. BP50 demonstrated a 28.92 ± 2.32% (P <0.0001), 38.61 ± 1.09% (P <0.0001), and 57.87 ± 2.70% (P <0.0001) AUC reduction with 1.1, 3.3, and 10 μM MMC treatment compared to the untreated control group (Figure 3A). BP52 demonstrated a 19.74 ± 3.64% (P <0.01), 37.78 ± 1.70% (P <0.0001), and 53.20 ± 1.09% (P <0.0001) decrease in the AUCs compared to the control after treatment with 1.1, 3.3, and 10 μM MMC, respectively (Figure 3B). Significant cytotoxicity was observed following 5-day treatment with 10 μM MMC in both SC cell lines.
Figure 3. Effects of MMC on SC cell proliferation.
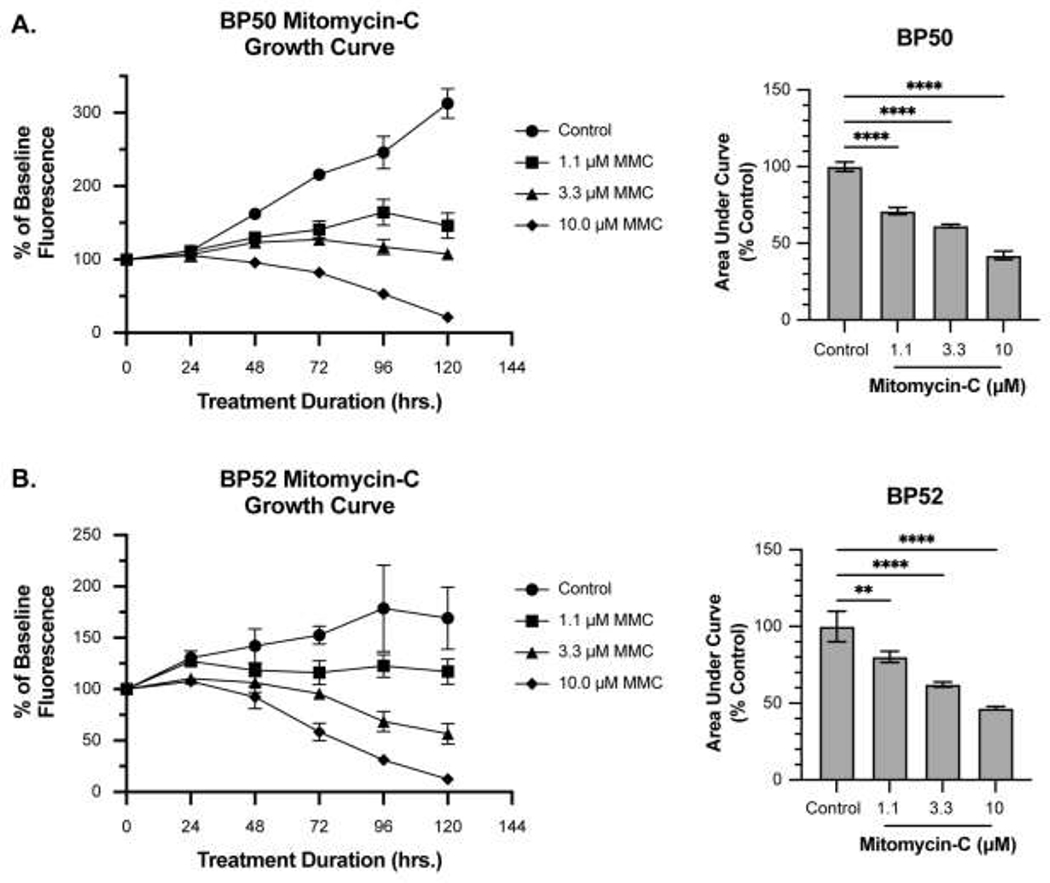
A-B) MMC inhibited cell proliferation for both BP50 and BP52 in a dose-dependent manner. The mean percent change in luminance compared to the untreated baseline was graphed. The mean percent change in area under the curves (AUC) for each treatment are plotted on the right. Prolonged treatment with MMC had cytotoxic effects. Error bars are standard deviations. ** P < 0.01, **** P < 0.0001.
5-FU also decreased SC cell proliferation in a dose-dependent manner. SC cells were treated with 10-fold serial dilutions (10, 100, and 1000 μM) of 5-FU. By day 5, BP50 demonstrated a 20.92 ± 1.64% (P <0.0001), 32.23 ± 1.85% (P <0.0001), and 50.13 ± 0.91% (P <0.0001) AUC reduction compared to the untreated control group with 10,100, and 1000 μM 5-FU, respectively (Figure 4A). BP52 demonstrated a 24.52 ± 1.56% (P <0.0001), 33.07 ± 1.02% (P <0.0001), and 50.43 ± 2.62% (P <0.0001) AUC reduction compared to the untreated control group with 10, 100, and 1000 μM 5-FU, respectively (Figure 4B). After 5-day treatment with 1000 μM 5-FU, the BP50 cell population decreased 5.25 ± 8.10% and the BP52 cell population decreased 15.32 ± 6.42% from baseline, indicating a minor cytotoxic effect at this dose and exposure duration.
Figure 4. Effects of 5-FU on SC cell proliferation.

A-B) 5-FU is a potent inhibitor of SC cell proliferation. 5-FU inhibited cell proliferation for both BP50 and BP52 in a dose-dependent manner. Values are the mean percent change in luminance compared to the untreated baseline. The mean percent change in area under the curves (AUC) for each treatment are plotted on the right. Error bars are standard deviations. **** P < 0.0001.
Migratory activity of SC cells after MMC or 5-FU treatment
To assess the effects of MMC and 5-FU on SC cell migration, a scratch assay was performed after 24-hour treatment. A cell-free area was created in a confluent monolayer to induce SC cells to migrate into the gap, thereby mimicking pagetoid spread. SC cells were observed for 24 hours after treatment to minimize the risk of conflating cell migration with cell proliferation.
Untreated BP50 cells exhibited a 92.86 ± 4.70% coverage of the acellular gap over a 24-hour period, whereas BP50 cells treated with MMC exhibited 67.02 ± 17.91% (P < 0.01), 55.72 ± 13.75% (P < 0.001), and 25.16 ± 1.78% (P < 0.0001) coverages after treatment with 1.1, 3.3, and 10 μM, respectively (Figure 5). Untreated BP52 cells exhibited an 84.43 ± 14.88% coverage of the acellular gap over a 24-hour period, whereas BP52 cells treated with MMC exhibited 67.35 ± 21.97% (ns), 57.65 ± 13.39% (P < 0.01), and 52.59 ± 10.21% (P < 0.01) coverages at 1.1, 3.3, and 10 μM, respectively (Figure 5).
Figure 5. MMC’s effects on SC cell migration.

BP50 and BP52 were pre-treated with MMC for 24 hours before the scratch. MMC inhibited migration of both SC cell lines in a dose-dependent manner after 24 hours. Values graphed are percent closure ± standard deviation. ** P < 0.01, *** P < 0.001, **** P < 0.0001.
5-FU did not display potent effects on SC cell migration. Treatment with 10, 100, and 1000 μM 5-FU did not result in significant differences in coverage of the acellular gap after 24 hours compared to the untreated controls (Figure 6A). Doses were increased to 10 mM (10,000 μM) and 100 mM (100,000 μM). Untreated BP50 cells exhibited an 89.61 ± 6.73% coverage of the acellular gap over a 24-hour period (Figure 6B), whereas BP50 treated with 10 mM and 100 mM 5-FU exhibited 37.18 ± 21.97% (P < 0.0001) and 3.52 ± 3.41% (P < 0.0001) coverages, respectively. Untreated BP52 cells exhibited an 89.11 ± 6.90% coverage of the acellular gap over a 24-hour period, whereas BP52 treated with 10 mM and 100 mM 5-FU displayed 35.28 ± 7.75% (P < 0.0001) and 1.613 ± 5.18% (P < 0.0001) coverages, respectively (Figure 6B).
Figure 6. 5-FU’s effects on SC cell migration.

BP50 and BP52 were treated with 5-FU for 24 hours prior to the scratch. A) 10, 100, or 1000 μM 5-FU for 24 hours did not inhibit the migration of either cell line. B) With 10 mM (10,000 μM) and 100 mM (100,000 μM) 5-FU, SC cell migration was inhibited in a dose-dependent manner after 24 hours. Values graphed are percent closure ± standard deviation. **** P < 0.0001.
DISCUSSION
Given their effectiveness with other ocular surface cancers, topical mitomycin-C (MMC) and 5-fluorouracil (5-FU) are often used for the management of ocular adnexal sebaceous carcinoma (SC) as either primary or adjuvant therapy. This practice is empirical as there is little to no biological data on these topical chemotherapeutic agents’ efficacy on SC. Animal models of SC have yet to be developed and large-scale, randomized controlled trials on the effectiveness, dosages, and schedules of these medications have not been performed. Shields et al. first reported topical 0.04% MMC as an adjuvant treatment for SC with pagetoid spread on four patients.15 Since then, there have only been a handful of case series that reflect a clinical effect of MMC, but no studies report rigorous laboratory experimental data.16–19 As far as topical 5-FU, to our knowledge, there are no reports on its effectiveness on ocular adnexal SC despite its empirical use.
Recently, we reported a technique to culture human ocular adnexal SC for use as an in vitro model.20 Using SC cell lines, we investigated the effects of MMC and 5-FU on three of the six cancer hallmarks of ocular adnexal SC: resistance to cell death, sustained proliferation, and cell migration.21
Our in vitro findings for MMC treatment on SC and LSC viability complement clinical observations. MMC caused SC cellular death in a dose-dependent manner (Figure 1A), which supports clinical reports on empirical topical MMC treatment as a means of clearing the tumor from the ocular surface. In addition to its antineoplastic effects, topical MMC causes severe adverse effects to the ocular surface, leading to pain, keratopathy, conjunctival scarring, and lid malposition.24,25 Long-term high-dose topical chemotherapy regimens can lead to limbal stem cell deficiency (LSCD) resulting in blindness.26 Our evaluation of MMC toxicity in cultured LSCs corroborates this clinical observation. Comparing the half-maximal effective concentrations (EC50) of MMC between the cultured SCs versus LSCs, we determined the EC50 to be 3-4-fold less for LSCs (EC50 = 3.24 μM) than for SC cells (EC50 = 10.4-11.8 μM). Our in vitro data expands on the finding that although topical MMC enacts a cytotoxic effect on SC, it does so at a concentration that is significantly toxic to the surrounding corneal LSCs.
Topical 5-FU is used empirically for ocular adnexal SC, presumably because it has excellent efficacy in ocular surface squamous neoplasia, tolerable side effects, and affordable costs.13,25 However, we found a large discrepancy comparing the 5-FU doses that reduce SC viability versus LSC viability (about 300-fold higher concentration to kill 50% of SC cells). In comparison to MMC, 5-FU is much less potent at killing SC in vitro (4,200-4,800-fold higher concentration to kill 50% of SC cells compared to MMC). Together, these findings reveal a considerable difference in SC cell sensitivities to 5-FU versus MMC. Our results suggest that the topical 5-FU concentration required to kill SC could also cause local or even systemic adverse effects. Our in vitro data underlines the cautious use of topical 5-FU as a means of tumor-killing in ocular adnexal SC.
A fundamental characteristic of cancer is its ability to sustain cell proliferation and tumor growth.21 Cell proliferation is the process that results in an increase in cell number due to a greater rate of cell division than cell loss. In addition to assessing the cytotoxic effects of MMC and 5-FU on SC cells via viability assays, we examined their effects on SC cell proliferation by quantifying ATP levels, which are directly proportional to cell number. Both MMC and 5-FU decreased SC cell proliferation in dose-dependent manners, even at concentrations tolerable to LSCs. Prolonged low-dose MMC treatment led not only to reduced cell proliferation but to significant SC cell death as well (Figure 3). Our data also suggest that 5-FU may have greater efficacy at reducing SC cell proliferation than at inducing cell death (Figure 4). Both MMC and 5-FU inhibit cellular division through multiple mechanisms, including the prevention of DNA synthesis. The prolonged exposure time (5 days) at lower dosages likely permits more efficient drug uptake and allows for more SC cells to be affected during the synthesis phase (S) of the cell cycle when DNA is replicated.
Pagetoid spread occurs when tumor cells migrate to the eyelid epidermis or conjunctival epithelium and form skip lesions separate from the original tumor mass. This occurs in 40-80% of SC cases and often necessitates orbital exenteration.9,27 Cell migration is a critical process in cancer progression, including in cases of pagetoid spread and possibly in metastatic disease. We performed the scratch assay to evaluate the effects of MMC and 5-FU on SC cell migration. We observed dose-dependent decreases in SC cell migration with MMC treatment (1.1 and 3.3 μM), even at concentrations tolerable to LSCs (EC50 of 3.24 δM) (Figure 5). 5-FU (10 and 100 mM) inhibited cell migration only at dilutions toxic to LSCs (>50% of cells are killed at 160 μM), which may suggest limited clinical effectiveness (Figure 6).
In summary, we demonstrate that MMC is more potent than 5-FU at killing cultured SC cells. MMC can decrease SC growth and migration at concentrations tolerable to LSCs. Concentrations of 5-FU required to kill SC cells or halt their migration are very toxic to LSCs, suggesting limited clinical utility. However, long-term low-dose 5-FU may exert cytostatic control of SC. Our results also suggest prolonged low-dose MMC as a means of tumor control, yet a further examination of this dosing strategy on LSCs is required. Future studies should explore the time-dose relationships for both MMC and 5-FU against SC and LSCs.
The largest limitation is in the in vitro nature of our study. This makes extrapolating in vivo dosing extremely difficult since cultured cells lack the diverse cell interactions and unique environment intrinsic to complex in vivo systems. Differences in drug exposure durations between in vitro and in vivo studies also make dosing extrapolation difficult. Clinically, the ocular surface is exposed to these agents transiently, with each topical application quickly draining into the nasolacrimal system. Additionally, these agents are used in a cyclical pattern with holidays that vary in duration, which may enable ocular surface recovery. We performed our experiments with prolonged exposures because our goal was to characterize and compare the efficacy of two common topical chemotherapeutic agents. Nevertheless, in vitro assays are valuable tools for extrapolating tumor behavior in vivo. Prior to the characterization of the SC cell line, no models existed from which to study SC. Therapies were created after use on only a few patients. Future studies exploring dosages, treatment schedules, and combinatorial treatment for SC are needed.
Little data are available on the effects of MMC or 5-FU on ocular adnexal SC due to the lack of reliable experimental platforms and animal models. Our findings provide the first insight into the biological efficacy of topical chemotherapy for advanced sebaceous carcinoma and its collateral effects on the surrounding limbal stem cells.
a. Funding/Support:
This work was supported in part by the Patrick P. Lee Foundation (Williamsville, NY, USA) and the Dr. Nasser Al-Rashid Orbital Research Endowment (Miami, FL, USA). Bascom Palmer Eye Institute is supported by NIH Center Core Grant P30EY014801 and a Research to Prevent Blindness Unrestricted Grant (New York, NY, USA). R.G. was supported in part by NIH Medical Scientist Training Program T32 GM112601.
c. Other Acknowledgements:
The authors thank the Florida Lions Eye Bank (Miami, FL, USA) for assisting with cornea tissue procurement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures: The authors have no financial disclosures.
REFERENCES
- 1.Kass LG, Hornblass A. Sebaceous carcinoma of the ocular adnexa. Surv Ophthalmol. May-Jun 1989;33(6):477–90. doi: 10.1016/0039-6257(89)90049-0 [DOI] [PubMed] [Google Scholar]
- 2.Nelson BR, Hamlet KR, Gillard M, Railan D, Johnson TM. Sebaceous carcinoma. J Am Acad Dermatol. Jul 1995;33(1):1–15; quiz 16-8. doi: 10.1016/0190-9622(95)90001-2 [DOI] [PubMed] [Google Scholar]
- 3.Shields JA, Demirci H, Marr BP, Eagle RC Jr., Shields CL. Sebaceous carcinoma of the ocular region: a review. Surv Ophthalmol. Mar-Apr 2005;50(2):103–22. doi: 10.1016/j.survophthal.2004.12.008 [DOI] [PubMed] [Google Scholar]
- 4.Margo CE, Lessner A, Stern GA. Intraepithelial sebaceous carcinoma of the conjunctiva and skin of the eyelid. Ophthalmology. Feb 1992;99(2):227–31. doi: 10.1016/s0161-6420(92)31988-8 [DOI] [PubMed] [Google Scholar]
- 5.Yeatts RP, Waller RR. Sebaceous carcinoma of the eyelid: pitfalls in diagnosis. Ophthalmic Plast Reconstr Surg. 1985;1(1):35–42. doi: 10.1097/00002341-198501000-00006 [DOI] [PubMed] [Google Scholar]
- 6.Wolfe JT 3rd, Yeatts RP, Wick MR, Campbell RJ, Waller RR. Sebaceous carcinoma of the eyelid. Errors in clinical and pathologic diagnosis. Am J Surg Pathol. Aug 1984;8(8):597–606. doi: 10.1097/00000478-198408000-00003 [DOI] [PubMed] [Google Scholar]
- 7.Brownstein S, Codere F, Jackson WB. Masquerade syndrome. Ophthalmology. Mar 1980;87(3):259–62. doi: 10.1016/s0161-6420(80)35245-7 [DOI] [PubMed] [Google Scholar]
- 8.McGrath LA, Currie ZI, Mudhar HS, Tan JHY, Salvi SM. Management of recurrent sebaceous gland carcinoma. Eye (Lond). Sep 2020;34(9):1685–1692. doi: 10.1038/s41433-019-0756-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen JL, Kibbi N, Worley B, et al. Sebaceous carcinoma: evidence-based clinical practice guidelines. Lancet Oncol. Dec 2019;20(12):e699–e714. doi: 10.1016/S1470-2045(19)30673-4 [DOI] [PubMed] [Google Scholar]
- 10.Frucht-Pery J, Pe’er J. Use of mitomycin C in the treatment of conjunctival primary acquired melanosis with atypia. Arch Ophthalmol. Oct 1996;114(10):1261–4. doi: 10.1001/archopht.1996.01100140461020 [DOI] [PubMed] [Google Scholar]
- 11.Kurli M, Finger PT. Topical mitomycin chemotherapy for conjunctival malignant melanoma and primary acquired melanosis with atypia: 12 years’ experience. Graefes Arch Clin Exp Ophthalmol. Nov 2005;243(11):1108–14. doi: 10.1007/s00417-004-1080-y [DOI] [PubMed] [Google Scholar]
- 12.Yeatts RP, Ford JG, Stanton CA, Reed JW. Topical 5-fluorouracil in treating epithelial neoplasia of the conjunctiva and cornea. Ophthalmology. Sep 1995;102(9):1338–44. doi: 10.1016/s0161-6420(95)30866-4 [DOI] [PubMed] [Google Scholar]
- 13.Joag MG, Sise A, Murillo JC, et al. Topical 5-Fluorouracil 1% as Primary Treatment for Ocular Surface Squamous Neoplasia. Ophthalmology. Jul 2016;123(7):1442–8. doi: 10.1016/j.ophtha.2016.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parrozzani R, Lazzarini D, Alemany-Rubio E, Urban F, Midena E. Topical 1% 5-fluorouracil in ocular surface squamous neoplasia: a long-term safety study. Br J Ophthalmol. Mar 2011;95(3):355–9. doi: 10.1136/bjo.2010.183244 [DOI] [PubMed] [Google Scholar]
- 15.Shields CL, Naseripour M, Shields JA, Eagle RC Jr. Topical mitomycin-C for pagetoid invasion of the conjunctiva by eyelid sebaceous gland carcinoma. Ophthalmology. Nov 2002;109(11):2129–33. doi: 10.1016/s0161-6420(02)01239-3 [DOI] [PubMed] [Google Scholar]
- 16.Tumuluri K, Kourt G, Martin P. Mitomycin C in sebaceous gland carcinoma with pagetoid spread. Br J Ophthalmol. May 2004;88(5):718–9. doi: 10.1136/bjo.2003.034215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudkin AK, Muecke JS. Mitomycin-C as adjuvant therapy in the treatment of sebaceous gland carcinoma in high-risk locations. Clin Exp Ophthalmol. May 2009;37(4):352–6. doi: 10.1111/j.1442-9071.2009.02048.x [DOI] [PubMed] [Google Scholar]
- 18.Russell HC, Chadha V, Lockington D, Kemp EG. Topical mitomycin C chemotherapy in the management of ocular surface neoplasia: a 10-year review of treatment outcomes and complications. Br J Ophthalmol. Oct 2010;94(10):1316–21. doi: 10.1136/bjo.2009.176099 [DOI] [PubMed] [Google Scholar]
- 19.Muqit MM, Foot B, Walters SJ, Mudhar HS, Roberts F, Rennie IG. Observational prospective cohort study of patients with newly-diagnosed ocular sebaceous carcinoma. Br J Ophthalmol. Jan 2013;97(1):47–51. doi: 10.1136/bjophthalmol-2012-302443 [DOI] [PubMed] [Google Scholar]
- 20.Rong AJ, Gallo RA, Zhang MG, et al. Establishment and Characterization of a Novel Human Ocular Adnexal Sebaceous Carcinoma Cell Line. Transl Vis Sci Technol. May 3 2021;10(6):34. doi: 10.1167/tvst.10.6.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. Mar 4 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 22.Di Veroli GY, Fornari C, Wang D, et al. Combenefit: an interactive platform for the analysis and visualization of drug combinations. Bioinformatics. Sep 15 2016;32(18):2866–8. doi: 10.1093/bioinformatics/btw230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. Jun 28 2012;9(7):676–82. doi: 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khong JJ, Muecke J. Complications of mitomycin C therapy in 100 eyes with ocular surface neoplasia. Br J Ophthalmol. Jul 2006;90(7):819–22. doi: 10.1136/bjo.2005.086850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Bayyat G, Arreaza-Kaufman D, Venkateswaran N, Galor A, Karp CL. Update on pharmacotherapy for ocular surface squamous neoplasia. Eye Vis (Lond). 2019;6:24. doi: 10.1186/s40662-019-0150-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dudney BW, Malecha MA. Limbal stem cell deficiency following topical mitomycin C treatment of conjunctival-corneal intraepithelial neoplasia. Am J Ophthalmol. May 2004;137(5):950–1. doi: 10.1016/j.ajo.2003.10.048 [DOI] [PubMed] [Google Scholar]
- 27.Chao AN, Shields CL, Krema H, Shields JA. Outcome of patients with periocular sebaceous gland carcinoma with and without conjunctival intraepithelial invasion. Ophthalmology. Oct 2001;108(10):1877–83. doi: 10.1016/s0161-6420(01)00719-9 [DOI] [PubMed] [Google Scholar]


