Abstract
Objectives
To investigate the effect of pH control through the use of a citrate-buffered saline diluent pH 7 on the degradation rate of piperacillin/tazobactam solutions for infusion and to determine if an extended shelf-life of up to 13 days fridge 2°C–8°C plus 24 hours ‘in-use’ at 32°C in two elastomeric devices: FOLFusor LV10 (Baxter Healthcare, Thetford, UK) and Easypump II (B. Braun Medical Ltd, Sheffield, UK) can be achieved.
Methods
Testing was as per the latest National Health Service (NHS) Pharmaceutical Quality Assurance Committee Yellow Cover Document (YCD) requirements.
A validated stability indicating high-performance liquid chromatography method was used for assessing the stability of the solutions of piperacillin/tazobactam at a combined concentration of 25 mg/mL and 90 mg/mL respectively. Solutions were tested in two batches in replicate (n=3) at five time points according to the requirements of the YCD.
Results
Piperacillin/tazobactam stability was significantly improved when 0.3% w/v citrate-buffered saline pH 7 was used as the diluent, compared with using 0.9% w/v saline as diluent. Greater than 95% of the zero-time concentration of both actives remained following storage at 2°C–8°C for up to 13 days plus 24 hours at 32°C in both devices. The data support extended storage of up to 13 days 2°C–8°C plus 24 hours at 32°C ‘in-use’ when using FOLFusor LV10 (Baxter) or Easypump II (B. Braun) pump devices.
Conclusions
The enhanced stability complies with UK national standards as stated in the YCD for stability testing of aseptically produced small molecules and supports the storage of piperacillin/tazobactam for up to 13 days 2°C–8°C plus 24 hours at 32°C ‘in-use’ within two elastomeric pump devices. The extended shelf-life provides a significant advantage over the stability of piperacillin/tazobactam solutions for infusion when reconstituted and diluted in 0.9% w/v saline as diluent. The data open up the possibility of a continuous infusion of piperacillin/tazobactam delivered by elastomeric pump devices over 24 hours in an outpatient parenteral antimicrobial therapy setting.
Keywords: drug stability, IV administration, shelf life, drug analysis, drug storage
Introduction
Multidrug resistant Gram-negative (MDRGN) infections are increasing and a significant proportion, with deep seated or complex infection, require prolonged intravenous antibiotic therapy.1 Many of these patients would be suitable for outpatient parenteral antimicrobial therapy (OPAT), to facilitate discharge from hospital. Piperacillin/tazobactam is a broad-spectrum penicillin/beta-lactamase inhibitor combination antibiotic with activity against a wide range of pathogens including MDRGN organisms such as Pseudomonas aeruginosa. The frequency of administration of piperacillin/tazobactam as an intermittent infusion makes it difficult to use in OPAT services. Continuous infusion in an elastomeric device would be desirable for OPAT services, but there is a requirement for assurance of the stability of the antibiotic in aqueous solution for the duration of the infusion. In the UK, the standards for stability assessment of small molecules such as piperacillin/tazobactam are set out in the ‘Yellow Cover Document’ (YCD).2 A review by Jenkins et al 3 failed to find any published data on the stability of antimicrobials as a 24-hour infusion in elastomeric devices that meets the YCD standards. Subsequently we have successfully identified an approach of pH control through the use of a citrate-buffered diluent for flucloxacillin,4 although we have also shown meropenem to be unsuitable for continuous 24-hour infusion in an elastomeric device.5
We undertook to assess piperacillin/tazobactam stability at circa 25 mg/mL (22 mg piperacillin/3 mg tazobactam) and 90 mg/mL (80 mg piperacillin/10 mg tazobactam) covering the range of clinically used concentrations (daily doses of 2.25 g three times per day to 4.5 g four times per day) in two different elastomeric devices: FOLFusor LV (Baxter, Thetford, UK) and Easypump II (B. Braun, Sheffield, UK).
Materials and methods
Materials
Chemicals used were as described previously.4 Piperacillin 4 g/tazobactam 500 mg powder for solution for infusion manufactured by Bowmed Ibisqus (Wrexham, UK) batch numbers 8H70TR and 8G56TR was used. Each vial was reconstituted according to the manufacturer’s instructions using citrate (0.3 % w/v) buffered 0.9% w/v sodium chloride solution pH 7 diluent. The citrate-buffered saline diluent was prepared by appropriate dilution of 4.5% w/v citrate-buffered saline pH 7 (Royal Free Hospital, London, UK, Lot SP1711004) in a 1-litre bag of 0.9% w/v sodium chloride for infusion (Royal Derby Hospital, Derby, UK).
Ambulatory infusion devices were elastomeric reservoirs (FOLFusor LV10, supplied by Baxter and Easypump II, supplied by B. Braun). To prepare the devices, a sufficient volume of each piperacillin/tazobactam solution was first prepared using 0.3% w/v citrate-buffered saline for reconstitution and dilution in a central 1-litre intravenous container. Vials were manually shaken to ensure complete dissolution. The nominal reservoir fill volume of 240 mL was transferred from this centralised stock solution to each device (n=3) using a 60 mL syringe, thus ensuring each of the devices contained the same drug concentration. In-line filters and flow restrictors were removed from the elastomeric devices for the study and the outlet was clamped using a suitable tube clamp (Fisher Scientific, Loughborough, UK). Devices were tested in triplicate. Filled devices were stored for 13 days at 2°C–8°C, followed by 24 hours at 32°C. At each test point, a 35 mL aliquot was taken via the administration tube for analysis.
Chromatographic apparatus and conditions
A stability indicating liquid chromatography method was developed for the analysis of piperacillin/tazobactam employing a Thermo Fisher Scientific Ultimate 3000 (U3000) UHPLC System with diode array detection and Chromeleon software V.6.80 (Thermo Fisher Scientific). The high-performance liquid chromatography (HPLC) column was a Kinetex EVO-C18 250×4.6 mm, 5 µm, 100A (Phenomenex, Macclesfield, UK); mobile phase: 0.02 M potassium dihydrogen orthophosphate adjusted to pH 2.5 with orthophosphoric acid (mobile phase A) and acetonitrile (mobile phase B) in gradient elution mode (acetonitrile held at 5% for the first 3 min of the run, increased linearly to reach 50% at 10 min, reduced linearly to reach 5% at 12 min and held at 5% until the end of the run at 15 min; flow rate 1.5 mL/min; injection volume 10 µL, dual wavelength detection mode: 210 nm for quantification of tazobactam and 280 nm for quantification of piperacillin; column temperature 25°C±1°C; autosampler temperature 5°C±1°C.
Preparation of test and quality control standard solutions for HPLC analysis
Piperacillin/tazobactam test solutions were diluted to 1 mg/mL (combined drug) concentration for the HPLC analysis. Freshly prepared piperacillin 0.89 mg/mL/tazobactam 0.11 mg/mL working standard solution was used for quantification of both active pharmaceutical ingredients (APIs). Two standard solutions were always prepared to demonstrate system suitability.
All standards (n=2) and test samples (n=3) were prepared and analysed in triplicate. Samples were stored at 5°C during HPLC analysis and were completed within 12 hours of sample preparation to avoid additional sample degradation.
Validation of the HPLC method
Forced degradation was undertaken using 0.02 M hydrochloric acid, 0.02 M sodium hydroxide and 0.15% hydrogen peroxide. Samples were prepared at 1 mg/mL combined drug concentration and stored at 30°C temperature for 6 hours. Samples were injected freshly prepared and every hour for the duration of the experiment.
In acid stored at 30°C temperature the piperacillin peak area reduced by 55% and tazobactam remained relatively stable with 97.9% remaining after 6 hours. In hydroxide both APIs were highly unstable with no piperacillin and only 45% tazobactam remaining when first injected 3 min after preparation, after 1 hour no traces of tazobactam were detected. In 0.15% (v/v) hydrogen peroxide, piperacillin was completely degraded after 120 min and tazobactam was >99% degraded after 6 hours at 30°C. All additional peaks resulting from degradation of the drug product were clearly resolved from the API peaks at the wavelengths used for quantification of piperacillin (280 nm) and tazobactam (210 nm).
Peak purity was determined using the spectral match factor across the tazobactam peak at relevant concentrations (from 50% to 100% of the initial concentration remaining) and remained at 1000 demonstrating high purity of the peak. Peak purity through spectral analysis of the piperacillin peak was complicated by detector saturation at lower wavelengths, therefore the peak purity was evaluated visually and based on peak symmetry. No evidence for overlaying peaks was observed.
pH was determined using a glass combination electrode and Orion 420A pH meter calibrated each day using the National Institute of Standards and Technology traceable standards (Thermo Fisher Scientific).
Subvisible particle counts were determined by light obscuration using the HIAC Royco 9703 liquid particle counting system (Beckman Coulter, High Wycombe, UK).
Testing schedule and storage conditions
On each of the time points approximately 35 mL sample aliquots were removed via the delivery tube (in-line filter removed) fitted to the device. A 25 mL aliquot was used for HIAC particle testing and the remaining 10 mL used for sample preparation for HPLC, pH and visual appearance testing.
Results
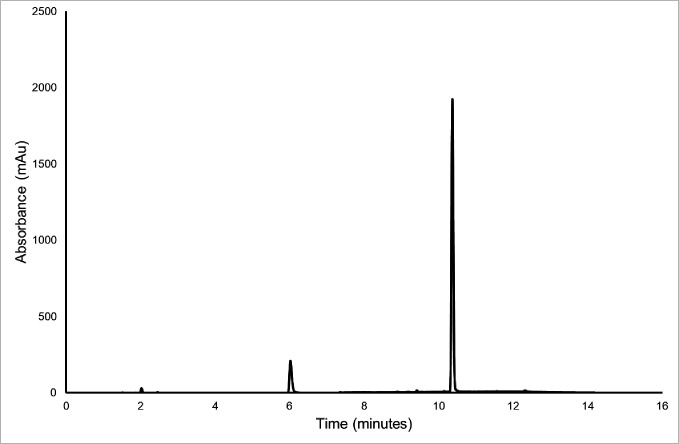
A stability indicating HPLC-diode array detector method was developed to fully separate all degradation species from the APIs: piperacillin and tazobactam. This was achieved using standard methods including forced degradation as described in the Methods section. An example of a typical chromatogram for the test solution is shown in figure 1. The dilution of the test solution did not influence the chromatogram in the final diluted sample.
Figure 1.
A typical chromatogram of test solution diluted to 0.11 mg/mL tazobactam (retention time 5.8 min) and 0.89 mg/mL piperacillin (retention time 10.2 min) at 210 nm.
Linearity of response for piperacillin was demonstrated from 0.45 mg/mL to 1.35 mg/mL with r2 values (correlation coefficient)≥0.99996. Linearity of response for tazobactam was demonstrated from 0.06 mg/mL to 0.17 mg/mL with R2 values (correlation coefficient)≥0.99993.
Precision and accuracy were tested at 0.89 mg/mL piperacillin/0.11 mg/mL tazobactam concentration. Maximum % Residual Standard Deviation between the replicate samples (n=3) was 1.8%, recovery was 99.9%–100.6% for piperacillin and 99.0%–100.6% for tazobactam.
Piperacillin when stored under acidic conditions at 30°C showed a peak area reduction of 55% whereas tazobactam remained relatively stable with 97.9% remaining after 6 hours. Under alkaline conditions however, both APIs were highly unstable with no piperacillin and only 45% tazobactam remaining 3 min after preparation. Under oxidising conditions (hydrogen peroxide, 0.15% (v/v), piperacillin was completely degraded after 120 min and tazobactam was >99% degraded after 6 hours at 30°C. All degradation peaks for both actives were clearly resolved from the API peaks at the wavelengths used for quantification, for piperacillin (280 nm) and tazobactam (210 nm).
The effect of pH on the stability of piperacillin/tazobactam was investigated prior to conducting the main stability study. Both piperacillin and tazobactam appeared to degrade more quickly at high pH compared with low pH. However tazobactam is inherently more stable than piperacillin. The optimum stability for both compounds appears to be around neutral pH 6.5–7.0. This is consistent with previous studies on flucloxacillin sodium solution for injection.4 The optimum stability appears to coincide with the pH of a commercially available citrate-buffered saline diluent available from a UK specials manufacturer (Royal Free Hospital) which provides a route of access to a suitable diluent for reconstitution and dilution of piperacillin/tazobactam powder for injection. Work was performed to demonstrate the effectiveness of citrate-buffered saline as suitable diluent for enhancing stability and extending practical shelf-life of piperacillin/tazobactam. The prestudy work compared levels of chemical degradation with and without pH control. pH control was achieved using citrate-buffered saline and demonstrated an improved stability profile for piperacillin when citrate-buffered saline was employed as diluent for reconstitution and dilution over the therapeutic range of drug concentrations 25 mg/mL to 90 mg/mL. The prestudy work demonstrated suitable stability to support a potential 14-day extension of practical shelf-life for the combination product.
The pH range for both concentrations of piperacillin/tazobactam at the start of the study was 6.38–6.73 pH units. A decrease in pH over time in a concentration-dependant manner was observed. All solutions at 25 mg/mL concentration remained within YCD limits which is±0.5 pH unit difference from the initial. For 90 mg/mL solutions after 13-day storage at fridge temperature followed by 24 hours at 32°C the pH slightly exceeded YCD limits for both devices tested.
For the Easypump II devices subvisible particle count data complied with the European Pharmacopoeia 2.19.9 limits at each time point. For the FOLFusor LV10 devices the 25 µm particle counts exceeded the limits at day 1 only in one device at the 25 mg/mL concentration, and at day 7 in two devices at 90 mg/mL concentration and day 10 in one device; however, there was no tendency for the subvisible particle counts to increase over time (trending).
There was no trend in the stability data over the five time points measured for both actives which appeared to remain intact throughout the study period of 14 days. The amount of both actives remaining at the end of the administration period was greater than the 95% required for YCD compliance.2
As with the data for the FOLFusor LV10 device, the Easypump II elastomeric pump appears to support extended stability of piperacillin/tazobactam solutions for infusion over the therapeutic concentration ranges studies. No trend was observed in the stability data and there was greater than 95% remaining of both actives at the end of the administration period. The data in tables 1 and 2 support compliance with YCD and an extended practical shelf-life for piperacillin/tazobactam solutions for infusion stored at 2°C–8°C for 13 days and a 24-hour ‘in use’ period at 32°C.
Table 1.
Piperacillin/tazobactam content during storage in FOLFusor LV10 (Baxter) elastomeric devices as determined by stability indicating HPLC assay
| Timepoint | 90 mg/mL* | 25 mg/mL† | ||
| PIP+TAZ, % remaining | 95% CI (%) | PIP+TAZ, % remaining | 95% CI (%) | |
| Day 0 | 100.0 (94.1±0.4 mg/mL) |
99.6 to 100.4 | 100 (27.1±0.2 mg/mL) |
99.1 to 100.9 |
| Day 7 | 101.3 | 100.8 to 101.8 | 101.5 | 101.2 to 101.8 |
| Day 10 | 99.7 | 99.5 to 99.9 | 100.3 | 99.9 to 100.7 |
| Day 13 | 98.7 | 98.3 to 99.1 | 99.7 | 99.4 to 100.1 |
| Day 13+1 | 98.0 | 97.8 to 98.2 | 100.9 | 100.3 to 101.5 |
*90 mg/ml total drug concentration: 80 mg piperacillin/10 mg tazobactam.
†25 mg/ml total drug concentration: 22 mg piperacillin/3 mg tazobactam.
PIP, piperacillin; TAZ, tazobactam.
Table 2.
Piperacillin/tazobactam content during storage in Easypump II (B. Braun) elastomeric devices as determined using a stability indicating HPLC assay
| Timepoint | 90 mg/mL* | 25 mg/mL† | ||
| PIP+TAZ, % remaining | 95% CI (%) | PIP+TAZ, % remaining | 95% CI (%) | |
| Day 0 | 100.0 (87.3±0.7 mg/mL) |
99.2 to 100.8 | 100 (24.6±0.5 mg/mL) |
97.9 to 102.1 |
| Day 7 | 101.7 | 101.6 to 101.8 | 100.8 | 100.42 to 101.18 |
| Day 11 | 101.1 | 99.83 to 102.37 | 102.2 | 102.13 to 102.27 |
| Day 13 | 100.7 | 100.6 to 100.8 | 98.9 | 97.35 to 100.45 |
| Day 13+1 | 100.8 | 100.22 to 101.38 | 101.0 | 100.91 to 101.09 |
*90 mg/ml total drug concentration: 80 mg piperacillin/10 mg tazobactam.
†25 mg/ml total drug concentration: 22 mg piperacillin/3 mg tazobactam.
PIP, piperacillin; TAZ, tazobactam.
Further results for pH, particle counts and HPLC assays can be found in online supplemental tables S1-S9.
ejhpharm-2020-002340supp001.pdf (85.1KB, pdf)
Discussion
It is essential that the stability of an antibiotic in a body worn infusion device can be demonstrated if it is to be considered for continuous 24-hour infusion via OPAT services. We have previously published data on the stability of flucloxacillin in the context of continuous infusion for OPAT,4 demonstrating the requirement for buffering to maintain neutral pH and reduce nucleophilic attack on the beta-lactam ring. Initial tests confirmed that when piperacillin/tazobactam was reconstituted and diluted in 0.9% w/v saline as diluent the solutions for infusion lacked the required stability. This was consistent with other published studies.6 7
Adelfred and Nejatbakhsh7 reported the stability of piperacillin/tazobactam at 12 g/1.5 g in an elastomeric device when reconstituted and diluted in 0.9% w/v saline with storage up to 7 days at fridge 2°C–8°C followed by 24 hours at 32°C. There was no reference to the amount of drug loss through degradation however, and in our hands, piperacillin was found to be too unstable without the use of citrate-buffered diluent.
Rigge and Jones6 reported the stability of piperacillin/tazobactam 45 mg/mL in polyvinyl chloride (PVC) and polyolefine laminate bags in 0.9% (w/v) sodium chloride at 7°C, 25°C and room temperature in the light (RTL). Stability at 7°C for up to 5 days and up to 4 days at 25°C and RTL was demonstrated for PVC bags while in polyolefine bags, the durations were 17, 4 and 3 days respectively. Stability was shown to improve in non-PVC bags when a buffered sodium chloride diluent was used for reconstitution and dilution, with storage durations of 58, 10 and 7 days respectively at 7°C, 25°C and RTL. Rigge and Jones6 did not consider any ‘in-use’ stability at the required temperature of 32°C and only considered one concentration value. These data do not meet the current YCD requirements.
Although the pH drop at the highest concentration of 90 mg/mL in both devices was recorded just outside of YCD limits at 0.6, this is acceptable for this type of active pharmaceutical ingredient and consistent with the amount of degradation. The subvisible counts recorded at greater than 10 µm and greater than 25 µm were within European Pharmacopoeia limits for the Easypump II device, but some data for FOLFusor LV10 was outside of the accepted limits. There was no trend in the data and often higher particle counts are seen at the start of the study due to reconstitution effects. There is an in-line 0.2 µm filter to protect the patient that was removed for the study testing. As such there is no concern for patient safety due to subvisible particulates as found in this study in either device.
Published work indicates that high metal ion concentrations and low pH can lead to subvisible particle formation and degradation of quality of piperacillin/tazobactam solutions for injection. In 2011 Wyeth Holdings Corporation patented a reformulated piperacillin/tazobactam product for infusion containing a chelating agent and citrate to support improved stability,8 highlighting the benefits of their product (Zosyn) versus generic versions of the same actives9 in a letter to the FDA.10 Viola et al 11 describe a general degradative pathway for penicillins from a stability study of piperacillin/tazobactam. The study identified an insoluble penicilloic acid-piperacillin dimer that is formed when EDTA/sodium citrate is included in the formulation and diluted in Ringer lactate solution, concluding that the role of EDTA is negligible and does not improve chemical stability of piperacillin/tazobactam.11
Despite the apparent confliction in data around inclusion of metal chelating agents it is clear from the literature that stabilisation of penicillins including piperacillin can be achieved using pH control and that citrate-buffered saline is a suitable diluent for reconstitution and dilution.
Our study has demonstrated the importance of buffering piperacillin/tazobactam with citrate to limit chemical degradation; and is consistent with other published studies. A published patent12 suggests that increased stability can be achieved for piperacillin/tazobactam when prepared in a citrate-buffered saline diluent providing up to 9 months extended shelf-life when stored frozen and 14 days when stored at fridge temperatures. While there are no published reports of stability of piperacillin-tazobactam in body worn infusion devices up to 32°C, the work performed by Rigge and Jones6 demonstrated improved stability of piperacillin/tazobactam when reconstituted and diluted in buffered saline and stored in non-PVC bags.
Our results add to the emerging literature on the stability of antimicrobial agents in elastomeric devices. Our testing methodology is based on the standards in the YCD used in the National Health Service in the UK, and gives assurance to OPAT centres based in the UK and worldwide that citrate-buffered piperacillin/tazobactam is suitable for use by continuous infusion in an outpatient setting using these devices. Piperacillin-tazobactam is a particularly useful antibiotic for the management of a number of complex Pseudomonal infections which require prolonged therapy, including malignant otitis externa and exacerbations of bronchiectasis (including complicating cystic fibrosis). Utilisation of a 24-hour infusion of piperacillin/tazobactam will significantly increase OPAT team capacity and also reduce the burden and complexity for patients, carers or nurses who would otherwise need to prepare and administer the antibiotic at home up to four times per day.
From the antimicrobial stewardship perspective, the availability of piperacillin/tazobactam via continuous infusion in OPAT has the additional advantage in reducing the reliance on other protected anti-Gram negative agents, including the commonly used carbapenem ertapenem, and the cephalosporins ceftriaxone and ceftazidime.13
Conclusions
This study confirms that when 0.3% w/v citrate-buffered saline pH 7.0 is used to reconstitute and dilute (compound) piperacillin/tazobactam at concentrations between circa 25 mg/mL and 90 mg/mL in two commercially available elastomeric devices, FOLFusor LV10 and Easypump II, the solutions are sufficiently stable to assign a shelf of 14 days including 24 hours at the recommended administration temperature of 32°C. The stability data include a designated warm up period for the devices when removed from the refrigerator prior to starting the administration period in accordance with the manufacturer’s instructions for use. All data comply with the latest YCD,2 British Pharmacopoeia14 and European Pharmacopoeia15 requirements where these apply.
What this paper adds.
What is already known on this subject
Multidrug resistant Gram-negative (MDRGN) infections are increasing and a significant proportion of patients, with deep seated or complex infection, require prolonged intravenous antibiotic therapy. Currently administration of piperacillin/tazobactam is via intermittent 6–8 hourly infusions and as such is challenging in the outpatient parenteral antimicrobial therapy (OPAT) setting; prolonged infusions may represent a more optimal administration method.
Piperacillin degrades in aqueous solution particularly at high and low pH and during extended storage without pH control. As such piperacillin/tazobactam solutions for infusion when reconstituted and diluted using 0.9% w/v saline are not sufficiently stable over a 14-day shelf life to support an OPAT service delivery model, according to Yellow Cover Document standards. There is therefore a requirement for additional stability data on piperacillin/tazobactam solutions for infusion with increased stability in elastomeric devices to support OPAT in the UK, and perhaps further afield.
What this study adds
The study presents new data on the improved chemical stability of piperacillin/tazobactam at 25 mg/mL and 90 mg/mL reconstituted and diluted using citrate-buffered saline as diluent within two elastomeric devices: FOLFusor LV10 (Baxter) and Easypump II (B. Braun).
The data supports an extended shelf life of up to 13 days fridge storage 2°C–8°C plus 24 hours at 32°C, facilitating its use as a one time per day infusion in OPAT.
Acknowledgments
The authors would like to thank (1) Baxter Healthcare Ltd. for supporting this study with a donation and also the in-kind provision of medical devices, (2) B. Braun Medical Ltd. for the in-kind provision of medical devices and (3) members of the BSAC Drug Stability Working Group for their contribution to the study and production of the manuscript.
Footnotes
Twitter: @BSTLNotts
Collaborators: The BSAC Drug Stability Working Party comprises CJ, (chair), MG, TH, Mark Santillo, RAS and representatives from the stability testing laboratory: LO and ASW.
Contributors: ASW and CJ planned the study. LO and ASW performed the study and collected the data. CJ, LO and ASW analysed the data and wrote the manuscript. FD, MG, TH and RAS critically reviewed the manuscript.
Funding: This study was commissioned and funded by the British Society for Antimicrobial Chemotherapy (BSAC) as part of their Drug Stability Testing Programme in the UK to support outpatient parenteral antimicrobial therapy (OPAT) services. Baxter Healthcare Ltd provided a donation to the study.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
the BSAC Drug Stability Testing Working Group:
Mark Gilchrist, Tim Hills, Mark Santillo, R. Andrew Seaton, Laima Ozolina, and Alan-Shaun Wilkinson
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Hawkey PM, Warren RE, Livermore DM, et al. Treatment of infections caused by multidrug-resistant gram-negative bacteria: report of the British Society for antimicrobial Chemotherapy/Healthcare infection Society/British infection association joint working Party†. J Antimicrob Chemother 2018;73:iii2–78. 10.1093/jac/dky027 [DOI] [PubMed] [Google Scholar]
- 2. NHS Pharmaceutical Quality Assurance Committee . A standard protocol for deriving and assessment of stability: part 1 - aseptic preparations (small molecules). Edition 5, 2019. Available: https://www.sps.nhs.uk/wp-content/uploads/2013/12/Stability-part-1-small-molecules-5th-Ed-Sept-19.pdf [Accessed 12 Mar 2020].
- 3. Jenkins A, Hills T, Santillo M, et al. Extended stability of antimicrobial agents in administration devices. J Antimicrob Chemother 2017;72:dkw556–1220. 10.1093/jac/dkw556 [DOI] [PubMed] [Google Scholar]
- 4. Allwood MC, Stonkute D, Wallace A, et al. Assessment of the stability of citrate-buffered flucloxacillin for injection when stored in two commercially available ambulatory elastomeric devices: INfusor LV (Baxter) and Accufuser (Woo young medical): a study compliant with the NHS yellow cover document (yCD) requirements. Eur J Hosp Pharm 2020;27:90–4. 10.1136/ejhpharm-2018-001515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jamieson C, Allwood MC, Stonkute D, et al. Investigation of meropenem stability after reconstitution: the influence of buffering and challenges to meet the NHS yellow cover document compliance for continuous infusions in an outpatient setting. Eur J Hosp Pharm 2020;27:e53–7. 10.1136/ejhpharm-2018-001699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rigge DC, Jones MF. Shelf lives of aseptically prepared medicines--stability of piperacillin/tazobactam in PVC and non-PVC bags. J Pharm Biomed Anal 2005;39:339–43. 10.1016/j.jpba.2005.03.013 [DOI] [PubMed] [Google Scholar]
- 7. Adelfred A, Nejatbakhsh Y. DD-001 Stability of piperacillin/tazobactam in elastomeric infusion pumps. Eur J Hosp Pharm Sci Prac 2014;21:A63. [Google Scholar]
- 8. Cohen J, Shah S, Ofslager C, et al. Compositions containing piperacillin and tazobactam useful for injection. United States patent US7915229B2. Available: http://patft.uspto.gov/netacgi/nph-Parser?d=PALL&p=1&u=%2Fnetahtml%2FPTO%2Fsrchnum.htm&r=1&f=G&l=50&s1=7915229.PN.&OS=PN/7915229&RS=PN/7915229 [Accessed 16 Mar 2020].
- 9. Wyeth Pharmaceuticals . Zosyn® (Piperacillin/Tazobactam For Injection USP [Glass Vials]) United States Prescribing Information and Wyeth Pharmaceuticals. 2007 Zosyn® (Piperacillin/Tazobactam For Injection USP [Galaxy® Bags]) United States Prescribing Information 2007.
- 10. Wyeth Pharmaceuticals . Wyeth pharmaceuticals letter to FDA, 2008. Available: https://www.regulations.gov/document?D=FDA-2006-P-0019-0018 [Accessed 13 Mar 2020].
- 11. Viola A, Ferrazzano L, Martelli G, et al. Novel insights into the chemistry of an old medicine: a general degradative pathway for penicillins from a piperacillin/tazobactam stability study. Eur J Pharm Sci 2019;136:104957. 10.1016/j.ejps.2019.104957 [DOI] [PubMed] [Google Scholar]
- 12. Thompson S, Chilamkurti R, Samuel M, et al. Premixed formulation of piperacillin sodium and tazobactam sodium injection. United States patent publication number US6207661B1, current assignee Baxter international Inc. first registered 2001.
- 13. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother 2015;70:965–70. 10.1093/jac/dku517 [DOI] [PubMed] [Google Scholar]
- 14. British Pharmacopoeia. Available: https://www.pharmacopoeia.com/ [Accessed 15 Apr 2020].
- 15. European Pharmacopoeia (EP) . Particulate contamination: sub-visible particles. edition 6, 2019. Available: http://uspbpep.com/ep60/2.9.19.%20particulate%20contamination-%20sub-visible%20particles%2020919e.pdf [Accessed 12 Mar 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ejhpharm-2020-002340supp001.pdf (85.1KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.