Abstract
Resistant starch (RS), a healthy dietary fiber, is a particular type of starch that has attracted much research attention in recent years. RS has important roles in reducing glycemic index, postprandial blood glucose levels, and serum cholesterol levels, thereby improving and preventing many diseases, such as diabetes, obesity, and cardiovascular disease. The formation of RS is influenced by intrinsic properties of starch (e.g., starch granule structure, starch crystal structure, and amylose-to-amylopectin ratio) and non-starch components (e.g., proteins, lipids, and sugars), as well as storage and processing conditions. Recent studies have revealed that several starch-synthesis-related genes (SSRGs) are crucial for the formation of RS during seed development. Several transcription factors and mRNA splicing factors have been shown to affect the expression or splicing of SSRGs that regulate RS content, suggesting their potential roles in RS formation. This review focuses mainly on recent research progress on the genetic regulation of RS content and discusses the emerging genetic and molecular mechanisms of RS formation in rice.
Keywords: rice, resistant starch, resistant starch formation, genetic regulation, starch-synthesis-related genes
Resistant starch (RS), a healthy functional food, has received significant research attention in recent years, although the genetic and molecular mechanisms of RS formation are still unclear. This review focuses mainly on recent research progress on the genetic regulation of RS content in rice, including direct and potential genetic factors that influence RS formation, and discusses how current knowledge may be used to improve RS content in crops.
Introduction
Resistant starch (RS) refers to the portion of starch that cannot be digested and absorbed in the small intestine of healthy human beings (Englyst et al., 1982; Asp and Björck, 1992). RS is also defined as a dietary fiber that is beneficial to health because it cannot be digested in the gastrointestinal tract (Haralampu, 2000; De Vries, 2003). However, RS can be fermented in the colon by the gut microbiota, thereby producing a mass of short-chain fatty acids (SCFAs) (e.g., butyrate, acetate, and propionate) that are beneficial to intestinal health (Nugent, 2005; Hu et al., 2016). RS with unique functional properties has a number of significant biological effects, such as improvement of fermentable properties and bacterial activities in the colon, reduction of colon cancer risk, prevention of gall stone formation, promotion of mineral absorption, and improvement of gut health (Govers et al., 1999; Brown, 2004; Bauer-Marinovic et al., 2006; Sajilata et al., 2006; Birt et al., 2013; Keenan et al., 2015). Thus, RS is gradually being recognized as a functional food that is beneficial for various conditions, such as inflammatory bowel disease, insulin resistance and type 2 diabetes, glycemic index, energy and weight management, cholesterol levels, and coronary heart disease (Higgins et al., 1996, 2011; Jacobasch et al., 1999; Martinez-Flores et al., 2004; Park et al., 2004; Han et al., 2005; Morita et al., 2005; Behall et al., 2006; Regina et al., 2006; Zhang et al., 2007). Increasing daily intake of RS is becoming an important target for improving public health. Therefore, RS, an emerging healthy food, has received continuous research attention in recent years.
Starch, a major storage carbohydrate, is a polysaccharide that is composed of single sugar units linked together with α-1,4- and α-1,6-glycosidic bonds (Hizukuri et al., 1989). Based on its in vitro digestibility rate, starch is classified into three categories: rapidly digestible starch (RDS), slowly digestible starch (SDS), and RS (Englyst and Hudson, 1996). RDS refers to the portion of starch that can be completely digested and absorbed in the small intestine; it is rapidly hydrolyzed and converted to single glucose molecules within 20 min by enzymatic digestion (Englyst et al., 1992). SDS is a fraction of starch that requires more time for digestion but can be completely digested after 120 min of enzymatic digestion (Englyst et al., 1992). RS is defined as the starch fraction that cannot be digested by enzymes within 120 min (Englyst et al., 1992).
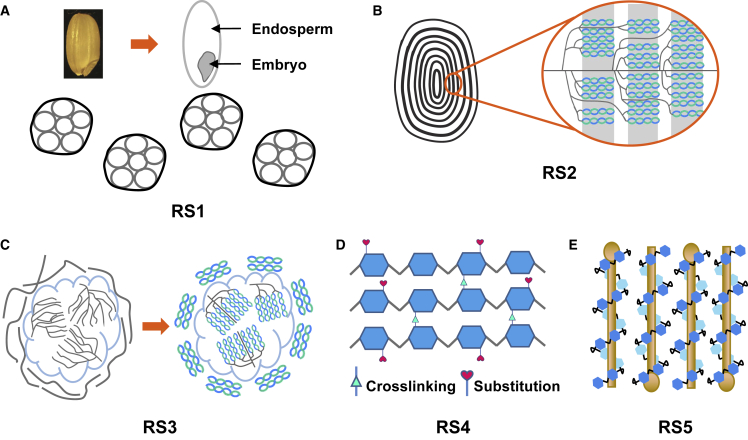
Based on its origin and resistance to enzymatic hydrolysis, RS is further classified into five subtypes: RS1, RS2, RS3, RS4, and RS5 (Figure 1) (Brown, 1996; Englyst et al., 1992; Guo et al., 2021; Raigond et al., 2015). RS1 is physically inaccessible to digestion, mainly because it is enclosed by cell walls or other tissues that prevent contact and reaction with amylase (Figure 1A) (Oladele, 2016). RS1 is unstable, and fine milling or deep processing will destroy its structure; it is generally found in partly milled grains, seeds, or tubers. RS2 refers to native starch granules with a compact structure that are relatively dehydrated (Figure 1B) (Sajilata et al., 2006). RS2 is tightly packed in a radial pattern in raw starch granules, restricting the accessibility of digestive enzymes and the majority of amylases. RS2 exists in raw potatoes, peas, and green bananas. RS3 is composed of retrograded starches that are principally recrystallized amylose produced after cooking and cooling of starchy foods (Figure 1C). RS3 belongs to the physically modified starches that are primarily formed via the gelatinization and retrogradation process during food processing and manufacturing (Haralampu, 2000). RS4 generally refers to chemically modified starch with altered molecular structure that contains new chemical bonds rather than α-1,4- or α-1,6-glycosidic bonds and thus exhibits increased resistance to amylolytic enzymes (Figure 1D) (Raigond et al., 2015). RS5 is a complex of amylose and lipids that forms a helical structure, preventing amylase digestion (Figure 1E) (Hasjim et al., 2010). The lipids bind to the amylose in the starch granule, preventing its expansion and producing resistance to enzymatic hydrolysis. Such amylose–lipid complexes are usually generated from high-amylose starches.
Figure 1.
Schematic drawing of different types of resistant starch and their structures.
(A) RS1, a type of RS that is physically inaccessible to digestion. The starch granules are enclosed by cell walls or other tissues that prevent contact and reaction with amylase.
(B) RS2, native starch granules with a compact crystalline structure that are relatively dehydrated. RS2 is tightly packed in a radial pattern in raw starch granules.
(C) RS3, retrograded starches that are primarily formed via the gelatinization and retrogradation process during food processing and manufacturing.
(D) RS4, chemically modified starch that generates new chemical bonds by substitution, esterification, or crosslinking. Through chemical modification, the molecular structure of starch is altered, thereby increasing its resistance to amylase.
(E) RS5, a new type of RS that forms a complex of amylose and lipid. RS5 is usually generated in high-amylose starch cereals.
Different crops contain distinct RS contents. For example, RS is most abundant in beans, ranging from 32% to 36%, compared with only 0.1% to 3.2% in cereal crops (Ragaee et al., 2006; Ambigaipalan et al., 2011). In recent years, several genes involved in starch biosynthesis have been reported to influence RS content in rice and other crops (Butardo et al., 2012; Matsumoto et al., 2012; Yang et al., 2012; Zhou et al., 2016; Guo et al., 2020). Breeding elite varieties with high RS content has become a new goal, owing to the significant biological effects of RS on human health. Several excellent reviews have provided very detailed information about the structure, classification, physiological effects, applications, and genetic improvement of RS (Nugent, 2005; Sajilata et al., 2006; Raigond et al., 2015; Ma and Boye, 2017; Xia et al., 2018; Bello-Perez et al., 2020; Jiang et al., 2020; Jukanti et al., 2020; Tian and Sun, 2020; Chang et al., 2021). In this review, we focus mainly on recent research progress on the genetic regulation of RS content in rice, including direct and potential genetic factors that influence RS formation, and we discuss how current knowledge may be used to improve RS content in crops.
Formation of resistant starch
Because RS is a special kind of starch that cannot be digested in the small intestine, RS formation is closely associated with starch biosynthesis. Based on the types of RS, the formation of RS is influenced by the intrinsic properties of starch (e.g., starch granule structure, starch crystal structure, and amylose-to-amylopectin ratio) and non-starch components (e.g., proteins, lipids, and sugars), as well as by storage and processing conditions (e.g., temperature, milling, and baking) (Sajilata et al., 2006; Raigond et al., 2015; Tian and Sun, 2020).
Intrinsic properties of starch influence RS formation
Structure of the starch granule influences RS formation
The size and structure of starch granules significantly affect the content of RS. Several studies have reported that the size of starch granules was negatively correlated with starch digestibility (Singh et al., 2010; Asare et al., 2011). Starch granules in a high-RS rice variety (TRS) were large, voluminous, and non-angular rounded bodies compared with those in a normal rice variety (TQ) (Wei et al., 2010a), indicating that the size of starch granules is related to RS content in rice. In general, starch granules with a large size and lower surface-to-volume ratio are more difficult to digest. The size of starch granules varies dramatically among different crops and organs. For instance, the diameter of starch granules in rice grains is generally less than 5 μm, whereas the diameter of starch granules in potato is more than 75 μm (Chakraborty et al., 2020). Therefore, potato starch is more resistant to digestion than most cereal starches, such as rice and wheat starches (Holm et al., 1988; Ring et al., 1988).
Crystallinity of starch influences RS formation
Based on X-ray diffraction characteristics, starch crystal structure can be divided into four types: A-, B-, C-, and V-type (Imberty et al., 1991; Buleon et al., 1998; Cheetham and Tao, 1998). The A-type starch crystal is usually present in cereal crops. Amylopectin with short lateral chains and closed branching points mainly determines the structure of A-type starch crystals (Buleon et al., 1998). The B-type starch crystal generally exists in raw potato and banana. Amylopectin with long side chains and distant branching points predominantly determines the structure of B-type starch crystals (Imberty et al., 1991). The C-type starch crystal is a mixture of A- and B-type and exists in smooth-seeded peas and beans (Bogracheva et al., 1998). It is generally accepted that the B-type starch crystal is more resistant to digestion than the A- and C-type crystals, and the C-type starch crystal shows more resistance to digestion than the A-type crystal (Ma and Boye, 2017). Consistent with this feature, raw potatoes with a high proportion of the B-type crystalline form have a higher RS content than cereal crops (Lunn and Buttriss, 2007). Starch granules in the high-RS rice line Goami 2 contain B-type crystals (Kang et al., 2003). It should be pointed out that chemical and physical treatments (such as cooking with high temperature) will eliminate the crystalline structure of this type of starch and lead to a reduction in RS content (Englyst and Cummings, 1986; Björck et al., 1989; Schweizer et al., 1990). Therefore, the crystallinity of starch influences its digestibility.
Amylose-to-amylopectin ratio influences RS formation
Starch consists of amylose and amylopectin. Amylose is a linear polymer of glucose residues mainly linked by α-1,4-glycosidic bonds. In general, amylose accounts for 0%–30% of the total starch in cereals. However, amylopectin is a highly branched glucose polymer linked by α-1,4-glycosidic bonds in linear chains and α-1,6-glycosidic bonds at branch points. Starch with a higher percentage composition of amylose has low digestibility, suggesting a positive correlation between amylose content (AC) and RS content (Berry, 1986; Sievert and Pomeranz, 1989). Consistent with this notion, several high-amylose rice mutants show high RS contents (Mizuno et al., 1993; Nishi et al., 2001; Itoh et al., 2003; Zhou et al., 2016; You et al., 2022). High amylose is also associated with high RS content in other cereals, such as wheat, maize, and barley (Granfeldt et al., 1995; Regina et al., 2006; Carciofi et al., 2012). It is generally accepted that a high amylose-to-amylopectin ratio leads to a high RS content, owing to the specific structure of amylose (Ahuja et al., 2013; Xia et al., 2018; Tian and Sun, 2020; Chang et al., 2021). The access of small intestine β-amylases to the two terminal glucose units of the amylose chain is limited because of the straight chains of amylose. By contrast, the highly branched structure of amylopectin provides numerous terminal glucose units that are conducive to access by β-amylases (Ahuja et al., 2013). Therefore, the amylose-to-amylopectin ratio is a major factor that affects the formation of RS2 and RS3 (Sajilata et al., 2006). Furthermore, the retrogradation of amylose also contributes to the formation of RS3 (Berry, 1986; Björck et al., 1990). In most cereals, retrograded amylose was found to be highly resistant to amylases owing to the formation of RS3 (Haralampu, 2000). Notably, varieties with similar AC can nonetheless differ in starch digestibility and RS content, suggesting that AC is an important factor—but not the only factor—in RS formation (Frei et al., 2003; Leeman et al., 2006).
Chain length of amylopectin influences RS formation
Chain length is another factor that affects the formation of RS. It is generally recognized that the medium and long chains of amylopectin are positively correlated with RS content (Ramadoss et al., 2019). This is mainly because long chains form much more stable helices in the starch crystal structure, thereby reducing digestibility (Lehmann and Robin, 2007). Consistent with this scenario, the percentage of medium and long amylopectin chains was increased in the high-RS rice mutants RSML 184 and RSML 278, whereas the content of short-chain amylopectin was reduced (Ramadoss et al., 2019). The opposite result was observed in the low-RS mutant RSML 352 (Ramadoss et al., 2019). A study showed that amylopectin retrogradation was affected by both external chain length and inter-block chain length (Vamadevan and Bertoft, 2018). Similarly, an increase in amylopectin with medium-length chains enhances retrogradation in maize, thereby increasing the RS content (Wu et al., 2017). These results suggest that medium- and long-chain amylopectin contributes to the formation of RS.
External factors influence RS formation
Non-starch components influence RS formation
Interactions of starch with different food components, such as proteins, lipids, dietary fibers, ions, and sugars, have been known to affect the formation of RS. Proteins can pack tightly around the surface of starch granules and prevent their accessibility to digestive enzymes, thereby resulting in increased RS1 content (Escarpa et al., 1997). By contrast, proteins can form hydrogen bonds with amylose during the retrogradation of starch, thereby influencing starch retrogradation and decreasing the yield of RS3 (Escarpa et al., 1997).
Lipids also significantly affect the formation of RS, as they can attach to the surfaces of starch granules and form an amylose–lipid complex, resulting in increased resistance to digestion (Hasjim et al., 2010). Consistent with this scenario, removal of lipids from rice flour can increase the digestibility of starch (Ye et al., 2018). A recent study showed that lipid content is positively associated with total RS content and negatively related to digestibility and glucose index (GI) in rice (Shen et al., 2021). In high-lipid mutants, the extent of digestion was significantly enhanced after non-starch-lipid removal, suggesting a decrease in RS content. However, this phenomenon was not observed in a low-lipid rice variety (Shen et al., 2021). In addition, during the retrogradation of starch, free lipids may competitively bind amylose to form amylose–lipid complexes, causing reductions in recrystallizable AC and RS3 yield (Eliasson et al., 1988; Eerlingen et al., 1994; Escarpa et al., 1997). Similarly, the presence of lipids on the surface or inside the starch granules may inhibit the gelatinization and retrogradation of starch in wheat, thereby decreasing the formation of RS3 (Wang et al., 2016). Although lipids can decrease the formation of RS3 during the retrogradation of starch, they can increase RS5 content due to the formation of amylose–lipid complexes.
Furthermore, calcium and potassium ions can also reduce the content of RS because these ions may prevent the formation of hydrogen bonds between amylose and amylopectin (Escarpa et al., 1997). Interaction between sugars and starch chains can increase the gelatinization temperature (GT) of starch, affecting the recrystallization of amylose and reducing the content of RS (Ianson et al., 1990; Kohyama and Nishinari, 1991). In addition, dietary fibers (e.g., polyphenols, phytic acid, tannic acid, and lectins) have been reported to affect the formation of RS (Thompson and Yoon, 1984; Björck and Nyman, 1987; Escarpa et al., 1997).
Storage and processing conditions influence RS formation
The content of RS is significantly affected by storage conditions such as temperature and time. In general, low-temperature storage is conducive to the formation of RS. Low-temperature storage has been reported to increase RS content in various cereals such as rice and wheat (Johansson et al., 1984; Perdon et al., 1999; Dhital et al., 2010; Yu et al., 2010). Moreover, the longer time that fully gelatinized starch paste is stored at 4°C, the more RS is produced (Chung et al., 2006), mainly owing to increased amylose retrogradation, which results in increased RS3.
Various treatments during food processing, such as milling, baking, extrusion cooking, frying, and autoclaving, affect the RS content of food (Siljestrom and Asp, 1985; Björck and Nyman, 1987; Siljestrom et al., 1989; Muir and O’Dea, 1992; Rabe and Sievert, 1992). Processing techniques affect the formation of RS by influencing the retrogradation processes of starch. During food processing, water and temperature are crucial factors that affect the formation of RS. The recrystallization of starch takes place within a certain range of moisture content (20%–90%), and the degree of recrystallization reaches a peak at 50% moisture content (Longton and Legrys, 1981). Cooking with high moisture and temperature conditions can substantially reduce RS contents by disrupting the crystalline structure. By contrast, extrusion and cooling can increase the yield of RS owing to the induction of amylose recrystallization (Haralampu, 2000). For example, the RS content was higher in parboiled rice than in raw rice because of the recrystallization of amylose (Marsono and Topping, 1993). In addition, stewing and baking affect the formation of RS. Compared with ordinary baking conditions, bread baked for a long time at a low temperature contains more RS (Liljeberg et al., 1996).
Genetic regulation of RS content
RS content is genetically controlled by a number of genes that are mainly involved in starch synthesis (Figure 2). Mutations in the genes encoding granule-bound starch synthase (GBSS), soluble starch synthase (SS), and starch-branching enzyme (SBE) have been reported to influence RS contents in rice, maize, and wheat (Zhou et al., 2016; Baysal et al., 2020; Li et al., 2021; Liu et al., 2021). These genetic factors that influence RS formation in rice are listed in Table 1. In addition, several transcription factors, mRNA splicing factors, and epigenome modifiers regulate the expression of starch-synthesis-related genes (SSRGs), potentially influencing RS content.
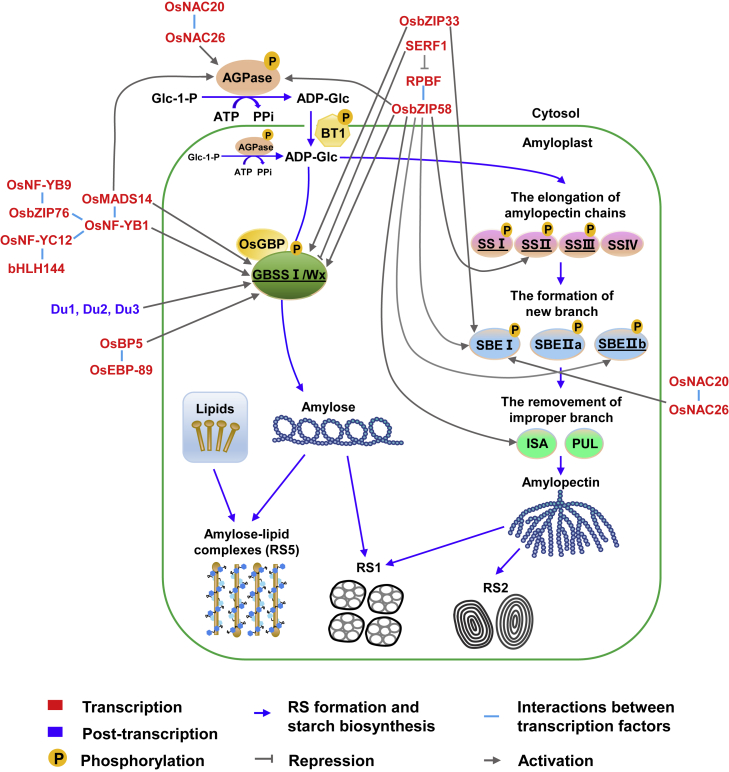
Figure 2.
Regulatory network of RS formation in the rice endosperm.
RS formation is a complicated process that incorporates a number of complex and highly regulated reactions. AGPase is a key rate-limiting enzyme that catalyzes the first step of starch biosynthesis, the production of adenosine diphosphoglucose (ADP-Glc) from glucose-1-phosphate (Glc-1-P). ADP-Glc, mainly synthesized in the cytosol and transported to the amyloplast by Brittle-1 (BT1), is the basic substrate for amylose and amylopectin synthesis. In the amyloplast, GBSSI interacts with GBSS-binding protein (OsGBP), which facilitates the localization of GBSSI to the surface of starch granules and promotes the synthesis of amylose. During amylopectin synthesis, SSI, SSII, and SSIII are commonly responsible for the elongation of α-1,4-glycosidic bonds in amylopectin, whereas SSIV is responsible for the initiation of starch granules. SBEs catalyze the production of α-1,6-glycosidic bonds and form branches. Isoamylase (ISA) and pullulanase (PUL) hydrolyze α-1,6-glycosidic bonds and remove incorrect branches to guarantee the orderly synthesis of amylopectin. SSREs (underlined) are direct regulators that are involved in RS formation. Starch granules enclosed by cell walls or other tissues are known as RS1. B-type starch granules with compact crystalline structure are known as RS2. Amyloses and lipids form amylose–lipid complexes (RS5). At the transcriptional level, several transcription factors that promote or suppress the expression of direct regulators (GBSSI, SSs, and SBEs) of RS formation are potential regulators. At the posttranscriptional level, Du genes (Du1, Du2, and Du3) have been reported to regulate amylose synthesis by specifically affecting the efficient splicing of the Wxb pre-mRNA. At the posttranslational level, phosphorylation regulates starch synthesis by affecting the formation of multi-enzyme complexes. Blue lines represent RS formation and starch biosynthetic processes. Gray lines represent potential regulations of RS formation.
Table 1.
Direct regulators of RS formation in the rice endosperm.
| Gene | Mutant/Allele | Accession number | RS content | Key references |
|---|---|---|---|---|
| GBSSI | Wxa | LOC_Os06g04200 | Increased | Cai et al. (1998); Isshiki et al. (1998); Itoh et al. (2003); You et al. (2022) |
| SSI | Os-076 | LOC_Os06g06560 | Increased | Raja et al. (2017) |
| Os-631 | Increased | |||
| Os-678 | Increased | |||
| SSIIa | SSIIai | LOC_Os06g12450 | Increased | You et al. (2022) |
| SSIIIa | b10 | LOC_Os08g09230 | Increased | Zhou et al. (2016) |
| ss3a-1/e1 | LOC_Os08g09230 | Increased | Fujita et al. (2007) | |
| ss3a-2 | ||||
| SBEIIb | sbe2b | LOC_Os02g32660 | Increased | Butardo et al. (2011) |
| sbeIIb | LOC_Os02g32660 | Increased | Guo et al. (2020) | |
| E15 | LOC_Os02g32660 | Increased | Baysal et al. (2020) | |
| be2b/ae | LOC_Os02g32660 | Increased | Nishi et al. (2001); Asai et al. (2014); Miura et al. (2021) | |
| sbeII | LOC_Os02g32660 | Increased | Sun et al. (2017) | |
| EM10 | LOC_Os02g32660 | Increased | Nishi et al. (2001); Asai et al. (2014) | |
| EM129 | LOC_Os02g32660 | Increased | Mizuno et al. (1993); Wada et al. (2018) | |
| sbe3-rs | LOC_Os02g32660 | Increased | Yang et al. (2012); Yang et al. (2006) | |
| GBSSI SBEIIb | wx ae | LOC_Os06g04200/LOC_Os02g32660 | Increased | Matsumoto et al. (2012) |
| SSI SSIIIa | ss1Lss3a | LOC_Os06g06560/LOC_Os08g09230 | Increased | Hayashi et al. (2015) |
| SSI SBEIIb | ss1Lbe2b | LOC_Os06g06560/LOC_Os02g32660 | Increased | Abe et al. (2014) |
| SSIIa SSIIIa | γ278 | LOC_Os06g12450/LOC_Os08g09230 | Increased | Gurunathan et al. (2019) |
| SSIIIa SSIVb | ss3a ss4b | LOC_Os08g09230/LOC_Os05g45720 | Increased | Toyosawa et al. (2016) |
| SSIIIa SBEI | ss3a be1 | LOC_Os08g09230/LOC_Os06g51084 | Increased | Tsuiki et al. (2016) |
| SSIIIa SBEIIb | ss3a be2b | LOC_Os08g09230/LOC_Os02g32660 | Increased | Asai et al. (2014) |
| SBEI SBEIIb | be1 be2b | LOC_Os06g51084/LOC_Os02g32660 | Increased | Miura et al. (2021) |
| TRS | Increased | Wei et al. (2010a, 2010b); Zhu et al. (2012) | ||
| Unknown | Goami 2/Goamy 2/Suweon 464 | Increased | Butardo et al. (2012); Kang et al. (2003); Kim et al. (2004); Kim et al. (2005a, 2005b); Lee et al. (2006); Nakaya et al. (2013) | |
| RSML 184 | Increased | Ramadoss et al. (2019) | ||
| RSML 278 | Increased | Ramadoss et al. (2019) | ||
| RSML 352 | Decreased | Ramadoss et al. (2019) | ||
| RS111 | Increased | Shu et al. (2006); Yang et al. (2006) | ||
| RS4 | Increased | Shu et al. (2009) |
Genetic factors that determine RS content
Wx alleles
GBSS is the key enzyme for amylose biosynthesis and catalyzes the conversion of individual ADP-glucose molecules into linear chains of glucose residues through α-1,4-glycosidic bonds (Tetlow, 2011). GBSSI, encoded by the Waxy (Wx) gene, is a key enzyme for amylose biosynthesis in the endosperm (Shure et al., 1983; Wang et al., 1990). The Wx gene has been reported to regulate RS contents in rice (Bao et al., 2017; Biselli et al., 2019; You et al., 2022). Several studies revealed that starch with a higher percentage of amylose has lower digestibility, indicating a positive correlation between AC and RS content in rice (Berry, 1986; Sievert and Pomeranz, 1989; Chen et al., 2017b). Consistent with this notion, the RS content of rice with the Wxa allele that produced a high AC was significantly higher than that of rice with the wx allele (Itoh et al., 2003; You et al., 2022). Moreover, a high amylose level is also associated with a high RS content in other cereals, such as wheat and maize (Li et al., 2008, 2020; Guzman and Alvarez, 2016; Lin et al., 2016). In rice, the broad diversity of AC is due mainly to allelic variation at the Wx locus (Tian et al., 2009; Zhang et al., 2019). In cultivated rice, there are two major functional Wx alleles, Wxa and Wxb, which are primarily found in indica and japonica cultivars, respectively (Sano, 1984; Sano et al., 1986; Hirano and Sano, 1998). Compared with the Wxa allele, the Wxb allele contains a G-to-T single base mutation at the 5′ splice site of the first intron, which results in lower mRNA and protein levels of Wx (Sano et al., 1986; Cai et al., 1998; Isshiki et al., 1998). In general, rice cultivars with the Wxa allele produce a large amount of amylose, whereas rice cultivars with the Wxb allele generate a small amount of amylose (Sano et al., 1986; Hirano and Sano, 1998; Isshiki et al., 1998; Larkin and Park, 2003). Consistent with this pattern, indica cultivars with the Wxa allele have higher RS than japonica cultivars with the Wxb allele (Bao et al., 2017). The increased RS in the Wxa background is mainly due to high AC that promotes the formation of RS3 and RS5. Moreover, several other natural alleles of Wx and genome-edited Wx mutants have been shown to influence the AC of rice (Liu et al., 2009; Ma et al., 2015; Zhang et al., 2019, 2021; Huang et al., 2020; Zeng et al., 2020; Zhou et al., 2021), but their effects on RS content have not been reported. Considering that AC is strongly related to RS content, it is possible that these natural Wx alleles and genome-edited mutants may also affect RS content in rice. It will be interesting to investigate the effects of these Wx natural alleles/mutants on RS content in the future.
SS family genes
SSs, which are mainly responsible for the extension of amylopectin chains, include four members (SSI, SSII, SSIII, and SSIV) with different substrate specificities in rice (Ohdan et al., 2005). SSI, SSII, and SSIII are commonly responsible for the elongation of α-1,4-glycoside bonds in amylopectin, whereas SSIV is required for the initiation of starch granules (Hirose and Terao, 2004; Dian et al., 2005; Ohdan et al., 2005; Jeon et al., 2010; Crofts et al., 2015; Guo et al., 2017; Abt and Zeeman, 2020). There are eight SS isoforms in rice (SSI, SSIIa, SSIIb, SSIIc, SSIIIa, SSIIIb, SSIVa, and SSIVb), and most of them are expressed in the endosperm during the filling stages (Ohdan et al., 2005; Tian et al., 2009). However, only a few SSs have been reported to influence RS formation (Zhou et al., 2016; Raja et al., 2017; You et al., 2022). Rice accessions carrying deleterious variants in the SSI gene have high RS contents (Raja et al., 2017), indicating that SSI is crucial for the formation of RS in rice. Downregulation of SSI in both japonica and indica rice cultivars significantly increases the AC and reduces the eating and cooking quality (Li et al., 2018a). Moreover, suppression of SSI in the rice cultivar Nipponbare influences the distribution of amylopectin chains and increases GBSS activity and AC (Zhao et al., 2019). These results suggest that SSI affects RS formation, possibly by influencing AC and the distribution of amylopectin chains.
SSIIa has been reported to affect RS formation in rice. SSIIa, which is encoded by the alkali degeneration (ALK) gene, is the major enzyme that controls GT in rice (Gao et al., 2003). Based on three key SNPs in exon 8 of SSIIa, three ALK alleles have been identified in rice cultivars. The ALKa and ALKb alleles (named SSIIaj) are found mainly in japonica cultivars and encode the SSIIa enzyme with about 10% activity, whereas the ALKc allele (named SSIIai) is found mainly in indica cultivars and encodes the active SSIIa enzyme (Nakamura et al., 2005; Waters et al., 2006; Gao et al., 2011; Chen et al., 2020). Recently, the novel ALK allele ALKd was identified, which can increase GT and improve retrogradation properties (Zhang et al., 2020). A recent study showed that the SSIIai allele produces higher RS content in indica cultivars than does the SSIIaj allele in japonica cultivars (You et al., 2022). Furthermore, the functional SSIIa produces high-crystallinity starch that is more resistant to enzymatic digestion, leading to a higher RS content (Bao et al., 2017). In rice, SSIIa is responsible for elongating short A and B1 chains of amylopectin (6 ≤ degree of polymerization [DP] ≤ 12) to long-type B1 chains (13 ≤ DP ≤ 24) (Nakamura et al., 2005), resulting in a higher accumulation of B1 chains in indica rice than in japonica rice. These results suggest that SSIIa regulates the formation of RS, possibly by increasing the number of medium amylopectin chains. Consistent with this notion, downregulation of SSIIa in rice significantly increases the short amylopectin chains (6 ≤ DP ≤ 10) and reduces the medium amylopectin chains (12 ≤ DP ≤ 24) (Zhang et al., 2011; Butardo et al., 2020). Moreover, the SSIIa-deficient mutant EM204 (ss2a) produced higher AC and shorter chain amylopectin (DP ≤ 12) and lower GT than the wild type (Miura et al., 2018). The SSII-3 single mutant s3, which was generated using CRISPR-Cas9 technology, also showed a significant increase in short amylopectin chains (5 ≤ DP ≤ 12) but a significant decrease in mid-length amylopectin chains (13 ≤ DP ≤ 24) (Huang et al., 2021a). In wheat and barley, the absence of SSIIa caused a significant increase in RS content, which was accompanied by significant increases in the AC and short branch chains of amylopectin (6 ≤ DP ≤ 10) and a decrease in the mid-length chains of amylopectin (11 ≤ DP ≤ 24) (Morell et al., 2003; Bird et al., 2004; Shimbata et al., 2012; Schoen et al., 2021; Yang et al., 2022). These results reveal that SSIIa can influence RS formation by changing the AC and the distribution of amylopectin chain length in rice and wheat.
SSIII consists of SSIIIa and SSIIIb, which are important for the formation of RS in rice. SSIIIa is mainly expressed in developing rice endosperm, whereas SSIIb is primarily expressed in leaves (Ohdan et al., 2005). The b10 mutant, which was isolated from the indica variety R7954, contains a G-to-A mutation at the 3′ splice site of the fifth intron in SSIIIa, which leads to a 4-base pair (bp) deletion in the coding sequence and introduces a premature stop codon (Zhou et al., 2016). The b10 mutant had significantly higher RS content compared with its parental line, R7954. Genetic analyses showed that the effect of the b10 mutant on RS content depends in part on the functional Wx gene (Wxa). Consistent with this finding, RS contents were only slightly higher in two transfer DNA (T-DNA) insertion mutants of SSIIIa in the japonica background (Wxb) (Ryoo et al., 2007; Zhou et al., 2016). During starch biosynthesis, a large complex is formed from a series of enzymes, including ADP-glucose pyrophosphorylase (AGPase), pyruvate orthophosphate dikinase (PPDK), SSIIa, SSIIIa, SBEIIa, and SBEIIb (Hennen-Bierwagen et al., 2009; Crofts et al., 2015). Loss of function of SSIIa disrupts this protein complex, which promotes the biosynthesis of lipids, and deficiency in SSIIIa decreases amylopectin synthesis and promotes amylose synthesis. Consequently, the increase in amylose and lipids in b10 results in an increase in the amylose–lipid complex RS5, thereby increasing the RS content (Zhou et al., 2016). Similarly, several other ss3a mutants have been reported to exhibit high RS content (Fujita et al., 2007; Ryoo et al., 2007; Tsuiki et al., 2016). Interestingly, rice ss2a ss3a double mutants have a higher RS content than the wild type and the ss2a and ss3a single mutants (Gurunathan et al., 2019), suggesting that SSIIa and SSIIIa may function redundantly to control RS content. In wheat and maize, mutations in SSII or SSIII dramatically increase the AC and change the distribution of amylopectin chain lengths, suggesting that they may also influence the content of RS (Zhang et al., 2004; Shimbata et al., 2012; Zhu et al., 2013).
SBE family genes
SBEs consist of three functional members, SBEI, SBEIIa, and SBEIIb, which catalyze the formation of α-1,6-glycosidic bonds and form branches in amylopectin (Han et al., 2007; Tian et al., 2009). SBEI and SBEIIb are specifically expressed in the developing endosperm, and SBEIIa shows a constitutive expression pattern in different rice tissues (Ohdan et al., 2005). Previous studies showed that SBEI is required mainly for the formation of medium and long amylopectin chains, whereas SBEIIb plays an important role in forming short amylopectin chains (Stitt and Zeeman, 2012; Sawada et al., 2018). Remarkably, SBEIIa is predominantly responsible for the formation of intermediate chains and partially compensates for the function of SBEI rather than the function of SBEIIb (Sawada et al., 2018). In rice, SBEIIb is a key gene that controls the formation of RS, but the roles of SBEI and SBEIIa in RS formation have not been reported. Several studies have shown that a deficiency in SBEIIb activity can significantly increase the RS content in the rice endosperm (Butardo et al., 2011; Baysal et al., 2020; Guo et al., 2020; Miura et al., 2021). In rice and maize, amylose extender (ae) mutants contain mutations in SBEIIb. In the japonica background, the ae mutant (EM10) showed a significant increase in RS content in raw rice flour, accompanied by a 2-fold increase in amylose and a marked increase in GT (Mizuno et al., 1993; Nishi et al., 2001; Asai et al., 2014; Miura et al., 2021). Similarly, another ae mutant (EM129) that exhibited similar starch properties to EM10 has been used to breed a rice cultivar, Chikushi-kona 85, with an RS content as high as 17.4% (Mizuno et al., 1993; Wada et al., 2018). The sbe3-rs mutant with a single amino acid mutation in SBEIIb is a mutant of SBEIIb, which contributes to high RS (11.6%) in Jiangtangdao 1 (Yang et al., 2006, 2012). In sbeIIb mutants generated by CRISPR-Cas9 in the japonica cultivar Kitaake, the RS content was dramatically enhanced because of the increase in AC and the high proportion of long de-branched amylopectin chains (Sun et al., 2017). Recently, the high-RS/low-glutelin rice germplasm sbeIIb/Lgc1 was generated by CRISPR-Cas9-induced site-specific mutations of SBEIIb in an elite low-glutelin japonica rice cultivar; it had a high RS content (6.36%) and a 1.8-fold increase in AC (Guo et al., 2020). In addition, downregulating the expression level of SBEIIb in rice endosperm by RNAi produced a high RS content (4.8%) in cooked rice, as well as an increase in long and intermediate amylopectin chains and AC (41.2%) (Butardo et al., 2011). Moreover, complete inhibition of SBEIIb activity produced increases of 0.2% to 17.2% in RS content and 19.6% to 27.4% in AC in the rice endosperm (Baysal et al., 2020). Interestingly, the significantly increased AC in the sbe2b mutant was not due to an increase in the relative proportion of amylose chains. Instead, it occurred because of an increase in long and intermediate de-branched amylopectin chains (Butardo et al., 2011). Furthermore, the increased proportion of long amylopectin chains led to the formation of B-type starch crystals in sbe2b mutant endosperms. The absence of SBEIIb also caused significant increases in sugar, lipid, and protein contents. Thus, all these changes contribute to the high RS content in the sbe2b mutant. In both winter and spring wheat, knockout of the three copies of SBEIIa by CRISPR-Cas9 resulted in significant increases in RS content (from 1.2% to 8.7%) and AC (from 22.6% to 38.7%) (Li et al., 2021). These results highlight the conserved function of SBEII in the control of RS content in cereal crops.
SSRG coordinators
Several SSRGs, such as GBSSI, SSIIa, SSIIIa, and SBEIIb, are known to influence the formation of RS. Moreover, combinatorial effects of SSRGs on RS content have been reported in rice. AMF18 is a wx ae double mutant that was generated by a cross between the wx mutant EM21 and the ae/sbe2b mutant EM16 (Kubo et al., 2008). The RS content in the wx ae double mutant was as high as 27.8%, higher than that in each single mutant and the wild type (Matsumoto et al., 2012; Nakaya et al., 2013). Although starches in the wx ae double mutant lacked amylose, they contained abundant long-unit chains of amylopectin that were conducive to the production of B-type starch crystals, which may contribute to high RS content in the double mutant (Kubo et al., 2010). This result strongly suggests that long amylopectin chains and starch structure also play important roles in promoting RS formation in rice.
The ss1L be2b double mutant generated by a cross between the leaky ss1 mutant and the ae mutant EM10 had approximately 35% RS content, much higher than that of the single mutants (Abe et al., 2014; Tsuiki et al., 2016). The ss3a be2b double mutant derived from a cross between the ss3a mutant e1 and the ae mutant EM10 exhibited high RS content (15%) (Asai et al., 2014; Tsuiki et al., 2016). Recently, a novel be1 be2b double mutant was generated by a cross between be1 (EM557) and be2b (EM10), resulting in a higher RS content (28.4%) than that of the be2b mutant (Nishi et al., 2001; Satoh et al., 2003; Miura et al., 2021). Similarly, simultaneous downregulation of both SBEI and SBEIIb expression led to a higher RS content (14.6%) compared with the wild type (Wei et al., 2010b; Zhu et al., 2012). In wheat, repressing the expression of both SBEIIa and SBEIIb caused a significant increase in RS content (16.6%) (Regina et al., 2015). Similar results have also been observed in durum wheat (Hazard et al., 2015). These studies reveal that combinations of different loss-of-function alleles of SSRGs can be used to generate ultra-high RS materials for RS breeding in rice and other crops.
Potential genetic factors controlling RS content
SSRGs that potentially influence RS content
In addition to the SSRGs such as GBSI, SSIIa, and SBEIIb that directly influence the formation of RS, other SSRGs may potentially affect RS formation. Several studies revealed that downregulation of SSIIb leads to significant decreases in AC and mid-length amylopectin chains, as well as an increase in short amylopectin chains, resulting in large starch granule size in the rice endosperm (Huang et al., 2021a; Li et al., 2018b; Xu et al., 2020). Moreover, suppression of SSIIb expression in both Wxa and Wxb backgrounds results in a decrease in AC and changes the distribution of amylopectin chain lengths, leading to better eating and cooking quality (Huang et al., 2021a). AC, amylopectin chain distribution, and starch granule size were markedly changed in the ss2b mutant, suggesting a potential role for SSIIb in RS formation. SSIV is mainly responsible for the initiation of starch granules in plants. To date, the function of SSIV in RS formation has not been reported. However, the RS content of the ss3a ss4b double mutant is higher than that of the wild type and ss3a, and the starch granules in the ss3a ss4b double mutant are small and spherical compared with those in the wild type and ss3a (Toyosawa et al., 2016; Tsuiki et al., 2016). These findings suggest the possibility that SSIVb might influence the formation of RS.
Transcription factors that potentially influence RS content
Several transcription factors have been reported to promote or suppress the expression of SSRGs that directly influence RS formation, such as basic leucine zipper (bZIP), nuclear factor-Y (NF-Y), myelocytomatosis (MYC), and MADS-box proteins (Zhu et al., 2003; Bello et al., 2019; Wang et al., 2020a; Niu et al., 2020, 2021; Feng et al., 2022), suggesting possible roles for these transcription factors in RS formation. In rice, the bZIP family member OsbZIP33 can associate with the ACGT motif in the promoter regions of Wx and SBEI genes and promote their expression (Yang et al., 2001; Cai et al., 2002). Another bZIP transcription factor, OsbZIP58, that interacts with the DNA binding with one finger (Dof) family transcription factor RPBF (rice prolamin box binding factor) can promote the expression of OsAGPL3, Wx, SSIIa, SBEI, SBEIIb, and ISA2 by directly binding to their promoter regions. The osbzip58 mutant had low total starch, AC, and lipid contents and showed a significant decrease in short amylopectin chains and an increase in long chains (Kawakatsu et al., 2009; Wang et al., 2013; Xu et al., 2020a; Yamamoto et al., 2006).
The dehydration-response element-binding (DREB)-type transcription factor SALT-RESPONSIVE ERF1 (SERF1) represses the expression of both Wx and RPBF by binding to their promoter regions. The contents of total starch and proteinogenic amino acids were clearly increased in the serf1 mutant (Schmidt et al., 2014). The MYC family member OsBP-5 can directly bind to the CAACGTG motif in the promoter region of the Wx gene and activate its transcription by interacting with the AP2/EREBP family member OsEBP-89 (Zhu et al., 2003). The AC of OsBP-5 RNAi lines was reduced compared with that of the wild type (Zhu et al., 2003). NF-YB1 interacts with NF-YC12 and bHLH144 to form a heterotrimeric complex and activates the expression of the Wx gene by directly binding to the G-box in the Wx promoter. Mutations in NF-YB1, NF-YC12, or bHLH144 reduced the total starch, AC, and lipid contents but increased the protein content in rice endosperm (Bello et al., 2019). Another study showed that OsbZIP76 interacts with the OsNF-YB family members OsNF-YB1 and OsNF-YB9 to regulate endosperm development in rice, further influencing the accumulation of amylose (Niu et al., 2020). The total starch and AC in osnf-yb9 mutant grains were significantly reduced compared with those in the wild type (Niu et al., 2021). Recent research showed that the MADS-box family member OsMADS14 interacts with NF-YB1 and directly binds to the CArG-box in the promoters of OsAGPL2 and Wx to promote their transcription. Mutations in OsMADS14 changed the shape and size of starch granules, resulting in a significant reduction in total starch and AC and an increase in soluble sugar content (Feng et al., 2022). Taken together, these transcription factors may form a complex regulatory network to regulate expression of the Wx gene, thereby affecting the synthesis of amylose and the fine structure of starch, potentially influencing RS content. The NAC family transcription factors OsNAC20 and OsNAC26 form heterodimers to regulate starch biosynthesis in the endosperm by directly binding to the promoters of SSRGs. The total starch and protein contents were significantly decreased in the osnac20/26 double mutant, whereas the AC and soluble sugar contents were strongly increased (Wang et al., 2020a), suggesting a potential role for OsNAC20 and OsNAC26 in RS formation. Considering that Wx, SSIIa, and SBEIIb have been known to influence the formation of RS, it is possible that these transcription factors may affect RS content by regulating the expression of Wx, SSIIa, and SBEIIb in rice. It will be interesting to investigate whether these transcription factors can affect RS formation in future research.
Splicing factors that potentially influence RS content
In rice, several dull endosperm (Du) genes, Du1, Du2, and Du3, have been reported to regulate amylose synthesis by specifically affecting the splicing of the Wxb pre-mRNA (Isshiki et al., 2000, 2008; Zeng et al., 2007). The splicing efficiency of the Wxb pre-mRNA is reduced in du mutants, resulting in a decrease in Wx protein and a dull endosperm with low amylose (Zeng et al., 2007; Isshiki et al., 2008). The Du1 gene encodes a member of the pre-mRNA processing (Prp1) family (Zeng et al., 2007). Du3 encodes the rice homolog of a cap-binding protein 20 kD subunit (CBP20) that is a component of the heterodimeric nuclear cap-binding complex (CBC), which plays a role in pre-mRNA splicing (Isshiki et al., 2008). Because Wx is a key gene for RS formation, it is possible that Du genes can affect RS content in rice.
Epigenetic modifications that potentially influence RS content
Epigenetic modification plays a crucial role in the regulation of plant growth and development. A previous study showed that DNA methylation levels of major SSRGs in rice endosperm were frequently lower than those in other tissues, suggesting that DNA methylation is crucial for starch synthesis (Zemach et al., 2010). Consistent with this, the promoter region of the Wx gene contains two neighboring CpG islands that may contribute to its level of DNA methylation, and the low DNA methylation level in its promoter is closely associated with high AC in the rice endosperm (Anacleto et al., 2019). These results indicate that DNA methylation may potentially affect RS formation. Recently, DNA methylation of SSRGs was also discovered in the maize endosperm, and the coding regions of SSRGs with low expression were highly methylated (Hu et al., 2021). Thus, these epigenetic modifications that are involved in starch synthesis could provide a novel strategy for fine-tuning RS content in rice.
Posttranslational modifications that potentially influence RS content
Phosphorylation, an important posttranslational protein modification, can modify the catalytic activities of starch-synthesis-related enzymes (SSREs) in cereals, thus influencing starch synthesis in the endosperm (Chen et al., 2016; Pang et al., 2018). In rice, oligomerization of GBSSI/Wx is crucial for its enzymatic activity, promoting amylose synthesis in the endosperm. However, phosphorylation represses the oligomerization of GBSSI (Liu et al., 2013). Moreover, nine phosphorylated sites were identified in GBSSI, and the Ser415 site is a key phosphorylated site that is strongly associated with the enzyme activity of GBSSI. The substitution of Ser415 with Pro415 in Wxlv results in high GBSSI activity compared with that of Wxa, leading to high AC in the rice endosperm (Zhang et al., 2019). This result suggests the possibility that phosphorylation might influence RS formation by changing the AC of the rice endosperm. In addition, several studies have revealed that phosphorylation is ubiquitous among SSREs in cereals (Tetlow et al., 2004; Walley et al., 2013; Makhmoudova et al., 2014; Cao et al., 2015; Chen et al., 2016, 2017a), demonstrating that phosphorylation plays an important role in starch biosynthesis. It is plausible that phosphorylation of SSREs might affect the formation of RS, although this possibility remains to be investigated in the future.
Other genetic factors that potentially influence RS content
Starch has been proposed to interact with different components, such as proteins, lipids, dietary fibers, ions, and sugars, to affect the formation of RS. Basically, genes involved in protein, lipid, and sugar biosynthesis and metabolism could influence RS content. However, researchers have not measured the effects of these genes on RS content in rice. As the second component of the rice endosperm, protein content is negatively related to starch content. In general, an increase in protein content is accompanied by a decrease in starch content, suggesting that genes related to protein synthesis may potentially influence RS formation. GPA3 (glutelin precursor accumulation 3) encodes a regulator of post-Golgi vesicular trafficking that interacts with the Rab5a-guanine exchange factor VPS9 to form a complex with Rab5a via VPS9. This complex regulates dense vesicle–mediated post-Golgi trafficking in rice. In the gpa3 mutant, AC was significantly reduced, whereas protein and lipid contents were increased (Ren et al., 2014). OsAAP10, an amino acid permease, is responsible for amino acid loading in the rice endosperm. The mutation of OsAAP10 led to decreases in both protein content and AC (Wang et al., 2020b). A recent study showed that lipid content is positively related to RS content in rice (Shen et al., 2021), further supporting the possibility that genetic modification of lipid-related genes might influence RS content. In addition, several genes involved in carbon metabolism influence starch and lipid contents. For example, rice OsPPDKB encodes a PPDK and regulates carbon metabolism and flow for starch and fat biosynthesis in the rice endosperm. Mutations in OsPPDKB caused significant decreases in both total starch and AC and an increase in lipid content (Kang et al., 2005; Zhang et al., 2018). To further understand the roles of genes involved in protein, lipid, and sugar biosynthesis, metabolism, and transport in RS formation, it will be important to measure RS content using mutants or transgenic lines in future research.
Challenges and perspectives
In recent years, research on RS has attracted significant scientific attention because of its promising physiological benefits for human health. Recent studies have identified several key genes that encode enzymes involved in RS formation, but our knowledge of RS formation in rice and other crops remains limited. To date, the genetic and molecular mechanisms of RS formation have not been well dissected in plants. In addition to these well-characterized SSREs, several studies have shown that other factors potentially regulate the formation of RS, including transcription factors, mRNA splicing factors, and protein posttranslational modifications (Isshiki et al., 2000, 2008; Zeng et al., 2007; Zhang et al., 2019; Xu et al., 2020b). However, the effect of these potential factors on RS content is still unclear. Therefore, a straightforward strategy is to test whether these potential genetic factors are really involved in RS formation by measuring the RS content of their loss-of-function mutants or overexpression lines. These studies will help us understand genetic and molecular mechanisms and the ways in which transcriptional, posttranscriptional, or posttranslational regulation of SSRGs determine RS formation. It is also necessary to identify novel genetic factors that determine RS content in rice and other crops using genetic screens, genome-wide association studies (GWAS) (Bao et al., 2017; Parween et al., 2020), and quantitative trait loci (QTL) mapping. Meanwhile, it is important to explore modern high-throughput methods, such as transcriptomics, proteomics, and metabolomics, to elucidate the genetic and molecular mechanisms by which these genes influence RS formation. The future challenge will be to construct the genetic and molecular regulatory network that underlies RS formation in crops.
Breeding novel rice varieties with high RS content has become a focus of breeders. Rice breeders are utilizing the SSRGs, such as SBEIIb, to cultivate new varieties with high RS content (Yang et al., 2012; Wada et al., 2018). Several studies revealed that RS content was significantly increased by combining different loss-of-function alleles of the SSRGs, as in the double mutants wx ae, ss1L be2b, and be1 be2b (Matsumoto et al., 2012; Nakaya et al., 2013; Abe et al., 2014; Tsuiki et al., 2016; Miura et al., 2021). It will be promising to explore genome editing technology to simultaneously edit multiple genes that are known to control RS content and generate their different mutant combinations for improving RS content in the future.
Increasing the RS content of rice usually causes decreases in grain yield, cooking and taste quality, and appearance quality (Butardo et al., 2011; Sun et al., 2017; Li et al., 2018b). Currently, a number of mutants and transgenic lines with high RS content have been developed, but their utilization is still limited because of decreased yield and taste quality (Crofts et al., 2012; Miura et al., 2018; Ramadoss et al., 2019; Baysal et al., 2020; Butardo et al., 2020). For example, suppression of SBEIIb or both SBEI and SBEIIb led to a significant increase in RS content and AC but caused a reduction in grain yield and poor cooking and eating quality (Zhu et al., 2012; Sun et al., 2017; Miura et al., 2021). Overexpression of GBSS or repression of SS and/or SBE causes high RS content but results in decreased starch granule size, stickiness, grain weight, and yield and increased grain chalkiness and hardness (Itoh et al., 2003; Zhang et al., 2011; Zhou et al., 2016). Obviously, seed yield and eating quality have undergone artificial selection during crop domestication. Therefore, it is promising to explore natural variations and find beneficial alleles that can increase both RS content and cooking and taste quality. Furthermore, fine-tuning the expression of SSRGs in the endosperm may be an effective way to generate elite varieties with high RS content and good quality. Creation of some weak alleles by genome editing technology and identification of upstream regulators and epigenetic modifications of SSRGs will help to fine-tune their expression. Therefore, the main challenge we now face is to understand the genetic and molecular mechanisms that control the balance between RS content, grain yield, and eating quality and to apply this knowledge for the improvement of RS, yield, and quality in crops.
Funding
This work is supported by grants from the National Key R&D Program of China (2021YFF1000202) and the Chinese Academy of Science (XDA24030504).
Acknowledgments
We would like to thank Anqi Wang for her helpful suggestions. We apologize to the colleagues whose work is not covered in this review because of limited space. No conflict of interest is declared.
Author contributions
L.S. wrote the manuscript, and Y.L. and J.L. revised the manuscript. All authors read and approved the final version of the manuscript.
Published: May 9, 2022
Footnotes
Published by the Plant Communications Shanghai Editorial Office in association with Cell Press, an imprint of Elsevier Inc., on behalf of CSPB and CEMPS, CAS.
Contributor Information
Jiayang Li, Email: jyli@genetics.ac.cn.
Yunhai Li, Email: yhli@genetics.ac.cn.
References
- Abe N., Asai H., Yago H., Oitome N.F., Itoh R., Crofts N., Nakamura Y., Fujita N. Relationships between starch synthase I and branching enzyme isozymes determined using double mutant rice lines. BMC Plant Biol. 2014;14:80. doi: 10.1186/1471-2229-14-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abt M.R., Zeeman S.C. Evolutionary innovations in starch metabolism. Curr. Opin. Plant Biol. 2020;55:109–117. doi: 10.1016/j.pbi.2020.03.001. [DOI] [PubMed] [Google Scholar]
- Ahuja G., Jaiswal S., Chibbar R.N. Resistant Starch. 2013. Starch biosynthesis in relation to resistant starch; pp. 1–22. [DOI] [Google Scholar]
- Ambigaipalan P., Hoover R., Donner E., Liu Q., Jaiswal S., Chibbar R., Nantanga K.K.M., Seetharaman K. Structure of faba bean, black bean and pinto bean starches at different levels of granule organization and their physicochemical properties. Food Res. Int. 2011;44:2962–2974. [Google Scholar]
- Anacleto R., Badoni S., Parween S., Butardo V.M., Misra G., Cuevas R.P., Kuhlmann M., Trinidad T.P., Mallillin A.C., Acuin C., et al. Integrating a genome-wide association study with a large-scale transcriptome analysis to predict genetic regions influencing the glycaemic index and texture in rice. Plant Biotechnol. J. 2019;17:1261–1275. doi: 10.1111/pbi.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai H., Abe N., Matsushima R., Crofts N., Oitome N.F., Nakamura Y., Fujita N. Deficiencies in both starch synthase IIIa and branching enzyme IIb lead to a significant increase in amylose in SSIIa-inactive japonica rice seeds. J. Exp. Bot. 2014;65:5497–5507. doi: 10.1093/jxb/eru310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asare E.K., Jaiswal S., Maley J., Baga M., Sammynaiken R., Rossnagel B.G., Chibbar R.N. Barley grain constituents, starch composition, and structure affect starch in vitro enzymatic hydrolysis. J. Agr Food Chem. 2011;59:4743–4754. doi: 10.1021/jf200054e. [DOI] [PubMed] [Google Scholar]
- Asp N.G., Björck I. Resistant starch. Trends Food Sci. Technol. 1992;3:111–114. doi: 10.1016/0924-2244(92)90153-n. [DOI] [Google Scholar]
- Bao J.S., Zhou X., Xu F.F., He Q., Park Y.J. Genome-wide association study of the resistant starch content in rice grains. Starch-Stärke. 2017;69:1600343. doi: 10.1002/star.201600343. [DOI] [Google Scholar]
- Bauer-Marinovic M., Florian S., Muller-Schmehl K., Glatt H., Jacobasch G. Dietary resistant starch type 3 prevents tumor induction by 1,2-dimethylhydrazine and alters proliferation, apoptosis and dedifferentiation in rat colon. Carcinogenesis. 2006;27:1849–1859. doi: 10.1093/carcin/bgl025. [DOI] [PubMed] [Google Scholar]
- Baysal C., He W.S., Drapal M., Villorbina G., Medina V., Capell T., Khush G.S., Zhu C.F., Fraser P.D., Christou P. Inactivation of rice starch branching enzyme IIb triggers broad and unexpected changes in metabolism by transcriptional reprogramming. P Natl. Acad. Sci. U S A. 2020;117:26503–26512. doi: 10.1073/pnas.2014860117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behall K.M., Scholfield D.J., Hallfrisch J.G., Liljeberg-Elmstahl H.G.M. Consumption of both resistant starch and beta-glucan improves postprandial plasma glucose and insulin in women. Diabetes Care. 2006;29:976–981. doi: 10.2337/dc05-2012. [DOI] [PubMed] [Google Scholar]
- Bello B.K., Hou Y., Zhao J., Jiao G., Wu Y., Li Z., Wang Y., Tong X., Wang W., Yuan W., et al. NF-YB1-YC12-bHLH144 complex directly activates Wx to regulate grain quality in rice (Oryza sativa L.) Plant Biotechnol. J. 2019;17:1222–1235. doi: 10.1111/pbi.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello-Perez L.A., Flores-Silva P.C., Agama-Acevedo E., Tovar J. Starch digestibility: past, present, and future. J. Sci. Food Agric. 2020;100:5009–5016. doi: 10.1002/jsfa.8955. [DOI] [PubMed] [Google Scholar]
- Berry C.S. Resistant starch: formation and measurement of starch that survives exhaustive digestion with amylolytic enzymes during the determination of dietary fibre. J. Cereal Sci. 1986;4:301–314. [Google Scholar]
- Bird A.R., Flory C., Davies D.A., Usher S., Topping D.L. A novel barley cultivar (Himalaya 292) with a specific gene mutation in starch synthase IIa raises large bowel starch and short-chain fatty acids in rats. J. Nutr. 2004;134:831–835. doi: 10.1093/jn/134.4.831. [DOI] [PubMed] [Google Scholar]
- Biselli C., Volante A., Desiderio F., Tondelli A., Gianinetti A., Finocchiaro F., Taddei F., Gazza L., Sgrulletta D., Cattivelli L., et al. GWAS for starch-related parameters in japonica rice (Oryza sativa L.) Plants. 2019;8:292. doi: 10.3390/plants8080292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birt D.F., Boylston T., Hendrich S., Jane J.L., Hollis J., Li L., McClelland J., Moore S., Phillips G.J., Rowling M., et al. Resistant starch: promise for improving human health. Adv. Nutr. 2013;4:587–601. doi: 10.3945/an.113.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björck I., Gunnarsson A., Ostergard K. A study of native and chemically modified potato starch .2. Digestibility in the rat intestinal-tract. Starch-Stärke. 1989;41:128–134. doi: 10.1002/star.19890410403. [DOI] [Google Scholar]
- Björck I., Eliasson A.C., Drews A., Gudmundsson M., Karlsson R. Some nutritional properties of starch and dietary fiber in barley genotypes containing different levels of amylose. Cereal Chem. 1990;67:327–333. https://www.cabdirect.org/cabdirect/abstract/19911433319 [Google Scholar]
- Björck I.M., Nyman M.E. In vitro effects of phytic acid and polyphenols on starch digestion and fiber degradation. J. Food Sci. 1987;52:1588–1594. doi: 10.1111/j.1365-2621.1987.tb05885.x. [DOI] [Google Scholar]
- Bogracheva T.Y., Morris V.J., Ring S.G., Hedley C.L. The granular structure of C-type pea starch and its role in gelatinization. Biopolymers. 1998;45:323–332. doi: 10.1002/(sici)1097-0282(19980405)45:4<323::aid-bip6>3.0.co;2-N. [DOI] [Google Scholar]
- Brown I. Complex carbohydrates and resistant starch. Nutr. Rev. 1996;54:S115–S119. doi: 10.1111/j.1753-4887.1996.tb03830.x. [DOI] [PubMed] [Google Scholar]
- Brown I.L. Applications and uses of resistant starch. J. Aoac Int. 2004;87:727–732. doi: 10.1093/jaoac/87.3.727. [DOI] [PubMed] [Google Scholar]
- Buleon A., Colonna P., Planchot V., Ball S. Starch granules: structure and biosynthesis. Int. J. Biol. Macromol. 1998;23:85–112. doi: 10.1016/s0141-8130(98)00040-3. [DOI] [PubMed] [Google Scholar]
- Jr V.M.B., Luo J., Li Z., Gidley M.J., Bird A.R., Tetlow I.J., Fitzgerald M., Jobling S.A., Rahman S. Functional genomic validation of the roles of soluble starch synthase IIa in japonica rice endosperm. Front. Genet. 2020;11:289. doi: 10.3389/fgene.2020.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butardo V.M., Fitzgerald M.A., Bird A.R., Gidley M.J., Flanagan B.M., Larroque O., Resurreccion A.P., Laidlaw H.K.C., Jobling S.A., Morell M.K., et al. Impact of down-regulation of starch branching enzyme IIb in rice by artificial microRNA- and hairpin RNA-mediated RNA silencing. J. Exp. Bot. 2011;62:4927–4941. doi: 10.1093/jxb/err188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butardo V.M., Jr., Daygon V.D., Colgrave M.L., Campbell P.M., Resurreccion A., Cuevas R.P., Jobling S.A., Tetlow I., Rahman S., Morell M., et al. Biomolecular analyses of starch and starch granule proteins in the high-amylose rice mutant Goami 2. J. Agric. Food Chem. 2012;60:11576–11585. doi: 10.1021/jf303205p. [DOI] [PubMed] [Google Scholar]
- Cai X.L., Wang Z.Y., Xing Y.Y., Zhang J.L., Hong M.M. Aberrant splicing of intron 1 leads to the heterogeneous 5' UTR and decreased expression of waxy gene in rice cultivars of intermediate amylose content. Plant J. 1998;14:459–465. doi: 10.1046/j.1365-313X.1998.00126.x. [DOI] [PubMed] [Google Scholar]
- Cai Y., Xie D.L., Wang Z.Y., Hong M.M. Interaction of rice bZIP protein REB with the 5'-upstream region of both rice sbe1 gene and waxy gene. Chin. Sci Bull. 2002;47:310–314. doi: 10.1360/02tb9074. [DOI] [Google Scholar]
- Cao H., Yan X., Chen G.X., Zhou J.W., Li X.H., Ma W.J., Yan Y.M. Comparative proteome analysis of A- and B-type starch granule-associated proteins in bread wheat (Triticum aestivum L.) and Aegilops crassa. J. Proteomics. 2015;112:95–112. doi: 10.1016/j.jprot.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Carciofi M., Blennow A., Jensen S.L., Shaik S.S., Henriksen A., Buléon A., Holm P.B., Hebelstrup K.H. Concerted suppression of all starch branching enzyme genes in barley produces amylose-only starch granules. BMC Plant Biol. 2012;12:223. doi: 10.1186/1471-2229-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty I., Pallen S., Shetty Y., Roy N., Mazumder N. Advanced microscopy techniques for revealing molecular structure of starch granules. Biophys. Rev. 2020;12:105–122. doi: 10.1007/s12551-020-00614-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q., Zheng B., Zhang Y., Zeng H. A comprehensive review of the factors influencing the formation of retrograded starch. Int. J. Biol. Macromol. 2021;186:163–173. doi: 10.1016/j.ijbiomac.2021.07.050. [DOI] [PubMed] [Google Scholar]
- Cheetham N.W.H., Tao L.P. Variation in crystalline type with amylose content in maize starch granules: an X-ray powder diffraction study. Carbohyd Polym. 1998;36:277–284. doi: 10.1016/S0144-8617(98)00007-1. [DOI] [Google Scholar]
- Chen Z., Lu Y., Feng L., Hao W., Li C., Yang Y., Fan X., Li Q., Zhang C., Liu Q. Genetic dissection and functional differentiation of ALKa and ALKb, two natural alleles of the ALK/SSIIa gene, responding to low gelatinization temperature in rice. Rice. 2020;13:39. doi: 10.1186/s12284-020-00393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.X., Zhen S.M., Liu Y.L., Yan X., Zhang M., Yan Y.M. In vivo phosphoproteome characterization reveals key starch granule-binding phosphoproteins involved in wheat water-deficit response. BMC Plant Biol. 2017;17:168. doi: 10.1186/s12870-017-1118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G.X., Zhou J.W., Liu Y.L., Lu X.B., Han C.X., Zhang W.Y., Xu Y.H., Yan Y.M. Biosynthesis and regulation of wheat amylose and amylopectin from proteomic and phosphoproteomic characterization of granule-binding proteins. Sci. Rep. 2016;6:33111. doi: 10.1038/srep33111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M.H., Bergman C.J., McClung A.M., Everette J.D., Tabien R.E. Resistant starch: variation among high amylose rice varieties and its relationship with apparent amylose content, pasting properties and cooking methods. Food Chem. 2017;234:180–189. doi: 10.1016/j.foodchem.2017.04.170. [DOI] [PubMed] [Google Scholar]
- Chung H.J., Lim H.S., Lim S.T. Effect of partial gelatinization and retrogradation on the enzymatic digestion of waxy rice starch. J. Cereal Sci. 2006;43:353–359. doi: 10.1016/j.jcs.2005.12.001. [DOI] [Google Scholar]
- Crofts N., Abe K., Aihara S., Itoh R., Nakamura Y., Itoh K., Fujita N. Lack of starch synthase IIIa and high expression of granule-bound starch synthase I synergistically increase the apparent amylose content in rice endosperm. Plant Sci. 2012;193-194:62–69. doi: 10.1016/j.plantsci.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Crofts N., Abe N., Oitome N.F., Matsushima R., Hayashi M., Tetlow I.J., Emes M.J., Nakamura Y., Fujita N. Amylopectin biosynthetic enzymes from developing rice seed form enzymatically active protein complexes. J. Exp. Bot. 2015;66:4469–4482. doi: 10.1093/jxb/erv212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries J.W. On defining dietary fibre. P Nutr. Soc. 2003;62:37–43. doi: 10.1079/PNS2002234. [DOI] [PubMed] [Google Scholar]
- Dhital S., Katawal S.B., Shrestha A.K. Formation of resistant starch during processing and storage of instant noodles. Int. J. Food Properties. 2010;13:454–463. doi: 10.1080/10942910802627091. [DOI] [Google Scholar]
- Dian W.M., Jiang H.W., Wu P. Evolution and expression analysis of starch synthase III and IV in rice. J. Exp. Bot. 2005;56:623–632. doi: 10.1093/jxb/eri065. [DOI] [PubMed] [Google Scholar]
- Eerlingen R.C., Jacobs H., Delcour J.A. Enzyme-resistant starch .V. Effect of retrogradation of waxy maize starch on enzyme susceptibility. Cereal Chem. 1994;71:351–355. [Google Scholar]
- Eliasson A.C., Finstad H., Ljunger G. A study of starch-lipid interactions for some native and modified maize starches. Starch-Stärke. 1988;40:95–100. doi: 10.1002/star.19880400304. [DOI] [Google Scholar]
- Englyst H., Wiggins H.S., Cummings J.H. Determination of the non-starch polysaccharides in plant foods by gas-liquid-chromatography of constituent sugars as alditol acetates. Analyst. 1982;107:307–318. doi: 10.1039/an9820700307. [DOI] [PubMed] [Google Scholar]
- Englyst H.N., Cummings J.H. Digestion of the carbohydrates of banana (musa-paradisiaca-sapientum) in the human small-intestine. Am. J. Clin. Nutr. 1986;44:42–50. doi: 10.1093/ajcn/44.1.42. [DOI] [PubMed] [Google Scholar]
- Englyst H.N., Hudson G.J. The classification and measurement of dietary carbohydrates. Food Chem. 1996;57:15–21. doi: 10.1016/0308-8146(96)00056-8. [DOI] [Google Scholar]
- Englyst H.N., Kingman S.M., Cummings J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992;46 Suppl 2:S33–S50. PMID: 1330528. [PubMed] [Google Scholar]
- Escarpa A., Gonzalez M.C., Morales M.D., SauraCalixto F. An approach to the influence of nutrients and other food constituents on resistant starch formation. Food Chem. 1997;60:527–532. doi: 10.1016/S0308-8146(97)00025-3. [DOI] [Google Scholar]
- Feng T., Wang L., Li L., Liu Y., Chong K., Theißen G., Meng Z. OsMADS14 and NF-YB1 cooperate in the direct activation of OsAGPL2 and Waxy during starch synthesis in rice endosperm. New Phytol. 2022;234:77–92. doi: 10.1111/nph.17990. [DOI] [PubMed] [Google Scholar]
- Frei M., Siddhuraju P., Becker K. Studies on the in vitro starch digestibility and the glycemic index of six different indigenous rice cultivars from the Philippines. Food Chem. 2003;83:395–402. doi: 10.1016/S0308-8146(03)00101-8. [DOI] [Google Scholar]
- Fujita N., Yoshida M., Kondo T., Saito K., Utsumi Y., Tokunaga T., Nishi A., Satoh H., Park J.H., Jane J.L., et al. Characterization of SSIIIa-deficient mutants of rice: the function of SSIIIa and pleiotropic effects by SSIIIa deficiency in the rice endosperm. Plant Physiol. 2007;144:2009–2023. doi: 10.1104/pp.107.102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z., Zeng D., Cui X., Zhou Y., Yan M., Huang D., Li J., Qian Q. Map-based cloning of the ALK gene, which controls the gelatinization temperature of rice. Sci. China Ser. C: Life Sci. 2003;46:661–668. doi: 10.1360/03yc0099. [DOI] [PubMed] [Google Scholar]
- Gao Z., Zeng D., Cheng F., Tian Z., Guo L., Su Y., Yan M., Jiang H., Dong G., Huang Y., et al. ALK, the key gene for gelatinization temperature, is a modifier gene for gel consistency in rice. J. Integr. Plant Biol. 2011;53:756–765. doi: 10.1111/j.1744-7909.2011.01065.x. [DOI] [PubMed] [Google Scholar]
- Govers M.J.A.P., Gannon N.J., Dunshea F.R., Gibson P.R., Muir J.G. Wheat bran affects the site of fermentation of resistant starch and luminal indexes related to colon cancer risk: a study in pigs. Gut. 1999;45:840–847. doi: 10.1136/gut.45.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granfeldt Y., Drews A., Bjorck I. Arepas made from high amylose corn flour produce favorably low glucose and insulin responses in healthy humans. J. Nutr. 1995;125:459–465. doi: 10.1093/jn/125.3.459. [DOI] [PubMed] [Google Scholar]
- Guo D., Ling X., Zhou X., Li X., Wang J., Qiu S., Yang Y., Zhang B. Evaluation of the quality of a high-resistant starch and low-glutelin rice (Oryza sativa L.) generated through CRISPR/Cas9-mediated targeted mutagenesis. J. Agric. Food Chem. 2020;68:9733–9742. doi: 10.1021/acs.jafc.0c02995. [DOI] [PubMed] [Google Scholar]
- Guo H.J., Liu Y.C., Li X., Yan Z.H., Xie Y.D., Xiong H.C., Zhao L.S., Gu J.Y., Zhao S.R., Liu L.X. Novel mutant alleles of the starch synthesis gene TaSSIVb-D result in the reduction of starch granule number per chloroplast in wheat. BMC Genomics. 2017;18:358. doi: 10.1186/s12864-017-3724-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Tan L., Kong L. Impact of dietary intake of resistant starch on obesity and associated metabolic profiles in human: a systematic review of the literature. Crit. Rev. Food Sci. Nutr. 2021;61:889–905. doi: 10.1080/10408398.2020.1747391. [DOI] [PubMed] [Google Scholar]
- Gurunathan S., Ramadoss B.R., Mudili V., Siddaiah C., Kalagatur N.K., Bapu J.R.K., Mohan C.D., Alqarawi A.A., Hashem A., Abd_Allah E.F. Single nucleotide polymorphisms in starch biosynthetic genes associated with increased resistant starch Concentration in rice mutant. Front. Genet. 2019;10:946. doi: 10.3389/fgene.2019.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman C., Alvarez J.B. Wheat waxy proteins: polymorphism, molecular characterization and effects on starch properties. Theor. Appl. Genet. 2016;129:1–16. doi: 10.1007/s00122-015-2595-9. [DOI] [PubMed] [Google Scholar]
- Han K.H., Iijuka M., Shimada K.I., Sekikawa M., Kuramochi K., Ohba K., Ruvini L., Chiji H., Fukushima M. Adzuki resistant starch lowered serum cholesterol and hepatic 3-hydroxy-3-methylglutaryl-CoA mRNA levels and increased hepatic LDL-receptor and cholesterol 7 alpha-hydroxylase mRNA levels in rats fed a cholesterol diet. Br. J Nutr. 2005;94:902–908. doi: 10.1079/Bjn20051598. [DOI] [PubMed] [Google Scholar]
- Han Y., Sun F.J., Rosales-Mendoza S., Korban S.S. Three orthologs in rice, Arabidopsis, and Populus encoding starch branching enzymes (SBEs) are different from other SBE gene families in plants. Gene. 2007;401:123–130. doi: 10.1016/j.gene.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Haralampu S.G. Resistant starch: a review of the physical properties and biological impact of RS3. Carbohyd Polym. 2000;41:285–292. [Google Scholar]
- Hasjim J., Lee S.O., Hendrich S., Setiawan S., Ai Y.F., Jane J.L. Characterization of a novel resistant-starch and its effects on postprandial plasma-glucose and insulin responses. Cereal Chem. 2010;87:257–262. [Google Scholar]
- Hayashi M., Kodama M., Nakamura Y., Fujita N. Thermal and pasting properties, morphology of starch granules, and crystallinity of endosperm starch in the rice SSI and SSIIIa double-mutant. J. Appl. Glycoscience. 2015;62:81–86. doi: 10.5458/jag.jag.JAG-2015_007. [DOI] [Google Scholar]
- Hazard B., Zhang X.Q., Naemeh M., Hamilton M.K., Rust B., Raybould H.E., Newman J.W., Martin R., Dubcovsky J. Mutations in durum wheat SBEII genes affect grain yield components, quality, and fermentation responses in rats. Crop Sci. 2015;55:2813–2825. doi: 10.2135/cropsci2015.03.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennen-Bierwagen T.A., Lin Q., Grimaud F., Planchot V., Keeling P.L., James M.G., Myers A.M. Proteins from multiple metabolic pathways associate with starch biosynthetic enzymes in high molecular weight complexes: a model for regulation of carbon allocation in maize amyloplasts. Plant Physiol. 2009;149:1541–1559. doi: 10.1104/pp.109.135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J.A., Brand Miller J.C., Denyer G.S. Development of insulin resistance in the rat is dependent on the rate of glucose absorption from the diet. J. Nutr. 1996;126:596–602. doi: 10.1093/jn/126.3.596. [DOI] [PubMed] [Google Scholar]
- Higgins J.A., Jackman M.R., Brown I.L., Johnson G.C., Steig A., Wyatt H.R., Hill J.O., MacLean P.S. Resistant starch and exercise independently attenuate weight regain on a high fat diet in a rat model of obesity. Nutr. Metab. 2011;8:49. doi: 10.1186/1743-7075-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano H.Y., Sano Y. Enhancement of Wx gene expression and the accumulation of amylose in response to cool temperatures during seed development in rice. Plant Cell Physiol. 1998;39:807–812. doi: 10.1093/oxfordjournals.pcp.a029438. [DOI] [Google Scholar]
- Hirose T., Terao T. A comprehensive expression analysis of the starch synthase gene family in rice (Oryza sativa L.) Planta. 2004;220:9–16. doi: 10.1007/s00425-004-1314-6. [DOI] [PubMed] [Google Scholar]
- Hizukuri S., Takeda Y., Maruta N., Juliano B.O. Molecular- structures of rice starch. Carbohyd Res. 1989;189:227–235. [Google Scholar]
- Holm J., Lundquist I., Bjorck I., Eliasson A.C., Asp N.G. Degree of starch gelatinization, digestion rate of starch in vitro, and metabolic response in rats. Am. J. Clin. Nutr. 1988;47:1010–1016. doi: 10.1093/ajcn/47.6.1010. [DOI] [PubMed] [Google Scholar]
- Hu Y., Le Leu R.K., Christophersen C.T., Somashekar R., Conlon M.A., Meng X.Q., Winter J.M., Woodman R.J., McKinnon R., Young G.P. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis. 2016;37:366–375. doi: 10.1093/carcin/bgw019. [DOI] [PubMed] [Google Scholar]
- Hu Y., Li Y., Weng J., Liu H., Yu G., Liu Y., Xiao Q., Huang H., Wang Y., Wei B., et al. Coordinated regulation of starch synthesis in maize endosperm by microRNAs and DNA methylation. Plant J. 2021;105:108–123. doi: 10.1111/tpj.15043. [DOI] [PubMed] [Google Scholar]
- Huang L., Sreenivasulu N., Liu Q. Waxy editing: old meets new. Trends Plant Science. 2020;25:963–966. doi: 10.1016/j.tplants.2020.07.009. [DOI] [PubMed] [Google Scholar]
- Huang L., Gu Z., Chen Z., Yu J., Chu R., Tan H., Zhao D., Fan X., Zhang C., Li Q., et al. Improving rice eating and cooking quality by coordinated expression of the major starch synthesis-related genes, SSII and Wx, in endosperm. Plant Mol. Biol. 2021;106:419–432. doi: 10.1007/s11103-021-01162-8. [DOI] [PubMed] [Google Scholar]
- Ianson K.J., Miles M.J., Morris V.J., Besford L.S., Jarvis D.A., Marsh R.A. The effects of added sugars on the retrogradation of wheat-starch gels. J. Cereal Sci. 1990;11:243–248. doi: 10.1016/S0733-5210(09)80168-9. [DOI] [Google Scholar]
- Imberty A., Buleon A., Tran V., Péerez S. Recent advances in knowledge of starch structure. Starch-Stärke. 1991;43:375–384. [Google Scholar]
- Isshiki M., Nakajima M., Satoh H., Shimamoto K. Dull: rice mutants with tissue-specific effects on the splicing of the waxy pre-mRNA. Plant J. 2000;23:451–460. doi: 10.1046/j.1365-313x.2000.00803.x. [DOI] [PubMed] [Google Scholar]
- Isshiki M., Matsuda Y., Takasaki A., Wong H.L., Satoh H., Shimamoto K. Du3, a mRNA cap-binding protein gene, regulates amylose content in Japonica rice seeds. Plant Biotechnol-Nar. 2008;25:483–487. [Google Scholar]
- Isshiki M., Morino K., Nakajima M., Okagaki R.J., Wessler S.R., Izawa T., Shimamoto K. A naturally occurring functional allele of the rice waxy locus has a GT to TT mutation at the 5' splice site of the first intron. Plant J. 1998;15:133–138. doi: 10.1046/j.1365-313X.1998.00189.x. [DOI] [PubMed] [Google Scholar]
- Itoh K., Ozaki H., Okada K., Hori H., Takeda Y., Mitsui T. Introduction of Wx transgene into rice wx mutants leads to both high- and low-amylose rice. Plant Cell Physiol. 2003;44:473–480. doi: 10.1093/pcp/pcg068. [DOI] [PubMed] [Google Scholar]
- Jacobasch G., Schmiedl D., Kruschewski M., Schmehl K. Dietary resistant starch and chronic inflammatory bowel diseases. Int. J. Colorectal Dis. 1999;14:201–211. doi: 10.1007/s003840050212. [DOI] [PubMed] [Google Scholar]
- Jeon J.S., Ryoo N., Hahn T.R., Walia H., Nakamura Y. Starch biosynthesis in cereal endosperm. Plant Physiol. Bioch. 2010;48:383–392. doi: 10.1016/j.plaphy.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Jiang F., Du C., Jiang W., Wang L., Du S.K. The preparation, formation, fermentability, and applications of resistant starch. Int. J. Biol. Macromol. 2020;150:1155–1161. doi: 10.1016/j.ijbiomac.2019.10.124. [DOI] [PubMed] [Google Scholar]
- Johansson C.G., Siljeström M., Asp N.G. Dietary fiber in bread and corresponding flours-formation of resistant starch during baking. Z. Lebensm Unters Forsch. 1984;179:24–28. doi: 10.1007/Bf01042850. [DOI] [PubMed] [Google Scholar]
- Jukanti A.K., Pautong P.A., Liu Q., Sreenivasulu N. Low glycemic index rice-a desired trait in starchy staples. Trends Food Sci. Technol. 2020;106:132–149. doi: 10.1016/j.tifs.2020.10.006. [DOI] [Google Scholar]
- Kang H.G., Park S., Matsuoka M., An G. White-core endosperm floury endosperm-4 in rice is generated by knockout mutations in the C4-type pyruvate orthophosphate dikinase gene (OsPPDKB) Plant J. 2005;42:901–911. doi: 10.1111/j.1365-313X.2005.02423.x. [DOI] [PubMed] [Google Scholar]
- Kang H.J., Hwang I.K., Kim K.S., Choi H.C. Comparative structure and physicochemical properties of Ilpumbyeo, a high-quality japonica rice, and its mutant, Suweon 464. J. Agric. Food Chem. 2003;51:6598–6603. doi: 10.1021/jf0344946. [DOI] [PubMed] [Google Scholar]
- Kawakatsu T., Yamamoto M.P., Touno S.M., Yasuda H., Takaiwa F. Compensation and interaction between RISBZ1 and RPBF during grain filling in rice. Plant J. 2009;59:908–920. doi: 10.1111/j.1365-313X.2009.03925.x. [DOI] [PubMed] [Google Scholar]
- Keenan M.J., Zhou J., Hegsted M., Pelkman C., Durham H.A., Coulon D.B., Martin R.J. Role of resistant starch in improving gut health, adiposity, and insulin resistance. Adv. Nutr. 2015;6:198–205. doi: 10.3945/an.114.007419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.S., Kang H.J., Hwang I.K., Hwang H.G., Kim T.Y., Choi H.C. Comparative ultrastructure of Ilpumbyeo, a high-quality japonica rice, and its mutant, Suweon 464: scanning and transmission electron microscopy studies. J. Agric. Food Chem. 2004;52:3876–3883. doi: 10.1021/jf049767r. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Hwang H.G., Kang H.J., Hwang I.K., Lee Y.T., Choi H.C. Ultrastructure of individual and compound starch granules in isolation preparation from a high-quality, low-amylose rice, ilpumbyeo, and its mutant, G2, a high-dietary fiber, high-amylose rice. J. Agr Food Chem. 2005;53:8745–8751. doi: 10.1021/jf051194a. [DOI] [PubMed] [Google Scholar]
- Kim K.S., Kang H.J., Hwang I.K., Hwang H.G., Kim T.Y., Choi H.C. Fibrillar microfilaments associated with a high-amylose rice, goami 2, a mutant of ilpumbyeo, a high-quality japonica rice. J. Agric. Food Chem. 2005;53:2600–2608. doi: 10.1021/jf047900+. [DOI] [PubMed] [Google Scholar]
- Kohyama K., Nishinari K. Effect of soluble sugars on gelatinization and retrogradation of sweet-potato starch. J. Agric. Food Chem. 1991;39:1406–1410. doi: 10.1021/jf00008a010. [DOI] [Google Scholar]
- Kubo A., Akdogan G., Nakaya M., Shojo A., Suzuki S., Satoh H., Kitamura S. Structure, physical, and digestive properties of starch from wx ae double-mutant rice. J. Agr Food Chem. 2010;58:4463–4469. doi: 10.1021/jf904074k. [DOI] [PubMed] [Google Scholar]
- Kubo T., Yamagata Y., Eguchi M., Yoshimura A. A novel epistatic interaction at two loci causing hybrid male sterility in an inter-subspecific cross of rice (Oryza sativa L.) Genes Genet. Syst. 2008;83:443–453. doi: 10.1266/ggs.83.443. [DOI] [PubMed] [Google Scholar]
- Larkin P.D., Park W.D. Association of waxy gene single nucleotide polymorphisms with starch characteristics in rice (Oryza sativa L.) Mol. Breed. 2003;12:335–339. doi: 10.1023/B:Molb.0000006797.51786.92. [DOI] [Google Scholar]
- Lee K.W., Song K.E., Lee H.S., Kim Y.K., Lee S.W., Kim D.J., Hwang W.S., Choe S.J., Kim Y.S., Kim T.Y. The effects of goami no. 2 rice, a natural fiber-rich rice, on body weight and lipid metabolism. Obesity. 2006;14:423–430. doi: 10.1038/oby.2006.56. [DOI] [PubMed] [Google Scholar]
- Margareta Leeman A., Leeman A.M., Karlsson M.E., Eliasson A.C., Bjorck I.M.E. Resistant starch formation in temperature treated potato starches varying in amylose/amylopectin ratio. Carbohyd Polym. 2006;65:306–313. [Google Scholar]
- Lehmann U., Robin F. Slowly digestible starch - its structure and health implications: a review. Trends Food Sci. Technol. 2007;18:346–355. doi: 10.1016/j.tifs.2007.02.009. [DOI] [Google Scholar]
- Li H.T., Sartika R.S., Kerr E.D., Schulz B.L., Gidley M.J., Dhital S. Starch granular protein of high-amylose wheat gives innate resistance to amylolysis. Food Chem. 2020;330:127328. doi: 10.1016/j.foodchem.2020.127328. [DOI] [PubMed] [Google Scholar]
- Li J., Jiao G., Sun Y., Chen J., Zhong Y., Yan L., Jiang D., Ma Y., Xia L. Modification of starch composition, structure and properties through editing of TaSBEIIa in both winter and spring wheat varieties by CRISPR/Cas9. Plant Biotechnol. J. 2021;19:937–951. doi: 10.1111/pbi.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Jiang H.X., Campbell M., Blanco M., Jane J.L. Characterization of maize amylose-extender (ae) mutant starches. Part I: relationship between resistant starch contents and molecular structures. Carbohyd Polym. 2008;74:396–404. doi: 10.1016/j.carbpol.2008.03.012. [DOI] [Google Scholar]
- Li Q., Liu X., Zhang C., Jiang L., Jiang M., Zhong M., Fan X., Gu M., Liu Q. Rice soluble starch synthase I: allelic variation, expression, function, and interaction with Waxy. Front. Plant Sci. 2018;9:1591. doi: 10.3389/fpls.2018.01591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.F., Huang L.C., Chu R., Li J., Jiang M.Y., Zhang C.Q., Fan X.L., Yu H.X., Gu M.H., Liu Q.Q. Down-regulation of SSSII-2 gene expression results in novel low-amylose rice with soft, transparent grains. J. Agric. Food Chem. 2018;66:9750–9760. doi: 10.1021/acs.jafc.8b02913. [DOI] [PubMed] [Google Scholar]
- Liljeberg H.G.M., Granfeldt Y.E., Bjorck I.M.E. Products based on a high fiber barley genotype, but not on common barley or oats, lower postprandial glucose and insulin responses in healthy humans. J. Nutr. 1996;126:458–466. doi: 10.1093/jn/126.2.458. [DOI] [PubMed] [Google Scholar]
- Lin L.S., Guo D.W., Huang J., Zhang X.D., Zhang L., Wei C.X. Molecular structure and enzymatic hydrolysis properties of starches from high-amylose maize inbred lines and their hybrids. Food Hydrocolloids. 2016;58:246–254. doi: 10.1016/j.foodhyd.2016.03.001. [DOI] [Google Scholar]
- Liu D.R., Huang W.X., Cai X.L. Oligomerization of rice granule-bound starch synthase 1 modulates its activity regulation. Plant Sci. 2013;210:141–150. doi: 10.1016/j.plantsci.2013.05.019. [DOI] [PubMed] [Google Scholar]
- Liu L., Ma X., Liu S., Zhu C., Jiang L., Wang Y., Shen Y., Ren Y., Dong H., Chen L., et al. Identification and characterization of a novel Waxy allele from a Yunnan rice landrace. Plant Mol. Biol. 2009;71:609–626. doi: 10.1007/s11103-009-9544-4. [DOI] [PubMed] [Google Scholar]
- Liu Q., Hu Y.P., Hu M.Y., Sun L.J., Chen X.Y., Li Q.Y., Wang P.N., Wang L.A., Zhang Y.J., Li H. Identification and molecular characterization of mutant line deficiency in three waxy proteins of common wheat (Triticum aestivum L.) Sci. Rep. 2021;11:3510. doi: 10.1038/s41598-021-82865-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longton J., Legrys G.A. Differential scanning calorimetry studies on the crystallinity of aging wheat-starch gels. Starch-Stärke. 1981;33:410–414. doi: 10.1002/star.19810331204. [DOI] [Google Scholar]
- Lunn J., Buttriss J.L. Carbohydrates and dietary fibre. Nutr. Bull. 2007;32:21–64. doi: 10.1111/j.1467-3010.2007.00616.x. [DOI] [Google Scholar]
- Ma X.L., Zhang Q.Y., Zhu Q.L., Liu W., Chen Y., Qiu R., Wang B., Yang Z.F., Li H.Y., Lin Y.R., et al. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol. Plant. 2015;8:1274–1284. doi: 10.1016/j.molp.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Ma Z., Boye J.I. Research advances on structural characterization of resistant starch and its structure-physiological function relationship: a review. Crit. Rev. Food Sci. Nutr. 2017;58:1059–1083. doi: 10.1080/10408398.2016.1230537. [DOI] [PubMed] [Google Scholar]
- Makhmoudova A., Williams D., Brewer D., Massey S., Patterson J., Silva A., Vassall K.A., Liu F., Subedi S., Harauz G., et al. Identification of multiple phosphorylation sites on maize endosperm starch branching enzyme IIb, a key enzyme in amylopectin biosynthesis. J. Biol. Chem. 2014;289:9233–9246. doi: 10.1074/jbc.M114.551093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsono Y., Topping D.L. Effects of particle size of rice on resistant starch and SCFA of the digesta in caecostomised pigs. Indonesian Food Nutr. Prog. 1993;6:44–51. [Google Scholar]
- Martinez-Flores H.E., Kil Chang Y., Martinez-Bustos F., Sgarbieri V. Effect of high fiber products on blood lipids and lipoproteins in hamsters. Nutr. Res. 2004;24:85–93. doi: 10.1016/j.nutres.2003.08.016. [DOI] [Google Scholar]
- Matsumoto K., Maekawa M., Nakaya M., Takemitsu H., Satoh H., Kitamura S. Wx/ae double-mutant brown rice prevents the rise in plasma lipid and glucose levels in mice. Biosci. Biotechnol. Biochem. 2012;76:2112–2117. doi: 10.1271/bbb.120501. [DOI] [PubMed] [Google Scholar]
- Miura S., Koyama N., Crofts N., Hosaka Y., Abe M., Fujita N. Generation and starch characterization of non-transgenic BEI and BEIIb double mutant rice (Oryza sativa) with ultra-high level of resistant starch. Rice. 2021;14:3. doi: 10.1186/s12284-020-00441-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura S., Crofts N., Saito Y., Hosaka Y., Oitome N.F., Watanabe T., Kumamaru T., Fujita N. Starch synthase IIa-deficient mutant rice line produces endosperm starch with lower gelatinization temperature than Japonica rice cultivars. Front. Plant Sci. 2018;9:645. doi: 10.3389/fpls.2018.00645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K., Kawasaki T., Shimada H., Satoh H., Kobayashi E., Okumura S., Arai Y., Baba T. Alteration of the structural properties of starch components by the lack of an isoform of starch branching enzyme in rice seeds. J. Biol. Chem. 1993;268:19084–19091. doi: 10.1016/S0021-9258(17)46738-X. [DOI] [PubMed] [Google Scholar]
- Morell M.K., Kosar-Hashemi B., Cmiel M., Samuel M.S., Chandler P., Rahman S., Buleon A., Batey I.L., Li Z. Barley sex6 mutants lack starch synthase IIa activity and contain a starch with novel properties. Plant J. 2003;34:173–185. doi: 10.1046/j.1365-313X.2003.01712.x. [DOI] [PubMed] [Google Scholar]
- Morita T., Kasaoka S., Kiriyama S., Brown I.L., Topping D.L. Comparative effects of acetylated and unmodified high-amylose maize starch in rats. Starch-Stärke. 2005;57:246–253. doi: 10.1002/star.200400373. [DOI] [Google Scholar]
- Muir J.G., O'Dea K. Measurement of resistant starch: factors affecting the amount of starch escaping digestion in vitro. Am. J. Clin. Nutr. 1992;56:123–127. doi: 10.1093/ajcn/56.1.123. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Francisco P.B., Hosaka Y., Sato A., Sawada T., Kubo A., Fujita N. Essential amino acids of starch synthase IIa differentiate amylopectin structure and starch quality between japonica and indica rice varieties. Plant Mol. Biol. 2005;58:213–227. doi: 10.1007/s11103-005-6507-2. [DOI] [PubMed] [Google Scholar]
- Nakaya M., Shojo A., Hirai H., Matsumoto K., Kitamura S. Hypolipidemic effects of starch and gamma-oryzanol from wx/ae double-mutant rice on BALB/c.KOR-Apoe(shl) mice. Biosci. Biotechnol. Biochem. 2013;77:1435–1440. doi: 10.1271/bbb.130087. [DOI] [PubMed] [Google Scholar]
- Nishi A., Nakamura Y., Tanaka N., Satoh H. Biochemical and genetic analysis of the effects of amylose-extender mutation in rice endosperm. Plant Physiol. 2001;127:459–472. doi: 10.1104/pp.010127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu B., Zhang Z., Zhang J., Zhou Y., Chen C. The rice LEC1-like transcription factor OsNF-YB9 interacts with SPK, an endosperm-specific sucrose synthase protein kinase, and functions in seed development. Plant J. 2021;106:1233–1246. doi: 10.1111/tpj.15230. [DOI] [PubMed] [Google Scholar]
- Niu B., Deng H., Li T., Sharma S., Yun Q., Li Q., E Z., Chen C. OsbZIP76 interacts with OsNF-YBs and regulates endosperm cellularization in rice (Oryza sativa) J. Integr. Plant Biol. 2020;62:1983–1996. doi: 10.1111/jipb.12989. [DOI] [PubMed] [Google Scholar]
- Nugent A.P. Health properties of resistant starch. Nutr. Bull. 2005;30:27–54. doi: 10.1111/j.1467-3010.2005.00481.x. [DOI] [Google Scholar]
- Ohdan T., Francisco P.B., Jr., Sawada T., Hirose T., Terao T., Satoh H., Nakamura Y. Expression profiling of genes involved in starch synthesis in sink and source organs of rice. J. Exp. Bot. 2005;56:3229–3244. doi: 10.1093/jxb/eri292. [DOI] [PubMed] [Google Scholar]
- Oladele E.O.P. Starch retrogradation and its impact on nutritional starch fractions in plantain (Musa AAB) foods. J. Food Meas. Charact. 2016;10:546–553. doi: 10.1007/s11694-016-9334-z. [DOI] [Google Scholar]
- Pang Y.H., Zhou X., Chen Y.L., Bao J.S. Comparative phosphoproteomic analysis of the developing seeds in two Indica rice (Oryza sativa L.) cultivars with different starch quality. J. Agr Food Chem. 2018;66:3030–3037. doi: 10.1021/acs.jafc.8b00074. [DOI] [PubMed] [Google Scholar]
- Park O.J., Kang N.E., Chang M.J., Kim W.K. Resistant starch supplementation influences blood lipid concentrations and glucose control in overweight subjects. J. Nutr. Sci. Vitaminol. 2004;50:93–99. doi: 10.3177/jnsv.50.93. PMID: 15242012. [DOI] [PubMed] [Google Scholar]
- Parween S., Anonuevo J.J., Butardo V.M., Misra G., Anacleto R., Llorente C., Kosik O., Romero M.V., Bandonill E.H., Mendioro M.S., et al. Balancing the double-edged sword effect of increased resistant starch content and its impact on rice texture: its genetics and molecular physiological mechanisms. Plant Biotechnol. J. 2020;18:1763–1777. doi: 10.1111/pbi.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdon A.A., Siebenmorgen T.J., Buescher R.W., Gbur E.E. Starch retrogradation and texture of cooked milled rice during storage. J. Food Sci. 1999;64:828–832. doi: 10.1111/j.1365-2621.1999.tb15921.x. [DOI] [Google Scholar]
- Rabe E., Sievert D. Effects of baking, pasta production, and extrusion cooking on formation of resistant starch. Eur. J. Clin. Nutr. 1992;46 Suppl 2:105–107. PMID: 1330513. [PubMed] [Google Scholar]
- Ragaee S., Abdel-Aal E.M., Noaman M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006;98:32–38. [Google Scholar]
- Raigond P., Ezekiel R., Raigond B. Resistant starch in food: a review. J. Sci. Food Agric. 2015;95:1968–1978. doi: 10.1002/jsfa.6966. [DOI] [PubMed] [Google Scholar]
- Raja R.B., Agasimani S., Jaiswal S., Thiruvengadam V., Sabariappan R., Chibbar R.N., Ram S.G. EcoTILLING by sequencing reveals polymorphisms in genes encoding starch synthases that are associated with low glycemic response in rice. BMC Plant Biol. 2017;17:13. doi: 10.1186/s12870-016-0968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss B.R., Gangola M.P., Agasimani S., Jaiswal S., Venkatesan T., Sundaram G.R., Chibbar R.N. Starch granule size and amylopectin chain length influence starch in vitro enzymatic digestibility in selected rice mutants with similar amylose concentration. J. Food Sci. Technol. 2019;56:391–400. doi: 10.1007/s13197-018-3500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regina A., Bird A., Topping D., Bowden S., Freeman J., Barsby T., Kosar-Hashemi B., Li Z.Y., Rahman S., Morell M. High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. P Natl. Acad. Sci. U S A. 2006;103:3546–3551. doi: 10.1073/pnas.0510737103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regina A., Berbezy P., Kosar-Hashemi B., Li S., Cmiel M., Larroque O., Bird A.R., Swain S.M., Cavanagh C., Jobling S.A., et al. A genetic strategy generating wheat with very high amylose content. Plant Biotechnol. J. 2015;13:1276–1286. doi: 10.1111/pbi.12345. [DOI] [PubMed] [Google Scholar]
- Ren Y., Wang Y., Liu F., Zhou K., Ding Y., Zhou F., Wang Y., Liu K., Gan L., Ma W., et al. GLUTELIN PRECURSOR ACCUMULATION3 encodes a regulator of post-Golgi vesicular traffic essential for vacuolar protein sorting in rice endosperm. The Plant Cell. 2014;26:410–425. doi: 10.1105/tpc.113.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring S.G., Gee J.M., Whittam M., Orford P., Johnson I.T. Resistant starch - its chemical form in foodstuffs and effect on digestibility invitro. Food Chem. 1988;28:97–109. doi: 10.1016/0308-8146(88)90139-2. [DOI] [Google Scholar]
- Ryoo N., Yu C., Park C.S., Baik M.Y., Park I.M., Cho M.H., Bhoo S.H., An G., Hahn T.R., Jeon J.S. Knockout of a starch synthase gene OsSSIIIa/Flo5 causes white-core floury endosperm in rice (Oryza sativa L.) Plant Cell Rep. 2007;26:1083–1095. doi: 10.1007/s00299-007-0309-8. [DOI] [PubMed] [Google Scholar]
- Sajilata M.G., Singhal R.S., Kulkarni P.R. Resistant starch - a review. Compr. Rev. Food Sci. F. 2006;5:1–17. doi: 10.1111/j.1541-4337.2006.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Sano Y. Differential regulation of Waxy gene-expression in rice endosperm. Theor. Appl. Genet. 1984;68:467–473. doi: 10.1007/Bf00254822. [DOI] [PubMed] [Google Scholar]
- Sano Y., Katsumata M., Okuno K. Genetic studies of speciation in cultivated rice. 5. Inter- and intraspecific differentiation in the waxy gene expression of rice. Euphytica. 1986;35:1–9. doi: 10.1007/Bf00028534. [DOI] [Google Scholar]
- Satoh H., Nishi A., Yamashita K., Takemoto Y., Tanaka Y., Hosaka Y., Sakurai A., Fujita N., Nakamura Y. Starch-branching enzyme I-deficient mutation specifically affects the structure and properties of starch in rice endosperm. Plant Physiol. 2003;133:1111–1121. doi: 10.1104/pp.103.021527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada T., Itoh M., Nakamura Y. Contributions of three starch branching enzyme isozymes to the fine structure of amylopectin in rice endosperm. Front. Plant Sci. 2018;9:1536. doi: 10.3389/fpls.2018.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Schippers J.H., Mieulet D., Watanabe M., Hoefgen R., Guiderdoni E., Mueller-Roeber B. SALT-RESPONSIVE ERF1 is a negative regulator of grain filling and gibberellin-mediated seedling establishment in rice. Mol. Plant. 2014;7:404–421. doi: 10.1093/mp/sst131. [DOI] [PubMed] [Google Scholar]
- Schoen A., Joshi A., Tiwari V., Gill B.S., Rawat N. Triple null mutations in starch synthase SSIIa gene homoeologs lead to high amylose and resistant starch in hexaploid wheat. BMC Plant Biol. 2021;21:74. doi: 10.1186/s12870-020-02822-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer T.F., Andersson H., Langkilde A.M., Reimann S., Torsdottir I. Nutrients excreted in ileostomy effluents after consumption of mixed diets with beans or potatoes. II. Starch, dietary fibre and sugars. Eur. J. Clin. Nutr. 1990;44:567–575. PMID: 2170104. [PubMed] [Google Scholar]
- Shen Y., Wu D., Fogliano V., Pellegrini N. Rice varieties with a high endosperm lipid content have reduced starch digestibility and increased γ-oryzanol bioaccessibility. Food Funct. 2021;12:11547–11556. doi: 10.1039/d1fo03039f. [DOI] [PubMed] [Google Scholar]
- Shimbata T., Ai Y.F., Fujita M., Inokuma T., Vrinten P., Sunohara A., Saito M., Takiya T., Jane J.L., Nakamura T. Effects of homoeologous wheat starch synthase IIa genes on starch properties. J. Agr Food Chem. 2012;60:12004–12010. doi: 10.1021/jf303623e. [DOI] [PubMed] [Google Scholar]
- Shu X., Jia L., Ye H., Li C., Wu D. Slow digestion properties of rice different in resistant starch. J. Agric. Food Chem. 2009;57:7552–7559. doi: 10.1021/jf900988h. [DOI] [PubMed] [Google Scholar]
- Shu X.L., Jiao G., Fitzgerald M.A., Yang C.Z., Shu Q.Y., Wu D.X. Starch structure and digestibility of rice high in resistant starch. Starch-Stärke. 2006;58:411–417. doi: 10.1002/star.200600501. [DOI] [Google Scholar]
- Shure M., Wessler S., Fedoroff N. Molecular-identification and isolation of the Waxy locus in maize. Cell. 1983;35:225–233. doi: 10.1016/0092-8674(83)90225-8. [DOI] [PubMed] [Google Scholar]
- Sievert D., Pomeranz Y. Enzyme-resistant starch .1. Characterization and evaluation by enzymatic, thermoanalytical, and microscopic methods. Cereal Chem. 1989;66:342–347. [Google Scholar]
- Siljeström M., Asp N.G. Resistant starch formation during baking-effect of baking time and temperature and variations in the recipe. Z. Lebensm Unters Forsch. 1985;181:4–8. doi: 10.1007/Bf01124798. [DOI] [Google Scholar]
- Siljestrom M., Eliasson A.C., Bjorck I. Characterization of resistant starch from autoclaved wheat-starch. Starch-Stärke. 1989;41:147–151. [Google Scholar]
- Singh J., Lelane C., Stewart R.B., Singh H. Formation of starch spherulites: role of amylose content and thermal events. Food Chem. 2010;121:980–989. doi: 10.1016/j.foodchem.2010.01.032. [DOI] [Google Scholar]
- Stitt M., Zeeman S.C. Starch turnover: pathways, regulation and role in growth. Curr. Opin. Plant Biol. 2012;15:282–292. doi: 10.1016/j.pbi.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Sun Y., Jiao G., Liu Z., Zhang X., Li J., Guo X., Du W., Du J., Francis F., Zhao Y., et al. Generation of high-amylose rice through CRISPR/Cas9 -mediated targeted mutagenesis of starch branching enzymes. Front. Plant Sci. 2017;8:298. doi: 10.3389/fpls.2017.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow I.J. Starch biosynthesis in developing seeds. Seed Sci. Res. 2011;21:5–32. [Google Scholar]
- Tetlow I.J., Wait R., Lu Z.X., Akkasaeng R., Bowsher C.G., Esposito S., Kosar-Hashemi B., Morell M.K., Emes M.J. Protein phosphorylation in amyloplasts regulates starch branching enzyme activity and protein-protein interactions. Plant Cell. 2004;16:694–708. doi: 10.1105/tpc.017400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L.U., Yoon J.H. Starch digestibility as affected by polyphenols and phytic acid. J. Food Sci. 1984;49:1228–1229. [Google Scholar]
- Tian S., Sun Y. Influencing factor of resistant starch formation and application in cereal products: a review. Int. J. Biol. Macromol. 2020;149:424–431. doi: 10.1016/j.ijbiomac.2020.01.264. [DOI] [PubMed] [Google Scholar]
- Tian Z., Qian Q., Liu Q., Yan M., Liu X., Yan C., Liu G., Gao Z., Tang S., Zeng D., et al. Allelic diversities in rice starch biosynthesis lead to a diverse array of rice eating and cooking qualities. P Natl. Acad. Sci. U S A. 2009;106:21760–21765. doi: 10.1073/pnas.0912396106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyosawa Y., Kawagoe Y., Matsushima R., Crofts N., Ogawa M., Fukuda M., Kumamaru T., Okazaki Y., Kusano M., Saito K., et al. Deficiency of starch synthase IIIa and IVb alters starch granule morphology from polyhedral to spherical in rice endosperm. Plant Physiol. 2016;170:1255–1270. doi: 10.1104/pp.15.01232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuiki K., Fujisawa H., Itoh A., Sato M., Fujita N. Alterations of starch structure lead to increased resistant starch of steamed rice: identification of high resistant starch rice lines. J. Cereal Sci. 2016;68:88–92. [Google Scholar]
- Vamadevan V., Bertoft E. Impact of different structural types of amylopectin on retrogradation. Food Hydrocolloids. 2018;80:88–96. [Google Scholar]
- Wada T., Yamaguchi O., Miyazaki M., Miyahara K., Ishibashi M., Aihara T., Shibuta T., Inoue T., Tsubone M., Toyosawa Y., et al. Development and characterization of a new rice cultivar, 'Chikushi-kona 85', derived from a starch-branching enzyme IIb-deficient mutant line. Breed. Sci. 2018;68:278–283. doi: 10.1270/jsbbs.17069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley J.W., Shen Z.X., Sartor R., Wu K.J., Osborn J., Smith L.G., Briggs S.P. Reconstruction of protein networks from an atlas of maize seed proteotypes. P Natl. Acad. Sci. U S A. 2013;110:E4808–E4817. doi: 10.1073/pnas.1319113110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen Z., Zhang Q., Meng S., Wei C. The NAC transcription factors OsNAC20 and OsNAC26 regulate starch and storage protein synthesis. Plant Physiol. 2020;184:1775–1791. doi: 10.1104/pp.20.00984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.C., Xu H., Zhu Y., Liu Q.Q., Cai X.L. OsbZIP58, a basic leucine zipper transcription factor, regulates starch biosynthesis in rice endosperm. J. Exp. Bot. 2013;64:3453–3466. doi: 10.1093/jxb/ert187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Yang Y., Guo M., Zhong C., Yan C., Sun S. Targeted mutagenesis of amino acid transporter genes for rice quality improvement using the CRISPR/Cas9 system. Crop J. 2020;8:457–464. doi: 10.1016/j.cj.2020.02.005. [DOI] [Google Scholar]
- Wang S.J., Wang J.R., Yu J.L., Wang S. Effect of fatty acids on functional properties of normal wheat and waxy wheat starches: a structural basis. Food Chem. 2016;190:285–292. doi: 10.1016/j.foodchem.2015.05.086. [DOI] [PubMed] [Google Scholar]
- Wang Z.Y., Wu Z.L., Xing Y.Y., Zheng F.G., Guo X.L., Zhang W.G., Hong M.M. Nucleotide-sequence of rice Waxy gene. Nucleic Acids Res. 1990;18:5898. doi: 10.1093/nar/18.19.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters D.L.E., Henry R.J., Reinke R.F., Fitzgerald M.A. Gelatinization temperature of rice explained by polymorphisms in starch synthase. Plant Biotechnol. J. 2006;4:115–122. doi: 10.1111/j.1467-7652.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- Wei C., Qin F., Zhu L., Zhou W., Chen Y., Wang Y., Gu M., Liu Q. Microstructure and ultrastructure of high-amylose rice resistant starch granules modified by antisense RNA inhibition of starch branching enzyme. J. Agric. Food Chem. 2010;58:1224–1232. doi: 10.1021/jf9031316. [DOI] [PubMed] [Google Scholar]
- Wei C., Qin F., Zhou W., Yu H., Xu B., Chen C., Zhu L., Wang Y., Gu M., Liu Q. Granule structure and distribution of allomorphs in C-type high-amylose rice starch granule modified by antisense RNA inhibition of starch branching enzyme. J. Agric. Food Chem. 2010;58:11946–11954. doi: 10.1021/jf103412d. [DOI] [PubMed] [Google Scholar]
- Wu C., Zhou X., Wei B., Tian Y., Xu X., Jin Z. Effects of α-maltotriohydrolase hydrolysis prior to debranching on the structure and digestibility of normal maize starch. Starch-Stärke. 2017;69:1600078. doi: 10.1002/star.201600078. [DOI] [Google Scholar]
- Xia J., Zhu D., Wang R., Cui Y., Yan Y. Crop resistant starch and genetic improvement: a review of recent advances. Theor. Appl. Genet. 2018;131:2495–2511. doi: 10.1007/s00122-018-3221-4. [DOI] [PubMed] [Google Scholar]
- Xu H., Li X.F., Zhang H., Wang L.C., Zhu Z.G., Gao J.P., Li C.S., Zhu Y. High temperature inhibits the accumulation of storage materials by inducing alternative splicing of OsbZIP58 during filling stage in rice. Plant Cell Environ. 2020;43:1879–1896. doi: 10.1111/pce.13779. [DOI] [PubMed] [Google Scholar]
- Xu Z., Yu M., Yin Y., Zhu C., Ji W., Zhang C., Li Q., Zhang H., Tang S., Yu H., et al. Generation of selectable marker-free soft transgenic rice with transparent kernels by downregulation of SSSII-2. Crop J. 2020;8:53–61. doi: 10.1016/j.cj.2019.05.006. [DOI] [Google Scholar]
- Yamamoto M.P., Onodera Y., Touno S.M., Takaiwa F. Synergism between RPBF Dof and RISBZ1 bZIP activators in the regulation of rice seed expression genes. Plant physiology. 2006;141:1694–1707. doi: 10.1104/pp.106.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C.Z., Shu X.L., Zhang L.L., Wang X.Y., Zhao H.J., Ma C.X., Wu D.X. Starch properties of mutant rice high in resistant starch. J. Agr Food Chem. 2006;54:523–528. doi: 10.1021/jf0524123. [DOI] [PubMed] [Google Scholar]
- Yang D.C., Wu L.Y., Hwang Y.S., Chen L.F., Huang N. Expression of the REB transcriptional activator in rice grains improves the yield of recombinant proteins whose genes are controlled by a Reb-responsive promoter. P Natl. Acad. Sci. U S A. 2001;98:11438–11443. doi: 10.1073/pnas.201411298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q., Ding J., Feng X., Zhong X., Lan J., Tang H., Harwood W., Li Z., Guzmán C., Xu Q., et al. Editing of the starch synthase IIa gene led to transcriptomic and metabolomic changes and high amylose starch in barley. Carbohyd Polym. 2022;285:119238. doi: 10.1016/j.carbpol.2022.119238. [DOI] [PubMed] [Google Scholar]
- Yang R., Sun C., Bai J., Luo Z., Shi B., Zhang J., Yan W., Piao Z. A putative gene sbe3-rs for resistant starch mutated from SBE3 for starch branching enzyme in rice (Oryza sativa L.) PLoS one. 2012;7:e43026. doi: 10.1371/journal.pone.0043026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J.P., Hu X.T., Luo S.J., McClements D.J., Liang L., Liu C.M. Effect of endogenous proteins and lipids on starch digestibility in rice flour. Food Res. Int. 2018;106:404–409. doi: 10.1016/j.foodres.2018.01.008. [DOI] [PubMed] [Google Scholar]
- You H., Liang C., Zhang O., Xu H., Xu L., Chen Y., Xiang X. Variation of resistant starch content in different processing types and their starch granules properties in rice. Carbohyd Polym. 2022;276:118742. doi: 10.1016/j.carbpol.2021.118742. [DOI] [PubMed] [Google Scholar]
- Yu S.F., Ma Y., Sun D.W. Effects of freezing rates on starch retrogradation and textural properties of cooked rice during storage. Lwt-food Sci. Technol. 2010;43:1138–1143. doi: 10.1016/j.lwt.2010.03.004. [DOI] [Google Scholar]
- Zemach A., Kim M.Y., Silva P., Rodrigues J.A., Dotson B., Brooks M.D., Zilberman D. Local DNA hypomethylation activates genes in rice endosperm. P Natl. Acad. Sci. U S A. 2010;107:18729–18734. doi: 10.1073/pnas.1009695107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng D., Yan M., Wang Y., Liu X., Qian Q., Li J. Du1, encoding a novel Prp1 protein, regulates starch biosynthesis through affecting the splicing of Wxb pre-mRNAs in rice (Oryza sativa L.) Plant Mol. Biol. 2007;65:501–509. doi: 10.1007/s11103-007-9186-3. [DOI] [PubMed] [Google Scholar]
- Zeng D.C., Liu T.L., Ma X.L., Wang B., Zheng Z.Y., Zhang Y.L., Xie X.R., Yang B.W., Zhao Z., Zhu Q.L., et al. Quantitative regulation of Waxy expression by CRISPR/Cas9-based promoter and 5'UTR-intron editing improves grain quality in rice. Plant Biotechnol. J. 2020;18:2385–2387. doi: 10.1111/pbi.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Yang Y., Chen Z., Chen F., Pan L., Lu Y., Li Q., Fan X., Sun Z., Liu Q. Characteristics of grain physicochemical properties and the starch structure in rice carrying a mutated ALK/SSIIa gene. J. Agric. Food Chem. 2020;68:13950–13959. doi: 10.1021/acs.jafc.0c01471. [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhu J., Chen S., Fan X., Li Q., Lu Y., Wang M., Yu H., Yi C., Tang S., et al. Wx, the ancestral allele of rice waxy gene. Mol. Plant. 2019;12:1157–1166. doi: 10.1016/j.molp.2019.05.011. [DOI] [PubMed] [Google Scholar]
- Zhang C.Q., Yang Y., Chen S.J., Liu X.J., Zhu J.H., Zhou L.H., Lu Y., Li Q.F., Fan X.L., Tang S.Z., et al. A rare Waxy allele coordinately improves rice eating and cooking quality and grain transparency. J. Integr. Plant Biol. 2021;63:889–901. doi: 10.1111/jipb.13010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.Y., Cheng Z.J., Zhang X., Guo X.P., Su N., Jiang L., Mao L., Wan J.M. Double repression of soluble starch synthase genes SSIIa and SSIIIa in rice (Oryza sativa L.) uncovers interactive effects on the physicochemical properties of starch. Genome. 2011;54:448–459. doi: 10.1139/G11-010. [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhao L., Lin L., Zhao L., Liu Q., Wei C. A novel mutation of OsPPDKB, encoding pyruvate orthophosphate dikinase, affects metabolism and structure of starch in the rice endosperm. Int. J. Mol. Sci. 2018;19:2268. doi: 10.3390/ijms19082268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W.W., Bi J.C., Yan X.Y., Wang H.L., Zhu C.L., Wang H.K., Wan J.M. In vitro measurement of resistant starch of cooked milled rice and physico-cheMical characteristics affecting its formation. Food Chem. 2007;105:462–468. doi: 10.1016/j.foodchem.2007.04.002. [DOI] [Google Scholar]
- Zhang X.L., Colleoni C., Ratushna V., Sirghie-colleoni M., Sirghle-Colleoni M., James M.G., Myers A.M. Molecular characterization demonstrates that the Zea mays gene sugary2 codes for the starch synthase isoform SSIIa. Plant Mol. Biol. 2004;54:865–879. doi: 10.1007/s11103-004-0312-1. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Du X.X., Han Z.Y., Ye Y., Pan G., Asad M.A.U., Zhou Q.F., Cheng F.M. Suppression of starch synthase I (SSI) by RNA interference alters starch biosynthesis and amylopectin chain distribution in rice plants subjected to high temperature. Crop J. 2019;7:573–586. doi: 10.1016/j.cj.2019.03.009. [DOI] [Google Scholar]
- Zhou H., Xia D., Zhao D., Li Y.H., Li P.B., Wu B., Gao G.J., Zhang Q.L., Wang G.W., Xiao J.H., et al. The origin of Wxla provides new insights into the improvement of grain quality in rice. J. Integr. Plant Biol. 2021;63:878–888. doi: 10.1111/jipb.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H.J., Wang L.J., Liu G.F., Meng X.B., Jing Y.H., Shu X.L., Kong X.L., Sun J., Yu H., Smith S.M., et al. Critical roles of soluble starch synthase SSIIIa and granule-bound starch synthase Waxy in synthesizing resistant starch in rice. P Natl. Acad. Sci. U S A. 2016;113:12844–12849. doi: 10.1073/pnas.1615104113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F., Bertoft E., Kallman A., Myers A.M., Seetharaman K. Molecular structure of starches from maize mutants deficient in starch synthase III. J. Agric. Food Chem. 2013;61:9899–9907. doi: 10.1021/jf402090f. [DOI] [PubMed] [Google Scholar]
- Zhu L.J., Gu M.H., Meng X.L., Cheung S.C.K., Yu H.X., Huang J., Sun Y., Shi Y.C., Liu Q.Q. High-amylose rice improves indices of animal health in normal and diabetic rats. Plant Biotechnol. J. 2012;10:353–362. doi: 10.1111/j.1467-7652.2011.00667.x. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Cai X.L., Wang Z.Y., Hong M.M. An interaction between a MYC protein and an EREBP protein is involved in transcriptional regulation of the rice Wx gene. J. Biol. Chem. 2003;278:47803–47811. doi: 10.1074/jbc.M302806200. [DOI] [PubMed] [Google Scholar]