Abstract
Isolated calf deep venous thrombosis (ICDVT) includes thrombosis located at the far end of the popliteal vein, such as the anterior tibial vein, posterior tibial vein, fibular vein, and intramuscular vein of the soleus and gastrocnemius. This type of thrombosis has the highest incidence, accounting for approximately half of all deep vein thrombosis (DVT) cases; however, there is no consistent recommendation for ICDVT treatment across countries, and there is also no optimal management strategy. In recent years, increasing evidence has shown that ICDVT can develop into proximal DVT, even causing pulmonary embolism (PE). Therefore, some experts suggest anticoagulant therapy for this type of DVT, while others hold an opposing attitude. Therefore, the treatment strategy for this type of DVT has become a hot and difficult research topic. The purpose of this review is to summarize the characteristics of ICDVT and the effects of different treatment strategies by analyzing recent and important classical works in the literature in an attempt to provide recommendations for the treatment of this most common type of DVT in orthopaedic clinics.
Keywords: Calf, Deep venous thrombosis, Fracture, Intramuscular vein, Treatment
A total of 726 relevant articles were retrieved, and 36 studies were ultimately included.
Introduction
Currently, the consistent theory holds that DVT of the lower extremities (popliteal vein and its proximal deep vein thrombosis) is prone to cause fatal PE due to the larger diameter of the veins. At present, the Chinese Orthopaedic Association (COA) 1 , 2 , American College of Chest Physicians (ACCP) 3 , and National Institute for Health and Care Excellence (NICE) 4 , 5 in England and other organizations in Europe have formulated relevant guidelines to elaborate anticoagulant methods for DVT of the proximal extremity, and it has been confirmed that the incidence of fatal PE can be reduced only strictly following the guidelines in clinical practice. However, for the treatment of ICDVT with a higher incidence (whether anticoagulation drugs are needed), there is still a lack of clear guidelines, or some suggestions based on a weak level of evidence, such as the 10th edition of VTE anticoagulant treatment guidelines from the ACCP. It was pointed out that, for patients with ICDVT who have no severe symptoms or risks of thrombus progression, the continuous check‐up of venous ultrasound for 2 weeks was recommended instead of anticoagulation (grade 2C). Anticoagulation therapy (grade 2C) was only recommended for patients with severe associated clinical symptoms or having risk factors for thrombus progression (grade 2C), and the anticoagulation protocol and duration were the same as for proximal DVT (grade 2C). The International Consensus Statement on Prevention and Treatment of Venous DVT recommended 3 months of oral anticoagulant therapy for all symptomatic ICDVT cases. The NICE guidelines would not have given the types of DVT treatment recommendations, which might be due to the previous view that this type of DVT rarely spreads proximally and is therefore less likely to cause fatal PE. In addition, the significant bleeding risk associated with anticoagulant therapy was also considered, and there was still heterogeneity in the risk‐benefit analysis of anticoagulant therapy in relevant pieces of literature. Therefore, to date, there is not enough evidence‐based medical evidence to support the need for anticoagulant therapy. However, if anticoagulation is needed, how to determine the optimal treatment interval, on the contrary, what are the nonanticoagulant treatment measures, so the best management measures of isolated distal ICDVT are still indefinite, and research on this issue has always been a hot spot and difficult point in orthopaedics. A large number of relevant studies are needed to illustrate the advantages and disadvantages of anticoagulant and nonanticoagulant therapies for ICDVT to effectively guide the treatment of the most common DVT after a fracture.
Due to the gradual deepening of research in this area, although there is still controversy over anticoagulation, many new pieces of research in recent years have indicated that attention should be given to the diagnosis of ICDVT. As a result, the detection rate should be improved, facilitating early appropriate treatment, and then monitoring its progress. To guide orthopaedic doctors in making more scientific clinical decisions, the subject of the ICDVT is reviewed in detail based on the relevant new and important literature at home and abroad. Therefore, this article focuses on the following issues: (i) the incidence rate and natural progression of ICDVT and the risk of secondary proximal DVT and PE; and (ii) whether anticoagulant therapy is needed. If necessary, what is the optimal anticoagulant duration and the associated probability of bleeding risk? In contrast, what are the other measures? Compared with anticoagulants, can these measures effectively reduce the complications?
Methods
First, we searched the Web of Science, PubMed, and Wanfang databases, as well as the China National Knowledge Infrastructure (CNKI), for related studies without language restrictions from June 2005 to June 2021. The following main search terms were used: (“isolated calf deep venous thrombosis” [Title] OR “venous thrombosis” [Title/MeSH Terms] OR “deep venous thrombosis” [Title/MeSH Terms]) AND (“fracture, bone” [Title/MeSH Terms] OR “calf ” [Title] OR “intramuscular vein” [Title] OR “soleus” [Title] OR “gastrocnemius muscle” [Title/MeSH Terms]) AND (“guideline” [Title/MeSH Terms] OR “expert advice” [Title Terms] OR “consultants” [Title/MeSH Terms]). Then, we also searched the same databases above with the following terms: “postthrombotic syndrome” [Title/MeSH Terms] AND “isolated calf deep venous thrombosis” [Title/MeSH Terms]. The relevant important references were also included, although they might belong to early research. A total of 726 relevant articles were retrieved, excluding duplicate articles, abstracts, letters, and articles unrelated to the theme. In addition to the relevant important references searched manually, 36 studies were ultimately included. The flow chart of the literature search and screening process is shown in Figure 1.
Fig. 1.
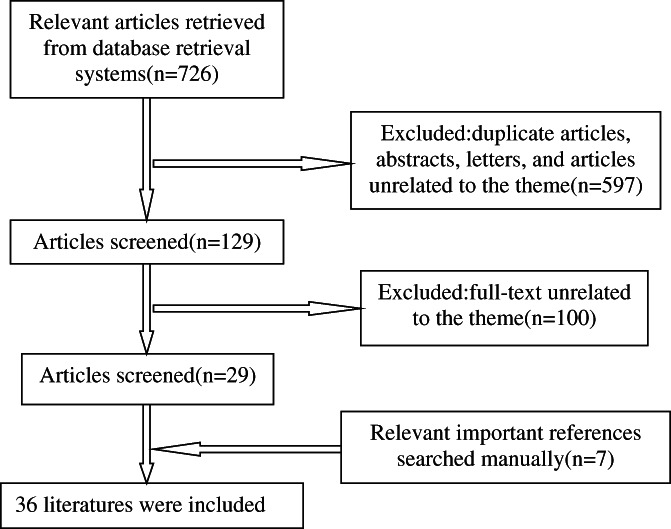
The flow chart of relevant literature retrieval from databases
Incidence, Outcome, and Secondary Risk of ICDVT
ICDVT is consistently considered to be the first step in the formation of proximal DVT, but the incidence rate obtained in each study may vary due to the different diagnostic methods used or different patient groups included. For example, the incidence of this type of DVT based on venography is usually higher than that of venous ultrasound, and the detection rate of symptomatic inpatients or outpatients with suspected DVT or PE was higher than that of asymptomatic patients. However, they generally supported the view that the incidence of ICDVT could reach half of all DVTs. It was estimated that, on average, approximately 300,000 ICDVT cases were detected in the United States each year, of which more than 100,000 required hospitalization. DVT and PE are the third leading cause of vascular death worldwide 6 . A French study 7 found that 56.8% of 1600 symptomatic patients with DVT had a distal calf DVT over 2 years. A relevant study in Japan 8 showed that solitary distal calf DVT accounts for 50% of all cases, many of which were asymptomatic, and was most common in patients undergoing surgery for lower limb fractures (60%–65.3%), followed by patients undergoing major abdominal surgery (20.8%), mainly affecting the distal vein below the popliteal vein. Considering that there might be more patients who have not undergone intravenous ultrasound examination, the actual incidence of asymptomatic distal DVT might be higher in high‐risk patients, including fracture patients and other perioperative patients. An examination of 969 patients suspected of having a DVT in the emergency room in the United Kingdom confirmed that less than 9% of patients were diagnosed with the disease, but acute ICDVT accounted for 49.6% of the total DVTs 9 .
Robert‐Ebadi et al. 10 reported that ICDVT accounted for 30%–50% of all lower extremity DVTs diagnosed by intravenous ultrasound, and the proportion was even higher in outpatients, approximately 60%–70%. However, in hospitalized patients, proximal deep vein thrombosis was dominant (80%), and distal DVT accounted for 20%. However, there were also different research results. For example, Horner 11 evaluated 951 outpatients suspected of having DVT and found that most of them were proximal DVT (104 cases), while ICDVTs were slightly less common, approximately 93 cases, with an incidence of approximately 10.0%. The study with different incidences due to different patient groups also included a study by Spencer et al. 12 , which included approximately 1500 patients in the Worcester community in the United States, with a population base of approximately 500,000 people, and found that most of them (88.9%) were diagnosed with proximal DVT, while only 166 patients (11.1%) had ICDVTs. Palareti et al. 13 performed venous ultrasound examinations of the whole leg in patients with suspected venous thrombus embolism (VTE), and the results showed that the prevalence of ICDVT was 4%–15% and 7%–11% in patients with suspected DVT and PE, respectively, while the proportion of patients diagnosed with DVT was 23%–59%. Another study 14 also suggested that if all patients with suspected DVT received full‐length venous ultrasound examinations of the lower limbs, the incidence of solitary distal calf DVT should account for half of all DVT diagnoses.
Ro 15 conducted an autopsy observation based on 100 patients who died of PE and found that 189 lower limbs had calf vein DVTs. Of these, nearly 50% of patients had ICDVTs, and most of them had fresh thrombi or organic thromboses. No isolated proximal DVTs were found, and the proximal DVTs were dominated by fresh thrombi. In addition, it was found that the incidence of DVTs in the soleus muscle vein was the highest among ICDVTs, which might be related to its frequent dilation and easy occurrence of venous stasis so that thrombosis was more easily formed. A similar study 16 was also found using CT venography (CTV) to detect DVTs in patients with suspected PE. ICDVTs were found in 33% (65/195) of 215 patients undergoing angiography, and calf muscular veins were the most common site. Yao et al. 17 studied the relationship between soleus vein thrombosis and joint replacement surgery and found that 78 (19.4%) of 402 patients had DVTs after total knee or hip replacement.
The main risk of ICDVTs lies in the spread of thrombosis to proximal DVTs or the loss of emboli leading to fatal PE. Therefore, it was necessary to clarify the natural history of this type of thrombosis or the outcome after anticoagulation treatment, as well as the probability of occurrence of the above risk. MacDonald et al. 18 conducted a 3‐month follow‐up of 135 patients with isolated intermuscular venous thrombosis of the calf who were not given anticoagulation therapy and confirmed that 16.3% of the patients had thrombosis progression, among which 3% had popliteal vein thrombosis and 13.3% had calf fibular vein and posterior tibial vein thrombosis. Gillet et al. 19 followed up 128 patients with isolated calf intermuscular venous thrombus for an average of 27 months and found that 19.0% of the patients eventually progressed to proximal DVT or PE (approximately 5.0% of which was PE).
A previous study 20 showed that approximately one‐quarter to one‐third of ICDVTs progressed proximally in the absence of treatment; however, according to the recent literature 21 , 22 , 23 , 24 , 25 , this proportion seemed too high, and the proportion of prolongation in untreated patients was 10% or 8%–15%. A prospective study 26 showed that >90% of patients with ICDVTs without anticoagulation therapy disappeared completely within 7 days after diagnosis, and only 3% of patients developed proximal progression. Singh et al. 27 studied the outcome of 180 ICDVTs in 156 patients with a total follow‐up period of 8 months and found that 9% of DVTs completely dissolved within 72 h and were recurrence‐free. At 1–3 months of follow‐up, 46% of thrombi disappeared completely. Additionally, there were 11 cases (7%) of proximal thrombotic progression and nine cases of PE, but these patients were in the high‐risk groups for secondary orthopaedic surgery, stroke, or malignancy. The remaining patients did not develop proximal DVT or PE at the 6‐ to 8‐month follow‐up. Shimabukuro et al. 8 observed the natural history of 127 cases of asymptomatic ICDVTs without anticoagulant treatment. They were followed up for 3 and 12 months, and ultrasound examinations were used to confirm the recurrence and approach to thrombosis. Finally, only two patients developed proximal DVTs. A Japanese study 28 showed that 3.6% of patients with ICDVTs had progression of proximal venous thrombosis, including some symptomatic patients with anticoagulant therapy. Therefore, it was believed that the incidence of secondary proximal DVTs was approximately 3%–3.7%.
However, recent studies have shown that the risk of proximal DVT or PE secondary to ICDVT might be higher than that in previous studies, although it was still well below that of proximal DVT. Garry et al. 29 summarized the results of five randomized clinical trials and 10 prospective cohort studies and found that the rate of ICDVT progressing to the proximal vein was up to 9%, while the rate of PE was close to 1.5%. They also found no reliable evidence that anticoagulant therapy could reduce the incidence of adverse outcomes. In a large registration‐based study 30 , 1885 patients with isolated symptomatic distal calf DVTs were found to have a 3‐month PE incidence of 0.7%, with a potentially fatal PE rate of 0.3%. In a recent literature report 13 , untreated ICDVTs had a proximal elongation rate between 10% and 15%.
Theoretically, patients with ICDVT had a lower risk of recurrence than patients with proximal DVT 31 . A recent multicenter and observational study showed that cumulative VTE recurrence was 4.8 times higher in patients with proximal DVT than in patients with distal DVT after 5 years of follow‐up 32 . In addition, bilateral isolated DVT has a worse prognosis, with more frequent recurrence, higher mortality, and a more frequent association with malignant tumors 33 , 34 , 35 , 36 . In addition, patients with isolated distal calf DVT also had fewer long‐term complications, such as PTS, than patients with proximal DVT. The study showed that, in the 5‐year long‐term follow‐up, approximately 37% of patients with symptomatic isolated distal calf DVT showed symptoms and signs of venous insufficiency, while 11% of patients developed PTS. However, a recent prospective study 36 showed that proximal DVT was more likely to cause long‐term PTS complications, and the risk was approximately 2.3 times higher than that of distal DVT.
However, there are still few studies on ICDVT following fracture of the lower limb. A multicenter prospective cohort study 37 investigated the three‐month incidence of symptomatic venous thrombosis in 1200 patients with knee‐to‐leg fractures without anticoagulant therapy. The majority of these patients were treated nonoperatively (treated with cast or splint immobilization), and only three patients were confirmed to have symptomatic ICDVT, with an incidence of 0.26%. A Danish nationwide registry study 38 of 57,619 patients undergoing surgery for fractures far from the knee found a 1.0% incidence of VTE within 180 days without anticoagulant therapy, suggesting that the incidence of isolated DVT in the distal leg was also less than 1.0%. Wang 39 studied the incidence of preoperative DVT in 1825 patients with isolated lower extremity fractures. On average, 3.5 days after injury, 64 of 159 patients with femoral shaft fractures developed DVT (40.3%), including 19 proximal DVTs (11.9%) and 45 distal DVTs (28.3%). Qu 40 found that the incidence of preoperative DVT for femoral fracture was 77.9%, and most were ICDVTs (93.6%). Li 41 found that, in 35 cases of femoral shaft fractures, DVT occurred in 10 cases (28.6%) within 24 hours before the operation, including seven cases of ICDVT. Zhang 42 found that nine of 32 femoral shaft fractures had DVTs (28.1%) before the operation, and the incidence was the highest in lower limb fractures. Guo 43 screened 39 cases of femoral shaft fracture with multi‐slice spiral CT venography (MSCTV) before surgery and found that 13 cases (33.3%) had DVT, most of which were ICDVTs. According to an epidemiological survey 44 in a large sample of fracture patients (24,049 cases, excluding hand and spine fractures), the incidence of DVT in femoral shaft fractures was 14.7% (178/1209), which was higher than 12.9% in hip fractures and 7.31% in pelvis and acetabulum fractures.
The incidence of preoperative DVT in the femoral shaft was more reported in the previous relevant literature, and the data were consistent. Most researchers believe that the probability of preoperative DVT is higher, even higher than that of hip fracture, and distal DVT is more common than proximal DVT.
There are three types of fractures around the knee: distal femoral fractures, tibial plateau fractures, and patellar fractures. Zhang 45 reported the incidence of preoperative DVT in 160 patients with distal femoral fractures over 65 years old. The incidence rate was 52.5% (84/160) of which ICDVT accounted for 26.3% and was the most common type. Lv 46 showed that the incidence of DVT around the knee was 11.6%, including 21.9% (21/96) of femoral intercondylar supracondylar fractures and 19.0% (19/248) of tibial plateau fractures, and ICDVTs were more common than proximal DVTs. Zang 44 found that the highest incidence of DVT was supracondylar and intercondylar fractures of the femur, which were 23.04% (165/716), even higher than 14.72% (178/1209) of femoral shaft fractures, and ICDVTs were also considered the most common. Another study 39 showed that the incidence of proximal DVT and ICDVT was 3.4% and 20.5%, respectively, and the total DVT rate was 23.9% in 176 tibial plateau fractures. The incidence of DVT was higher in tibial plateau fractures without prophylaxis, up to 43% 47 . Xiao 48 compared the preoperative incidence of DVT in tibial plateau fractures (50 cases) and patellar fractures (50 cases) and found that the incidence in tibial plateau fractures was 52%, while it was 30% in patellar fractures. The thrombosis was mostly located in the anterior tibial vein and posterior tibial vein. A study 49 based on preoperative ultrasound examination of 114 patients with patellar fractures showed that 25 cases (21.9%) had DVT, including 24 cases (96.0%) of ICDVT and only one case of proximal DVT.
Gao 50 found that 51 of 178 (28.65%) cases with tibiofibular fracture developed DVT, and ICDVTs accounted for 86.0% of them. Another study 51 with a similar number of cases showed that 39 (21.7%) patients had preoperative DVTs, and 38 patients had ICDVTs. Other studies 41 , 42 , 43 reported that the incidence was lower, approximately 2.86%–11.5%.
In conclusion, lower extremity venous thrombosis is closely related to the specific fracture site and type and may even be related to individual factors of the patient. Different patients will have very different incidences of thrombosis; however, current studies consistently believe that patients with fractures have a higher incidence of ICDVTs than proximal DVTs; therefore, the progression of ICDVTs requires close follow‐up, especially for fracture types prone to ICDVTs, such as femur and hip fractures. The incidence of ICDVT at different fracture sites is shown in Table 1.
TABLE 1.
The incidence of ICDVT at different fracture sites
| Fracture types | Literature numbers | Cases of fractures | Incidence of preoperative ICDVT (%) |
|---|---|---|---|
| Femoral shaft fracture | 6 | 3249 | 14.7–72.9 |
| Fracture around knee joint | 7 | 1610 | 11.6–43.0 |
| Fracture of tibia and fibula | 5 | 502 | 2.86–24.6 |
| Ankle fracture | 2 | 58,819 | 0.26–1.00 |
Abbreviation: ICDVT, isolated calf deep venous thrombosis.
Optimal Treatment: Anticoagulant or Nonanticoagulant
Whether anticoagulant therapy is needed for ICDVT is the focus of current debate, but there is no optimal treatment strategy. Some scholars have recommended anticoagulant therapy, while others believe that anticoagulant therapy was not needed. Schwarz et al. 52 carried out the first prospective study of low molecular weight heparin therapy for ICDVT; 52 patients in the treatment group were treated with low molecular weight heparin, while 32 patients in the control group were treated with conventional compression stockings only. At 3 months of follow‐up, the results showed no proximal spread of thrombosis in the anticoagulation group, while the proximal spread risk and recurrence rate in the control group were significantly higher than those in the anticoagulant group; no hemorrhagic events or PEs occurred in the two groups. Therefore, short‐term anticoagulation was recommended for these patients. Utter 53 studied the relationship between anticoagulant therapy and reducing the risk of proximal spread or PE caused by ICDVT. The author treated 243 patients with warfarin or low molecular weight heparin, and the remaining 141 patients with no anticoagulant therapy. The follow‐up period was 180 days, and four cases (1.6%) in the anticoagulation group and seven cases (5.0%) in the control group developed proximal DVT. PE occurred in four patients in the anticoagulant group and six patients in the control group. Overall, proximal DVT or PE occurred in 13 patients (9.2%) in the control group and eight patients (3.3%) in the anticoagulant group. Anticoagulant therapy was associated with a lower risk of proximal DVT or PE, with an RR of 0.36. Therefore, the author believed that anticoagulation therapy could indeed significantly reduce the risk of VTE within 180 days in these patients but might increase the risk of bleeding. Franco et al. 54 found that, compared with patients who did not receive anticoagulant therapy, patients who received anticoagulation therapy had a lower recurrence rate of venous thromboembolism and a lower incidence of PE (2936 patients; OR 0.50, 95% CI 0.31–0.79), without an increased risk of major bleeding (OR 0.64, 95% CI 0.15–2.73). Therefore, anticoagulant therapy for more than 6 weeks was recommended. Another study 11 showed that the incidence of major clinical outcomes (proximal thrombosis spread, pulmonary embolism, or major bleeding) within 90 days in 35 ICDVT anticoagulant groups was 11% and 0%, respectively, compared with the same number of patients in the conservative treatment group. The anticoagulation group showed a significant advantage without the related risk of major bleeding. Lautz et al. 55 studied the treatment effect of 406 isolated gastrocnemii and soleus venous thrombosis (IGSVT) with a follow‐up time of 7.5–8 months. The results showed that the incidence of VTE was the lowest among patients receiving the treated amount of anticoagulants, which was approximately 12% (23/188), which was significantly lower than 27% (13/48) in the prophylactic anticoagulation group and 30% (36/119) in the nonanticoagulation group. It was believed that such patients should be treated with anticoagulation. The study of Yoon et al. 56 also obtained similar results. Their study on 647 cases of ICDVT showed that the complications of VTE in the treatment anticoagulant group, prevention anticoagulant group, and nonanticoagulant group increased successively, accounting for 10%, 30%, and 35%, respectively, with statistical significance (p = 0.0003). It was suggested that anticoagulant therapy could significantly reduce the incidence of VTE.
However, some scholars did not support anticoagulant therapy. A similar study by Sales et al. 57 included the anticoagulant efficacy of 141 patients with intermuscular venous thrombosis of the calf muscles and found that anticoagulant therapy had no clinical advantage. The authors suggested that intravenous ultrasound monitoring should be strengthened for such patients to detect the proximal spread of DVT as early as possible and to get out of bed at an early stage. Righini et al. 58 conducted a randomized, double‐blind, placebo‐controlled trial involving multiple national medical centers, in which 122 patients with ICDVT were treated with nadroparin anticoagulation vs 130 patients treated with 0.9% normal saline in the control group. At the end of follow‐up (6 weeks), there was no significant difference in the incidence of proximal DVT or PE between the two groups; the incidence was 3% in the anticoagulant group and 5% in the placebo group. However, the bleeding rate was 4% in the anticoagulant group and 0% in the placebo group; therefore, the authors concluded that anticoagulant therapy did not reduce the secondary risk of symptomatic DVT but rather increased the risk of bleeding. The result of another randomized controlled trial 59 showed that the clinical effect of low molecular weight heparin plus compression stockings was comparable to that of compression stockings alone in treating low‐risk calf DVT for 3 months, and the former showed no significant advantage. This trial included a total of 107 patients with calf muscle venous DVT, most of whom were outpatients (89%), and only 11% of patients were hospitalized. After 3 months of treatment, proximal DVT occurred in two patients (3.7%) in the heparin group and 3.8% in the nonanticoagulant group, with no hemorrhagic events between the two groups.
Some scholars recommend selective anticoagulation. The study by Singh et al. 27 included 156 patients with 180 ICDVTs, and all patients were treated with low molecular weight heparin for anticoagulation. During 1–3 months of follow‐up, 11 patients (7.1%) developed proximal DVTs, and nine patients (5.8%) developed PEs. However, these patients were all at high risk for DVT, including complications of cerebrovascular diseases, malignant tumors, long‐term immobilization, or orthopaedic surgery. Therefore, the authors recommended anticoagulation therapy for these patients, while asymptomatic patients did not need anticoagulation. Shimabukuro 8 also supported the former view and followed up 127 patients with asymptomatic ICDVT who did not receive anticoagulant treatment. After 3 months and 1 year, ultrasound examination confirmed that there was no recurrence of venous thromboembolism, and only two patients had proximal DVT spread. Therefore, because of the good prognosis of asymptomatic ICDVTs, uniform anticoagulant therapy was not necessary. A recent high‐quality study by Palareti 14 stated the strategy for the treatment of ICDVT in the University Hospital of Bologna, Italy, and the author denied the current treatment model and anticoagulation time, that was, ultrasound monitoring for 2 weeks or anticoagulant therapy only if the proximal spread was detected. Once anticoagulation was started, patients were maintained on an anticoagulant for 3 months. He reviewed and summarized the experience of his medical institutions in the treatment of such patients and thus proposed a relatively new treatment strategy: all patients with symptomatic ICDVT were given anticoagulant treatment, but the choice of anticoagulant drugs and treatment time were different, combined with wearing elastic socks under the knees. The specific plans were as follows: (i) patients with the following conditions need to be given low molecular weight heparin and warfarin combined anticoagulation for 3 months, such as those with a previous history of VTE, primary thrombosis or secondary thrombosis requiring prolonged immobilization; thrombus during pregnancy or puerperium; thrombosis of more than one calf vein; thrombosis in both legs; combined with cancer or undergoing chemotherapy; combined with susceptible diseases, such as inflammatory bowel disease, or a known tendency to embolize; and (ii) patients with the following conditions would be given a short‐term anticoagulation strategy (first, given a sufficient amount of low molecular weight heparin for 7–10 days, then reduced to half dose, with anticoagulation treatment for 30 days): limb immobility secondary to surgery or other factors, such as plaster fixation, trauma or prolonged travel, oral contraceptives, or hormone replacement drugs. Finally, the author believed that, in the future, the use of oral direct anticoagulant drugs, such as rivaroxaban and other single‐drug therapies, would be superior to combination drugs, but the optimal dose and duration time of this drug still need to be further studied. Horner 9 , another expert from the University Hospital of Manchester, UK, also believed that ICDVTs accounted for half of all DVTs, and in the conservative treatment of patients, the extension rate was as high as 10%. Additionally, the embolization rate was 1%–3%, so he also tended to use direct oral anticoagulant agents (DOACs) with fewer complications, such as bleeding, in high‐risk patients with cancer, pregnancy, or previous primary thrombosis for at least 6 weeks. We agree with Palareti and Horner that individualized treatment should be taken for different fracture patients according to their characteristics. Patients at high risk of ICDVT should be given anticoagulant therapy with the same duration of treatment as a proximal DVT. Anticoagulant therapy is not recommended for the rest of the patients at low risk for ICDVT, but early physical activity and periodic review should be encouraged to monitor their progress until at least 6 weeks.
At present, for patients with ICDVT who have contraindications to anticoagulant drugs, elastic compression stockings (ECSs) are currently generally used as adjuvant treatments, which aim to reduce limb swelling, pain, and the incidence of post‐thrombotic syndrome (PTS). A previous study 60 showed that wearing ECS providing 40 mmHg of foot and ankle pressure within 2–3 weeks after a diagnosis of DVT could significantly reduce the incidence of PTS in patients. Among 96 patients who wore ECS, 30 (31%) developed PTS after 2 years of follow‐up, while the incidence of PTS in patients without ECS was as high as 70%. Recent studies 61 , 62 , 63 have shown relatively consistent results in that wearing compression stockings providing a pressure of 30–40 mmHg after a diagnosis of thrombosis could halve the incidence of PTS after 2 years (from approximately 50.0% to 24.5%). However, a recent 64 randomized placebo‐controlled trial involving 806 patients with DVT at 24 centers in the United States and Canada yielded opposite results. Among them, 410 patients were treated with ECS for 2 years, and 396 patients were used as controls. At the end of follow‐up, the ECS group was not superior to the nonwearing group in the prevention of PTS, with incidences of 14.2% (ESC group) and 12.7% (placebo group), respectively. Therefore, it was not recommended to routinely wear ECS after DVT to prevent PTS.
Because the current relationship between ICDVT and PTS is unclear or there is heterogeneity in related studies, it is generally agreed in clinical practice that ECS can significantly reduce the symptoms of pain and edema of the affected limb in such patients after diagnosis 64 , 65 . However, whether this measure can reduce the incidence of PTS in patients with ICDVT in the long term is still unclear and is still being studied. Therefore, the evidence for using ECS to reduce the complications of PTS in such patients is insufficient. At present, for patients with ICDVT who have contraindications to anticoagulant drugs, ECS is still recommended to alleviate related symptoms.
In conclusion, ICDVT is a common complication in orthopaedic trauma, and the incidence can reach half of all DVTs. Although it is less likely to cause fatal PE than proximal DVT, it is not rare. Theoretically, proximal extension or secondary PE can also occur and may also cause difficult clinical treatment of PTS complications in the long term. At present, there are clear guidelines for the treatment strategies of proximal DVTs at home and abroad, but for this more common type of DVT, there is no unified optimal treatment management strategy due to the lack of evidence‐based medical evidence. However, it is gratifying that an increasing number of experts in various countries have paid more attention to the treatment of this type of thrombosis, and corresponding research is also being vigorously carried out. Any research that helps to formulate the best management strategy is welcome, and it is believed that there will be specific guidelines for the treatment of ICDVT soon.
In this article, by reviewing the previous relevant literature, we summarized and analyzed the relevant research results about ICDVT, which is more common in orthopaedic clinics, and there is no unified optimal management opinion in the guidelines. We recommend individualized treatment according to the characteristics of different fracture patients. Patients at high risk of ICDVT should receive the same course of anticoagulant therapy as proximal DVT. For other patients with a low risk of ICDVT, anticoagulant therapy is not recommended, but early physical activity should be encouraged, and attention should be given to regular review to monitor their progress until at least 6 weeks. The review will help orthopaedic surgeons gain a deeper understanding of this disease or enlighten and broaden the horizons of follow‐up research.
Author Contributions
Weiguang Zhao and Yingze Zhang conceived the idea for the study; Weiguang Zhao designed the study. Wei‐guang Zhao, Ji‐ying Yan,Xiao‐lei Li, Cai‐ying Shi, Zhi‐yun Wang, Wei Guo, Kai Zhang,Wei‐li Zhang, Xiao‐chuan Jia, Shu‐bei Cui collected the relevant data. Li‐qiang Jiang, Jian‐long Zhao, Zhen‐wu Liu, Zhao‐hui Yang, Li Liu interpreted the data and contributed to preparation of the manuscript.
Conflict of Interest
All the authors stated they had no conflicts of interest.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Acknowledgments
We thank doctors who took part in this study. We also thank all friends for their kind assistance in the revision of the article.
References
- 1. The group of orthopedics of trauma, Chinese orthopedics Association, the group of external fixation and limb reconstruction, Chinese orthopedics Association, the working committee of trauma experts, Chinese orthopedics Association,the group of trauma infection, Chinese orthopedics Association , Lin Q, Yang M, etc. Guidelines for the prevention of venous thromboembolism in Chinese orthopedic trauma patients (2021). Chinese Journal of orthopedic trauma, 2021;23:185–192. [Google Scholar]
- 2. Orthopedics Branch of Chinese Medical Association . Guidelines for prevention of venous thromboembolism in major orthopedic surgery in China. Chin J Orthop. 2016;2:65–71. [Google Scholar]
- 3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 4. Venous thromboembolic diseases: diagnosis, management, and thrombophilia testing. London: National Institute for Health and Care Excellence (UK); 2020. p. 26. [Google Scholar]
- 5. Venous thromboembolism in over 16s: reducing the risk of hospital‐acquired deep vein thrombosis or pulmonary embolism. London: National Institute for Health and Care Excellence (UK); 2019. p. 13. [PubMed] [Google Scholar]
- 6. Nicholson M, Chan N, Bhagirath V, Ginsberg J. Prevention of Venous Thromboembolism in 2020 and Beyond. J Clin Med. 2020;9:2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galanaud JP, Sevestre‐Pietri MA, Bosson JL, et al. Comparative study on risk factors and early outcome of symptomatic distal versus proximal deep vein thrombosis: results from the OPTIMEV study. Thromb Haemost. 2009;102:493–500. [DOI] [PubMed] [Google Scholar]
- 8. Shimabukuro N, Mo M, Hashiyama N, et al. Clinical course of asymptomatic isolated distal deep vein thrombosis of the leg: a single‐institution study. Ann Vasc Dis. 2019;12:487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horner D, Hogg K, Body R. Should we be looking for and treating isolated calf vein thrombosis? Emerg Med J. 2016;33:431–7. [DOI] [PubMed] [Google Scholar]
- 10. Robert‐Ebadi H, Righini M. Management of distal deep vein thrombosis. Thromb Res. 2017;149:48–55. [DOI] [PubMed] [Google Scholar]
- 11. Horner D, Hogg K, Body R, Nash MJ, Baglin T, Mackway‐Jones K. The anticoagulation of calf thrombosis (ACT) project: results from the randomized controlled external pilot trial. Chest. 2014;146:1468–77. [DOI] [PubMed] [Google Scholar]
- 12. Spencer FA, Kroll A, Lessard D, et al. Isolated calf deep vein thrombosis in the community setting: the Worcester Venous Thromboembolism Study. J Thromb Thrombolysis. 2012;33:211–7. [DOI] [PubMed] [Google Scholar]
- 13. Palareti G, Sartori M. Treatment of isolated below the knee deep vein thrombosis. Curr Atheroscler Rep. 2016;18:37. [DOI] [PubMed] [Google Scholar]
- 14. Palareti G. How I treat isolated distal deep vein thrombosis (IDDVT). Blood. 2014;123:1802–9. [DOI] [PubMed] [Google Scholar]
- 15. Ro A, Kageyama N. Clinical significance of the soleal vein and related drainage veins, in calf vein thrombosis in autopsy cases with massive pulmonary thromboembolism. Ann Vasc Dis. 2016;9:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshimura N, Hori Y, Horii Y, Takano T, Ishikawa H, Aoyama H. Where is the most common site of DVT? Evaluation by CT venography. Jpn J Radiol. 2012;30:393–7. [DOI] [PubMed] [Google Scholar]
- 17. Yao Y, Qiao L, Song K, et al. Preoperative evaluation of soleal vein diameter by ultrasound is beneficial for prophylaxis of deep vein thrombosis after total knee or hip arthroplasty. Biomed Res Int. 2018;19:3417648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Macdonald PS, Kahn SR, Miller N, Obrand D. Short‐term natural history of isolated gastrocnemius and soleal vein thrombosis. J Vasc Surg. 2003;37(3):523–7. [DOI] [PubMed] [Google Scholar]
- 19. Gillet JL, Perrin MR, Allaert FA. Short‐term and mid‐term outcome of isolated symptomatic muscular calf vein thrombosis. J Vasc Surg. 2007;46:513–9. discussion 519. [DOI] [PubMed] [Google Scholar]
- 20. Kearon C. Natural history of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I22–30. [DOI] [PubMed] [Google Scholar]
- 21. Patel P, Patel P, Bhatt M, et al. Systematic review and meta‐analysis of outcomes in patients with suspected pulmonary embolism. Blood Adv. 2021;5:2237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel P, Patel P, Bhatt M, et al. Systematic review and meta‐analysis of outcomes in patients with suspected deep vein thrombosis. Blood Adv. 2020;4:2779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chopard R, Albertsen IE, Piazza G. Diagnosis and treatment of lower extremity venous thromboembolism: a review. JAMA. 2020;24:1765–76. [DOI] [PubMed] [Google Scholar]
- 24. Tritschler T, Kraaijpoel N, Le Gal G, Wells PS. Venous thromboembolism: advances in diagnosis and treatment. JAMA. 2018;320:1583–94. [DOI] [PubMed] [Google Scholar]
- 25. Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet. 2021;10:S0140‐6736(20)32658‐1. [DOI] [PubMed] [Google Scholar]
- 26. Righini M, Bounameaux H. Clinical relevance of distal deep vein thrombosis. Curr Opin Pulm Med. 2008;14:408–13. [DOI] [PubMed] [Google Scholar]
- 27. Singh K, Yakoub D, Giangola P, et al. Early follow‐up and treatment recommendations for isolated calf deep venous thrombosis. J Vasc Surg. 2012;55:136–40. [DOI] [PubMed] [Google Scholar]
- 28. Schellong SM, Goldhaber SZ, Weitz JI, et al. Isolated distal deep vein thrombosis: perspectives from the GARFIELD‐VTE registry. Thromb Haemost. 2019;119:1675–85. [DOI] [PubMed] [Google Scholar]
- 29. Garry J, Duke A, Labropoulos N. Systematic review of the complications following isolated calf deep vein thrombosis. Br J Surg. 2016;103:789–96. [DOI] [PubMed] [Google Scholar]
- 30. Galanaud JP, Quenet S, Rivron‐Guillot K, et al. Comparison of the clinical history of symptomatic isolated distal deep‐vein thrombosis vs. proximal deep vein thrombosis in 11 086 patients. J Thromb Haemost. 2009;7:2028–34. [DOI] [PubMed] [Google Scholar]
- 31. Ageno W, Mantovani LG, Haas S, et al. Patient management strategies and long‐term outcomes in isolated distal deep‐vein thrombosis versus proximal deep‐vein thrombosis: findings from XALIA. TH Open. 2019;3:e85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schellong S, Ageno W, Casella IB, et al. Profile of patients with isolated distal deep vein thrombosis versus proximal deep vein thrombosis or pulmonary embolism: RECOVERY DVT/PE study. Semin Thromb Hemost. 2021;10: Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Barco S, Corti M, Trinchero A, et al. Survival and recurrent venous thromboembolism in patients with first proximal or isolated distal deep vein thrombosis and no pulmonary embolism. J Thromb Haemost. 2017;15:1436–42. [DOI] [PubMed] [Google Scholar]
- 34. Dentali F, Pegoraro S, Barco S, et al. Clinical course of isolated distal deep vein thrombosis in patients with active cancer: a multicenter cohort study. J Thromb Haemost. 2017;15:1757–63. [DOI] [PubMed] [Google Scholar]
- 35. Ng S, Carrier M. Prevention and treatment of cancer‐associated thrombosis. Curr Oncol. 2020;27:275–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ten Cate‐Hoek AJ. Prevention and treatment of the post‐thrombotic syndrome. Res Pract Thromb Haemost. 2018;2:209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Selby R, Geerts WH, Kreder HJ, Crowther MA, Kaus L, Sealey F. Symptomatic venous thromboembolism uncommon without thromboprophylaxis after isolated lower‐limb fracture: the knee‐to‐ankle fracture (KAF) cohort study. J Bone Joint Surg Am. 2014;96:e83. [DOI] [PubMed] [Google Scholar]
- 38. Wahlsten LR, Eckardt H, Lyngbæk S, et al. Symptomatic venous thromboembolism following fractures distal to the knee: a nationwide Danish cohort study. J Bone Joint Surg Am. 2015;97:470–7. [DOI] [PubMed] [Google Scholar]
- 39. Wang H, Kandemir U, Liu P, et al. Perioperative incidence and locations of deep vein thrombosis following specific isolated lower extremity fractures. Injury. 2018;49:1353–7. [DOI] [PubMed] [Google Scholar]
- 40. Qu SW, Cong YX, Wang PF, et al. Deep vein thrombosis in the uninjured lower extremity: a retrospective study of 1454 patients with lower extremity fractures. Clin Appl Thromb Hemost. 2021;27:1076029620986862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li S, Zhang K, Heng L, Feng D, Cai X, Tian D. Incidence and risk factors of deep venous thrombosis of lower extremities within 24 hours after closed fractures of lower extremities. Int J Orthop. 2019;40:306–10. [Google Scholar]
- 42. Zhang J, Yang H, Zhang Z, Li R, Li J, Huang W. Influential factors analysis and epidemiological study on deep venous thrombosis(DVT) of lower limb closed fracture before operation. J Pract Orthop. 2015;21:988–92. [Google Scholar]
- 43. Guo Y, Zhao Q, Lin F, Cai X, Ying X, Lin L. Preoperative risk factors for deep vein thrombosis in patients with long bone fractures of lower extremity. Chin J Bone Joint Injury. 2015;30:618–21. [Google Scholar]
- 44. Zang J, Ma X, Ma J, Jiang H, Li P, Li Y. Epidemiological study on the incidence of deep vein thrombosis associated with fracture sites. Chin J Orthop. 2016;36:540–5. [Google Scholar]
- 45. Zhang J, Zhao K, Li J, Meng H, Zhu Y, Zhang Y. Age over 65 years and high levels of C‐reactive protein are associated with the risk of preoperative deep vein thrombosis following closed distal femur fractures: a prospective cohort study. J Orthop Surg Res. 2020;15:559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lv B, Xue F, Tang G, Pan M, Luo H, Wang Y. Risk factors of deep vein thrombosis after lower limb fractures. Int J Orthop. 2018;39:373–7. [Google Scholar]
- 47. Abelseth G, Buckley RE, Pineo GE, Hull R, Rose MS. Incidence of deep vein thrombosis in patients with fractures of the lower extremity distal to the hip. J Orthop Trauma. 1996;10:230–5. [DOI] [PubMed] [Google Scholar]
- 48. Xiao L, Li J, Zhang X, Huang M. Analysis of related factors between knee joint injury and acute deep venous thrombosis of the lower extremity. Chin J Gen Surg. 2017;32:336–9. [Google Scholar]
- 49. Li J, Wang P, Zhang B, Zhuang Y, Xue H, Yang N. Analysis of the incidence and risk factors of deep vein thrombosis during hospitalization of patients with simple patella fracture. Chin J Bone Joint Injury. 2019;34:390–2. [Google Scholar]
- 50. Gao F, Wang G, Wang D, et al. Characteristics and risk factors of deep vein thrombosis in tibiofibular fractures. Orthop J China. 2020;28:1085–8. [Google Scholar]
- 51. Li J, Wang Q, Wang P, Lu Y, Zhang B, Li Z. Analysis of incidence and risk factors of perioperative DVT in patients with tibiofibular fractures. Chin J Bone Joint Injury. 2019;34:813–7. [Google Scholar]
- 52. Schwarz T, Schmidt B, Beyer J, Schellong SM. Therapy of isolated calf muscle vein thrombosis with low‐molecular‐weight heparin. Blood Coagul Fibrinolysis. 2001;12:597–9. [DOI] [PubMed] [Google Scholar]
- 53. Utter GH, Dhillon TS, Salcedo ES, et al. Therapeutic anticoagulation for isolated calf deep vein thrombosis. JAMA Surg. 2016;151:e161770. [DOI] [PubMed] [Google Scholar]
- 54. Franco L, Giustozzi M, Agnelli G, Becattini C. Anticoagulation in patients with isolated distal deep vein thrombosis: a meta‐analysis. J Thromb Haemost. 2017;15:1142–54. [DOI] [PubMed] [Google Scholar]
- 55. Lautz TB, Abbas F, Walsh SJ, et al. Isolated gastrocnemius and soleal vein thrombosis: should these patients receive therapeutic anticoagulation? Ann Surg. 2010;251:735–42. [DOI] [PubMed] [Google Scholar]
- 56. Yoon DY, Riaz A, Teter K, et al. Surveillance, anticoagulation, or filter in calf vein thrombosis. J Vasc Surg Venous Lymphat Disord. 2017;5:25–32. [DOI] [PubMed] [Google Scholar]
- 57. Sales CM, Haq F, Bustami R, Sun F. Management of isolated soleal and gastrocnemius vein thrombosis. J Vasc Surg. 2010;52:1251–4. [DOI] [PubMed] [Google Scholar]
- 58. Righini M, Galanaud JP, Guenneguez H, et al. Anticoagulant therapy for symptomatic calf deep vein thrombosis (CACTUS): a randomised, double‐blind, placebo‐controlled trial. Lancet Haematol. 2016;3:e556–62. [DOI] [PubMed] [Google Scholar]
- 59. Schwarz T, Buschmann L, Beyer J, Halbritter K, Rastan A, Schellong S. Therapy of isolated calf muscle vein thrombosis: a randomized, controlled study. J Vasc Surg. 2010;52:1246–50. [DOI] [PubMed] [Google Scholar]
- 60. Appelen D, van Loo E, Prins MH, Neumann MH, Kolbach DN. Compression therapy for prevention of post‐thrombotic syndrome. Cochrane Database Syst Rev. 2017;9:CD004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kabashneh S, Singh V, Alkassis S. A comprehensive literature review on the management of distal deep vein thrombosis. Cureus. 2020;12(5):e8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Galanaud JP, Genty‐Vermorel C, Rolland C, et al. Compression stockings to prevent postthrombotic syndrome: literature overview and presentation of the CELEST trial. Res Pract Thromb Haemost. 2020;4:1239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kahn SR, Shapiro S, Wells PS, et al. Compression stockings to prevent post‐thrombotic syndrome: a randomised placebo‐controlled trial. Lancet. 2014;383:880–8. [DOI] [PubMed] [Google Scholar]
- 64. Avila ML, Montoya M, Lumia C, Marson A, Brandão LR, Tomlinson G. Compression stockings to prevent post‐thrombotic syndrome in adults, a Bayesian meta‐analysis. Thromb Res. 2019;182:20–6. [DOI] [PubMed] [Google Scholar]
- 65. Makedonov I, Kahn SR, Galanaud JP. Prevention and management of the post‐thrombotic syndrome. J Clin Med. 2020;9:923. [DOI] [PMC free article] [PubMed] [Google Scholar]


