Abstract
The complete picture regarding transmission modes of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is unknown. This review summarises the available evidence on its transmission modes, our preliminary research findings and implications for infection control policy, and outlines future research directions. Environmental contamination has been reported in hospital settings occupied by infected patients, and is higher in the first week of illness. Transmission via environmental surfaces or fomites is likely, but decontamination protocols are effective in minimising this risk. The extent of airborne transmission is also unclear. While several studies have detected SARS-CoV-2 ribonucleic acid in air samples, none has isolated viable virus in culture. Transmission likely lies on a spectrum between droplet and airborne transmission, depending on the patient, disease and environmental factors. Singapore’s current personal protective equipment and isolation protocols are sufficient to manage this risk.
Keywords: airborne, environment, infection control, SARS-CoV-2, transmission
INTRODUCTION
The COVID-19 epidemic accelerated at an alarming pace since it was first reported on 31 December 2019,(1) affecting more than five million patients globally.(2) Singapore was affected in the early stages of the epidemic and has reported more than 30,000 confirmed cases at the time of writing.(3)
While a large proportion of patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative virus, remain clinically well,(4,5) a small proportion will develop severe illness requiring intensive care unit (ICU) admission and mechanical ventilation. Reported case fatality rates have varied across countries, likely affected by testing rate, availability of intensive care and other healthcare resources, and variations in demographics.(6)
If the epidemic continues to progress unmitigated, the strain on healthcare systems will be tremendous. Decreased healthcare resource availability was reported to be associated with an increased mortality rate in China, possibly accounting for the disproportionate case fatality rate in the outbreak epicentre of Wuhan, Hubei, China (more than 3% in Wuhan as compared to an average of 0.7% in provinces outside Hubei).(7) It is, thus, of paramount importance to make every effort in containing the outbreak to flatten the epidemic curve and prevent disease burden from outpacing the expansion of healthcare resource capacity.
Nosocomial transmission of SARS-CoV-2 was also reported in China, implicated in up to 41% of patients in a single-centre case series.(8) Cohort studies of healthcare workers in outbreak settings have found infection rates of between 1.8% and 18%.(9,10) Previous large outbreaks of Middle East respiratory syndrome (MERS) and severe acute respiratory syndrome (SARS) have similarly been driven by nosocomial spread,(11-13) highlighting the importance of effective hospital infection control policies to mitigate risk.
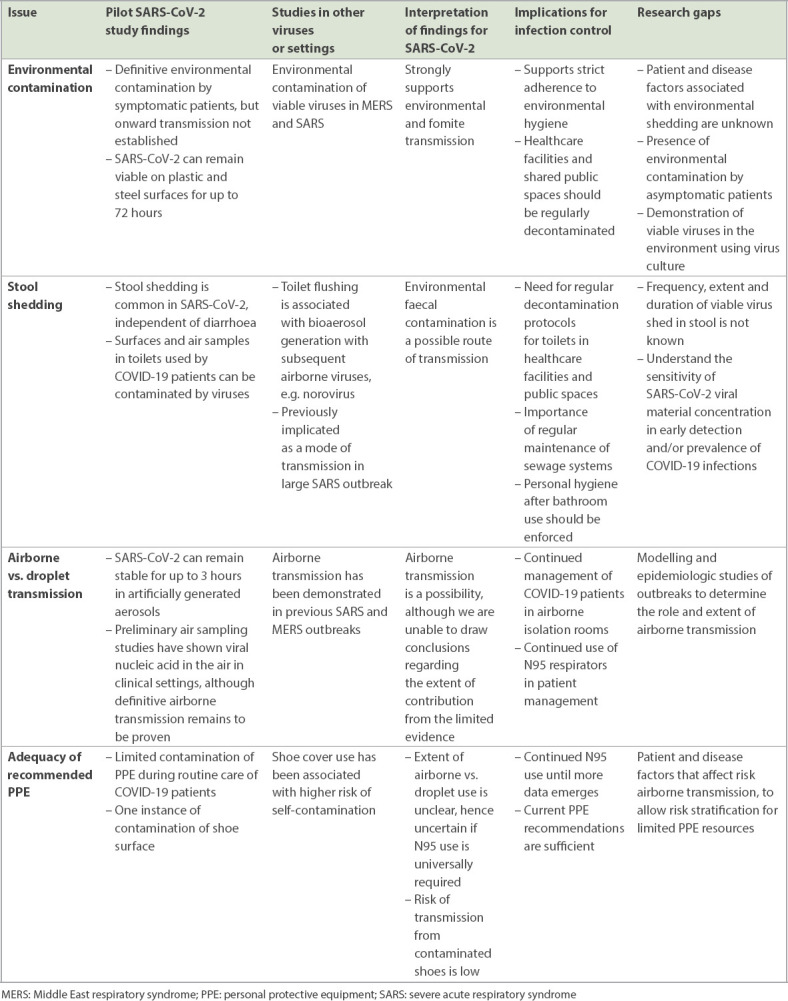
Infection control and personal protective equipment (PPE) recommendations are evolving as the outbreak progresses. Numerous research gaps and unanswered questions remain, and policymakers are faced with the difficult task of balancing scientific evidence, resource availabilities, and the attitudes and well-being of healthcare workers (HCWs) and patients. In this review, we summarise the available evidence on the modes of transmission of SARS-CoV-2, including our preliminary research findings on air and environmental sampling,(14,15) and implications on infection control policy. We also outline the future directions of research (Table I).
Table I.
Summary of available evidence, implications and research gaps related to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) transmission and infection control.
FOMITE AND ENVIRONMENTAL TRANSMISSION
In our pilot study, we demonstrated extensive environmental contamination of the hospital room and attached bathroom by a COVID-19 patient with mild upper respiratory tract disease, with 13 (87%) of 15 room sites and 3 (60%) of five toilet sites testing positive for SARS-CoV-2 nucleic acid by polymerase chain reaction (PCR).(14) Several other studies have since confirmed these findings of environmental contamination in hospital settings.(16-19) Our follow-up study evaluating environmental contamination in 30 rooms (27 general wards and three ICUs) confirmed that environmental contamination was common (17/30, 57%), and occurred in rooms of both symptomatic, pauci-symptomatic and asymptomatic patients.(15) Patients in the first week of illness had significantly more environmental contamination, which declined after Day 8 of the disease. This finding is consistent with previous data showing that viral shedding from the respiratory tract is higher in the first week of illness.(20,21)
These studies strongly suggest that the environment can serve as a medium of transmission of SARS-CoV-2, through touch contamination and subsequent self-inoculation of mucous membranes by a non-infected individual coming into contact with a contaminated environmental surface or fomite. Previous studies on MERS and SARS have shown that these viruses extensively contaminate the environment, and have successfully isolated viruses in environmental samples on viral culture,(22-24) clearly demonstrating the potential of this mode of transmission. Importantly, while a laboratory study has shown that SARS-CoV-2 can remain viable on plastic and stainless steel surfaces for up to 72 hours (median 16 hours and 13 hours, respectively),(25) no study has isolated viable virus from the environment in a natural clinical setting. Attempts to isolate viable SARS-CoV-2 virions in viral culture from the environment are ongoing, and if successful, will prove to be the smoking gun in favour of some extent of environmental transmission. However, the odds are stacked against successful virus isolation from environmental samples. Unlike laboratory settings, ambient environmental conditions vary and can lead to virus structural changes primarily via desiccation and ultimately, loss of viability. Additionally, the low viral loads typically seen in these samples would reduce chances of virion attachment to cell culture, which is required to establish an infection.
The impact of these findings on infection control and public health policy is significant. First, strict adherence to environmental hygiene protocols should be ensured in all healthcare settings to minimise the risk of environment-mediated transmission. Reassuringly, all post-cleaning samples in the pilot study were negative, suggesting that the current decontamination protocols are sufficient.(14) Second, regular cleaning and decontamination should be carried out in shared public places to minimise the risk of environmental transmission.
Further research should elucidate in greater detail the patient and disease factors affecting the extent of environmental contamination. If more data confirms that the degree of environmental contamination decreases beyond the first week of illness, this can allow prioritisation of limited resources (e.g. isolation rooms) for patients early in the disease course and inform de-isolation or cohorting policies.
STOOL SHEDDING AND FAECAL TRANSMISSION
Stool shedding of SARS-CoV-2 by COVID-19 patients has been reported. While infrequent, isolation of the virus in cell culture from stool samples has been reported, indicating the viability of viruses shed from the gastrointestinal tract.(26,27) 4 (50%) out of eight patients in a case series from Singapore were found to have positive stool samples for SARS-CoV-2 by PCR,(20) which did not correlate with the presence of diarrhoea or other gastrointestinal symptoms. Furthermore, COVID-19 patients have been reported to shed virus in stools even a few days after all respiratory symptoms have disappeared.(28,29)
In our pilot environmental study, samples from the toilet bowl, sink and door handle were positive for SARS-CoV-2, as tested by PCR.(14) The follow-up environmental study also found contamination of the toilet bowl in 5 (18.5%) out of 27 rooms.(15) Toilet contamination can be explained by two possible mechanisms: (a) respiratory droplets contaminating the toilet environment while in use by the patient or (b) environmental faecal contamination. In the pilot study, SARS-CoV-2 PCR was also found in two of the patient’s clinical stool samples, possibly supporting the hypothesis of environmental faecal contamination.
Flushing of toilets has been shown to generate bioaerosols containing faecal micro-organisms,(30) and other viruses such as norovirus have been detected in hospital air samples, presumably through bioaerosol spread.(31) Viral stool shedding has been demonstrated in SARS,(32) and was implicated in at least one large outbreak in a private apartment building in Hong Kong, hypothesised to be mediated through contamination of the sewage system and spread via faeces-contaminated droplets.(33,34) A preliminary study from China found positive air samples by PCR in a mobile toilet used by a COVID-19 patient, suggesting that similar aerosolisation of virus shed in stool can also occur with SARS-CoV-2.(35)
Horizontal transmission can occur should an uninfected individual touch contaminated toilet surfaces and self-inoculate his/her mucous membranes, or even via the respiratory route should there be faecal bioaerosolisation. However, the frequency, extent and duration of shedding of viable virus in stool is still unclear. Environmental faecal contamination as a route of transmission, while plausible, remains hypothetical, and we are unable to quantify this risk with the currently available evidence.
These findings raise the possibility of viable SARS-CoV-2 shed in stool contaminating the toilet environment, although respiratory droplet contamination remains a possible alternate hypothesis. Further studies should expand the sample size and recruit patients with a broad range of symptoms, examining the extent of contamination of patient rooms and toilets and its correlation with respiratory symptoms to tease out the difference between these two mechanisms of toilet contamination.
AIRBORNE (AEROSOL) AND DROPLET TRANSMISSION
Airborne transmissibility of SARS-CoV-2, which has recently been debated, could have significant ramifications for patient disposition, allocation of healthcare infrastructure, PPE use and public health policy. Unfortunately, it is not straightforward, and based on the currently available evidence, the extent of airborne transmission of SARS-CoV-2 is unknown.
Whether a virus can spread via the airborne route is largely determined by the decay rate of the virus in an aerosolised state and the ability of an infected individual to efficiently shed the virus in respiratory droplets or other bodily fluids. It is understood that large respiratory droplets tend to settle by force of gravity within a 1–2-m radius from the source. By contrast, smaller droplets are carried more efficiently through the air and droplets < 5 μm are respirable.(36,37) Diseases can be classified as obligate (e.g. tuberculosis), preferential (e.g. varicella, measles) or opportunistic (e.g. influenza, SARS) airborne diseases.(38) This classification depends on whether the primary means of spread is airborne or if airborne transmission only arises in specific situations (e.g. during aerosol-generating procedures).(37)
The need to consider the human infectious dose (if known), as well as the dose-response relationship of the virus, adds to the difficulty in characterising the airborne transmissibility of a virus. Molecular identification of viral nucleic acids in aerosols is usually the first line of scientific evidence of potential airborne transmission of a virus. Still, it does not mean that the contained virus can generate a clinical infection.(39) Furthermore, airborne transmission is strongly influenced by environmental factors such as airflow, ventilation, temperature and humidity, which can affect droplet desiccation and movement as well as virus stability.(40)
Several studies have attempted to detect SARS-CoV-2 in air samples (Table II). In two recent air sampling pilot studies, SARS-CoV-2 nucleic acid was not detected in the air in a COVID-19 patient room or from an exhaled breath sample from an infected patient.(14,16) However, four subsequent studies detected SARS-CoV-2 contamination in air samples collected in healthcare settings. In addition to the positive aerosol samples collected near the patient toilet in the study by Liu et al,(35) low concentrations of aerosolised virus were detected in both patient and staff areas, including the designated room for PPE removal. Quantification of droplet size showed a bimodal size distribution of 0.25–1.0 μm and > 2.5 μm, which is suggestive of potential airborne transmission. However, whether these low concentrations of aerosolised virus (1–42 copies/m3 of air) are clinically significant or can result in onward transmission is unclear.
Table II.
Summary of key studies testing air samples for severe acute respiratory syndrome coronavirus 2.
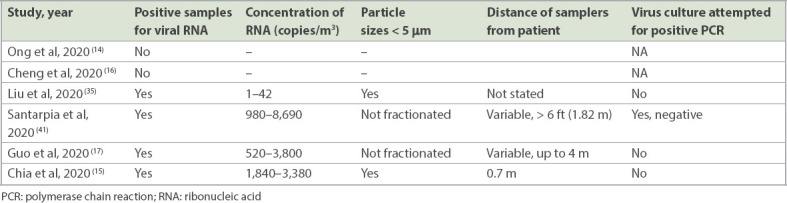
Another study by Santarpia et al found that 8 (66.7%) out of 12 air samples collected from patient rooms and hallways were positive for SARS-CoV-2, with viral concentrations of 0.98–8.69 copies/L of air (or 980–8,690 copies/m3; mean concentration 2,860 copies/m3).(41) Fractionation of droplet size was not performed, and the distance between the air samplers and the source patient varied, although at least two of the positive air samples were from samplers more than 6 ft (1.83 m) from the patient.
Guo et al have also detected SARS-CoV-2 ribonucleic acid (RNA) in air samples (14/40 samples from the ICU and 2/16 samples from the general ward), including from a sampling site up to 4 m from the patient.(17) Concentrations were 0.52–3.8 copies/L (520–3,800 copies/m3), and droplet size fractionation was not performed.
Our second air sampling study in Singapore detected two positive air samples from patients early in the course of illness (Day 5) with high viral loads (clinical cycle threshold values of 18.45 and 20.11) and viral concentrations of 1,840 and 3,380 RNA copies/m3 of air, consistent with the findings in the aforementioned studies.(15) Importantly, size fractionation was performed using National Institute for Occupational Safety and Health (NIOSH) air samplers, identifying SARS-CoV-2 in aerosols 1–4 μm in diameter, which are capable of transmission beyond short distances.
Importantly, none of these studies demonstrated virus viability by isolating virions from air samples in virus culture. An in vitro experiment has shown that SARS-CoV-2 can remain viable in artificially generated aerosols for up to three hours,(25) but whether this can be demonstrated in a real-world clinical setting is unknown. Identification of viable viruses in air samples from real-world environments would provide more convincing evidence for an airborne transmission hypothesis.
Future directions of research should include modelling and epidemiologic studies of real-life outbreaks, for example, by demonstrating evidence of transmission between individuals in which no other discernible route of transmission can be identified. The current estimates of the reproductive number (R0) of SARS-CoV-2, ranging from 1.0 to 2.5,(42,43) seem to suggest a largely close-range droplet transmission (with potential opportunistic airborne transmission, e.g. during aerosol-generating procedures), as primarily airborne pathogens tend to give rise to a much higher R0. Anecdotal evidence of healthcare workers exposed to confirmed cases without developing infection despite inadequate protection against airborne transmission also suggests that the degree of airborne transmission may not be extensive.(44) Routine use of surgical masks in the community has also prevented transmission from symptomatic COVID-19-infected hair stylists to a large number of customers.(45) On the other hand, several super-spreading events have been reported, wherein the high attack rate may suggest the role of aerosolisation and airborne transmission.(46,47)
Recent mathematical modelling indicates that short-range airborne transmission may be the dominant route of respiratory virus transmission among close contacts,(48) which is contrary to the historical understanding of how respiratory viruses are primarily transmitted. Nevertheless, epidemiologic evidence should supplement mechanistic studies to serve as additional confirmation of airborne transmission in natural settings,(49) as previously demonstrated with SARS and measles.(50,51)
ADEQUACY OF PERSONAL PROTECTIVE EQUIPMENT
In Singapore, the PPE for healthcare workers includes gloves, disposable gown, respiratory protection (at least as protective as a fit-tested NIOSH-certified disposable N95 filtering facepiece respirator) and eye protection (e.g. goggles or disposable face shield) for all healthcare workers in contact with patients with known or suspected COVID-19, if resources permit, similar to the recommendation of the United States Centers for Disease Control and Prevention.(52) By contrast, the World Health Organization (WHO) recommends droplet and contact precautions with a surgical mask, eye or face protection, disposable gown and gloves for regular patient contact.(53) The WHO continues to recommend airborne precautions for settings in which aerosol-generating procedures are performed, according to risk assessment.
As discussed, the dichotomy of airborne versus droplet transmission is an inaccurate distinction, and SARS-CoV-2 seems to fall within a spectrum of potential for airborne transmission. Likewise, the choice between a surgical mask and an aerosol-filtering respirator (e.g. N95 mask) is not merely dependent on the matter of airborne versus droplet transmission, but has to take into account patient factors (the necessity for aerosol-generating procedures), user factors (fit-testing, comfort, training and familiarity) and resource factors (availability of N95 respirators).
Given the current paucity of data on SARS-CoV-2 and its transmission routes, no conclusions can be drawn as to whether N95 respirators or surgical masks are superior in the protection of healthcare personnel. Nonetheless, we err on the side of caution and advise N95 respirator use for all contact with known or suspected COVID-19 patients. For aerosol-generating procedures (e.g. intubation or bronchoscopy), powered air-purifying respirators may be used (if HCWs are trained and familiar with its use), taking into account the high proportion of nosocomial transmission to HCWs associated with these procedures during SARS.(54,55) However, these recommendations are contingent on the continued availability of PPE resources, which were both scarce and expensive during SARS.(56,57) Should the epidemic progress unabated, with resultant pressure on PPE stockpiles, respirator use may have to be allocated based on situational risk. Further studies are urgently needed to determine whether surgical masks are sufficient and to identify patient, environmental and viral factors that necessitate up-triaging of PPE recommendations.
In our preliminary study, PPE samples taken from the gown, gloves, visor mask and N95 mask were negative for SARS-CoV-2 by PCR.(14) However, one swab from the front surface of shoes of a HCW was positive after contact with a known COVID-19 patient, raising uncertainty about the necessity of shoe covers. Implementing a policy of routine shoe cover use requires a balance between the risk of infection transmission without shoe covers and the risks of shoe cover use. We assess the risk of infectious transmission from contaminated shoes to be negligible, especially since the risk of contamination of shoes in itself is insignificant. To our knowledge, no case of transmission of respiratory viruses mediated by contaminated shoes has been reported. Conversely, the routine use of shoe covers comes with its attendant issues – the safe doffing of shoe covers is a complicated process requiring additional infrastructure in the doffing area (e.g. chair, step stool or handgrips), which offers further avenues for contamination.(58) A previous study auditing the rate of self-contamination by HCWs during donning and doffing of PPE found that removal of shoe covers was a vulnerable step in the doffing process, with a 65.5% (19/29 incidents) rate of self-contamination.(59)
A follow-up study sampling HCWs’ PPE after contact with COVID-19 patients, which tested 90 samples (N95 mask, goggles and shoe front) from 30 HCWs (doctors, nurses and cleaners), did not detect any PPE contamination by SARS-CoV-2, providing reassurance that the possibility of PPE contamination is low during regular contact with patients.(60) Weighing the risks and benefits, we do not recommend the use of shoe covers given the low assessed risk of contaminated shoes and the increased risk of self-contamination during the doffing process.
In view of the expected global shortages of PPE supplies, if the pandemic continues, research has been conducted to evaluate the feasibility and efficacy of disinfection of surgical masks and N95 respirators for reuse. Studies have evaluated the use of dry heat, steam microwave, ethanol and hydrogen peroxide vapour for decontamination of masks;(61-64) however, varying results have been reported with regard to mask integrity post sterilisation. Furthermore, all these studies tested the adequacy of sterilisation with in vitro inoculation of PPE, and these methods have yet to be tested in real clinical settings. Until larger studies evaluating the implementation of such decontamination protocols are carried out, we cannot recommend their routine use while PPE supplies are still available.
CONCLUSION
The COVID-19 pandemic poses a significant threat to human health, healthcare systems, the economy and the society. Unmitigated progression will likely overwhelm workforce and resource capacity at considerable cost to mortality. Containment of the outbreak should be a foremost priority for all public health authorities, and strict infection control recommendations are paramount to achieving this goal.
Although we do not comprehensively understand the transmission routes of SARS-CoV-2, emerging evidence suggests that transmission can occur via multiple routes. COVID-19 patients can shed viable viruses from both the respiratory and gastrointestinal tracts, resulting in secondary infection either directly via droplet and opportunistic aerosol generation or indirectly via contamination of the environment or fomites.
Current isolation, PPE and decontamination protocols are likely to be sufficient for the management of confirmed or suspected COVID-19 patients, but the progression of the outbreak and resultant resource constraints may necessitate a redrawing and rationalisation of these protocols. Further studies to clarify the extent and relative importance of each of these transmission routes, as well as the patient, disease and environmental factors that affect each medium, are urgently needed to allow policymakers to risk-stratify and tailor infection control recommendations.
REFERENCES
- 1.World Health Organization. Novel Coronavirus –China. [Accessed June 1, 2020]. Available at: https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/
- 2.World Health Organization. Coronavirus disease (COVID-2019) situation reports. [Accessed June 1, 2020]. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports .
- 3.Ministry of Health, Singapore. Updates on COVID-19 (Coronavirus Disease 2019) local situation. [Accessed June 1, 2020]. Available at: https://www.moh.gov.sg/covid-19 .
- 4.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China:summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–42. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 6.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323:1775–6. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 7.Ji Y, Ma Z, Peppelenbosch MP, Pan Q. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020;8:e480. doi: 10.1016/S2214-109X(20)30068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korth J, Wilde B, Dolff S, et al. SARS-CoV-2-specific antibody detection in healthcare workers in Germany with direct contact to COVID-19 patients. J Clin Virol. 2020;128:104437. doi: 10.1016/j.jcv.2020.104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keeley AJ, Evans C, Colton H, et al. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25:2000433. doi: 10.2807/1560-7917.ES.2020.25.14.2000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim KH, Tandi TE, Choi JW, Moon JM, Kim MS. Middle East respiratory syndrome coronavirus (MERS-CoV) outbreak in South Korea, 2015:epidemiology, characteristics and public health implications. J Hosp Infect. 2017;95:207–13. doi: 10.1016/j.jhin.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen M, Leo YS, Ang B, Heng BH, Choo P. The outbreak of SARS at Tan Tock Seng Hospital--relating epidemiology to control. Ann Acad Med Singap. 2006;35:317–25. [PubMed] [Google Scholar]
- 13.Chowell G, Abdirizak F, Lee S, et al. Transmission characteristics of MERS and SARS in the healthcare setting:a comparative study. BMC Med. 2015;13:210. doi: 10.1186/s12916-015-0450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong SWX, Tan YK, Chia PY, et al. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–2. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chia PY, Coleman KK, Tan YK, et al. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun. 2020;11:2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng VCC, Wong SC, Chen JHK, et al. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. 2020;41:493–8. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583–91. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye G, Lin H, Chen S, et al. Environmental contamination of SARS-CoV-2 in healthcare premises. J Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Wang Y, Jin X, et al. Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. Am J Infect Control. 2020;48:910–4. doi: 10.1016/j.ajic.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323:1488–94. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–9. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Chang SY, Sung M, et al. Extensive viable Middle East respiratory syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis. 2016;63:363–9. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bin SY, Heo JY, Song MS, et al. Environmental contamination and viral shedding in MERS patients during MERS-CoV outbreak in South Korea. Clin Infect Dis. 2016;62:755–60. doi: 10.1093/cid/civ1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Booth TF, Kournikakis B, Bastien N, et al. Detection of airborne severe acute respiratory syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J Infect Dis. 2005;191:1472–7. doi: 10.1086/429634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–7. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Chen C, Zhu S, et al. Isolation of 2019-nCoV from a stool specimen of a laboratory-confirmed case of the coronavirus disease 2019 (COVID-19) China CDC Weekly. 2020;2:123–4. [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao F, Sun J, Xu Y, et al. Infectious SARS-CoV-2 in feces of patient with severe COVID-19. Emerg Infect Dis. 2020;26:1920–2. doi: 10.3201/eid2608.200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian Y, Rong L, Nian W, He Y. Review article:gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843–51. doi: 10.1111/apt.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol. 2020;5:434–5. doi: 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knowlton SD, Boles CL, Perencevich EN, Diekema DJ, Nonnenmann MW CDC Epicenters Program. Bioaerosol concentrations generated from toilet flushing in a hospital-based patient care setting. Antimicrob Resist Infect Control. 2018;7:16. doi: 10.1186/s13756-018-0301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonifait L, Charlebois R, Vimont A, et al. Detection and quantification of airborne norovirus during outbreaks in healthcare facilities. Clin Infect Dis. 2015;61:299–304. doi: 10.1093/cid/civ321. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Tang F, Fontanet A, et al. Long-term SARS coronavirus excretion from patient cohort, China. Emerg Infect Dis. 2004;10:1841–3. doi: 10.3201/eid1010.040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH. The SARS epidemic in Hong Kong. J Epidemiol Community Health. 2003;57:652–4. doi: 10.1136/jech.57.9.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKinney KR, Gong YY, Lewis TG. Environmental transmission of SARS at Amoy Gardens. J Environ Health. 2006;68:26–30. quiz 51-2. [PubMed] [Google Scholar]
- 35.Liu Y, Ning Z, Chen Y, et al. Aerodynamic analysis of SARS-CoV-2 in two Wuhan hospitals. Nature. 2020;582:557–60. doi: 10.1038/s41586-020-2271-3. [DOI] [PubMed] [Google Scholar]
- 36.Shiu EYC, Leung NHL, Cowling BJ. Controversy around airborne versus droplet transmission of respiratory viruses:implication for infection prevention. Curr Opin Infect Dis. 2019;32:372–9. doi: 10.1097/QCO.0000000000000563. [DOI] [PubMed] [Google Scholar]
- 37.Seto WH. Airborne transmission and precautions:facts and myths. J Hosp Infect. 2015;89:225–8. doi: 10.1016/j.jhin.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roy CJ, Milton DK. Airborne transmission of communicable infection--the elusive pathway. N Engl J Med. 2004;350:1710–2. doi: 10.1056/NEJMp048051. [DOI] [PubMed] [Google Scholar]
- 39.Teunis PF, Brienen N, Kretzschmar ME. High infectivity and pathogenicity of influenza A virus via aerosol and droplet transmission. Epidemics. 2010;2:215–22. doi: 10.1016/j.epidem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Nicas M, Nazaroff WW, Hubbard A. Toward understanding the risk of secondary airborne infection:emission of respirable pathogens. J Occup Environ Hyg. 2005;2:143–54. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santarpia JL, Rivera DN, Herrera V, et al. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. medRxiv. 2020 Mar 23; https://doi.org/10.1101/2020.03.23.20039446. Preprint. [Google Scholar]
- 42.Zhang S, Diao M, Yu W, et al. Estimation of the reproductive number of novel coronavirus (COVID-19) and the probable outbreak size on the Diamond Princess cruise ship:a data-driven analysis. Int J Infect Dis. 2020;93:201–4. doi: 10.1016/j.ijid.2020.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kucharski AJ, Russell TW, Diamond C, et al. Early dynamics of transmission and control of COVID-19:a mathematical modelling study. Lancet Infect Dis. 2020;20:553–8. doi: 10.1016/S1473-3099(20)30144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng K, Poon BH, Puar TH, et al. COVID-19 and the risk to health care workers:a case report. Ann Intern Med. 2020;172:766–7. doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hendrix MJ, Walde C, Findley K, Trotman R. Absence of apparent transmission of SARS-CoV-2 from two stylists after exposure at a hair salon with a universal face covering policy - Springfield, Missouri, May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:930–2. doi: 10.15585/mmwr.mm6928e2. [DOI] [PubMed] [Google Scholar]
- 46.Hamner L, Dubbel P, Capron I, et al. High SARS-CoV-2 attack rate following exposure at a choir practice - Skagit County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:606–10. doi: 10.15585/mmwr.mm6919e6. [DOI] [PubMed] [Google Scholar]
- 47.Ghinai I, Woods S, Ritger KA, et al. Community transmission of SARS-CoV-2 at two family gatherings - Chicago, Illinois, February-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:446–50. doi: 10.15585/mmwr.mm6915e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen W, Zhang N, Wei J, Yen HL, Li Y. Short-range airborne route dominates exposure of respiratory infection during close contact. Build Environ. 2020;176:106859. [Google Scholar]
- 49.Tellier R, Li Y, Cowling BJ, Tang JW. Recognition of aerosol transmission of infectious agents:a commentary. BMC Infect Dis. 2019;19:101. doi: 10.1186/s12879-019-3707-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu IT, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350:1731–9. doi: 10.1056/NEJMoa032867. [DOI] [PubMed] [Google Scholar]
- 51.Remington PL, Hall WN, Davis IH, Herald A, Gunn RA. Airborne transmission of measles in a physician's office. JAMA. 1985;253:1574–7. [PubMed] [Google Scholar]
- 52.US Centers for Disease Control and Prevention. Infection Control Guidance for Healthcare Professionals about Coronavirus (COVID-19) [Accessed June 1, 2020]. Available at: https://www.cdc.gov/coronavirus/2019-nCoV/hcp/infection-control.html .
- 53.World Health Organization. Country &technical guidance - coronavirus disease (COVID-19) [Accessed June 1, 2020]. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/infection-prevention-and-control .
- 54.Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers:a systematic review. PLoS One. 2012;7:e35797. doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raboud J, Shigayeva A, McGeer A, et al. Risk factors for SARS transmission from patients requiring intubation:a multicentre investigation in Toronto, Canada. PLoS One. 2010;5:e10717. doi: 10.1371/journal.pone.0010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tambyah PA. Severe acute respiratory syndrome from the trenches, at a Singapore university hospital. Lancet Infect Dis. 2004;4:690–6. doi: 10.1016/S1473-3099(04)01175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ho PL, Tang XP, Seto WH. SARS:hospital infection control and admission strategies. Respirology. 2003;8(Suppl(Suppl 1)):S41–5. doi: 10.1046/j.1440-1843.2003.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.DuBose JR, Matić Z, Sala MFW, et al. Design strategies to improve healthcare worker safety in biocontainment units:learning from ebola preparedness. Infect Control Hosp Epidemiol. 2018;39:961–7. doi: 10.1017/ice.2018.125. [DOI] [PubMed] [Google Scholar]
- 59.Lim SM, Cha WC, Chae MK, Jo IJ. Contamination during doffing of personal protective equipment by healthcare providers. Clin Exp Emerg Med. 2015;2:162–7. doi: 10.15441/ceem.15.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ong SWX, Tan YK, Sutjipto S, et al. Absence of contamination of personal protective equipment (PPE) by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Infect Control Hosp Epidemiol. 2020;41:614–6. doi: 10.1017/ice.2020.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saini V, Sikri K, Batra SD, Kalra P, Gautam K. Development of a highly effective low-cost vaporized hydrogen peroxide-based method for disinfection of personal protective equipment for their selective reuse during pandemics. Gut Pathog. 2020;12:29. doi: 10.1186/s13099-020-00367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiang Y, Song Q, Gu W. Decontamination of surgical face masks and N95 respirators by dry heat pasteurization for one hour at 70°C. Am J Infect Control. 2020;48:880–2. doi: 10.1016/j.ajic.2020.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zulauf KE, Green AB, Nguyen Ba AN, et al. Microwave-generated steam decontamination of N95 respirators utilizing universally accessible materials. mBio. 2020;11:e00997–20. doi: 10.1128/mBio.00997-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grinshpun SA, Yermakov M, Khodoun M. Autoclave sterilization and ethanol treatment of re-used surgical masks and N95 respirators during COVID-19:impact on their performance and integrity. J Hosp Infect. 2020;105:608–14. doi: 10.1016/j.jhin.2020.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


