Abstract
Background
Postoperative pulmonary complications (PPCs) have an impact on the recovery of adults after surgery. It is therefore important to establish whether preoperative respiratory rehabilitation can decrease the risk of PPCs and to identify adults who might benefit from respiratory rehabilitation.
Objectives
Our primary objective was to assess the effectiveness of preoperative inspiratory muscle training (IMT) on PPCs in adults undergoing cardiac or major abdominal surgery. We looked at all‐cause mortality and adverse events.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014, Issue 10), MEDLINE (1966 to October 2014), EMBASE (1980 to October 2014), CINAHL (1982 to October 2014), LILACS (1982 to October 2014), and ISI Web of Science (1985 to October 2014). We did not impose any language restrictions.
Selection criteria
We included randomized controlled trials that compared preoperative IMT and usual preoperative care for adults undergoing cardiac or major abdominal surgery.
Data collection and analysis
Two or more review authors independently identified studies, assessed trial quality, and extracted data. We extracted the following information: study characteristics, participant characteristics, intervention details, and outcome measures. We contacted study authors for additional information in order to identify any unpublished data.
Main results
We included 12 trials with 695 participants; five trials included participants awaiting elective cardiac surgery and seven trials included participants awaiting elective major abdominal surgery. All trials contained at least one domain judged to be at high or unclear risk of bias. Of greatest concern was the risk of bias associated with inadequate blinding, as it was impossible to blind participants due to the nature of the study designs. We could pool postoperative atelectasis in seven trials (443 participants) and postoperative pneumonia in 11 trials (675 participants) in a meta‐analysis. Preoperative IMT was associated with a reduction of postoperative atelectasis and pneumonia, compared with usual care or non‐exercise intervention (respectively; risk ratio (RR) 0.53, 95% confidence interval (CI) 0.34 to 0.82 and RR 0.45, 95% CI 0.26 to 0.77). We could pool all‐cause mortality within postoperative period in seven trials (431 participants) in a meta‐analysis. However, the effect of IMT on all‐cause postoperative mortality is uncertain (RR 0.40, 95% CI 0.04 to 4.23). Eight trials reported the incidence of adverse events caused by IMT. All of these trials reported that there were no adverse events in both groups. We could pool the mean duration of hospital stay in six trials (424 participants) in a meta‐analysis. Preoperative IMT was associated with reduced length of hospital stay (MD ‐1.33, 95% CI ‐2.53 to ‐0.13). According to the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) Working Group guidelines for evaluating the impact of healthcare interventions, the overall quality of studies for the incidence of pneumonia was moderate, whereas the overall quality of studies for the incidence of atelectasis, all‐cause postoperative death, adverse events, and duration of hospital stay was low or very low.
Authors' conclusions
We found evidence that preoperative IMT was associated with a reduction of postoperative atelectasis, pneumonia, and duration of hospital stay in adults undergoing cardiac and major abdominal surgery. The potential for overestimation of treatment effect due to lack of adequate blinding, small‐study effects, and publication bias needs to be considered when interpreting the present findings.
Plain language summary
Breathing training before surgery for reducing lung complications after surgery in adults undergoing heart and major abdominal surgery
Background and review question
Despite advances regarding patient care in the last few decades, breathing complications as a result of lung injury after surgery such as pneumonia are the leading cause of sickness and death in adults undergoing heart and major abdominal surgery. Training of breathing muscles using a small device at home before surgery seems to make breathing easier and helps strengthen muscles of respiration after surgery. This training may help reduce breathing complications after surgery and may lead to improved patient care and overall health care cost savings for the public health system. We wanted to establish whether training of breathing muscles before surgery can reduce the risk of lung complications and to identify who in particular might benefit from such training.
Objective
We reviewed the evidence about the effects of breathing training before surgery on lung complications after surgery in adults undergoing heart or major abdominal surgery.
Study characteristics
We included 12 trials with 695 participants. Five of the 12 studies included participants awaiting planned heart surgery, and seven studies included participants awaiting planned major abdominal surgery. The evidence is current to October 2014.
Key results
This review showed that training of breathing muscles before surgery reduced the risk of some lung complications (atelectasis and pneumonia) after surgery and the length of hospital stay, compared with usual care. However, the effect of this training on in‐hospital death after surgery is unclear and needs further investigation. The trials did not report any undesirable effects associated with training of breathing muscles, and no study reported on costs resulting from breathing training using a device.
Quality of evidence and conclusion
Although the available evidence is insufficient in terms of the quality and size of trials, we can conclude that training of breathing muscles before surgery prevents lung complications after surgery. This training is easily performed at home under the supervision of a physiotherapist. The training of breathing muscles therefore appears to be a suitable option as one of the preparations for planned surgery, especially for adults awaiting high‐risk heart and abdominal surgery. Other surgeries, such as oesophageal resection (removal of part of the gastrointestinal tract 'food pipe'), should be evaluated; cost‐effectiveness and patient‐reported outcomes should be reported. The potential for overestimation of treatment effect needs to be considered when interpreting the present findings, as the quality of evidence is low to moderate.
Summary of findings
Summary of findings for the main comparison. Preoperative inspiratory muscle training compared to usual care, non‐exercise intervention for adults on waiting list for cardiac and major abdominal surgery.
| Preoperative inspiratory muscle training compared to usual care, non‐exercise intervention for adults on waiting list for cardiac and major abdominal surgery | ||||||
| Patient or population: adult participants undergoing cardiac and major abdominal surgery Settings: hospital Intervention: preoperative inspiratory muscle training | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Preoperative inspiratory muscle training | |||||
| Postoperative atelectasis original authors’ definitions | Study population | RR 0.53 (0.34 to 0.82) | 443 (7 studies) | ⊕⊕⊝⊝ low1,2,3 | I2 statistic = 0%, P value = 0.004 | |
| 207 per 1000 | 110 per 1000 (70 to 170) | |||||
| Moderate | ||||||
| 263 per 1000 | 139 per 1000 (89 to 216) | |||||
| Postoperative pneumonia original authors’ definitions | Study population | RR 0.45 (0.26 to 0.77) | 675 (11 studies) | ⊕⊕⊕⊝ moderate1,2,3,4 | I2statistic = 0%, P value = 0.004 | |
| 117 per 1000 | 53 per 1000 (31 to 90) | |||||
| Moderate | ||||||
| 83 per 1000 | 37 per 1000 (22 to 64) | |||||
| Mechanical ventilation > 48 hours | Study population | RR 0.55 (0.03 to 9.2) | 338 (3 studies) | ⊕⊝⊝⊝ very low1,2,3,5,6 | I2 statistic = 56%, P value = 0.68, effect is uncertain | |
| 36 per 1000 | 20 per 1000 (1 to 327) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| All‐cause mortality within 30 days during postoperative period | Study population | RR 0.4 (0.04 to 4.23) | 431 (7 studies) | ⊕⊝⊝⊝ very low1,3,5,6,7 | I2 statistic = 59%, P value = 0.09, effect is uncertain | |
| 41 per 1000 | 17 per 1000 (2 to 175) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Adverse events | See comment | See comment | Not estimable | 462 (8 studies) | ⊕⊕⊝⊝ low1,3 | 8 studies reported on adverse events, however all these studies reported that there were no adverse events in both groups |
| Duration of hospital stay | ‐ | The mean duration of hospital stay in the intervention groups was 1.33 lower (2.53 to 0.13 lower) | ‐ | 424 (6 studies) | ⊕⊕⊝⊝ low1,3 | I2 statistic = 7%, P value = 0.03 |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 About half of studies were at unclear risk of bias in sequence generation and allocation concealment. (Downgraded one level for risk of bias.) 2 Different definition of postoperative pulmonary complications was used in each trial. (Downgraded one level for risk of bias.) 3 Small‐study effects may be present. (Downgraded one level for publication bias.) 4 RR < 0.5 (+1 for large effect) 5 I2 statistic ≥ 50% and P value < 0.1. (Downgraded one level for inconsistency.) 6 Confidence interval is wide and includes important benefit and potential harm. (Downgraded one level for imprecision.) 7 Even though six studies reported this outcome, three studies had zero events, and therefore only three studies could be pooled in the analysis (Ferreira 2009; Hulzebos 2006b; Soares 2013).
Background
Description of the condition
Despite advances in perioperative care in the last few decades, postoperative pulmonary complications (PPCs) are probably the leading cause of morbidity and mortality in adults undergoing chest and abdominal surgery. PPCs and cardiac complications are commonly regarded as the two major causes of perioperative problems in selected groups of patients undergoing these high‐risk surgical procedures (Mendez‐Tellez 2008). However, PPCs are more common than postoperative cardiac complications and play a bigger role in mortality and healthcare costs (Fleischmann 2003; Shander 2011; Smetana 2009). Despite these factors, the natural history of PPCs and the necessity of preventive strategies have not been well recognized in studies to date.
PPCs are comprised of atelectasis, pneumonia, bronchospasm, pleural effusion, pulmonary oedema, and respiratory failure (Brooks‐Brunn 1995). Differences in the definition of PPCs across studies have contributed to a wide range in the reported incidences (6% to 76%) (Chumillas 1998). Various changes in the respiratory system occur during the postoperative period that increase the risk of these complications, including consequences from the residual anaesthetic effect, the surgical procedure itself, and the effects of any premorbid conditions (Mendez‐Tellez 2008).
A number of recognized risk factors predispose the person to developing PPCs. These risk factors can be classified into procedure‐related and patient‐related risk factors (Mendez‐Tellez 2008; Qaseem 2006). Procedure‐related risk factors include the type of surgery (abdominal, thoracic, neuro, head and neck, vascular, aortic aneurysm repair, and emergency surgery are all associated with higher risk of PPCs), the site of incision, prolonged operative time (exceeding three hours), and the type of anaesthesia (Guimarães 2009; Qaseem 2006; Smetana 2009). Patient‐related risk factors include advanced age (equal to or greater than 60 years), pre‐existing disease (for example chronic obstructive pulmonary disease, congestive heart failure), current smoking, alcohol consumption, functional dependence, impaired sensorium, recent marked weight loss, and American Society of Anesthesiologists (ASA) Physical Status (PS) Classification System class 2 (mild systemic disease without functional limitations) or severer status (Mendez‐Tellez 2008; Qaseem 2006). It is important to consider these risk factors at the time of the preoperative evaluation.
Description of the intervention
Prevention of PPCs can be more effective than treatment of already established PPCs, and it is incumbent on the surgeon to ensure that all necessary measures have been taken to prevent PPCs in their patient. For example, patients at high risk of PPCs who are scheduled to undergo elective surgery should be considered for preoperative respiratory rehabilitation strategies. These strategies can be performed in the outpatient setting (for example at home or in a local rehabilitation clinic) under the supervision of a physiotherapist. Hospitalized patients should also be commenced on a respiratory rehabilitation strategy immediately before surgery.
Respiratory rehabilitation is now considered to be the standard of care for surgical patients, the techniques of which include deep‐breathing exercises, thoracic physiotherapy, incentive spirometry (IS), and preoperative inspiratory muscle training (IMT; see Types of interventions). To date, four systematic reviews have focused on IS for the prevention of PPCs after abdominal surgery (do Nascimento 2014; Lawrence 2006; Overend 2001; Thomas 1994). The most recent review, which compared the effect of IS with no therapy or physiotherapy on PPCs, concluded that there is no evidence to support the effectiveness of IS for the prevention of PPCs after upper abdominal surgery (do Nascimento 2014). As more meticulous perioperative management is required in high‐risk patients, preoperative IMT has recently received wide research attention as a means to preventing PPCs (Hulzebos 2006b; Kulkarni 2010).
How the intervention might work
Respiratory muscle strength has not been reported to correlate with the routinely examined pulmonary function (for example vital capacity (VC), forced expired volume in one second (FEV1)) (Nomori 1994). However, people with respiratory muscle weakness have a higher risk of PPCs (Nomori 1994). This is thought to be due to inspiratory muscle fatigue leading to collapse of alveoli (Kulkarni 2010). Several authors have reported that preoperative IMT resulted in significant improvement in mean inspiratory muscle strength and endurance after thoracic and abdominal surgery without causing adverse effects (Hulzebos 2006b; Kulkarni 2010; Nomori 1994). Thus, it is reasonable to assume that an increase in inspiratory muscle strength and endurance through preoperative IMT can lead to better‐quality deep breathing after surgery, which would in turn result in a decreased incidence of PPCs (Valkenet 2011).
In summary, preoperative IMT is a feasible intervention and seems to have a prophylactic effect against PPCs (Hulzebos 2006b; Valkenet 2011). This strategy to reduce PPCs can lead to improved perioperative patient care, better resource utilization, and overall cost savings (for example save extra invasive stresses and drugs, shorten the length of hospital stay and mechanical ventilation) for the public health system (Shander 2011).
Why it is important to do this review
PPCs affect the quality, effectiveness, and efficiency of postoperative patient care. It is therefore important to establish whether preoperative respiratory rehabilitation can decrease the risk of PPCs and to identify patients who might benefit from respiratory rehabilitation.
Some published trials have demonstrated the advantages of preoperative IMT in participants undergoing cardiac surgery, in Hulzebos 2006a, Hulzebos 2006b, and Weiner 1998, or major abdominal surgery, in Dronkers 2008 and Kulkarni 2010. One systematic review summarized current evidence regarding the effectiveness of preoperative exercise therapy, including physical rehabilitation, on the postoperative complication rate in general (Valkenet 2011). However, to our knowledge no systematic review has focused on preoperative IMT alone for the prevention of PPCs. This systematic review will thus investigate whether preoperative IMT in participants undergoing cardiac or major abdominal surgery is an effective intervention for the prevention of PPCs. We hope that this review will help guide perioperative management in the future.
Objectives
Our primary objective was to assess the effectiveness of preoperative IMT on PPCs in adults undergoing cardiac or major abdominal surgery. We looked at all‐cause mortality and adverse events.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) that compared preoperative IMT and usual preoperative care for adults undergoing cardiac or major abdominal surgery. We excluded quasi‐randomized studies, historically controlled trials, cohort studies, and case series. However, we planned to include cluster‐randomized trials if the intracluster correlation coefficient was reported.
Types of participants
We included adults (equal to or greater than 18 years of age) awaiting elective cardiac or major abdominal surgery.
We defined cardiac surgery as any surgical procedure involving the heart (for example coronary artery bypass graft surgery, valve surgery, and correction of congenital defects).
We defined major abdominal surgery as deliberate breach of peritoneum or retroperitoneum, including abdominal aortic surgery, gastrointestinal surgery, and urological surgery.
We excluded participants undergoing chest surgery that may involve the lung per se other than cardiac surgery because such procedures are likely to have a deep influence on pulmonary complications. We also excluded participants if they had undergone surgery within two weeks of initial contact. We excluded participants if they were ASA PS 5, or had a suspected or established respiratory infection.
Types of interventions
The intervention included a tailored training programme (called 'inspiratory muscle training', or IMT) designed to increase the strength and endurance of the inspiratory muscles. IMT consists of two distinct types of specific respiratory muscle training (that is respiratory muscle strength (resistive/threshold) and endurance (hyperpnoea) training). Training occurred five to seven times a week for at least two weeks before the actual date of surgery. Each session consisted of 15 to 30 minutes of IMT, performed under supervision by either a physiotherapist, physician, or researcher. The participants were trained to use an inspiratory threshold‐loading device with a load of 10% to 60% of maximal inspiratory muscle strength (Pi‐max). The participants in the intervention group also received usual care as defined below. We excluded participants who also received IMT after the surgery.
Our comparative intervention was usual care, a non‐exercise intervention, or no intervention. The usual‐care group received care as usual the day before surgery (instruction on deep‐breathing manoeuvres, IS, coughing, and early mobilization may be provided).
We included trials that required participants to undergo rehabilitation, as long as both groups (control and intervention) received similar IS, chest physiotherapy, and mobilization after surgery.
Types of outcome measures
Primary outcomes
PPCs. We defined clinically significant PPCs as two or more items in the grade 2 complications or one item in the grade 3 or 4 complications (Appendix 1).
All‐cause mortality within 30 days during postoperative period.
Evidence of adverse events from IMT.
Secondary outcomes
Maximal inspiratory muscle strength (Pi‐max) and endurance (Pm‐peak/Pi‐max). (Pm‐peak: Maximal negative pressure produced by the participant handling incremental resistance during the endurance test; inspiratory muscle strength and endurance assessed with respiratory pressure meter).
Duration of hospital stay.
Other types of complications within 30 days during postoperative period: cardiac complications, neurological complications, and surgical‐site infections (see Appendix 2 for detailed descriptions of each complication).
Total drop‐out from the study for any reason, as surrogate measure of overall acceptability.
Quality of life: patient‐reported health‐related quality of life such as the 12‐Item Short Form Health Survey, 36‐Item Short Form Health Survey, Health of the Nation Outcome Scales, and World Health Organization Quality of Life.
Cost analysis.
Search methods for identification of studies
Electronic searches
We contacted the Trials Search Co‐ordinator to search the Cochrane Anaesthesia, Critical and Emergency CareGroup's Trials Register (October 2014). We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2014 Issue 10, Appendix 3), MEDLINE (Ovid SP, 1966 to October 2014, Appendix 4), EMBASE (Ovid SP, 1980 to October 2014, Appendix 5), CINAHL (EBSCO host, 1982 to October 2014, Appendix 6), LILACS (via BIREME, 1982 to October 2014, Appendix 7), and ISI Web of Science (1985 to October 2014, Appendix 8).
We employed a sensitive search strategy and search using both subject headings and free‐text words. We used search strategies that are optimal for identifying RCTs, together with specific subject terms. We developed a search strategy for use in MEDLINE and revised it appropriately for use in all other databases in conjunction with the Cochrane Anaesthesia, Critical and Emergency Care Group. We used the cited and citing reference searching method in the Web of Science to find more recent articles that update earlier research. We imposed no language restrictions.
Searching other resources
We checked the reference lists of the included articles. We also manually searched the journals Clinical Rehabilitation,Physiotherapy, and Physical Therapy from 1987 to October 2014. We attempted to contact relevant trial authors or experts in the field to identify any additional ongoing trials or unpublished data. We searched the conference proceedings of important meetings and abstracts. We also searched the databases of ongoing trials such as www.controlled‐trials.com/ and www.clinicaltrials.gov/. We contacted companies who supply respiratory muscle training devices in order to identify possible ongoing trials.
Data collection and analysis
Selection of studies
Two review authors (MK, AK) screened the titles and abstracts of all the publications obtained by the search strategy and independently selected trials that met our inclusion criteria.
We obtained the full text of all identified articles and any deemed unclear for inclusion from the titles and abstracts. We (MK, AK) independently examined the full‐text articles for inclusion. In case of disagreements, we consulted with a third independent review author (TT).
Data extraction and management
Two review authors (MK, AK) independently, and in duplicate, extracted data, including the following information.
Quality assessment of the study (risk of bias).
Participants' characteristics.
Intervention details and control agents.
Outcomes (types of outcome measures, timing of outcomes, effect differences).
We resolved any disagreements in consultation with a third review author (TT). We collected data manually on paper extraction forms and entered it into intermediate software (Microsoft Excel for Windows), ensuring accurate transfer by using double entry, before entering it into Review Manager 5 (RevMan 5.3). This allowed for any necessary statistical conversions.
Assessment of risk of bias in included studies
We assessed the risk of bias in the included studies using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (MK, AK) independently assessed trial quality. A third review author (TT) arbitrated and resolved any disagreements. We assessed risk of bias of all included studies across the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, incomplete outcome data, selective reporting, and other bias. For each included study, we assigned each of these domains one of three ratings: low risk of bias, high risk of bias, or unclear risk of bias (unclear indicating either lack of information or uncertainty over the potential for bias). We constructed a 'Risk of bias' graph and 'Risk of bias' summary figure using Review Manager (RevMan 5.3).
Measures of treatment effect
We performed all comparisons between the preoperative IMT and usual preoperative care groups. We used the following measures of the effect of intervention: we calculated the risk ratio for dichotomous outcomes and the mean difference (when all the outcomes are measured in the same unit) or standardized mean difference (when different scales are used for measuring the same construct) for continuous outcomes.
Unit of analysis issues
We took into account the level at which randomization occurred. We made an assessment of the randomization into simple parallel groups and the variations of this such as cluster‐randomized trials. Two review authors (MK, AK) reviewed the unit of analysis issues according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any differences by discussion. For trials that had a cross‐over design, we considered the results only from the first randomization periods. We included cluster‐randomized trials if the intracluster correlation coefficient was reported.
Dealing with missing data
We took the following steps for handling any missing data:
we calculated the standard deviation (SD) from study statistics (e.g. 95% confidence intervals (CIs), standard errors, t values, P values, F values) for missing SDs of continuous outcome data;
where possible, we contacted the original investigators of the study to obtain the missing data;
we addressed the potential impact of missing data on the findings of the review in the Discussion section;
when we could not calculate the exact SD, we imputed them from other studies in the meta‐analysis (Furukawa 2006).
We a priori planned to impute the missing SDs from other studies, but some studies reported quite different SDs of Pi‐max. We therefore did not pool the study in the meta‐analysis if we could not calculate the exact SD.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is we attempted to include all participants randomized to each group in the analyses, and analyse all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. We calculated and reported the percentage lost to follow‐up if there was a discrepancy between the numbers randomized and analysed in each treatment group. If there were dropouts, we looked for reasons for the dropouts in the publication itself or obtained additional information from the authors of the publications as to the cause of the drop‐outs. We then carried out analyses with knowledge of the precise outcomes because it was possible to make an informed guess about dropouts. Finally, we repeated the meta‐analysis imputing the missing data and the dropouts as best‐ and worst‐possible outcomes in a sensitivity analysis.
Assessment of heterogeneity
We used clinical heterogeneity to describe differences in participants, interventions, and outcomes that might reasonably affect recruitment strategies. We initially assessed heterogeneity by visual inspection of the results in the forest plots from a meta‐analysis of the studies. We measured statistical heterogeneity using the I2 and Cochran’s Q statistics (Higgins 2002; Higgins 2003). If we identified significant heterogeneity (I2 statistic ≥ 50% and P value < 0.1), we investigated potential sources of the heterogeneity through subgroup and sensitivity analysis. We also undertook quality‐control checks of data extraction and input and reviewed the clinical and methodological aspects of the study trials.
Assessment of reporting biases
Funnel plot asymmetry may be caused by: selection bias (publication or location bias), poor methodological quality of smaller studies (design, analysis, fraud), true heterogeneity (variation with effect size), or artefact or chance (Egger 1997). In order to determine the level of publication bias, we performed a funnel plot analysis to visually assess whether small‐study effects may be present in the meta‐analysis, since sufficient numbers of trials allowed for a meaningful analysis.
Data synthesis
As the trials were sufficiently homogeneous, and clinically similar trials were available, we were able to pool the results in the meta‐analysis. We pooled data using the random‐effects model for dichotomous and continuous data because the intervention in question represents a complex, variable method and is expected to be associated with some clinical heterogeneity and also because the random‐effects model is known to be more conservative (i.e. is less likely to lead to type I error) than the fixed‐effect model.
Dichotomous data
We calculated the risk ratio as an effect measure and its associated 95% confidence interval (CI) because it was clinically interpretable and generalizable (Furukawa 2002). Where possible, we calculated the number needed to treat for an additional beneficial outcome and its 95% CI, assuming the observed average control event rate.
Continuous data
We calculated the mean difference and its associated 95% CI. We used the standardized mean difference for data that we could not convert to a uniform scale.
If no meta‐analysis was possible or appropriate due to substantive clinical or statistical (or both) heterogeneity, skewed distribution, missing data, or otherwise, we qualitatively summarized and described the identified trials.
Subgroup analysis and investigation of heterogeneity
Where possible, we performed the following subgroup analyses. We were unable to perform some subgroup analyses due to lack of data.
Type of surgery: cardiac surgery or major abdominal surgery as subgroup analysis because the differences in operative procedure (procedure‐related risk factors) may affect the postoperative status of the participants.
High‐risk participants as subgroup analysis because patient‐related risk factors may affect the postoperative status of the participants. We included high‐risk participants if the trial reported the results of high‐risk participants separately. If the trial did not report these results separately, we attempted to contact relevant trial authors in order to identify any additional unpublished data about the participants' risk factors. We also included the trials in which more than 80% of participants had at least one risk factor. However, we anticipated that various studies would have used variable definitions of 'high‐risk participants'. We accepted the study authors' definitions.
Type of intervention as subgroup analysis.
Sensitivity analysis
To check the robustness of the observed findings, we performed the following sensitivity analyses using Stata, Stata 11, and Review Manager, RevMan 5.3.
Repeating meta‐analysis after exclusion of studies with high or unclear risk of bias in allocation concealment.
Repeating meta‐analysis after exclusion of studies in which the primary outcome evaluation was not blinded.
Repeating meta‐analysis imputing missing data and dropouts as best‐ and worst‐possible outcomes.
Comparing the difference of pooling analysis results by using a fixed‐effect and a random‐effects model.
Repeating meta‐analysis after exclusion of studies that used original authors' definition of PPCs and included no outcome based on our definition.
Summary of findings
We used the principles of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system to assess the quality of the body of evidence associated with the following specific outcomes in our review and constructed a Table 1 (Guyatt 2008):
PPCs;
all‐cause mortality within 30 days' postoperative period;
evidence of adverse events from IMT;
duration of hospital stay.
The GRADE approach assesses the quality of a body of evidence based on the extent to which there can be confidence that an estimate of effect or association reflects the item being assessed. This assessment considers the study methodological quality, directness of the evidence, heterogeneity of the data, precision of the effect estimates, and the risk of publication bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies.
Results of the search
We identified a total of 3218 citations from the database searches (see Figure 1 for selection process). Based on title and abstracts, we obtained copies of 61 full‐text studies that were potentially eligible for inclusion. After reading the full‐text papers, we excluded 46 studies from this review. We have provided the reasons for exclusion in Characteristics of excluded studies. We judged 12 trials (15 articles) eligible for inclusion (see Characteristics of included studies). We also identified two ongoing trials (see Characteristics of ongoing studies). We contacted the corresponding authors of the two ongoing studies and, at the time this review was submitted for editorial approval, we are still awaiting a reply.
1.

Study flow diagram.
Included studies
We included 12 trials in this systematic review (Barbalho‐Moulim 2011; Carvalho 2011; Da Cunha 2013; Dronkers 2008; Dronkers 2010; Ferreira 2009; Heynen 2012; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010; Soares 2013; Weiner 1998). Three of the 12 trials were reported as a conference result only, including an abstract and poster presentation (Carvalho 2011; Da Cunha 2013; Heynen 2012). We attempted to contact the authors of all 12 included trials for missing information; authors of nine trials responded, all of whom provided at least some of the desired information (Barbalho‐Moulim 2011; Carvalho 2011; Da Cunha 2013; Dronkers 2008; Dronkers 2010; Heynen 2012; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010).
Design
We included RCTs and cluster‐RCTs. All trials were single‐institution RCTs.
Sample size
In the majority of the trials, the sample sizes per arm were small. Seven of the 12 trials recruited fewer than 20 participants per arm (Barbalho‐Moulim 2011; Carvalho 2011; Dronkers 2008; Ferreira 2009; Heynen 2012; Hulzebos 2006a; Soares 2013). Three trials recruited 20 to 40 participants per arm (Dronkers 2010; Kulkarni 2010; Weiner 1998), and one trial recruited less than 10 participants per arm (Da Cunha 2013). Only one trial recruited over 100 participants to each arm (Hulzebos 2006b).
Setting
In six of the 12 included trials the setting was unclear (Carvalho 2011; Da Cunha 2013; Heynen 2012; Kulkarni 2010; Soares 2013; Weiner 1998). Of the remaining six trials, three were conducted in university hospital settings (Ferreira 2009; Hulzebos 2006a; Hulzebos 2006b), and three in non‐university hospital settings (Barbalho‐Moulim 2011; Dronkers 2008; Dronkers 2010). Of the 12 trials, five were performed in the Netherlands (Dronkers 2008; Dronkers 2010; Heynen 2012; Hulzebos 2006a; Hulzebos 2006b); five in Brazil (Barbalho‐Moulim 2011; Carvalho 2011; Da Cunha 2013; Ferreira 2009; Soares 2013); one in the United Kingdom (Kulkarni 2010); and one in Israel (Weiner 1998).
Participants
Proportion of women
Three trials did not report the number of female participants (Da Cunha 2013; Heynen 2012; Weiner 1998). One trial recruited only women (Barbalho‐Moulim 2011). In the remaining eight trials, the proportion of females ranged between 22% and 78%.
Age
Ten of the 12 included trials provided the mean (or median) age of participants. The remaining two trials did not provide this information (Da Cunha 2013; Heynen 2012).
One trial recruited young participants, whose mean age was 35 (Barbalho‐Moulim 2011). Seven trials recruited middle‐age participants, whose mean age was in the 50s to 60s (Carvalho 2011; Dronkers 2008; Ferreira 2009; Hulzebos 2006b; Kulkarni 2010; Soares 2013; Weiner 1998). The remaining two trials recruited elderly individuals only, whose mean age was in the 70s (Dronkers 2010; Hulzebos 2006a).
Participants' risk factors for PPCs
Nine of the 12 included trials reported the proportion of participants' risk factors for PPCs, such as smoking, chronic obstructive pulmonary disease, diabetes, hypertension, and body mass index. Three of the 12 trials did not report participants' risk factors for PPCs (Da Cunha 2013; Heynen 2012; Weiner 1998).
Three trials included only participants with "high risk" factors for PPCs (the definition of "high risk" depended on the trial) (Dronkers 2008; Hulzebos 2006a; Hulzebos 2006b). Five trials included participants at both high and low risk for PPCs. One trial included "high risk" participants, but the authors did not define "high risk" (Carvalho 2011).
Type of surgery
Five trials included participants awaiting elective cardiac surgery (Carvalho 2011; Ferreira 2009; Hulzebos 2006a; Hulzebos 2006b; Weiner 1998); the remaining seven trials included participants awaiting elective major abdominal surgery (Barbalho‐Moulim 2011; Da Cunha 2013; Dronkers 2008; Dronkers 2010; Heynen 2012; Kulkarni 2010; Soares 2013).
Of the five cardiac surgery trials, four were only coronary artery bypass graft surgery (Carvalho 2011; Hulzebos 2006a; Hulzebos 2006b; Weiner 1998), and one was both myocardial revascularization and valvuloplasty (Ferreira 2009).
The seven major abdominal surgery trials were: open Roux‐en‐Y gastric bypass surgery (Barbalho‐Moulim 2011), aneurysm of the abdominal aorta (Dronkers 2008), colon surgery (Dronkers 2010), oesophagogastric surgery (Da Cunha 2013; Heynen 2012), and major abdominal or urological surgery (Kulkarni 2010; Soares 2013).
Intervention
In 10 trials, the intervention was a simple preoperative IMT with threshold device (Barbalho‐Moulim 2011; Carvalho 2011; Da Cunha 2013; Dronkers 2008; Ferreira 2009; Heynen 2012; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010; Weiner 1998) (see Characteristics of included studies). In two trials, the intervention was a mixed training programme (Dronkers 2010; Soares 2013). The mixed training programme consisted of IMT with threshold‐loading device and exercise training of trunk and extremity. Kulkarni 2010 was a four‐arm RCT. The three different interventions were deep‐breathing exercises, incentive spirometry, and specific IMT with threshold‐loading device.
Comparisons
In eight trials (Barbalho‐Moulim 2011; Da Cunha 2013; Dronkers 2008; Ferreira 2009; Heynen 2012; Hulzebos 2006a; Hulzebos 2006b; Soares 2013), the control interventions were usual care or a non‐exercise intervention. In one trial, the control group underwent sham training, which consisted of breathing training through the same trainer but with no resistance (Weiner 1998). In another trial, the control group underwent home‐based exercise advice, which consisted of emphasizing the importance of the participant's physical condition to the postoperative course and encouragement to be active for minimally 30 minutes a day in the period prior to hospital admission (Dronkers 2010). In one trial, details of the control group were not described (Carvalho 2011). One trial was a four‐arm RCT in which participants were allocated to one of four groups (Group A, no training; Group B, deep‐breathing exercises; Group C, incentive spirometry; and Group D, specific IMT) (Kulkarni 2010). As described in the Methods section of our protocol (Katsura 2013), Group A, B, and C were the interventions of comparative group as usual care in our protocol.
Outcomes
All 12 included trials reported data on PPCs. Definitions for PPCs were author‐defined and varied across the trials. Of the 12 included trials, six did not report detailed definitions for PPCs (Carvalho 2011; Da Cunha 2013; Ferreira 2009; Kulkarni 2010; Heynen 2012; Weiner 1998). Seven trials reported on all‐cause mortality (Barbalho‐Moulim 2011; Da Cunha 2013; Dronkers 2008; Ferreira 2009; Heynen 2012; Hulzebos 2006b; Soares 2013). Eight trials reported on adverse events (Carvalho 2011; Da Cunha 2013; Dronkers 2008; Dronkers 2010; Ferreira 2009; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010).
As for secondary outcomes, all trials reported on maximal inspiratory muscle strength (Pi‐max), whereas only four trials reported respiratory muscle endurance (Pm‐peak/Pi‐max) (Dronkers 2008; Hulzebos 2006b; Soares 2013; Weiner 1998). Seven trials reported on the duration of hospital stay (Barbalho‐Moulim 2011; Carvalho 2011; Da Cunha 2013; Dronkers 2010; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010). Two trials reported other types of early postoperative complications, that is cardiac or neurological or surgical site infection (Barbalho‐Moulim 2011; Ferreira 2009). Ten trials reported on total drop‐out from the study. One trial reported on quality of life (Dronkers 2010). No trial reported on cost analysis.
Excluded studies
Of the 61 trials retrieved for more detailed evaluation (full‐paper review), 46 trials did not meet our inclusion criteria and were excluded because of one of the following reasons: non‐randomized design including review articles (12 trials), interventions not relevant (33 trials), and other types of outcomes (one trial) (for full details see Characteristics of excluded studies).
Ongoing studies
We identified two ongoing studies from searching the database www.clinicaltrials.gov/ (NCT01321983; NCT01828632). (See Characteristics of ongoing studies.)
Studies awaiting classification
There are no studies awaiting classification.
Risk of bias in included studies
See Figure 2 and Figure 3 for data on study quality.
2.
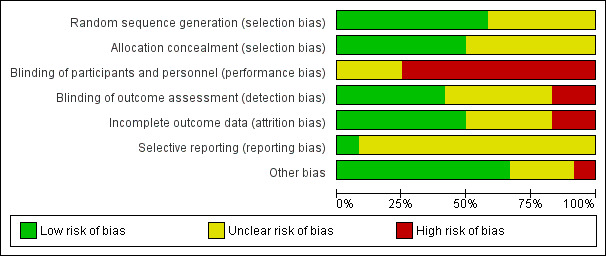
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
See Characteristics of included studies.
Allocation
Seven trials used an adequate method of generating random sequences (Barbalho‐Moulim 2011; Dronkers 2008; Dronkers 2010; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010; Soares 2013). Three trials provided no description on random sequence generation (Carvalho 2011; Ferreira 2009; Weiner 1998). We contacted three authors for details regarding allocation concealment (Ferreira 2009; Hulzebos 2006a; Weiner 1998), and one replied with the requested information (Hulzebos 2006a). Seven trials used an adequate method of allocation concealment (Barbalho‐Moulim 2011; Dronkers 2008; Dronkers 2010; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010; Soares 2013). Five trials used sealed envelopes (Barbalho‐Moulim 2011; Dronkers 2008; Hulzebos 2006b; Kulkarni 2010; Soares 2013). One study used a computer‐generated allocation table (Hulzebos 2006a). Five trials provided no description.
Blinding
Due to the nature of the study designs, it was impossible to blind the participants in all trials. Of the 12 included trials, five trials adequately blinded the outcome assessors (Barbalho‐Moulim 2011; Dronkers 2008; Dronkers 2010; Hulzebos 2006a; Hulzebos 2006b). In two of the five trials, the radiologist who evaluated the images of pulmonary status was unaware of the allocation or participant information (Barbalho‐Moulim 2011; Dronkers 2008). In the third trial, an investigator who was unaware of the treatment allocation assessed preoperative and postoperative measures (Dronkers 2010). In the fourth trial, a physical therapist who was blinded from the participant allocation took the outcome measures (Hulzebos 2006a). In the fifth trial, the incidence of PPCs was scored by a blinded independent investigator, and other data were evaluated by a blinded microbiologist (Hulzebos 2006b).
In two of the 12 included trials, the researchers and outcome assessors were identical (Kulkarni 2010; Soares 2013). The other five trials provided no information (Carvalho 2011; Da Cunha 2013; Ferreira 2009; Heynen 2012; Weiner 1998).
Incomplete outcome data
Nine trials adequately reported the information regarding the loss to follow‐up. In five trials, all participants completed the study (Barbalho‐Moulim 2011; Da Cunha 2013; Dronkers 2008; Ferreira 2009; Hulzebos 2006a). In three trials, the proportion of loss to follow‐up was small and similar between the groups (Da Cunha 2013; Dronkers 2010; Hulzebos 2006b).
One trial that compared four groups provided information on missing outcome data, but the proportion of loss to follow‐up was higher than 20% in two of the groups (Kulkarni 2010).
The remaining three studies provided no description.
Selective reporting
Only one trial adequately prespecified and reported the primary outcome (Hulzebos 2006b). The other trials either did not prespecify the primary outcome, or the protocols were unavailable.
Other potential sources of bias
Three trials did not reveal the participants' baseline data (Da Cunha 2013; Heynen 2012; Weiner 1998). The other nine trials revealed the participants' baseline data, and no baseline imbalance was found. One trial had a potential bias in its design: the intervention group was instructed to keep a diary, whereas the control group did not receive the same instruction (Dronkers 2008). This might have made the intervention group subject to the Hawthorne effect (Parsons 1974).
Effects of interventions
See: Table 1
Overall, 12 trials involving 695 participants were available for assessing the effects of interventions. In one of the 12 trials (Kulkarni 2010), participants were allocated to one of four groups (Group A, control; Group B, deep‐breathing exercises; Group C, incentive spirometry; Group D, specific IMT). Following our protocol (Katsura 2013), we combined Groups A, B, and C into a single control group, which was compared with Group D, as an intervention group. Therefore, the results of the present systematic review included 320 participants allocated to the intervention group and 359 participants allocated to the control group. (See Table 1.) We pooled data using the random‐effects model because the intervention in question represents a complex, variable method and statistical significance were more conservative (i.e. is less likely to lead to type I error) than the fixed‐effect model.
Primary outcomes
1. PPCs
Different original authors' definitions for PPCs were used in all trials. The definition of Kroenke 1992, which we described in our protocol (Katsura 2013), was used in only one trial (Hulzebos 2006b). We therefore pooled data for the major three types of PPCs (atelectasis, pneumonia, and mechanical ventilation exceeding 48 hours) according to the original authors' definitions in a meta‐analysis, respectively.
1.1. PPCs; atelectasis
Seven trials involving 443 participants reported on postoperative atelectasis (Barbalho‐Moulim 2011; Carvalho 2011; Dronkers 2008; Heynen 2012; Hulzebos 2006a; Hulzebos 2006b; Soares 2013). Pooled analysis showed preoperative IMT was associated with a reduction of postoperative atelectasis, compared with usual care or non‐exercise intervention (risk ratio (RR) 0.53; 95% confidence interval (CI) 0.34 to 0.82; P value = 0.004; I2 statistic = 0%) (Figure 4). Although we noted no heterogeneity between the trials, small‐study effects may be present. We therefore downgraded the outcome for risk of bias and publication bias and rated the quality of evidence as low quality.
4.

Forest plot of comparison: 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, outcome: 1.1 PPC; Atelectasis.
1.2. PPCs; pneumonia
Eleven trials involving 675 participants reported on postoperative pneumonia (Barbalho‐Moulim 2011; Carvalho 2011; Da Cunha 2013; Dronkers 2010; Ferreira 2009; Heynen 2012; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010; Soares 2013; Weiner 1998). Pooled analysis showed preoperative IMT was associated with a reduction of postoperative pneumonia, compared with usual care or non‐exercise intervention (RR 0.45; 95% CI 0.26 to 0.77; P value = 0.004; I2 statistic = 0%) (Figure 5; Figure 6). Although we noted no heterogeneity between the trials, small‐study effects may be present. We downgraded the outcome for risk of bias and publication bias, but upgraded it for large effect (RR < 0.5). As a result, we rated the quality of evidence as moderate quality.
5.

Forest plot of comparison: 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, outcome: 1.2 PPC; Pneumonia (Type of surgery).
6.

Forest plot of comparison: 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, outcome: 1.3 PPC; Pneumonia (Type of intervention).
1.3. PPCs; mechanical ventilation exceeding 48 hours
Four trials reported on postoperative length of mechanical ventilation (Barbalho‐Moulim 2011; Ferreira 2009; Hulzebos 2006b; Weiner 1998). One trial reported on postoperative ventilation dependence exceeding 24 hours (Weiner 1998). PPCs grade 4 is defined as the ventilator failure, which is postoperative ventilator dependence exceeding 48 hours (Appendix 1), according to Kroenke 1992's definition. We therefore pooled data for postoperative mechanical ventilation exceeding 48 hours from the remaining three trials in a meta‐analysis (Barbalho‐Moulim 2011; Ferreira 2009; Hulzebos 2006b). Pooled analysis showed there was no evidence of a reduction of postoperative mechanical ventilation exceeding 48 hours in the groups receiving preoperative IMT (RR 0.55; 95% CI 0.03 to 9.20; P value = 0.68; I2 statistic = 56%) (Analysis 1.4). We noted a large degree of heterogeneity and wide confidence intervals, including important benefit and potential harm, so we downgraded this outcome for inconsistency and imprecision. As with the other outcomes regarding PPCs, small‐study effects may be present; we therefore also downgraded this outcome for publication bias. As a result, we rated the quality of evidence as very low quality and effect is uncertain.
1.4. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 4 PPC; mechanical ventilation > 48 hours.
2. All‐cause mortality within 30 days during postoperative period
Seven trials reported on all‐cause mortality within the postoperative period (Barbalho‐Moulim 2011; Da Cunha 2013; Dronkers 2008; Ferreira 2009; Heynen 2012; Hulzebos 2006b; Soares 2013). In four trials, there were zero events in both groups (Barbalho‐Moulim 2011; Da Cunha 2013; Dronkers 2008; Heynen 2012). In the remaining three trials, we could pool all‐cause postoperative death in a meta‐analysis (Ferreira 2009; Hulzebos 2006b; Soares 2013), because Ferreira 2009 reported all‐cause mortality within a 30‐days postoperative period, whereas Hulzebos 2006b and Soares 2013 reported all‐cause postoperative mortality. The analysis found the effect of IMT on all‐cause postoperative death is uncertain (RR 0.40; 95% CI 0.04 to 4.23; P value = 0.09; I2 statistic = 59%) (Analysis 1.5). We noted a large degree of heterogeneity and wide confidence intervals, including important benefit and potential harm, so we downgraded the outcome for inconsistency and imprecision. We also downgraded the outcome for publication bias because small‐study effects may be present. As a result, we rated the quality of evidence as very low quality and effect is uncertain.
1.5. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 5 All‐cause postoperative death.
3. Adverse events
Eight trials provided reports of adverse events (Carvalho 2011; Da Cunha 2013; Dronkers 2008; Dronkers 2010; Ferreira 2009; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010). All these trials reported that there were no adverse events in both groups (Analysis 1.6).
1.6. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 6 Adverse events.
Secondary outcomes
1.1. Maximal inspiratory muscle strength; Pi‐max
Eleven trials reported the data on maximal inspiratory muscle training strength (Pi‐max) (Barbalho‐Moulim 2011; Carvalho 2011; Da Cunha 2013; Dronkers 2008; Dronkers 2010; Ferreira 2009; Heynen 2012; Hulzebos 2006a; Kulkarni 2010; Soares 2013; Weiner 1998). We considered the clinically relevant outcome to be the change of Pi‐max between baseline and three postoperative days, rather than the final value of Pi‐max after the surgery. We therefore planned to pool the change scores of Pi‐max in the meta‐analysis. Six trials reported the data on Pi‐max, both before and after surgery (Barbalho‐Moulim 2011; Carvalho 2011; Da Cunha 2013; Ferreira 2009; Kulkarni 2010; Soares 2013). Three trials provided sufficient information, such as the P value or standard deviations (SDs) of the Pi‐max at both time points, to calculate the change scores (Barbalho‐Moulim 2011; Da Cunha 2013; Ferreira 2009). Three trials reported the mean or median of Pi‐max, however the mean and SDs of the change score were unavailable despite our contacting the authors (Carvalho 2011; Kulkarni 2010; Soares 2013). We a priori planned to impute the missing SDs from other trials, but as the remaining two trials reported quite different SDs, we therefore did not pool this one trial in the meta‐analysis. Our analysis found that preoperative IMT was not associated with improved Pi‐max (mean difference (MD) ‐7.87; 95% CI ‐21.36 to 5.61; P value = 0.25; I2 statistic = 0%) (Analysis 1.7).
1.7. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 7 Maximal inspiratory muscle strength; Pi‐max (postoperative change from preoperative baseline).
1.2. Respiratory muscle endurance; Pm‐peak/Pi‐max
Three trials reported the data about respiratory muscle endurance (Pm‐peak/Pi‐max) (Hulzebos 2006b; Soares 2013; Weiner 1998). Only one trial reported the change scores from baseline to postoperative status (Weiner 1998). However, as SDs of the change score were unavailable, we did not pool this one trial in the meta‐analysis.
2. Duration of hospital stay
Eight trials reported duration of hospital stay (Barbalho‐Moulim 2011; Carvalho 2011; Da Cunha 2013; Dronkers 2010; Heynen 2012; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010). However, in two trials the median and range of hospital stay between groups were reported, and it was impossible to calculate the mean and SD of hospital stays (Heynen 2012; Kulkarni 2010). We could therefore pool the mean duration of hospital stay in the remaining six trials in a meta‐analysis. The analysis found that preoperative IMT was associated with reduced length of hospital stay (MD ‐1.33; 95% CI ‐2.53 to ‐0.13; P value = 0.03; I2 statistic = 7%) (Figure 7). Although we noted no heterogeneity between trials, small‐study effects may be present. We therefore downgraded the outcome for publication bias in addition to risk of bias and rated the quality of evidence as low quality.
7.

Forest plot of comparison: 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, outcome: 1.8 Duration of hospital stay.
3. Other types of complications within 30 days during postoperative period
One trial reported the occurrence of other types of early postoperative complication, that is cardiac complications, neurological complications, or surgical‐site infections (Ferreira 2009). This trial reported that there is no statistically significant difference regarding postoperative heart failure; experimental 1/15 (67%) versus control 1/15 (67%), P value = 0.65.
4. Total drop‐out from the study for any reason, as surrogate measure of overall acceptability
Ten trials reported on dropouts (Barbalho‐Moulim 2011; Da Cunha 2013; Dronkers 2008; Dronkers 2010; Ferreira 2009; Heynen 2012; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010; Soares 2013). In five of the 10 trials there is no drop‐out participant between both groups (Barbalho‐Moulim 2011; Da Cunha 2013; Ferreira 2009; Hulzebos 2006a; Hulzebos 2006b). In the remaining five trials that reported on drop‐out (Dronkers 2008; Dronkers 2010; Heynen 2012; Kulkarni 2010; Soares 2013;), the proportion of loss to follow‐up was higher than 20% in two trials (Kulkarni 2010; Soares 2013). All trials that reported dropouts described the reasons for drop‐out in each case. There was no difference between participants allocated to the intervention group and those allocated to the control group for discontinuation from trials due to any cause (RR 0.74; 95% CI 0.36 to 1.51; P value = 0.40; I2 statistic = 0%) (Analysis 1.9). As we mentioned earlier, five trials reported zero events, therefore we conducted post‐hoc analysis using risk difference (RD) instead of RR because the RD method can include trials with zero events in both arms into the analysis. The analysis found no evidence of significant difference between groups (RD ‐0.00; 95% CI ‐0.01 to 0.01; P value = 0.95; I2 statistic = 0%).
1.9. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 9 Total drop‐out from the study.
5. Quality of life
One trial with 42 participants reported on quality of life (Dronkers 2010). In this trial, there were no significant differences across the groups as measured by the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ‐ C30 version 3 designed for patients with cancer (Kaasa 1995)) that evaluated quality of life based on global health status/functional scale/symptom scale (see Table 2). It was impossible to conduct a meta‐analysis of this data due to the small number of studies and participants.
1. Quality of life.
| Baseline (before intervention) | Outcome (after surgery) | ||
| EORTC QLQ‐C30/GH | intervention group | 70 | 72 |
| (Global Health Status) | control group | 71 | 68 |
| EORTC QLQ‐C30/FS | intervention group | 408 | 413 |
| (Functional Scale) | control group | 427 | 425 |
| EORTC QLQ‐C30/SC | intervention group | 154 | 119 |
| (Symptom Scale) | control group | 130 | 155 |
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30: a quality‐of‐life instrument for use in international clinical trials in oncology
6. Cost analysis
No trial provided reports of cost analysis.
Subgroup analysis
We performed pre‐planned subgroup analysis for our primary outcome only when a meaningful number of relevant trials had been identified.
1. Type of surgery
We performed subgroup analysis according to the type of surgery, that is cardiac surgery and major abdominal surgery. In the cardiac surgery subgroup, pooled analysis showed preoperative IMT was associated with a reduction of postoperative atelectasis and pneumonia compared with usual care or non‐exercise intervention (Figure 4; Figure 5). On the other hand, in the major abdominal surgery subgroup, pooled analysis showed that preoperative IMT was associated with a reduction of postoperative atelectasis, still not associated with a reduction of postoperative pneumonia even though a number of pooled trials were not meaningful for this subgroup analysis (Figure 4; Figure 5). Test for subgroup differences: Chi2 statistic = 0.57, df = 1 (P value = 0.45), I2 statistic = 0% (postoperative atelectasis) and Chi2 statistic = 0.00, df = 1 (P value = 0.96), I2 statistic = 0% (postoperative pneumonia).
2. High‐risk participants
Three trials included only participants with "high risk" factors for PPCs (the definition of "high risk" depended on the trial) (Dronkers 2008; Hulzebos 2006a; Hulzebos 2006b). The other trials did not restrict inclusion to high‐risk participants and did not report the results of high‐risk participants separately. We attempted to contact the relevant trial authors to identify the risk factors of the included participants, but were unable to obtain this information. It was therefore not meaningful to carry out this pre‐planned subgroup analysis because of the small number of trials.
3. Type of intervention
In 10 of the 12 included trials, the intervention was simply preoperative IMT with threshold device (Barbalho‐Moulim 2011; Carvalho 2011; Da Cunha 2013; Dronkers 2008; Ferreira 2009; Heynen 2012; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010; Weiner 1998). In the other two trials, the intervention was a mixed training programme including IMT (Dronkers 2010; Soares 2013). Dronkers 2010 reported only data on pneumonia for the primary outcome. We therefore performed subgroup analysis about postoperative pneumonia according to the type of intervention, that is simple IMT intervention and mixed training programme including IMT. In the simple IMT intervention subgroup, pooled analysis showed preoperative simple IMT was associated with a reduction of postoperative pneumonia compared with usual care or non‐exercise intervention (RR 0.49; 95% CI 0.27 to 0.88; P value = 0.02) (Figure 6). Test for subgroup differences: Chi2 statistic = 0.67, df = 1 (P value = 0.41), I2 statistic = 0% (postoperative pneumonia).
Sensitivity analysis
We performed pre‐planned sensitivity analysis about the primary outcome only when we had identified a meaningful number of relevant trials. None of the sensitivity analyses led to a change in the main findings regarding the PPCs.
1. Excluding trials with high or unclear risk of bias in allocation concealment
After excluding the trials with high or unclear risk of bias with respect to allocation concealment, we included six trials in this analysis (Barbalho‐Moulim 2011; Dronkers 2008; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010; Soares 2013). Five of those six trials adequately reported postoperative atelectasis (Barbalho‐Moulim 2011; Dronkers 2008; Hulzebos 2006a; Hulzebos 2006b; Soares 2013), and five of six trials adequately reported postoperative pneumonia (Barbalho‐Moulim 2011; Hulzebos 2006a; Hulzebos 2006b; Kulkarni 2010; Soares 2013). The result was consistent with the main findings regarding the incidence of postoperative atelectasis (Analysis 1.10) and of postoperative pneumonia (Analysis 1.11).
1.10. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 10 Sensitivity analysis; PPC; Atelectasis (Excluding studies with high risk or unclear risk of bias in allocation concealment).
1.11. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 11 Sensitivity analysis; PPC; Pneumonia (Excluding studies with high risk or unclear risk of bias in allocation concealment).
2. Excluding trials in which the primary outcome evaluation was not blinded
After excluding trials with a high or unclear risk of bias with respect to the blinding of outcome assessment, five trials adequately blinded the outcome assessors (Barbalho‐Moulim 2011; Dronkers 2008; Dronkers 2010; Hulzebos 2006a; Hulzebos 2006b). Of these five trials, we finally included in this analysis four that adequately reported postoperative atelectasis (Barbalho‐Moulim 2011; Dronkers 2008; Hulzebos 2006a; Hulzebos 2006b) and four that adequately reported postoperative pneumonia (Barbalho‐Moulim 2011; Dronkers 2010; Hulzebos 2006a; Hulzebos 2006b). The main findings regarding the incidence of postoperative atelectasis (Analysis 1.12) and of postoperative pneumonia (Analysis 1.13) did not change.
1.12. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 12 Sensitivity analysis; PPC; Atelectasis (Excluding studies with high risk or unclear risk of bias in blinding of outcome assessment).
1.13. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 13 Sensitivity analysis; PPC; Pneumonia (Excluding studies with high risk or unclear risk of bias in blinding of outcome assessment).
3. Performing the worst‐ and best‐case scenario analysis
Intention‐to‐treat analysis of RCTs regarding the incidence of postoperative pneumonia is the best‐case scenario. In a worst‐case‐scenario analysis, participants who drop out are treated as incident cases of postoperative pneumonia. Pooled analysis of this worst‐case scenario was consistent with the main findings regarding the incidence of postoperative atelectasis (Analysis 1.14) and of postoperative pneumonia (Analysis 1.15).
1.14. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 14 Sensitivity analysis; PPC; Atelectasis (worst‐case scenario analysis).
1.15. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 15 Sensitivity analysis; PPC; Pneumonia (worst‐case scenario analysis).
4. Fixed‐effect or random‐effects models
Repetition of the analysis using a fixed‐effect model was consistent with the main findings regarding the incidence of PPCs.
5. Excluding trials that used original authors' definition of PPCs and included no outcome based on our definition
It was not meaningful to carry out this pre‐planned sensitivity analysis because all the trials used different original authors' definitions for PPCs.
Funnel plot analysis
As stated in the protocol for this review, we conducted funnel plot analysis for the incidence of pneumonia (Katsura 2013). The funnel plot showed asymmetrical appearance with a gap in the bottom corner of the graph (Figure 8), but Egger's test was not statistically significant (P value = 0.22). When we carried out the trim‐and‐fill imputation as a sensitivity check, the overall results did not change materially (the imputed RR = 0.41).
8.

Funnel plot of comparison: 1 Preoperative inspiratory muscle training versus usual care, non‐exercise intervention, outcome: 1.2 PPC; Pneumonia (Type of surgery).
Discussion
Summary of main results
In this systematic review and meta‐analysis, we compared the effectiveness of preoperative IMT and usual preoperative care on PPCs in adult participants undergoing cardiac or major abdominal surgery. We identified and pooled the data of 12 trials, of which five included participants awaiting elective cardiac surgery and seven included participants awaiting elective major abdominal surgery. The included trials did not report on all of the outcomes that were described in the protocol of this review (Katsura 2013). The present review showed that preoperative IMT was associated with a reduction of postoperative atelectasis and pneumonia, compared with usual care or non‐exercise intervention (respectively; RR 0.53; 95% CI 0.34 to 0.82 and RR 0.45; 95% CI 0.26 to 0.77). However, the effect of preoperative IMT on all‐cause postoperative mortality is uncertain (RR 0.40; 95% CI 0.04 to 4.23) and needs further investigation. We found no trial that reported the incidence of adverse events caused by preoperative IMT.
When we considered secondary outcomes, the analysis found that preoperative IMT was associated with reduced length of hospital stay (MD ‐1.33; 95% CI ‐2.53 to ‐0.13). On the other hand, we found no evidence of a significant difference in the change score regarding Pi‐max between the preoperative baseline before intervention and postoperative period (MD ‐7.87; 95% CI ‐21.36 to 5.61); however, there were only three pooled trials.
We have presented the main findings of this review in Table 1. However, several points need to be considered when interpreting the present findings, as the majority of studies were small and rated as being at an unclear or high risk of bias.
Overall completeness and applicability of evidence
Although this review answered our research questions, the number of included trials was small for some of the outcomes. It is known that most large treatment effects emerge from small‐sized trials, and all included trials but one were small‐sized. The salutary effect of the intervention might have derived from small‐sized trials (Pereira 2012), and so cautious interpretation and application are necessary. Our review included 10 trials from around the world, but six of them were from high‐income countries, including five from the Netherlands and one from the United Kingdom. The accessibility to the threshold device might limit the applicability of IMT with threshold device into the clinical practice.
Quality of the evidence
We included 12 trials with 695 participants in our review. Of the 12 trials we pooled 11 in the meta‐analysis on the incidence of pneumonia and 10 on the meta‐analysis on the incidence of drop‐out. Meta‐analyses on the other outcomes included a small number of trials (three to seven). The sample size of each of the 12 trials was small, except for one trial that comprised 276 participants (Hulzebos 2006b). The incidence of PPCs was relatively small in each trial. We assessed only one trial as at low risk of bias in all domains except the domain of performance bias (Hulzebos 2006b). We assessed all trials as at unclear or high risk of bias for the domain of performance bias. According to the GRADE Working Group guidelines for evaluating the impact of healthcare interventions, the overall quality of the trials for the incidence of pneumonia was moderate, whereas the overall quality of the trials for the incidence of atelectasis, all‐cause postoperative death, and duration of hospital stay was low or very low.
Potential biases in the review process
In order to minimize the risk of publication bias, we conducted an extensive search without language restrictions, and attempted to include three conference proceedings (Carvalho 2011; Da Cunha 2013; Heynen 2012). We also attempted to contact all of the authors of the included trials for information that was not reported in the published articles or proceedings. We gained necessary information regarding some outcomes, but were unable to obtain all of the information regarding other outcomes and trial designs. We also contacted the companies who supply respiratory muscle training devices, but information regarding any ongoing trials was unavailable. This limitation might introduce a source of bias. It is also possible that, as the funnel plot for the incidence of pneumonia suggests (Figure 8), there remains the possibility of small‐study effects and publication bias for this outcome.
Another concern is that the largest trial, Hulzebos 2006b, weighed nearly 50% in some primary analyses. It is possible that these outcomes were heavily influenced by this one trial (Hulzebos 2006b). However, this trial is free of any major bias and is large in the sample size, and may therefore accurately represent the necessity of preoperative IMT. We therefore decided to include this trial in the analysis.
We needed to make several changes to the protocol methods (Katsura 2013). Firstly, we included all reported data on PPCs, because most trials used their own definitions of PPCs, and because only a limited number of trials reported the outcomes of interest to us. Secondly, we also considered trials that used interventions that differed slightly from those in our original protocol (Katsura 2013): a trial where participants were required to have IMT five days a week, in Da Cunha 2013, and a trial where participants were assigned to start from 10% of maximal inspiratory pressure and to work incrementally, in Dronkers 2010. We believed that including these trials might enhance the applicability and understanding of preoperative IMT and find enough credibility in the clinical community. Thirdly, we also included two trials that employed complex mixed interventions consisting of preoperative IMT and exercise training (Dronkers 2010; Soares 2013). We subsequently examined the efficacy of these trials as well as those trials that met our original eligibility criteria in a subgroup analysis; while we found the statistical significance only in the simple IMT intervention subgroup, the direction of the effect size in the subgroup of the complex mixed intervention was the same (Figure 6).
While we found no heterogeneity between trials in the analysis of postoperative atelectasis, pneumonia, and duration of hospital stay, we found a large degree of heterogeneity in the analysis of mechanical ventilation exceeding 48 hours and all‐cause postoperative death. There were not enough trials in these analyses to enable subgroup analysis or meta‐regression analysis to be performed to investigate the cause of heterogeneity. Given the small number of trials for these outcomes, the effect is uncertain and the generalizability on these outcomes might also be limited.
Agreements and disagreements with other studies or reviews
As far as we know, there exist two other recent systematic reviews focusing on preoperative physical therapy including IMT (Hulzebos 2012; Valkenet 2011). These reviews evaluated the efficacy of preoperative physical therapy with an exercise component. Hulzebos 2012 included participants awaiting cardiac surgery, while Valkenet 2011 included participants awaiting invasive surgery including cardiac, abdominal, or joint replacement surgery. Both studies concluded that preoperative physical therapy prevents some postoperative complications including atelectasis and pneumonia. We understand that their studies will have a few RCTs in common with ours, but we think that our proposed title can still be meaningful for the following reasons. We are interested in "inspiratory muscle training for major cardiac or abdominal surgery", and the other two review authors are interested in "physical exercise training for cardiac surgery or invasive surgery". We believe that the effectiveness of IMT is homogeneous across cardiac or abdominal surgeries (which is why we included the two in our review), and that excluding abdominal surgeries would make our estimate of the effectiveness of IMT less precise (that is with wider confidence intervals). Furthermore, "physical exercise training" will include therapeutic exercise programme and muscle endurance training in addition to IMT, and they will not directly answer our clinical question of whether we should regularly recommend IMT to our patients. The other reviews could perform subgroup analyses on IMT for abdominal surgeries, but then again such subgroup analyses will suffer from unnecessarily wide confidence intervals.
Authors' conclusions
Implications for practice.
In high‐volume centres, many patients usually wait longer than two weeks for their elective surgery. Preoperative IMT could be easily performed in the outpatient setting (for example at home or in a local rehabilitation clinic) under the supervision of a physiotherapist, and with no adverse events reported. We found evidence that preoperative IMT is associated with a reduction of postoperative atelectasis, pneumonia, and duration of hospital stay. Preoperative IMT therefore appears to be a suitable preparation for elective surgery, especially for people on the waiting list for high‐risk major cardiac and abdominal surgery. It is important to note that as the quality of evidence is low to moderate, the potential for overestimation of treatment effect needs to be considered when interpreting the present findings.
Implications for research.
More high‐quality RCTs are needed to evaluate the postoperative benefit of preoperative IMT, especially for major abdominal surgery. Further studies should be more specific in their definition of PPCs and uniform in their descriptions regarding the change score of Pi‐max and the risk of bias. Other types of surgery associated with a high risk of PPCs, such as oesophagogastric resection and other non‐cardiac surgery, should be evaluated. Future studies need to assess whether a further increase of the dose and intensity of IMT influences its effect in reducing PPCs. Evaluations of the cost‐effectiveness of preoperative IMT and patient‐reported outcomes are also greatly desired.
Notes
We would like to thank Mathew Zacharias (content editor), Cathal Walsh (statistical editor), and Rik Gosselink and Andrezza Lemos (peer reviewers) for their help and editorial advice during the preparation of the protocol, Katsura 2013, for the systematic review.
Acknowledgements
We would like to thank Jane Cracknell (Managing Editor, Cochrane Anaesthesia, Critical and Emergency Care Group) and Karen Hovhannisyan (Trials Search Co‐ordinator) for their help and editorial advice during the preparation of the protocol, Katsura 2013, and this systematic review.
We would like to thank Rodrigo Cavallazzi (content editor), Cathal Walsh (statistical editor), Peter Hartley and Tom J Overend (peer reviewers), and Anne Lyddiatt (consumer referee) for their help and editorial advice during the preparation of this systematic review.
We would also like to thank Tales de Carvalho, Dra Marcia Souza Volpe, Meindert Sosef, Marie‐Céline Schraepen, Marcela Cangussu Barbalho‐Moulim, Sachin Kulkarni, Jaap Dronkers, and Erik Hulzebos for providing unpublished information on their studies.
Appendices
Appendix 1. Definition of postoperative pulmonary complications
| Grade | Definition | |
| 1 | Cough, dry | |
| Microatelectasis | Abnormal lung findings and temperature > 37.5°C without other documented cause; results of chest radiograph either normal or unavailable | |
| Dyspnoea | Not due to other documented cause | |
| 2 | Cough, productive | Not due to other documented cause |
| Bronchospasm | New wheezing or pre‐existing wheeze resulting in change in therapy | |
| Hypoxaemia | Alveolar‐arterial gradient > 29 and symptoms of dyspnoea or wheezing | |
| Atelectasis | Radiological confirmation plus temperature > 37.5°C or abnormal lung findings | |
| Hypercarbia, transient | Requiring treatment, such as naloxone or increased manual or mechanical ventilation | |
| Adverse reaction to pulmonary medication | ||
| 3 | Pleural effusion | Resulting in thoracentesis |
| Pneumonia, suspected | Radiological evidence without bacteriological confirmation | |
| Pneumonia, proved | Radiological evidence and documentation of pathological organism by Gram stain or culture | |
| Pneumothorax | ||
| Re‐intubation | Postoperative or intubation, period of ventilator dependence does not exceed 48 hours | |
| 4 | Ventilatory failure | Postoperative ventilator dependence exceeding 48 hours, or re‐intubation with subsequent period of ventilator dependence exceeding 48 hours |
We will define postoperative pulmonary complications (PPCs) according to clinical (symptoms and physical examination) plus radiological criteria as atelectasis and pneumonia. When there are only radiological alterations, without clinical symptoms or alterations in auscultation, we will consider the complications to be subclinical, that is grade 1 PPCs (Chumillas 1998).
Many trials have employed different definitions for PPCs because of difficulty of their measurement. We will use a valid and reliable definition according to Kroenke 1992. If the study authors use different definitions for pulmonary complications, we will contact the original authors to ascertain whether they have data available that matches our definition. If they do not, or if they fail to respond, we will use the original authors' definition for the moderate to severe complications. We will examine the influence of including such studies in a sensitivity analysis.
Appendix 2. Definition of postoperative complications
a) Cardiac complications
The cardiac complications were classified into the following categories (Wijeysundera 2009):
myocardial infarction: definition as per individual study;
myocardial ischaemias: as detected on an electrocardiogram or trans‐oesophageal echocardiogram;
supraventricular tachyarrhythmia: supraventricular tachycardia, atrial fibrillation, or atrial flutter;
congestive heart failure: clinical diagnosis of congestive heart failure or a requirement for intra‐aortic balloon pump support.
b) Neurological complications
The neurological complications were classified into two categories: type I neurological complications were defined as focal injury (e.g. stroke), stupor, or coma at discharge, while type II neurological complications were defined as deterioration in intellectual function, memory deficits, or seizures (Roach 1996).
c) Surgical site infections
We will use the criteria developed by the Centers for Disease Control and Prevention for defining surgical site infections. These criteria define surgical site infections as infections related to the operative procedure that occur at or near the surgical incision within 30 days of an operative procedure or within one year if an implant is left in place (Horan 1992).
Appendix 3. Search strategy for CENTRAL
#1 (Preoperative near ((inspiratory or muscle) and train*)) or IMT:ti,ab or (inspiratory and muscle and train*) or physiotherapy:ti,ab or ((presurgical or perioperative or preoperative) near train*):ti,ab or preconditioning #2 MeSH descriptor: [Breathing Exercises] explode all trees #3 MeSH descriptor: [Physical Therapy Modalities] this term only #4 MeSH descriptor: [Preoperative Care] this term only #5 #1 or #2 or #3 or #4 #6 (postoperative pulmonary complication*) or ((cardiac or valve or aort* or gastrointestinal or urological) near (surg* or operat*)):ti,ab or CABG or (correct* near (congenital defect*)) #7 MeSH descriptor: [Cardiac Surgical Procedures] this term only #8 MeSH descriptor: [Coronary Artery Bypass] this term only #9 MeSH descriptor: [Digestive System Surgical Procedures] this term only #10 MeSH descriptor: [Urologic Surgical Procedures] this term only #11 #6 or #7 or #8 or #9 or #10 #12 #5 and #11
Appendix 4. Search strategy for MEDLINE (Ovid SP)
1. (Preoperative adj3 ((inspiratory or muscle) and train*)).af. or IMT.ti,ab. or (inspiratory and muscle and train*).af. or exp Breathing Exercises/ or Physical Therapy Modalities/ or Preoperative Care/ or physiotherapy.ti,ab. or ((presurgical or perioperative or preoperative) adj3 train*).ti,ab. or preconditioning.mp. 2. postoperative pulmonary complication*.mp. or Cardiac Surgical Procedures/ or ((cardiac or valve or aort* or gastrointestinal or urological) adj3 (surg* or operat*)).ti,ab. or Coronary Artery Bypass/ or CABG.mp. or (correct* adj3 congenital defect*).mp. or Digestive System Surgical Procedures/ or Urologic Surgical Procedures/ 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 5. EMBASE (Ovid SP) search strategy
1. (preoperative adj3 ((inspiratory or muscle) and train*)).af. or IMT.ti,ab. or (inspiratory and muscle and train*).af. or exp breathing exercise/ or physiotherapy/ or preoperative care/ or physiotherapy.ti,ab. or ((presurgical or perioperative or preoperative) adj3 train*).ti,ab. or preconditioning.mp. 2. postoperative pulmonary complication*.mp. or heart surgery/ or ((cardiac or valve or aort* or gastrointestinal or urological) adj3 (surg* or operat*)).ti,ab. or coronary artery bypass graft/ or CABG.mp. or (correct* adj3 congenital defect*).mp. or abdominal surgery/ or urologic surgery/ 3. 1 and 2 4. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 6. CINAHL (EBSCO host) search strategy
S1 ( (MH "Breathing Exercises") OR (MH "Physical Therapy") OR (MH "Preoperative Care") ) OR ( (preoperative N3 ((inspiratory or muscle) and train*)) or IMT or (inspiratory and muscle and train*) or physiotherapy or ((presurgical or perioperative or preoperative) N3 train*) or preconditioning ) S2 ( (MH "Coronary Artery Bypass") OR (MH "Surgery, Urogenital") OR (MH "Surgery, Cardiovascular") OR (MH "Surgery, Digestive System") ) OR ( postoperative pulmonary complication* or ((cardiac or valve or aort* or gastrointestinal or urological) N3 (surg* or operat*)) or CABG or (correct* N3 congenital defect*) ) S3 S1 and S2 S4 (random* or ((clinical or controlled) and trial*) or placebo* or multicenter* or prospective) or ((blind* or mask*) and (single or double or triple or treble)) S5 S3 and S4
Appendix 7. LILACS (BIREME) search strategy
((preoperative and ((inspiratory or muscle) and train$)) or IMT or (inspiratory and muscle and train$) or physiotherapy or ((presurgical or perioperative or preoperative) and train$) or preconditioning) and (postoperative pulmonary complication$ or ((cardiac or valve or aort$ or gastrointestinal or urological) and (surg$ or operat$)) or CABG or (correct$ and congenital defect$))
Appendix 8. ISI Web of Science search strategy
#1 TS=((preoperative SAME ((inspiratory or muscle) and train*)) or IMT or (inspiratory and muscle and train*) or physiotherapy or ((presurgical or perioperative or preoperative) SAME train*) or preconditioning) #2 TS=(postoperative pulmonary complication* or ((cardiac or valve or aort* or gastrointestinal or urological) SAME (surg* or operat*)) or CABG or (correct* SAME congenital defect*)) #3 #2 AND #1 #4 TS=((random* or ((clinical or controlled) and trial*) or placebo* or multicenter* or prospective) or ((blind* or mask*) and (single or double or triple or treble))) #5 #3 and #4
Data and analyses
Comparison 1. Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PPC; Atelectasis | 7 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.34, 0.82] |
| 1.1 cardiac surgery | 3 | 334 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.35, 1.00] |
| 1.2 major abdominal surgery | 4 | 109 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.19, 0.90] |
| 2 PPC; Pneumonia (Type of surgery) | 11 | 675 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.26, 0.77] |
| 2.1 cardiac surgery | 5 | 448 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.23, 0.83] |
| 2.2 major abdominal surgery | 6 | 227 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.16, 1.33] |
| 3 PPC; Pneumonia (Type of intervention) | 11 | 675 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.26, 0.77] |
| 3.1 simple IMT | 9 | 596 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.27, 0.88] |
| 3.2 mixed training programme including IMT | 2 | 79 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.06, 1.11] |
| 4 PPC; mechanical ventilation > 48 hours | 3 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.03, 9.20] |
| 5 All‐cause postoperative death | 7 | 431 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.04, 4.23] |
| 6 Adverse events | 8 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Maximal inspiratory muscle strength; Pi‐max (postoperative change from preoperative baseline) | 3 | 80 | Mean Difference (IV, Random, 95% CI) | ‐7.87 [‐21.36, 5.61] |
| 8 Duration of hospital stay | 6 | 424 | Mean Difference (IV, Random, 95% CI) | ‐1.33 [‐2.53, ‐0.13] |
| 9 Total drop‐out from the study | 10 | 579 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.36, 1.51] |
| 10 Sensitivity analysis; PPC; Atelectasis (Excluding studies with high risk or unclear risk of bias in allocation concealment) | 5 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.33, 0.88] |
| 11 Sensitivity analysis; PPC; Pneumonia (Excluding studies with high risk or unclear risk of bias in allocation concealment) | 5 | 451 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.76] |
| 12 Sensitivity analysis; PPC; Atelectasis (Excluding studies with high risk or unclear risk of bias in blinding of outcome assessment) | 4 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.30, 0.96] |
| 13 Sensitivity analysis; PPC; Pneumonia (Excluding studies with high risk or unclear risk of bias in blinding of outcome assessment) | 4 | 376 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.21, 0.81] |
| 14 Sensitivity analysis; PPC; Atelectasis (worst‐case scenario analysis) | 7 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.41, 0.98] |
| 15 Sensitivity analysis; PPC; Pneumonia (worst‐case scenario analysis) | 11 | 675 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.34, 0.81] |
1.1. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 1 PPC; Atelectasis.
1.2. Analysis.
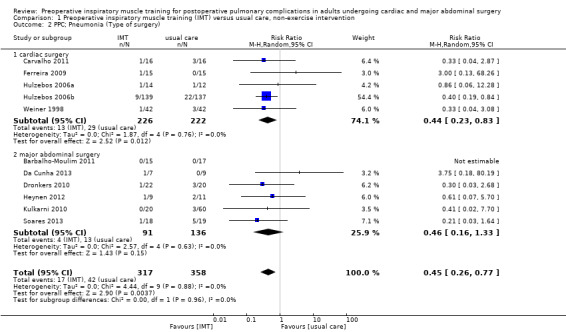
Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 2 PPC; Pneumonia (Type of surgery).
1.3. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 3 PPC; Pneumonia (Type of intervention).
1.8. Analysis.

Comparison 1 Preoperative inspiratory muscle training (IMT) versus usual care, non‐exercise intervention, Outcome 8 Duration of hospital stay.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barbalho‐Moulim 2011.
| Methods |
Design: RCT Setting: single centre, Brazil Recruitment period: not described |
|
| Participants |
Sample size: 32 individuals were eligible and randomized into 2 groups Inclusion criteria: Obese individuals who were candidates for elective open Roux‐en‐Y gastric bypass surgery. Only females over 18 years of age who did not smoke and did not have respiratory disease were included Exclusion criteria: a history of prior abdominal surgery; unable to understand and perform the tests properly; refused to sign the informed consent form Number of participants ‐ Experimental: 15 ‐ Control: 17 Mean age (SD) (NS) ‐ Experimental: 36.1 (8.1) ‐ Control: 34.8 (9.5) Gender (NS) ‐ Experimental: 100% female ‐ Control: 100% female Hypertension (NS) ‐ Experimental: 60% ‐ Control: 41% Diabetes (NS) ‐ Experimental: 20% ‐ Control: 18% Dyslipidemia (NS) ‐ Experimental: 13% ‐ Control: 12% BMI (NS) ‐ Experimental: 41.6 ‐ Control: 42.1 |
|
| Interventions |
Experimental: IMT with threshold device during 2 to 4 weeks before the surgery. The programme consisted of 1 daily 15‐minute session, 6 times per week under supervision of a physiotherapist. The initial load was calculated at 30% of their MIP. Control: usual care |
|
| Outcomes |
Primary outcomes 1. PPCs 1.1 Atelectasis ‐ Experimental: 0 ‐ Control: 0 1.2 Pneumonia ‐ Experimental: 0 ‐ Control: 0 1.3 Mechanical ventilation > 48 hrs ‐ Experimental: 0 ‐ Control: 0 2. All‐cause mortality within 30 days during postoperative period ‐ Experimental: 0 ‐ Control: 0 3. Adverse events: not reported Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max ‐ Experimental: Pi‐max increased significantly; 93.3 cmH2O vs 120.0 cmH2O between baseline and after training preoperatively (P value < 0.05), and decreased significantly after the operation (63.3 cmH2O) ‐ Control: Pi‐max was unchanged before the operation; 92.9 cmH2O vs 91.8 cmH2O between baseline and after training preoperatively, and decreased significantly after the operation (48.8 cmH2O) 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max: not reported 2. Mean duration of hospital stay (SD) ‐ Experimental: 2.00 (0.0) ‐ Control: 2.11 (0.33) ‐ P value = 0.571 3. Other types of complications within 30 days during postoperative period ‐ Experimental: 0 ‐ Control: 0 4. Total drop‐out from the study ‐ Experimental: 0 ‐ Control: 0 5. Quality of life: not reported 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: daily physiotherapy (diaphragmatic breathing, incentive spirometry, assisted cough, circulatory exercises, early ambulation) Definition of PPCs: pneumonia (body temperature ≥ 38℃, productive cough with purulent sputum, presence of pulmonary infiltration on chest X‐ray, increased leukocyte count), atelectasis with clinical implications and acute respiratory failure The author kindly provided additional data on the number of participants with atelectasis, pneumonia, prolonged ventilation, all‐cause mortality, and other types of early complications |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly assigned by opening a sealed envelope". |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly assigned by opening a sealed envelope". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is impossible to blind participants in a preoperative training programme |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The images were analysed by the same radiologist, who was blinded to the information regarding to which group each patient belonged". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Postoperative assessments of all included participants were reported |
| Selective reporting (reporting bias) | Unclear risk | The protocol was unavailable, and so it was difficult to discern which was the primary outcome |
| Other bias | Low risk | No baseline imbalance between the groups |
Carvalho 2011.
| Methods | This study was reported as a conference result (only including an abstract and poster presentation) Design: RCT Setting: not reported, probably single centre, Brazil Recruitment period: not described |
|
| Participants |
Sample size: 32 individuals were eligible and randomized into 2 groups Inclusion criteria: individuals who were candidates for elective coronary artery bypass graft surgery, with a high risk for pulmonary complications (unspecified) Exclusion criteria: not described Number of participants ‐ Experimental: 16 ‐ Control: 16 Mean age (SD) (NS) ‐ Experimental: 62.0 (9.9) ‐ Control: 62.0 (10.9) Gender (NS) ‐ Experimental: 38% female ‐ Control: 31% female Hypertension (NS) ‐ Experimental: 93.8% ‐ Control: 87.5% Smokers (NS) ‐ Experimental: 25% ‐ Control: 37.5% Diabetes (NS) ‐ Experimental: 37.5% ‐ Control: 37.5% Left ventricular ejection fraction > 50% (NS) ‐ Experimental: 75.0% ‐ Control: 87.5% |
|
| Interventions |
Experimental: IMT with threshold device during 2 weeks before the surgery. The programme consisted of 7 days a week, twice a day, 3 sets of 10 repetitions. The work load was calculated at 30% of their MIP Control: not specified |
|
| Outcomes |
Primary outcomes 1. PPCs 1.1 Atelectasis ‐ Experimental: 3 ‐ Control: 7 ‐ P value = 0.02 1.2 Pneumonia ‐ Experimental: 1 ‐ Control: 3 ‐ P value = 0.04 2. All‐cause mortality within 30 days during postoperative period: not reported 3. Adverse events: no adverse events in both groups. None of the participants presented discomfort, stable angina, or any other changes during IMT Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max ‐ Experimental: Pi‐max increased; 70.0 cmH2O vs 92.7 cmH2O between baseline and after training preoperatively, and decreased on the 7th postoperative day (64.1 cmH2O) ‐ Control: Pi‐max was unchanged before the operation; 75.9 cmH2O vs 66.6 cmH2O between baseline and after training preoperatively, and decreased on the 7th postoperative day (47.1 cmH2O) 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max: not reported 2. Mean duration of hospital stay (SD) ‐ Experimental: 8 (3.1) ‐ Control: 8 (2.9) ‐ P value = 0.892 3. Other types of complications within 30 days during postoperative period: not reported 4. Total drop‐out from the study: not reported 5. Quality of life: not reported 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: not described Definition of PPCs: Pneumonia was confirmed by X‐thorax Additional data was obtained from a review article, Hulzebos 2012, and from author contact |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Da Cunha 2013.
| Methods | This study was reported as a conference result (only including an abstract and e‐poster presentation) Design: RCT Setting: hospital in Brazil Recruitment period: not described |
|
| Participants |
Sample size: 15 individuals were eligible and randomized into 2 groups Inclusion criteria: individuals with megaoesophagus or oesophageal cancer, in the preoperative of oesophagectomy or Heller myotomy, waiting period of 2 to 3 weeks Exclusion criteria: not described Number of participants ‐ Experimental: 7 ‐ Control: 9 Baseline participant characteristics: Both groups had similar age, BMI, and gender |
|
| Interventions |
Experimental: Only the experimental group received preoperatively inspiratory and expiratory muscle training (IMT). Each training consisted of 3 series of 12 repetitions performed using threshold devices, at a resistance equal to 60% of their maximal inspiratory and expiratory pressures. The participants trained daily, 5 times a week for at least 2 weeks before surgery. Their resistance was increased incrementally, based on the rate of perceived exertion. Control: Both groups received usual care characterized by instructions to perform breathing exercises associated to upper and lower limbs exercises. |
|
| Outcomes |
Primary outcomes 1. PPCs 1.1 Atelectasis not reported 1. 2 Pneumonia ‐ Experimental: 1 ‐ Control: 0 2. All‐cause mortality within 30 days during postoperative period ‐ Experimental: 0 ‐ Control: 0 3. Adverse events: no adverse events in both groups Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max Pi‐max increased in the preoperative in the IMT group (although only Pi‐max increment was significant), but not in the control group. In the postoperative period, Pi‐max behaved similarly in the 2 groups. Lung function was decreased in the postoperative day 2 and returned to baseline values in the postoperative day 30 in both groups 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max: not reported 2. Mean duration of hospital stay (SD) ‐ Experimental: 9 (4.9) ‐ Control: 7 (7.6) 3. Other types of complications within 30 days during postoperative period: not reported 4. Total drop‐out from the study ‐ Experimental: 0 ‐ Control: 0 5. Quality of life: not reported 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: not reported Definition of PPCs: not reported The author kindly provided additional data on the number of participants with pneumonia, all‐cause mortality, adverse events, and total drop‐out from the study. The author also provided data about the mean and SD of hospital stay and the change score of Pi‐max between the preoperative baseline and postoperative day 2 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Dronkers 2008.
| Methods |
Design: Single‐blind RCT Setting: single centre, the Netherlands Recruitment period: not described |
|
| Participants |
Sample size: 20 individuals were eligible and randomized into 2 groups Inclusion criteria: High‐risk individuals who were candidates for elective surgery for aneurysm of the abdominal aorta with a scheduled delay until surgery of at least 2 weeks, with at least 1 of the following risk factors: age > 65 years, smoking < 2 months before surgery, COPD, and overweight (BMI > 27 kg/m2) Exclusion criteria: individuals with a history of cerebrovascular disorders, immunosuppressive treatment < 30 days before the operation, neuromuscular diseases, prior lung surgery, cardiovascular instability, and treatment by a physical therapist within 8 weeks before elective surgery Number of participants ‐ Experimental: 10 ‐ Control: 10 Mean age (SD) (NS) ‐ Experimental: 70 (6) ‐ Control: 59 (6) Gender (NS) ‐ Experimental: 80% female ‐ Control: 70% female Smokers (NS) ‐ Experimental: 60% ‐ Control: 60% COPD (NS) ‐ Experimental: 10% ‐ Control: 10% BMI (NS) ‐ Experimental: 26 ‐ Control: 25 |
|
| Interventions |
Experimental: IMT with threshold‐loading device during at least 2 weeks before the surgery. The programme consisted of 15 minutes of IMT (6 sessions, 6 days per week), 1 session/week was supervised by a physical therapist. The initial load was calculated at 20% of their MIP, and the resistance was increased incrementally Control: usual care, consisting of instruction in (a) diaphragmatic breathing, (b) deep inspirations with the aid of incentive spirometer, and (c) coughing and "forced expiration techniques" |
|
| Outcomes |
Primary outcomes 1. PPCs 1.1 Atelectasis ‐ Experimental: 3 (30%) ‐ Control: 8 (80%) ‐ P value = 0.07 2. All‐cause mortality within 30 days during postoperative period ‐ Experimental: 0 ‐ Control: 0 3. Adverse events: no adverse events in both groups Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max ‐ Experimental: Pi‐max increased; 68 cmH2O vs 72 cmH2O between baseline and after training preoperatively (P value = 0.32), and decreased to the pretreatment levels after the operation (42 to 55 cmH2O). ‐ Control: Pi‐max was unchanged; 83 cmH2O vs 80 cmH2O between baseline and after training preoperatively (P value = 0.60), and decreased after the operation (39 to 67 cmH2O). The mean postoperative Pi‐max (as the percentage of the MIP at baseline) was 10% higher in the intervention group (P value = 0.36) 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max ‐ Experimental: Pm‐peak/Pi‐max increased; 32 cmH2O vs 43 cmH2O between baseline and after training preoperatively (P value = 0.05) ‐ Control: Pm‐peak/Pi‐max was unchanged before the operation; 39 cmH2O vs 43 cmH2O between baseline and after training preoperatively (P value = 0.36) 2. Mean duration of hospital stay: not reported 3. Other types of complications within 30 days during postoperative period: 2 participants in the intervention group underwent acute reoperation for blood vessel occlusion in the leg. 1 of these 2 participants developed sepsis and renal insufficiency and died 35 days after surgery 4. Total drop‐out from the study ‐ Experimental: 0 ‐ Control: 2 5. Quality of life: not reported 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: daily physical therapy (simulating deep inspirations, diaphragmatic inspiration, "forced expiration techniques" and coughing, early mobilization) Definition of PPCs: operationalized as atelectasis. A blinded radiologist evaluated radiographs of the lung base for the presence of atelectasis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "independent research assistant randomly assigned the patients by opening a sealed and numbered envelope". |
| Allocation concealment (selection bias) | Low risk | Quote: "independent research assistant randomly assigned the patients by opening a sealed and numbered envelope". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is impossible to blind participants in a preoperative training programme. Quote: "In this single‐blind randomized controlled trial" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The main postoperative outcome was atelectasis as diagnosed at the base of X‐rays by a blinded radiologist". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included participants were reported on. 2 participants in the control group dropped out because they were not registered at the department of physical therapy when they were admitted to hospital, and thus follow‐up was not possible. Quote: "Intention‐to‐treat analyses were used to compare the preoperative outcomes and the incidence of atelectasis between the two groups". |
| Selective reporting (reporting bias) | Unclear risk | The protocol was not available, and it was difficult to discern which was the primary outcome |
| Other bias | High risk | The participants in the intervention group were assigned to keep a diary. This might be subject to the Hawthorne effect, or the participants might have become willing to comply with the intervention |
Dronkers 2010.
| Methods |
Design: Single‐blind RCT Setting: single centre, the Netherlands Recruitment period: not described |
|
| Participants |
Sample size: 42 individuals were eligible and randomized into 2 groups Inclusion criteria: Elderly individuals who were candidates for elective colon surgery (waiting period minimally 2 weeks and first surgical intervention for this pathology), age ≥ 65 years, and adequate cognitive functioning Exclusion criteria: individuals with a history of heart disease that prohibits or impedes exercise, severe systemic illness, recent embolism, thrombophlebitis, uncontrolled diabetes (fasting blood glucose of > 400 mg/dL), severe orthopaedic conditions that prohibit or impede exercise, and wheelchair dependence Number of participants ‐ Experimental: 22 ‐ Control: 20 Mean age (SD) (NS) ‐ Experimental: 71.1 (6.3) ‐ Control: 68.8 (6.4) Gender (NS) ‐ Experimental: 32% female ‐ Control: 20% female Smokers (NS) ‐ Experimental: 16% ‐ Control: 43% COPD (NS) ‐ Experimental: 14% ‐ Control: 18% Diabetes ‐ Experimental: 57% ‐ Control: 5% BMI (SD) (NS) ‐ Experimental: 26.6 (3.6) ‐ Control: 25.7 (3.1) |
|
| Interventions |
Experimental: 1. Training in the outpatient department ‐ resistance training of the lower limb extensors ‐ IMT; breathed against a variable resistance (10% to 60% of the MIP) for about 15 minutes ‐ aerobic training ‐ training of functional activities according to participant's capabilities and interest 2. Home‐based training programme ‐ walking ‐ IMT with threshold‐loading device for 15 minutes per day. The initial load was calculated at 20% of their MIP, and the resistance was increased incrementally Control: 1. Home‐based exercise advice They were told of the importance of their physical condition to the postoperative course and were encouraged to be active for minimally 30 minutes a day in the period prior to hospital admission |
|
| Outcomes |
Primary outcomes 1. PPCs PPCs (atelectasis, hypoxia, or pneumonia) ‐ Experimental: 5 (24%) ‐ Control: 5 (25%) ‐ P value = 0.93 Pneumonia ‐ Experimental: 1 (5%) ‐ Control: 3 (15%) ‐ P value = 0.27 2. All‐cause mortality within 30 days during postoperative period: not reported 3. Adverse events: no adverse events in both groups Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max ‐ Experimental: Pi‐max increased significantly; 78 cmH2O vs 92 cmH2O between baseline and after training preoperatively (P value < 0.001) ‐ Control: Pi‐max was unchanged; 93 cmH2O vs 98 cmH2O between baseline and after training preoperatively (P value = 0.33) 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max: not reported 2. Mean duration of hospital stay (SD) ‐ Experimental: 16.2 (11.5) ‐ Control: 21.6 (23.7) ‐ P value = 0.31 3. Other types of complications within 30 days during postoperative period Total postoperative complication ‐ Experimental: 9 (45%) ‐ Control: 8 (38%) ‐ P value = 0.65 4. Total drop‐out from the study ‐ Experimental: 3 in the preoperative measurement, 1 in the postoperative measurement ‐ Control: 1 in the preoperative measurement, 0 in the postoperative measurement 5. Quality of life (EORTC QLQ‐C30/GH/FS/SC) There was no significant difference between the 2 groups 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: received instruction in diaphragmatic breathing, deep inspirations with the aid of incentive spirometry and coughing and "forced expiration techniques" Definition of PPCs: classified as hypoxia (defined as need for additional oxygen), atelectasis (diagnosed by a radiologist), pneumonia (defined according to classification of Arozullah 2001 et al.), and respiratory failure (defined as need for artificial respiration) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomly assigned (block randomization) using prepared envelopes". |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is impossible to blind participants in a preoperative training programme |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Preoperative outcome measures and the postoperative course were assessed by an investigator who was unaware of the treatment allocation until after data analysis was completed". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included participants were reported on. One participant of the intervention group decided not to have the operation. Quote: "Intention‐to‐treat analyses were used to compare outcomes between the two groups". |
| Selective reporting (reporting bias) | Unclear risk | The protocol was unavailable, and it was difficult to discern which outcome was the primary |
| Other bias | Low risk | No baseline imbalance between the groups |
Ferreira 2009.
| Methods |
Design: RCT Setting: single centre, Brazil Recruitment period: not described |
|
| Participants |
Sample size: 30 individuals were eligible and randomized into 2 groups Inclusion criteria: individuals age 50 years or older who were candidates for elective myocardial revascularization surgery or valvuloplasty Exclusion criteria: individuals with a history of unstable angina pectoris, congestive heart failure, lack of physical or intellectual capacity to adequately perform the prescribed exercise, complex ventricular and uncontrolled arrhythmia, uncontrolled high blood pressure (> 140/90 mm/Hg), myocardial infarction or cerebrovascular accident less than 3 years ago, high‐grade atrioventricular blockage, or exercise‐induced bronchial spasm. Participants submitted to surgical procedures before completing a minimum of 2 weeks of respiratory muscle training were excluded Number of participants ‐ Experimental: 15 ‐ Control: 15 Mean age (SD) (NS) ‐ Experimental: 62.5 (6.1) ‐ Control: 63.1 (7.9) Gender ‐ Experimental: 40% female ‐ Control: 13.3% female Smokers (NS) ‐ Experimental: 20% ‐ Control: 6.7% COPD (NS) ‐ Experimental: 6.7% ‐ Control: 6.7% Diabetes ‐ Experimental: 40% ‐ Control: 40% BMI (SD) (NS) ‐ Experimental: 26.6 (5.2) ‐ Control: 28.3 (3.2) |
|
| Interventions |
Experimental: Training program for the inspiratory musculature ‐ At least 2 weeks of preoperative training ‐ 5 series of 10 calm and deep inspirations with at least 1‐minute intervals between series with threshold‐loading device ‐ With a load of 40% of their MIP ‐ The series were to be repeated thrice daily, while waiting for the surgery Control: General information and usual care |
|
| Outcomes |
Primary outcomes 1. PPCs Pneumonia ‐ Experimental: 1 (6.7%) ‐ Control: 0 (0%) ‐ P value = 1.00 Mechanical ventilation > 48 hrs ‐ Experimental: 1 (6.7%) ‐ Control: 0 (0%) ‐ P value = 1.00 2. All‐cause mortality within 30 days during postoperative period ‐ Experimental: 3 (20%) ‐ Control: 1 (6.7%) ‐ P value = 0.598 3. Adverse events: no adverse events in both groups Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max There was no significant difference between groups concerning Pi‐max. 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max: not reported 2. Mean duration of hospital stay: not reported 3. Other types of complications within 30 days during postoperative period Renal failure ‐ Experimental: 3 (20%) ‐ Control: 0 (0%) ‐ P value = 0.224 Heart failure ‐ Experimental: 1 (6.7%) ‐ Control: 1 (6.7%) ‐ P value = 0.65 4. Total drop‐out from the study ‐ Experimental: 0 ‐ Control: 0 5. Quality of life: not reported 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: daily physical therapeutic procedure (Deep‐inspiration exercises without special equipment and daily walks up to their own limits) Definition of PPCs: definition of PPC and pneumonia not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is impossible to blind participants in a preoperative training programme |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There were no missing values for any collected data". |
| Selective reporting (reporting bias) | Unclear risk | The protocol was unavailable, and it was difficult to discern which outcome was the primary |
| Other bias | Low risk | No baseline imbalance between the groups |
Heynen 2012.
| Methods | This study was reported as a conference result only including an abstract Design: RCT Setting: not reported, probably single centre, the Netherlands Recruitment period: not described |
|
| Participants |
Sample size: 20 individuals were eligible and randomized into 2 groups Inclusion criteria: individuals suffering from oesophageal cancer who were candidates for elective oesophagectomy in combination with neoadjuvant therapy (unspecified) Exclusion criteria: not described Number of participants ‐ Experimental: 9 ‐ Control: 11 Baseline participant characteristics: not described |
|
| Interventions |
Experimental: daily IMT at home, supervised physical therapy twice a week, nutritional support once a week, and psychological support if necessary. Participants instructed to train at home 7 days a week until surgery with a minimum of 2 weeks. IMT was performed with threshold device, and inspiratory load was set at 60% of the measured MIP. The load was incrementally increased. The intervention took place in the period between chemoradiation therapy and surgery Control: usual care |
|
| Outcomes |
Primary outcomes 1. PPCs 1.1 Atelectasis ‐ Experimental: 1 ‐ Control: 2 1.2 Pneumonia ‐ Experimental: 1 ‐ Control: 2 2. All‐cause mortality within 30 days during postoperative period ‐ Experimental: 0 ‐ Control: 0 3. Adverse events: not described Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max There was a significant improvement in Pi‐max in the entire group (paired t‐test, P value = 0.050), differences between the groups were not significant 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max: not reported 2. Mean duration of hospital stay (range) ‐ Experimental: 15.71 (6 to 54 days) ‐ Control: 12.72 (4 to 39 days) 3. Other types of complications within 30 days during postoperative period: not reported 4. Total drop‐out from the study ‐ Experimental: 2 ‐ Control: 2 5. Quality of life: still in progress 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: not described Definition of PPCs: definition of PPC and pneumonia not mentioned The author kindly provided additional data on the number of participants with atelectasis, pneumonia, and all‐cause mortality |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
Hulzebos 2006a.
| Methods |
Design: Single‐blind RCT Setting: single centre, the Netherlands Recruitment period: from October to December 2002 |
|
| Participants |
Sample size: 26 individuals were eligible and randomized into 2 groups Inclusion criteria: individuals who were candidates for elective coronary artery bypass graft surgery and had the following risk factors: age ≥ 70 years, productive cough, history of smoking, diabetes mellitus, inspiratory vital capacity < 75% of predicted, and maximal expiratory pressure < 75% of predicted Exclusion criteria: individuals with a history of cerebrovascular accident, use of immunosuppressive medication 30 days before surgery, the presence of a neuromuscular disorder, previous pulmonary surgery, cardiovascular instability, or the existence of an aneurysm Number of participants ‐ Experimental: 14 ‐ Control: 12 Mean age (SD) (NS) ‐ Experimental: 70.1 (9.9) ‐ Control: 70.5 (10.1) Gender (NS) ‐ Experimental: 50% female ‐ Control: 50% female Smokers (NS) ‐ Experimental: 29% ‐ Control: 25% COPD (NS) ‐ Experimental: 43% ‐ Control: 17% Diabetes (NS) ‐ Experimental: 14% ‐ Control: 25% BMI (SD) (NS) ‐ Experimental: 26.1 (2.9) ‐ Control: 28.3 (3.5) |
|
| Interventions |
Experimental: ‐ 2 to 4 weeks of preoperative inspiratory muscle training with threshold‐loading device ‐ trained daily at home, 7 times a week, each session 20 minutes long The initial load was calculated at 30% of their MIP, and the resistance was increased incrementally Control: usual care |
|
| Outcomes |
Primary outcomes 1. PPCs 1.1 Atelectasis ‐ Experimental: 2 (14%) ‐ Control: 6 (50%) ‐ P value = 0.05 1.2 Pneumonia ‐ Experimental: 1 (7%) ‐ Control: 1 (8%) ‐ P value > 0.05 2. All‐cause mortality within 30 days during postoperative period: not reported 3. Adverse events: no adverse events in both groups Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max ‐ Experimental: Pi‐max increased significantly; 64.6 cmH2O vs 87.6 cmH2O between baseline and after training preoperatively (P value = 0.00) ‐ Control: Pi‐max increased; 66.8 cmH2O vs 76.8 cmH2O between baseline and after training preoperatively (P value = 0.18) The effect size between the intervention group and control group 1 day before surgery was medium (effect size = 0.38; 95% confidence interval ‐0.39 to 1.15) 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max: not reported 2. Mean duration of hospital stay (SD) ‐ Experimental: 7.93 (1.94) ‐ Control: 9.92 (5.78) ‐ P value > 0.05 3. Other types of complications within 30 days during postoperative period: not reported 4. Total drop‐out from the study ‐ Experimental: 0 ‐ Control: 0 5. Quality of life: not reported 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: no information Definition of PPCs: defined according to clinical (symptoms and physical examinations) and radiological criteria such as bronchitis, atelectasis, and pneumonia. When there were only radiological alterations without clinical symptoms, the complications were considered to be subclinical |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated randomized blocked design". |
| Allocation concealment (selection bias) | Low risk | Information from the author contact: "A computer‐generated allocation table was used". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was impossible to blind the participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All measurements were taken by a physical therapist who was blinded to the participant allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | The protocol was unavailable, and it was difficult to discern which outcome was the primary |
| Other bias | Low risk | No baseline imbalance between the groups |
Hulzebos 2006b.
| Methods |
Design: Single‐blind RCT Setting: single centre, the Netherlands Recruitment period: between July 2002 and August 2005 |
|
| Participants |
Sample size: 276 individuals were eligible and randomized into 2 groups Inclusion criteria: individuals who were candidates for elective coronary artery bypass graft surgery and had risk factors for developing PPC: age, productive cough, diabetes mellitus, history of smoking, COPD, BMI, and pulmonary function tests Exclusion criteria: individuals with a history of cerebrovascular accident, use of immunosuppressive medication 30 days before surgery, the presence of a neuromuscular disorder, cardiovascular instability, or the existence of an aneurysm Participants submitted to surgical procedures before completing a minimum of 2 weeks of respiratory muscle training were excluded Number of participants ‐ Experimental: 139 ‐ Control: 137 Mean age (SD) (NS) ‐ Experimental: 66.5 (9.0) ‐ Control: 67.3 (9.2) Gender (NS) ‐ Experimental: 22.3% female ‐ Control: 21.9% female Smokers (NS) ‐ Experimental: 32.4% ‐ Control: 38.0% COPD (NS) ‐ Experimental: 19.4% ‐ Control: 21.9% Diabetes (NS) ‐ Experimental: 43.9% ‐ Control: 32.8% BMI (SD) (NS) ‐ Experimental: 28.3 (5.5) ‐ Control: 28.1 (3.2) |
|
| Interventions |
Experimental: daily (7 times a week) IMT with threshold‐loading device during at least 2 weeks before the surgery. The programme consisted of 20 min of IMT; 6 sessions (6 days a week) without supervision, and 1 session (once a week) was supervised by a physical therapist The initial load was calculated at 30% of their MIP, and the resistance was increased incrementally Control: usual care (instruction on deep‐breathing manoeuvres, coughing, and early mobilization) |
|
| Outcomes |
Primary outcomes 1. PPCs Atelectasis ‐ Experimental: 14 ‐ Control: 18 ‐ P value = 0.02 Pneumonia ‐ Experimental: 9 ‐ Control: 22 ‐ P value = 0.01 Mechanical ventilation > 48 hrs ‐ Experimental: 1 ‐ Control: 6 PPC grade 2 ‐ Experimental: 14 ‐ Control: 18 ‐ P value = 0.02 PPC grade 3 ‐ Experimental: 10 ‐ Control: 24 ‐ P value = 0.01 PPC grade 4 ‐ Experimental: 1 ‐ Control: 6 ‐ P value = 0.09 PPC grade ≥ 2 ‐ Experimental: 25 ‐ Control: 48 ‐ P value = 0.02 2. All‐cause mortality ‐ Experimental: 0 ‐ Control: 4 3. Adverse events: no adverse events in both groups Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max ‐ Experimental: Pi‐max increased significantly; 81.1 cmH2O vs 95.6 cmH2O between baseline and after training preoperatively (P value < 0.001) ‐ Control: Pi‐max was unchanged before the operation; 80.3 cmH2O vs 79.5 cmH2O between baseline and after training preoperatively 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max ‐ Experimental: Pm‐peak/Pi‐max increased significantly; 48.8% vs 56.0% between baseline and after training preoperatively (P value < 0.001) ‐ Control: Pm‐peak/Pi‐max was unchanged before the operation; 50.7% vs 51.8% between baseline and after training preoperatively (P value = 0.24) 2. Mean duration of hospital stay (SD) ‐ Experimental: 7.89 (2.17) ‐ Control: 9.94 (8.27) ‐ P value = 0.02 3. Other types of complications within 30 days during postoperative period: not reported 4. Total drop‐out from the study ‐ Experimental: 0 ‐ Control: 0 5. Quality of life: not reported 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: Both groups received similar incentive spirometry, chest physical therapy, and mobilization scheme after operation. Definition of PPCs: an ordinal scale of 1 to 4, using the operational definition of Kroenke 1992 et al. They defined a clinically significant PPC as 2 or more items in the grade 2 complications or 1 item in the grade 3 or 4 complications (Appendix 1). When there were only radiological alterations without clinical symptoms, the complications were considered to be subclinical. Additional data was obtained from a review article, Hulzebos 2012. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer generated randomization table was used". |
| Allocation concealment (selection bias) | Low risk | Quote: "An external investigator blinded to the allocation sequence picked consecutive allocation envelopes for consecutive participants". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was impossible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "the incidence of PPCs was scored by a blinded independent investigator" and "A microbiologist, who was independent and blinded to patients' allocation, collected data from the medical chart and clinical records, assessed bacteriology samples, and evaluated other data indicative for bronchitis, pneumonia, or both". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proportion of lost to follow‐up was small and similar between the groups |
| Selective reporting (reporting bias) | Low risk | The primary outcome was reported in the text and figure |
| Other bias | Low risk | Baseline data, except the duration of mechanical ventilation, was similar between the groups |
Kulkarni 2010.
| Methods |
Design: RCT Setting: single centre, United Kingdom Recruitment period: not described |
|
| Participants |
Sample size: 80 individuals were eligible and randomized into 4 groups Inclusion criteria: ‐ Over 18 years of age ‐ Undergoing major abdominal general surgery with ASA grade Ⅰ‐Ⅳ Exclusion criteria: ‐ ASA grade Ⅴ ‐ surgery to be performed within 2 weeks of initial assessment ‐ a history of previous spontaneous pneumothorax ‐ were unable to give informed consent Number of participants ‐ Group A (no training): 20 ‐ Group B (deep breathing): 20 ‐ Group C (incentive spirometry): 20 ‐ Group D (specific IMT): 20 Mean age ‐ Group A: 65 ‐ Group B: 64 ‐ Group C: 65 ‐ Group D: 60 Gender ‐ Group A: 40% female ‐ Group B: 50% female ‐ Group C: 75% female ‐ Group D: 45% female Smokers ‐ Group A: 15% ‐ Group B: 10% ‐ Group C: 5% ‐ Group D: 10% Respiratory disease ‐ Group A: 15% ‐ Group B: 15% ‐ Group C: 15% ‐ Group D: 15% Diabetes ‐ Group A: 15% ‐ Group B: 10% ‐ Group C: 5% ‐ Group D: 10% Hypertension ‐ Group A: 30% ‐ Group B: 40% ‐ Group C: 20% ‐ Group D: 15% ASA grade (median) ‐ Group A: 2 ‐ Group B: 2 ‐ Group C: 2 ‐ Group D: 2 |
|
| Interventions |
Experimental: Participants were expected to train for 2 weeks minimum, and instructed in technique by the researcher and asked to train twice daily, each session lasting 15 min, at home up to the day before surgery ‐ Group B: deep‐breathing exercises ‐ Group C: incentive spirometry ‐ Group D: specific IMT with threshold‐loading device (the initial load was calculated at 20% to 30% of their MIP, and the resistance was increased incrementally) Control: ‐ Group A: no training and usual care |
|
| Outcomes |
Primary outcomes 1. PPCs Chest infections requiring antibiotics treatment (pneumonia) ‐ Group A: 2 ‐ Group B: 1 ‐ Group C: 0 ‐ Group D: 0 Other pulmonary complications ‐ Group A: 0 ‐ Group B: 2 ‐ Group C: 0 ‐ Group D: 0 2. All‐cause mortality within 30 days during postoperative period: not reported 3. Adverse events: no adverse events in both groups Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max (median) ‐ Group A: 55 cmH2O vs 48 cmH2O between baseline and after training preoperatively (P value = 0.25) ‐ Group B: 55 cmH2O vs 48 cmH2O between baseline and after training preoperatively (P value = 0.04) ‐ Group C: 50.5 cmH2O vs 44 cmH2O between baseline and after training preoperatively (P value = 0.19) ‐ Group D: 51.5 cmH2O vs 68.5 cmH2O (median) between baseline and after training preoperatively (P value < 0.001) Postoperatively, groups A, B, and C showed a fall in Pi‐max, and only in group D did the Pi‐max remain more than pre‐training level 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max: not reported 2. Duration of hospital stay (median, range) ‐ Group A: 6 (1 to 14) ‐ Group B: 5 (1 to 10) ‐ Group C: 4 (2 to 22) ‐ Group D: 4 (1 to 13) 3. Other types of complications within 30 days during postoperative period: not reported 4. Total drop‐out from the study ‐ Group A: 3 ‐ Group B: 3 ‐ Group C: 5 ‐ Group D: 3 5. Quality of life: not reported 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: not reported Definition of PPCs: not reported The author kindly provided additional data on the number of adverse events, and “chest infections requiring antibiotics treatment” is same as pneumonia according to author definition |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were allocated to four groups by computer‐generated, random numbers placed in sequentially numbered sealed envelopes". |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were allocated to four groups by computer‐generated, random numbers placed in sequentially numbered sealed envelopes". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was impossible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The researchers and outcome assessors were identical |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The lack of postoperative assessment was similar across groups, but its proportion was higher than 20% in Group C |
| Selective reporting (reporting bias) | Unclear risk | The protocol was unavailable, and it was difficult to discern which outcome was the primary |
| Other bias | Low risk | Baseline data were similar across groups (Table 1) |
Soares 2013.
| Methods |
Design: non‐blind RCT Setting: tertiary public hospital and private university, Brazil Recruitment period: not described |
|
| Participants |
Sample size: 37 individuals were eligible and randomized into 2 groups Inclusion criteria: participants who were candidates for elective open abdominal surgery (defined as opening of the peritoneal cavity), waiting period of at least 2 weeks and age ≥ 40 years Exclusion criteria: individuals with a history of cerebrovascular disease, use of immunosuppressive medication within 30 days of surgery, cardiovascular instability, or physical therapy within 8 weeks preceding study enrolment Number of participants ‐ Experimental: 18 ‐ Control: 19 Median age ‐ Experimental: 58.5 ‐ Control: 55.0 Gender ‐ Experimental: 50% female ‐ Control: 43.8% female Smokers ‐ Experimental: 56.3% ‐ Control: 68.8% Body mass index (median) ‐ Experimental: 23.6 ‐ Control: 24.2 Cancer diagnosis ‐ Experimental: 75.0% ‐ Control: 81.2% |
|
| Interventions |
Experimental: 50‐min physical therapy sessions per week during the 2 to 3 weeks preceding their surgical procedure, which consisted of: ‐ stretching exercise, trunk rotation, active upper and lower extremity exercises, walking (10‐min walk on flat ground), and relaxation ‐ deep breathing and guidance and training on coughing and huffing ‐ respiratory muscle training Participants used an inspiratory threshold‐loading device for 15 min daily; the initial load was calculated at 15% of their MIP, and the resistance was increased incrementally Control: Participants did not receive any physical therapy intervention in the preoperative period |
|
| Outcomes |
Primary outcomes 1. PPCs Atelectasis ‐ Experimental: 2 ‐ Control: 5 Pneumonia ‐ Experimental: 1 ‐ Control: 5 Respiratory failure ‐ Experimental: 1 ‐ Control: 4 2. All‐cause mortality ‐ Experimental: 0 ‐ Control: 4 3. Adverse events: not reported Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max ‐ Experimental: Pi‐max increased significantly; 62 cmH2O vs 88 cmH2O between baseline and after training preoperatively (P value = 0.028) ‐ Control: Pi‐max was unchanged before the operation; 72 cmH2O vs 64 cmH2O between baseline and after training preoperatively 1.2 Respiratory muscle endurance On the 7th postoperative day, MIP (expressed as percentage of predictive value) was significantly higher in the treatment group (65% of predicted value) than in the control group (47% of predicted value), P value < 0.05 2. Mean duration of hospital stay: not reported 3. Other types of complications within 30 days during postoperative period: not reported 4. Total drop‐out from the study ‐ Experimental: 3 ‐ Control: 6 5. Quality of life: not reported 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: A physical therapy protocol was implemented in both groups and followed until the 7th postoperative day or hospital discharge (when the length of stay was < 7 days) Definition of PPCs: defined as pulmonary complications treated and noted in medical records until the 7th postoperative day:
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly allocated, by means of sealed envelope randomization, to the treatment or control group". |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly allocated, by means of sealed envelope randomization, to the treatment or control group". "Neither patients nor physical therapists were blinded to group assignment". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It is impossible to blind participants in a preoperative training programme. Quote: "Non‐blind randomized controlled trial" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Neither patients nor physical therapists were blinded to group assignment, and the investigators responsible for data collection were aware of allocation". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The proportion of participants excluded from the analysis was not small and outcome data of excluded participants was incomplete |
| Selective reporting (reporting bias) | Unclear risk | The protocol was unavailable, and it was difficult to discern which outcome was the primary |
| Other bias | Low risk | No baseline imbalance between the groups |
Weiner 1998.
| Methods |
Design: RCT Setting: single centre, Israel Recruitment period: not described |
|
| Participants |
Sample size: 84 individuals were eligible and randomized into 2 groups Inclusion criteria: individuals who were candidates for elective coronary artery bypass graft surgery Exclusion criteria: not reported Number of participants ‐ Experimental: 42 ‐ Control: 42 Mean age (SD) (NS) ‐ Experimental: 59.2 (3.8) ‐ Control: 63.8 (3.1) There was no difference between the 2 groups for the operative technique |
|
| Interventions |
Experimental: daily (6 times a week) IMT with threshold‐loading device for 2 to 4 weeks before the surgery. The program consisted of 30 min of IMT, which was performed under the supervision of a physician. The initial load was calculated at 15% of their MIP, and the resistance was increased incrementally up to 60% of their MIP Control: Breathing through the same trainer but with no resistance |
|
| Outcomes |
Primary outcomes 1. PPCs Pneumonia ‐ Experimental: 1 ‐ Control: 3 Pleural effusion ‐ Experimental: 5 ‐ Control: 3 Hemidiaphragmatic paralysis ‐ Experimental: 2 ‐ Control: 3 Mechanical ventilation > 24 hrs ‐ Experimental: 2 ‐ Control: 11 2. All‐cause mortality within 30 days during postoperative period: not reported 3. Adverse events: not reported Secondary outcomes 1.1 Maximal inspiratory muscle strength; Pi‐max ‐ Experimental: Pi‐max increased significantly; 88.0 cmH2O vs 101.9 cmH2O between baseline and after training preoperatively (P value < 0.005), and decreased to the pretreatment levels after the operation (90.4 cmH2O) ‐ Control: Pi‐max was unchanged before the operation; 94.1 cmH2O vs 91.2 cmH2O between baseline and after training preoperatively, and decreased significantly after the operation (74.4 cmH2O) 1.2 Respiratory muscle endurance; Pm‐peak/Pi‐max ‐ Experimental: Pm‐peak/Pi‐max increased significantly; 76.1% vs 87.0% between baseline and after training preoperatively (P value < 0.001), and decreased to the pretreatment levels after the operation (80.4%) ‐ Control: Pm‐peak/Pi‐max was unchanged before the operation; 77.8% vs 75.6% between baseline and after training preoperatively, and decreased significantly after the operation (51.9%). 2. Mean duration of hospital stay: not reported 3. Other types of complications within 30 days during postoperative period: not reported 4. Total drop‐out from the study: not reported 5. Quality of life: not reported 6. Cost analysis: not reported |
|
| Notes |
Postoperative care for both groups: not described Definition of PPCs: definition of PPC and pneumonia not mentioned, just that PPCs were "detected by chest radiography". |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was impossible to blind participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information was provided |
| Selective reporting (reporting bias) | Unclear risk | It was impossible to discern which outcome was the primary one, and some results were embedded in figures |
| Other bias | Low risk | Baseline data were similar between groups (Table 1) |
ASA: American Society of Anesthesiologists BMI: body mass index COPD: chronic obstructive pulmonary disease EORTC QLQ‐C30/GH/FS/SC: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30/Global Health Status/Functional Scales/Symptom Scales IMT: inspiratory muscle training MIP: maximal inspiratory pressure NS: not significant PPCs: postoperative pulmonary complications RCT: randomized controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alper 2007 | Not a RCT, observational study |
| Ambrosino 2010 | Not a RCT, observational study |
| Arthur 2000 | Trial with a non‐relevant intervention |
| Barros 2010 | Trial with a non‐relevant intervention: postoperative intervention |
| Borghi‐Silva 2005 | Trial with a non‐relevant intervention |
| Casali 2011 | Trial with a non‐relevant intervention: from the 2nd until 30th postoperative day |
| Celli 1984 | Trial with a non‐relevant intervention |
| Christensen 1991 | Trial with a non‐relevant intervention: postoperative regimen |
| Chumillas 1998 | Trial with a non‐relevant intervention |
| Dias 2011 | Trial with a non‐relevant intervention |
| Dull 1983 | Trial with a non‐relevant intervention: postoperative regimen |
| Fagevik Olsén 1997 | Trial with a non‐relevant intervention |
| Fagevik Olsén 1999 | Trial with a non‐relevant intervention |
| Fagevik Olsén 2002 | Trial with a non‐relevant intervention: postoperative breathing exercise |
| Gastaldi 2008 | Trial with a non‐relevant intervention |
| Grams 2012 | Review article |
| Heisterberg 1979 | Trial with a non‐relevant intervention: chest physiotherapy after surgery |
| Herdy 2008 | Trial with a non‐relevant intervention: inadequate preoperative intervention with postoperative intervention |
| Hulzebos 2012 | Cochrane systematic review |
| Ingwersen 1993 | Trial with a non‐relevant intervention: mask physiotherapy after surgery |
| Jenkins 1989 | Trial with a non‐relevant intervention |
| Jenkins 1990 | Trial with a non‐relevant intervention: postoperative regimen |
| Johnson 1996 | Trial with a non‐relevant intervention: chest physiotherapy after surgery |
| Larsen 1994 | Trial with a non‐relevant intervention |
| Larsen 1995 | Trial with a non‐relevant intervention |
| Larsen 1997 | Trial with a non‐relevant intervention |
| Matheus 2012 | Trial with a non‐relevant intervention: daily during the first 3 postoperative days |
| Mendes 2006 | Trial with a non‐relevant intervention |
| Miranda 2011 | Review article |
| Morran 1983 | Trial with a non‐relevant intervention: chest physiotherapy after surgery |
| Murphy 2006 | Not a RCT, observational study |
| Nava 2007 | Not a RCT, letter article |
| Olsén 2012 | Review article |
| Pasquina 2003 | Not a RCT, review article |
| Pasquina 2006 | Not a RCT, review |
| Rajendran 1998 | Not a RCT, observational study |
| Rideout 2012 | Trial with a non‐relevant intervention |
| Roukema 1988 | Trial with a non‐relevant intervention |
| Savci 2011 | Trial with a non‐relevant intervention: 5 days in postoperative period |
| Silva 2013 | Trial with a non‐relevant postoperative intervention: physiotherapy‐directed early mobilization |
| Stiller 1994 | Trial with a non‐relevant intervention: chest physiotherapy after surgery |
| Trevisan 2010 | Trial with a non‐relevant intervention |
| Valkenet 2011 | Review article |
| van Adrichem 2014 | Trial with a non‐relevant intervention: to compare high‐intensity preoperative IMT with normal‐intensity training |
| van den Buijs 2004 | Aspects of the study design meant that this study did not address the review question |
| Walther 2010 | Trial with a non‐relevant intervention and unclear description in the abstract |
IMT: inspiratory muscle training RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01321983.
| Trial name or title | Effects of preoperative IMT in obese women undergoing open bariatric surgery |
| Methods |
Design: Randomized controlled trial Setting: Meridional Hospital, Cariacica/ES, Brazil |
| Participants | Obese female participants undergoing open bariatric surgery Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Experimental: preoperative IMT Control: usual care |
| Outcomes | Respiratory muscle strength (maximal inspiratory pressure and maximal expiratory pressure), lung volumes, and diaphragmatic excursion |
| Starting date | Not provided |
| Contact information | Universidade Federal de Sao Carlos |
| Notes | The study has been completed, but the information on ClinicalTrials.gov has not been updated |
NCT01828632.
| Trial name or title | Effects of preoperative respiratory physical therapy on postoperative respiratory function after bariatric surgery |
| Methods |
Design: Randomized controlled trial Setting: Hospital Clínico Universitario, Valencia, Spain |
| Participants | Morbidly obese participants undergoing laparoscopic bariatric surgery Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
Experimental: Respiratory physical therapy: a programme of IMT, combined with the incentive spirometer for a period of 30 days before the actual date of surgery Control: usual care |
| Outcomes |
Primary outcome: Intergroup difference in arterial oxygen partial pressure/inspired oxygen fraction ratio (PaO2/FiO2) Secondary outcomes: Intragroup and intergroup differences in maximal inspiratory pressure; intergroup difference in arterial carbon dioxide; intragroup and intergroup spirometry values (force vital capacity and forced expiratory volume in 1 second) in the postoperative period |
| Starting date | March 2012 |
| Contact information | Julio Llorens, Fundación para la Investigación del Hospital Clínico de Valencia |
| Notes | The study has been completed, but the information on ClinicalTrials.gov has not been updated |
IMT: inspiratory muscle training
Differences between protocol and review
We made the following changes to the protocol (Katsura 2013).
We removed the word 'patients' from the title.
We a priori planned to impute the missing SDs from other studies, but as some studies reported quite different SDs of Pi‐max, we did not pool the study in the meta‐analysis if we could not calculate the exact SD.
We made a subtle change to the eligibility criteria regarding the frequency of IMT and the range of threshold‐loading, which was set in the protocol. We had decided in the protocol to include trials where participants were required to have IMT six or seven times a week and to use an inspiratory threshold‐loading device with the load of 15% to 60% of maximal inspiratory muscle strength. We found two trials that were close to our eligibility criteria; Da Cunha 2013 assigned the training program for five days a week, and Dronkers 2010 required participants in the intervention group to work at 10% to 60% of the maximal inspiratory pressure. However, we considered these differences to be minor; the difference in the frequency between our protocol and the Da Cunha 2013 trial represented only one day per week, and the Dronkers 2010 trial required participants to start from 10% of the maximal inspiratory pressure and to work incrementally. We felt that including these similar trials would make the findings of our review relevant to the readers' clinical practice, and might also make the intervention more useable. We thus decided to include these trials with minor differences from our original protocol.
We deleted one secondary outcome (other types of complications within 30 days during postoperative period) from 'Summary of findings' table 1. We believed it was not meaningful to describe this pre‐planned secondary outcome because only one study reported its occurrence.
Contributions of authors
Morihiro Katsura (MK), Akira Kuriyama (AK), Taro Takeshima (TT), Shunichi Fukuhara (SF), Toshi A Furukawa (TAF)
Conceiving the review: MK
Designing the review: MK, TAF
Co‐ordinating the review: MK
Undertaking manual searches: MK, TT, AK
Screening search results: MK, TT, AK
Organizing retrieval of papers: SF, TAF
Screening retrieved papers against inclusion criteria: MK, TT, AK
Appraising quality of papers: SF, TAF
Abstracting data from papers: MK, TT, AK
Writing to authors of papers for additional information: MK, AK
Providing additional data about papers: MK, AK
Obtaining and screening data on unpublished studies: MK, AK
Data management for the review: MK, AK
Entering data into Review Manager (RevMan 5.3): MK, AK
Review Manager statistical data: MK, AK, TAF
Other statistical analysis not using Review Manager: MK, AK, TAF
Double entry of data: (data entered by person one: MK; data entered by person two: AK)
Interpretation of data: MK, AK, SF, TAF
Statistical inferences: TAF
Writing the review: MK, AK, TAF
Providing guidance on the review: TAF
Securing funding for the review: not applicable
Performing previous work that was the foundation of the present study: MK, TAF
Guarantor for the review (one review author): MK
Persons responsible for reading and checking review before submission: MK, AK, SF, TAF
Sources of support
Internal sources
New Source of support, Other.
External sources
New Source of support, Other.
Declarations of interest
Morihiro Katsura: None known.
Akira Kuriyama: None known.
Taro Takeshima: None known.
Shunichi Fukuhara: None known.
Toshi A Furukawa has received lecture fees from Eli Lilly, Meiji, Mochida, MSD, Pfizer, and Mitsubishi Tanabe, and consultancy fees from Sekisui and Takeda Science Foundation. He is diplomate of the Academy of Cognitive Therapy. He has received royalties from Igaku‐Shoin, Seiwa‐Shoten, and Nihon Bunka Kagakusha. None of these companies manufacture drugs or products for preoperative IMT. The Japanese Ministry of Education, Science, and Technology; the Japanese Ministry of Health, Labor and Welfare; and the Japan Foundation for Neuroscience and Mental Health have funded his research projects.
New
References
References to studies included in this review
Barbalho‐Moulim 2011 {published and unpublished data}
- Barbalho‐Moulim MC, Miguel GP, Forti EM, Campos Fdo A, Costa D. Effects of preoperative inspiratory muscle training in obese women undergoing open bariatric surgery: respiratory muscle strength, lung volumes, and diaphragmatic excursion. Clinics 2011;66(10):1721‐7. [PUBMED: 22012043] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carvalho 2011 {published and unpublished data}
- Carvalho T, Bonorino KC, Panigas TF. Preoperative respiratory muscle training reduces complications in coronary artery bypass surgery. European Heart Journal. European Society of Cardiology, ESC Congress 2011 Paris France, 2011; Vol. 32 Supp:328.
Da Cunha 2013 {published and unpublished data}
- Cunha FMR, Ruas G, Fanan JMV, Crema E, Volpe MS. Effects of preoperative respiratory muscle training on early and late postoperative outcome of patients undergoing esophageal surgery. Conference abstract; 26th Annual Congress of the European Society of Intensive Care Medicine, Paris France Oct 5‐9, 2013. [Google Scholar]
Dronkers 2008 {published data only}
- Dronkers J, Veldman A, Hoberg E, Waal C, Meeteren N. Prevention of pulmonary complications after upper abdominal surgery by preoperative intensive inspiratory muscle training: a randomized controlled pilot study. Clinical Rehabilitation 2008;22(2):134‐42. [PUBMED: 18057088] [DOI] [PubMed] [Google Scholar]
Dronkers 2010 {published data only}
- Dronkers JJ, Lamberts H, Reutelingsperger IM, Naber RH, Dronkers‐Landman CM, Veldman A, et al. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clinical Rehabilitation 2010;24(7):614‐22. [PUBMED: 20530651] [DOI] [PubMed] [Google Scholar]
Ferreira 2009 {published data only}
- Ferreira PE, Rodrigues AJ, Evora PR. Effects of an inspiratory muscle rehabilitation program in the postoperative period of cardiac surgery. Arquivos Brasileiros De Cardiologia 2009;92(4):275‐82. [PUBMED: 19565135] [DOI] [PubMed] [Google Scholar]
Heynen 2012 {published and unpublished data}
- Heynen H, Jonge C, Kerkkamp H, Willms J, Sosef M. Preconditioning in patients undergoing esophagectomy: A randomized controlled pilot study. Conference: 13th World Congress of the International Society for Diseases of the Esophagus, Venice, Italy. October 15‐17, 2012; Vol. programme:36.
Hulzebos 2006a {published data only}
- Hulzebos EH, Meeteren NL, Buijs BJ, Bie RA, Brutel de la Rivière A, Helders PJ. Feasibility of preoperative inspiratory muscle training in patients undergoing coronary artery bypass surgery with a high risk of postoperative pulmonary complications: a randomized controlled pilot study. Clinical Rehabilitation 2006;20(11):949‐59. [PUBMED: 17065538] [DOI] [PubMed] [Google Scholar]
Hulzebos 2006b {published and unpublished data}
- Hulzebos EH, Helders PJ, Favié NJ, Bie RA, Brutel de la Rivière A, Meeteren NL. Fewer lung complications following inspiratory muscle training in patients undergoing coronary bypass surgery: a randomized trial. Nederlands Tijdschrift Voor Geneeskunde 2007;151(45):2505‐11. [PubMed] [Google Scholar]
- Hulzebos EH, Helders PJ, Favié NJ, Bie RA, Brutel de la Rivière A, Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high‐risk patients undergoing CABG surgery: a randomized clinical trial. Nederlands Tijdschrift Voor Fysiotherapie 2007;117(1):2‐9. [DOI] [PubMed] [Google Scholar]
- Hulzebos EH, Helders PJ, Favié NJ, Bie RA, Brutel de la Riviere A, Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high‐risk patients undergoing CABG surgery: a randomized clinical trial. JAMA 2006;296(15):1851‐7. [PUBMED: 17047215] [DOI] [PubMed] [Google Scholar]
Kulkarni 2010 {published and unpublished data}
- Kulkarni SR, Fletcher E, McConnell AK, Poskitt KR, Whyman MR. Pre‐operative inspiratory muscle training preserves postoperative inspiratory muscle strength following major abdominal surgery ‐ a randomised pilot study. Annals of the Royal College of Surgeons of England 2010;92(8):700‐7. [PUBMED: 20663275 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Soares 2013 {published data only}
- Soares SM, Nucci LB, Silva MM, Campacci TC. Pulmonary function and physical performance outcomes with preoperative physical therapy in upper abdominal surgery: a randomized controlled trial. Clinical Rehabilitation 2013;27(7):616‐27. [PUBMED: 23405020] [DOI] [PubMed] [Google Scholar]
Weiner 1998 {published data only}
- Weiner P, Zeidan F, Zamir D, Pelled B. Prophylactic inspiratory muscle training before coronary artery bypass graft. Harefuah 1995;129(7‐8):225‐8. [PubMed] [Google Scholar]
- Weiner P, Zeidan F, Zamir D, Pelled B, Waizman J, Beckerman M, et al. Prophylactic inspiratory muscle training in patients undergoing coronary artery bypass graft. World Journal of Surgery 1998;22(5):427‐31. [PUBMED: 9564282] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alper 2007 {published data only}
- Alper BS. Evidence‐based medicine. Inspiratory muscle training reduces pulmonary complications in high‐risk CABG patients. Clinical Advisor for Nurse Practitioners 2007;10(3):179. [Google Scholar]
Ambrosino 2010 {published data only}
- Ambrosino N, Gabbrielli L. Physiotherapy in the perioperative period. Clinical Anaesthesiology 2010;24(2):283‐9. [PUBMED: 20608563] [DOI] [PubMed] [Google Scholar]
Arthur 2000 {published data only}
- Arthur HM, Daniels C, McKelvie R, Hirsh J, Rush B. Effect of a preoperative intervention on preoperative and postoperative outcomes in low‐risk patients awaiting elective coronary artery bypass graft surgery. A randomized, controlled trial. Annals of Internal Medicine 2000;133(4):253‐62. [PUBMED: 10929164] [DOI] [PubMed] [Google Scholar]
Barros 2010 {published data only}
- Barros GF, Santos Cda S, Granado FB, Costa PT, Limaco RP, Gardenghi G. Respiratory muscle training in patients submitted to coronary arterial bypass graft. Revista Brasileira De Cirurgia Cardiovascular 2010;25(4):483‐90. [PUBMED: 21340377] [DOI] [PubMed] [Google Scholar]
Borghi‐Silva 2005 {published data only}
- Borghi‐Silva A, Mendes RG, Costa Fde S, Lorenzo VA, Oliveira CR, Luzzi S. The influences of positive end expiratory pressure (PEEP) associated with physiotherapy intervention in phase I cardiac rehabilitation. Clinics 2005;60(6):465‐72. [PUBMED: 16358136] [DOI] [PubMed] [Google Scholar]
Casali 2011 {published data only}
- Casali CC, Pereira AP, Martinez JA, Souza HC, Gastaldi AC. Effects of inspiratory muscle training on muscular and pulmonary function after bariatric surgery in obese patients. Obesity Surgery 2011;21(9):1389‐94. [PUBMED: 21229331] [DOI] [PubMed] [Google Scholar]
Celli 1984 {published data only}
- Celli BR, Rodriguez KS, Snider GL. A controlled trial of intermittent positive pressure breathing, incentive spirometry, and deep breathing exercises in preventing pulmonary complications after abdominal surgery. The American Review of Respiratory Disease 1984;130(1):12‐5. [PUBMED: 6377994] [DOI] [PubMed] [Google Scholar]
Christensen 1991 {published data only}
- Christensen EF, Schultz P, Jensen OV, Egebo K, Engberg M, Gron I, et al. Postoperative pulmonary complications and lung function in high‐risk patients: a comparison of three physiotherapy regimens after upper abdominal surgery in general anesthesia. Acta Anaesthesiologica Scandinavica 1991;35(2):97‐104. [PUBMED: 2024569] [DOI] [PubMed] [Google Scholar]
Chumillas 1998 {published data only}
- Chumillas S, Ponce JL, Delgado F, Viciano V, Mateu M. Prevention of postoperative pulmonary complications through respiratory rehabilitation: a controlled clinical study. Archives of Physical Medicine and Rehabilitation 1998;79(1):5‐9. [PUBMED: 9440408] [DOI] [PubMed] [Google Scholar]
Dias 2011 {published data only}
- Dias CM, Vieira Rde O, Oliveira JF, Lopes AJ, Menezes SL, Guimarães FS. Three physiotherapy protocols: effects on pulmonary volumes after cardiac surgery. Jornal Brasileiro De Pneumologia 2011;37(1):54‐60. [PUBMED: 21390432] [DOI] [PubMed] [Google Scholar]
Dull 1983 {published data only}
- Dull JL, Dull WL. Are maximal inspiratory breathing exercises or incentive spirometry better than early mobilization after cardiopulmonary bypass?. Physical Therapy 1983;63(5):655‐9. [PUBMED: 6844410] [DOI] [PubMed] [Google Scholar]
Fagevik Olsén 1997 {published data only}
- Fagevik Olsén M, Hahn I, Nordgren S, Lönroth H, Lundholm K. Randomized controlled trial of prophylactic chest physiotherapy in major abdominal surgery. The British Journal of Surgery 1997;84(11):1535‐8. [PUBMED: 9393272 ] [DOI] [PubMed] [Google Scholar]
Fagevik Olsén 1999 {published data only}
- Fagevik Olsén M, Josefson K, Lönroth H. Chest physiotherapy does not improve the outcome in laparoscopic fundoplication and vertical‐banded gastroplasty. Surgical Endoscopy 1999;13(3):260‐3. [PUBMED: 10064759] [DOI] [PubMed] [Google Scholar]
Fagevik Olsén 2002 {published data only}
- Fagevik Olsén M, Wennberg E, Johnsson E, Josefson K, Lönroth H, Lundell L. Randomized clinical study of the prevention of pulmonary complications after thoracoabdominal resection by two different breathing techniques. British Journal of Surgery 2002;89(10):1228‐34. [PUBMED: 12296888] [DOI] [PubMed] [Google Scholar]
Gastaldi 2008 {published data only}
- Gastaldi AC, Magalhaes CMB, Barauna MA, Silva EMC, Souza HCD. Benefits of postoperative respiratory kinesiotherapy following laparoscopic cholecystectomy. Revista Brasileira De Fisioterapia 2008;12(2):100‐6. [Google Scholar]
Grams 2012 {published data only}
- Grams ST, Ono LM, Noronha MA, Schivinski CIS, Paulin E. Breathing exercises in upper abdominal surgery: a systematic review and meta‐analysis. Revista Brasileira De Fisioterapia 2012;16(5):345‐53. [PUBMED: 23060237] [DOI] [PubMed] [Google Scholar]
Heisterberg 1979 {published data only}
- Heisterberg L, Johansen TS, Larsen HW, Holm M, Andersen B. Postoperative pulmonary complications in upper abdominal surgery. A randomized clinical comparison between physiotherapy and blow‐bottles. Acta Chirurgica Scandinavica 1979;145(8):505‐7. [PUBMED: 120091] [PubMed] [Google Scholar]
Herdy 2008 {published data only}
- Herdy AH, Marcchi PL, Vila A, Tavares C, Collaço J, Niebauer J, et al. Pre‐ and postoperative cardiopulmonary rehabilitation in hospitalized patients undergoing coronary artery bypass surgery: a randomized controlled trial. American Journal of Physical Medicine & Rehabilitation 2008;87(9):714‐9. [PUBMED: 18716482] [DOI] [PubMed] [Google Scholar]
Hulzebos 2012 {published data only}
- Hulzebos EH, Smit Y, Helders PP, Meeteren NL. Preoperative physical therapy for elective cardiac surgery patients. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010118.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingwersen 1993 {published data only}
- Ingwersen UM, Larsen KR, Bertelsen MT, Kiil‐Nielsen K, Laub M, Sandermann J, et al. Three different mask physiotherapy regimens for prevention of post‐operative pulmonary complications after heart and pulmonary surgery. Intensive Care Medicine 1993;19(5):294‐8. [PUBMED: 8408940] [DOI] [PubMed] [Google Scholar]
Jenkins 1989 {published data only}
- Jenkins SC, Soutar SA, Loukota JM, Johnson LC, Moxham J. Physiotherapy after coronary artery surgery: are breathing exercises necessary?. Thorax 1989;44(8):634‐9. [PUBMED: 2799743] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jenkins 1990 {published data only}
- Jenkins SC, Soutar SA, Loukota JM, Johnson LC, Moxham JA. A comparison of breathing exercises, incentive spirometry and mobilisation after coronary artery surgery. Physiotherapy Theory and Practice 1990;6(3):117‐26. [Google Scholar]
Johnson 1996 {published data only}
- Johnson D, Kelm C, Thomson D, Burbridge B, Mayers I. The effect of physical therapy on respiratory complications following cardiac valve surgery. Chest 1996;109(3):638‐44. [PUBMED: 8617070] [DOI] [PubMed] [Google Scholar]
Larsen 1994 {published data only}
- Larsen KR, Ingwersen U, Bertelsen MT, Kiil‐Nielsen K, Laub MS, Bach KS, et al. Prevention of postoperative pulmonary complications after heart‐lung surgery. Comparison of 3 different mask physiotherapy regimens. Ugeskrift for Laeger 1994;156(39):5689‐92. [PUBMED: 7985254] [PubMed] [Google Scholar]
Larsen 1995 {published data only}
- Larsen KR, Ingwersen U, Thode S, Jakobsen S. Mask physiotherapy in patients after heart surgery ‐ a controlled study. Intensive Care Medicine 1995;21(6):469‐74. [PUBMED: 7560489 ] [DOI] [PubMed] [Google Scholar]
Larsen 1997 {published data only}
- Larsen KR, Ingwersen U, Thode S, Jakobsen SH. Mask physiotherapy for prevention of pulmonary complications after heart surgery. A controlled study. Ugeskrift for Laeger 1997;159(14):2096‐9. [PUBMED: 9148534] [PubMed] [Google Scholar]
Matheus 2012 {published data only}
- Matheus GB, Dragosavac D, Trevisan P, Costa CE, Lopes MM, Ribeiro GC. Inspiratory muscle training improves tidal volume and vital capacity after CABG surgery. Revista Brasileira de Cirurgia Cardiovascular 2012;27(3):362‐9. [PUBMED: 23288176] [DOI] [PubMed] [Google Scholar]
Mendes 2006 {published data only}
- Mendes RG, Borghi‐Silva A. Efficacy of physiotherapy intervention associated to intermittent positive pressure breathing after cardiac surgery with cardiopulmonary bypass. Fisioterapia Em Movimento 2006;19(4):73‐82. [Google Scholar]
Miranda 2011 {published data only}
- Miranda RC, Padulla SA, Bortolatto CR. Respiratory physiotherapy and its application in preoperative period of cardiac surgery. Revista Brasileira de Cirurgia Cardiovascular 2011;26(4):647‐52. [PUBMED: 22358282] [DOI] [PubMed] [Google Scholar]
Morran 1983 {published data only}
- Morran CG, Finlay IG, Mathieson M, McKay AJ, Wilson N, McArdle CS. Randomized controlled trial of physiotherapy for postoperative pulmonary complications. British Journal of Anaesthesia 1983;55(11):1113‐7. [PUBMED: 6357256] [DOI] [PubMed] [Google Scholar]
Murphy 2006 {published data only}
- Murphy SA. Intensive inspiratory training and post‐CABG pulmonary complications. ACC Cardiosource Review Journal 2006;15(12):16. [Google Scholar]
Nava 2007 {published data only}
- Nava S, Culp WC Jr, Beyer EA, Takagi H, Kawai N, Umemoto T, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high‐risk patients undergoing CABG surgery: a randomized clinical trial. JAMA 2007;297(7):697‐9. [PUBMED: 17312286] 17312286 [Google Scholar]
Olsén 2012 {published data only}
- Olsén MF, Anén H. Effects of training interventions prior to thoracic or abdominal surgery: a systematic review. Physical Therapy Reviews 2012;17(2):124‐31. [Google Scholar]
Pasquina 2003 {published data only}
- Pasquina P, Tramer MR, Walder B. Prophylactic respiratory physiotherapy after cardiac surgery: systematic review. BMJ 2003;327(7428):1379‐81. [PUBMED: 14670881] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasquina 2006 {published data only}
- Pasquina P, Tramer MR, Granier JM, Walder B. Respiratory physiotherapy to prevent pulmonary complications after abdominal surgery: a systematic review. Chest 2006;130(6):1887‐99. [PUBMED: 17167013] [DOI] [PubMed] [Google Scholar]
Rajendran 1998 {published data only}
- Rajendran AJ, Pandurangi UM, Murali R, Gomathi S, Vijayan VK, Cherian KM. Pre‐operative short‐term pulmonary rehabilitation for patients of chronic obstructive pulmonary disease undergoing coronary artery bypass graft surgery. Indian Heart Journal 1998;50(5):531‐4. [PUBMED: 10052279] [PubMed] [Google Scholar]
Rideout 2012 {published data only}
- Rideout A, Lindsay G, Godwin J. Patient mortality in the 12 years following enrolment into a pre‐surgical cardiac rehabilitation programme. Clinical Rehabilitation 2012;26(7):642‐7. [PUBMED: 22172922] [DOI] [PubMed] [Google Scholar]
Roukema 1988 {published data only}
- Roukema JA, Carol EJ, Prins JG. The prevention of pulmonary complications after upper abdominal surgery in patients with noncompromised pulmonary status. Archives of Surgery 1988;123(1):30‐4. [PUBMED: 3337652] [DOI] [PubMed] [Google Scholar]
Savci 2011 {published data only}
- Savci S, Degirmenci B, Saglam M, Arikan H, Inal‐Ince D, Turan HN, et al. Short‐term effects of inspiratory muscle training in coronary artery bypass graft surgery: A randomized controlled trial. Scandinavian Cardiovascular Journal 2011;45(5):286‐93. [PUBMED: 21793631] [DOI] [PubMed] [Google Scholar]
Silva 2013 {published data only}
- Silva YR, Li SK, Rickard MJ. Does the addition of deep breathing exercises to physiotherapy‐directed early mobilisation alter patient outcomes following high‐risk open upper abdominal surgery? Cluster randomised controlled trial. Physiotherapy 2013;99(3):187‐93. [PUBMED: 23206316] [DOI] [PubMed] [Google Scholar]
Stiller 1994 {published data only}
- Stiller K, Montarello J, Wallace M, Daff M, Grant R, Jenkins S, et al. Efficacy of breathing and coughing exercises in the prevention of pulmonary complications after coronary artery surgery. Chest 1994;105(3):741‐7. [PUBMED: 8131535] [DOI] [PubMed] [Google Scholar]
Trevisan 2010 {published data only}
- Trevisan ME, Soares JC, Rondinel TZ. Effects of two respiratory incentive techniques on chest wall mobility after upper abdominal surgery. Fisioterapia e Pesquisa 2010;17(4):322‐6. [Google Scholar]
Valkenet 2011 {published data only}
- Valkenet K, Port IG, Dronkers J, Vries WR, Lindeman E, Backx FJ. The effects of preoperative exercise therapy on postoperative outcome: a systematic review. Clinical Rehabilitation 2011;25(2):99‐111. [PUBMED: 21059667] [DOI] [PubMed] [Google Scholar]
van Adrichem 2014 {published data only}
- Adrichem EJ, Meulenbroek RL, Plukker JT, Groen H, Weert E. Comparison of two preoperative inspiratory muscle training programs to prevent pulmonary complications in patients undergoing esophagectomy: a randomized controlled pilot study. Annals of Surgical Oncology 2014;21(7):2353‐60. [PUBMED: 24604584] [DOI] [PubMed] [Google Scholar]
van den Buijs 2004 {published data only}
- Buijs BJ, Hulzebos HJ, Bie RA AB, Helders PJM, Meeteren NLU. Preoperative inspiratory muscle training in patients due to undergo open‐heart surgery: a pilot study. Nederlands Tijdschrift Voor Fysiotherapie 2004;114(4):104‐9. [Google Scholar]
Walther 2010 {published data only}
- Walther C, Fiess A. Preoperative exercise training is associated with less peri‐ and postoperative adverse events but similar long term outcome in patients with stable coronary artery disease. European Journal of Cardiovascular Prevention and Rehabilitation 2010;17:S59. [Google Scholar]
References to ongoing studies
NCT01321983 {unpublished data only}
- Effects of preoperative IMT in obese women undergoing open bariatric surgery. Ongoing study Not provided.
NCT01828632 {unpublished data only}
- Effects of preoperative respiratory physical therapy on postoperative respiratory function after bariatric surgery. Ongoing study March 2012.
Additional references
Arozullah 2001
- Arozullah AM, Khuri SF, Henderson WG, Daley J. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Annals of Internal Medicine 2001;135(10):847‐57. [DOI] [PubMed] [Google Scholar]
Brooks‐Brunn 1995
- Brooks‐Brunn JA. Postoperative atelectasis and pneumonia: risk factors. American Journal of Critical Care 1995;4:340‐9. [PUBMED: 7489036] [PubMed] [Google Scholar]
do Nascimento 2014
- do Nascimento Junior P, Módolo NSP, Andrade S, Guimarães MMF, Braz LG, Dib R. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD006058; PUBMED: 24510642] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis is detected by a simple graphical test. BMJ 1997;31(5):629–34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleischmann 2003
- Fleischmann KE, Goldman L, Young B, Lee TH. Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. The American Journal of Medicine 2003;115:515‐20. [PUBMED: 14599629] [DOI] [PubMed] [Google Scholar]
Furukawa 2002
- Furukawa TA, Guyatt GH, Griffith LE. Can we individualize the 'number needed to treat'? An empirical study of summary effect measures in meta‐analyses. International Journal of Epidemiology 2002;31(1):72‐6. [PUBMED: 11914297] [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. [PUBMED: 16360555] [DOI] [PubMed] [Google Scholar]
Guimarães 2009
- Guimarães MM, Dib R, Smith AF, Matos D. Incentive spirometry for prevention of postoperative pulmonary complications in upper abdominal surgery. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006058.pub2] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, GRADE Working Group. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336:995‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horan 1992
- Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. American Journal of Infection Control 1992;20:271‐4. [PUBMED: 1332552] [DOI] [PubMed] [Google Scholar]
Kaasa 1995
- Kaasa S, Bjordal K, Aaronson N, Moum T, Wist E, Hagen S, et al. The EORTC core quality of life questionnaire (QLQ‐C30): validity and reliability when analysed with patients treated with palliative radiotherapy. European Journal of Cancer 1995;31A:2260‐3. [DOI] [PubMed] [Google Scholar]
Kroenke 1992
- Kroenke K, Lawrence VA, Theroux JF, Tuley MR. Operative risk in patients with severe obstructive pulmonary disease. Archives of Internal Medicine 1992;152:967–71. [PUBMED: 1580723] [PubMed] [Google Scholar]
Lawrence 2006
- Lawrence VA, Cornell JE, Smetana GW, American College of Physicians. Strategies to reduce postoperative pulmonary complications after noncardiothoracic surgery: systematic review for the American College of Physicians. Annals of Internal Medicine 2006;144:596‐608. [PUBMED: 16618957] [DOI] [PubMed] [Google Scholar]
Mendez‐Tellez 2008
- Mendez‐Tellez PA, Dorman T, Cameron JL (editor). Current Surgical Therapy. 9th Edition. Philadelphia: Mosby, 2008. [Google Scholar]
Nomori 1994
- Nomori H, Kobayashi R, Fuyuno G, Morinaga S, Yashima H. Preoperative respiratory muscle training. Assessment in thoracic surgery patients with special reference to postoperative pulmonary complications. Chest 1994;105:1782‐8. [PUBMED: 8205877] [DOI] [PubMed] [Google Scholar]
Overend 2001
- Overend TJ, Anderson CM, Lucy SD, Bhatia C, Jonsson BI, Timmermans C. The effect of incentive spirometry on postoperative pulmonary complications: a systematic review. Chest 2001;120:971‐8. [PUBMED: 11555536] [DOI] [PubMed] [Google Scholar]
Parsons 1974
- Parsons HM. What happened at Hawthorne?: New evidence suggests the Hawthorne effect resulted from operant reinforcement contingencies. Science 1974;183(4128):922‐32. [PUBMED: 17756742] [DOI] [PubMed] [Google Scholar]
Pereira 2012
- Pereira TV, Horwitz RI, Ioannidis JP. Empirical evaluation of very large treatment effects of medical interventions. JAMA 2012;308(16):1676‐84. [PUBMED: 23093165] [DOI] [PubMed] [Google Scholar]
Qaseem 2006
- Qaseem A, Snow V, Fitterman N, Hornbake ER, Lawrence VA, Smetana GW, et al. Risk assessment for and strategies to reduce perioperative pulmonary complications for patients undergoing noncardiothoracic surgery: a guideline from the American College of Physicians. Annals of Internal Medicine 2006;144(8):575‐80. [PUBMED: 16618955] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roach 1996
- Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. The New England Journal of Medicine 1996;335:1857‐63. [PUBMED: 8948560] [DOI] [PubMed] [Google Scholar]
Shander 2011
- Shander A, Fleisher LA, Barie PS, Bigatello LM, Sladen RN, Watson CB. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk‐reducing interventions, and preventive strategies. Critical Care Medicine 2011;39:2163‐72. [PUBMED: 21572323] [DOI] [PubMed] [Google Scholar]
Smetana 2009
- Smetana GW. Postoperative pulmonary complications: an update on risk assessment and reduction. Cleveland Clinic Journal of Medicine 2009;76(Suppl 4):S60‐5. [PUBMED: 19880838] [DOI] [PubMed] [Google Scholar]
Stata 11 [Computer program]
- StataCorp. Stata Statistical Software. Version Release 11. College Station, Texas: StataCorp, 2009.
Thomas 1994
- Thomas JA, McIntosh JM. Are incentive spirometry, intermittent positive pressure breathing, and deep breathing exercises effective in the prevention of postoperative pulmonary complications after upper abdominal surgery? A systematic overview and meta‐analysis. Physical Therapy 1994;74:3‐10. [PUBMED: 8265725] [DOI] [PubMed] [Google Scholar]
Wijeysundera 2009
- Wijeysundera DN, Bender JS, Beattie WS. Alpha‐2 adrenergic agonists for the prevention of cardiac complications among patients undergoing surgery. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004126.pub2; PUBMED: 19821319] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Katsura 2013
- Katsura M, Kuriyama A, Takeshima T, Fukuhara S, Furukawa TA. Preoperative inspiratory muscle training for postoperative pulmonary complications in adult patients undergoing cardiac and major abdominal surgery. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD010356] [DOI] [PMC free article] [PubMed] [Google Scholar]


