Abstract
Objective
This study aims to assess the proportions of complex regional pain syndrome type I (CRPS I) in radial head fracture patients undergoing unilateral arthroplasty and to explore associated factors.
Methods
This is a prospective observational study. From March 2016 to May 2019, a total of 221 adult patients with radial head fracture patients were included in consecutive studies and completed the 1‐year follow‐up. All patients were treated by unilateral arthroplasty. At each follow‐up visit, the visual analogue scale was used to measure patients' pain level. Occurrence of CRPS I, which was diagnosed by Budapest criteria, was the main outcome collected at baseline and the 1‐, 3‐, 6‐, and 9‐month follow‐ups. The baseline data were collected before surgery and included demographic and clinical data. Independent t‐tests and χ 2 tests were used as univariate analyses to compare the baseline data of patients with and without CRPS I. Multivariate analysis (Backword‐Wald) was used to identify factors independently associated with CRPS I.
Results
The proportion of CRPS I cases among radial head fracture patients undergoing unilateral arthroplasty was 11% (n = 24). A total of 19 (79%) patients were diagnosed with CRPS I within 1 month after surgery. Multivariable logistic regression analysis revealed that female gender (odds ratios [OR]: 1.537; 95% confidence interval [CI]: 1.138–2.072), age younger than 60 years (OR: 1.682; 95% CI: 1.246–2.267), moderate and severe Mayo Elbow Performance Score (MEPS) pain (OR: 3.229; 95% CI: 2.392–4.351) and anxiety (OR: 83.346; 95% CI: 61.752–112.320) were independently associated with CRPS I.
Conclusions
This exploratory study reported that the incidence of CRPS I developing after radial head arthroplasty was 11%. Female sex, younger age, moderate and severe MEPS pain and anxiety patients seems more likely to develop CRPS I.
Keywords: Arthroplasty, Complex regional pain syndromes, Fractures, Logistic models, Radial heads

CRPS I often appears in the first 1 month after radial head arthroplasty. The risk factors for developing CRPS I after radial head arthroplasty include female sex, younger age, moderate and severe MEPS pain and anxiety.
Introduction
Radial head fractures account for 1.7% to 5.4% of all fractures. 1 Radial head arthroplasty is a method commonly employed to restore the stability of the elbow joint, preserve the range of motion, and maintain the radial length. 2 However, chronic pain conditions after arthroplasty may occur, which is one of the reasons for patients' poor functional outcomes and dissatisfaction. Clohisy et al. 3 reported that hip pain is common among developmental dysplasia of the hip patients after arthroscopic. Most of the pain presents without an obvious cause. Complex regional pain syndrome type I (CRPS I) is one of the causes of some chronic pain syndromes and is most frequently induced by fracture. 4
According to two population‐based studies, the national incidence of CRPS I was 5.5 and 26.2 per 100,000 person‐years in North America 4 and Europe, 5 respectively. CRPS I is a chronic neuropathic condition that includes continuing pain, hyperalgesia, temperature asymmetry, edema changes, and motor dysfunction. 6 Varenna et al. 7 reported that trauma events were the most common triggering event CRPS I cases. According to Budapest criteria, the common symptoms of CRPS include abnormal sensation, hyperalgesia or allodynia, edema, sudomotor and vasomotor changes. 8 The clinical signs include four categories: the evidence of sensory; vasomotor; edema and trophic. The diagnosis of CRPS I involves the presence of at least two clinical signs included in the four categories and at least three symptoms in its four categories. 9 For the treatment of CRPS I, evidence has revealed that there may be greater potential gains from comprehensive approach, which includes physical therapy, educational interventions, and neurorehabilitation. 9
Many studies reported that fracture was the most common trigger of CRPS I. 10 , 11 , 12 Jellad et al. 13 reported that CRPS I occurred in 32.2% of distal radius fracture patients. The injury mechanism of distal radius fractures was similar to radial head fractures. However, few studies have focused on the incidence of CRPS I secondary to radial head fractures.
However, acutely injured patients often experience secondary injury, mostly caused by ongoing tissue trauma during surgical preparation, related inflammatory reaction, hypovolemia due to blood loss and other causes. In addition, surgical methods may have an impact on incidence of CRPS I. 14 , 15 , 16 Jo et al. 17 reported that the incidence of CRPS‐1in distal radius fractures patients was higher after open reduction than after closed reduction. Therefore, we focused on the incidence of CRPS I after radial head arthroplasty.
Our hypothesis was that incidence and associated factors among patients after radial head arthroplasty would be different compared to other fracture types. The aim of this prospective observational study was to determine: (i) the incidence of CRPS I after radial head arthroplasty; and (ii) if previously reported risk factors are actually associated with the development of CRPS I.
Methods
This prospective observational study was performed from March 2016 to May 2019 in two level II regional trauma centers. The sample size of this study was calculated based on the number of variables. The lower limit of the number of included individuals was at least 10 times the number of events per variable (EPV). 18 In this study, 10 variables were eligible for multivariate logistic regression, and the lower limit of the number of included individuals was 100 individuals. Convenient sampling was used as a sampling method. Written informed consents were obtained before the trial began. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Our research team explained the study to all participants. The study was registered with Clinicaltrials.gov; Trial registration number (ChiCTR2100050694).
The inclusion criteria were prespecified according to the PICO criteria: (i) types of participants—radial head fractures patients; (ii) types of interventions—patients undergoing unilateral arthroplasty were included; and (iii) types of outcomes—the primary outcomes were the occurrence of CRPS I, other observation index (independent variable) was shown in the section “Evaluations”.
The exclusion criteria were as follows: (i) patients who received conservative treatment or other operation methods; (ii) patients with multiple trauma; (iii) age ≤ 18 years; (iv) associated neurovascular injury; (v) associated injuries that will impede postoperative rehabilitation training; (vi) previous diagnosis of CRPS I and other chronic pain conditions because it has been demonstrated that a history of CRPS is a risk factor for recurrence 19 ; (vii) patients who developed complications, such as infection, heterotopic ossification, etc., because these complication may influence the accuracy of results; and (viii) patients who declined to participate in the study.
The dropout criteria were as follows: participants who are unable to comply with this study, or who experience severe changes in this condition during treatment, were dropped from the study.
Treatments and Diagnosis
All patients underwent axillary brachial plexus block by one anesthesiologist (MJ). All treatments were performed by the same surgical team using the lateral approach. We used the same radial head prosthesis (Wright Medical Technology, Memphis, TN, USA) in this study (Figure 1). All patients received the same postoperative rehabilitation. 20
Fig. 1.
Postoperative anteroposterior (A) and lateral (B) radiographs (radial head prosthesis; Wright Medical Technology, Memphis, TN, USA).
At each follow‐up visit, a pain specialist (YW) who was blind to baseline questionnaire scores measured the patient's pain level using the visual analogue scale (VAS). Previous studies found that few patients undergoing radial head arthroplasty reported greater pain (VAS > 50 points) 1 month after surgery. Therefore, we regard 50 points as the cut‐off scores of the pain level. 21 , 22 For patients who present disproportionate pain in the operated limb, we initially attempted to exclude other potential causes. Second, we diagnosed CRPS I according to Budapest criteria, 23 which includes four categories (sensory, vasomotor, sudomotor/edema, motor/trophic). The specific process is shown in Figure 2.
Fig. 2.
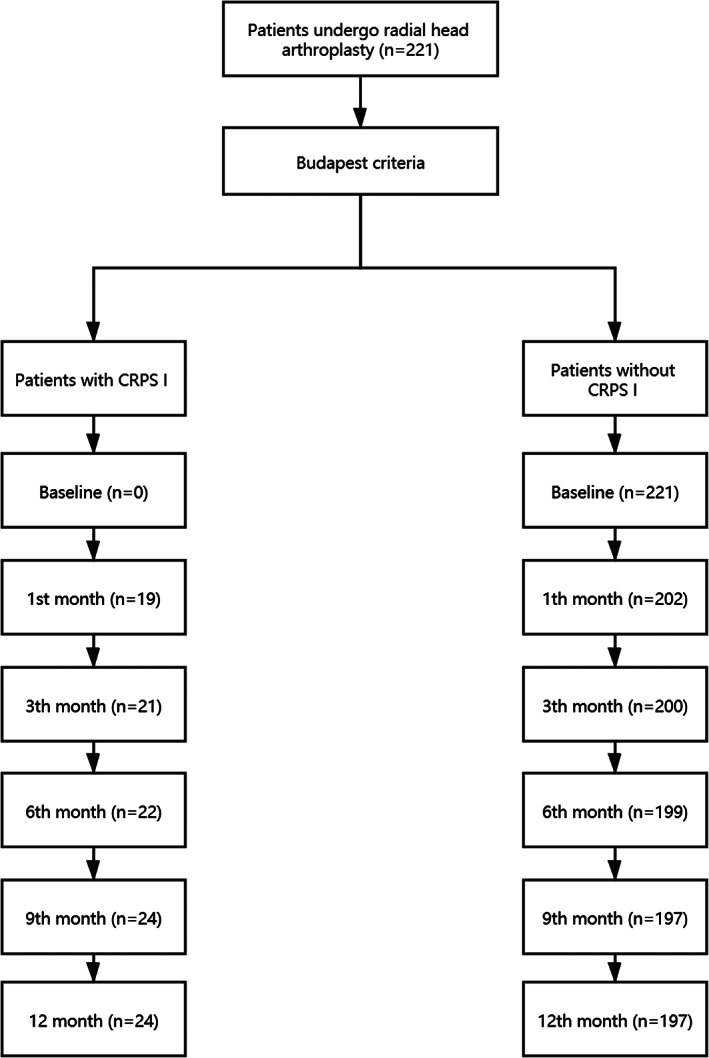
Flow chart of patient inclusion in the present study: radial head fracture patients underwent prosthesis treatment and completed the 1‐year follow‐up.
Evaluations
General Data
Preoperatively, the injuries were classified into high energy (motor vehicle collision/fall from height >1 m), medium energy (fall from <1 m), and low energy (ground‐level fall) types based on the mechanism. Elbow range of motion and strength was assessed by one expert physician. We measured elbow range of motion using a goniometer (Longhua Medical Company, Shijiazhuang, China). We measured elbow flexion strength and grip strength of the hand using a dynamometer (Dongxing Medical Company, Wuxi, Jiangsu, China). The strength scores of the nondominant hand were increased by 5% to exclude the discrepancy between dominant and nondominant hand strength. 24 , 25 Elbow pain was assesses using the 100‐mm VAS.
Intraoperatively, we recorded incision length, operative time, and intraoperative blood loss. According to the soaked gauze weight and the aspirated fluids, the surgeon and the anesthesiologist measured the intraoperative blood loss at the end of the operation. In order to measure the skin incision, digital photography was used to image each wound daily with a ruler included for scale at the end of surgery.
Functional Evaluation
We assessed elbow function using the Mayo Elbow Performance Score (MEPS) 26 (<60, poor; 60–74, fair; 75–89; and 90–100, excellent). Elbow pain was also assessed using the MEPS (none = 45; mild = 30; moderate = 15; severe = 0) 27 We assess elbow disability in activities of daily living using the Patient‐rated Elbow Evaluation (PREE) questionnaire 28 , 29 (<70, poor; 70–80 fair, 80–90 is good and 90–100 excellent. The Shortened Disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) was used to evaluate the patients' upper limb function. The scale scores had a minimum of 0 points (no disability) and a maximum of 100 points (most severe disability). The Chinese version of the Short‐Form Health Survey (SF‐12) was used to evaluate patients' quality of life, 30 The score standard had a maximum of 100 points (best possible outcome).
Mental Status Assessment
We evaluated the patient's psychological conditions using the Hospital Anxiety and Depression Scale (HADS). 31 The scale included the anxiety subscale and depression subscale, with each scale including seven questions. The cut‐off scores of depression and anxiety were eight points. The patients were divided into present cases (depressed or anxious) and absent cases (nondepressed/nonanxious) according to the responses of the questions 32 , 33 (Figure 3).
Fig. 3.
Determinants of complex regional pain syndrome type I among radial head fracture patients with unilateral arthroplasty.
Statistical Analyses
The statistical analyses were performed using Statistical Package for the Social Sciences (SPSS, version 25, Chicago, IL, USA). The mean and standard deviation results are presented for symmetric distribution variables. Independent t‐tests and χ 2 tests were used as univariate analyses to compare the baseline data of patients with and without CRPS I. Multivariate analysis (Backword‐Wald) was used to identify factors independently associated with CRPS I, including two types of predictors. The first type included predictors with statistically significant results (p < 0.1) in univariate analysis, and the second type included clinically relevant variables reported in previous studies. The Pearson correlation coefficient statistic, the −2 log‐likelihood ratio test and Hosmer–Lemeshow goodness‐of‐fit chi‐square test were used to assess the multicollinearity, overall significance and fit of the model. This model also used the estimated values and Pearson and deviation residuals to explore the outliers and detect the influential observations. 34 The results of logistic regression are expressed as ORs and 95% confidence intervals (CIs). All reported p‐values were two‐tailed. Differences were considered statistically significant at p < 0.05.
Results
A total of 461 patients visited our trauma center. In total, 255 patients were recruited in this study. The sample size of this study met the standard of 10 EPV. The reason for non‐participation were as follows: 121 patients have received conservative treatment or other operation methods; 37 patients were diagnosed with multiple trauma; five patients were younger than 18 years old; 31 patients had CRPS I or other chronic pain conditions; and 12 patients declined to participate in this study. 221 patients completed the 1‐year follow‐up. At the end of the follow‐up period, the dropout rate was 13.3% (34 patients: 21 refuse to follow in the study and 13 were unreachable). The demographic characteristics are summarized in Table 1.
TABLE 1.
Demographic characteristics of study sample
| Characteristics | Patients with CRPS I (n = 24) | Patients without CRPS I (n = 197) | T value/x value2 | p value |
|---|---|---|---|---|
| Age (year) | 51.5 ± 14.2 | 58.7 ± 16.8 | −2.013 | 0.045* |
| Gender, n (%) | ||||
| Male | 2 | 54 | ||
| Female | 22 | 143 | 4.116 | 0.042* |
| Dominant hand, n (%) | ||||
| Left | 7 | 35 | ||
| Right | 17 | 162 | 1.806 | 0.179 |
| Injured side | ||||
| Dominant | 20 | 141 | ||
| Non‐dominant | 4 | 56 | 1.496 | 0.221 |
| Body mass index (kg/m2) | 21.3 ± 1.4 | 19.9 ± 3.8 | 1.787 | 0.075 |
| Marital status, n (%) | ||||
| Married | 17 | 138 | ||
| Single | 1 | 0 | ||
| Divorced | 2 | 43 | ||
| Widowed | 4 | 16 | 11.849 | 0.008 |
| Education, n (%) | ||||
| University | 15 | 130 | ||
| Primary and middle | 7 | 63 | ||
| Illiterate | 2 | 4 | 3.224 | 0.200 |
| Job status, n (%) | ||||
| Unemployed (%) | 8 | 71 | ||
| Employed | 16 | 126 | 0.068 | 0.794 |
| Socioeconomic status, n (%) | ||||
| High | 9 | 65 | ||
| Medium | 10 | 87 | ||
| Low | 5 | 45 | 0.198 | 0.906 |
| Type of trauma | ||||
| High energy | 2 | 57 | ||
| Medium | 12 | 104 | ||
| Low energy | 10 | 36 | 9.057 | 0.011* |
| Tobacco use, n (%) | 5 | 39 | 0.014 | 0.904 |
| Alcohol use, n (%) | 1 | 24 | 1.370 | 0.242 |
| Medical problems | ||||
| Hypertension, n (%) | 9 | 51 | 1.458 | 0.227 |
| Diabetes mellitus, n (%) | 5 | 43 | 0.012 | 0.911 |
p < 0.05.
General Results
There were 56 male and 165 female patients. The mean time interval from injury to radial head arthroplasty was 5 days (range, 3–10 days). A total of 24 (11%) patients were diagnosed with CRPS I during the first year after surgery. The average time from operation to onset of CRPS I was 2.7 ± 1.8 weeks. In our series, 19 (79%) patients were diagnosed with CRPS I within 1 month after surgery. There was no significant difference for intraoperative results in all patients, which includes the incision length, operation time and blood loss (p > 0.05). The mean incision length was 8.07 ± 1.02 versus 7.91 ± 1.15 cm (t = 0.651, p = 0.516). The mean operation time was 79. 3 ± 17.9 versus 77.6 ± 18.5min (t = 0.426, p = 0.670), and intraoperative blood loss was 72.5 ± 14.3 versus 74.2 ± 16.9 mL (t = −0.472, p = 0.637). Significant differences in old age, younger than 60 years, high energy trauma. We present clinical characteristics of the patients in Table 2.
TABLE 2.
Clinical characteristics of study sample
| Characteristics | Patients with CRPS I (n = 24) | Patients without CRPS I (n = 197) | T value/x value2 | p value |
|---|---|---|---|---|
| Pain at rest (VAS) (out of 100) | 18.7 ± 9.4 | 12.6 ± 3.2 | −0.437 | 0.664 |
| Pain at activity (VAS) (out of 100) | 57.6 ± 21.5 | 48.3 ± 16.4 | 2.529 | 0.012* |
| Elbow strength (mm) | 91 ± 19.5 | 96 ± 11.2 | −1.875 | 0.062 |
| Quick DASH | 28.3 ± 8.2 | 30.9 ± 6.5 | −1.795 | 0.074 |
| Maximum displacement (mm) | 2.1 ± 0.6 | 2.2 ± 0.3 | −1.344 | 0.180 |
| Number of free fragments | 1.6 ± 0.2 | 1.5 ± 0.6 | 0.810 | 0.419 |
| Elbow range of motion (°) | ||||
| Flexion | 119 ± 23 | 123 ± 19 | −0.951 | 0.343 |
| Extension | 18 ± 5 | 15 ± 8 | 1.793 | 0.074 |
| Pronation | 59 ± 10 | 64 ± 13 | −1.818 | 0.070 |
| Supination | 61 ± 13 | 58 ± 16 | 0.883 | 0.378 |
| SF‐12 (points) | ||||
| Physical (0–100) | 52 ± 6 | 56 ± 7 | −2.681 | 0.008* |
| Mental (0–100) | 52 ± 4 | 55 ± 2 | −6.05 | <0.001* |
| Strength (kg) | ||||
| Flexion | 8.4 ± 2.1 | 8.9 ± 1.8 | −1.261 | 0.209 |
| Grip | 33.6 ± 11.7 | 31.5 ± 13.6 | 0.724 | 0.470 |
| MEPS (points) | ||||
| Pain (0–45) | 28 ± 3 | 24 ± 5 | 3.831 | <0.001* |
| Arc of motion (0–20) | 15 ± 2 | 16 ± 3 | −1.589 | 0.114 |
| Stability (0–15) | 7 ± 3 | 8 ± 3 | −1.542 | 0.125 |
| Daily function (0–20) | 11 ± 5 | 10 ± 2 | 1.857 | 0.065 |
| Total (out of 100) | 61 ± 6 | 59 ± 4 | 2.174 | 0.031* |
| PREE (points) | ||||
| Pain (0–50) | 31 ± 19 | 22 ± 8 | 4.267 | <0.001* |
| Function (0–50) | 22 ± 12 | 23 ± 7 | −0.602 | 0.548 |
| Total (0–100) | 51 ± 21 | 49 ± 18 | 0.504 | 0.614 |
| HADS | ||||
| Depression | 7.4 ± 3.1 | 6.9 ± 2.5 | 0.900 | 0.369 |
| Anxiety | 7.1 ± 3.8 | 6.0 ± 2.2 | 2.104 | 0.037* |
Non‐dominant hand values increased by 5%.
MEPS, Mayo Elbow Performance Score; PREE, Patient‐rated Elbow Evaluation; Quick DASH, Quick Disabilities of the Arm, Shoulder, and Hand; VAS, 100‐mm visual analogue scale.
p < 0.05.
Functional Evaluation
According to the MEPS, we measured the patients' pain level at rest and activity. During the resting state, the VAS scores were 18.7 ± 9.4 in patients with CRPS I to 12.6 ± 3.2 in patients without CRPS I (t = −0.437, p = 0.664). During the activity state, the VAS scores were 57.6 ± 21.5 in patients with CRPS I to 48.3 ± 16.4 in patients without CRPS I (t = 2.529, p < 0.05). Significant difference was observed in the total scores and pain aspect of the MEPS score system (61 ± 6 vs. 59 ± 4, t = 2.174, p < 0.05; 28 ± 3 vs. 24 ± 5, t = 3.831, p < 0.001) There was no significant difference between the total scores of PREE between the two groups (t = 0.504, p > 0.05). However, significant difference was observed in the pain aspect of the PREE score system (31 ± 19 vs. 22 ± 8, t = 4.267, p < 0.01). The mean points of two groups were 28.3 and 30.9. No significant difference was observed in QuickDASH Scores (t = −1.795, P > 0.05).
Mental Status Assessment
The depression scores were 7.4 ± 3.1 and 6.9 ± 2.5 respectively in the two group patients (t = 0.900, p = 0.369), and the anxiety scores were 7.1 ± 3.8 and 6.0 ± 2.2 (t = 2.104, p < 0.05), respectively. A significant difference was observed in anxiety scores between the two groups.
Independently Associated Factors
Significant differences in higher scores of pain at activity, high SF‐12 physical and mental points, higher MEPS pain and total points, and higher PREE pain points were noted between the patients with CRPS I (n = 24) and without CRPS I (n = 197). The parameters with significant differences were regarded as dependent variables and included in multivariable logistic analysis to identify the independently associated factors of developing CRPS I after radial head fractures, which included female sex (OR: 1.537; 95% CI: 1.138–2.072), age younger than 60 years (OR: 1.682; 95% CI: 1.246–2.267), moderate and severe MEPS pain (OR: 3.229; 95% CI: 2.392–4.351) and anxiety (OR: 83.346; 95% CI: 61.752–112.320) (Table 3).
TABLE 3.
Logistic regression for variables predictive factors of occurrence of CRPS I
| Variable | β | Odds ratio | 95% CI | p value |
|---|---|---|---|---|
| Gender (female) | 0.430 | 1.537 | 1.138–2.072 | 0.043* |
| Age (younger than 60 years) | 0.520 | 1.682 | 1.246–2.267 | 0.018* |
| MEPS (moderate and severe pain) | 1.172 | 3.229 | 2.392–4.351 | 0.022* |
| Anxious personality | 4.423 | 83.346 | 61.752–112.320 | 0.011* |
Multivariable logistic analysis was used.
MEPS, Mayo Elbow Performance Score.
p < 0.05.
Discussion
This study reported that the incidence of CRPS I after radial head arthroplasty was 11%. According to the multivariable logistic analysis, the independently associated factors were female sex, age younger than 60 years, moderate and severe MEPS pain and anxiety.
The Incidence of CRPS I after Radial Head Arthroplasty
Jellad et al. 13 reported that, the incidence of CRPS I was 32.2% for distal radius fracture patients, which is higher than our study. The disparity of fracture types and could well explain this difference. Our studies included radial head fracture patients. Compared to the distal radius fracture, radial head fracture may even have more effect on functional recovery after fracture. Another possible explanation of this lower incidence is the difference in therapeutic schedule. The patients included in their study got conservative treatment and our study focused on the patients undergoing unilateral arthroplasty. We also found that the majority of patients met the criteria of CRPS I within 1 month after surgery. Field et al. 35 showed that CRPS I often occurs within 2 weeks in patients who suffered distal radius fractures. In general, elbow pain, swelling, and reduced movement are considered normal features within 7 days after surgery. If the pain persists for a long time and shows no evidence of decreasing or reappears after relief, doctors should consider the possibility of CRPS I.
The Associated Factors of CRPS I
Similarly, some previous studies reported the same results as this study, in which female sex, young age, moderate and severe MEPS pain were the independent risk factors for the development of CRPS I. 4 , 36 , 37 Women seem to exhibit an increased prevalence of radial head fractures compared with men (1.3:1). 37 Another possible cause of this phenomenon is vitamin D deficiency. Women are considered at‐risk populations with a high prevalence of vitamin D deficiency. Previous studies 38 have verified that vitamin D deficiency is the potential reason for neuropathic pain. Yoon et al. 39 reported that younger age was associated with worse postoperative outcomes and more complications, which may partly explain the increased incidence of CRPS I. However, Hastie et al. 40 reported that basal pain sensitivity and modulation vary widely in different patients. Many studies have reported that perioperative pain is one of the risk factors for CRPS I, which may be ascribed to sensitization of the nervous system. 41 , 42 Therefore, orthopedists should pay more attention to perioperative pain.
In our study, there is no significant difference in the depression scores between the patients with and without CRPS I, which was different from those observed in a previous study. 43 In that study, depression may contribute to the development of CRPS. Reverse causation may serve as a potential limitation of the study. Causality cannot be determined from the logistic regression model. On the same hand, it is difficult to explain the changes and abnormalities in the tissues by psychiatric factors only. However, anxiety was also a risk factor for the development of CRPS I. A prospective study performed in the United States verified that high preoperative anxiety levels predict the development of CRPS I in patients undergoing total knee arthroplasty. 44 The increased catecholamine activity could account for the relationship between anxiety and CRPS I. Harden et al. 45 verified that catecholamine levels in the injured limb were significantly increased compared with normal control levels. Additionally, catecholamine levels are associated with the patient's anxiety status. 45
There is no significant difference of the post‐operation elbow function in 9‐month of follow‐up between two groups of patients. This phenomenon may be attributed to the characteristics of CRPS I, because the regional pain of CRPS I is disproportionate in time or severity to the trauma events. 9 However, a previous study by Reimer et al. drew different conclusions, which reported that motor dysfunction, sensory symptoms and mild pain persisted in CRPS‐I. 46 The factors responsible for the seemingly conflicting results of these prior studies are unclear but may be related to the small sample size of their study (n = 19) resulting in larger variance around outcome estimates. Further investigation into the effects of CRPS‐I on post‐operation elbow function is needed to draw a more precise conclusion.
Our study only included patients undergoing radial head arthroplasty. Given the lack of a control conservative treatment group, we cannot determine whether radial head arthroplasty exerts an influence on the progression of CRPS I. In 2021, Jacques et al. 47 verified that the incidence of CRPS I after total knee arthroplasty was 13%. Therefore, they suggested that doctors should closely monitor CRPS I and provide appropriate interventions as early as possible in patients undergoing arthroplasties. For radial head fracture patients, a vigilance medical care could lead to greater potential gain, which includes careful medical history taking, optimal postoperative pain management, and prompt intervention providing. Medical history taking should more focus on patients' biological, psychological, and social condition, which may identify associated risk factors. For suspected CRPS I cases, postoperative pain management should be provided by a multidisciplinary team, including physical therapy, prompt intervention providing, and psychological counseling.
Limitations
The limitations of this study were as follows. First, our study is based on patients who underwent radial head arthroplasty, and the results may not be universally applicable to patients who undergo other treatments. Second, the findings of this study are limited to the small sample size, which limits the generalizability of our results. Third, some factors, such as radiological parameters of fractures and the cost of surgery, were not included in this study. The strength of this study was the use of a multivariate analysis regression model and the availability of demographic and clinical data to CRPS I in radial head fracture patients undergoing unilateral arthroplasty. As far as know, this is the first study to explore the incidence of CRPS I in radial head fracture patients undergoing unilateral arthroplasty. Another important point of this study was the identification of associations between baseline data, clinical data and CRPS I in radial head fracture patients undergoing unilateral arthroplasty.
Conclusion
In this prospective observational study, the proportion of CRPS I in radial head fracture patients undergoing unilateral arthroplasty was 11% and to explore associated factors. The risk factors for developing CRPS I after radial head arthroplasty include female sex, younger age, moderate and severe MEPS pain and anxiety. Future research needs to base upon more large survey sample to assess the actual prevalence of CRPS I in radial head fracture patients with different surgical methods.
Acknowledgements
The authors are grateful to all participants who willingly participated in their study and their biostatistics department.
Disclosure: The authors declare that they have no conflict of interest.
References
- 1. Ha AS, Petscavage JM, Chew FS. Radial head arthroplasty: a radiologic outcome study. AJR Am J Roentgenol. 2012;199(5):1078–82. [DOI] [PubMed] [Google Scholar]
- 2. Madsen JE, Flugsrud G. Radial head fractures: indications and technique for primary arthroplasty. Eur J Trauma Emerg Surg. 2008;34(2):105–12. [DOI] [PubMed] [Google Scholar]
- 3. Schmitz MR, Murtha AS, Clohisy JC, Group AS . Developmental dysplasia of the hip in adolescents and young adults. J Am Acad Orthop Surg. 2020;28(3):91–101. [DOI] [PubMed] [Google Scholar]
- 4. de Mos M. , de Bruijn AGJ, Huygen FJPM, Dieleman JP, Stricker BH, Sturkenboom MC. The incidence of complex regional pain syndrome: a population‐based study. Pain, 2007, 129(1–2):12–20. [DOI] [PubMed] [Google Scholar]
- 5. Sandroni P, Benrud‐Larson LM, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted county, a population‐based study. Pain. 2003;103(1–2):199–207. [DOI] [PubMed] [Google Scholar]
- 6. Birklein F, Dimova V. Complex regional pain syndrome–up‐to‐date. Pain Rep. 2017;2(6):e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Varenna M, Crotti C, Ughi N, Zucchi F, Caporali R. Determinants of diagnostic delay in complex regional pain syndrome type 1: an observational study of 180 consecutive new cases. J Clin Rheumatol. 2021;27(8):e491–5. [DOI] [PubMed] [Google Scholar]
- 8. Mos M, Sturkenboom MCJM, Huygen FJPM. Current understandings on complex regional pain syndrome. Pain Pract. 2009;9(2):86–99. [DOI] [PubMed] [Google Scholar]
- 9. Iolascon G, Sire A, Moretti A, Gimigliano F. Complex regional pain syndrome (CRPS) type I: historical perspective and critical issues. Clin Cases Miner Bone Metab. 2015;12(Suppl 1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chapman KB, Spiegel MA, Dickerson DM, Billet B, Patel KV, Hunter C, et al. A paramedian approach for dorsal root ganglion stimulation placement developed to limit lead migration and fracture. Pain Pract. 2021;21(8):991–1000. [DOI] [PubMed] [Google Scholar]
- 11. Zafereo J, Jones S, Jarrett RB, Frost S, Noe C. Improved symptoms of complex regional pain syndrome after novel lymphatic treatment and interdisciplinary pain management. Complement Ther Clin Pract. 2022;46:101512. [DOI] [PubMed] [Google Scholar]
- 12. Sahbaie P, Li W‐W, Guo T‐Z, Shi XY, Kingery WS, Clark JD. Autonomic regulation of nociceptive and immunologic changes in a mouse model of complex regional pain syndrome. J Pain. 2021;46:101512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jellad A, Salah S, Frih ZBS. Complex regional pain syndrome type I: incidence and risk factors in patients with fracture of the distal radius. Arch Phys Med Rehabil. 2014;95(3):487–92. [DOI] [PubMed] [Google Scholar]
- 14. Harada M, Takahara M, Mura N, Yuki L, Tsuruta D, Takagi M. Risk factors related to complications of the fingers and hand after arthroscopic rotator cuff repair—carpal tunnel syndrome, flexor tenosynovitis, and complex regional pain syndrome. JSES Int. 2021;5(6):1077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoon D, Xu Y, Cipriano PW, Alam LS, Aparici CM, Tawfik V, et al. Neurovascular, muscle, and skin changes on [18F]FDG PET/MRI in complex regional pain syndrome of the foot: a prospective clinical study. Pain Med. 2022;23(2):339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moretti A, Gimigliano F, Paoletta M, Bertone M, Liguori S, Toro G, et al. Complex regional pain syndrome type I following non‐orthopedic surgery: case report and narrative review. Diagnostics (Basel). 2021;11(9):1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jo Y‐H, Kim K, Lee B‐G, Kim J‐H, Lee C‐H, Lee K‐H. Incidence of and risk factors for complex regional pain syndrome type 1 after surgery for distal radius fractures: a population‐based study. Sci Rep. 2019;9(1):4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mv S, Moons KG, Groot JA, Collins GS, Altman DG, Eijkmans MJ, et al. Sample size for binary logistic prediction models: beyond events per variable criteria. Stat Methods Med Res. 2019;28(8):2455–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Satteson ES, Harbour PW, Koman LA, Smith BP, Li Z. The risk of pain syndrome affecting a previously non‐painful limb following trauma or surgery in patients with a history of complex regional pain syndrome. Scand J Pain. 2017;14:84–8. [DOI] [PubMed] [Google Scholar]
- 20. Fusaro I, Orsini S, Kantar SS, Sforza T, Benedetti MG, Bettelli G, et al. Elbow rehabilitation in traumatic pathology. Musculoskelet Surg. 2014;98(Suppl 1):95–102. [DOI] [PubMed] [Google Scholar]
- 21. Catellani F, Caro F, Biase CF, Perrino VR, Usai L, Triolo V, et al. Radial head resection versus arthroplasty in unrepairable comminuted fractures Mason type III and type IV: a systematic review. Biomed Res Int. 2018;2018:4020625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eyberg BA, McKee MD. Indications and clinical results of radial head replacement: has anything changed? J Orthop Trauma. 2019;33(Suppl 8):S1–6. [DOI] [PubMed] [Google Scholar]
- 23. Harden NR, Bruehl S, Perez RSGM, Birklein F, Marinus J, Maihofner C, et al. Validation of proposed diagnostic criteria (the "Budapest criteria") for complex regional pain syndrome. Pain. 2010;150(2):268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chung KC, Pillsbury MS, Walters MR, Hayward RA. Reliability and validity testing of the Michigan hand outcomes questionnaire. J Hand Surg Am. 1998;23(4):575–87. [DOI] [PubMed] [Google Scholar]
- 25. Chung KC, Hamill JB, Walters MR, Hayward RA. The Michigan hand outcomes questionnaire (MHQ): assessment of responsiveness to clinical change. Ann Plast Surg. 1999;42(6):619–22. [DOI] [PubMed] [Google Scholar]
- 26. Cusick MC, Bonnaig NS, Azar FM, Mauck BM, Smith RA, Throckmorton TW. Accuracy and reliability of the Mayo elbow performance score. J Hand Surg Am. 2014;39(6):1146–50. [DOI] [PubMed] [Google Scholar]
- 27. Valone LC, Waites C, Tartarilla AB, Whited A, Sugimoto D, Bae DS, Bauer AS. Functional elbow range of motion in children and adolescents. J Pediatr Orthop. 2020;40(6):304–9. [DOI] [PubMed] [Google Scholar]
- 28. MacDermid JC. Outcome evaluation in patients with elbow pathology: issues in instrument development and evaluation. J Hand Ther. 2001;14(2):105–14. [DOI] [PubMed] [Google Scholar]
- 29. Vincent JI, MacDermid JC, King GJ, Grewal R. Validity and sensitivity to change of patient‐reported pain and disability measures for elbow pathologies. J Orthop Sports Phys Ther. 2013;43(4):263–74. [DOI] [PubMed] [Google Scholar]
- 30. Fan ZJ, Smith CK, Silverstein BA. Assessing validity of the QuickDASH and SF‐12 as surveillance tools among workers with neck or upper extremity musculoskeletal disorders. J Hand Ther. 2008;21(4):354–65. [DOI] [PubMed] [Google Scholar]
- 31. Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–70. [DOI] [PubMed] [Google Scholar]
- 32. Beaton DE, Wright JG, Katz JN. Development of the QUICKDASH: comparison of three item‐reduction approaches. J Bone Joint Surg Am. 2005;87(5):1038–46. [DOI] [PubMed] [Google Scholar]
- 33. Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full‐length DASH. BMC Musculoskelet Disord. 2006;7:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hayes AF, Matthes J. Computational procedures for probing interactions in OLS and logistic regression: SPSS and SAS implementations. Behav Res Methods. 2009;41(3):924–36. [DOI] [PubMed] [Google Scholar]
- 35. Field J, Atkins RM. Algodystrophy is an early complication of Colles' fracture. What are the implications? J Hand Surg Br. 1997;22(2):178–82. [DOI] [PubMed] [Google Scholar]
- 36. Veldman PH, Goris RJ. Surgery on extremities with reflex sympathetic dystrophy. Unfallchirurg. 1995;98(1):45–8. [PubMed] [Google Scholar]
- 37. Klug A, Gramlich Y, Wincheringer D, Hoffmann R, Schmidt‐Horlohe K. Epidemiology and treatment of radial head fractures: a database analysis of over 70,000 inpatient cases. J Hand Surg Am. 2021;46(1):27–35. [DOI] [PubMed] [Google Scholar]
- 38. Lee S‐U, Na K‐T, Lee Y‐M, Park JH, Joo SY. Low vitamin D levels in post‐menopausal women are associated with complex regional pain syndrome type I in surgically treated distal radius fractures. J Orthop Surg Res. 2020;15(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yoon A, King GJW, Grewal R. Is ORIF superior to nonoperative treatment in isolated displaced partial articular fractures of the radial head? Clin Orthop Relat Res. 2014;472(7):2105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hastie BA, Riley JL, Robinson ME, Glover T, Campbell CM, Staud R, et al. Cluster analysis of multiple experimental pain modalities. Pain. 2005;116(3):227–37. [DOI] [PubMed] [Google Scholar]
- 41. Perez RS, Zollinger PE, Dijkstra PU, Thomassen‐Hilgersom IL, Zuurmond WW, Rosenbrand KC, et al. Evidence based guidelines for complex regional pain syndrome type 1. BMC Neurol. 2010;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martini ML, Caridi JM, Zeldin L, Neifert SN, Nistal DA, Kim JD, et al. Perioperative outcomes of spinal cord stimulator placement in patients with complex regional pain syndrome compared with patients without complex regional pain syndrome. World Neurosurg. 2020;137:e106–17. [DOI] [PubMed] [Google Scholar]
- 43. Puchalski P, Zyluk A. Complex regional pain syndrome type 1 after fractures of the distal radius: a prospective study of the role of psychological factors. J Hand Surg Br. 2005;30:574–80. [DOI] [PubMed] [Google Scholar]
- 44. Harden NR, Bruehl S, Stanos S, Brander V, Chung OY, Saltz S, et al. Prospective examination of pain‐related and psychological predictors of CRPS‐like phenomena following total knee arthroplasty: a preliminary study. Pain. 2003;106(3):393–400. [DOI] [PubMed] [Google Scholar]
- 45. Harden RN, Rudin NJ, Bruehl S, Kee W, Parikh DK, Kooch J, et al. Increased systemic catecholamines in complex regional pain syndrome and relationship to psychological factors: a pilot study. Anesth Analg. 2004;99(5):1478–85. [DOI] [PubMed] [Google Scholar]
- 46. Reimer M, Rempe T, Diedrichs C, Baron R, Gierthmühlen J. Sensitization of the nociceptive system in complex regional pain syndrome. PLoS One. 2016;11(5):e0154553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jacques H, Jérôme V, Antoine C, Lucile S, Valerie D, Amandine L, et al. Prospective randomized study of the vitamin C effect on pain and complex pain regional syndrome after total knee arthroplasty. Int Orthop. 2021;45(5):1155–62. [DOI] [PubMed] [Google Scholar]


