Abstract
INTRODUCTION
Stroke is a leading cause of death and disability, with the administration of recombinant transcriptase-plasminogen activator (rtPA) improving outcomes in a time-dependent manner. Only 52.3% of eligible stroke patients at our institution received rtPA within 60 minutes of arrival. We aimed to improve the percentage of acute stroke patients receiving rtPA within 60 minutes of arrival at the emergency department (ED).
METHODS
This study presents results from the first year of a clinical practice improvement project that implemented quality improvement interventions. The primary outcome measure was percentage of acute ischaemic stroke patients receiving rtPA within 60 minutes of arrival at the ED. Secondary outcome measures included components of total door-to-needle (DTN) time and factors for delay to thrombolysis. Interventions were establishment of standardised acute stroke activation guidelines, screening question at ED registration, prehospital notification of stroke activation, public education, scripting for thrombolysis consent and easy access to equipment.
RESULTS
The percentage of patients thrombolysed within 60 minutes increased to 60.6% (p = 0.27), and DTN time decreased from 59 minutes to 54.5 minutes (p = 0.15). This was attributable to reduced door-to-physician time, door-to-imaging time and decision time, although the results were not significant. There was no significant increase in symptomatic intracranial haemorrhage or mortality secondary to stroke. Length of stay was significantly reduced by 1.5 days (p < 0.048).
CONCLUSION
The interventions resulted in an increasing but non-significant trend of acute stroke patients receiving thrombolysis within 60 minutes. Outcomes will be monitored for a longer duration to demonstrate trends and sustainability.
Keywords: acute ischaemic stroke, acute thrombotic stroke, door-to-door treatment, quality improvement, thrombolytic therapy
INTRODUCTION
Intravenous thrombolysis for the management of acute ischaemic stroke is now routine treatment in developed countries and its benefit is time-dependent.(1-3) The American Heart Association/American Stroke Association (AHA/ASA) guidelines for the early management of ischaemic stroke(4) have a Class 1 recommendation to complete stroke evaluation and administration of thrombolytic agent within 60 minutes of arrival at the emergency department (ED), and for 80% of these patients to meet this standard.(5) As per our hospital’s stroke registry, only 52.3% of acute stroke patients in our ED received thrombolysis within 60 minutes of arrival.
This article describes the outcomes of a clinical practice improvement project (CPIP) driven by the ED, neurology and neuroradiology departments at Tan Tock Seng Hospital (TTSH), Singapore. The aim was to create systemic improvements to increase the percentage of acute stroke patients receiving intravenous recombinant transcriptase-plasminogen activator (rtPA) within 60 minutes of arrival at the ED from the existing 52.3% to 80%. We herein describe the interventions, report on the results and discuss challenges faced during the first year of the quality improvement project.
METHODS
TTSH is a 1,400-bed tertiary urban hospital that houses the National Neuroscience Institute, Singapore. Together, they form a major stroke centre in Singapore. The hospital has a dedicated neuroimaging suite that operates at all hours. The study was set up in two phases – the pre-intervention phase (1 January 2014–30 June 2015) and intervention phase (1 July 2015–30 June 2016, the first year of intervention). All ED patients who received rtPA for acute ischaemic stroke during this period were included in the study. Patients who were transferred from other hospitals for thrombolysis were excluded. Data was extracted from the Singapore Stroke Registry and ED electronic medical records. Data collected included patient demographics, mode of presentation, nursing triage acuity, door-to-needle (DTN) time, reasons for delay, complications from thrombolysis and mortality. DTN time was further subdivided into door-to-physician (DTP) time, door-to-imaging (DTI) time and decision time (DT). The study was approved by the National Healthcare Group ethics review board.
Prior to this project, acute stroke patients were managed by the emergency team arbitrarily. The neurology team was activated for acute stroke for patients presenting with symptom duration of 3–4.5 hours. The sets of inclusion and exclusion criteria applied were not standardised. These patients were then reviewed in the resuscitation room before proceeding for imaging. Subsequently, once the imaging did not show any contraindications to thrombolysis, the family was counselled before the drug was administered.
A series of interventions were introduced in our project. First, a multidisciplinary stroke team was formed, comprising a neurologist, neurology advanced nurse practitioner, emergency physician, emergency nurse and neuroradiology nurse. Second, the pre-existing work process was reviewed and CPIP methodology was applied to analyse, design and implement interventions that targeted systemic factors resulting in prolonged DTN times. Within the ED, debriefs were held after every thrombolysed patient, and feedback was sought from the managing team. The core study team then convened every month to discuss patients for whom DTN exceeded 60 minutes and to review plan-do-study-act (PDSA) cycles. A PDSA cycle is a tool used for quality improvement, where the team plans a change, trials it on a small scale, studies the results and refines the change based on what was learned from the trial.
The interventions introduced involved: (a) establishment of standardised stroke activation guidelines; (b) screening for ‘one-sided weakness’ at registration; (c) introduction of prehospital centralised notification for stroke activation; (d) display of multilingual posters at prominent locations in patient care areas; (e) rapid neurological assessment and scripting of consent; and (f) easy access to equipment for thrombolysis.
A survey of ED doctors and nurses showed that the decision of triage to resuscitation and/or stroke activation was arbitrary, with a time frame of 3–4.5 hours and application of variable exclusion criteria. This ambiguity increased time to stroke activation. We decided to standardise the activation criterion to ‘any patient with stroke symptoms presenting within five hours of onset’, which aligned it with prehospital EMS (Emergency Medical Services) criterion. Reminders were broadcast during staff meetings. The ED electronic system was programmed to remind staff of the activation window upon first contact with relevant patients.
ED administrative personnel screened all patients at registration. Screening allowed early identification of time-sensitive emergencies (e.g. all patients who screened positive for chest pain underwent an electrocardiogram before triage, allowing expedient diagnosis of ST-elevation acute myocardial infarction). Triage nurses immediately attended to any patients who screened positive for one-sided weakness to determine the onset of symptoms. Patients with symptom onset within five hours were up-triaged to the resuscitation area for stroke activation. This screening symptom was selected based on the ease of interpretation and translation by non-medically-trained staff and patients. Under this intervention, the hospital’s call centre would broadcast all prehospital notifications for acute stroke to the neurology resident-on-call and neurology advanced nurse practitioner. This made it possible to assemble the stroke team prior to the patient’s arrival for prehospital standby cases and upon the patient’s arrival for non-standby cases.
Posters in the dominant four languages of Singapore’s multiethnic society were placed at prominent locations in the patient care areas to increase patient awareness of stroke. This was in line with a nationwide campaign to increase stroke awareness and the importance of prompt treatment.
The consultant neurologist, who was also the head of the stroke service, coached all neurology senior residents. These sessions were akin to direct observation of procedural skills, where the resident was observed during real-life assessment of a stroke patient. Residents were also equipped with a script for counselling and consent for thrombolysis. Consent for thrombolysis involved verbal consent. This was the first known implementation of verbal consent for thrombolysis in Singapore. A thrombolysis kit was placed in the radiology suite, with instructions and precalculated dosages of rtPA according to body weight.
Throughout the study period, staff meetings were held in participating departments to educate key players and raise awareness of the time-dependent treatment efficacy. The aim was to ingrain a Pavlovian sense of urgency in the staff towards the management of all acute strokes. At the end of the intervention phase, a clinical pathway for the management of acute ischaemic stroke with rtPA was designed and put into practice. Figs. 1 and 2 show the post-intervention workflow for acute stroke.
Fig. 1.
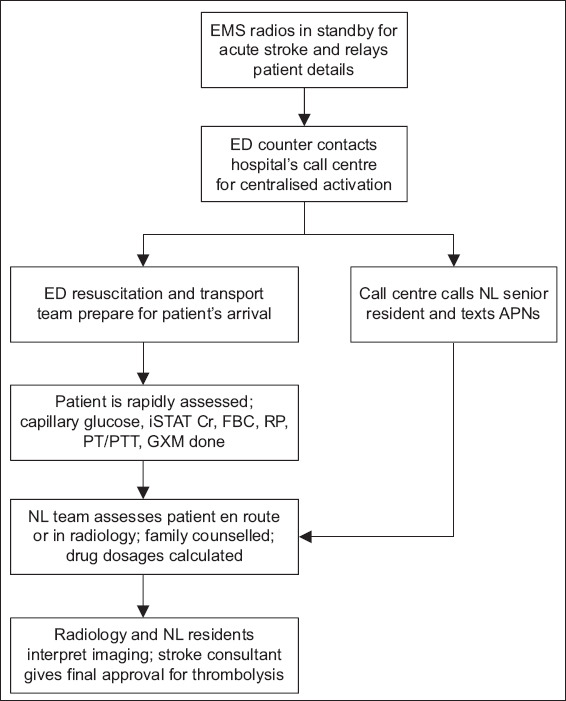
Flowchart shows the resuscitation workflow. APN: advanced practice nurse; Cr: creatinine; ED: emergency department; EMS: Emergency Medical Services; FBC: full blood count; GXM: group and crossmatch; NL: neurology; iSTAT: point-of-care testing iSTAT system; PT/PTT: prothrombin time/partial thromboplastin time; RP: renal panel (consisting of electrolyte, urea and creatinine levels)
Fig. 2.
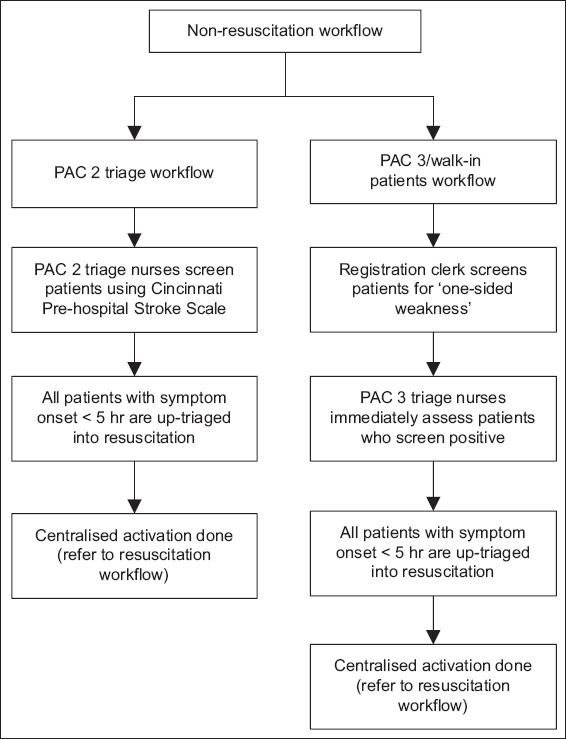
Flowchart shows the non-resuscitation workflow. PAC: patient acuity care
The primary outcome measure was the percentage of acute ischaemic stroke patients receiving rtPA within 60 minutes of arrival at the ED. Secondary outcome measures included the time periods that made up the total DTN (i.e. DTP, DTI and DT), complication rates and mortality. Target DTP, DTI and DT times were ≤ 10 minutes, ≤ 25 minutes and ≤ 20 minutes, respectively. DTP and DTI times were based on AHA/ASA recommendations. Complications included the rates of symptomatic intracranial haemorrhage and other haemorrhagic complications. Symptomatic intracranial haemorrhage was defined as haemorrhage that required reversal agents or neurosurgical intervention, or that led to death within 72 hours. Qualitative analyses were conducted to determine the reasons for delayed thrombolysis in patients who received rtPA during the pre-intervention and intervention phases.
Statistical analyses were conducted using IBM SPSS Statistics version 24.0 (IBM Corp, Armonk, NY, USA). Categorical data was presented as numbers and percentages, while non-normal continuous data was presented as medians and interquartile ranges (IQRs). Chi-square test was used to compare data, with p < 0.05 being considered statistically significant.
RESULTS
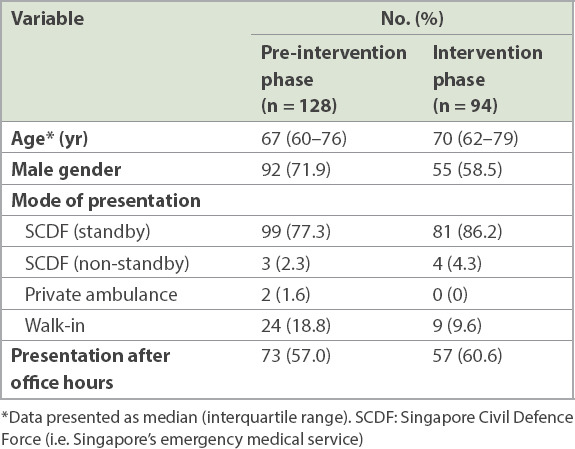
A total of 222 patients received rtPA during the entire study period: 128 patients received it in the pre-intervention phase and 94 patients during the intervention phase. The median age of the patients was 68.5 (IQR 60–77) years and 147 (66.2%) patients were men. Table I shows the patient demographics. Baseline characteristics were similar among patients who received rtPA during the pre-intervention phase and those who received it during the intervention phase.
Table I.
Patient demographics.
The percentage of patients who were thrombolysed within 60 minutes of arrival at the ED increased by 8.3%, from 52.3% in the pre-intervention phase to 60.6% in the intervention phase. The percentage of patients receiving thrombolysis within 45 minutes and 30 minutes of arrival at the ED also increased by 9.9% and 3.7%, respectively. There was a decrease in median DTN time of 4.5 (7.6%) minutes. DTI time decreased by 3.7%, while DT decreased by 15.2%. Unfortunately, DTP time increased by 16.7% (i.e. one minute). To ensure that these interventions did not compromise patient safety, variables such as length of stay, complication rates and mortality were compared. There were no significant differences in symptomatic intracranial haemorrhage and mortality between the patient groups. In the pre-intervention phase, five patients developed other haemorrhagic complications (forehead haematoma, retroperitoneal haematoma, flank haematoma, postsurgical wound bleeding and pelvic intramuscular haematoma); in the intervention phase, six patients developed other haemorrhagic complications (haemoptysis, arachnoid cyst bleed, haemorrhage from scalp laceration, gum bleeding, intravenous cannula site haematoma, back haematoma and groin haematoma). Length of hospital stay was significantly reduced by 1.5 days (p = 0.048). Table II and Fig. 3 show the outcomes and safety measures.
Table II.
Outcomes and safety measures.
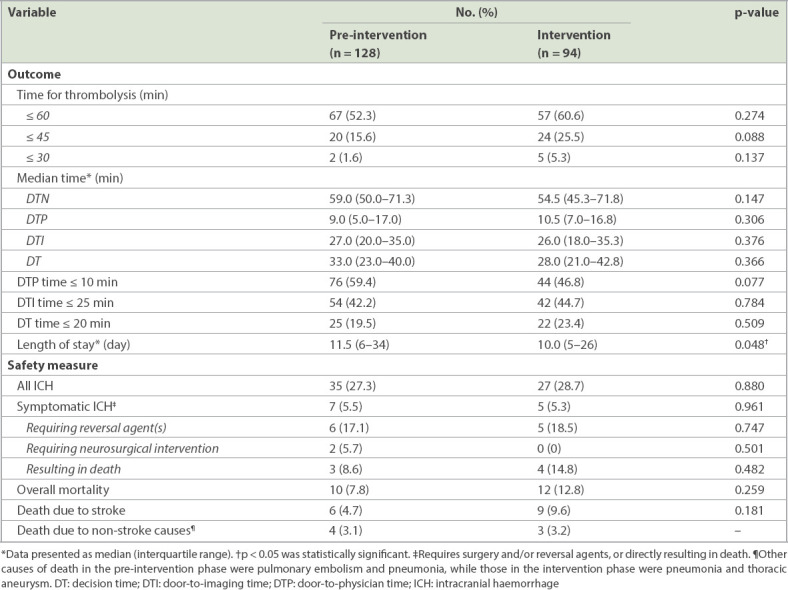
Fig. 3.
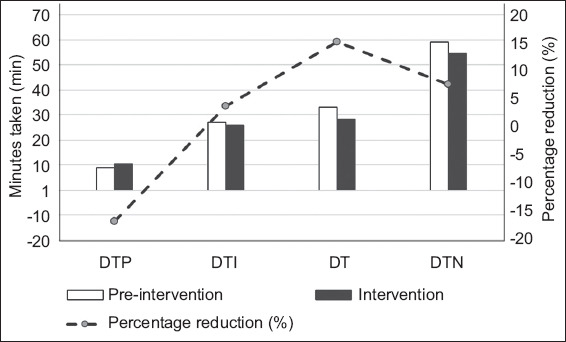
Chart (y1-axis) shows the time taken for DTP, DTI, DT and overall DTN in the pre-intervention and intervention periods. Chart (y2-axis) shows the percentage reduction in DTP, DT, DT and overall DTN in the pre-intervention and intervention periods. DT: decision time; DTI: door-to-imaging time; DTN: door-to-needle time; DTP: door-to-physician time
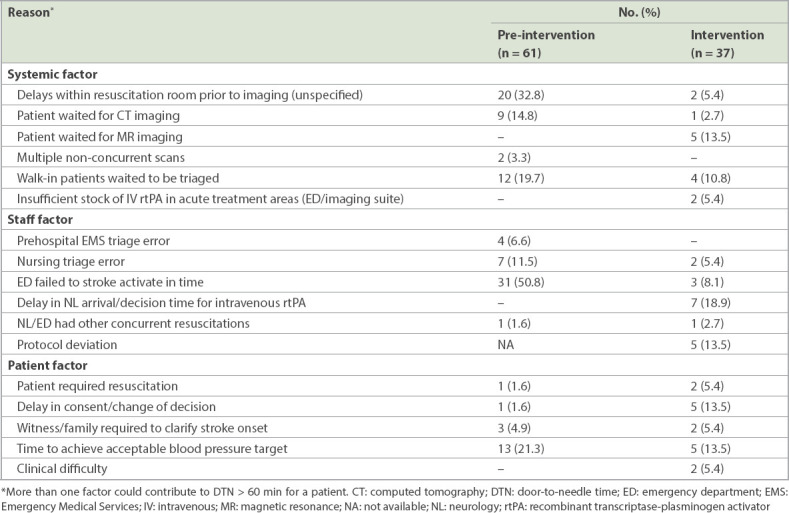
In the pre-intervention phase, 61 (47.7%) patients did not receive thrombolysis within 60 minutes of arrival at the ED. The most common reasons for delayed DTN times were failure of timely activation by the ED (24.2%) and delays within the resuscitation room prior to transfer to neuroimaging (15.6%).
In the intervention phase, 37 (39.4%) patients did not receive thrombolysis within 60 minutes of arrival at the ED. The main reason for the delay was prolonged decision time (18.9%). Other reasons included waiting for magnetic resonance imaging (13.4%), protocol deviation (13.4%), delay or change in consent (13.4%), and time taken to lower blood pressure to within safety limits for thrombolysis (13.4%). Table III shows the reasons for DTN of > 60 minutes.
Table III.
Reasons for DTN > 60 minutes.
DISCUSSION
Many studies have shown that application of quality improvement processes and a standardised operating procedure has improved DTN timings for acute stroke patients. Interventions can be divided into three categories: prehospital, preimaging and postimaging. In the prehospital phase, the following interventions have been implemented – prehospital notification of stroke team,(6-9) patient preregistration in hospital computer systems,(6) conveyance of history from the patient or patient’s family via phone to the neurologist,(6) insertion of the intravenous cannula in preparation for drug administration, and even premixing of rtPA.(6) Upon arrival at the hospital, the patient is often transported directly for computed tomography (CT) scanning, where the assessment takes place.(6,9,10) Many centres have had their CT scanners relocated to the ED.(6) In one study, a countdown clock was attached to the patient trolley as a visual cue for the team.(11) The traditional laboratory coagulation profile was replaced by point-of-care international normalised ratio testing.(6,8-10) After non-contrast CT of the brain was performed, thrombolysis was administered on-table, provided that there were no contraindications.(6-9) Specialised imaging was only performed for patients with diagnostic uncertainty or questionable risk-benefit ratio.(6,9) In recent years, there have also been studies involving prehospital mobile stroke units, which have further reduced the onset-to-thrombolysis timing.(12)
During our CPIP, the team created a flowchart of the process from patient arrival to ED assessment, imaging and thrombolysis. A brainstorming session was held to identify various reasons for delays. Reasons were grouped under general headings and an Ishikawa diagram was created. Root cause analysis was applied and the dominant root causes in the Pareto chart were targeted for intervention. Some of our interventions (e.g. standardisation of stroke activation guidelines, prehospital centralised stroke activation and easy access to equipment) were similar to those in many studies.(6-9)
The addition of a screening question by the registration clerk was unique to our hospital. Due to ease of public access to the hospital, between 10% and 20% of our patients who received thrombolysis were walk-ins. The median triage wait time for our patients was approximately ten minutes. Hence, it was imperative to reduce the triage wait for such patients. The percentage of walk-in patients who were potentially eligible for thrombolysis (if any) was unclear in previous studies.(6-10) This new intervention could be potentially useful for other hospitals with similar patient profiles.
Our results show that the CPIP methodology resulted in a small improvement in the percentage of eligible stroke patients who received thrombolysis within 60 minutes of arrival at the ED and a small reduction in DTN time. However, these improvements were not significant. Of note, the DT was reduced by 15.2%. DT is the time taken for image interpretation, the neurology resident’s phone discussion of the decision for thrombolysis with a neurologist, and drug preparation. As thrombolysis is not without its risks, we decided that it was prudent for the neurologist to make the final decision instead of the neurology resident. The interventions targeted at reducing DT were consent scripting and improving access to thrombolysis equipment. The former directly impacted DT, while the latter was aimed at reducing the time taken to transfer the patient to the imaging suite (i.e. DTI time). No intervention was targeted at the time for image interpretation. Hence, it was likely that the reduction in DT in our study was largely due to the scripting of explanation and consent for rtPA into the residents’ communications with patients and their families. At the same time, the residents gave feedback of increased confidence in their interactions with patients and their families in a fast-paced, highly stressful situation. Studies have shown that a standardised, concise and easily understandable explanation of thrombolysis helps the patient to make an informed decision more rapidly and confidently.(13) In emergent situations where patients are unable to provide informed consent, medical care should be rendered according to the patient’s best interests. Nevertheless, Asian communities are unique in that families are close-knit and often participate in the decision-making process. Hence, consent scripting is extremely useful when counselling family members about treatment. Accepting consent given in a verbal format helped to reduce DT, as families could provide consent over the telephone instead of being physically present.
As this study only examined the data of patients presenting in the first year of intervention, limiting factors included the small study size and retrospective analysis of factors leading to delayed thrombolysis in the pre-intervention group. As data was collected retrospectively, we were unable to define the exact cause of delay for the reasons ‘delays within resuscitation room prior to imaging’ and ‘ED failed to stroke activate in time’. We spoke to the ED staff to gather input and subsequently ran a root cause analysis. The results were similar to those by Ford et al(10) and Schrock and Lum.(14) During the intervention phase, it is possible that the full potential of these interventions was not realised, since the data was collected over a period of 12 months. A longer post-intervention study period would be necessary to assess for results and sustainability.
There were also a number of interventions that did not materialise due to administrative challenges, such as preregistration of patients, prehospital centralised notification for the neuroradiologist and radiographer, as well as a direct-to-CT protocol. The distance between the ED and the radiology suite remained a challenge in our study, as we were unable to make changes to the infrastructure to reduce it.
In conclusion, the intervention applied in our exploratory effort using the CPIP methodology resulted in a trend of improvement of patients’ access to timely thrombolysis following acute stroke. However, the results were not significant, and the outcomes need to be monitored over the longer term to assess their sustainability. Departments need to approach current problems with novel approaches to achieve seamless, rapid and safe care for acute stroke patients, especially given the time-dependent nature of treatment efficacy for this group.
About the First Author

Dr Chiu Li Qi is a Senior Consultant and Emergency Physician at Tan Tock Seng Hospital, Singapore. Her interests are in acute trauma care and trauma education. She completed a fellowship in Trauma and Acute Care Medicine at Sunnybrook Health Sciences Centre and St Michael’s Hospital in Toronto, Canada. Apart from clinical work, she also spends time in quality improvement and patient safety. She is actively involved in the management of quality improvement initiatives in the hospital.
REFERENCES
- 1.Hacke W, Kaste M, Bluhmki E, et al. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.Sandercock P, Wardlaw JM, et al. IST-3 collaborative group. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischemic stroke (the third international stroke trial [IST-3]):a randomised controlled trial. Lancet. 2012;379:2352–63. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke:an updated systematic review and meta-analysis. Lancet. 2012;379:2364–72. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jauch EC, Saver JL, Adams HP Jr, et al. Guidelines for the early management of patients with acute ischemic stroke:a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. doi: 10.1161/STR.0b013e318284056a. [DOI] [PubMed] [Google Scholar]
- 5.Alberts MJ, Hademenos G, Latchaw RE, et al. Recommendations for the establishment of primary stroke centers. Brain Attack Coalition. JAMA. 2000;283:3102–9. doi: 10.1001/jama.283.23.3102. [DOI] [PubMed] [Google Scholar]
- 6.Meretoja A, Strbian D, Mustanoja S, et al. Reducing in-hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79:306–13. doi: 10.1212/WNL.0b013e31825d6011. [DOI] [PubMed] [Google Scholar]
- 7.Kendall J, Dutta D, Brown E. Reducing delay to stroke thrombolysis--lessons learnt from the Stroke 90 Project. Emerg Med J. 2015;32:100–4. doi: 10.1136/emermed-2013-202993. [DOI] [PubMed] [Google Scholar]
- 8.Van Schaik SM, Van der Veen B, Van den Berg-Vos RM, Weinstein HC, Bosboom WM. Achieving a door-to-needle time of 25 minutes in thrombolysis for acute ischemic stroke:a quality improvement project. J Stroke Cerebrovasc Dis. 2014;23:2900–6. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Busby L, Owada K, Dhungana S, et al. CODE FAST:a quality improvement initiative to reduce door-to-needle times. J Neurointerv Surg. 2016;8:661–4. doi: 10.1136/neurintsurg-2015-011806. [DOI] [PubMed] [Google Scholar]
- 10.Ford AL, Williams JA, Spencer M, et al. Reducing door-to-needle times using Toyota's lean manufacturing principles and value stream analysis. Stroke. 2012;43:3395–8. doi: 10.1161/STROKEAHA.112.670687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swartz RH, Sicard MN, Silver FL, et al. University of Toronto Stroke Program Investigators. The CLOQS trial protocol:a cluster-randomized trial evaluating a simple, low-cost intervention to reduce treatment times in acute stroke. Int J Stroke. 2014;9:525–8. doi: 10.1111/ijs.12066. [DOI] [PubMed] [Google Scholar]
- 12.Ebinger M, Kunz A, Wendt M, et al. Effects of golden hour thrombolysis:a Prehospital Acute Neurological Treatment and Optimization of Medical Care in Stroke (PHANTOM-S) substudy. JAMA Neurol. 2015;72:25–30. doi: 10.1001/jamaneurol.2014.3188. [DOI] [PubMed] [Google Scholar]
- 13.Choi HY, Kim EH, Yoo J, et al. Decision-making support using a standardized script and visual decision aid to reduce door-to-needle time in stroke. J Stroke. 2016;18:239–41. doi: 10.5853/jos.2016.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schrock JW, Lum M. Drill down analysis of door-to-needle time of acute ischemic stroke patients treated with intravenous tissue plasminogen activator. Am J Emerg Med. 2014;32:1330–3. doi: 10.1016/j.ajem.2014.08.002. [DOI] [PubMed] [Google Scholar]


