Abstract
Background
Inflammation is a common feature of many kidney diseases. The implicated inflammatory mediators and their underlying molecular mechanisms however are often not clear.
Summary
suPAR is the soluble form of urokinase-type plasminogen activator receptor (uPAR), associated with inflammation and immune activation. It has evolved into a unique circulating kidney disease factor over the last 10 years. In particular, suPAR has multiple looks due to enzymatic cleavage and alternative transcriptional splicing of the uPAR gene. Most recently, suPAR has emerged as a systemic mediator for COVID-19 infection, associated with lung as well as kidney dysfunction. Like membrane-bound uPAR, suPAR could interact with integrins (e.g., αvβ3 integrin) on podocytes, providing the molecular basis for some glomerular kidney diseases. In addition, there have been numerous studies suggesting that suPAR connects acute kidney injury to chronic kidney disease as a special kidney risk factor. Moreover, the implication of circulating suPAR levels in kidney transplantation and plasmapheresis not only indicates its relevance in monitoring for recurrence but also implies suPAR as a possible therapeutic target. In fact, the therapeutic concept of manipulating suPAR function has been evidenced in several kidney disease experimental models.
Key Messages
The last 10 years of research has established suPAR as a unique inflammatory mediator for kidneys. While open questions remain and deserve additional studies, modulating suPAR function may represent a promising novel therapeutic strategy for kidney disease.
Keywords: Soluble urokinase-type plasminogen activator receptor, Kidney, Integrin, COVID-19, Biomarker, Therapeutics
Introduction
Over the last decade, the concept of kidney inflammation by circulating mediators has centered on soluble urokinase-type plasminogen activator receptor (suPAR), an innate immune effector molecule. Manipulating suPAR function has therapeutic implications in kidney disease. While suPAR's role as a circulating kidney disease factor is established, there are still some open questions that warrant further studies. In this review, we will discuss these intriguing topics including the origin, biology, and function of suPAR; its role in initiating or aggravating kidney disease under various pathological conditions such as the current severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic; and lastly, the diagnostic and therapeutic modalities centered around suPAR. The current review is intended to provide some insights guiding present and future work on this fascinating molecule.
uPAR/suPAR Biology and Function
Urokinase-type plasminogen activator receptor (uPAR), also known as CD87, is a heavily glycosylated membrane-bound receptor, devoid of any transmembrane and intracellular domains, lipid-anchored on the surface of multiple cell types such as immune cells and vascular endothelial cells [1]. uPAR harbors three consecutive LY6/uPAR (LU) repeats, namely DI, DII, and DIII (as numbered from the N-terminus), with a linker sequence between DI and DII in particular [2]. The receptor is fettered to the lipid bilayer of the cell membrane by a C-terminal glycosyl-phosphatidylinositol (GPI) anchor. While the N-terminal DI domain allows the binding of uPAR to its cognate ligand, urokinase-type plasminogen activator (uPA), the three-domain intact uPAR is required for high-affinity binding [3]. Interestingly, the full-length uPAR not only can bind to uPA but also to other moieties like vitronectin. The receptor-bound uPA catalyzes the conversion of plasminogen precursor into activated plasmin, a protease that mediates degradation of extracellular matrix, facilitating cell adhesion, migration, invasion, and tissue remodeling [4].
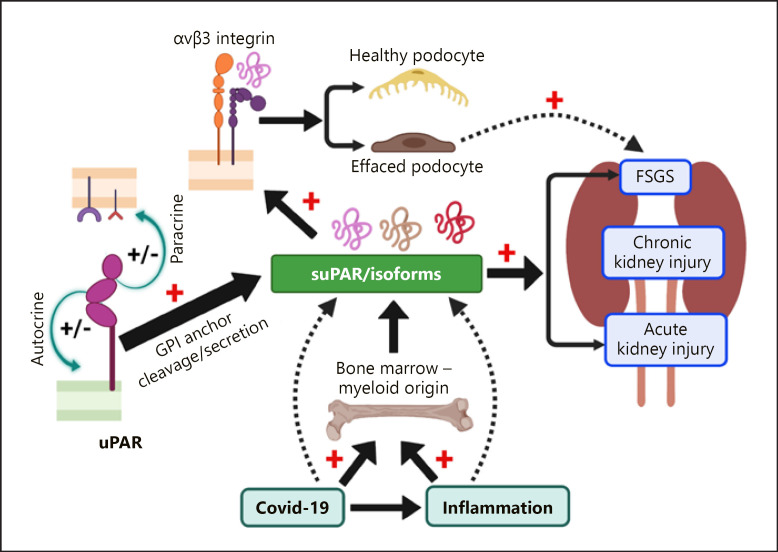
uPAR is released as a soluble multidomain signaling molecule, so called suPAR [5, 6] upon cleavage of the GPI anchor by a GPI-specific phospholipase C [7], or cathepsin G [8]. Further, the linker region between the DI and DII domain in both suPAR and membrane-bound uPAR is highly susceptible to proteolytic cleavage by enzymes like plasmin, chymotrypsin, elastase, several matrix metalloproteases, or uPA [5]. Thus, different suPAR fragments generated by enzymatic cleavage (suPAR DI-DII-DIII, suPAR DII-D3, suPAR DI) are detected in circulation or other bodily fluids [9, 10, 11]. These properties make uPAR/suPAR a multifaceted molecule. Although the DII-DIII fragment is incompetent to bind uPA, vitronectin, and most other ligands except formyl peptide receptor-like 1 and 2 (FPRL 1 and 2) [12], it still confers biological activity owing to its chemotactic properties [13, 14]. We have initially defined a role of podocyte uPAR in mediating kidney filtration barrier function [15], followed by many studies delineating the implication of suPAR as a circulating factor in kidney disease [16]. While circulating suPAR acts systemically on the kidney via a multitude of receptors, kidney cell-expressed uPAR likely participates in autologous cell signaling (Fig. 1).
Fig. 1.
suPAR and the kidney − an evolving story. Membrane-bound uPAR (e.g., podocyte uPAR) could mediate cell signaling in an “autocrine” or “paracrine” manner. uPAR has multiple isoforms due to alternative transcription. Cleavage of the GPI anchor from the cell membrane or direct secretion from cells generates suPAR that circulates in blood. Infection and/or inflammation such as COVID-19 promotes suPAR production from bone marrow myeloid cells. High circulating suPAR levels are associated with both AKI and CKD as a kidney disease factor. In certain circumstance, suPAR alone (i.e., dimerized form) or together with other risk factors (such as CD40 autoantibody, or APOL1 risk variants) causes FSGS-like changes.
uPAR Exists in Multiple Isoforms
uPAR, encoded canonically by seven exons, undergoes alternative splicing to generate isoforms with distinct characteristics and localization. Unlike the mouse uPAR (muPAR) that has two isoforms [17], the human uPAR (huPAR) exists in at least four major isoforms [18]. Identification of a full-length canonical form (muPAR1) of the muPAR in the luminal epithelial cells and a shorter secreted form (muPAR2) in the basal epithelial cells of the gastrointestinal tract provided the first evidence for the existence of uPAR isoforms [17]. While muPAR1 contains 3 intact domains (DI, DII, and DIII) with 7 predicted sites of glycosylation, muPAR2 possess only a complete DI (encoded by exons 2 and 3) and a partial DII domain (encoded by exon 4). The muPAR2, lacking a GPI anchor, represents the secretory version of the protein, without DIII, and a part of DII (encoded by exons 5–7) also missing [19].
Analogous to the canonical muPAR1, the human isoform 1 (huPAR1) contains three intact domains, five N-linked glycosylation sites, and the GPI anchor [20]. Similarly, the human isoform 2 (huPAR2) is the equivalent of muPAR2 in that it lacks the GPI anchor and exon 7, which encodes the C-terminal portion of DIII. Consequently, huPAR2 would likely generate the secretory form of huPAR. The human isoform 3 (huPAR3) is characterized by deletion of exon 5, which results in the truncation of DII at its C-terminus. Lastly, an in-frame deletion of exon 6 generates the human isoform 4 (huPAR4). Although this deletion retains the C-terminal of DIII and the GPI anchor, a portion of N-terminus in the DIII is missing.
Despite the availability of detailed information on uPAR isoforms, an outstanding question still remains, “what is the implication of these different uPAR isoforms in human kidney disease”? Expression of muPAR2 in mice via electroporation or use of muPAR2 transgenic mice induced proteinuric kidney disease that resembled FSGS [16, 21]. Since huPAR3 resembles closely with the muPAR2 in its protein structure [21], we can speculate some similarity in its function. With some promising data indicating the presence of different huPAR isoforms, it will be exciting and important to demonstrate if huPAR isoforms are pathogenic for human kidney diseases. For example, would huPAR3 cause FSGS?
suPAR and Integrins: When and Where
suPAR/uPAR has multiple ligands and coreceptors [4]. We specifically focus on integrins in this review as activation of integrins on diverse kidney cells under different pathological conditions is known to play an important role in the progression of kidney diseases [22]. Integrins, heterodimers of noncovalently associated α and β subunits, each of which is a single-pass type I transmembrane protein, are a major family of cell surface adhesion receptors expressed essentially in all tissues. Most integrins contain large extracellular but short cytoplasmic domains. Integrins can perform bidirectional signaling. Signals from inside the cell activate the binding of integrin to extracellular ligands (inside-out signaling), which in turn triggers intracellular signaling (outside-in signaling). Obviously, integrins are implicated in renal pathobiology which is dependent on both integrin expression and level of activation. Studies have revealed that different integrins are expressed differentially on various renal cell types like tubular epithelial cells (TECs) [23], fibroblasts [24], and podocytes [25]. TECs, under normal conditions, express mostly αv and β1 integrins but switch to αvβ6 subtype under tubular injury [26]. Fibroblasts normally express integrin α1, α4, α5, and β1 which turn into integrin α5, β1, and αv as dominant subtypes under fibrotic conditions [24, 27]. For podocytes, one of the best characterized integrin dimers is α3β1, knockout of which causes the absence of foot process (FP) formation, and downregulation leads to FP effacement in the adult kidney [28]. Another major integrin that is expressed on the surface of podocytes is αvβ3, albeit its activity level is low under normal conditions. In consistent with a low baseline activation, depleting αvβ3 did not cause an overt renal phenotype, yet its absence mitigated its activation conferring protection from LPS-mediated podocyte FP effacement [15].
Most integrins exhibit an ability to bind a wide range of ligands [29]. Many studies have unequivocally demonstrated that integrins can bind with noncanonical molecules to orchestrate downstream signaling pathways [30]. For example, some integrins bind and activate TGF-β to elicit Smad2/3 signaling, which promotes interstitial fibrosis and suppress TEC proliferation [31]. There are also many studies suggesting that uPAR/suPAR works as a ligand for integrins. An intriguing question that had always befuddled researchers was how uPAR, a molecule without typical transmembrane structure, can mediate cellular signaling? Initially, it was believed that there must be some “transmembrane adapter molecules” which connect the extracellular uPAR with the intracellular signaling molecules to transduce the signaling cascade [32]. However, it has become apparent that uPAR can form dynamic signaling complexes on the cell surface that may include integrins [33], epidermal growth factor receptor [34], platelet-derived growth factor receptor [35], the internalizing receptor lipoprotein receptor-related protein [36], caveolin [37], vitronectin [38], and potentially other molecules as well. Specifically, uPAR has been shown to interact with β1, β2, and β3 families of integrin receptor [39, 40, 41]. An interesting study by Tarui et al. [42] demonstrated that the GPI-anchored uPAR specifically bound to integrins on the opposing cells; thus, uPAR-integrin engagement could facilitate a trans cell-cell interaction. A seminal study from our group unveiled the ability of suPAR to initiate signaling by binding to αvβ3 integrin on the podocyte surface, contributing to FSGS [16]. In 2015, Alfano et al. [43] demonstrated that interaction of full-length suPAR with αvβ3 integrin on podocytes resulted in down-modulation of nephrin and WT-1, which potentially could explain the cause for kidney dysfunction in pathologies associated with increased suPAR concentration. Furthermore, we identified high-affinity interactions between suPAR, apolipoprotein L1 (APOL1), and αvβ3 integrin, whereby APOL1 G1 and G2 protein variants exhibited higher affinity for suPAR-activated αvβ3 integrin than the APOL1 reference G0 protein, demonstrating an example of risk factor aggravation in kidney disease [25]. In addition, suPAR has been shown to bind to TECs through integrin β6 under injured conditions leading to Rac1 activation followed by an onset of CD44/Smad3 signaling, culminating in interstitial fibrosis [44]. When and where suPAR interacts with which integrin(s) in different disease setting will be a fascinating area for future studies.
suPAR Connects Acute Kidney Injury to Chronic Kidney Disease
Chronic Kidney Disease
Glomerular disease is often characterized by podocyte dysfunction, injury, or loss, which in turn is attributed to many factors [45]. Circulating systemic factors, intraglomerular mediators, mediators within the podocyte itself, or a combination of all these can impose structural changes on podocyte's FPs [46]. Upon prolonged and continual injury such as in FSGS, podocyte loss drives disease progression and deterioration into chronic kidney disease (CKD) and eventually end-stage renal disease. Although podocyte gene defects are a known cause of some FSGS in humans [47], occurrence of FSGS even in the absence of gene defects or recurrence of proteinuria within hours or days after renal transplantation have incited the researchers to believe involvement of certain causative circulating factor, which was popularly known as “FSGS permeability factor.” This belief was further strengthened by evidence demonstrating recurrence of FSGS after transplantation [48]. In particular, an important study led by Dr. Savin showed that sera from FSGS patients could cause proteinuria in rats [49]. The proposed circulating factor was expected to be smaller in size than albumin and removable by plasmapheresis [50] or immunoadsorption [51]. Another supporting evidence that provided further credence to the “permeability factor” theory was the case report of transient nephrotic syndrome in a newborn whose mother had FSGS, indicating the transmissibility of the glomerular permeability factor [52]. In 2011, we identified suPAR as a causative permeability factor [16]. Our studies demonstrate that in proteinuric kidney diseases, especially in primary and recurrent FSGS, suPAR concentrations in the plasma are elevated; increase of circulating suPAR levels cause FSGS-like disease in mouse models [16]. Bone marrow-derived immature myeloid cells were found to be a main cellular source of circulating suPAR contributing to the proteinuric kidney disease [53]. A recent study documents activated neutrophils as source of circulating suPAR during systemic inflammation [54]. Notably, suPAR has also been shown to interact with other molecules to synergistically induce podocyte damage and mediate progression to CKD in different disease settings. For instance, CD40 autoantibodies augmented suPAR-mediated effects in FSGS [55]; and levels of acid sphingomyelinase-like phosphodiesterase 3b modulated the effect of suPAR in diabetic nephropathy (DN) [56]. Elevated suPAR levels have been associated with DN in other studies as well [57]. Having said that, it is noteworthy to mention that although many elegant studies demonstrate the causative role for suPAR in the incidence of FSGS, there have been controversies around this issue as some clinical reports could not ascertain the relationship between the circulating suPAR levels and FSGS [58, 59, 60, 61, 62, 63, 64]. For instance, a recent study by Sun et al. [65] reported that the plasma and urinary suPAR levels did not correlate with any of their clinical and pathological parameters like albumin, serum creatinine, eGFR, urine total protein, C-reactive protein (CRP), and glomerular global sclerosis or segmental sclerosis.
Obviously, the mechanisms that underlie the involvement of suPAR in each different kidney disease entity need further investigation. The question regarding the circulating suPAR levels and kidney function however has prompted a series of studies assessing suPAR as a biomarker for kidney disease. To evaluate the utility of suPAR as a potential biomarker for CKD, we investigated the relationship between baseline suPAR levels and decline in eGFR over time in a large and heterogenous cohort study of patients with chest pain [66]. We found that participants who were in the two higher quartiles of suPAR levels (≥3,040 pg/mL) had a significantly greater decline in the eGFR in comparison to those in the two lower quartiles (<3,040 pg/mL). Moreover, over a period of 5 years, the decline in the eGFR was 7.3% in the two lower quartiles, as compared with 14.5% in the third quartile and 20.4% in the fourth quartile. Congruent to the eGFR decline, the incident rate of CKD was found to be 7% at 1 year and 41% at 5 years in participants with a suPAR level of ≥3,040 ng/mL (third and fourth quartiles), as compared with 1% and 12%, respectively, among participants with a suPAR level of <3,040 ng/mL (first and second quartiles) [66]. These data clearly indicate an association between high circulating suPAR levels and both a decline in the eGFR and the development of CKD. This association between circulating suPAR levels and declining kidney function was observed in patients with normal baseline kidney function as well and was independent of conventional risk factors for kidney and cardiovascular disease. Besides, circulating suPAR levels have been shown to have independent association with an increased risk of progression to end-stage renal disease in Chinese [67] and African American [68] CKD patients. In addition to adult patients, suPAR as an independent risk factor for CKD progression has also been demonstrated in pediatric cohorts [69, 70, 71]. With certain concerns of renal retention, many studies have analyzed the correlation of suPAR to eGFR [72]. It turns out that suPAR is not correlated to eGFR in people with eGFR above 90 mL/min/1.73 m2. In lower eGFR ranges, suPAR shows a weak correlation to eGFR but still not enough to attribute any major part of suPAR rise in circulation to a simple renal filtration decrease-incurred suPAR accumulation rather than its increased production. In consistent with this finding, Ngo et al. [73] showed that renal clearance of suPAR is very low when measuring suPAR concentration in renal artery and renal vein.
Acute Kidney Disease
Acute kidney injury (AKI) is characterized by an abrupt or rapid decline in kidney function, encompassing both structural damage and dysregulation of excretory functions but without a sole distinct pathophysiology as with CKD. Hayek et al. [26] recently showed that suPAR was associated with AKI across three cohorts in different clinical contexts (patients who were exposed to intra-arterial contrast material for coronary angiography, who underwent cardiac surgery, or who were critically ill and admitted to the ICU). Mechanistically, suPAR sensitizes the kidney proximal tubules to injury through modulation of cellular bioenergetics and increased oxidative stress, suggesting a causative role for suPAR in AKI as well [26]. Similar association between the development of AKI and suPAR levels was documented in cardiac surgery patients by Mossanen et al. [74]. In other independent studies, suPAR has been proposed to be a better marker of infection than CRP in critically ill patients with AKI stage 2/3 [75], or an applicable marker in predicting AKI among older patients (≥65 years) in the emergency department. In particular, a recent study suggests suPAR along with neutrophil gelatinase-associated lipocalin, a protein which is produced in the kidney after ischemic or nephrotoxic injury, as a biomarker for early detection of AKI [72, 76, 77]. The results overall demonstrated that suPAR and neutrophil gelatinase-associated lipocalin levels were independently associated with incident AKI and its severity, but their combination yielded improved discriminatory power for risk determination of AKI [78].
suPAR Predicts AKI and Disease Severity in COVID-19 Patients
COVID-19, caused by the SARS-CoV-2, has emerged into a global pandemic, upending millions of lives as well as damaging the economy. The disease can progress unpredictably with patients suddenly deteriorating into multi-organ failure including severe respiratory failure, AKI, and death [79]. Thus, identification of biomarkers for disease progression and timely onset of targeted therapies are of paramount importance [80]. Understanding of the viral physiology and host response has uncovered a gamut of potential biomarkers which are used as the indicators of either pathological processes or pharmacological responses to the therapeutic intervention. Examples include hematological (neutrophil-to-lymphocyte ratio, neutrophil-to-monocyte ratio, lymphopenia, neutrophilia), inflammatory (cytokines: IL-1β, IL-2, IL-8, IL-17, G-CSF, GMCSF, IP-10, MCP-1, CCL3, and TNFα; chemokines, growth factors, CRP, procalcitonin, lactate dehydrogenase), coagulation (D-dimer, fibrinogen, fibrin degradation products), and biochemical (aspartate aminotransferase, alanine aminotransferase, bilirubin, albumin, ferritin, muscle creatinine kinase, myoglobin, cardiac troponin, brain natriuretic peptide) markers [81, 82, 83, 84, 85, 86, 87, 88, 89]. However, since the pandemic is evolving with the emergence of new strains that results in varying disease severity and symptoms, discovering “the best” biomarkers for COVID-19 could provide not only convincing but also objective information to the clinicians in: (a) predicting the severity and progression of the disease, (b) monitoring and recognition of complications, (c) management and disposition of patients, (d) identification and classification of high-risk cohorts, (e) predicting and improving the prognosis, and (f) rationalizing the therapies and assessing the subsequent response.
suPAR has been shown to be dramatically elevated in patients with severe COVID-19 [90] and stands out as a predictor of overall disease severity and outcome [91, 92, 93, 94] including severe respiratory failure [95] and AKI [96]. In SARS-CoV-2-infected individuals with low levels of suPAR (<4 ng/mL) upon admission, the risk of needing mechanical ventilation and the 14-day mortality was small, while levels between 4 and 6 ng/mL and especially >6 ng/mL were associated with a significantly increased risk [97]. Recently, a study reported the ability of suPAR in independently predicting the severity of COVID-19 disease, the length of hospital stays along with the need for supplemental oxygen therapy for these patients [98]. These studies demonstrate that suPAR might function as a “crystal ball” in predicting the host response to COVID-19 infection. Another particularly interesting study called suPAR-guided Anakinra treatment for Validation of the risk and Early Management Of seveRE respiratory failure by COVID-19 (SAVE-MORE) was a phase 3, double-blind randomized controlled trial that evaluated the efficacy and safety of early initiation of anakinra treatment (an IL-1α/β inhibitor) in hospitalized patients with moderate or severe COVID-19. This trial evaluated a novel approach for the management of COVID-19, which relied on early identification of patients at risk for unfavorable outcome using suPAR as the parameter [99]. Taken together, all these studies indicate the implication of suPAR in COVID-19-incurred infection. However, is suPAR merely a biomarker or a causative factor as well awaits further studies. For example, as we know that suPAR could be induced as a result of immune cell activation, would elevated suPAR then trigger further organ damage? Would an elevated baseline suPAR level facilitate or aggravate the SARS-CoV-2 virus-induced infection? Would suPAR and other cytokines rather than SARS-CoV-2 virus itself be the culprit for extrapulmonary organ damage?
suPAR and Its Implications in Transplantation
While the prognostic relevance of suPAR has been recognized in various kidney diseases, its role in transplantation-specific outcomes is mounting. We initially observed that higher levels of suPAR before transplantation are associated with an increased risk of recurrence of FSGS in the allograft [16]. Jehn et al. [100] recently investigated the prognostic significance of suPAR in a cohort of 100 patients, before and 1 year after kidney transplantation. They revealed a strong correlation between suPAR levels at 1-year mark post-transplantation and eGFR loss: suPAR levels above 6,212 pg/mL were associated with an accelerated eGFR loss of >30%, which is almost twice as fast as in patients with suPAR ≤6,212 pg/mL [100]. Interestingly in another study with post-transplantation-recurrent FSGS patients, reduction in the suPAR levels has been identified as a biomarker to gauge the success and outcome of therapeutic plasma exchange (TPE) in combination with rituximab. TPE caused a significant reduction in serum suPAR levels with a concomitant decrease in proteinuria and suPAR-induced podocyte αvβ3 integrin activity. Considering variables analyzed including eGFR, baseline serum creatinine, age at diagnosis and transplantation, TPE course numbers, notably, only a reduction in suPAR stood out as the strongest predictor for proteinuria and response to therapy [101]. Discrepancy however was reported in a pilot trial where high suPAR levels were not indicative of severity in patients with kidney transplant and infectious complications [102]. Similarly, another study demonstrated a significant decrease of suPAR levels post-transplantation, but no correlation of suPAR levels and transplanted graft function could be confirmed or established [103]. Thus, considering confounding factors like demographic variation within the study cohort, small sample size when working with human subjects, and different detection methods to assess suPAR levels, in particular ELISA-based assays versus proteomic assays, can lead to conflicting results [104]. The growing importance for suPAR in kidney transplant patients has just been demonstrated once again. Morath et al. [105] showed that suPAR levels in 1,023 kidney transplant patients (measured at transplantation or 1 year after) predicted cardiac death.
Modulation of suPAR Levels and Its Function Is a Therapeutic Approach
Discovered more than three decades ago, the pleiotropic uPAR has been firmly established as a promising and versatile molecular target for the treatment of inflammatory diseases and several malignancies [106]. Robust expression of uPAR in many human cancerous tissues versus sparse expression in their healthy and quiescent counterparts renders uPAR as an attractive target for cancer therapeutics [9]. To impair and eradicate uPAR-expressing cells selectively, approaches developed to date have focused on neutralizing uPAR function, primarily by interfering with its gene expression using antisense RNA or oligonucleotides [107, 108] or interaction with its ligand uPA [109]. One of the initial challenges faced by the researchers in developing therapeutics that target the binding of uPA to uPAR was the stringent species specificity of the uPA-uPAR interaction [110]. Mouse uPA would bind to huPAR very poorly and vice versa, which posed a great obstacle to test the efficacy of any antagonists in mouse xenograft tumor models. However, when uPAR was later found to interact with many different ligands in addition to uPA, abrogation of uPA-uPAR interaction was accomplished by using anti-uPAR monoclonal antibodies [111, 112], uPA-derived peptides like UPARANT [113, 114, 115] or small molecules [116, 117], and the amino-terminal fragment of uPA (which contains the receptor-binding domain) with decent to moderate success in combating cancer [118].
In the context of kidney disease, the therapeutic implication of suPAR modulation has already come to light. Many studies from us and others have demonstrated the encouraging effect of functional blocking of suPAR by uPAR antibodies in different kidney disease animal models. For example, we showed that administration of blocking antibody could ameliorate suPAR-caused kidney damage in mice [16]. Dal Monte et al. [119] reported the therapeutic effect of small peptide uPARANT in STZ-induced DN in rats. More recently, our group demonstrated that pretreatment with a uPAR monoclonal antibody attenuated contrast-induced kidney injury in suPAR-overexpressing mice [26]. Clinically, the effect of modulating circulating suPAR levels has been shown with patients receiving plasmapheresis and/or immunoadsorption treatment, making it an effective therapy for some transplant FSGS patients [16, 91, 112, 113]. Imminent trial from Miltenyi Biotec utilizing uPAR antibody-coated columns seems promising. In the settings where immunoadsorption or plasmapheresis is applied, a suPAR antibody-coated column should remove excess suPAR from plasma and provide much advantage over general immunoadsorption or plasmapheresis. An injectable suPAR-neutralizing antibody would be even more preferable especially for a broader range of patients without any needs for plasmapheresis or immunoadsorption.
Since uPAR/suPAR signals through podocyte αvβ3 integrin and thus mediates downstream cellular injury in glomerular kidney disease, modulating αvβ3 integrin activity could possibly represent another therapeutic avenue [14, 15]. While lack of efficacy and/or side effects observed with small-molecule and/or antibody inhibitors of αv integrins, including MK-0429, cilengitide (EMD121974), and vitaxin (LM609), have prevented them for potential use in cancer treatment, researchers have not given up testing integrin inhibitors in kidney disease. In line with this, Janssen/Vascular Therapeutics in 2017 has also developed an antibody, VPI-2690B that blocks αvβ3 signaling for the purpose of treating DN [120], but the results have not been disclosed.
In conclusion, we have witnessed very exciting progress in our understanding of suPAR's multifaceted roles in kidney disease, even though open questions remain. Further suPAR studies will not only shed more light on its role in kidney disease but also bring about suPAR modifying therapeutics which may prove valuable to many patients suffering from kidney disease.
Conflict of Interest Statement
J.R. reports personal fees from Biomarin, Visterra, Astellas, Genentech, Merck, Gerson Lehrman Group, and Massachusetts General Hospital. He is the recipient of grants from Nephcure Kidney International and Thermo BCT. J.R.'s lab is the recipient of fee-for-service funds from Walden Biosciences. J.R. is cofounder, scientific advisory board co-chair, and shareholder of Walden Biosciences, a kidney therapeutic company. Other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Sources
This work was supported by RO1DK125858, RO1DK109720, R01DK113761 to J.R. and C.W.
Author Contributions
Yashwanth Reddy Sudhini wrote the first draft of the manuscript. Changli Wei and Jochen Reiser edited the manuscript. All the authors contributed to the article and approved the final submitted version.
References
- 1.Thuno M, Macho B, Eugen-Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27((3)):157–72. doi: 10.3233/DMA-2009-0657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fowler B, Mackman N, Parmer RJ, Miles LA. Binding of human single chain urokinase to Chinese Hamster Ovary cells and cloning of hamster u-PAR. Thromb Haemost. 1998;80((1)):148–54. [PubMed] [Google Scholar]
- 3.Behrendt N, Ronne E, Dano K. Domain interplay in the urokinase receptor. Requirement for the third domain in high affinity ligand binding and demonstration of ligand contact sites in distinct receptor domains. J Biol Chem. 1996;271((37)):22885–94. doi: 10.1074/jbc.271.37.22885. [DOI] [PubMed] [Google Scholar]
- 4.Blasi F, Carmeliet P. uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol. 2002;3((12)):932–43. doi: 10.1038/nrm977. [DOI] [PubMed] [Google Scholar]
- 5.Montuori N, Visconte V, Rossi G, Ragno P. Soluble and cleaved forms of the urokinase-receptor: degradation products or active molecules? Thromb Haemost. 2005;93((2)):192–8. doi: 10.1160/TH04-09-0580. [DOI] [PubMed] [Google Scholar]
- 6.Ronne E, Pappot H, Grondahl-Hansen J, Hoyer-Hansen G, Plesner T, Hansen NE, et al. The receptor for urokinase plasminogen activator is present in plasma from healthy donors and elevated in patients with paroxysmal nocturnal haemoglobinuria. Br J Haematol. 1995;89((3)):576–81. doi: 10.1111/j.1365-2141.1995.tb08366.x. [DOI] [PubMed] [Google Scholar]
- 7.van Veen M, Matas-Rico E, van de Wetering K, Leyton-Puig D, Kedziora KM, De Lorenzi V, et al. Negative regulation of urokinase receptor activity by a GPI-specific phospholipase C in breast cancer cells. Elife. 2017;6:e23649. doi: 10.7554/eLife.23649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ploug M, Rønne E, Behrendt N, Jensen AL, Blasi F, Danø K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem. 1991;266((3)):1926–33. [PubMed] [Google Scholar]
- 9.Mahmood N, Mihalcioiu C, Rabbani SA. Multifaceted role of the urokinase-type plasminogen activator (uPA) and its receptor (uPAR): diagnostic, prognostic, and therapeutic applications. Front Oncol. 2018;8:24. doi: 10.3389/fonc.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sidenius N, Sier CF, Blasi F. Shedding and cleavage of the urokinase receptor (uPAR): identification and characterisation of uPAR fragments in vitro and in vivo. FEBS Lett. 2000;475((1)):52–6. doi: 10.1016/s0014-5793(00)01624-0. [DOI] [PubMed] [Google Scholar]
- 11.Mustjoki S, Sidenius N, Sier CF, Blasi F, Elonen E, Alitalo R, et al. Soluble urokinase receptor levels correlate with number of circulating tumor cells in acute myeloid leukemia and decrease rapidly during chemotherapy. Cancer Res. 2000;60((24)):7126–32. [PubMed] [Google Scholar]
- 12.Iribarren P, Zhou Y, Hu J, Le Y, Wang JM. Role of formyl peptide receptor-like 1 (FPRL1/FPR2) in mononuclear phagocyte responses in Alzheimer disease. Immunol Res. 2005;31((3)):165–76. doi: 10.1385/IR:31:3:165. [DOI] [PubMed] [Google Scholar]
- 13.Fazioli F, Resnati M, Sidenius N, Higashimoto Y, Appella E, Blasi F. A urokinase-sensitive region of the human urokinase receptor is responsible for its chemotactic activity. EMBO J. 1997;16((24)):7279–86. doi: 10.1093/emboj/16.24.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoyer-Hansen G, Behrendt N, Ploug M, Dano K, Preissner KT. The intact urokinase receptor is required for efficient vitronectin binding: receptor cleavage prevents ligand interaction. FEBS Lett. 1997;420((1)):79–85. doi: 10.1016/s0014-5793(97)01491-9. [DOI] [PubMed] [Google Scholar]
- 15.Wei C, Moller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14((1)):55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 16.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17((8)):952–60. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kristensen P, Eriksen J, Blasi F, Danø K. Two alternatively spliced mouse urokinase receptor mRNAs with different histological localization in the gastrointestinal tract. J Cell Biol. 1991;115((6)):1763–71. doi: 10.1083/jcb.115.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pyke C, Eriksen J, Solberg H, Nielsen BS, Kristensen P, Lund LR, et al. An alternatively spliced variant of mRNA for the human receptor for urokinase plasminogen activator. FEBS Lett. 1993;326((1–3)):69–74. doi: 10.1016/0014-5793(93)81763-p. [DOI] [PubMed] [Google Scholar]
- 19.Suh TT, Nerlov C, Danø K, Degen JL. The murine urokinase-type plasminogen activator receptor gene. J Biol Chem. 1994;269((42)):25992–8. [PubMed] [Google Scholar]
- 20.Wei C, Spear R, Hahm E, Reiser J. suPAR, a circulating kidney disease factor. Front Med. 2021;8:745838. doi: 10.3389/fmed.2021.745838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei C, Li J, Adair BD, Zhu K, Cai J, Merchant M, et al. uPAR isoform 2 forms a dimer and induces severe kidney disease in mice. J Clin Invest. 2019;129((5)):1946–59. doi: 10.1172/JCI124793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozzi A, Zent R. Integrins in kidney disease. J Am Soc Nephrol. 2013;24((7)):1034–9. doi: 10.1681/ASN.2013010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu H, Liao J, Zhou X, Hong X, Song D, Hou FF, et al. Tenascin-C promotes acute kidney injury to chronic kidney disease progression by impairing tubular integrity via alphavbeta6 integrin signaling. Kidney Int. 2020;97((5)):1017–31. doi: 10.1016/j.kint.2020.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bon H, Hales P, Lumb S, Holdsworth G, Johnson T, Qureshi O, et al. Spontaneous extracellular matrix accumulation in a human in vitro model of renal fibrosis is mediated by alphaV integrins. Nephron. 2019;142((4)):328–50. doi: 10.1159/000499506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hayek SS, Koh KH, Grams ME, Wei C, Ko YA, Li J, et al. A tripartite complex of suPAR, APOL1 risk variants and alphavbeta3 integrin on podocytes mediates chronic kidney disease. Nat Med. 2017;23((8)):945–53. doi: 10.1038/nm.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayek SS, Leaf DE, Samman Tahhan A, Raad M, Sharma S, Waikar SS, et al. Soluble urokinase receptor and acute kidney injury. N Engl J Med. 2020;382((5)):416–26. doi: 10.1056/NEJMoa1911481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norman JT, Fine LG. Progressive renal disease: fibroblasts, extracellular matrix, and integrins. Exp Nephrol. 1999;7((2)):167–77. doi: 10.1159/000020597. [DOI] [PubMed] [Google Scholar]
- 28.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122((11)):3537–47. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- 29.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119((Pt 19)):3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen AR, Zhong X, Tang TT, Wang C, Jing J, Liu BC, et al. Integrin, exosome and kidney disease. Front Physiol. 2020;11:627800. doi: 10.3389/fphys.2020.627800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Q, Ren GL, Wei B, Jin J, Huang XR, Shao W, et al. Conditional knockout of TGF-betaRII/Smad2 signals protects against acute renal injury by alleviating cell necroptosis, apoptosis and inflammation. Theranostics. 2019;9((26)):8277–93. doi: 10.7150/thno.35686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resnati M, Guttinger M, Valcamonica S, Sidenius N, Blasi F, Fazioli F. Proteolytic cleavage of the urokinase receptor substitutes for the agonist-induced chemotactic effect. EMBO J. 1996;15((7)):1572–82. [PMC free article] [PubMed] [Google Scholar]
- 33.Kugler MC, Wei Y, Chapman HA. Urokinase receptor and integrin interactions. Curr Pharm Des. 2003;9((19)):1565–74. doi: 10.2174/1381612033454658. [DOI] [PubMed] [Google Scholar]
- 34.Jo M, Thomas KS, Marozkina N, Amin TJ, Silva CM, Parsons SJ, et al. Dynamic assembly of the urokinase-type plasminogen activator signaling receptor complex determines the mitogenic activity of urokinase-type plasminogen activator. J Biol Chem. 2005;280((17)):17449–57. doi: 10.1074/jbc.M413141200. [DOI] [PubMed] [Google Scholar]
- 35.Kiyan J, Kiyan R, Haller H, Dumler I. Urokinase-induced signaling in human vascular smooth muscle cells is mediated by PDGFR-beta. EMBO J. 2005;24((10)):1787–97. doi: 10.1038/sj.emboj.7600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb DJ, Nguyen DH, Gonias SL. Extracellular signal-regulated kinase functions in the urokinase receptor-dependent pathway by which neutralization of low density lipoprotein receptor-related protein promotes fibrosarcoma cell migration and matrigel invasion. J Cell Sci. 2000;113((Pt 1)):123–34. doi: 10.1242/jcs.113.1.123. [DOI] [PubMed] [Google Scholar]
- 37.Stahl A, Mueller BM. The urokinase-type plasminogen activator receptor, a GPI-linked protein, is localized in caveolae. J Cell Biol. 1995;129((2)):335–44. doi: 10.1083/jcb.129.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madsen CD, Sidenius N. The interaction between urokinase receptor and vitronectin in cell adhesion and signalling. Eur J Cell Biol. 2008;87((8–9)):617–29. doi: 10.1016/j.ejcb.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Wei Y, Lukashev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, et al. Regulation of integrin function by the urokinase receptor. Science. 1996;273((5281)):1551–5. doi: 10.1126/science.273.5281.1551. [DOI] [PubMed] [Google Scholar]
- 40.Xue W, Kindzelskii AL, Todd RF, 3rd, Petty HR. Physical association of complement receptor type 3 and urokinase-type plasminogen activator receptor in neutrophil membranes. J Immunol. 1994;152((9)):4630–40. [PubMed] [Google Scholar]
- 41.Xue W, Mizukami I, Todd RF, 3rd, Petty HR. Urokinase-type plasminogen activator receptors associate with beta1 and beta3 integrins of fibrosarcoma cells: dependence on extracellular matrix components. Cancer Res. 1997;57((9)):1682–9. [PubMed] [Google Scholar]
- 42.Tarui T, Mazar AP, Cines DB, Takada Y. Urokinase-type plasminogen activator receptor (CD87) is a ligand for integrins and mediates cell-cell interaction. J Biol Chem. 2001;276((6)):3983–90. doi: 10.1074/jbc.M008220200. [DOI] [PubMed] [Google Scholar]
- 43.Alfano M, Cinque P, Giusti G, Proietti S, Nebuloni M, Danese S, et al. Full-length soluble urokinase plasminogen activator receptor down-modulates nephrin expression in podocytes. Sci Rep. 2015;5:13647. doi: 10.1038/srep13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han R, Hu S, Qin W, Shi J, Hou Q, Wang X, et al. C3a and suPAR drive versican V1 expression in tubular cells of focal segmental glomerulosclerosis. JCI Insight. 2019;4((13)):e122912. doi: 10.1172/jci.insight.130986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reiser J, Sever S. Podocyte biology and pathogenesis of kidney disease. Annu Rev Med. 2013;64:357–66. doi: 10.1146/annurev-med-050311-163340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reiser J. Circulating permeability factor suPAR: from concept to discovery to clinic. Trans Am Clin Climatol Assoc. 2013;124:133–8. [PMC free article] [PubMed] [Google Scholar]
- 47.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N Engl J Med. 2006;354((13)):1387–401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 48.Recurrence of idiopathic nephrotic syndrome after renal transplantation. 1972. J Am Soc Nephrol. 2001;12((9)):1994–2002. doi: 10.1681/ASN.V1291994. [DOI] [PubMed] [Google Scholar]
- 49.Sharma M, Sharma R, Reddy SR, McCarthy ET, Savin VJ. Proteinuria after injection of human focal segmental glomerulosclerosis factor. Transplantation. 2002;73((3)):366–72. doi: 10.1097/00007890-200202150-00009. [DOI] [PubMed] [Google Scholar]
- 50.Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334((14)):878–83. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 51.Haas M, Godfrin Y, Oberbauer R, Yilmaz N, Borchhardt K, Regele H, et al. Plasma immunadsorption treatment in patients with primary focal and segmental glomerulosclerosis. Nephrol Dial Transplant. 1998;13((8)):2013–6. doi: 10.1093/ndt/13.8.2013. [DOI] [PubMed] [Google Scholar]
- 52.Kemper MJ, Wolf G, Müller-Wiefel DE. Transmission of glomerular permeability factor from a mother to her child. N Engl J Med. 2001;344((5)):386–7. doi: 10.1056/NEJM200102013440517. [DOI] [PubMed] [Google Scholar]
- 53.Hahm E, Wei C, Fernandez I, Li J, Tardi NJ, Tracy M, et al. Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med. 2017;23((1)):100–6. doi: 10.1038/nm.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gussen H, Hohlstein P, Bartneck M, Warzecha KT, Buendgens L, Luedde T, et al. Neutrophils are a main source of circulating suPAR predicting outcome in critical illness. J Intensive Care. 2019;7:26. doi: 10.1186/s40560-019-0381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delville M, Sigdel TK, Wei C, Li J, Hsieh SC, Fornoni A, et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med. 2014;6((256)):256ra136. doi: 10.1126/scitranslmed.3008538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yoo TH, Pedigo CE, Guzman J, Correa-Medina M, Wei C, Villarreal R, et al. Sphingomyelinase-like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol. 2015;26((1)):133–47. doi: 10.1681/ASN.2013111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Theilade S, Lyngbaek S, Hansen TW, Eugen-Olsen J, Fenger M, Rossing P, et al. Soluble urokinase plasminogen activator receptor levels are elevated and associated with complications in patients with type 1 diabetes. J Intern Med. 2015;277((3)):362–71. doi: 10.1111/joim.12269. [DOI] [PubMed] [Google Scholar]
- 58.Meijers B, Maas RJ, Sprangers B, Claes K, Poesen R, Bammens B, et al. The soluble urokinase receptor is not a clinical marker for focal segmental glomerulosclerosis. Kidney Int. 2014;85((3)):636–40. doi: 10.1038/ki.2013.505. [DOI] [PubMed] [Google Scholar]
- 59.Bock ME, Price HE, Gallon L, Langman CB. Serum soluble urokinase-type plasminogen activator receptor levels and idiopathic FSGS in children: a single-center report. Clin J Am Soc Nephrol. 2013;8((8)):1304–11. doi: 10.2215/CJN.07680712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J, Liu G, Zhang YM, Cui Z, Wang F, Liu XJ, et al. Plasma soluble urokinase receptor levels are increased but do not distinguish primary from secondary focal segmental glomerulosclerosis. Kidney Int. 2013;84((2)):366–72. doi: 10.1038/ki.2013.55. [DOI] [PubMed] [Google Scholar]
- 61.Spinale JM, Mariani LH, Kapoor S, Zhang J, Weyant R, Song PX, et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int. 2015;87((3)):564–74. doi: 10.1038/ki.2014.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sinha A, Bajpai J, Saini S, Bhatia D, Gupta A, Puraswani M, et al. Serum-soluble urokinase receptor levels do not distinguish focal segmental glomerulosclerosis from other causes of nephrotic syndrome in children. Kidney Int. 2014;85((3)):649–58. doi: 10.1038/ki.2013.546. [DOI] [PubMed] [Google Scholar]
- 63.Cathelin D, Placier S, Ploug M, Verpont MC, Vandermeersch S, Luque Y, et al. Administration of recombinant soluble urokinase receptor per se is not sufficient to induce podocyte alterations and proteinuria in mice. J Am Soc Nephrol. 2014;25((8)):1662–8. doi: 10.1681/ASN.2013040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harel E, Shoji J, Abraham V, Miller L, Laszik ZG, King A, et al. Further evidence that the soluble urokinase plasminogen activator receptor does not directly injure mice or human podocytes. Transplantation. 2020;104((1)):54–60. doi: 10.1097/TP.0000000000002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun P, Yu L, Huang J, Wang S, Zou W, Yang L, et al. Soluble urokinase receptor levels in secondary focal segmental glomerulosclerosis. Kidney Dis. 2019;5((4)):239–46. doi: 10.1159/000497353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373((20)):1916–25. doi: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lv L, Wang F, Wu L, Wang JW, Cui Z, Hayek SS, et al. Soluble urokinase-type plasminogen activator receptor and incident end-stage renal disease in Chinese patients with chronic kidney disease. Nephrol Dial Transplant. 2020;35((3)):465–70. doi: 10.1093/ndt/gfy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo S, Coresh J, Tin A, Rebholz CM, Chen TK, Hayek SS, et al. Soluble urokinase-type plasminogen activator receptor in Black Americans with CKD. Clin J Am Soc Nephrol. 2018;13((7)):1013–21. doi: 10.2215/CJN.13631217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaefer F, Trachtman H, Wuhl E, Kirchner M, Hayek SS, Anarat A, et al. Association of serum soluble urokinase receptor levels with progression of kidney disease in children. JAMA Pediatr. 2017;171((11)):e172914. doi: 10.1001/jamapediatrics.2017.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weidemann DK, Abraham AG, Roem JL, Furth SL, Warady BA. Plasma soluble urokinase plasminogen activator receptor (suPAR) and CKD progression in children. Am J Kidney Dis. 2020;76((2)):194–202. doi: 10.1053/j.ajkd.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soltysiak J, Zachwieja J, Benedyk A, Lewandowska-Stachowiak M, Nowicki M, Ostalska-Nowicka D. Circulating suPAR as a biomarker of disease severity in children with proteinuric glomerulonephritis. Minerva Pediatr. 2019;71((1)):4–11. doi: 10.23736/S0026-4946.16.04461-3. [DOI] [PubMed] [Google Scholar]
- 72.Ronco C. N-GAL: diagnosing AKI as soon as possible. Crit Care. 2007;11((6)):173. doi: 10.1186/cc6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ngo D, Wen D, Gao Y, Keyes MJ, Drury ER, Katz DH, et al. Circulating testican-2 is a podocyte-derived marker of kidney health. Proc Natl Acad Sci U S A. 2020;117((40)):25026–35. doi: 10.1073/pnas.2009606117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mossanen JC, Pracht J, Jansen TU, Buendgens L, Stoppe C, Goetzenich A, et al. Elevated soluble urokinase plasminogen activator receptor and proenkephalin serum levels predict the development of acute kidney injury after cardiac surgery. Int J Mol Sci. 2017;18((8)):1662. doi: 10.3390/ijms18081662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hall A, Crichton S, Varrier M, Bear DE, Ostermann M. suPAR as a marker of infection in acute kidney injury: a prospective observational study. BMC Nephrol. 2018;19((1)):191. doi: 10.1186/s12882-018-0990-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martensson J, Bellomo R. The rise and fall of NGAL in acute kidney injury. Blood Purif. 2014;37((4)):304–10. doi: 10.1159/000364937. [DOI] [PubMed] [Google Scholar]
- 77.Mishra J, Ma Q, Prada A, Mitsnefes M, Zahedi K, Yang J, et al. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J Am Soc Nephrol. 2003;14((10)):2534–43. doi: 10.1097/01.asn.0000088027.54400.c6. [DOI] [PubMed] [Google Scholar]
- 78.Walls AB, Bengaard AK, Iversen E, Nguyen CN, Kallemose T, Juul-Larsen HG, et al. Utility of suPAR and NGAL for AKI risk stratification and early optimization of renal risk medications among older patients in the emergency department. Pharmaceuticals. 2021;14((9)):843. doi: 10.3390/ph14090843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bal A, Agrawal R, Vaideeswar P, Arava S, Jain A. COVID-19: an up-to-date review − from morphology to pathogenesis. Indian J Pathol Microbiol. 2020;63((3)):358–66. doi: 10.4103/IJPM.IJPM_779_20. [DOI] [PubMed] [Google Scholar]
- 80.Samprathi M, Jayashree M. Biomarkers in COVID-19: an up-to-date review. Front Pediatr. 2020;8:607647. doi: 10.3389/fped.2020.607647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheng Y, Zhang Y, Chi H, Chen S, Peng M, Luo L, et al. The hemocyte counts as a potential biomarker for predicting disease progression in COVID-19: a retrospective study. Clin Chem Lab Med. 2020;58((7)):1106–15. doi: 10.1515/cclm-2020-0377. [DOI] [PubMed] [Google Scholar]
- 82.Hu JJ, Nie SM, Gao Y, Yan XS, Huang JX, Li TL, et al. [The correlations and prognostic value of neutrophil to lymphocyte ratio, immunophenotype and cytogenetic abnormalities in patients with newly diagnosed multiple myeloma] Zhonghua Xue Ye Xue Za Zhi. 2019;40((12)):1044–6. doi: 10.3760/cma.j.issn.0253-2727.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma A, Cheng J, Yang J, Dong M, Liao X, Kang Y. Neutrophil-to-lymphocyte ratio as a predictive biomarker for moderate-severe ARDS in severe COVID-19 patients. Crit Care. 2020;24((1)):288. doi: 10.1186/s13054-020-03007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lagunas-Rangel FA. Neutrophil-to-lymphocyte ratio and lymphocyte-to-C-reactive protein ratio in patients with severe coronavirus disease 2019 (COVID-19): a meta-analysis. J Med Virol. 2020;92((10)):1733–4. doi: 10.1002/jmv.25819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther Adv Respir Dis. 2020;14:1753466620937175. doi: 10.1177/1753466620937175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen R, Sang L, Jiang M, Yang Z, Jia N, Fu W, et al. Longitudinal hematologic and immunologic variations associated with the progression of COVID-19 patients in China. J Allergy Clin Immunol. 2020;146((1)):89–100. doi: 10.1016/j.jaci.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu T, Zhang J, Yang Y, Ma H, Li Z, Zhang J, et al. The role of interleukin-6 in monitoring severe case of coronavirus disease 2019. EMBO Mol Med. 2020;12((7)):e12421. doi: 10.15252/emmm.202012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.D'Ardes D, Boccatonda A, Rossi I, Guagnano MT, Santilli F, Cipollone F, et al. COVID-19 and RAS: unravelling an unclear relationship. Int J Mol Sci. 2020;21((8)):3003. doi: 10.3390/ijms21083003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Catanzaro M, Fagiani F, Racchi M, Corsini E, Govoni S, Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct Target Ther. 2020;5((1)):84. doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kerget B, Kerget F, Aksakal A, Aşkın S, Uçar EY, Sağlam L. Evaluation of the relationship between KIM-1 and suPAR levels and clinical severity in COVID-19 patients: a different perspective on suPAR. J Med Virol. 2021;93((9)):5568–73. doi: 10.1002/jmv.27099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chalkias A, Mouzarou A, Samara E, Xanthos T, Ischaki E, Pantazopoulos I. Soluble urokinase plasminogen activator receptor: a biomarker for predicting complications and critical care admission of COVID-19 patients. Mol Diagn Ther. 2020;24((5)):517–21. doi: 10.1007/s40291-020-00481-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang M, Li L, Shen J, Wang Y, Wang R, Yuan C, et al. Plasma levels of the active form of suPAR are associated with COVID-19 severity. Crit Care. 2020;24((1)):704. doi: 10.1186/s13054-020-03336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oulhaj A, Alsuwaidi AR, Suliman A, Gasmelseed H, Khan S, Alawi S, et al. Admission levels of soluble urokinase plasminogen activator receptor (suPAR) are associated with the development of severe complications in hospitalised COVID-19 patients: a Prospective Cohort Study. Int J Infect Dis. 2021;107:188–94. doi: 10.1016/j.ijid.2021.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Velissaris D, Lagadinou M, Paraskevas T, Oikonomou E, Karamouzos V, Karteri S, et al. Evaluation of plasma soluble urokinase plasminogen activator receptor levels in patients with COVID-19 and non-COVID-19 pneumonia: an observational cohort study. J Clin Med Res. 2021;13((9)):474–8. doi: 10.14740/jocmr4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rovina N, Akinosoglou K, Eugen-Olsen J, Hayek S, Reiser J, Giamarellos-Bourboulis EJ. Soluble urokinase plasminogen activator receptor (suPAR) as an early predictor of severe respiratory failure in patients with COVID-19 pneumonia. Crit Care. 2020;24((1)):187. doi: 10.1186/s13054-020-02897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Azam TU, Shadid HR, Blakely P, O'Hayer P, Berlin H, Pan M, et al. soluble urokinase receptor (SuPAR) in COVID-19-related AKI. J Am Soc Nephrol. 2020;31((11)):2725–35. doi: 10.1681/ASN.2020060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Altintas I, Eugen-Olsen J, Seppala S, Tingleff J, Stauning MA, El Caidi NO, et al. suPAR cut-offs for risk stratification in patients with symptoms of COVID-19. Biomark Insights. 2021;16:11772719211034685. doi: 10.1177/11772719211034685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Enocsson H, Idoff C, Gustafsson A, Govender M, Hopkins F, Larsson M, et al. Soluble urokinase plasminogen activator receptor (suPAR) independently predicts severity and length of hospitalisation in patients with COVID-19. Front Med. 2021;8:791716. doi: 10.3389/fmed.2021.791716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kyriazopoulou E, Poulakou G, Milionis H, Metallidis S, Adamis G, Tsiakos K, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27((10)):1752–60. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jehn U, Schütte-Nütgen K, Henke U, Pavenstädt H, Suwelack B, Reuter S. Soluble urokinase-type plasminogen activator receptor (suPAR) is a risk indicator for eGFR loss in kidney transplant recipients. Sci Rep. 2021;11((1)):3713. doi: 10.1038/s41598-021-83333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alachkar N, Li J, Matar D, Vujjini V, Alasfar S, Tracy M, et al. Monitoring suPAR levels in post-kidney transplant focal segmental glomerulosclerosis treated with therapeutic plasma exchange and rituximab. BMC Nephrol. 2018;19((1)):361. doi: 10.1186/s12882-018-1177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rapetskaya NV, Komissarov KS, Kalachik OV. SuPAR-based choice of the management strategy in kidney transplant recipients with infection. Almanac Clin Med. 2020;48((3)):187–92. [Google Scholar]
- 103.Macionyte RS, Bardauskas M, Vaiciuniene R, Bumblyte IA. Changes of soluble urokinase plasminogen activator receptor (Supar) after cadaveric kidney transplantation and association with kidney function. Nephrol Dial Transplant. 2021;36(1) [Google Scholar]
- 104.Winnicki W, Sunder-Plassmann G, Sengolge G, Handisurya A, Herkner H, Kornauth C, et al. Diagnostic and prognostic value of soluble urokinase-type plasminogen activator receptor (suPAR) in focal segmental glomerulosclerosis and impact of detection method. Sci Rep. 2019;9((1)):13783. doi: 10.1038/s41598-019-50405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morath CHS, Döhler B, Nusshag C, Sommerer C, Zeier M, Reiser J, et al. Soluble urokinase receptor and mortality in kidney transplant recipients. Transplant Int. 2022;35:10071. doi: 10.3389/ti.2021.10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Montuori N, Pesapane A, Rossi FW, Giudice V, De Paulis A, Selleri C, et al. Urokinase type plasminogen activator receptor (uPAR) as a new therapeutic target in cancer. Transl Med UniSa. 2016;15:15–21. [PMC free article] [PubMed] [Google Scholar]
- 107.Margheri F, D'Alessio S, Serrati S, Pucci M, Annunziato F, Cosmi L, et al. Effects of blocking urokinase receptor signaling by antisense oligonucleotides in a mouse model of experimental prostate cancer bone metastases. Gene Ther. 2005;12((8)):702–14. doi: 10.1038/sj.gt.3302456. [DOI] [PubMed] [Google Scholar]
- 108.Rao JS, Gondi C, Chetty C, Chittivelu S, Joseph PA, Lakka SS. Inhibition of invasion, angiogenesis, tumor growth, and metastasis by adenovirus-mediated transfer of antisense uPAR and MMP-9 in non-small cell lung cancer cells. Mol Cancer Ther. 2005;4((9)):1399–408. doi: 10.1158/1535-7163.MCT-05-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yuan C, Guo Z, Yu S, Jiang L, Huang M. Development of inhibitors for uPAR: blocking the interaction of uPAR with its partners. Drug Discov Today. 2021;26((4)):1076–85. doi: 10.1016/j.drudis.2021.01.016. [DOI] [PubMed] [Google Scholar]
- 110.Mazar AP, Ahn RW, O'Halloran TV. Development of novel therapeutics targeting the urokinase plasminogen activator receptor (uPAR) and their translation toward the clinic. Curr Pharm Des. 2011;17((19)):1970–8. doi: 10.2174/138161211796718152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pass J, Jogi A, Lund IK, Rono B, Rasch MG, Gardsvoll H, et al. Murine monoclonal antibodies against murine uPA receptor produced in gene-deficient mice: inhibitory effects on receptor-mediated uPA activity in vitro and in vivo. Thromb Haemost. 2007;97((6)):1013–22. [PubMed] [Google Scholar]
- 112.Xu X, Cai Y, Wei Y, Donate F, Juarez J, Parry G, et al. Identification of a new epitope in uPAR as a target for the cancer therapeutic monoclonal antibody ATN-658, a structural homolog of the uPAR binding integrin CD11b (alphaM) PLoS One. 2014;9((1)):e85349. doi: 10.1371/journal.pone.0085349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bifulco K, Longanesi-Cattani I, Gargiulo L, Maglio O, Cataldi M, De Rosa M, et al. An urokinase receptor antagonist that inhibits cell migration by blocking the formyl peptide receptor. FEBS Lett. 2008;582((7)):1141–6. doi: 10.1016/j.febslet.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 114.Carriero MV, Bifulco K, Minopoli M, Lista L, Maglio O, Mele L, et al. UPARANT: a urokinase receptor-derived peptide inhibitor of VEGF-driven angiogenesis with enhanced stability and in vitro and in vivo potency. Mol Cancer Ther. 2014;13((5)):1092–104. doi: 10.1158/1535-7163.MCT-13-0949. [DOI] [PubMed] [Google Scholar]
- 115.Tarighi P, Montazeri H, Khorramizadeh MR, Sobhani AM, Ostad SN, Ghahremani MH. uPAR peptide antagonist alters regulation of MAP kinases and Bcl-2 family members in favor of apoptosis in MDA-MB-231 cell line. Res Pharm Sci. 2015;10((3)):200–5. [PMC free article] [PubMed] [Google Scholar]
- 116.Chaurasia P, Mezei M, Zhou MM, Ossowski L. Computer aided identification of small molecules disrupting uPAR/alpha5beta1: integrin interaction − a new paradigm for metastasis prevention. PLoS One. 2009;4((2)):e4617. doi: 10.1371/journal.pone.0004617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kobayashi H, Yagyu T, Kondo T, Kurita N, Inagaki K, Haruta S, et al. Suppression of urokinase receptor expression by thalidomide is associated with inhibition of nuclear factor kappaB activation and subsequently suppressed ovarian cancer dissemination. Cancer Res. 2005;65((22)):10464–71. doi: 10.1158/0008-5472.CAN-04-3789. [DOI] [PubMed] [Google Scholar]
- 118.Metrangolo V, Ploug M, Engelholm LH. The urokinase receptor (uPAR) as a “Trojan Horse” in targeted cancer therapy: challenges and opportunities. Cancers. 2021;13((21)):5376. doi: 10.3390/cancers13215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dal Monte M, Cammalleri M, Pecci V, Carmosino M, Procino G, Pini A, et al. Inhibiting the urokinase-type plasminogen activator receptor system recovers STZ-induced diabetic nephropathy. J Cell Mol Med. 2019;23((2)):1034–49. doi: 10.1111/jcmm.14004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maile LA, Busby WH, Gollahon KA, Flowers W, Garbacik N, Garbacik S, et al. Blocking ligand occupancy of the alphaVbeta3 integrin inhibits the development of nephropathy in diabetic pigs. Endocrinology. 2014;155((12)):4665–75. doi: 10.1210/en.2014-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]