Abstract
Some types of actinomycetes produce many different secondary metabolites of fatty acids, hydrocarbons, or other compounds. Many of these products play an important role in various medical fields. This study aims to extract natural compounds from actinomycetes after their isolation from the soil and their identification as antimicrobials. Soil samples were collected from different regions after being treated by known methods. Colonies that indicate actinomycetes were replanted and provided with suitable conditions for growth, and then tested against a number of pathogenic microbes. Isolate 3-D is more effective than others. D-3 was exposed to ultraviolet rays for greater production of antimicrobials. The compounds obtained from the isolates were extracted by the column chromatography technique. To identify the compounds resulting from the extract, the Gas Chromatography-Mass Spectrometry (GC–MS) technique was used. Ten compounds have been identified by GC–MS. Some of the compounds are of fatty acid nature, and some are hydrocarbons. These compound includes Hexadecane, 2,6,11,15- Tetramethyl – Octacosane - Dodecanoic Acid, 1,2,3- Propane-triyl ester – Hexatriacontane – Heptacosane - Eicosyl Acetate – Tritetracontane - Tetracosane, 2,6,10,15,19,23-Hexamethyl - Myristic Acid vinyl ester – Tetratetracontane. All of these extracts are of medical importance. Some of them are anti-bacterial, some are anti-allergic, anti-inflammatory, anti-cancer, anti-fungal, antioxidants, and some of them are essential ingredients in cosmetics. The current study showed that isolated D-3 actinomycetes from soil have the ability to produce antimicrobials against a variety of gram-negative and gram-positive bacteria, which are important in the medical field.
Keywords: Actinomycetes, Fermentation, Antimicrobials, GC/MS, Extraction, Compounds
1. Introduction
Gram-positive hyphal bacteria with aerial mycelia and substrate are known as actinomycetes. They are rich in bioactive secondary metabolites such as enzymes, antibiotics, and anti-oxidants. (Barka et al., 2016). Actinomycetes produce more than 20,000 microbial biologically effective secondary metabolites and 40% of the 160 microbe-derived antibiotics. (Rajaram et al., 2020). It was proposed that screening soil isolates yielded the majority of new antibiotics. Baniya et al. (2018). Moreover, due to the emergence of multidrug-resistant microbes, resistance to antibiotics has become a critical concern in the treatment of human diseases, so further study is required to find new and more effective antibiotics that aid in the control of the problem (Pathalam et al., 2017, Roitch et al., 2017). Natural products derived from microorganisms are effective against diseases (Newman and Cragg, 2016, Sebak et al., 2021). Streptomyces species are responsible for more than 70% of the identified active ingredients isolated from actinobacteria (Undabarrena et al., 2016, Sebak et al., 2021). Most of these substances are essential in ecological systems because they inhibit microbial competition in their surroundings in order to preserve their food sources. The production of these active components by Streptomyces sp. shows highly disciplined and synchronized metabolic processes, allowing them to dominate among other ecosystem inhabitants (Ilic-Tomic et al., 2015, Chevrette et al., 2019). Many of the bioactive compounds secreted by Streptomyces sp. have already been used as antimicrobial drugs against drug-resistant pathogens in animals and humans (Sebak et al., 2021). GC–MS is a technique for identifying the components of a mixture, such as hydrocarbons, essential oils, and solvents. Even at very low concentrations, the electron capture detector and the flame ionization detector can quantify the materials. It is widely used, particularly in biochemistry, due to its simplicity, sensitivity, and effectiveness in quantitative and qualitatively separating components of mixtures to fix thermochemical standards such as heats of solution and vaporization, vaporpressure, activity coefficients, and compound purification. The GC–MS is useful in pharmaceutical analysis, pharmacognosy, pharmaceutical biotechnology, and pharmaceutical process control (Al-rubaye et al., 2020). The goal of this research is to detect the biological activity of actinomycetes isolated from different soil samples..
2. Materials and methods
A large number of actinomycetes were studied prospectively in this study. Dried sandy soils with pH 7.0 were collected from various locations in Alkharj city were used to isolate samples. Actinomycetes were separated using a variety of media and screened for antimicrobial activity against a diverse selection of gram-negative bacteria, gram-positive bacteria, and yeast. Various fermentation factors have been enhanced for effective antimicrobial production.
2.1. Collection of samples
Sterile polythene bags and other equipment were used to collect soil samples from various locations throughout Alkharj city. Before being used to isolate actinomycetes, these specimens were air-dried for one week. (Sharma, 2016).
2.2. Soil treatment and actinomycete isolation
In 9 ml of sterile DW, 1 gm of soil was dissolved. After settling on 10–5 dilutions, 0.1 ml of 10-2, 10-3, 10-4, and 10-5 dilutions were added to starch casein agar. C, D, E, F, and G are the names of the actinomycete colonies that were isolated from the crowded plate for further study.
2.3. Sub-cultivation of the isolates
Colonies with a dry, chalky appearance were chosen after 7 days of incubation at 28 °C. After being divided into sections, each of the distinct actinomycete colonies was sub cultured in the Starch Casein Plate (SCA). The plates were incubated at 28 °C for 7 days. Following the formation of colonies, they were sub-cultured in a test tube containing SCA slants, incubated at 28 °C for 7 days, and then kept in the fridge as the stock. To prevent the cultures from dying out, the isolates were subculture again every 2–3 weeks (Budhathoki and Shrestha, 2020).
2.4. Primary screening isolates
The duplicate perpendicular streak method was used for the preliminary screening of inhibition activity of isolated strains against bacterial species. The isolated bacteria were streaked in the middle of a Mueller-Hinton agar plate and incubated at 28 °C till actinomycetes were visible as an adherent vertical colony line. Following incubation, each of the standard organisms (Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus vulgaris, Salmonella typhimurium, Aspergillus niger, and Candida albicans) were streaked 1 cm apart and 2 mm on the side of an actinomycetes colony on nutrient agar, vertical to the colony, and then incubated for 24 h at 37 °C. Plates were examined after incubation to see if the growth of test organisms was inhibited. Growth inhibition was observed as a reduction in the number of test organisms growing on the streak line (Ganesan et al., 2017).
2.5. Characteristics at the microscopic level
The slide culture method, the Gram staining technique, and the direct method were all employed. The isolates exhibited actinomycete characteristics (Ibnouf, 2021).
2.6. Fermentation for the production of antibiotics
In a conical flask, Yeast Extract-Malt Extract broth (ISP-2) was prepared. The colony of selected actinomycetes that showed the most activity against test organisms in primary screening was cut from their pure culture and placed in the broth. For 8 days, the conical flasks were placed in a rotary shaker incubator at 28 °C (Budhathoki and Shrestha, 2020).
2.7. Extraction of antibiotics
Following incubation, the broth was obtained and centrifuged for 30 min at 4000 rpm. After collecting the supernatant, an equal amount of ethyl acetate was added. For one hour, the two phases were vortexed. The solutions were placed in a separate funnel for 5 min to separate the aqueous and organic phases. The lower aqueous phase was removed, and the upper organic phase was obtained and evaporated in a 40 °C water bath (Ghorbani et al., 2013). To solubilize crude antibiotic extract, the residue was weighed and mixed with a small amount of pH 7 phosphate buffer (Gopinath et al., 2013a, Gopinath et al., 2013b).
2.8. Second screening
For the second screening against tested strains, the agar well diffusion method was utilized. The test strains were placed in the Mueller-Hinton broth and incubated for 4 h at 37 °C. After incubation, the turbidity of the broth was measured and compared to the McFarland standard of 0.5. Test organisms were cultured on Nutrient Agar plates from McFarland adjusted broth cultures on the lawn or carpet. Five millimeter wells were cut in Nutrient Agar plates with a sterile agar borer, and 40 μl of ethyl acetate-extracted antibiotic fractions were added to the wells. Before incubating at 37 °C for 24 h, plates were left at room temperature for 20–30 min to allow antibiotic fractions to diffuse. Plates were examined after 24 h, as well as the diameter of the inhibition zone around each well (Gopinath et al., 2013a, Gopinath et al., 2013b).
2.9. UV exposure
On the selected soil isolate, the UV mutagenesis method was used to obtain hyper-antibiotic producing stable UV mutants (D-3). Actinomycete (D-3) isolate was spread on the surface of actinomycete agar plates. The plates were then exposed to UV light at 254 and 324 nm at a distance of 25 cm for 5, 10, 15, 30, 60, 90, 120, 150, 180, 240, 300 s and more, before being incubated at 27 °C for 24 h. (Butler et al., 2003).
2.10. GC–Ms
The GC–MS analysis was performed on a Perkin Elmer Clarus 600 gas chromatograph linked to a mass spectrometer (Turbo mass). An aliquot of 2 µl of extract was injected into the Elite-5MS column of 30 m, 0.25 mm film thickness, and 0.25 µm internal diameter. capillary column using the following temperature program:
Initial oven temperature of 40 °C for 5 min, then 240 °C at a rate of 5 °C for 2 min, followed by 300 °C at a rate of 5 °C for 5 min. The injector temperature was maintained at 220 OC. The interface temperature was 200 °C. At a flow rate of 1.0 ml/min, helium was used as a mobile phase. Scanning at 40 to 600 m/z was used for mass spectral detection in the electron ionization mode. Finally, unidentified compounds were recognized by comparison of the spectra to those in the National Institute of Standards and Technology library. A single sample took 61 min to analyze in total.
3. Results
3.1. Antimicrobial activity of actinomycetes extracted from soil samples
The isolated actinomycetes isolates were assessed for therapeutic potential against 7 bacteria, 1 fungal strain, and 1 yeast strain using the perpendicular streak method (Table 1). Eleven isolates (C-5), (C-8), (C-19), (D-1), (D-3), (D-5), (D-6), (E-3), (E-9), (E-11), and (G-6) displayed high antimicrobial activity against gram-negative and/or gram-positive pathogens. (D-3), on the other hand, demonstrated a very wide range with higher scores than all other strains. Six actinomycetes were chosen from the eleven strains for taxonomic classification based on antimicrobial activity testing (Table 2). The susceptibility of the same microbes to the most commonly used antibiotics was recorded (Table 3).
Table 1.
Sensitivity of different microorganisms towards the soil isolates by perpendicular streak method.
| Isolates | Staphylococcus aureus ATCC 29,213 | Bacillus subtilis ATCC 10,400 | Escherichia coli ATCC 13,706 | Klebsiella pneumoniae ATCC 10,031 | Pseudomonas aeruginosa ATCC 15,442 | Proteus vulgaris ATCC 6380 | Salmonella typhimurium ATCC 25,241 | Aspergillus niger ATCC 16,404 | Candida albicans ATCC 10,231 |
|---|---|---|---|---|---|---|---|---|---|
| C-5 | ++ | + | + | – | – | + | – | ++ | + |
| C-8 | +++ | + | + | ++ | – | ++ | + | + | + |
| C-19 | – | ++ | – | + | + | + | – | ++ | – |
| D-1 | ++ | + | + | – | – | + | – | +++ | + |
| D-3 | +++ | +++ | +++ | ++ | + | ++ | – | ++ | +++ |
| D-5 | – | – | + | + | ++ | – | + | + | + |
| D-6 | ++ | + | ++ | ++ | – | – | ++ | + | +++ |
| E-3 | + | + | – | – | – | ++ | – | ++ | + |
| E-9 | – | – | + | + | – | – | + | ++ | + |
| E-11 | + | + | – | – | + | + | + | +++ | ++ |
| G-6 | +++ | +++ | – | ++ | + | ++ | – | +++ | +++ |
(+++) = High Inhibition, (++) = Medium Inhibition, (+) = Low Inhibition, (-) = No Inhibition.
Table 2.
Taxonomical characterization of the six soil isolates.
| Isolates | Hydrolysis of starch | Proteolytic activity | Melanoid formation | Nitrate reduction |
Production of acid |
Liquefaction of gelatin | Carbohydrate assimilation | Hydrogen sulfide production | Utilization of citrate | Methyl red | Indole |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C-5 | – | + | – | + | + | + | – | – | – | + | + |
| C-8 | – | – | + | + | + | + | + | – | – | + | + |
| D-1 | + | – | – | + | + | + | – | – | – | + | – |
| D-3 | + | + | + | + | + | + | + | – | – | + | + |
| D-6 | + | + | + | + | + | + | – | + | – | – | + |
| G-6 | + | + | + | + | + | + | – | – | – | + | + |
(+): Positive test, (-): Negative test.
Table 3.
Activity of some antibiotics against standard organisms compared with the isolate.
| Antimicrobial | Content (µg) | Diameter of Zone of Inhibition (mm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Staphylococcus aureus ATCC 29,213 |
Bacillus subtilis ATCC 10,400 |
Escherichia coli ATCC 13,706 |
Klebsiella pneumonaie ATCC 10,031 |
Pseudomonas aeruginosa ATCC 15,442 |
Proteus vulgaris ATCC 6380 |
Salmonella typhi ATCC 25,241 |
Aspergillus niger ATCC 16,404 |
Candida albicans ATCC 10,231 |
||
| D-3 isolate extract | Whole extract | 21 | 21 | 21 | 18 | 11 | 18 | N. Z | 18 | 21 |
| AN | 30 | 17 | 21 | 19 | 18 | 10 | 15 | 16 | 20 | 19 |
| AM | 10 | 10 | N. Z | N. Z | N. Z | N. Z | N. Z | N. Z | N. Z | N. Z |
| E | 15 | N. Z | 15 | N. Z | N. Z | N. Z | N. Z | N. Z | N. Z | N. Z |
| P | 10 | 20 | 18 | N. Z | N. Z | N. Z | N. Z | 12 | N. Z | N. Z |
| GE | 10 | N. Z | 16 | 13 | N. Z | 10 | N. Z | N. Z | N. Z | N. Z |
AN = Amikacin (30 µg) P = Penicillin (10 µg).
AM = Ampicillin (10 µg) GE = Gentamicin (10 µg).
E = Erythromycin (15 µg) N. Z = No zone of inhibition.
3.2. Taxonomic classification
Six selected isolates were subjected to biochemical tests. All six strains (C-5, C-8, D-1, D-3, D-6, and G-6) demonstrated positive results in gelatin liquefaction and nitrate reduction tests. The strains (C-8), (D-3), (D-6) and (G-6) showed positive results in pigment production tests in Waksman medium, and (C-5), (D-1) showed negative results in melanoid formation tests, but they produced good growth in the above medium. Except for the strain (D-1) that tested positive for indole, all of the strains were able to produce acid in the acid production test. Except for the strains (C-8) and (D-1), all of the strains tested positive for proteolytic activity. All of the strains showed positive results for methyl red except the strain (D-6). Except for the strain (D-6), all of the six strains performed poorly in the H2S production test. Except for (C-5) and (C-8), all of the strains are positive for starch hydrolysis (Table 2). These physicochemical characteristics help to differentiate the actinomycete genus. Among all the isolates, (D-3) showed the most promising results for effective antibiotic production. Nutritional sources were investigated in order to boost antibiotic production.
3.3. Cultural and morphological properties of the (D-3) isolate
On different media for actinomycetes, the cultural and morphological characteristics of the isolate (D-3) were investigated (Table 4, Fig. 1). The investigation showed that the (D-3) isolate is a hyphal gram-positive organism. It was discovered that the (D-3) isolate belongs to the genus Actinomycetes after studying its taxonomic, morphological, and cultural characteristics.
Table 4.
Morphological and cultural characters of the isolate (D-3) on different media.
| S. N | Medium | Growth | Features of (D-3) |
|---|---|---|---|
| 1 | Carbon utilization agar (ISP-9) | Excellent | Thin yellowish golden colored colonies |
| 2 | Czapek’s sucrose agar | Good | Cretaceous colonies |
| 3 | Glycerol-arginine medium (ISP-5) | Good | Whitish colored, thin colonies and striated surface |
| 4 | Glucose yeast extract agar | Good | Brown colored |
| 5 | Glucose agar | Excellent | Whitish colored |
| 6 | Starch agar | Moderate | Yellowish colonies |
| 7 | Starch casein agar | Good | Dark brown colored |
Fig. 1.
Cultural characteristic of the isolate on glycerol yeast extract agar (GYE) and Sabouraud dextrose agar (SDA) media.
3.4. UV mutation
The isolate (D-3) was genetically modified using the UV irradiation method. When tested against standard organisms, it was discovered that the strain that was exposed to UV irradiation (324 nm) for 4 min improved (Table 5).
Table 5.
The effect of UV on antimicrobial production of D-3 isolate using four standard organisms.
| Time/s | Zone of inhibition (mm) before UV (324) exposing |
Zone of inhibition (mm) after UV (324 nm) exposing |
||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | C. albicans | S. aureus | B. subtilis | E. coli | C. albicans | |
| 5 | 21 | 23 | 20 | 19 | 21 | 22 | 21 | 20 |
| 10 | 23 | 22 | 24 | 21 | 24 | 22 | 24 | 22 |
| 15 | 23 | 22 | 23 | 20 | 23 | 21 | 22 | 20 |
| 30 | 22 | 23 | 23 | 21 | 24 | 23 | 25 | 23 |
| 60 | 24 | 23 | 24 | 22 | 25 | 24 | 24 | 23 |
| 90 | 24 | 22 | 25 | 23 | 26 | 25 | 23 | 24 |
| 120 | 23 | 23 | 24 | 21 | 26 | 26 | 24 | 25 |
| 150 | 24 | 23 | 24 | 22 | 26 | 27 | 25 | 25 |
| 180 | 23 | 22 | 23 | 22 | 27 | 27 | 26 | 25 |
| 240 | 24 | 24 | 23 | 22 | 33 | 34 | 32 | 30 |
| 300 | 24 | 23 | 23 | 22 | 31 | 32 | 30 | 28 |
3.5. Exposed isolate characteristics (D-3)
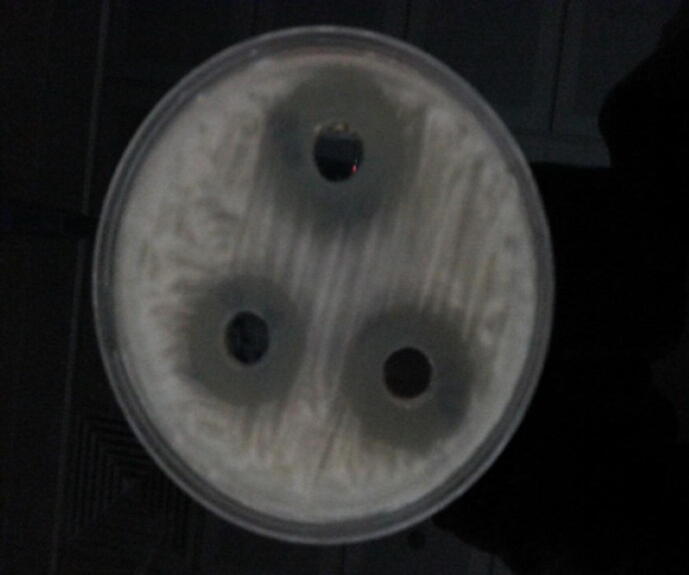
On different actinomycete media, the cultural and morphological characteristics of the exposed (D-3) isolate were investigated (Table 6). The inhibition zone was shown in Fig. 2.
Table 6.
Morphological and Cultural Characterization of the (D-3) exposed Strain on Different Media.
| S. N | Medium | Growth | Features of (D-3) Exposed |
|---|---|---|---|
| 1 | Carbon utilization agar (ISP-9) | Excellent | Thick cream colored colonies |
| 2 | Czapek’s sucrose agar | Excellent | White colored colonies |
| 3 | Glycerol-arginine medium (ISP-5) | Good | Cream colored colonies |
| 4 | Glucose yeast extract agar | Excellent | No pigmentation observed. |
| 5 | Glucose agar | Excellent | Brown colonies |
| 6 | Starch agar | Good | Yellow colonies |
| 7 | Starch casein agar | Excellent | Brown color |
Fig. 2.
Well diffusion method showing a zone of inhibition.
3.6. GC–Ms
After using the GC–MS to identify the compounds extracted from the isolate (D-3) we found that there are ten active compounds that have antimicrobial effect and other biological activity such as anticancer, antioxidant and cosmetics as shown in Fig. 3 and Table 7, Table 8.
Fig. 3.
GC–MS for the isolate D-3.
Table 7.
Compounds extracted from D-3 fraction.
| No. | Name of the compound | RT | Area % |
|---|---|---|---|
| 1 | Hexadecane, 2,6,11,15- Tetramethyl | 45.18 | 1.03 |
| 2 | Octacosane | 49.54 | 5.98 |
| 3 | Dodecanoic Acid, 1,2,3- Propane-triyl ester | 50.36 | 15.38 |
| 4 | Hexatriacontane | 50.90 | 8.57 |
| 5 | Heptacosane | 52.22 | 9.72 |
| 6 | Eicosyl Acetate | 54.04 | 0.70 |
| 7 | Tritetracontane | 54.80 | 9.58 |
| 8 | Tetracosane, 2,6,10,15,19,23-Hexamethyl | 59.26 | 36.24 |
| 9 | Myristic Acid vinyl ester | 60.32 | 10.44 |
| 10 | Tetratetracontane | 51.04 | 6.65 |
Table 8.
Chemical composition and biological activity of compounds extracted from D-3 using GC–MS.
| No. | Compound name | Molecular formula | Nature | Biological activity | Reference |
|---|---|---|---|---|---|
| 1 | Hexadecane, 2,6,11,15- Tetramethyl | C20H42 | Hydrocarbon | Flavoring agent | Pammi et al., (2021) |
| 2 | Octacosane | C28H58 | Hydrocarbon | Antimicrobial activity, Insecticidal activity | Khatua et al., (2016) |
| 3 | Dodecanoic Acid, 1,2,3- Propane -triyl ester | C39H74O6 | Fatty acid | hepatoprtective. b5-α reductase inhibitor, Flavour, Lubricant, Hypocholesterolemic, Cosmetic, | Achi and Ohaeri, (2015) |
| 4 | Hexatriacontane | C36H74 | Hydrocarbon | Antibacterial, antiviral, anticancer | Ibnouf, (2021) |
| 5 | Heptacosane | C27H56 | Hydrocarbon | Anti-oxidant activity, Antibacterial, Insecticidal | Khatua et al., (2016) |
| 6 | Eicosyl Acetate | C22H44O2 | Fatty acid | Anti-cancer | Ali et al., (2021) |
| 7 | Tritetracontane | C43H88 | Hydrocarbon | For treating wounds, ulcers, burns, scars and keloids |
Ibnouf, (2021) |
| 8 | Tetracosane, 2,6,10,15,19,23-Hexamethyl | C30H62 | Hydrocarbon | Pesticide, Chemopreventive, Immunostimulant, Cancer preventive, Antitumor, Antioxidant, Antibacterial, | Sudha et al., (2013) |
| 9 | Myristic Acid vinyl ester | C16H30O2 | Fatty acid | Antimicrobial, anticancer, cosmetics | Sujatha et al., (2020) |
| 10 | Tetratetracontane | C44 H90 | Hydrocarbon | Antibacterial | Kumari and Menghani (2021) |
4. Discussion
Actinomycetes are gram-positive, fungus-like hyphal bacteria that continue to be the most prolific natural antimicrobial producers (Sapkota et al., 2020). A number of chemical compounds were extracted from isolate D-3, and they have a biological effect on many microbes. We also found them to be highly effective against some types of microbes compared to some of the antibiotics used. Exposing isolate D-3 to ultraviolet rays increased the production of antimicrobial substances extracted from the isolate. Exposing isolate D-3 to ultraviolet rays increased the production of antimicrobial substances extracted from the isolate. This is consistent with Ibnouf (2021) when he treated his isolate O-7 with ultraviolet rays, which increased the production of antimicrobials. Also, the results are in agreement with Ashok et al. (2014).
Dodecanoic Acid, 1,2,3- Propane -triyl ester is one of the compounds that have been extracted from the isolate as an antimicrobial. Ibnouf (2021) extracted the same compound from isolates O-7 that belong to actinomycetes, and this compound acts as an antimicrobial as it is used in the manufacture of soap and shampoo. Sujatha et al. (2020) extracted the same compound from the plant Phymatosorus scolopendria, and he mentioned that this plant possesses bronchodilator activity, anti-inflammatory, and insect repellent properties. Achi and Ohaeri, (2015) extracted this compound from the leaves of the plant Cnidoscolus aconitifolius and mentioned the benefits of this compound in folkloric medicine.
The heptacosane compound is among the compounds that we extracted from isolate D-3 as an antimicrobial. Ibnouf (2021) extracted the same compound from actinomycetes as an antioxidant and antimicrobial agent. These results agree with the findings of Elsayed et al. (2020) when he extracted heptacosane with other compounds from actinomycetes. He stated that this compound had an anti-bacterial effect when he tested it against Bacillus cereus (ATCC33018) and E. coli O157 (ATCC93111) as model strains of gram-positive and gram-negative species. Lulamba et al. (2021) mentioned that he extracted this compound, and he also indicated that this compound is pathogenic to a wide range of insects.
Hexadecane, 2,6,11,15-Tetramethyl is one of the compounds that we extracted from isolate D-3, which showed antimicrobial activity. Subramanian et al. (2020) has extracted this compound from the Gymnema sylvestre plant, one of the medicinal plants found in the Indian subcontinent and Sri Lanka. This plant is well known for Diabetes mellitus, weight-reducing, against cancer, and anti-bacterial properties.
Kumari and Menghani (2021) extracted tetratetracontane compound from Rhizospheric Actinomycetes along with a number of other compounds, and it was found that these extracts, including tetratetracontane, have a very effective effect against some types of bacteria, and this is consistent with what we found in our research. Siddharthan et al. (2020) isolated the same compound and other compounds, including tetratetracontane, from Streptomyces diastaticus. These compounds exhibited activity against Candida albicans.
Our study in consistent with many previous studies revealed that actinomycetes secrete secondary metabolites with a variety of valuable properties, including the production of antibiotics, nutritional materials, cosmetics, enzymes, antitumor agents, enzyme inhibitors, immune modulators, and vitamins, as well as playing an important role in the recycling of organic matter in soil. As a result, they provide many important bioactive compounds with high commercial value and are routinely screened for new bioactive substances (Velayudham and Murugan, 2012, Deepa et al., 2014, Gopinath et al., 2013a, Gopinath et al., 2013b). Approximately two-thirds of naturally occurring antibiotics have been isolated from Actinomycetes with the majority of them coming from the genera Streptomyces and Micromonospora (Pandey et al., 2011).
5. Conclusion
The current study discovered that actinomycetes isolated from soil can produce antimicrobials susceptible to a broad spectrum of bacterial and fungal infections. In addition, the isolate D-3 outperformed the other isolates in terms of efficacy.
Funding information
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project number 2021/03/18770.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This publication was supported by the Deanship of scientific research at Prince Sattam bin Abdulaziz University.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Elmutasim O. Ibnouf, Email: eo.ahmed@psau.edu.sa.
Mohammed F. Aldawsari, Email: moh.aldawsari@psau.edu.sa.
Hisham Ali Waggiallah, Email: h.waggiallah@psau.edu.sa.
References
- Achi N.K., Ohaeri O.C. GC-MS determination of bioactive constituents of the methanolic fractions of Cnidoscolus aconitifolius. J. Pharm. Res. Int. 2015;5(3):163–172. [Google Scholar]
- Ali A., Ali A., Warsi M.H., Ahmad W. Chemical characterization, antidiabetic and anticancer activities of Santolina chamaecyparissus. Saudi J. Biol. Sci. 2021;28(8):4575–4580. doi: 10.1016/j.sjbs.2021.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-rubaye T.S., Risan M.H., Al-Rubaye D. Gas chromatography-mass-spectroscopy analysis of bioactive compounds from Streptomyces spp. isolated from Tigris river sediments in Baghdad city. J. Biotech. Res. Cent. 2020;14(1):63–71. [Google Scholar]
- Ashok G., Karthikeyan P., Panneerselvam A., Senthilkumar G. Effect of antimicrobial activity of UV mutated Actinomycetes SP isolated from mangroves. Eur. J. Exp. Biol. 2014;4(5):46–52. [Google Scholar]
- Baniya A., Singh S., Singh M., Nepal P., Adhikari M., Aryal S., Adhikari A. Isolation and screening of antibiotics producing Streptomyces spp from the soil collected around the root of Alnus nepalensis from Godawari. Nep. J. Biotech. 2018;6(1):46–56. [Google Scholar]
- Barka E.A., Vatsa P., Sanchez L., Gaveau-Vaillant N., Jacquard C., Klenk H.-P., Clément C., Ouhdouch Y., van Wezel G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016;80(1):1–43. doi: 10.1128/MMBR.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budhathoki S., Shrestha A. Screening of Actinomycetes from soil for antibacterial activity. Nep. J. Biotech. 2020;8(3):102–110. [Google Scholar]
- Butler M.J., Takano E., Bruheim P., Jovetie S., Marinelli F., Bibb M.J. Deletion of scb A enhancer antibiotic production in Streptomyces lividans. Appl. Microbiol. Biotech. 2003;61:512–516. doi: 10.1007/s00253-003-1277-8. [DOI] [PubMed] [Google Scholar]
- Chevrette M.G., Carlson C.M., Ortega H.E., Thomas C., Ananiev G.E., Barns K.J., Currie C.R. The antimicrobial potential of Streptomyces from insect microbiomes. Nat. Commun. 2019;10(1):1–11. doi: 10.1038/s41467-019-08438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deepa S., Kanimozhi K., Panneerselvam A. 16S rDNA Phylogenetic Analysis of Actinomycetes Isolated from Marine Environment Associated with Antimicrobial Activities. J. Drug Med. 2014;5:43–50. [Google Scholar]
- Elsayed T.R., Galil D.F., Sedik M.Z., Hassan H.M., Sadik M.W. Antimicrobial and anticancer activities of actinomycetes isolated from egyptian soils. Int. J. Curr. Microbiol. Appl. Sci. 2020;9(9):1689–1700. [Google Scholar]
- Ganesan P., Host R., Antony D., Appadurai D.R., Gandhi M.R., Paulraj M.G., Ignacimuthu S., Al-Dhabi N.A. Isolation and molecular characterization of actinomycetes with antimicrobial and mosquito larvicidal properties. BJBAS. 2017;6(2):209–217. [Google Scholar]
- Ghorbani N.R., Greiner R., Alikhani H.A., Hamedi J., Yakhchali B. Distribution of actinomycetes in different soil ecosystems and effect of media composition on extracellular phosphatase activity. J. Soil Sci. Plant Nutr. 2013;13(1):223–236. [Google Scholar]
- Gopinath B.V., Vootla P.K., Jyothi R., Reddy S.K. Antimicrobial Activity of Actinomycetes Isolated from Coal Mine Soils of Godavari Belt Region, A.P, India. Asia. J. Exper. Biol. Sci. 2013;4:518–523. [Google Scholar]
- Gopinath B.V., Vootla P.K., Jyothi R., Reddy K.S. Antimicrobial activity of Actinomycetes isolated from Coal Mine soils of Godavari Belt Region, A.P., India. Asia. J. Exp. Biol. Sci. 2013;4(4):518–523. [Google Scholar]
- Ibnouf E.O. Screening of O-7 Isolate Actinomycete Producing Antimicrobials in Different Growth Conditions against Selected Pathogens. IJPPR. 2021;11(2):13–23. [Google Scholar]
- Ilic-Tomic T., Genčić M.S., Živković M.Z., Vasiljevic B., Djokic L., Nikodinovic-Runic J., Radulović N.S. Structural diversity and possible functional roles of free fatty acids of the novel soil isolate Streptomyces sp. NP10. Appl. Microbiol. Biotechnol. 2015;99(11):4815–4833. doi: 10.1007/s00253-014-6364-5. [DOI] [PubMed] [Google Scholar]
- Khatua S., Pandey A., Biswas S.J. Phytochemical evaluation and antimicrobial properties of Trichosanthes dioica root extract. J. Pharmacog. Phytochem. 2016;5(5):410–413. [Google Scholar]
- Kumari N., Menghani E. Evaluation of antibacterial activity and identification of bioactive metabolites by GCMS technique from Rhizospheric Actinomycetes. Indian J. Nat. Prod. Resour. (IJNPR) [Formerly Natural Product Radiance (NPR)] 2021;11(4):287–294. [Google Scholar]
- Lulamba T.E., Green E., Serepa-Dlamini M.H. Photorhabdus sp. ETL Antimicrobial Properties and Characterization of Its Secondary Metabolites by Gas Chromatography-Mass Spectrometry. Life. 2021;11(8):787–800. doi: 10.3390/life11080787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Pammi N., Bhukya K.K., Lunavath R.K., Bhukya B. Bioprospecting of Palmyra Palm (Borassus flabellifer) Nectar: Unveiling the Probiotic and Therapeutic Potential of the Traditional Rural Drink. Front Microbiol. 2021;12:683996–684112. doi: 10.3389/fmicb.2021.683996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A., Imran A., Kailash S.B., Tanushri C., Vidyottma S. Isolation and Characterization of Actinomycetes from Soil and Evaluation of Antibacterial Activities of Actinomycetes against Pathogens. Int. J. App. Biol. Pharm. Tech. 2011;2:384–392. [Google Scholar]
- Pathalam G., Rajendran H.A.D., Appadurai D.R., Gandhi M.R., Michael G.P., Savarimuthu I., Naif A.A.D. Isolation and molecular characterization of actinomycetes with antimicrobial and mosquito larvicidal properties. BJBAS. 2017;6(2):209–217. [Google Scholar]
- Rajaram S.K., Ahmad P., Keerthana S.S.S., Cressida P.J., Moorthy I.G., Suresh R.S. Extraction and purification of an antimicrobial bioactive element from lichen associated Streptomyces olivaceus LEP7 against wound inhabiting microbial pathogens. JKSUS. 2020;32(3):2009–2015. [Google Scholar]
- Roitch M.C., Magiri E., Bii C., Maina N. Bioprospecting for broad spectrum antibiotic producing Actinomycetes isolated from virgin soils in Kericho country. Kenya Adv. Microbiol. 2017;7(1):56–70. [Google Scholar]
- Sapkota A., Thapa A., Budhathoki A., Sainju M., Shrestha P., Aryal S. Isolation, characterization, and screening of antimicrobial-producing actinomycetes from soil samples. Int. J. Microbiol. 2020;2020:2716584–2716591. doi: 10.1155/2020/2716584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebak M., Saafan A.E., Abdelghani S., Bakeer W., Moawad A.S., El-Gendy A.O. Isolation and optimized production of putative antimicrobial compounds from Egyptian soil isolate Streptomyces sp. MS. BJBAS. 2021;10(1):1–12. [Google Scholar]
- Sharma S. Isolation and Screening of Actinomycetes from Soil Sample of Dal Lake (Kashmir) Against Selected Pathogens. Int. J. Eng. Sci. Adv. Res. 2016;2(1):51–54. [Google Scholar]
- Siddharthan S., Rajamohamed B.S., Gopal V. Streptomyces diastaticus isolated from the marine crustacean Portunus sanguinolentus with potential antibiofilm activity against Candida albicans. Arch. Microbiol. 2020;202(7):1977–1984. doi: 10.1007/s00203-020-01918-8. [DOI] [PubMed] [Google Scholar]
- Subramanian S., Dowlath M.J.H., Karuppannan S.K., Saravanan M., Arunachalam K.D. Effect of Solvent on the Phytochemical Extraction and GC-MS Analysis of Gymnema sylvestre. Pharmacognosy J. 2020;12(4):749–761. [Google Scholar]
- Sudha T., Chidambaram Pillai S., Mohan V.R. GC-MS analysis of bioactive components of aerial parts of Fluggea leucopyrus Willd (Euphorbiaceae) J. Appl. Pharm. Sci. 2013;3(5):126–130. [Google Scholar]
- Sujatha S., Sara S.C., Gayathiri M., Roselin I.R., Ruby R.G.D. Analysis of Bioactive Compounds Present in Methanolic Extract of Phymatosorus Scolopendria (Burm. F.) Pic. Serm. Through Gas Chromatography and Mass Spectroscopy. IJPSR. 2020;11(7):3294–3299. [Google Scholar]
- Undabarrena A., Beltrametti F., Claverías F.P., González M., Moore E.R.B., Seeger M., Cámara B. Exploring the diversity and antimicrobial potential of marine actinobacteria from the comau fjord in Northern Patagonia, Chile. Front. Microbiol. 2016;7:1135–1151. doi: 10.3389/fmicb.2016.01135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayudham S., Murugan K. Diversity and Antibacterial Screening of Actinomycetes from Javadi Hill Forest Soil, Tamilnadu, India. J. Microbiol. Res. 2012;2:41–46. [Google Scholar]