Abstract
Objective
To determine whether more precise cup positioning can be achieved with robot‐assisted total hip arthroplasty (THA) as compared to conventional THA.
Methods
In this study, between July 2019 and May 2021, 93 patients aged 23–75 years with osteonecrosis of the femoral head (ONFH) and adult developmental dysplasia of hip who underwent first hip surgery were included in the study. They were randomly assigned to either the robotic‐assisted THA group (n = 45) or the conventional THA group (n = 48). After the operation, all patients were given routine rapid rehabilitation guidance. The duration of operation was recorded to estimate the learning curve through cumulative summation analysis. We compared the demographics, duration of operation, cup positioning, leg length discrepancy, hip offset, and Harris Hip Score between robot‐assisted THA and manual THA. Precision in the positioning of the acetabular prosthesis using the MAKO system was also compared between the two groups.
Results
The mean duration of operation for the robot‐assisted THA group was 91.37 ± 17.34 min (range: 63 to 135 min), which was significantly higher than that for the conventional THA group. When the number of procedures was increased to 13, the duration of operation in the robot‐assisted group decreased significantly and gradually became stable. In terms of duration of operation, robot‐assisted THA was associated with a learning curve of 13 cases. The mean amount of bleeding in the robot‐assisted THA group was not significantly different from that in conventional THA group (328 ± 210 ml vs 315 ± 205 ml) (p = 0.741). There was no significant difference in the proportion of prostheses located within Lewinnek's safe zone between robot‐assisted THA group and conventional THA group (69.81% vs 64.41%). The leg length discrepancy (LLD) was significantly smaller in the robot‐assisted THA group than in the conventional THA group (p < 0.001), but both were within acceptable limits (10 mm). The inclination and anteversion angles of the acetabular prosthesis planned before operations were correlated with the actual measurement (r = 0.857 p < 0.001, r = 0.830, p < 0.001). After surgery, none of the patients experienced hip dislocation, aseptic loosening, or periprosthetic infection during the 3 months of follow‐up.
Conclusion
The proportion of acetabular prostheses in the Lewinnek's safety zone was higher and the extent of LLD was significantly lower in the robot‐assisted THA group, as compared to the same metrics in the conventional THA group. The MAKO robot improved the accuracy of implant placement in THA.
Keywords: cup positioning, learning curve, robot‐assisted surgery, total hip arthroplasty
A total of 91 patients were enrolled in this study (robot‐assisted THA group: 44 cases, manual THA group: 47 cases). For operating time, robot‐assisted THA was associated with a learning curve of 13 cases. Located within Lewinnek's safe zone, the two groups had no significant differences (robot‐assisted THA 88.67%, manual THA 64.41%). MAKO robot can enhance the accuracy of implant placement in THA.

Introduction
Total hip arthroplasty (THA) is one of the most common orthopaedic interventions for reducing pain, improving function, and enhancing the quality of life of patients with debilitating hip disease 1 . More than 600,000 people undergo THA annually worldwide, in 2018 400,000 people were treated with THA in China alone. This number is projected to increase in the near future, with about 1 million procedures expected to be performed annually worldwide by 2030 2 .
The success of this treatment strongly depends on the accuracy of implant placement. Poor acetabular component orientation may lead to various modes of early failures, increased rates of dislocation, impingement, pelvic osteolysis, acetabular migration, and polyethylene wear 3 . Hip offset and leg length are important external performance metrics for this treatment. Risks associated with THA include leg length discrepancy (LLD), hip dislocation, and nerve injury 4 , 5 . A large, population‐based study reported that malpositioning exceeded the acceptable range in a little more than 50% of the cases (917 out of 1823) 6 . While the malpositioning of cups is associated with surgical approach, volume, and obesity (body mass index >30) in conventional THA 6 , misalignment is probably the single most important variable. Overall, acetabular orientation is a key parameter that affects the success of THA. Dobzyniak et al. have reported that 291 revisions (39% of their sample size) were performed during the first 5 years after THA surgery. A third of these revision surgeries were performed due to instability 7 . Lewinnek et al. defined the safe zone of cup inclination and version to minimize dislocation as 45° ± 10° inclination and 15° ± 10° of anteversion. Most surgeons perform THA on the basis of this Lewinnek safe zone 8 . Najarian et al. have demonstrated the use of navigation and robot‐assisted technologies to aid and improve the positioning and biomedically accurate recovery of the hip prosthesis 9 . The MAKO Robotic Arm Interactive Orthopaedic System (Stryker Corporation; Kalamazoo, MI, USA) is the latest semi‐active robotic system available to assist with THA. Based on CT scans, it can suggest adjustments in the distance from the superior border of the lesser trochanter to the teardrop line (hip length) from the initial preoperative level during preoperative planning as well as during the operation 10 . Robotic assistance is an excellent tool to facilitate successful implant placement during THA 11 , 12 , 13 , 14 . MAKO Robotic Arm Interactive Orthopaedic System has been recently introduced in China. There is a non‐trivial learning curve associated with achieving proficiency in the use of this system for robot‐assisted surgery 15 , 16 . Some reports suggest that post learning curve, robot‐assisted surgery has an advantage over conventional technique in cup positioning 15 . However, currently there are only a limited number of reports available in the literature about the learning curve for the adoption phase of the technology to draw generalized conclusions. As of now, the value of robot‐assisted THA, as compared to conventional THA, remains unclear 17 .
The purpose of this study was: (i) to assess the learning curve of surgeons using MAKO robot‐assisted THA in terms of duration of operation; (ii) to evaluate whether higher accuracy can be achieved in cup positioning by MAKO robot‐assisted THA than by conventional THA.
Materials and Methods
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (i) patients aged between 18 and 75 years; (ii) patients suffering from osteonecrosis of the femoral head (ONFH) in ARCO stage III or IV 18 or Crowe type I or II adult developmental dysplasia of hip (DDH) 19 ; (iii) patients who underwent first hip surgery.
The exclusion criteria were as follows: (i) patients with incomplete clinical data; (ii) patients with nonstandard radiographs; (iii) patients with pathologic fractures or inflammatory arthritis; (iv) patients with neurological or severe systemic diseases affecting musculoskeletal function.
Study Design
The Ethical Committee of Cangzhou Hospital of Integrated TCM‐WM (Hebei, China) approved this study (No. 2015/065/010). Written, informed consent was also obtained from all patients. The MAKO Robotic Arm Interactive Orthopaedic System was introduced into our hospital in 2019. We enrolled patients diagnosed with avascular necrosis of the femoral head or osteoarthritis, and who had undergone THA between July 2019 and May 2021. During the study period, a total of 133 patients were hospitalized for THA. According to a sequence of computer‐generated random numbers, 93 patients who met the criteria and agreed to participate were randomly assigned to either robot‐assisted THA group (n = 45) or conventional THA group (n = 48). In the conventional THA group, no computer navigation system was employed. Each of the robot‐assisted THA group and the conventional THA group had one patient who failed to complete the follow‐up. All procedures were performed by the same team led by a chief physician who had more than 10 years of experience in conventional THA, but with no clinical experience either in robot‐assisted or CT‐based navigation prior to this study. The video of the sequence of operations was recorded in the robot‐assisted THA group, to enable assessment of the learning curve.
Preoperative Preparations
In the conventional THA group, patients underwent preoperative hip radiographs in the supine position 20 and standard pelvic radiographs. We used anteroposterior pelvic radiographs of the patients in standing position and the software TraumaCad® (Brainlab Company, USA) for preoperative template measurements to plan the type and position of the surgical prosthesis. Preoperative 3D CT scans of the hip (including that of the whole pelvis) and knee were performed on patients in the robot‐assisted THA group. This data was uploaded into the MAKO robot‐assisted total hip system (Stryker Orthopaedics, Mahwah, NJ) to create a 3D model for preoperative planning and design. All acetabular cups were placed at 40° inclination and 20° anteversion. The surgeon could choose between various types of femoral stem to optimize bone contact, hip length, and offset.
Surgical Techniques
Anesthesia and Position
All surgical procedures were performed by the same specialist surgical team. During the procedure the patients were placed under general anesthesia, lying on their sides on the operating table.
In the robot‐assisted THA group, the camera stand and robotic arm were placed in a suitable position beside the patient's head and on the opposite side of the main surgeon, operating according to the rapid workflow of the MAKO system.
Approach
All patients were treated with THA through a posterolateral approach without trochanteric osteotomy, using Stryker Howmedica Osteonics™ Trident® cup with an Accolade™ femoral component (Stryker Orthopaedics, Mahwah, NJ). The duration of operation was defined as the time from the initial skin incision to the final skin suture.
Femoral Neck Osteotomy and Prosthetic Implantation
Figure 4 shows the detailed flow chart of the operation for patients in the robot‐assisted THA group. After installing the pelvic locator, the standard posterolateral hip approach was selected. Before dislocation of the hip, the proximal and distal femur checkpoints were collected. The length of lower limb and hip eccentricity was recorded before operation. Subsequently, the surgeon dislocated the joint and performed femoral neck osteotomy. The posterior edge, anterior edge, and upper edge of the acetabulum were marked, and a total of 32 registration points were accurately noted. The reamer was inserted into the acetabulum, and the appropriate volume of bone tissue was removed as per the plan using the haptic arm. The acetabular cup was inserted into the appropriate position. Although the MAKO system did provide a robot‐assisted installation mode, the femoral stem was manually implanted. The robot‐assisted mode in the MAKO system was time‐consuming. It involved exposing the proximal femur, manual reaming, installing the femoral handle to test the model, then repositioning to confirm the stability and range of motion (ROM) of the hip joint, recording the length of the lower limbs and the eccentricity of the hip joint, and finally installing the real femoral prosthesis. No drainage was placed.
Fig. 4.
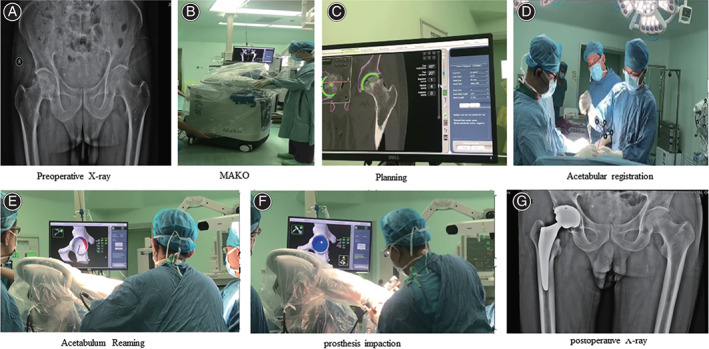
A robot‐assisted THA process for a patient with necrosis of the femoral head. A 67‐year‐old man complained that he had pain in his right hip joint when he was walking, which bothered him for 2 years. The X‐ray plain film of the hip joint showed necrosis of the right femoral head. In 2019 the patient underwent Mako robot‐assisted THA on the right side. The patient reported good recovery, no pain, and good mobility. (A) preoperative X‐ray of the hip; (B) MAKO Robotic Arm Interactive Orthopaedic System; (C) preoperative planning; (D) acetabular registration; (E) acetabulum reaming; (F) prosthesis impaction; (G) postoperative X‐ray of the hip
In the conventional THA group, femoral neck osteotomy was performed for the implantation of prosthesis according to preoperative template measurement. Intraoperatively, the LLD was evaluated and adjusted according to the inferior patellar pole in the lateral recumbent position.
Postoperative Management and Follow‐up
After the operation, all patients received routine antibiotic treatment, deep vein thrombosis (DVT) prophylaxis, multi‐mode analgesics, and rapid rehabilitation guidance. The patients were able to walk with crutches the next day. Follow‐up examination, including X‐ray imaging of the hip joint, was done on outpatients for 3 months after the operation.
Clinical Functional Assessments
The functioning of the hip was evaluated using Harris hip score (HHS) and Western Ontario and McMaster Universities Arthritis Index (WOMAC) Osteoarthritis assessment techniques.
Harris hip score
HHS is a collection of numerical rating criteria put forward by Harris, which is widely used to evaluate hip joint function. The evaluation included pain, function, ROM, and deformity. With a maximum possible HHS score of 100, scores in the range 90–100 were rated as excellent, scores in the range 80–89 were rated as good, scores in the range 70–79 were rated as fair, and scores less than 70 were rated poor 21 .
WOMAC Osteoarthritis Scoring
This scale assesses the structure and function of the joint on 24 items in three areas: pain, stiffness, and function. Higher WOMAC scores indicate more severe arthritis. Scores less than 80 indicate mild arthritis, scores in the range 80–120 indicate moderate arthritis and scores more than 120 indicate severe arthritis.
Radiological Assessments
Detailed radiological evaluation of the hip, including LLD, anteversion and inclination of acetabular cup, was carried out postoperatively using X‐ray imaging and CT scans. All patient‐identifying information was removed from the radiographs.
Leg Length Discrepancy (LLD)
LLD, the vertical distance between the bilateral lesser trochanter and bilateral teardrop 22 , was measured based on the anterior and posterior pelvic X‐ray images taken after operation. LLD exceeding 10 mm was considered unacceptable.
Inclination Angle
The inclination angle is defined as the angle between the long axis of the acetabular cup and the bilateral teardrop line. Inclination angle or forward inclination angle in the range of the angular value targeted in the surgical plan ±10° were considered acceptable.
Anteversion Angle
The anteversion angle is calculated by acetabular cup shadow projection method, as calculated by the expression below. The acceptable range was the planned angle ±5°. anteversion angle = arc‐sin (short axis/major axis).
The anteversion angle and the inclination angle of each patient were compared with the Lewinnek's safe zone (inclination angle 30°–50°, anteversion angle 5–25°) to verify that the placement angle of the prosthesis was appropriate. 8
Statistical Analysis
All statistical analyses were performed using SPSS, Version 26 (SPSS Inc., Chicago, Illinois). Quantitative data were recorded as X¯ ± SD and analyzed by the t‐test, while the counts were analyzed either with the χ2‐test or Fisher's exact probability method.
The learning curve was evaluated using the cumulative summation (CUSUM) method. This was used to sort all cases according to the operation date, and the deviation between the observed value of each sample and the target value (the mean duration of operation in all cases in the robot‐assisted THA group) was calculated. Progressive curve fitting was used on the scatter plots obtained by the CUSUM method. The end of the learning curve was determined as the point where the slope of the curve changed from positive to negative.
Two competent surgeons, blinded to the patient grouping, as well as to each other's judgments, independently determined HHS and WOMAC Osteoarthritis scores, as well as reviewed the patients' radiography results. The inter‐observer consistency was analyzed using intraclass correlation coefficient (ICC) test.
The correlation between the MAKO surgical plan and postoperative measurement of anteversion angle, inclination angle, and acetabular reconstruction position was assessed by Pearson correlation test. All P values were two‐sided. p‐Values less than 0.05 were considered statistically significant.
Results
General Results
In this study, 44 patients (53 hips) underwent robot‐assisted THA. This included 24 males and 20 females. There were 20 cases (25 hips) of ONFH and 24 cases (28 hips) of DDH. The conventional THA group consisted of 47 patients (59 hips) (24 males, 23 females), including 24 cases (30 hips) of ONFH, and 23 cases (29 hips) of DDH. There was no evidence of aseptic loosening and periprosthetic joint infection. No significant difference was noted in demographic characteristics and hip function between the two groups (Table 1).
TABLE 1.
Comparison of the clinical data between robot‐assisted THA and conventional THA
| Parameter | Robot‐assisted THA (n = 44) | Conventional THA (n = 47) | T value/χ2value | p value |
|---|---|---|---|---|
| Number of hips (left/right) | 53(25/27) | 59(28/31) | 0.01 | 0.337 |
| Age (years), | 53.2 ± 12.5 | 52.7 ± 11.8 | 0.19 | 0.885 |
| Male (%) | 24(54.54) | 24(51.06) | 0.11 | 0.819 |
| BMI (kg/m2) | 24.93 ± 2.74 | 25.27 ± 3.15 | 0.54 | 0.686 |
| Diagnoses (%) | 0.15 | 0.807 | ||
| DDH (hips) | 28 | 29 | 0.01 | 0.900 |
| ARCO stage III | 13 | 14 | ||
| ARCO stage IV | 15 | 15 | ||
| ONFH (hips) | 25 | 30 | 0.01 | 0.863 |
| Crowe type I | 12 | 14 | ||
| Crowe type II | 13 | 16 | ||
| WOMAC | 70.35 ± 6.37 | 71.25 ± 6.54 | 0.66 | 0.562 |
| HHS | 54.80 ± 4.72 | 55.10 ± 3.76 | 0.33 | 0.769 |
| LLD (mm) | 9.20 ± 5.60 | 9.60 ± 4.70 | 0.37 | 0.786 |
Note: Results are expressed as mean ± SD or number of individuals (percentages).
Abbreviations: BMI, body mass index; DDH, developmental hip dysplasia; HHS, Harris Hip Score; LLD, leg length discrepancy; ONHF, osteonecrosis of the femoral head; SD, standard deviation; WOMAC, Western Ontario and McMaster osteoarthritis index.
The mean amount of bleeding in the robot‐assisted THA group was greater than but not significantly different from that in conventional THA group [(328 ± 210) ml. vs. (315 ± 205) ml)] (p = 0.741). The length of hospital stays also did not differ significantly between the two groups (p = 0.409) (Table 2).
TABLE 2.
Comparison of the postoperative clinical data between proficient robot‐assisted THA and conventional THA (°)
| Parameter | Robot‐assisted THA (53 hips) | Conventional THA (59 hips) | t value | p value |
|---|---|---|---|---|
| Duration of operation (min) | 91.37 ± 17.34 | 77.52 ± 6.04 | 5.76 | <0.001 |
| Amount of bleeding (ml) | 328 ± 210 | 315 ± 205 | 0.33 | 0.741 |
| LOH (d) | 4.32 ± 1.69 | 4.68 ± 1.35 | 1.25 | 0.409 |
| HHS | 84.52 ± 5.37 | 85.61 ± 8.73 | 0.78 | 0.531 |
| WOMAC | 16.78 ± 2.15 | 17.07 ± 1.05 | 0.92 | 0.359 |
| Cup anteversion (°) | 21.13 ± 5.65 | 17.29 ± 8.46 | 2.79 | 0.006 |
| Cup inclination (°) | 41.48 ± 4.20 | 40.48 ± 6.39 | 0.96 | 0.338 |
| Lewinnek's safe zone (%) | 37 (69.81) | 38 (64.41) | 0.36 | 0.544 |
| LLD (mm) | 3.22 ± 2.71 | 6.95 ± 3.02 | 6.84 | <0.001 |
| Offset‐D (mm) | 3.23 ± 1.32 | 3.65 ± 1.57 | 1.52 | 0.311 |
Note: Results are expressed as mean ± SD or number of individuals (percentages).
Abbreviations: HHS, Harris hip score; LLD, leg length discrepancy; LOH, postoperative length of hospitalization; Offset‐D, offset discrepancy.
Learning Curve
In the present study, the operations in both groups were completed successfully, and the incisions healed well. The duration of operation was significantly higher in the robot‐assisted THA group than those in the conventional THA group [(91.37 ± 17.34) min vs (77.52 ± 6.17) min] (p < 0.001) (Table 2). However, after 13 cases, the duration of operation in the robot‐assisted group decreased significantly and gradually became stable (Figure 1). The amount of bleeding decreased and stabilized as the duration of the operation stabilized.
Fig. 1.
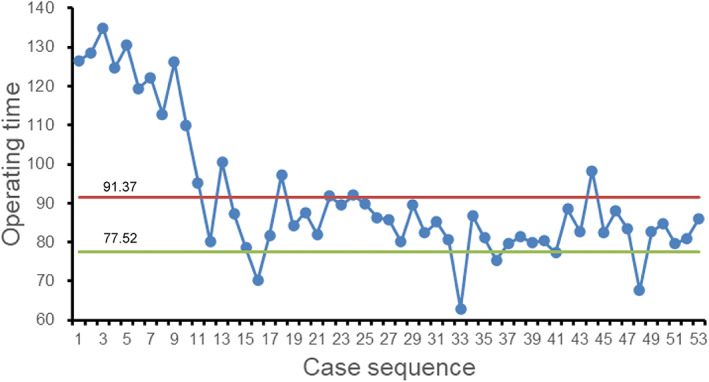
Scatter plot between the duration of operation in robot‐assisted THA. (The solid red line represents the mean duration of operation of robot‐assisted THA; the solid green line represents the mean duration of operation of conventional THA)
CUSUM analysis of duration of operation in robot‐assisted THA group identified an inflection point after the initial 13 patients, which suggested two different stages on the learning curve (Figure 2).
Fig. 2.
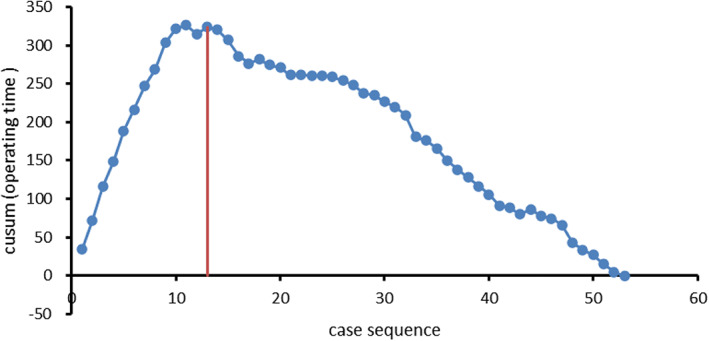
The LC‐CUSUM analysis of the duration of operation in robot‐assisted THA group
Implant Positioning and Orientation
The intra‐group correlation coefficient between observers was 0.91 (95% CI 0.88–0.94), which indicated good consistency. The final value was the mean of the values determined by the two surgeons.
Clinical Functional Assessments
Postoperatively, the WOMAC decreased and the HHS increased in both groups compared with the preoperative period, but the differences between the groups were not statistically significant (p > 0.05, Table 2).
Radiological Assessments
There were no significant differences in acetabular inclination angle and offset discrepancies between groups during follow‐up (p > 0.05) (Table 2). Mean acetabular anteversion angle was higher in the robot‐assisted THA group (22.78 ± 5.49), as compared with conventional THA group (15.76 ± 8.02, p < 0.001). The proportion of acetabular prosthesis in Lewinnek's safe zone was higher in robot‐assisted THA group than in conventional THA group [69.81% (37/ 53) vs 64.41% (38/59)] with no significant difference (p = 0.544, Figure 3). The difference of LLD was significantly smaller in robot‐assisted THA group than in conventional THA group (p < 0.001), which, however, were within the acceptable limit (10 mm) Figure 4.
Fig. 3.
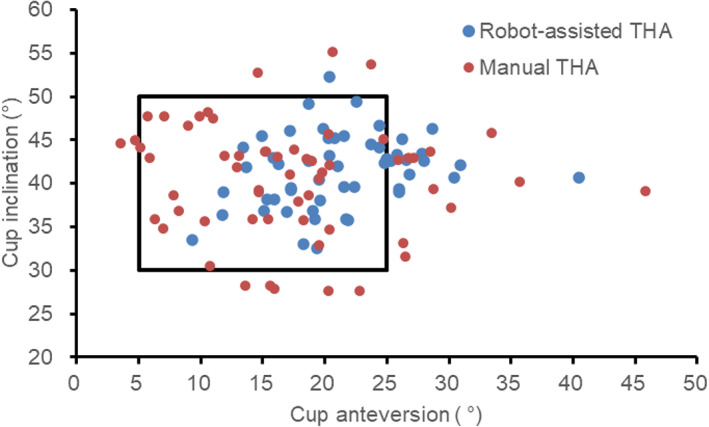
The scatter plot of cup positioning in robot‐assisted THA and conventional THA
Accuracy of Robot‐Assisted THA
The inclination and anteversion angle of the acetabular prosthesis planned before operations were correlated with the actual measurement (r = 0.857, p < 0.001, r = 0.830, p < 0.001). The difference between the planned and the actual measurement was 1.43 ± 0.15° and 1.47 ± 0.11°, respectively (Table 3).
TABLE 3.
Comparison of the planned implant positions by MAKO robot and the actual positions
| Parameter | Planned | Actual | Difference | r | p value |
|---|---|---|---|---|---|
| Cup inclination (°) | 40.72 ± 1.81 | 41.48 ± 4.20 | 1.43 ± 0.15 | 0.857 | <0.001 |
| Cup anteversion (°) | 20.57 ± 2.37 | 21.13 ± 5.65 | 1.47 ± 0.11 | 0.830 | <0.001 |
| Horizontal distance (mm) | 31.57 ± 4.97 | 29.32 ± 5.38 | 1.56 ± 0.17 | 0.983 | <0.001 |
| Vertical distance (mm) | 21.95 ± 5.73 | 20.02 ± 3.84 | 1.62 ± 0.16 | 0.969 | <0.001 |
Note: Results are expressed as mean ± SD.
The distance between the center of hip rotation and the teardrop was measured by X‐ray imaging after the operation. This was related to the planned position of MAKO (r = 0.983, p < 0.001; r = 0.969, p < 0.001). The dimensional differences in horizontal and vertical directions were (1.56 ± 0.17) mm and (1.62 ± 0.16) mm, respectively (Table 3).
Complications
Postoperatively, all incisions healed in the first stage with early recovery and satisfactory recovery of hip function without complications such as hip dislocation, aseptic loosening, and periprosthetic infection. One patient in each group developed lower limb deep vein thrombosis that was resolved by pharmacological thrombolysis.
Discussion
Main Findings of the Study
This study found that learning curve for robot‐assisted THA based on the duration of surgery was 13 cases for a surgeon with 10 years of experience in hip arthroplasty. Despite the existence of a learning curve, there was no statistical difference between the proportion of prostheses placed within Lewinnek's safe zone for patients in the robot‐assisted THA group and that for patients in the conventional THA group. Moreover, our study found that the MAKO robot performed well in achieving the accuracy of preoperative planning.
Learning Curve for Robot‐Assisted Surgery
For conventional THA, the learning curve to achieve optimal cup position for surgeons trained for 1 year was up to 50 attempts 23 . In contrast, the learning curve for robot‐assisted surgery was much shorter, 13 cases. The advantages of robots are less likely to be offset by the experience of surgeons. In another report on the learning curve for MAKO robot‐assisted THA, the duration of operation stabilized after the initial 14 cases. Nonetheless, there was no significant difference in the complications during or after robot‐assisted THA between learning and proficient phases 13 . However, a longer duration of operation was associated with an increased risk of blood loss during surgery and surgical trauma, while a shorter surgery would reduce the risks. The duration of operation of robot‐assisted surgery was more than that of conventional operation, due to the extra time needed to set parameters of the robot surgery system, such as loose placement of locator, failure in acetabular registration. Our study found that with an increased number of surgical procedures, the surgical team became more familiar with the system, and as a result, the duration of operation was decreased significantly, almost as low as the durations for the conventional THA group.
Precise Cup Positioning
We have shown that the robot‐assisted implantation of the acetabular cup could get more accurate anteversion, a more reasonable LLD, and a higher proportion of the prosthesis position in the Lewinnek's safe zone. Robot‐assisted THA surgery has been a reliable surgical technique in recent years 13 , 24 , 25 , 26 , 27 . Ilmen et al. reported that robotic‐assisted THA improved acetabular component accuracy compared with conventional THA. In the robotic‐assisted THA group, the rate of acetabular component placement within Lewinnek safe zone was 77% 28 . Redmond et al. claimed that robotic‐assisted THA group had a more acceptable radiographic leg length and offset data 29 . They also found that intraoperative data from robot‐assisted THA and acetabular implants could accurately predict the location of postoperative radiographic acetabular component. Our research also showed that the actual postoperative measurements did not deviate significantly from the preoperative plan. Therefore, preoperative planning in MAKO robot system is vital for obtaining good clinical results.
A study reported that in the absence of a robotic system, cups were placed beyond the bounds of the safe zone as defined by Lewinnek and Callanan in approximately 43.47% and 69.57% of patients, respectively 25 . An effective way to improve the implant placement accuracy during THA is to tailor the implants to the patient's anatomy 30 . Surgeons' intuition is gradually being replaced by computerized precision to minimize human error like LLD or over‐resection of bone. MAKO operates through a robotic arm that is governed by a 3D computer model derived from a CT scan. The MAKO robot can assist the surgeon “hand in hand” in acetabular grinding and acetabular prosthesis installation. The acetabulum and femur are registered via a series of intraoperative checkpoints, which enables the real‐time model to guide acetabular reaming and installation 31 . Moreover, the robotic arm provides tactile resistance, when its position breaches boundaries defined in the surgical plan.
Limitations
This study has several limitations. First, the data from the case records could potentially introduce some bias due to measurement errors. Nevertheless, the imaging parameters were measured by two trained physicians who were blinded to surgical grouping, thereby reducing the bias to a certain extent. Second, we established a learning curve based solely on the duration of operation. The method of establishing the learning curve of the surgical process is based on operation outcome and duration of operation. However, in our study, we did not find any statistically significant difference in complications like unacceptable cup positioning, excessive LLD, or offsets. Previous studies have shown that cup position, LLD, and offset in the robot‐assisted THA group are not adversely affected during the learning curve 15 . Third, the follow‐up period of this study is short. Patients in this cohort study will be followed up to evaluate long‐term outcomes.
Conclusion
Robot‐assisted THA can provide more accurate acetabular cup prosthesis implantation. A learning curve is present, which, however, does not affect the therapeutic effect, but only increases the duration of operation. With the familiarity of the robot‐assisted system, the duration of operation tends to be stable, and the short‐term therapeutic effect is non‐inferior to that of conventional implantation. Long‐term follow‐up is needed to confirm the long‐term effect of surgical procedure.
Ethics Approval and Consent to Participate
This study was approved by the Ethical Committee of Cangzhou Hospital of Integrated Traditional Chinese and Western Medicine of Hebei Province. All patients had written informed consent.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Authors' Contributions
Dong‐Hui Guo initiated the study, analyzed the data, wrote the first draft of the manuscript. Yun‐chao Zhao and Chao Qi collected data. Shi‐Qiang Ma helped with the first draft of the manuscript. Xiao‐Ming Li and Yuan Xue designed the study and contributed significantly to the final draft of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Data Availability Statement
The generated data sets are available from the corresponding author on reasonable request.
References
- 1. Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet. 2007;370(9597):1508–19. [DOI] [PubMed] [Google Scholar]
- 2. Buj‐Corral I, Vidal D, Tejo‐Otero A, Padilla JA, Xuriguera E, Fenollosa‐Artés F. Characterization of 3D printed yttria‐stabilized zirconia parts for use in prostheses. Nanomaterials. 2021;11(11):2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harrison CL, Thomson AI, Cutts S, Rowe PJ, Riches PE. Research synthesis of recommended acetabular cup orientations for total hip arthroplasty. J Arthroplasty. 2014;29(2):377–82. [DOI] [PubMed] [Google Scholar]
- 4. Patterson DC, Grelsamer RP, Bronson MJ, Moucha CS. Lawsuits after primary and revision total hip arthroplasty: a malpractice claims analysis. J Arthroplasty. 2017;32(10):2958–62. [DOI] [PubMed] [Google Scholar]
- 5. Zengerink I, Reijman M, Mathijssen NMC, Eikens‐Jansen MP, Bos PK. Hip arthroplasty malpractice claims in The Netherlands: closed claim study 2000–2012. J Arthroplasty. 2016;31(9):1890–3.e4. [DOI] [PubMed] [Google Scholar]
- 6. Callanan MC, Jarrett B, Bragdon CR, Zurakowski D, Rubash HE, Freiberg AA, et al. The John Charnley award: risk factors for cup malpositioning: quality improvement through a joint registry at a tertiary hospital. Clin Orthop Relat Res. 2011;469(2):319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dobzyniak M, Fehring TK, Odum S. Early failure in total hip arthroplasty. Clin Orthop Relat Res. 2006;447:76–8. [DOI] [PubMed] [Google Scholar]
- 8. Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip‐replacement arthroplasties. J Bone Joint Surg Am. 1978;60(2):217–20. [PubMed] [Google Scholar]
- 9. Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24(1):15–21. [DOI] [PubMed] [Google Scholar]
- 10. Austin MS, Hozack WJ, Sharkey PF, Rothman RH. Stability and leg length equality in total hip arthroplasty. J Arthroplasty. 2003;18(3 Suppl 1):88–90. [DOI] [PubMed] [Google Scholar]
- 11. Ellapparadja P, Mahajan V, Atiya S, Sankar B, Deep K. Leg length discrepancy in computer navigated total hip arthroplasty—how accurate are we? Hip Int. 2016;26(5):438–43. [DOI] [PubMed] [Google Scholar]
- 12. Nodzo SR, Chang CC, Carroll KM, Barlow BT, Banks SA, Padgett DE, et al. Intraoperative placement of total hip arthroplasty components with robotic‐arm assisted technology correlates with postoperative implant position: a CT‐based study. Bone Joint J. 2018;100B(10):1303–9. [DOI] [PubMed] [Google Scholar]
- 13. Domb BG, Chen JW, Lall AC, Perets I, Maldonado DR. Minimum 5‐year outcomes of robotic‐assisted primary total hip arthroplasty with a nested comparison against manual primary total hip arthroplasty: a propensity score‐matched study. J Am Acad Orthop Surg. 2020;28(20):847–56. [DOI] [PubMed] [Google Scholar]
- 14. Reininga IH, Zijlstra W, Wagenmakers R, Boerboom AL, Huijbers BP, Groothoff JW, et al. Minimally invasive and computer‐navigated total hip arthroplasty: a qualitative and systematic review of the literature. BMC Musculoskelet Disord. 2010;11:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kong X, Yang M, Jerabek S, Zhang G, Chen J, Chai W. A retrospective study comparing a single surgeon's experience on manual versus robot‐assisted total hip arthroplasty after the learning curve of the latter procedure—a cohort study. Int J Surg. 2020;77:174–80. [DOI] [PubMed] [Google Scholar]
- 16. Kayani B, Konan S, Huq SS, Ibrahim MS, Ayuob A, Haddad FS. The learning curve of robotic‐arm assisted acetabular cup positioning during total hip arthroplasty. Hip Int. 2021;31(3):311–9. [DOI] [PubMed] [Google Scholar]
- 17. Hsiue PP, Chen CJ, Villalpando C, Ponzio D, Khoshbin A, Stavrakis AI. Trends and patient factors associated with technology‐assisted total hip arthroplasty in the United States from 2005 to 2014. Arthroplast Today. 2020;6(1):112–7.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sultan AA, Mohamed N, Samuel LT, Chughtai M, Sodhi N, Krebs VE, et al. Classification systems of hip osteonecrosis: an updated review. Int Orthop. 2019;43(5):1089–95. [DOI] [PubMed] [Google Scholar]
- 19. Crowe JF, Mani VJ, Ranawat CS. Total hip replacement in congenital dislocation and dysplasia of the hip. J Bone Joint Surg Am. 1979;61(1):15–23. [PubMed] [Google Scholar]
- 20. Polesello GC, Nakao TS, de Queiroz MC, Daniachi D, Ricioli W Jr, Guimarães RP, et al. Proposal for standardization of radiographic studies on the hip and pelvis. Rev Bras Ortop. 2011;46(6):634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamara E, Robinson J, Bas MA, Rodriguez JA, Hepinstall MS. Adoption of robotic vs. fluoroscopic guidance in total hip arthroplasty: is acetabular positioning improved in the learning curve? J Arthroplasty. 2017;32(1):125–30. [DOI] [PubMed] [Google Scholar]
- 22. Woolson ST, Hartford JM, Sawyer A. Results of a method of leg‐length equalization for patients undergoing primary total hip replacement. J Arthroplasty. 1999;14(2):159–64. [DOI] [PubMed] [Google Scholar]
- 23. Lee YK, Biau DJ, Yoon BH, Kim TY, Ha YC, Koo KH. Learning curve of acetabular cup positioning in total hip arthroplasty using a cumulative summation test for learning curve (LC‐CUSUM). J Arthroplasty. 2014;29(3):586–9. [DOI] [PubMed] [Google Scholar]
- 24. Sousa PL, Sculco PK, Mayman DJ, Jerabek SA, Ast MP, Chalmers BP. Robots in the operating room during hip and knee arthroplasty. Curr Rev Musculoskelet Med. 2020;13(3):309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shapira J, Diulus SC, Rosinsky PJ, Maldonado DR, Lall AC, Domb BG. Robotics and navigation as learning tools for fellows training in hip arthroplasty. J Am Acad Orthop Surg. 2021;29(4):176–81. [DOI] [PubMed] [Google Scholar]
- 26. Perazzini P, Trevisan M, Sembenini P, Alberton F, Laterza M, Marangon A, et al. The Mako™ robotic arm‐assisted total hip arthroplasty using direct anterior approach: surgical technique, skills and pitfals. Acta Biomed. 2020;91(4‐s):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Renkawitz T, Haimerl M, Dohmen L, Gneiting S, Wegner M, Ehret N, et al. Minimally invasive computer‐navigated total hip arthroplasty, following the concept of femur first and combined anteversion: design of a blinded randomized controlled trial. BMC Musculoskelet Disord. 2011;12:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Illgen RLN, Bukowski BR, Abiola R, Anderson P, Chughtai M, Khlopas A, et al. Robotic‐assisted total hip arthroplasty: outcomes at minimum two‐year follow‐up. Surg Technol Int. 2017;30:365–72. [PubMed] [Google Scholar]
- 29. Redmond JM, Gupta A, Hammarstedt JE, Petrakos A, Stake CE, Domb BG. Accuracy of component placement in robotic‐assisted Total hip arthroplasty. Orthopedics. 2016;39(3):193–9. [DOI] [PubMed] [Google Scholar]
- 30. Perets I, Mu BH, Mont MA, Rivkin G, Kandel L, Domb BG. Current topics in robotic‐assisted total hip arthroplasty: a review. Hip Int. 2020;30(2):118–24. [DOI] [PubMed] [Google Scholar]
- 31. Tarwala R, Dorr LD. Robotic assisted total hip arthroplasty using the MAKO platform. Curr Rev Musculoskelet Med. 2011;4(3):151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The generated data sets are available from the corresponding author on reasonable request.


