Abstract
INTRODUCTION
Few studies have examined the changes in the prevalence of comorbidity of mental and physical disorders in recent years. The present study sought to examine whether the prevalence of comorbidity of mental and physical disorders in Singapore showed any changes between 2010 and 2016.
METHODS
We extracted data from two repeated nationally representative cross-sectional surveys conducted among resident adults aged ≥ 18 years in Singapore. Significant changes were tested using pooled multinomial logistic regression analyses.
RESULTS
The prevalence of comorbid mental and physical disorders increased significantly from 5.8% in 2010 to 6.7% in 2016. Among those with physical disorders, there were significant increases over time in the prevalence of comorbid generalised anxiety disorder (GAD) (0.1% vs. 0.4%) and obsessive-compulsive disorder (OCD) (1.4% vs. 3.9%) in diabetes mellitus, and alcohol dependence in cardiovascular disorders (0.1% vs. 1.3%). Among those with mental disorders, there were significant increases over time in the prevalence of comorbid diabetes mellitus in OCD (4.1% vs. 10.9%), cancer in major depressive disorder (0.4% vs. 2.4%), and cardiovascular disorders in GAD (0.4% vs. 6.7%) and alcohol dependence (0.9% vs. 11.8%). Significant changes in the overall prevalence of comorbid mental and physical disorders were also observed across age group, education and employment status.
CONCLUSION
The prevalence of comorbid mental and physical disorders increased significantly over time. This finding supports the need for more appropriate clinical management with better integration between mental health and general medical care professionals across all aspects of the healthcare system to treat this comorbidity in Singapore.
Keywords: Asian, comorbidity, mental disorder, physical disorder, survey
INTRODUCTION
Comorbidity of mental and physical disorders – defined as the presence of two or more mental and physical disorders in the same person, regardless of the chronological order in which they occur(1) – has been recognised as one of the most important challenges affecting healthcare systems in the 21st century.(2) Treatment for those with comorbidity requires more intensive clinical management, with better integration of mental health and general medical care professionals across all aspects of the healthcare system.(2-4) Comorbidity of mental and physical disorders has been linked to a variety of adverse health outcomes, including greater role disability, loss of quality of life, and increase in total healthcare use and cost.(5-9) Inclusion of comorbidity in studies examining the burden of mental and physical disorders has been recommended owing to its substantial influence on disease burden and health outcomes.(9) It has been hypothesised that the synergistic effects of comorbid mental and physical disorders on health outcomes can be explained by their underlying shared pathophysiology(10) and the bidirectionality of the relationship between the two conditions.(11) Many epidemiological studies have examined the prevalence of comorbidity of mental and physical disorders, and their sociodemographic correlates and negative impact in the general population. In the United States, a representative epidemiological survey – the National Comorbidity Survey Replication, conducted between 2001 and 2003 – revealed that more than 68% of adults with a mental disorder (diagnosed with a structured clinical interview) reported having at least one physical disorder and that 29% of those with a physical disorder had a comorbid mental disorder.(12) In New Zealand, about 67.7% of the population with mental disorders had at least one chronic physical disorder, as compared to 52.9% in those without mental disorders.(13) In Australia, of those with 12-month mental disorders, 34% had at least one chronic physical disorder. Patients with both comorbid mental and physical disorders reported higher days out of role (5.5 days), as compared to those with either mental (3.2 days) or physical (2.5 days) disorders alone.(14) In Korea, it was found that those with any mental disorder showed a significantly higher prevalence of chronic physical disorders and medical risk factors, including smoking, heavy drinking, overweight and hypertension.(15) The first Singapore Mental Health Study reported higher rates of physical disorder among those with mental disorders as compared to the general population, and that mental illnesses comorbid with other chronic physical illnesses led to significantly greater reductions in health-related quality of life than any mental or medical illness alone.(5) It is also evident that mental and physical disorders are linked to significant losses in quality-adjusted life-years(16) for the society as a whole, and role disability for individuals.(17)
Although several studies have investigated the prevalence, impact and risk factors of comorbidity of mental and physical disorders in the general population, little is known regarding how this prevalence may have changed in recent years. Singapore, a country in Southeast Asia with a resident population of approximately 5.64 million, has one of the world’s fastest ageing multi-ethnic populations, consisting of 74.3% Chinese, 13.4% Malay, 9.0% Indian and 3.2% of other ethnicities.(18) With a rapidly ageing population and the attendant increasing risk of chronic diseases, data on changes in the prevalence of comorbid mental and physical disorders is important for monitoring the evolution and burden of these conditions in the Singapore population. This provides useful information for developing effective health policies and improving resource allocation decisions. Therefore, the current study aimed to examine the changes in the prevalence of comorbidity of mental and physical disorders between two repeated nationally representative surveys – the Singapore Mental Health Study 2010 (SMHS-2010) and the Singapore Mental Health Study 2016 (SMHS-2016).
METHODS
Data was extracted from the SMHS-2010 and SMHS-2016 surveys – serial nationally representative cross-sectional surveys conducted among resident adults aged ≥ 18 years in Singapore. The surveys’ study designs and characteristics of the samples have been described in detail elsewhere.(19,20) In brief, both studies applied a disproportionate stratified random sampling design where face-to-face interviews were conducted with eligible respondents over a one-year period. In the disproportionate stratified random sampling design, 16 strata were constructed based on four ethnic groups (Chinese, Malay, Indian and Others) and four age groups (18–34, 35–49, 50–64 and ≥ 65 years). The sample was randomly selected from each stratum with unequal probabilities and without replacement. Residents aged ≥ 65 years, Malays and Indians were over-sampled to ensure that a sufficient sample size would be achieved to improve the reliability of estimates for the subgroup analysis. In all, 12,742 Singapore citizens and permanent residents participated in both surveys. The studies were approved by the National Healthcare Group’s Domain Specific Review Board. Written informed consent was obtained from all participants and the parents or legally acceptable representatives of those aged below 21 years.
Respondents from both surveys were administered the World Mental Health Composite International Diagnostic Interview (WMH-CIDI),(12) a fully structured diagnostic interview to assess mental disorders and their treatment. Diagnostic modules for lifetime and 12-month prevalence of mood disorders were included: major depressive disorder (MDD); dysthymia and bipolar disorder; anxiety disorders, including generalised anxiety disorder (GAD) and obsessive-compulsive disorder (OCD); and alcohol abuse and dependence. Mental disorders were diagnosed using the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition.(21) WMH-CIDI hierarchy rules were applied to all diagnoses.
All respondents were asked to report any of the physical disorders listed in the checklist using a modified version of the WMH-CIDI checklist of chronic medical disorders. The question was read as, “I’m going to read to you a list of health problems some people have. Has a doctor ever told you that you have any of the following…?” This was followed by a list of chronic disorders that were considered prevalent in Singapore’s population. In order to ensure that the list of disorders was comparable between the two surveys, we included data from 17 individual disorders that was available from both surveys and then re-classified these disorders into ten types of physical disorders: (1) asthma; (2) diabetes mellitus (DM); (3) hypertension and high blood pressure; (4) chronic pain (arthritis or rheumatism, back problems including disk or spine, migraine headaches); (5) cancer; (6) cardiovascular disorders (stroke or major paralysis, heart attack, coronary heart disease, angina, congestive heart failure or other heart disease); (7) ulcer and chronic inflamed bowel (stomach ulcer, chronic inflamed bowel, enteritis or colitis); (8) thyroid disease; (9) neurological condition (epilepsy, convulsions, fainting spells or Parkinson’s disease); and (10) chronic lung disease (chronic bronchitis or emphysema, excluding asthma).
We collected data on gender, age group (18–34, 35–49, 50–64 and ≥ 65 years), ethnicity (Chinese, Malay, Indian and Others), marital status (never married, married, divorced/separated and widowed), educational level (primary and below, secondary, vocational/Institute of Technical Education [ITE], pre-university/junior college/diploma and university) and employment status (employed, unemployed and economically inactive [i.e. students, homemakers and retirees]).
All estimates were weighted to adjust for oversampling and non-response, and post-stratified for age and ethnicity distributions between the survey sample and the Singapore resident population. Descriptive analyses were performed to establish the prevalence of comorbidity of mental and physical disorders, as well as to describe the sociodemographic profile of the SMHS-2010 and SMHS-2016 study populations. Respondents were divided into four mutually exclusive subgroups that were defined as: comorbidity of mental and physical disorders; any mental disorder only; any chronic physical disorder only; and controls (no mental or physical disorder). To examine whether the overall prevalence rate of comorbidity was significantly different between the two surveys, data from the SMHS-2016 study was analysed using a similar sampling weight as that for the SMHS-2010 study. Significant changes in the overall prevalence of comorbidity were tested using chi-square tests, followed by pooled multinomial logistic regression analysis. Year of survey (SMHS-2010 = 0, SMHS-2016 = 1) and sociodemographic variables (including age cohort, gender, ethnicity, marital status, education and employment status) were included as predictors. Subsequently, a significant change in the overall prevalence of comorbidity between the two surveys in terms of sociodemographic groups was further tested in the pooled multinomial logistic regression analyses by adding interaction terms between the year of survey and each demographic variable, with adjustment of sociodemographic factors. Standard errors and significance tests were estimated using the Taylor series linearisation method. All statistical analyses were performed using SAS version 9.3 (SAS Institute Inc, Cary, NC, USA).
RESULTS
The demographic characteristics of the total sample in the two surveys are shown in the Appendix (Supplementary Table I). The proportion of the four mutually exclusive subgroups (comorbidity of mental and physical disorders, mental disorder only, chronic physical disorder only and control) in the SMHS-2016 population was 6.7%, 7.1%, 42.1% and 44.1%, respectively. The proportion of the corresponding four conditions in the SMHS-2010 population was 5.8%, 6.2%, 35.9% and 52.1%, respectively. We found that the prevalence rates of comorbidity of mental and physical disorders, mental disorder only and chronic physical disorder only increased significantly from 2010 to 2016 (p < 0.05; Table I). The year of survey variable remained significant after controlling for all sociodemographic factors in the multinomial logistic regression (Appendix, Supplementary Table II).
Table I.
Proportion of residents with comorbidity of mental and physical disorders, mental disorder and chronic physical disorder in the Singapore Mental Health Study (SMHS) 2010 and 2016.
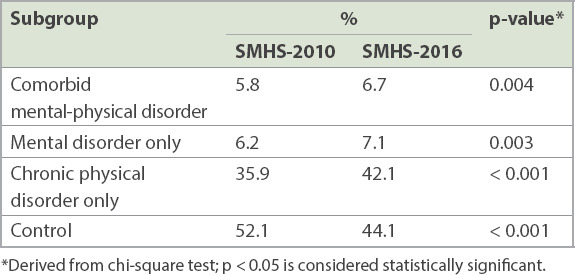
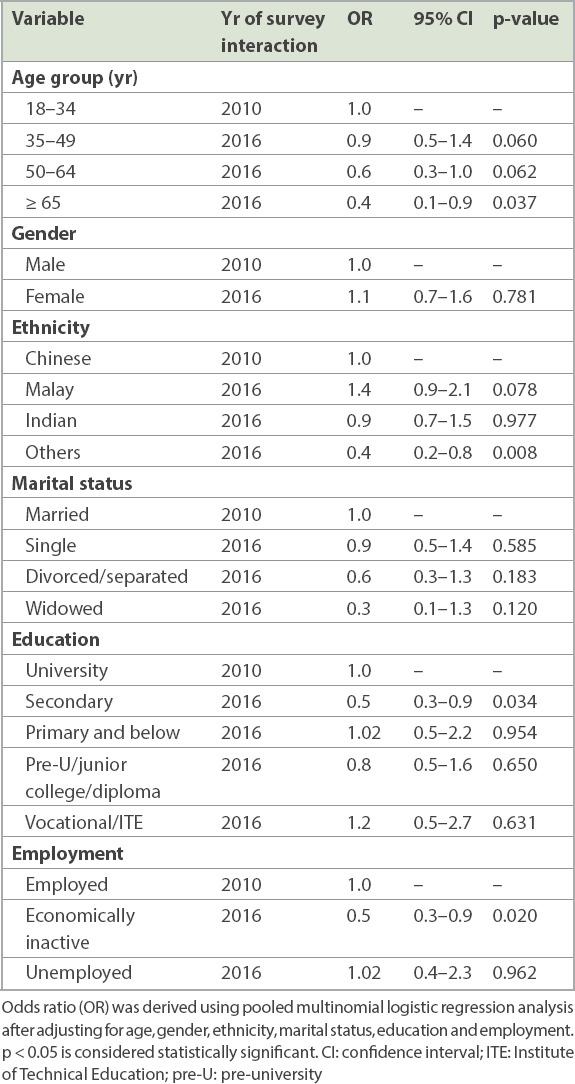
We investigated whether changes in the prevalence of comorbidity of mental and physical disorders over time occurred within the subgroups of demographic variables (Appendix, Supplementary Table III). In the univariate analyses, a significant increase in the prevalence of comorbidity of mental and physical disorders was observed within these subgroups: (a) younger age group (18–49 years); (b) female gender; (c) Chinese, Malay and Indian ethnicities; (d) never married and married statuses; (e) vocational/ITE and university education; and (f) employed status. By contrast, there was a significant decrease in the prevalence of comorbidity of mental and physical disorders among the ‘Others’ ethnic group (Table II). After controlling for all covariates in the multinomial logistic regression, the odds of having comorbidity over time were significantly lower among the following subgroups than their respective reference groups (Table III): (a) aged ≥ 65 years vs. 18–34 years (odds ratio [OR] 0.4, 95% confidence interval [CI] 0.1–0.9); (b) secondary vs. university education (OR 0.5, 95% CI 0.3–0.9); and (c) economically inactive vs. employed (OR 0.5, 95% CI 0.3–0.9).
Table II.
Changes in the prevalence of comorbidity of mental and physical disorders across selected sociodemographic factors.

Table III.
Interaction between year of survey and sociodemographic variables in relation to comorbidity of mental and physical disorders.
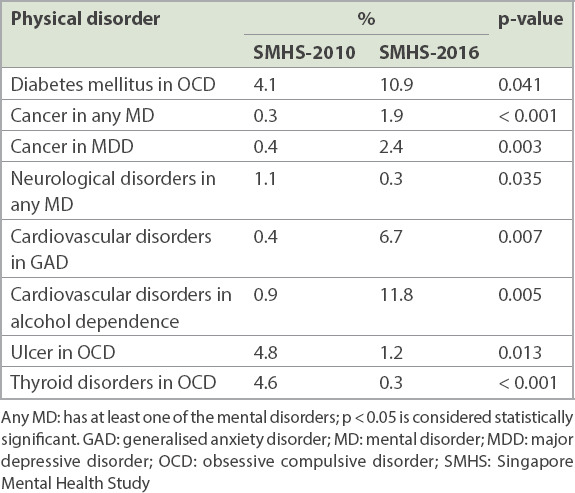
Changes in the prevalence of comorbid physical disorders among individuals with mental disorders are shown in the Appendix (Supplementary Table IV). Among those with mental disorders, the most common comorbid physical disorder in 2016 and 2010 was chronic pain (28.3% and 24.9%, respectively). There was a significant increase over time in the prevalence of comorbid DM in OCD (4.1% vs. 10.9%, p = 0.041); cancer in any mental disorder (0.3% vs. 1.9%, p < 0.001) and MDD (0.4% vs. 2.4%, p = 0.003); and cardiovascular disorders in GAD (0.4% vs. 6.7%, p = 0.007) and in alcohol dependence (0.9% vs. 11.8%, p = 0.005). There was a significant decrease over time in the prevalence of comorbid neurological disorders in any mental disorder (1.1% vs. 0.3%, p = 0.035); ulcer in OCD (4.8% vs. 1.2%, p = 0.013) and thyroid disorders in OCD (4.6% vs. 0.3%, p < 0.001; Table IV).
Table IV.
Changes in the prevalence of chronic physical disorders among individuals with mental disorders.
Among those with physical disorders, the most common comorbid mental disorder in 2016 and 2010 was MDD (6.0% and 6.8%, respectively), as shown in the Appendix (Supplementary Table V). There was a significant increase over time in the prevalence of comorbid GAD in DM (0.1% vs. 0.4%, p = 0.046), OCD in DM (1.4% vs. 3.9%, p = 0.033) and alcohol dependence in cardiovascular disorders (0.1% vs. 1.3%, p = 0.019). By contrast, a significant decrease over time was observed in the prevalence of comorbid dysthymia in asthma (1.0% vs. 0.2%, p = 0.014), OCD in ulcer (6.9% vs. 1.9%, p = 0.024), OCD in thyroid disorders (5.4% vs. 0.4%, p < 0.001), alcohol dependence in asthma (1.3% vs. 0.4%, p = 0.033) and alcohol dependence in ulcer (2.7% vs. 0.2%, p = 0.013; Table V).
Table V.
Changes in the prevalence of mental disorders among individuals with chronic physical disorders.

DISCUSSION
Between 2010 and 2016, we found that the prevalence of overall comorbidity of mental and physical disorders increased significantly (5.8% in 2010 to 6.7% in 2016; p = 0.004). After controlling for all sociodemographic factors in multinomial logistic regression, we found that the prevalence remained significantly higher, suggesting that the increase was not influenced by population growth or changing demographics. This finding is consistent with an earlier analysis, which found that the lifetime prevalence of any mental disorder (i.e. mood, anxiety or alcohol use disorder) increased significantly between 2010 and 2016.(20) Consistent with our findings, cohort studies conducted in the United Kingdom between 2000 and 2014 and in the Netherlands between 1995 and 2010 reported an increase in the prevalence of comorbidity among patients with cardiovascular disease and colorectal cancer over time.(22,23) Significant changes in the overall prevalence were also observed within the subgroups of key sociodemographic variables, including age, education and employment status. Results from this study suggest that the increase in overall prevalence was driven by a younger cohort, those with university education and those who were employed. The working population, especially younger adults aged 18–49 years, was found to have a disproportionately large increase in comorbidity of mental and physical disorders in 2016, as compared to their counterparts in 2010. The significant change in these subgroups is not easy to explain owing to the many factors that may have played a role. Previous studies have suggested that a significant change in comorbidity could be attributable to changes in lifestyle factors, such as increased smoking, low physical activity, heavy alcohol use and unhealthy diet over the years.(24-26) In Singapore, the most common disease burden among young people when they enter late childhood, adolescence and adulthood is non-communicable disease.(27) It was reported that mental disorder, which is a non-communicable disease, represented the largest contributor to disease burden for younger Singaporeans aged 10–34 years, with the leading risk factors of disease burden being diet, tobacco consumption and high blood pressure.(27) Hence, these findings suggest that the increase in comorbidity of mental and physical disorders among the younger population might be associated with changes in behavioural factors, as well as an increasing prevalence of mental disorders in this group. Interestingly, our data shows that those with a comorbid chronic physical condition were less likely to have sought treatment for their mental disorder.(28) This finding might be another possible reason for the increase in the prevalence of comorbidity among young adults, which needs to be further explored in future studies. Lastly, it is plausible that those belonging to the younger age groups may have been particularly vulnerable to economic and psychological stresses, as compared to their counterparts. In addition, we cannot rule out the possibility of factors at the macro level (i.e. rapid changes in the economy and rising unemployment rates in certain sectors), which may have played a significant role; again, further research is needed to confirm this assumption. However, the significant changes in our data, with an increasing prevalence of comorbidity of mental and physical disorders among working-age young adults, certainly deserve further consideration.
An encouraging finding is that the change in comorbidity of mental and physical disorders among older adults decreased significantly from 2010 to 2016, which may reflect increasing access to health services in the older age group and the effectiveness of government initiatives in the Action Plan for Successful Ageing programme, which encourages seniors to stay active, healthy and socially engaged.(29)
Changes in the prevalence of comorbidity of mental and physical disorders of individual disorders showed mixed results. Chronic pain is the most common comorbid physical disorder among individuals with mental illness in Singapore, as shown in both surveys. This finding is consistent with that of previous epidemiological studies, which demonstrated chronic pain as a common problem among the general population.(30,31) However, although more than one in four individuals with mental illness in the population were affected by chronic pain, we found no significant change in the prevalence of this condition over time. Meanwhile, among those with any chronic physical condition, MDD was the most common comorbid mental disorder in Singapore between the two survey periods. This finding is consistent with almost all epidemiological surveys conducted in various countries, which found MDD to be a common and disabling mental disorder.(32) In the current study, we found no significant change in the prevalence of comorbid MDD over time, which is consistent with an earlier analysis that reported no significant increase in the prevalence of MDD between 2010 and 2016.(20) Similarly, Patten et al found that the prevalence of MDD did not increase significantly between 2002 and 2012 in Canada.(33) This was also reported in a study carried out as part of the epidemiological modelling for Global Burden of Disease 2010, which found no evidence for increasing prevalence of depressive disorders between 1990 and 2010.(34) The study postulated that the lack of increase in prevalence over time could be due to the fact that while some risk factors for mental disorders have increased (e.g. relationship breakdowns), others have been mitigated to some degree (e.g. improved living standards leading to higher rates of education, lower levels of illness due to communicable disease and malnutrition).
We found that there was a significant increase in the prevalence of comorbid GAD and DM. Connections between DM and anxiety disorders have fascinated both endocrinologists and mental health professionals for years, ever since a bidirectional association (i.e. both influencing each other in multiple ways) between DM and mental illness has been suggested in the literature.(35) Several studies have also reported that the prevalence of anxiety disorders among patients with DM is considerably higher than that among the general population.(36,37) Increase in the prevalence of comorbid anxiety among individuals with DM could be explained by several factors. Anxiety has been found to be a significant risk factor for the development of DM.(38,39) Ducat et al(40) have suggested that increased anxiety among patients with DM could occur both at the point of diagnosis and when the patient has diabetes-related complications as a result of feeling overwhelmed with the long-term implications and management of the condition and its complications. It has also been suggested that pre-existing anxiety about injections or blood draws may lead to severe anxiety or panic disorders when an individual is diagnosed with DM.(40) Doherty also suggested that anxiety may worsen the prognosis and interfere with an individual’s ability to carry out self-management of DM.(41) For example, anxiety may exacerbate the disabling effect of DM through its influence on treatment adherence and health behaviours.(42) In light of these relationships, the integration of screening into clinical settings with appropriate referrals to qualified mental health professionals has been recommended by international treatment guidelines to improve outcomes among individuals with DM. For example, the American Diabetes Association recommended that DM healthcare providers should consider screening for anxiety in individuals exhibiting anxiety and worries regardless of DM complications and insulin use, and also consider the need for blood glucose awareness training for individuals with hypoglycaemia unawareness, which can co-occur with fear of hypoglycaemia.(43) We also found that there was a significant increase in the prevalence of comorbid OCD and DM. Little research has been conducted to examine the link between OCD and DM. A study that attempted to identify the biological mechanism underlying the aetiology of OCD has linked the condition with dysregulated peripheral insulin signalling (i.e. DM), suggesting that insulin may be linked to several neuropsychiatric disorders.(44) It has also been recommended that clinicians should consider potential drug interaction between the patient’s medical and psychiatric medications during treatment. Paroxetine, a type of selective serotonin reuptake inhibitor prescribed for OCD, has been reported to be associated with weight gain and, hence, is not recommended as the first choice for patients with DM.(45) Similarly, the use of antipsychotics that can worsen glucose control and cause weight gain needs to be carefully considered in this population. Given that the rate of comorbidity between DM and anxiety disorders has increased over time, relevant treatment guidelines should be developed to address comorbidity.
This study has a few limitations that should be considered when interpreting its findings. First, the assessment of chronic physical conditions among the respondents was based on self-report rather than clinical assessment or medical reports. This method of data collection, which has been widely used in other epidemiological studies,(46,47) is found to have acceptable and good concordance with objective data (e.g. general practitioner-reported information and medical records).(48,49) Second, to ensure the comparability of analyses across surveys, we did not include all the chronic physical disorders in the current analyses. However, the excluded disorders made a small contribution to the overall prevalence of comorbidity, while those included in the current analyses accounted for a substantial proportion of comorbidity. Finally, as both studies were cross-sectional surveys, we were unable to attribute causality for the comorbidity between mental and physical disorders.
Despite these limitations, the current study has important implications. The increasing prevalence of comorbidity of mental and physical disorders suggests that these conditions are important contributors to the burden of disease in Singapore’s population. It also supports the need to build systems that treat those with comorbidity appropriately, with better integration between mental health and general medical care professionals across all aspects of the healthcare system to improve the care of comorbidity in Singapore.
ACKNOWLEDGEMENT
The study was funded by the Ministry of Health Singapore and Temasek Foundation.
SUPPLEMENTARY MATERIAL
The Appendix is available online at https://doi.org/10.11622/smedj.2020124.
REFERENCES
- 1.Valderas JM, Starfield B, Sibbald B, Salisbury C, Roland M. Defining comorbidity:implications for understanding health and health services. Ann Fam Med. 2009;7:357–63. doi: 10.1370/afm.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sartorius N. Comorbidity of mental and physical disorders:a key problem for medicine in the 21st century. Acta Psychiatr Scand. 2018;137:369–70. doi: 10.1111/acps.12888. [DOI] [PubMed] [Google Scholar]
- 3.Ngo VK, Rubinstein A, Ganju V, et al. Grand challenges:integrating mental health care into the non-communicable disease agenda. PLoS Med. 2013;10:e1001443. doi: 10.1371/journal.pmed.1001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhugra D, Tasman A, Pathare S, et al. The WPA-Lancet Psychiatry Commission on the Future of Psychiatry. Lancet Psychiatry. 2017;4:775–818. doi: 10.1016/S2215-0366(17)30333-4. [DOI] [PubMed] [Google Scholar]
- 5.Chong SA, Abdin E, Nan L, Vaingankar JA, Subramaniam M. Prevalence and impact of mental and physical comorbidity in the adult Singapore population. Ann Acad Med Singapore. 2012;41:105–14. [PubMed] [Google Scholar]
- 6.Scott KM, Von Korff M, Alonso J, et al. Mental-physical co-morbidity and its relationship with disability:results from the World Mental Health Surveys. Psychol Med. 2009;39:33–43. doi: 10.1017/S0033291708003188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merikangas KR, Ames M, Cui L, et al. The impact of comorbidity of mental and physical conditions on role disability in the US adult household population. Arch Gen Psychiatry. 2007;64:1180–8. doi: 10.1001/archpsyc.64.10.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alonso J, Vilagut G, Chatterji S, et al. Including information about co-morbidity in estimates of disease burden:results from the World Health Organization World Mental Health Surveys. Psychol Med. 2011;41:873–86. doi: 10.1017/S0033291710001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gadermann AM, Alonso J, Vilagut G, Zaslavsky AM, Kessler RC. Comorbidity and disease burden in the National Comorbidity Survey Replication (NCS-R) Depress Anxiety. 2012;29:797–806. doi: 10.1002/da.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodyear IM. The hypothalamic-pituitary-adrenal axis:cortisol, DHEA and mental and behavioral function. In: Steptoe A, editor. Depression and Physical Illness. London: Cambridge Unversity Press; 2007. pp. 280–98. [Google Scholar]
- 11.Cohen S, Rodriguez MS. Pathways linking affective disturbances and physical disorders. Health Psychol. 1995;14:374–80. doi: 10.1037//0278-6133.14.5.374. [DOI] [PubMed] [Google Scholar]
- 12.Kessler RC, Ustün TB. The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI) Int J Methods Psychiatr Res. 2004;13:93–121. doi: 10.1002/mpr.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott KM, Oakley Browne MA, McGee MA, Wells JE New Zealand Mental Health Survey Team. Mental-physical comorbidity in Te Rau Hinengaro:the New Zealand Mental Health Survey. Aust N Z J Psychiatry. 2006;40:882–8. doi: 10.1080/j.1440-1614.2006.01907.x. [DOI] [PubMed] [Google Scholar]
- 14.Teesson M, Slade T, Mills K. Comorbidity in Australia:findings of the 2007 National Survey of Mental Health and Wellbeing. Aust N Z J Psychiatry. 2009;43:606–14. doi: 10.1080/00048670902970908. [DOI] [PubMed] [Google Scholar]
- 15.Kim JH, Chang SM, Bae JN, et al. Mental-physical comorbidity in Korean adults:results from a nationwide general population survey in Korea. Psychiatry Investig. 2016;13:496–503. doi: 10.4306/pi.2016.13.5.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramaniam M, Abdin E, Vaingankar JA, et al. Impact of psychiatric disorders and chronic physical conditions on health-related quality of life:Singapore Mental Health Study. J Affect Disord. 2013;147:325–30. doi: 10.1016/j.jad.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 17.Abdin E, Ong C, Chong SA, Vaingankar JA, Subramaniam M. Days out of role due to mental and physical conditions:results from the Singapore Mental Health Study. PLoS One. 2016;11:e0148248. doi: 10.1371/journal.pone.0148248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Statistics, Singapore. Population Trends 2018. Department of Statistics, Ministry of Trade &Industry, Republic of Singapre. ISSN:2591-8028. [Accessed June 20, 2019]. Available at: https://www.singstat.gov.sg/-/media/files/publications/population/population2018.pdf .
- 19.Chong SA, Abdin E, Vaingankar JA, et al. A population-based survey of mental disorders in Singapore. Ann Acad Med Singapore. 2012;41:49–66. [PubMed] [Google Scholar]
- 20.Subramaniam M, Abdin E, Vaingankar JA, et al. Tracking the mental health of a nation:prevalence and correlates of mental disorders in the second Singapore mental health study. Epidemiol Psychiatr Sci. 2019 Apr 5; doi: 10.1017/S2045796019000179. doi:10.1017/S2045796019000179. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 22.Tran J, Norton R, Conrad N, et al. Patterns and temporal trends of comorbidity among adult patients with incident cardiovascular disease in the UK between 2000 and 2014:a population-based cohort study. PLoS Med. 2018;15:e1002513. doi: 10.1371/journal.pmed.1002513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Leersum NJ, Janssen-Heijnen ML, Wouters MW, et al. Increasing prevalence of comorbidity in patients with colorectal cancer in the South of the Netherlands 1995-2010. Int J Cancer. 2013;132:2157–63. doi: 10.1002/ijc.27871. [DOI] [PubMed] [Google Scholar]
- 24.Fortin M, Haggerty J, Almirall J, et al. Lifestyle factors and multimorbidity:a cross sectional study. BMC Public Health. 2014;14:686. doi: 10.1186/1471-2458-14-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agborsangaya CB, Lau D, Lahtinen M, Cooke T, Johnson JA. Multimorbidity prevalence and patterns across socioeconomic determinants:a cross-sectional survey. BMC Public Health. 2012;12:201. doi: 10.1186/1471-2458-12-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguglia A, Signorelli MS, Albert U, Maina G. The impact of general medical conditions in obsessive-compulsive disorder. Psychiatry Investig. 2018;15:246–53. doi: 10.30773/pi.2017.06.17.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Epidemiology and Disease Control Division, Ministry of Health, Singapore;Institute for Health Metrics and Evaluation. The burden of disease in Singapore, 1990-2017:an overview of the Global Burden of Disease Study 2017 results. Seattle, WA: Institute for Health Metrics and Evaluation; 2019. [Google Scholar]
- 28.Subramaniam M, Abdin E, Vaingankar JA, et al. Minding the treatment gap:results of the Singapore Mental Health Study. Soc Psychiatry Psychiatr Epidemiol. 2019 Jul 17; doi: 10.1007/s00127-019-01748-0. doi:10.1007/s00127-019-01748-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ministry of Health, Singapore. $3 billion action plan to enable Singaporeans to age successfully. [Accessed June 20, 2019]. Available at: https://www.moh.gov.sg/news-highlights/details/3billion-action-plan-to-enable-singaporeans-to-age-successfully .
- 30.Ng KF, Tsui SL, Chan WS. Prevalence of common chronic pain in Hong Kong adults. Clin J Pain. 2002;18:275–81. doi: 10.1097/00002508-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries:gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–91. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 32.Alonso J, Angermeyer MC, Bernert S, et al. ESEMeD/MHEDEA 2000 Investigators, European Study of the Epidemiology of Mental Disorders (ESEMeD) Project. Prevalence of mental disorders in Europe:results from the European Study of the Epidemiology of Mental Disorders (ESEMeD) project. Acta Psychiatr Scand Suppl. 2004;(420):21–7. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- 33.Patten SB, Williams JV, Lavorato DH, et al. Major depression in Canada:what has changed over the past 10 years? Can J Psychiatry. 2016;61:80–5. doi: 10.1177/0706743715625940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baxter AJ, Scott KM, Ferrari AJ, et al. Challenging the myth of an “epidemic”of common mental disorders:trends in the global prevalence of anxiety and depression between 1990 and 2010. Depress Anxiety. 2014;31:506–16. doi: 10.1002/da.22230. [DOI] [PubMed] [Google Scholar]
- 35.Balhara YP. Diabetes and psychiatric disorders. Indian J Endocrinol Metab. 2011;15:274–83. doi: 10.4103/2230-8210.85579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CJ, Chiu HC, Lee MH, Wang SY. Prevalence and incidence of anxiety disorders in diabetic patients:a national population-based cohort study. Gen Hosp Psychiatry. 2011;33:8–15. doi: 10.1016/j.genhosppsych.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Hermanns N, Kulzer B, Krichbaum M, Kubiak T, Haak T. Affective and anxiety disorders in a German sample of diabetic patients:prevalence, comorbidity and risk factors. Diabet Med. 2005;22:293–300. doi: 10.1111/j.1464-5491.2005.01414.x. [DOI] [PubMed] [Google Scholar]
- 38.Engum A. The role of depression and anxiety in onset of diabetes in a large population-based study. J Psychosom Res. 2007;62:31–8. doi: 10.1016/j.jpsychores.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 39.Chien IC, Lin CH. Increased risk of diabetes in patients with anxiety disorders:a population-based study. J Psychosom Res. 2016;86:47–52. doi: 10.1016/j.jpsychores.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Ducat L, Philipson LH, Anderson B. The mental health comorbidity of diabetes. JAMA. 2014;312:691–2. doi: 10.1001/jama.2014.8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doherty AM. Psychiatric aspects of diabetes mellitus. BJPsych Advances. 2015;21:407–16. [Google Scholar]
- 42.Bickett A, Tapp H. Anxiety and diabetes:innovative approaches to management in primary care. Exp Biol Med (Maywood) 2016;241:1724–31. doi: 10.1177/1535370216657613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Young-Hyman D, de Groot M, Hill-Briggs F, et al. Psychosocial care for people with diabetes:a position statement of the American Diabetes Association. Diabetes Care. 2016;39:2126–40. doi: 10.2337/dc16-2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van de Vondervoort I, Poelmans G, Aschrafi A, et al. An integrated molecular landscape implicates the regulation of dendritic spine formation through insulin-related signalling in obsessive-compulsive disorder. J Psychiatry Neurosci. 2016;41:280–5. doi: 10.1503/jpn.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Arlington, VA: American Psychiatric Association; 2007. p. 23. [PubMed] [Google Scholar]
- 46.Alonso J, Petukhova M, Vilagut G, et al. Days out of role due to common physical and mental conditions:results from the WHO World Mental Health surveys. Mol Psychiatry. 2011;16:1234–46. doi: 10.1038/mp.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kessler RC, Aguilar-Gaxiola S, Alonso J, et al. The global burden of mental disorders:an update from the WHO World Mental Health (WMH) surveys. Epidemiol Psichiatr Soc. 2009;18:23–33. doi: 10.1017/s1121189x00001421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kriegsman DM, Penninx BW, van Eijk JT, Boeke AJ, Deeg DJ. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients'self-reports and on determinants of inaccuracy. J Clin Epidemiol. 1996;49:1407–17. doi: 10.1016/s0895-4356(96)00274-0. [DOI] [PubMed] [Google Scholar]
- 49.Hansen H, Schäfer I, Schön G, et al. Agreement between self-reported and general practitioner-reported chronic conditions among multimorbid patients in primary care - results of the MultiCare Cohort Study. BMC Fam Pract. 2014;15:39. doi: 10.1186/1471-2296-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]


