Abstract
Objective
To compare the clinical outcomes of culture‐negative periprosthetic joint infection (CN PJI) with those of culture‐positive periprosthetic joint infection (CP PJI).
Methods
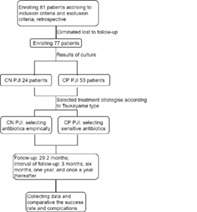
This study retrospectively examined data from 77 patients who underwent revision surgery due to periprosthetic joint infection (PJI) after hip and knee arthroplasty at our center from January 2012 to June 2017. There were 37 males and 40 females, with an average age of 63.6 year. All patients were classified by Tsukayama type, according to the bacterial culture results of synovial fluid and pre‐ and intraoperative tissues, 24 cases were included in the CN PJI group, and 53 cases were included in the CP PJI group. All patients underwent routine blood tests, liver, renal function tests, erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP) measurements. The remission rates of CN PJI and CP PJI were compared. The effects of the culture results on the curative effect were further compared by survival analysis.
Results
The patients were followed regularly with an average of 29.2 months (range, 12–76 months). In total, there were 24 cases of CN PJI, with an incidence of 29.63%. The overall success rate of CN PJI group was 86.4% (19/22), and overall success rate of CP PJI group was 87.5% (42/48). The relative efficacy of various surgical options was: one‐stage revision 100% (7/7), two‐stage revision 96.3% (26/27), debridement and implant retention 64.3% (9/14), respectively. There was no significant difference in the success rate between the CN PJI group and the CP PJI group. The incidence of antibiotic‐related complications for the CN PJI group was significantly higher than that of the CP PJI group, with 58.3% for CN PJI and 11.3% for CP PJI, respectively.
Conclusion
When CN PJI was treated according to the strict standards for the diagnosis and treatment, the success rate of treatment for the CN PJI group was similar to that for the CP PJI group. The incidence of antibiotic‐related complications from the CN PJI group was higher than that from the CP PJI group.
Keywords: Antibiotic, Culture negative, Culture positive, Operative, Periprosthetic joint infection
This study showed that if CN PJI patients were treated strictly according to Tsukayama type, with a combination of 2–6 weeks of intravenous vancomycin and ceftazidime or carbapenem antibiotic administration with oral quinolone antibiotics for 6–10 weeks, the overall success rate was similar to that of CP PJI, and there was no significant difference between the success rates of various surgical strategies in CN PJI and CP PJI.
Introduction
Total joint arthroplasty (TJA) has been proved to be one of the effective treatments for the severe hip and knee disease. It can effectively relieve the pain of the diseased joint, restore the joint function, and greatly improve the quality of life of patients. 1 With the advent of an aging society, serious degenerative changes of hip and knee joint are becoming more and more common, which leads to an increasing number of cases of joint arthroplasty. With the increasing number of cases of TJA, surgeons are constantly worried about its complications, including: prosthesis dislocation, periprosthetic fracture, particles disease, postoperative pain, periprosthetic joint infection (PJI) and so on. PJI is one of the most devastating complications after TJA. The diagnosis of PJI is difficult and the treatment is complex, which seriously affects the quality of surgery. Once it occurs, it will not only increase the length of stay and economic expenditure of patients, but also bring a great burden to social medical resources. 2 According to the literature, the incidence of infection after the primary joint replacement is 1%–3%, in which the incidence of PJI after the primary total knee arthroplasty (TKA) is 1%–4%, and the incidence of PJI after the primary total hip arthroplasty (THA) is 1%–2%. 3 Although PJI is considered to be a rare complication of TJA, with the significant increase in the number of TJA patients, the total number of PJI is also gradually increasing, which poses a great challenge to joint surgeons.
The identification of pathogenic microorganisms is the gold standard for the diagnosis of PJI and can provide reference for the treatment of sensitive antibiotics. 4 , 5 The treatment of PJI includes surgery (debridement antibiotics irrigation and implant retention, one‐stage revision and two‐stage revision) and systemic application of antibiotics based on microbial culture and drug sensitivity results. Therefore, the culture results of pathogenic bacteria affect the whole chain of clinical intervention of PJI. With the continuous optimization of technology, a number of strategies have been used to improve the detection rate of pathogens, including ultrasonic lysis of the extracted prosthesis, prolonged incubation, enhanced culture media, metagenomic next‐generation sequencing, etc., 6 but culture‐negative PJI (CN PJI) is not uncommon in clinic. It is reported that the incidence of CN PJI is 0%–42.1%. 7 The high incidence of negative results is due to a number of reasons, including: early use of antibiotics, infection of low‐virulent organisms and the effects of biofilms. In the case of early use of antibiotics, the probability of negative culture can be as high as 50%–60%. 8 CN PJI lacks independent clinical manifestations, and negative culture results complicate the already challenging diagnosis and treatment of PJI.
For the surgical treatment of CN PJI, the current report is more inclined to use the method of two‐stage revision9, 10 which includes the first stage debridement: removal of artificial prosthesis, complete debridement, implantation of spacer, antibiotic treatment and second‐stage revision surgery: removal of spacer, re‐debridement and reimplantation of revision joint prosthesis. In the first stage of operation, the use of a bone cement placeholder containing antibiotics to maintain local high concentration of antibiotics and eliminate bacteria is an important part of infection control. 11 According to the patient's condition and wishes, other surgical strategies are sometimes chosen, including: debridement, antibiotics, irrigation, and retention (DAIR) plus polyethylene prosthesis replacement, one‐stage revision, permanent joint fusion and so on. The use of antibiotics in addition to surgical treatment is necessary for the effective treatment of PJI, but due to the lack of reliable data on pathogenic bacteria, it is extremely difficult to choose the antibiotic regimen for CN PJI. At present, it is considered that the combined use of vancomycin and the third‐generation cephalosporins or carbapenem is the most suitable scheme for the treatment of CN PJI. 7 However, the duration of antibiotic use and the choice of oral antibiotics after intravenous antibiotics are still unclear, which make patients and surgeons feel uneasy. In addition, long‐term use of broad‐spectrum antibiotics and combined with a variety of antibiotics caused by myelosuppression, liver and kidney function damage and other complications are constantly causing concern among surgeons.
Although it has been reported that the application of two‐stage revision and reasonable antibiotic regimens make the treatment success rate of CN PJI reach 70%–100%, 8 but, the diversity of surgical options and the uncertainty of antibiotic application make it difficult to make decisions on the treatment of CN PJI. The lack of standardized guidelines confuses the clinical management of CN PJI. And, as far as we know, there are few reports of complications in the use of CN PJI antibiotics.
Therefore, we have carried out this study, the purpose of this study is: (i) to compare the success rate of treatment and the incidence of antibiotic‐related complications between CN PJI and culture‐positive periprosthetic joint infection (CP PJI); and (ii) the clinical effects of different surgical methods and antibiotic regimens in the treatment of CN PJI.
Materials and Methods
Patient Selection and Clinical Data Collection
This retrospective case–control study has been approved by the Ethics Committee of our hospital (MTCA, ECFAH of FMU [2015]084‐1). All data was derived from the electronic medical record system. Inclusion criteria: (i) patients who were diagnosed as PJI according to MSIS criteria; (ii) patients who underwent surgery in our center due to PJI and follow‐up regularly; and (iii) patients with complete medical data. Exclusion criteria: patients who had immunosuppressive disease, infectious disease in other part, or malignant tumors. Patients with negative culture during treatment were defined as CN PJI, and patients with pathogenic bacteria cultured in more than one sample were defined as CP PJI.
From January 2012 to June 2017, 81 patients who underwent revision surgery due to PJI after hip and knee arthroplasty in our center were retrospectively collected. Four patients who failed to follow‐up were excluded (bacterial culture results were positive and underwent two‐stage revision). A diagnosis for PJI were made according to the Musculoskeletal Infection Society (MSIS) criteria for PJI. 12 , 13 All patients were classified by Tsukayama type. 14 , 15
Age, sex, body mass index (BMI), preoperative complications, American Association of Anesthesiologists (ASA) physical status, laboratory tests (routine blood test, erythrocyte sedimentation rate (ESR), C‐reactive protein (CRP)), preoperative and intraoperative synovial fluid white blood cell (SF‐WBC) count and percentage of polymorphonuclear leukocytes (SF‐PMN%), bacterial culture results and intraoperative frozen section results of periprosthetic tissue were collected. The incidence of PJI after total joint arthroplasty (type, sinus, pathogenic microorganism), surgical strategies (one‐stage revision, two‐stage revision and DAIR), antibiotic regimens and clinical results were also documented.
Surgical Strategies
We routinely use Tsukayama type classification as a reference to choose the operation plan. Generally, for cases of Tsukayama type II or III without sinus, DAIR was selected. For cases classified as Tsukayama type I or IV without sinus and where multiresistance bacteria was isolated from the preoperative synovial fluid, as well as cases involving elderly populations and poor economic conditions combined with the wishes of the patients, one‐stage revision surgery was chosen. For cases classified as Tsukayama type IV with poor soft tissue conditions and where multiresistance bacteria was isolated from preoperative synovial fluid as well as the existence of sinus, two‐stage revision was selected. And patient's willingness was also taken into consideration.
All operations were performed by the same surgical team and were performed under general or spinal anesthesia. Synovial fluid and diseased tissues were routinely collected during the operation and submitted to flow cytometry and pathogenic microorganism culture. For DAIR, a new polyethylene liner was implanted in the joint after complete debridement. For the one‐stage revision, the prosthesis was removed, thoroughly debridement, and directly implanted into the new joint prosthesis. For two‐stage revision, including the first stage of surgery: removal of the prosthesis, complete debridement, implantation of the spacer and second‐stage surgery: removal of the spacer, re‐debridement and implantation of a new joint prosthesis. Drainage tubes are generally not placed except for special needs. When antibiotic bone cement is needed, the formula is: 2 g of vancomycin per 40 g of bone cement.
Antibiotic Regimens
For the CN PJI group, the administration of vancomycin and third‐generation cephalosporin or carbapenem antibiotics was combined intravenously. For the CP PJI group, antibiotics were selected based on drug sensitivity tests. All patients received intravenous antibiotics for 2–6 weeks (mean time: 20 days for DAIR, 16 days for one‐stage revision, and 31 days for two‐stage revision). After that, the antibiotic regimens were switched to oral antibiotics. Rifampicin combined with sensitive antibiotics was selected for staphylococcal infections and only sensitive antibiotics for other positive cultures, quinolone antibiotics were selected for negative culture, and the course of antibiotics administered was 6–10 weeks, with a total of 12 weeks. Antibiotics were administered intravenously for 7–14 days after the second stage of reimplantation. If the pathogenic microorganism culture was positive during reimplantation, antibiotics were orally taken for 8–10 weeks after 2–4 weeks of sensitive antibiotic administration according to drug‐sensitive veins, with a total course of treatment of 12 weeks.
Follow‐up
All patients were followed regularly (3 months, 6 months, 1 year after the operation, and once a year thereafter, with a minimum follow‐up of 2 years). The ESR and CRP level, liver and renal function were reexamined at each follow‐up.
Outcome Evaluation
Success of treatment was evaluated by the International Consensus in 2013, 16 included three parts: (i) infection eradication, characterized by good wound healing and no exudation, sinus or wound pain; (ii) no infection‐related surgical intervention after reimplantation; and (iii) no infection‐related death (caused by septicemia, necrotizing fasciitis, etc.).
Antibiotic‐related complications include: (i) myelosuppression—a preoperative routine WBC count of >4 × 109/L and a maximum value of WBCs during intravenous or oral antibiotics <3 × 109/L; (ii) liver function damage—before the operation, the liver function alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were all normal, and the peak value of ALT or AST during intravenous or oral antibiotics increased more than 1.5 times; and (iii) renal function damage—the creatinine level characteristic of renal function was within the normal range before the operation, and the creatinine value increased more than 1.5 times the initial value during intravenous or oral antibiotic administration.
Statistical Analysis
The χ 2 test and F‐test were used to compare the differences in demographic characteristics and comorbidities between CN PJI and CP PJI. Continuous variables such as age, body mass index and ASA between groups were analyzed by student t test if they obeyed a normal distribution; otherwise, the Mann–Whitney U test was performed. Kaplan–Meier survival analysis and log‐rank tests were used to compare the success rates of CN PJI and CP PJI. The χ 2 test and F‐test were used to compare the incidence of antibiotic complications. All statistical analyses were performed in SPSS 20.0 (IBM, Armonk, NY, USA). The statistical significance level was set at p < 0.05.
Results
Demographic Characteristics
Seventy‐seven PJI cases of the hip and knee were enrolled. According to the bacterial culture results of synovial fluid and pre‐ and intraoperative tissues, 24 cases were included in the CN PJI group, 53 cases were included in the CP PJI group. All patients were followed up for an average of 29.2 months (12–76 months).
There was no statistical difference in age, sex, body mass index (BMI), preoperative complications or ASA grade between two groups (Table 1). Nineteen patients in the CN PJI group had used antibiotics prior to surgery, which was significantly different from that in the CP PJI group. The administration of antibiotics prior to surgery might be the reason for the negative culture. The microbiology of CP PJI group included methicillin‐resistant Staphylococcus (MRS) in 25 cases, methicillin‐sensitive Staphylococcus (MSS) in 16 cases, Streptococcus in four cases, gram‐negative bacilli in three cases, fungi in three cases, and multiple microbial infection in two cases. Six patients with CN PJI underwent one‐stage revision, 11 patients underwent two‐stage revision (two patients did not undergo reimplantation of the prosthesis due to poor economy), and seven patients underwent DAIR. Seven patients with CP PJI underwent one‐stage revision, 32 patients underwent two‐stage revision (five patients did not undergo reimplantation of the prosthesis due to other reasons), and 14 patients underwent DAIR.
TABLE 1.
Demographic characteristics of all patients
| Variable | CN PJI (n = 24) | CP PJI (n = 53) | Statistic value | p value |
|---|---|---|---|---|
| Sex (male) | 12 | 25 | 0.053 a | 0.818 |
| Age | 62.50 | 64.15 | −0.508 c | 0.613 |
| BMI | 23.84 | 24.13 | −0.347 c | 0.729 |
| ASA | 0.92 | 0.79 | −0.521 b | 0.602 |
| Hypertension | 14 | 33 | 0.107 a | 0.743 |
| Diabetes | 4 | 14 | 0.876 a | 0.349 |
| Sinus | 7 | 16 | 0.008 a | 0.928 |
| Intraoperative suppuration | 15 | 37 | 0.403 a | 0.526 |
| Joint involved | 1.291 a | 0.256 | ||
| Hip | 17 | 28 | ||
| Knee | 7 | 25 | ||
| Administration of preoperative antibiotics | 19 | 29 | 4.206 a | 0.040 |
| Surgical strategies | 2.032 a | 0.362 | ||
| DAIR | 7 | 14 | ||
| One‐stage revision | 6 | 7 | ||
| Two‐stage revision | 11 | 32 | ||
| Antibiotic‐related complications | 14 | 6 | 18.989 a | <0.001 |
Abbreviations: ASA, American society of Anesthesiologists; BMI, Body Mass Index; CN PJI, culture‐negative periprosthetic joint infection; CP PJI, culture‐positive periprosthetic joint infection; DAIR, Debridement, Antibiotics and Implant Retention.
Chi‐squared test.
Mann–Whitney U test.
Independent‐ samples t‐ test.
Comparison of Success Rate of Treatment
Because the definition of successful treatment in this study was aimed at patients who underwent reimplantation of the prosthesis, the patients who did not received reimplantation (two patients with CN PJI and two patients with CP PJI) were excluded when analyzing the success rate of treatment. The overall success rate of CN PJI group treatment was 86.4% (19/22). In one of the failed cases, the wound continued to ooze fluid after reimplantation surgery, and DAIR was performed 11 days after reimplantation. One patient experienced infection reoccurrence 4 months after one‐stage revision, and another patient experienced infection reoccurrence 6 months after DAIR (Figure 1). The overall success rate of CP PJI group treatment was 87.5% (42/48). Among the failed cases, three patients received additional DAIR within 2 weeks after primary DAIR due to wound discharge, and the other two patients received additional DAIR treatment 35 days and 13 months after primary DAIR due to infection recurrence. One patient received DAIR 5 months after two‐stage revision, and no signs of infection recurrence were found till now. The difference between the overall success rate of CN PJI group and CP PJI group (Figure 2) and the success rate among various surgical strategies did not reach statistical significance (Table 2).
Fig. 1.
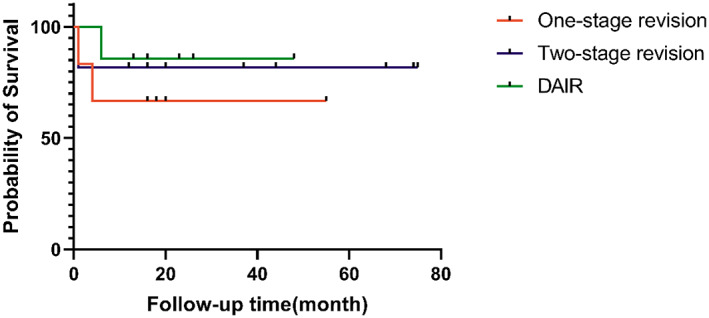
Kaplan–Meier survival analysis showing the success rate of culture‐negative periprosthetic joint infection treated with various surgical strategies
Fig. 2.
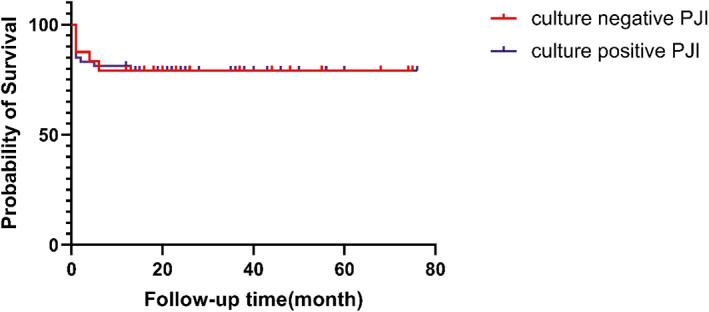
Kaplan–Meier survival analysis showing that the overall success rates of culture‐negative periprosthetic joint infection and culture‐positive periprosthetic joint infection are similar (p = 0.897)
TABLE 2.
Comparison of the treatment success rates of PJI
| Surgical strategies | CN PJI (n = number of successful, success rate) | CP PJI (n = number of successful, success rate) | χ2 value | p value |
|---|---|---|---|---|
| Total | 22(19, 86.4%) | 48(42, 87.5%) | 0.017 | 0.897 |
| One‐stage revision | 6(4, 66.7%) | 7(7, 100%) | 2.560 | 0.110 |
| Two‐stage revision | 9(9, 100%) | 27(26, 96.3%) | 0.333 | 0.564 |
| DAIR | 7(6, 85.7%) | 14(9, 64.3%) | 1.079 | 0.299 |
Abbreviations: CN PJI, culture‐negative periprosthetic joint infection; CP PJI, culture‐positive periprosthetic joint infection; DAIR, Debridement, Antibiotics and Implant Retention; PJI, periprosthetic joint infection.
Comparison of the Incidence of Antibiotic‐related Complications
CN PJI group was associated with an overall antibiotic complication rate of 58.3% (14/24): myelosuppression occurred in five cases (20.8%), renal dysfunction in four (16.7%), liver dysfunction in one (4.0%), simultaneous liver and renal dysfunction in three (12.5%), and simultaneous myelosuppression and renal dysfunction in one (4.2%). CP PJI group was associated with an overall antibiotic complication rate of 11.3% (6/53): liver injury occurred in four cases (7.5%) and renal function damage in two (3.7%) without concurrent damage. The incidence of CN PJI group and CP PJI group antibiotic‐related complications was significantly different, and the incidence of CN PJI group antibiotic‐related complications was higher than that of CP PJI group antibiotic‐related complication.
Typical Cases
Case 1
A 54‐year‐old woman underwent one‐stage revision of the left hip joint with CN PJI. There was no recurrence after follow‐up so far. The antibiotic regimen was: third‐generation cephalosporin and vancomycin were given intravenously for 4 weeks, then quinolones were taken orally for 6 weeks. There was renal damage, but the side effects disappeared after stopping the use of antibiotics (Figure 3).
Fig. 3.
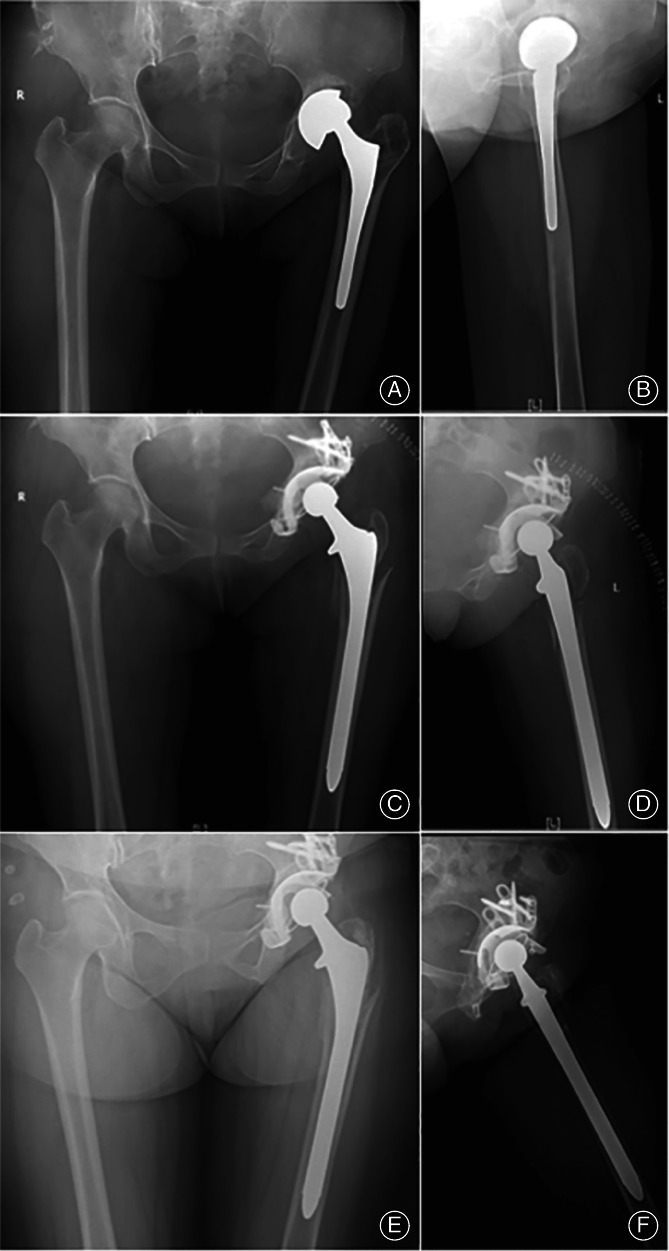
Female, 54‐year‐old, underwent one‐stage revision of the left hip joint with culture‐negative periprosthetic joint infection. (A, B) preoperative X‐ray: prosthesis loosening; (C, D) postoperative X‐ray; (E, F) reexamination X‐ray 2 years after operation
Case 2
A 56‐year‐old woman had CP PJI of the right knee. The pathogen was MRS, for two‐stage revision, and no recurrence was found in the follow‐up so far. According to the drug sensitivity test, the antibiotic regimen was determined by intravenous administration of vancomycin for 6 weeks, followed by oral administration of linezolid for 1 week, and myelosuppression occurred, but the side effects disappeared after stopping the use of antibiotics (Figure 4).
Fig. 4.
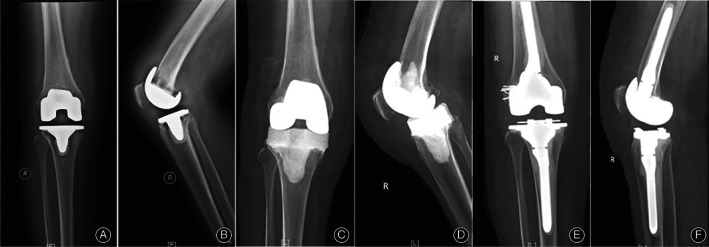
Female, 56‐year‐old, underwent two‐stage revision of the right knee joint with culture‐positive periprosthetic joint infection. (A, B) preoperative X‐ray: prosthesis loosening; (C, D) one‐step postoperative X‐ray; (E, F) second‐step postoperative X‐ray
Case 3
A 71‐year‐old male diagnosed with CN PJI of the left knee. It relapsed after 4 months of one‐stage revision. Antibiotic regimen: third‐generation cephalosporin and vancomycin were given intravenously for 4 weeks, then quinolones were taken orally for 8 weeks, and there were no side effects of antibiotics (Figure 5).
Fig. 5.
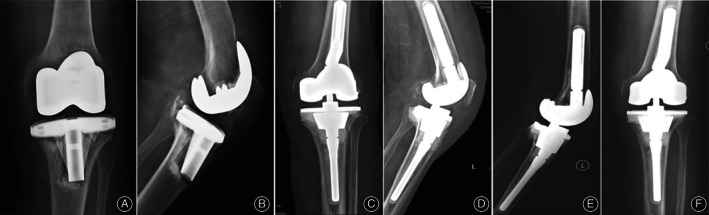
Male, 71‐year‐old, diagnosed with culture‐negative periprosthetic joint infection of the left knee. It relapsed after 4 months of one‐stage revision. (A, B) preoperative X‐ray: prosthesis loosening; (C, D) X‐ray 1 day after operation; (E, F) X‐ray 4 months after operation
Discussion
The Success Rate of CN PJI was Similar to that of CP PJI
A total of 77 cases of hip and knee PJIs were included in the study. Nineteen patients in the CN PJI group had used antibiotics prior to surgery, which was significantly different from the CP PJI group. This may be the main cause of the negative culture results, so it is necessary to stop antibiotics for a few weeks before specimen collection for culturing. In the present study, the overall treatment success rate of PJI was 87.1%, and the average follow‐up period was 29.2 months (12–76 months). The overall treatment success rates of the CN PJI group and the CP PJI group were 86.4% and 87.5%, respectively. There was no significant difference between the two groups (p = 0.897), which was consistent with the conclusions reported in most previous studies. 17 , 18 , 19 , 20 It has been reported that DAIR and two‐stage revision are the two main surgical strategies for CN PJI, and the rate of infection eradication can reach 73%–94%. 20 There was no significant difference in the success rate of one‐stage revision between the groups. However, the sample size was small in the present work, and whether one‐stage revision is applicable for CN PJI needs to be further investigated. In the present work, the antibiotic regimens for CN PJI group that were typically used were an intravenous combination of vancomycin with third‐generation cephalosporin or carbapenem. This kind of antibiotic regimen is effective for all pathogenic microorganisms except Mycobacterium tuberculosis and fungi (including G + /G−/ drug‐resistant bacteria), 20 which might be the reason why the success rate in the CN PJI group was similar to that in the CP PJI group in the present work.
The Incidence of Antibiotic‐related Complications in CN PJI was Higher than that in CP PJI
The total incidence rate of antibiotic‐related complications was 25.9%, and the incidence rates of the CN PJI and CP PJI groups were 58.3% and 11.3%, respectively; the difference was statistically significant. In the CN PJI group, the incidence rate of bone marrow suppression complicated by antibiotics was 20.8%, which was higher than the 2%–8% reported in previous studies. 21 The incidence rate of concurrent renal impairment was 16.7%, and the incidence of renal impairment related to vancomycin treatment alone has been reported in the literature to be 6.5%–10.7%. 22 , 23 Some scholars have also shown that the incidence of renal damage increases from 6% to 21% with vancomycin treatment intravenously for more than 1 week, and the incidence of renal failure can reach 30% when the antibiotic treatment course is extended to more than 2 weeks. 24 , 25 Although there was no significant difference in clinical outcomes between the CN PJI group and the CP PJI group, the incidence of antibiotic‐related complications in the CN group was relatively higher than that in the CP PJI group. Therefore, the isolation of pathogenic bacteria and the proper administration of antibiotics were of great significance in reducing the incidence of antibiotic‐related complications.
The cases of PJI in this study were included from January 2012 to June 2017, with uniform diagnosis and treatment standards, and the surgery was performed by doctors of the same medical team. The definition of successful treatment was judged by the international consensus made in 2013 and was well representative. This study is the first to compare the incidence of antibiotic‐related complications between CN PJI and CP PJI as an evaluation of clinical outcome.
Limitations
There were also some limitations of this study. (i) This study involved a single center, so the sample size was small. In addition, the minimum follow‐up time was 1 year, and a few cases of recurrent infection may have been missed. (ii) This study focused on the short‐term and medium‐term clinical outcomes of CN PJI and CP PJI, and we should prolong the follow‐up time to compare whether there are differences in long‐term clinical outcomes. (iii) In the present study, the patients who did not undergo reimplantation of their prosthesis were excluded when comparing the success rate, and four patients in the CP PJI group who were treated with two‐stage revision were lost to follow‐up, which may have affected the results. (iv) For multiple microbial infection, we did not analyze them further due to their too small sample size, which is one of our limitations.
Conclusion
In conclusion, this study showed that if CN PJI patients were treated strictly according to Tsukayama type, with a combination of 2–6 weeks of intravenous vancomycin and ceftazidime or carbapenem antibiotic administration with oral quinolone antibiotics for 6–10 weeks, the overall success rate was similar to that of CP PJI, and there was no significant difference between the success rates of various surgical strategies in CN PJI and CP PJI. However, the incidence rate of antibiotic‐related complications in CN PJI was higher than that of antibiotic‐related complication in CP PJI. Due to a lack of etiological basis, the selection of suitable antibiotics is unclear for CN PJI. The empirical administration of a combination of vancomycin and ceftazidime or carbapenem antibiotics, which covers almost all pathogenic microorganisms except Mycobacterium tuberculosis and fungi, can achieve a good treatment success rate while at the same time also increasing the incidence rate of antibiotic‐related complications.
Author Contribution
All authors contributed to the study conception and design. Experiment design, data collection and analysis were performed by Zhiyang Xu, Changyu Huang and Yiming Lin. The first draft of the manuscript was written by Zhiyang Xu and Changyu Huang, and translated by Yongfa Chen, Xinyu Fang, Zida Huang and Chaofan Zhang, final edition was completed by Zhenzhen Zhang and Wenming Zhang. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This paper is supported by National Natural Science Foundation of China (82072458), the Joint Funds for the innovation of science and Technology, Fujian Province, China (2019Y9136).
The authors thank all patients in this study.
Zhiyang Xu, Changyu Huang are co‐first authors and contributed equally to this work.
REFERENCES
- 1. Walker DJ, Heslop PS, Chandler C, Pinder IM. Measured ambulation and self‐reported health status following total joint replacement for the osteoarthritic knee. Rheumatology. 2002;41(7):755–8. [DOI] [PubMed] [Google Scholar]
- 2. Kong L, Cao J, Zhang Y, Ding W, Shen Y. Risk factors for periprosthetic joint infection following primary total hip or knee arthroplasty: a meta‐analysis. Int Wound J. 2017;14(3):529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Phillips JE, Crane TP, Noy M, Elliott TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15‐year prospective survey. J Bone Joint Surg Br. 2006;88(7):943–8. [DOI] [PubMed] [Google Scholar]
- 4. Morgenstern C, Cabric S, Perka C, Trampuz A, Renz N. Synovial fluid multiplex PCR is superior to culture for detection of low‐virulent pathogens causing periprosthetic joint infection. Diagn Microbiol Infect Dis. 2018;90(2):115–9. [DOI] [PubMed] [Google Scholar]
- 5. Peel TN, Sedarski JA, Dylla BL, Shannon SK, Amirahmadi F, Hughes JG, et al. Laboratory workflow analysis of culture of periprosthetic tissues in blood culture bottles. J Clin Microbiol. 2017;55(9):2817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang Z, Li W, Lee GC, Fang X, Xing L, Yang B, et al. Metagenomic next‐generation sequencing of synovial fluid demonstrates high accuracy in prosthetic joint infection diagnostics: mNGS for diagnosing PJI. Bone Joint Res. 2020;9(7):440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yoon HK, Cho SH, Lee DY, Kang BH, Lee SH, Moon DG, et al. A review of the literature on culture‐negative periprosthetic joint infection: epidemiology, diagnosis and treatment. Knee Surg Relat Res. 2017;29(3):155–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tirumala V, Smith E, Box H, van den Kieboom J, Klemt C, Kwon YM. Outcome of debridement, antibiotics, and implant retention with modular component exchange in acute culture‐negative periprosthetic joint infections. J Arthroplasty. 2021;36(3):1087–93. [DOI] [PubMed] [Google Scholar]
- 9. Shanmugasundaram S, Ricciardi BF, Briggs TW, Sussmann PS, Bostrom MP. Evaluation and management of periprosthetic joint Infection—an international, multicenter study. HSS J. 2014;10(1):36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Masters JP, Smith NA, Foguet P, Reed M, Parsons H, Sprowson AP. A systematic review of the evidence for single stage and two stage revision of infected knee replacement. BMC Musculoskelet Disord. 2013;14:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parvizi J, Gehrke T. International consensus group on periprosthetic joint infection. Definition of periprosthetic joint infection. J Arthroplasty. 2014;29(7):1331. [DOI] [PubMed] [Google Scholar]
- 12. Parvizi J, Zmistowski B, Berbari EF, Bauer TW, Springer BD, Della Valle CJ, et al. New definition for periprosthetic joint infection: from the workgroup of the musculoskeletal infection society. Clin Orthop Relat Res. 2011;469(11):2992–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Parvizi J, Tan TL, Goswami K, Higuera C, Della Valle C, Chen AF, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence‐based and validated criteria. J Arthroplasty. 2018;33(5):1309–1314.e2. [DOI] [PubMed] [Google Scholar]
- 14. Tsukayama DT, Estrada R, Gustilo RB. Infection after total hip arthroplasty. A study of the treatment of one hundred and six infections. J Bone Joint Surg Am. 1996;78(4):512–23. [DOI] [PubMed] [Google Scholar]
- 15. Tsukayama DT, Goldberg VM, Kyle R. Diagnosis and management of infection after total knee arthroplasty. J Bone Joint Surg Am. 2003;85‐A(Suppl 1):S75–80. [DOI] [PubMed] [Google Scholar]
- 16. Diaz‐Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi‐based international multidisciplinary consensus. Clin Orthop Relat Res. 2013;471(7):2374–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim YH, Kulkarni SS, Park JW, Kim JS, Oh HK, Rastogi D. Comparison of infection control rates and clinical outcomes in culture‐positive and culture‐negative infected total‐knee arthroplasty. J Orthop. 2015;12(Suppl 1):S37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li H, Ni M, Li X, Zhang Q, Li X, Chen J. Two‐stage revisions for culture‐negative infected total knee arthroplasties: a five‐year outcome in comparison with one‐stage and two‐stage revisions for culture‐positive cases. J Orthop Sci. 2017;22(2):306–12. [DOI] [PubMed] [Google Scholar]
- 19. Kang JS, Shin EH, Roh TH, Na Y, Moon KH, Park JH. Long‐term clinical outcome of two‐stage revision surgery for infected hip arthroplasty using cement spacer: culture negative versus culture positive. J Orthop Surg. 2018;26(1):2309499017754095. [DOI] [PubMed] [Google Scholar]
- 20. Wang J, Wang Q, Shen H, Zhang X. Comparable outcome of culture‐negative and culture‐positive periprosthetic hip joint infection for patients undergoing two‐stage revision. Int Orthop. 2018;42(3):469–77. [DOI] [PubMed] [Google Scholar]
- 21. Morris A, Ward C. High incidence of vancomycin‐associated leucopenia and neutropenia in a cardiothoracic surgical unit. J Infect. 1991;22(3):217–23. [DOI] [PubMed] [Google Scholar]
- 22. Wood MJ. Comparative safety of teicoplanin and vancomycin. J Chemother. 2000;12(Suppl 5):21–5. [DOI] [PubMed] [Google Scholar]
- 23. Finch RG, Eliopoulos GM. Safety and efficacy of glycopeptide antibiotics. J Antimicrob Chemother. 2005;55(Suppl 2):ii5–ii13. [DOI] [PubMed] [Google Scholar]
- 24. Kalil AC, Murthy MH, Hermsen ED, Neto FK, Sun J, Rupp ME. Linezolid versus vancomycin or teicoplanin for nosocomial pneumonia: a systematic review and meta‐analysis. Crit Care Med. 2010;38(9):1802–8. [DOI] [PubMed] [Google Scholar]
- 25. Lodise TP, Patel N, Lomaestro BM, Rodvold KA, Drusano GL. Relationship between initial vancomycin concentration‐time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis. 2009;49(4):507–14. [DOI] [PubMed] [Google Scholar]


