Abstract
Background
Spinal cord injury (SCI) is one of the most debilitating disorders throughout the world, causing persistent sensory-motor dysfunction, with no effective treatment. Oxidative stress and inflammatory responses play key roles in the secondary phase of SCI. Naringenin (NAR) is a natural flavonoid with known anti-inflammatory and antioxidative properties. This study aims at evaluating the effects of intrathecal NAR administration on sensory-motor disability after SCI.
Methods
Animals underwent a severe compression injury using an aneurysm clip. About 30 minutes after surgery, NAR was injected intrathecally at the doses of 5, 10, and 15 mM in 20 µL volumes. For the assessment of neuropathic pain and locomotor function, acetone drop, hot plate, inclined plane, and Basso, Beattie, Bresnahan tests were carried out weekly till day 28 post-SCI. Effects of NAR on matrix metalloproteinase (MMP)-2 and MMP-9 activity was appraised by gelatin zymography. Also, histopathological analyses and serum levels of glutathione (GSH), catalase and nitrite were measured in different groups.
Results
NAR reduced neuropathic pain, improved locomotor function, and also attenuated SCI-induced weight loss weekly till day 28 post-SCI. Zymography analysis showed that NAR suppressed MMP-9 activity, whereas it increased that of MMP-2, indicating its anti-neuroinflammatory effects. Also, intrathecal NAR modified oxidative stress related markers GSH, catalase, and nitrite levels. Besides, the neuroprotective effect of NAR was corroborated through increased survival of sensory and motor neurons after SCI.
Conclusions
These results suggest intrathecal NAR as a promising candidate for medical therapeutics for SCI-induced sensory and motor dysfunction.
Keywords: Anti-Inflammatory Agents, Catalase, Glutathione, Inflammation, Matrix Metalloproteinase, Motor Disorders, Naringenin, Neuralgia, Nitrites, Oxidative Stress, Pain, Spinal Cord Injuries
INTRODUCTION
Spinal cord injury (SCI), as an enfeebling central nervous system disorder affecting around 2.5 million people throughout the globe, results from trauma, degeneration, and diseases. It impairs main sensory-motor, autonomic, and reflex functions, and thus significantly affects life expectancy and quality as well as health expenses [1]. Up to around 60% of patients with SCI experience neuropathic pain which is secondary to the nervous system lesion. Neuropathic pain is regarded as a substantial detriment to the life quality of SCI patients [2].
Two phases ensue from SCI, the first being related to direct cell death as a result of traumatic injury that affects the local cells and the secondary phase originating from cellular and molecular events such as inflammation which exacerbates the injuries caused by the primary damage [3]. This secondary phase is characterized by increased blood-brain/spinal cord barrier permeability, apoptosis of neuronal and glial cells, neurotransmitter accumulation, and mitochondrial dysfunction which give rise to oxidative stress and neuroinflammation [4,5]. This phase can last for months and even years and lead to sensory-motor dysfunction. Although the accurate mechanisms pertinent to secondary injury have not been fully elucidated, oxidative stress and inflammation are considered to be key underlying mechanisms accounting for debilitating post-SCI complications [6,7]. Current therapeutic strategies to reduce pain and improve motor function include palliative approaches and surgical decompression which are associated with low efficacy in most SCI patients [8,9]. In spite of recent advances, there is still no effective and definite therapy to improve sensory-motor function and ameliorate other SCI complications. Therefore, finding novel therapeutics for these patients is of high importance.
Naringenin (NAR, 4’,5,7-trihydroxyflavanone) is a flavonoid abundantly found in grapefruit and other citrus fruits [10]. This compound is widely consumed by humans and is, because of its good bioavailability, easily detected in the serum following intake [11]. Along with its anti-inflammatory and antioxidant features and low toxicity, NAR possesses the potential to be employed as a therapeutic agent [12]. Previous studies have pointed to the analgesic and neuroprotective properties of NAR in neuropathic pain models and neurodegenerative disorders. NAR has shown therapeutic potential in multiple models of inflammatory pain [13–17]. It has been shown that NAR attenuates the pain-like behavior caused by inflammatory stimuli as acetic acid, carrageenan, complete Freund’s adjuvant, formalin, and phenyl-p-benzoquinone [12]. NAR can attenuate mechanical allodynia and thermal hyperalgesia as well as inhibit glial cell activation and the expression of inflammatory mediators [13].
The present study aimed at exploring the impacts of NAR on the modulation of sensory-motor dysfunctions and histopathological changes in relation to its antioxidant and anti-inflammatory capabilities.
MATERIALS AND METHODS
1. Animals
Totally, 35 male Wistar rats (weight: 230–270 g) were obtained from Kermanshah University of Medical Sciences (KUMS) and fed ad libitum from seven days prior to the experiments. They were maintained at room temperature (22°C ± 2°C) and under standard conditions including 12 h/12 h light/dark cycles and a relative humidity of 60%. This study was accomplished as per the National Institutes of Health Guidelines on the care and use of laboratory animals confirmed by KUMS (IR.KUMS.REC.1399.381). We tried to utilize the minimum number of rats in the study and all surgeries were performed under aseptic conditions.
2. Experimental groups
The animals were divided randomly into five groups with each group consisting of seven rats. The first group (Sham) underwent laminectomy without compression injury. The second group (SCI) consisted of animals undergoing laminectomy and compression injury followed by treatment with 5% dimethylsulfoxide (DMSO), as a vehicle. The remaining three groups were NAR-treated groups (NAR), which received intrathecal injections of NAR at the doses of 5, 10, 15 mM, just 30 minutes post-injury. Behavioral tests were carried out before surgery (day 0) and on days 7, 14, 21, and 28 after surgery. Animals were anesthetized on day 28 using an intraperitoneal (i.p.) injection of a ketamine/xylazine mixture (100/20 mg/kg), and blood samples were taken for assessment of matrix metalloproteinase (MMP)-2 and MMP-9 activity and also measurement of glutathione (GSH), catalase, and nitrite levels. Finally, spinal cord sections were obtained for histopathological evaluations.
3. SCI
All rats were deeply anesthetized by i.p. injection of ketamine/xylazine (60/10 mg/kg). Then, surgical sites were shaved and disinfected using 70% ethanol. By means of a micro rongeur (Fine Science Tools, Foster City, CA), laminectomy was performed at the T8-T9 level. The injury to the T8-T9 region of the spinal cord is the most commonly injured site during human accidents. Therefore, we made an in vivo model of SCI in rats through T8-T9 compression [2,3,18]. Accordingly, an aneurysm clip (Aesculap, Tuttlingen, Germany) was closed (closing force 90 g) for 1 minute around the spinal cord, rendering all rats paraplegic, a complete paralysis of the lower half of the body with involvement of both legs. During such compression SCI both sides of the spinal cord was simultaneously compressed. This produces paralysis with sensory functions in rats; something like Grade B of the American Spinal Cord Injury Association (ASIA) Impairment Scale in human (sensory function, but not motor function). Following sham or SCI surgery, skin and muscles were sutured, and animals were allowed to recover. To rehydrate and avoid the infection of the urinary tract, rats were given saline (2 mL, twice a day, subcutaneous) and cefazoline (40 mg/kg, twice on the surgery day, i.p.), respectively. Twice a day, the urinary bladder of the animals was manually evacuated until the recovery of the bladder-emptying reflex.
4. Intrathecal NAR injection
For intrathecal NAR injection, we followed the method of Mestre et al. [19] with some changes. NAR (Sigma-Aldrich, St. Louis, MO) was dissolved in DMSO (5%, as vehicle) to final concentrations of 5, 10, 15 mM. Just 30 minutes after SCI surgery on the anesthetized animals, NAR or the vehicle were intrathecally injected in 20 µL volumes using a 25 µL Hamilton microsyringe at the L5-L6 spine level over 10 seconds. The needle was kept for a few more seconds to prevent the outflow of NAR or vehicle. The correct site of injection was corroborated by the tail reflex.
5. Behavioral assessments
1) Acetone drop test for cold allodynia
The animals were located in acrylic cages over a wire mesh grid in order to have access to the paws. After acclimatization for 45 minutes, 100 µL of acetone was applied, from a 2 cm distance, to test the cold stimulation reaction of the hind paw. In the case of hind paw withdrawal, a response was recorded. The responses scores were 0, 1, 2, 3, 4, and 5 which correspond respectively to: 0) no reaction, 1) a startled response (with no paw withdrawal), 2) brief paw withdrawal, 3) prolonged paw withdrawal (for 5–30 seconds), 4) repetitive and prolonged withdrawal (for 30 seconds), and 5) flinching/licking [20]. Cold allodynia was tested by an observer in a blinded manner.
2) Hot plate test for heat hyperalgesia
To assess heat hyperalgesia, the hind paw of rat was thermally stimulated [21,22]. Rats were accustomed to the room for 1 hour followed by testing for baseline latency to paw licking or jumping. Analgesia was evaluated by a hot plate apparatus (45°C–52°C, Harvard Apparatus, Holliston, MA) [2]. Animals were placed on an analgesia meter and the latency to the start of pain-related behaviors, such as paw licking, was recorded. The mean time obtained from three repeats of the experiment (5 minutes intervals) was considered as the animal’s withdrawal latency. To prevent tissue injury, a 60 seconds cut-off was used.
3) Basso, Beattie, and Bresnahan test for motor activity
To determine the motor behavior, we conducted the Basso, Beattie, and Bresnahan (BBB) scale method of locomotor assessment [21]. Briefly, animals’ movements were appraised in a 90 cm2 box with surrounding walls of 10 cm for 5 minutes. The BBB test scores ranged from 0 (for full paralysis of the hind limbs) to 21 (for normal walking), using the 21-point BBB scale with all operational definitions applied as previously described [23]. Each score represents a specific set of characteristics shown by rats during locomotion. The average scores related to both right and left hind paws, which were used to acquire the average.
4) Inclined plane test for motor activity
The motor efficiency of animals was measured by means of a wood plane with 60 cm × 40 cm dimensions capable of inclining at the angle range of 0° (horizontal) to 70° [24]. Two blinded observers analyzed the biggest angle on which animals could stay for 5 seconds and the mean angles at the same time points were determined.
6. Gelatin zymography
Gelatin zymography was employed to assess MMP-2 and MMP-9 gelatinolytic activity. Sodium dodecyl sulfate-polyacrylamide gels were copolymerized with 0.1% gelatin. Then, serum samples (equivalent to total protein content of 100 µg as quantified by Bradford assay) were loaded and electrophoresis at a fixed voltage of 150 V was carried out using mini-gel slab device Mini Protean III (Bio-Rad, Hercules, CA). The gel underwent washing in renaturation buffer containing 2.5% Triton X-100 (in 50 mM Tris-HCl) on a shaker for 1 hour. Thereafter, the gel was incubated at 37°C in incubation buffer comprising of 10 mM CaCl2, 0.02% NaN3, and 0.15 NaCl in Tris-HCl (50 mM) for 18 hours. The gel was stained in Coomassie blue followed by destaining in acetic acid (5%) and methanol (7%). Clear bands representing gelatinolytic activity appeared on the gel. ImageJ software (National Institutes of Health, Bethesda, MD) was used to relatively quantify the intensities of the bands.
7. Nitrite assay
Nitrite levels were measured in serum samples by Griess assay which determines the concentration of nitrite, as an inducible nitric oxide (iNOS) byproduct. Therefore, the assay is indirectly indicative of the nitrite level. Griess reagents involving sulfanilamide (dissolved in 5% H3PO4) and naphthyl ethylene diamine dihydrochloride (NEDD; 0.1% in distilled water) solutions were prepared. Concisely, 100 µL of serum sample was mixed with 50 µL of sulfonamide solution in a 96-well plate and incubated in the dark for 5 minutes. Then, NEDD solution (50 µL) was added. The plate was once more incubated in the dark for 5 minutes. Optical density (OD) was read at 540 nm. Concurrently, different concentrations of sodium nitrite were used to plot a standard curve.
8. GSH/catalase assay
The changes in the levels of GSH and catalase were measures to assess the antioxidant levels. This assay is based on the reduction of GSH (as the reduced form) with 5, 5’-dithio-bis (2-nitrobenzoic acid) (DTNB), known as Ellman’s reagent. This reaction generates oxidized glutathione-TNB adduct (GS-TNB) and the TNB chromophore, whose maximal absorbance is at 412 nm. TNB formation rate is proportional to GSH concentration of the sample [25]. Briefly, phosphate buffered saline (PBS, 50 µL) and serum samples (40 µL) were mixed in the wells of a 96-well plate. Thereafter, 100 µL DTNB was added and the plate was incubated for 10 minutes at 37°C. Finally, OD of each well was measured at 412 nm by a plate reader.
Consequently, the method of Aebi [26] was used for catalase activity measurement. First, 20 µL of serum samples were added into 96-well plate wells. Then, 100 µL of hydrogen peroxide (H2O2, 65 mM) was added followed by incubation at 25°C for 4 minutes. The reaction was ended by adding 100 µL ammonium molybdate (32.4 mM). Finally, ODs were measured at 405 nm using a plate reader. The difference of ODs (in %) between treatment groups and the control group was reported in both the GSH and catalase assay, using the following formula:
In the above formula, sample is SCI or NAR.
9. Histological analysis
Animals underwent deep anesthesia using i.p. administration of xylazine (20 mg/kg) and ketamine (100 mg/kg) and were then euthanized. Then, 200 mL of PBS (0.1 M) followed by 200 mL of paraformaldehyde (4%) in 0.1 M PBS (pH 7.4) were transcardially infused. Spinal cord tissues, on the order of about 1.5 cm, were removed and processed in a tissue processor followed by embedding in paraffin. Afterwards, serial sections of SCI lesion underwent H & E staining [27], and dehydration with alcohol and xylene was carried out. For visualization, the light microscope (Nikon E600; Nikon, Tokyo, Japan) with an optical camera at 40× magnifications was used. To quantify the mean number of sensory neurons of the dorsal and ventral horns, ImageJ software (National Institutes of Health) was utilized. The neural morphology and the shape of the nucleus were used to determine the survival or damage to the neurons. The mean numbers of surviving neurons were counted on the gray matter of the dorsal horns. Repeated measures one-way analysis of variance (ANOVA) followed by Tukey post-hoc tests were used for data analysis.
10. Measurement of weight change
The animals were weighed at weekly intervals from the onset of the study. Animal weight changes in each group were calculated by the formula that follows:
Weight difference = weight of animal on days after the surgery – weight of the animal before the surgery.
11. Statistical analysis
Data are expressed as mean ± standard error of the mean, by GraphPad Prism software (version 8.4.3; GraphPad Software, San Diego, CA), using repeated measures one-way and two-way ANOVA together with Tukey’s and Bonferroni post-hoc analysis. P < 0.05 was considered to be a difference of statistically significance.
RESULTS
1. Sensory outcomes
1) Acetone drop test
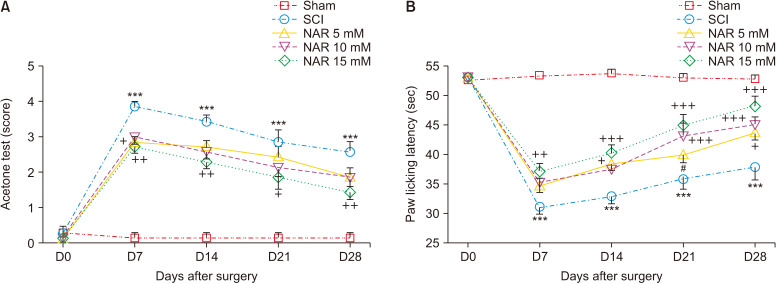
The results showed that the paw withdrawal reflex in response to cold stimulus (acetone) in all animals of the sham group was identical and these rats were unresponsive. The animals of the SCI group developed a drastic cold hypersensitivity at all evaluated days post-injury compared to the sham group as indicated by the mean acetone test score [interaction: F (16, 150) = 7.001, P < 0.001, times: F (4, 150) = 89.16, P < 0.001, groups: F (4, 150) = 90.60, P < 0.001]. Treatment with NAR could improve animals’ responses to cold stimulation in comparison to the SCI group and the best result was acquired following treatment with 15 mM NAR (Fig. 1A).
Fig. 1.
Effects of naringenin (NAR) on heat hyperalgesia and cold allodynia following compression spinal cord injury (SCI) in Wistar rats using (A) acetone drop, and (B) hot plate tests. The data are reported as mean ± standard error of the mean (n = 7). Repeated measures two-way analysis of variance followed by Bonferroni post-hoc tests were used for data analysis. ***P < 0.001 vs. sham group. +P < 0.05, ++P < 0.01, and +++P < 0.001, respectively in the comparisons between NAR groups with the SCI group. #P < 0.05 vs. NAR 15 mM.
2) Hot plate test
According to the obtained data (Fig. 1B), paw licking latency (indicative of pain threshold) in the sham group did not decrease and remained unchanged from day one to day 28 post-surgery. In addition, SCI resulted in significantly reduced paw licking latency (P < 0.001) from day 7 to 28 compared to the sham group. The treatment group receiving 15 mM NAR showed increased paw licking latency from day 7 to 28 in comparison to the SCI group [interaction: F (16, 150) = 9.333, P < 0.001, times: F (4, 150) = 107.7, P < 0.001, groups: F (4, 150) = 103.7, P < 0.001] indicating that NAR could attenuate SCI-induced thermal hyperalgesia.
2. Motor outcome
1) BBB test
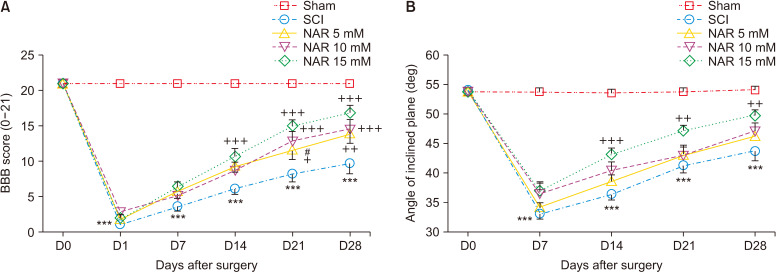
BBB score variation was observed for the sham group during the 28-day follow up of the animals (score: 21 at all time intervals) indicating no motor dysfunction after laminectomy. For the SCI and NAR-treated groups, we observed a BBB score of about 0 (complete paralysis) on the first day post-surgery which was significantly different from the sham group (P < 0.001). In the treatment groups, BBB score showed an increasing trend over time so that the 15 mM NAR-receiving group had the highest difference compared to the SCI group (Fig. 2A) group [interaction: F (20, 180) = 16.05, P < 0.001, times: F (5, 180) = 227, P < 0.001, groups: F (4, 180) = 226, P < 0.001].
Fig. 2.
Effects of naringenin (NAR) on improving motor dysfunction following compression spinal cord injury (SCI) in rats as assessed by (A) Basso, Beattie, Bresnahan (BBB), and (B) inclined plane test. The data are reported as mean ± standard error of the mean (n = 7). Repeated measures two-way analysis of variance followed by Bonferroni post-hoc tests were used for data analysis. ***P < 0.001 vs. sham group. +P < 0.05, ++P < 0.01, and +++P < 0.001, respectively in the comparisons between NAR groups with the SCI group. #P < 0.05 vs. NAR 15 mM.
2) Inclined plane test
Based on the results, sham group rats maintained a normal angle of stay on the inclined-planed device (55°) at the evaluated time intervals post-laminectomy which indicates no locomotor impairment after the surgery. Animals of the SCI group exhibited a decrease in the mean angle of stay on the device which was significantly different form that of the sham group at all-time intervals (P < 0.001). However, treatment with NAR considerably improved the locomotor recovery of rats from day 7 post-SCI in comparison to the SCI group (Fig. 2B) [interaction: F (16, 150) = 8.406, P < 0.001, times: F (4, 150) = 121.8, P < 0.001, groups: F (4, 150) = 85.50, P < 0.001].
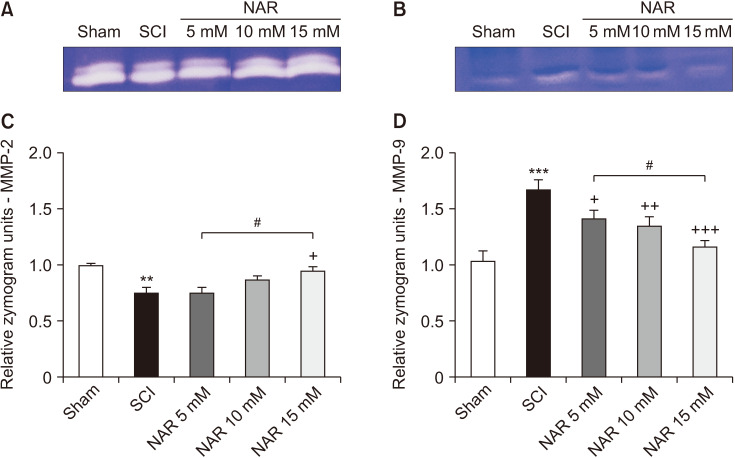
3. Gelatin zymography
The results of evaluating MMP gelatinase activity in different groups demonstrated that MMP-2 activity was significantly decreased in the SCI group in comparison to the sham group (P = 0.006). In addition, the NAR-treated group (15 mM) showed a significantly higher MMP-2 in comparison to the SCI group (P = 0.027). Also, the comparison of MMP-2 activity among NAR-receiving groups revealed a statistically significant difference between the 15 mM NAR-treated group and the 5 mM NAR-treated group [F (4, 10) = 9.035, P = 0.002]. These findings are illustrated in Fig. 3. Regarding MMP-9, the SCI group showed a significantly increased MMP-9 activity relative to the sham group rats (P < 0.001). Besides, NAR (10 and 15 mM) could decrease MMP-9 gelatinase activity significantly (P = 0.002 and P < 0.001, respectively) in comparison to the SCI group. Also, the group receiving 15 mM NAR showed a statistically significant difference with the groups treated with 5 mM (P = 0.014) NAR, as depicted in Fig. 3 [F (4, 10) = 32.14, P < 0.001].
Fig. 3.
Effects of naringenin (NAR) on matrix metalloproteinase (MMP)-2 and MMP-9 gelatinase activities following compression spinal cord injury (SCI) in rats. (A) and (B) illustrate the gelatin zymograms of MMP-2 and MMP-9, respectively. The data presented in (C) and (D) are expressed as mean ± standard error of the mean (n = 3). Repeated measures one-way analysis of variance followed by Tukey post-hoc tests were used for data analysis. **P < 0.01 and ***P < 0.001 vs. sham group. +P < 0.05, ++P < 0.01, and +++P < 0.001, respectively vs. the SCI group. #P < 0.05 in the comparisons between different NAR groups.
4. Oxidative stress analysis
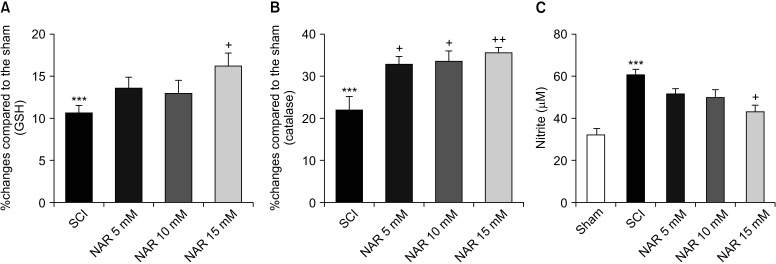
1) GSH/catalase assay
GSH assay results are shown in Fig. 4A. Comparison of GSH levels between SCI and NAR-treated groups elucidated the capability of NAR in modulating the levels of this antioxidant so that the GSH level was significantly higher in the group of animals receiving 15 mM NAR compared to the SCI group [F (4, 10) = 30.06, P < 0.001].
Fig. 4.
Effects of naringenin (NAR) on serum glutathione (GSH) (A), catalase (B), and nitrite (C) levels on day 28 post-spinal cord injury (SCI). Treatment with NAR (15 mM) was associated with significantly decreased serum catalase, GSH and nitrite compared to the SCI group. The data are expressed as mean ± standard error of the mean (n = 3). Repeated measures one-way analysis of variance followed by Tukey post-hoc tests were used for data analysis. ***P < 0.001 vs. sham group. +P < 0.05 and ++P < 0.01, respectively vs. the SCI group.
Serum catalase activity in the SCI and different treatment groups were measured, and the results are presented in Fig. 4B. Statistically significant differences were found between the treatment groups and the SCI group that remained untreated. It was found that treatment with NAR increased catalase levels in comparison to the SCI group. The highest effect of NAR on increasing catalase activity was observed in the group which received 15 mM NAR (P = 0.006). Additionally, animals treated with lower doses of NAR, i.e., 1 and 5 mM, also showed increased catalase activity compared to the SCI group [F (4, 10) = 53.93, P < 0.001].
2) Nitrite assay
According to our analysis (Fig. 4C), the SCI group had higher serum nitrite levels relative to the sham group (P < 0.001). Treatment of spinal cord injured rats with NAR was shown to reduce serum nitrite levels. Although rats receiving 5 and 10 mM NAR showed decreased serum nitrite, the differences with the SCI rats without treatment were not significant statistically (P = 0.250 , P = 0.140). However, NAR at the dose of 15 mM was found to decrease nitrite levels significantly [F (4, 10) = 13.54, P < 0.001].
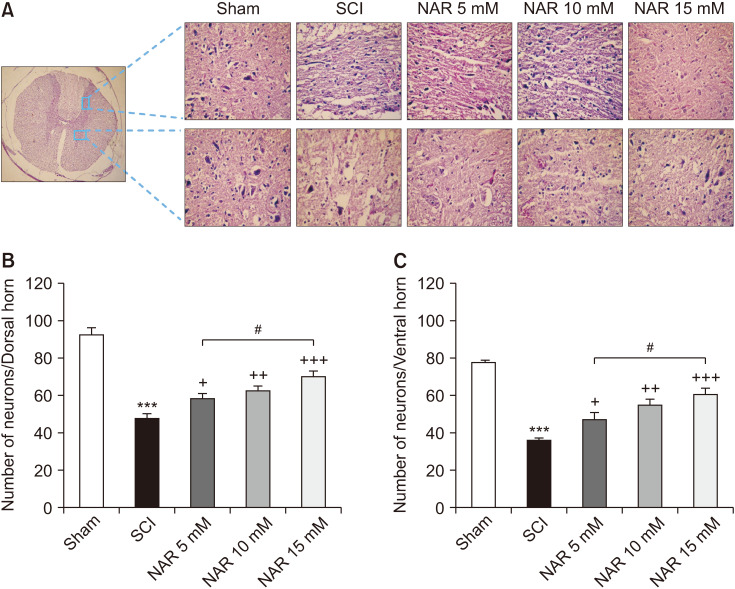
5. Histopathological analysis
Histopathological analysis of the spinal cord sections revealed reduced numbers of both dorsal [F (4, 25) = 46.25, P < 0.001] and ventral [F (4, 25) = 34.98, P < 0.001] horn neurons in the SCI group in comparison to the sham group. Besides, treatment with NAR at all three doses was found to meaningfully increase the number of neurons at both horns, so that the highest count was observed in rats that received 15 mM NAR. Also, as demonstrated in Fig. 5, a statistically significant difference was observed between the treatment group administered with 15 mM NAR in comparison to that receiving 5 mM NAR (Pdorsal = 0.015, Pventral = 0.110).
Fig. 5.
(A) Effects of naringenin (NAR) on H & E staining of dorsal and ventral transverse sections of spinal cord on day 28 post-spinal cord injury (SCI) in rats (40× magnification). (B) and (C) represent the mean numbers of survived neurons at dorsal and ventral horns, respectively. The data are expressed as mean ± standard error of the mean (n = 6). Repeated measures one-way analysis of variance followed by Tukey post-hoc tests were used for data analysis. ***P < 0.001 vs. sham group. +P < 0.05, ++P < 0.01, and +++P < 0.001, respectively vs. the SCI group. #P < 0.05 in the comparisons between different NAR groups.
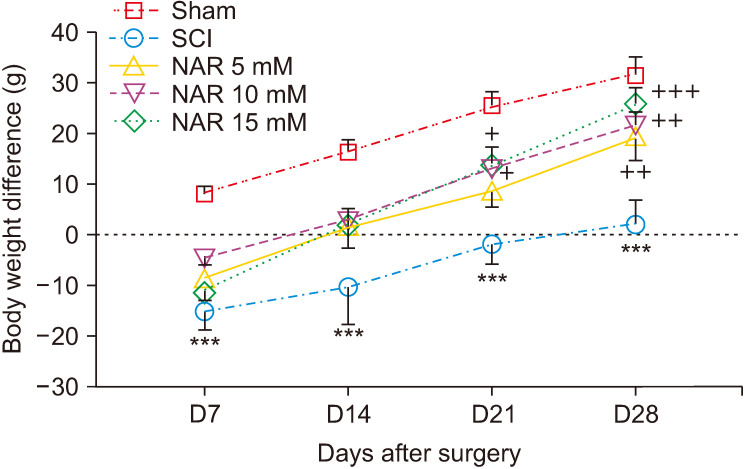
6. Body weight changes
Differences in animals’ body weights on days 7, 14, 21, and 21 after surgery were monitored in different groups. The findings indicated that only the sham group exhibited a pattern of weight gain on day 7 post-surgery while SCI and NAR-treated groups showed weight loss. Over time, though monitoring of body weight showed an increasing trend, SCI group animals had lower body weight differences in comparison to the sham group. Additionally, weight differences in NAR-treated groups showed an increasing trend from day 7 to 28 post-surgery and these groups had higher weight difference values compared to the SCI group. The highest weight increase, in comparison to the SCI group, was observed in the group receiving 15 mM NAR [interaction: F (12, 120) = 0.774, P = 0.675, times: F (3, 120) = 53.88, P < 0.001, groups: F (4, 120) = 29.87, P < 0.001] (Fig. 6). This finding indicates that NAR treatment can improve weight gain following SCI injury.
Fig. 6.
Effects of naringenin (NAR) on body weight changes of rats over time after clip compression spinal cord injury (SCI). The data are expressed as mean ± standard error of the mean (n = 7). Repeated measures two-way analysis of variance followed by Bonferroni post-hoc tests were used for data analysis. ***P < 0.001 vs. sham group. +P < 0.05, ++P < 0.01, and +++P < 0.001, respectively vs. the SCI group.
DISCUSSION
The present study unveiled a promising role for NAR, when administered intrathecally, in counteracting post-SCI sensory-motor dysfunction in rats. To the best of the authors’ knowledge, the current study is the first one to apply intrathecal injection of NAR for exploring its effects on the amelioration of sensory-motor disabilities, with respect to some oxidative stress and inflammation associated markers, following compression SCI. One-time injection of NAR could reduce pain thresholds as evidenced by hot plate and acetone drop tests aimed at evaluating heat hyperalgesia and cold allodynia, respectively. Also, NAR was shown to improve SCI-induced motor dysfunction based on the results obtained from the BBB and inclined plane tests. Besides, due to the critical roles of inflammation and oxidative stress in mediating post-SCI complications, as well as being on the way toward gaining more insights on the possible mechanisms of actions of this natural compound, the authors evaluated its effects on several markers related to inflammation (MMP-2 and MMP-9) and oxidative stress (GSH, catalase, and nitrite). On the other hand, as sensory-motor dysfunction is intimately linked with neuron loss after injury, we also conducted histopathological analysis for assessing NAR effects on the number of surviving neurons in the dorsal and ventral horns. According to our data, NAR could modify the levels of inflammatory and oxidative stress markers in favor of neuroprotective activity and improvement of post-SCI complications.
Unfortunately, there is still no U.S. Food and Drug Administration approved medication for SCI. Since SCI is associated with intricate pathophysiological mechanisms bringing about diverse complications, a multi-target treatment is demanded for more efficient management of such patients [2]. Recent research has underscored the auspicious potential of phytochemicals derived from natural plants in combating neurodegenerative diseases. Among various classes of phytochemicals, flavonoids are deemed to be multi-target therapeutic agents capable of modulating various pathogenic mechanisms related to neurodegeneration. In this regard, given the capacity of NAR (as a natural flavonoid) in targeting various signaling pathways linked with neurodegeneration, this phytocompound has been the focus of many studies. The neuroprotective effects of NAR in various models of neurodegenerative diseases highlight its potential application in the management of patients suffering from such unpleasant disorders [28].
Three key biological phenomena with major contributions to post-SCI pathological events are: inflammation, oxidative stress, and apoptosis [18]. Based on our data and also previous reports, NAR can ameliorate these processes and hence these effects of NAR could partly account for its therapeutic efficacy in the context of SCI. In this regard, MMPs have been shown to have key roles in the destructive inflammatory reactions involved in SCI-induced sensory-motor dysfunctionality [29]. MMPs are capable of digesting the components of extracellular matrix including proteoglycans, collagen, and laminin, and thus their excessive production and activation can lead to neuronal damage [30,31].
Apart from SCI, these metalloproteinases have been reported to be implicated in many other central nervous system disorders, like multiple sclerosis, stroke, Alzheimer’s disease, and amyotrophic lateral sclerosis [32–34]. Inflammatory cells secrete MMPs, especially MMP-9, which then penetrate the blood-spinal cord barrier (BSB) [35]. MMP-2 and MMP-9 impart a key role in the maintenance of BSB and the blood-brain barrier, and also in the amplification of spinal cord inflammation [36]. It has been verified that these enzymes are involved in the pathology of post-traumatic injuries so that in a rodent SCI model, 11 MMPs were found to be considerably upregulated [37]. MMPs, by digesting the components of basal lamina, disrupt BSB integrity thereby potentiating demyelination, oxidative stress, and progressive neuro-inflammatory reactions [38,39]. Augmented production of MMP-9 after SCI contributes to BSB integrity disruption, resulting in neuro-inflammatory responses and cell death. MMP-9 is highly upregulated in glial cells, macrophages, and endothelial cells following damage to the central nervous system, while neuronal cells, including motor neurons of the anterior horn, show low levels of MMP-9 activation [40].
Our results indicated that intrathecal injection of NAR (10 and 15 mM) alleviated MMP-9 expression/activity following SCI in comparison to the control SCI group. MMP-9 can be activated through reactive oxygen species (ROS) and pro-inflammatory mediators. For example, tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β induce MMP-9 through the nuclear factor-κB (NF-κB) pathway [18]. Previous studies have demonstrated the anti-inflammatory and antioxidant effects of NAR through attenuation of TNF-α, IL-1β, IL-6, iNOS, NF-κB, and ROS levels, which could partly account for the observed effect of this flavonoid on the inhibition of MMP-9 activation. In this line, in the study of Bai et al. [41] it was elucidated that pre-treatment with NAR (100 mg/kg) substantially reduced neurological impairment and the water content of the brain in a rat model of cerebral ischemia. In that study, NAR administration resulted in considerably decreased expression of MMP-9, NF-κB, nucleotide-binding oligomerization domain 2, and receptor interacting protein-2.
In contrast, a somewhat different role could be considered for MMP-2 in SCI. It has been demonstrated that MMP-2 activation/expression could improve locomotor function and wound healing following SCI by regulating axonal plasticity, glial scar formation, as well as white matter sparing [42]. From this perspective, increased activation of MMP-2 after intrathecal injection of NAR (15 mM) might have contributed to the improved functional recovery of rats following SCI. Nevertheless, it is worth mentioning that MMP-2 activity can result in detrimental effects in the acute phase of SCI, as well. Shi et al. [43], in 2016, explored the protective effects of NAR in rats undergoing SCI with an emphasis on neutrophil mediated neuro-inflammatory mechanisms. In comparison to the present study, they administered NAR orally and used 50 mg/kg and 100 mg/kg doses once daily for 11 successive days starting from three days before surgery to seven days post-surgery. They reported that NAR restrained SCI-induced neutrophil activation by repressing miR-223.
Oxidative stress and apoptosis play key roles in secondary damage from SCI. Compelling evidence over recent decades has highlighted augmented formation of ROS and the ensuing oxidative stress as key events concerned with SCI. Spinal cord neurons and glia are particularly vulnerable to oxidative stress owing to many factors, such as their high contents of polyunsaturated fatty acids and low antioxidant capacities, persuading researchers in this field to consider oxidative stress as a hallmark of SCI secondary phase injuries. On such a basis, it can be inferred that oxidative stress could be a therapeutic target in SCI treatment [6].
In this study, the effects of intrathecal administration of NAR on serum GSH and catalase (as antioxidant factors), as well as nitrite (as a kind of ROS) levels after SCI were investigated. Our findings showed that intrathecal administration of NAR at the dose of 15 mM could significantly increase serum GSH levels compared to the SCI group. In the case of catalase, all NAR-treated groups showed significantly higher catalase in comparison to the SCI group. In addition, treatment with NAR could significantly ameliorate serum nitrite level only in the group which received the dose of 15 mM, while nitrite decrease in other treatment groups was not statistically significant. Also, our histopathological results point to the neuroprotective and pro-survival effect of this interesting flavonoid.
In a mechanistic study, it was demonstrated that pre-supplementation with NAR increased GSH and superoxide dismutase (SOD) as antioxidant markers. Also, NAR was shown to alleviate NF-κB-mediated inflammatory mediators including IL-1β, cyclooxygenase-2, TNF-α, and iNOS [44]. It has been reported that NAR decreases malondialdehyde (MDA), H2O2, and 4-hydroxynonenal in the treated animals while increasing GSH, catalase, SOD, and glutathione peroxidase levels [28]. Consistently, NAR treatment has been shown to enhance Hippocampal GSH, catalase, SOD, and Nrf2 levels and mitigate Hippocampal MDA level [45].
Pre-treatment with NAR was shown to reverse methyl mercury (MeHg)-induced pyramidal cell damage associated with decreased alleviated oxidative stress as evidenced by increased GSH and glutathione S-tranferase levels accompanied with a notable decrease of MDA [46]. Chtourou et al. [47], in 2014, reported the neuroprotective effects of NAR against iron-induced neurotoxicity through attenuation of ROS and promotion of antioxidant capacity by increasing GSH, catalase, and SOD. In another study, NAR treatment following carbaryl-induced toxicity could improve the survival of mouse neuroblastoma cells, mitigated ROS levels and hindered apoptosis via upregulation of Bcl-2 and inhibition of Bax and caspase-3 [48]. A previous study has reported pro-survival and anti-apoptotic impacts of NAR against glutamate-induced cytotoxicity through upregulation of phosphorylated Akt and ERK in the treated primary hippocampal neurons of mice. NAR was demonstrated to actuate brain-derived neurotrophic factor and also other neuroprotective cytokines to improve the survival of neurons [49].
Change in body weight is considered as another post-SCI complication. Patients with SCI experience weight loss over time that can be due to metabolic alterations, muscle atrophy and mineral loss [50]. In animal models of SCI, a prolonged body weight loss following SCI is observed. The most important reasons for this complication are early stage hypoplasia, long-lasting changes in the alimentary system, as well as homeostatic mechanisms related to later stages. Herein, treatment with NAR could ameliorate SCI-induced weight loss and promoted the process of weight gain in spinal cord injured animals. This finding could be partly ascribed to the improvement by NAR of sensory-motor performance, attenuation of pain, and other SCI-induced deficits which made animals able to take food and water.
In conclusion, the current study addressed the ameliorative and neuroprotective effects of intrathecal NAR in a rodent model of SCI. In this study, intrathecal administration of NAR was shown to attenuate SCI-induced neuropathic pain, as well as improve motor dysfunction and neuronal loss in the spinal cord injured rats. This efficacy of NAR could be mediated by decreasing MMP-9 activation, increasing MMP-2 activation, modification of oxidative stress through upregulation of GSH and catalase, and downregulation of nitrite as well as pro-survival effects on spinal cord neurons. This study shed more light on the potential application of NAR in human SCI. However, further investigations are required to delineate in more detail other responsible mechanisms governing the effects of NAR and confirm its clinical use for SCI patients as a novel treatment.
Footnotes
DATA AVAILABILITY
The datasets supporting the findings of this study are available from the corresponding author upon reasonable request.
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
FUNDING
The authors acknowledged Pharmaceutical Sciences Research Center, Kermanshah University of Medical Sciences, for the special Grant No. 4000170.
REFERENCES
- 1.Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–43. doi: 10.1038/nrn1955. Erratum in: Nat Rev Neurosci 2006; 7: 902. [DOI] [PubMed] [Google Scholar]
- 2.Fakhri S, Dargahi L, Abbaszadeh F, Jorjani M. Effects of astaxanthin on sensory-motor function in a compression model of spinal cord injury: Involvement of ERK and AKT signalling pathway. Eur J Pain. 2019;23:750–64. doi: 10.1002/ejp.1342. [DOI] [PubMed] [Google Scholar]
- 3.Fakhri S, Dargahi L, Abbaszadeh F, Jorjani M. Astaxanthin attenuates neuroinflammation contributed to the neuropathic pain and motor dysfunction following compression spinal cord injury. Brain Res Bull. 2018;143:217–24. doi: 10.1016/j.brainresbull.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Naseem M, Parvez S. Role of melatonin in traumatic brain injury and spinal cord injury. ScientificWorldJournal. 2014;2014:586270. doi: 10.1155/2014/586270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito E, Genovese T, Caminiti R, Bramanti P, Meli R, Cuzzocrea S. Melatonin regulates matrix metalloproteinases after traumatic experimental spinal cord injury. J Pineal Res. 2008;45:149–56. doi: 10.1111/j.1600-079X.2008.00569.x. [DOI] [PubMed] [Google Scholar]
- 6.Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord. 2012;50:264–74. doi: 10.1038/sc.2011.111. [DOI] [PubMed] [Google Scholar]
- 7.Faden AI, Wu J, Stoica BA, Loane DJ. Progressive inflammation-mediated neurodegeneration after traumatic brain or spinal cord injury. Br J Pharmacol. 2016;173:681–91. doi: 10.1111/bph.13179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franz S, Finnerup NB. Diagnostics and treatment of pain in spinal cord injury. In: Weidner N, Rupp R, Tansey K, editors. Neurological aspects of spinal cord injury. Springer; Cham: 2017. pp. 283–302. https://doi.org/10.1007/978-3-319-46293-6_12. [DOI] [Google Scholar]
- 9.Backonja MM, Irving G, Argoff C. Rational multidrug therapy in the treatment of neuropathic pain. Curr Pain Headache Rep. 2006;10:34–8. doi: 10.1007/s11916-006-0007-1. [DOI] [PubMed] [Google Scholar]
- 10.Hare JT, Elliott DP. Grapefruit juice and potential drug interactions. Consult Pharm. 2003;18:466–72. [PubMed] [Google Scholar]
- 11.Palma-Duran SA, Caire-Juvera G, Robles-Burgeño Mdel R, Ortega-Vélez MI, Gutiérrez-Coronado Mde L, Almada Mdel C, et al. Serum levels of phytoestrogens as biomarkers of intake in Mexican women. Int J Food Sci Nutr. 2015;66:819–25. doi: 10.3109/09637486.2015.1092019. [DOI] [PubMed] [Google Scholar]
- 12.Pinho-Ribeiro FA, Zarpelon AC, Fattori V, Manchope MF, Mizokami SS, Casagrande R, et al. Naringenin reduces inflammatory pain in mice. Neuropharmacology. 2016;105:508–19. doi: 10.1016/j.neuropharm.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 13.Hu CY, Zhao YT. Analgesic effects of naringenin in rats with spinal nerve ligation-induced neuropathic pain. Biomed Rep. 2014;2:569–73. doi: 10.3892/br.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Rejaie SS, Aleisa AM, Abuohashish HM, Parmar MY, Ola MS, Al-Hosaini AA, et al. Naringenin neutralises oxidative stress and nerve growth factor discrepancy in experimental diabetic neuropathy. Neurol Res. 2015;37:924–33. doi: 10.1179/1743132815Y.0000000079. [DOI] [PubMed] [Google Scholar]
- 15.Kaulaskar S, Bhutada P, Rahigude A, Jain D, Harle U. Effects of naringenin on allodynia and hyperalgesia in rats with chronic constriction injury-induced neuropathic pain. Zhong Xi Yi Jie He Xue Bao. 2012;10:1482–9. doi: 10.3736/jcim20121223. [DOI] [PubMed] [Google Scholar]
- 16.Pinho-Ribeiro FA, Zarpelon AC, Mizokami SS, Borghi SM, Bordignon J, Silva RL, et al. The citrus flavonone naringenin reduces lipopolysaccharide-induced inflammatory pain and leukocyte recruitment by inhibiting NF-κB activation. J Nutr Biochem. 2016;33:8–14. doi: 10.1016/j.jnutbio.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Manchope MF, Calixto-Campos C, Coelho-Silva L, Zarpelon AC, Pinho-Ribeiro FA, Georgetti SR, et al. Naringenin inhibits superoxide anion-induced inflammatory pain: role of oxidative stress, cytokines, Nrf-2 and the NO-cGMP-PKG-KATP channel signaling pathway. PLoS One. 2016;11:e0153015. doi: 10.1371/journal.pone.0153015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fakhri S, Kiani A, Jalili C, Abbaszadeh F, Piri S, Farzaei MH, et al. Intrathecal administration of melatonin ameliorates the neuroinflammation- mediated sensory and motor dysfunction in a rat model of compression spinal cord injury. Curr Mol Pharmacol. 2021;14:646–57. doi: 10.2174/1874467213666201230101811. [DOI] [PubMed] [Google Scholar]
- 19.Mestre C, Pélissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. J Pharmacol Toxicol Methods. 1994;32:197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 20.Kauppila T. Cold exposure enhances tactile allodynia transiently in mononeuropathic rats. Exp Neurol. 2000;161:740–4. doi: 10.1006/exnr.1999.7287. [DOI] [PubMed] [Google Scholar]
- 21.Hama AT, Plum AW, Sagen J. Antinociceptive effect of ambroxol in rats with neuropathic spinal cord injury pain. Pharmacol Biochem Behav. 2010;97:249–55. doi: 10.1016/j.pbb.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janicki P, Libich J. Detection of antagonist activity for narcotic analgesics in mouse hot-plate test. Pharmacol Biochem Behav. 1979;10:623–6. doi: 10.1016/0091-3057(79)90244-2. [DOI] [PubMed] [Google Scholar]
- 23.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 24.Samini F, Samarghandian S, Borji A, Mohammadi G, bakaian M. Curcumin pretreatment attenuates brain lesion size and improves neurological function following traumatic brain injury in the rat. Pharmacol Biochem Behav. 2013;110:238–44. doi: 10.1016/j.pbb.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–65. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 26.Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. 2nd ed. Academic Press; New York: 1974. pp. 673–84. https://doi.org/10.1016/B978-0-12-091302-2.50032-3 . [DOI] [Google Scholar]
- 27.Masoudi A, Dargahi L, Abbaszadeh F, Pourgholami MH, Asgari A, Manoochehri M, et al. Neuroprotective effects of astaxanthin in a rat model of spinal cord injury. Behav Brain Res. 2017;329:104–10. doi: 10.1016/j.bbr.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 28.Nouri Z, Fakhri S, El-Senduny FF, Sanadgol N, Abd-ElGhani GE, Farzaei MH, et al. On the neuroprotective effects of naringenin: pharmacological targets, signaling pathways, molecular mechanisms, and clinical perspective. Biomolecules. 2019;9:690. doi: 10.3390/biom9110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao L, Wang H, Qiao L, Wang X. Disruption of Nrf2 enhances the upregulation of nuclear factor-kappaB activity, tumor necrosis factor-α, and matrix metalloproteinase-9 after spinal cord injury in mice. Mediators Inflamm. 2010;2010:238321. doi: 10.1155/2010/238321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alghamdi BS. The neuroprotective role of melatonin in neurological disorders. J Neurosci Res. 2018;96:1136–49. doi: 10.1002/jnr.24220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stetler-Stevenson WG. Dynamics of matrix turnover during pathologic remodeling of the extracellular matrix. Am J Pathol. 1996;148:1345–50. [PMC free article] [PubMed] [Google Scholar]
- 32.Shibata N, Ohnuma T, Higashi S, Usui C, Ohkubo T, Kitajima A, et al. Genetic association between matrix metalloproteinase MMP-9 and MMP-3 polymorphisms and Japanese sporadic Alzheimer's disease. Neurobiol Aging. 2005;26:1011–4. doi: 10.1016/j.neurobiolaging.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Lorenzl S, Albers DS, Narr S, Chirichigno J, Beal MF. Expression of MMP-2, MMP-9, and MMP-1 and their endogenous counterregulators TIMP-1 and TIMP-2 in postmortem brain tissue of Parkinson's disease. Exp Neurol. 2002;178:13–20. doi: 10.1006/exnr.2002.8019. [DOI] [PubMed] [Google Scholar]
- 34.Beuche W, Yushchenko M, Mäder M, Maliszewska M, Felgenhauer K, Weber F. Matrix metalloproteinase-9 is elevated in serum of patients with amyotrophic lateral sclerosis. Neuroreport. 2000;11:3419–22. doi: 10.1097/00001756-200011090-00003. [DOI] [PubMed] [Google Scholar]
- 35.Noble LJ, Donovan F, Igarashi T, Goussev S, Werb Z. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22:7526–35. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang R, Guo L, Wang P, Huang L, Tang Y, Wang W, et al. Epidemiology of spinal cord injuries and risk factors for complete injuries in Guangdong, China: a retrospective study. PLoS One. 2014;9:e84733. doi: 10.1371/journal.pone.0084733.9bcaf3aad5fb45dea562cdd91b88f449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells JE, Rice TK, Nuttall RK, Edwards DR, Zekki H, Rivest S, et al. An adverse role for matrix metalloproteinase 12 after spinal cord injury in mice. J Neurosci. 2003;23:10107–15. doi: 10.1523/JNEUROSCI.23-31-10107.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Chang M, Hansen CN, Basso DM, Noble-Haeusslein LJ. Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics. 2011;8:206–20. doi: 10.1007/s13311-011-0038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piao MS, Lee JK, Jang JW, Hur H, Lee SS, Xiao L, et al. Melatonin improves functional outcome via inhibition of matrix metalloproteinases-9 after photothrombotic spinal cord injury in rats. Acta Neurochir (Wien) 2014;156:2173–82. doi: 10.1007/s00701-014-2119-4. [DOI] [PubMed] [Google Scholar]
- 40.Jang JW, Lee JK, Kim SH. Activation of matrix metalloproteinases-9 after photothrombotic spinal cord injury model in rats. J Korean Neurosurg Soc. 2011;50:288–92. doi: 10.3340/jkns.2011.50.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai X, Zhang X, Chen L, Zhang J, Zhang L, Zhao X, et al. Protective effect of naringenin in experimental ischemic stroke: down-regulated NOD2, RIP2, NF-κB, MMP-9 and up-regulated claudin-5 expression. Neurochem Res. 2014;39:1405–15. doi: 10.1007/s11064-014-1326-y. [DOI] [PubMed] [Google Scholar]
- 42.Hsu JY, McKeon R, Goussev S, Werb Z, Lee JU, Trivedi A, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. J Neurosci. 2006;26:9841–50. doi: 10.1523/JNEUROSCI.1993-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shi LB, Tang PF, Zhang W, Zhao YP, Zhang LC, Zhang H. Naringenin inhibits spinal cord injury-induced activation of neutrophils through miR-223. Gene. 2016;592:128–33. doi: 10.1016/j.gene.2016.07.037. [DOI] [PubMed] [Google Scholar]
- 44.Puspitasari V, Gunawan PY, Wiradarma HD, Hartoyo V. Glial fibrillary acidic protein serum level as a predictor of clinical outcome in ischemic stroke. Open Access Maced J Med Sci. 2019;7:1471–4. doi: 10.3889/oamjms.2019.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khajevand-Khazaei MR, Ziaee P, Motevalizadeh SA, Rohani M, Afshin-Majd S, Baluchnejadmojarad T, et al. Naringenin ameliorates learning and memory impairment following systemic lipopolysaccharide challenge in the rat. Eur J Pharmacol. 2018;826:114–22. doi: 10.1016/j.ejphar.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 46.Krishna Chandran AM, Christina H, Das S, Mumbrekar KD, Satish Rao BS. Neuroprotective role of naringenin against methylmercury induced cognitive impairment and mitochondrial damage in a mouse model. Environ Toxicol Pharmacol. 2019;71:103224. doi: 10.1016/j.etap.2019.103224. [DOI] [PubMed] [Google Scholar]
- 47.Chtourou Y, Fetoui H, Gdoura R. Protective effects of naringenin on iron-overload-induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol Trace Elem Res. 2014;158:376–83. doi: 10.1007/s12011-014-9948-0. [DOI] [PubMed] [Google Scholar]
- 48.Muthaiah VP, Venkitasamy L, Michael FM, Chandrasekar K, Venkatachalam S. Neuroprotective role of naringenin on carbaryl induced neurotoxicity in mouse neuroblastoma cells. J Pharmacol Pharmacother. 2013;4:192–7. doi: 10.4103/0976-500X.114599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu XH, Ma CM, Han YZ, Li Y, Liu C, Duan ZH, et al. Protective effect of naringenin on glutamate-induced neurotoxicity in cultured hippocampal cells. Arch Biol Sci. 2015;67:639–46. doi: 10.2298/ABS140811023X. https://doi.org/10.2298/ABS140811023X. [DOI] [Google Scholar]
- 50.Giangregorio L, McCartney N. Bone loss and muscle atrophy in spinal cord injury: epidemiology, fracture prediction, and rehabilitation strategies. J Spinal Cord Med. 2006;29:489–500. doi: 10.1080/10790268.2006.11753898. [DOI] [PMC free article] [PubMed] [Google Scholar]