Abstract
The receptor for advanced glycation end-products (RAGE) is a multiligand binding and single-pass transmembrane protein which actively participates in several chronic inflammation-related diseases. RAGE, in addition to AGEs, has a wide repertoire of ligands, including several damage-associated molecular pattern molecules or alarmins such as HMGB1 and members of the S100 family proteins.
Over the last years, a large and compelling body of evidence has revealed the active participation of the RAGE axis in tumor biology based on its active involvement in several crucial mechanisms involved in tumor growth, immune evasion, dissemination, as well as by sculpturing of the tumor microenvironment as a tumor-supportive niche. In the present review, we will detail the consequences of the RAGE axis activation to fuel essential mechanisms to guarantee tumor growth and spreading.
Keywords: advanced glycation, alarmins, receptor advanced glycation end-products, tumor biology, tumor microenvironment
Introduction
Tumor biology is characterized by a complex spectrum of alterations, ranging from aberrant intracellular events to those involving communication between cells and with neighboring tissues. In this setting, the tumor microenvironment (TME) is a dynamic niche where complex and reciprocal interactions are established, not only among cancer cells but also with a myriad of infiltrating immune and stromal cells, as well as the surrounding extracellular matrix (ECM) [1].
Under healthy conditions, pro- and anti-inflammatory signals are maintained in a state of balance, referred to as inflammatory homeostasis [2]. Dysregulation of this balance leads to the onset and development of numerous human diseases, including cancer, where chronic inflammation is a widely recognized contributor to shaping a supportive microenvironment for tumor growth and development [3–5].
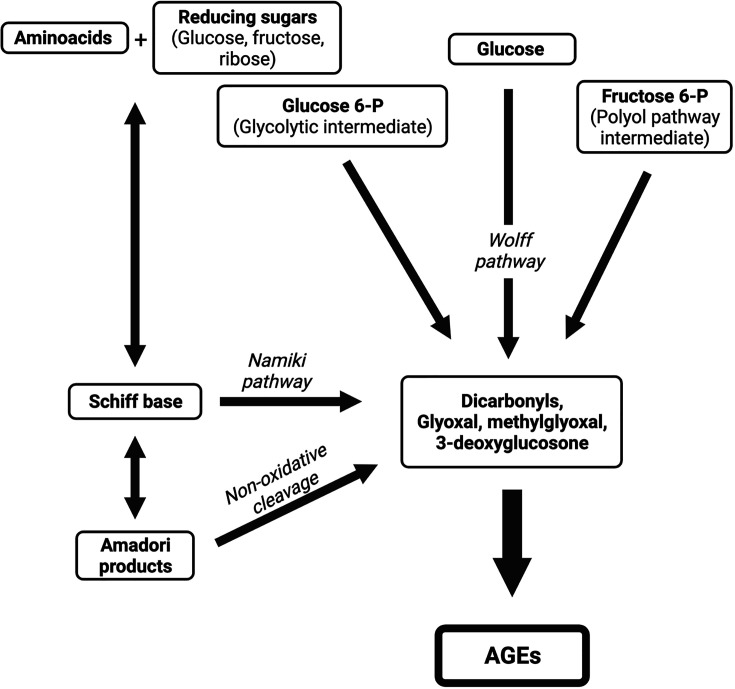
The protein, receptor for advanced glycation end-products (RAGE), was initially reported as the receptor for advanced glycation end-products (AGEs) [6], which are a broad and heterogeneous group of compounds derived from structural modifications of proteins, lipids, and nucleic acids, which become non-enzymatically glycated by reducing sugars [7]. Although these compounds were originally described in the well-known Maillard reaction, AGEs are also endogenously synthesized by a non-enzymatic reaction involving a glycation/condensation process between reducing sugars, such as glucose and fructose, and the free amino group of different biomolecules, such as proteins, lipids, and nucleic acids, to initially form Schiff bases, which are subsequently converted to intermediate glycation products, known as Amadori products [8].
Degradation of Schiff bases and Amadori products generates highly reactive short-chain carbonyl compounds, known as α-dicarbonyls, or α-oxaldehydes, such as oxoaldehydes glyoxal, methylglyoxal (MGO), and 3-deoxyglucosone, which can also be formed during glycolysis, as well as by glucose autoxidation in the presence of catalytic metals [9].
These α-dicarbonyl compounds are highly reactive, and participate in the formation of intra- or inter-protein cross-links. The production of α-dicarbonyls proceeds by different pathways, including the Namiki pathway, when a Schiff base degrades and forms glyoxal, and the Wolff pathway, which involves the autoxidation of monosaccharides. In addition, other metabolic intermediates have been implicated in α-dicarbonyl production, including glycolytic intermediates (glucose-6-phosphate, glyceraldehyde-3-phosphate, and dihydroxyacetone phosphate) and fructose-6-phosphate (polyol pathway intermediate) [10,11] (Figure 1).
Figure 1. Different pathways are involved in advanced glycation end-product formation.
AGEs are endogenously synthesized by a non-enzymatic reaction involving a glycation/condensation process between reducing sugars and the free amino group of different biomolecules to form Schiff bases, which are subsequently converted to Amadori products. Degradation of both Schiff bases (Nakimi pathway) and Amadori products (non-oxidative cleavage) renders the main intermediate compounds in the formation of AGEs. These highly reactive intermediates are also formed by other metabolic intermediates
Data raised from both in vitro and in vivo studies supported that MGO-driven biological activities are positively related to cancer onset and progression. MGO is considered a genotoxic agent based on its capacity to produce oxidative damage to DNA and DNA adducts as well [12,13]. MGO-modified proteins, such as heat shock protein (Hsp) are responsible to protect cancer cells from apoptosis, as reported in non-small cell lung cancer and gastrointestinal neoplasia [14,15]. Furthermore, accumulation of MGO adducts are associated with tumor aggressiveness in colorectal cancer [16].
Although AGEs were initially recognized as formed in excess in diabetes, due to hyperglycemia, exogenous AGEs, mostly derived from dietary intake, are important contributors to the physiological AGEs pool [11,17]. In this setting, emerging data support a potential role for high dietary AGEs intake in human carcinogenesis, particularly of gallbladder cancer, because of their pro-inflammatory and pro-oxidative properties [18].
The formation of AGEs also occurs in cancer cells through the ‘Warburg effect’, which leads to increased production of MGO, a reactive dicarbonyl known to be the major precursor of AGEs. This increased formation and accumulation of MGO in cancer cells promotes tumor development and progression [19–21].
Soon afterward its discovery, the binding capacity of RAGE was found to be extended to other ligands, beyond AGEs, such as the alarmin, high mobility group box 1 (HMGB1), and members of the S-100 calgranulins family. These molecules are abundant in the TME of most solid tumors, thus increasing the complexity of intracellular signaling networks involving RAGE [22–26].
Engagement of RAGE by its ligands initiates complex signaling pathways, including activation of NADPH oxidase, p21ras GTPase, several kinases (such as ERK1/2 (p44/p42) MAP kinases, p38 and SAPK/JNKMAP kinases, and phosphoinositol-3 kinase (PI3K)), and rho GTPases, as well as JAK/STAT pathways, with crucial downstream inflammatory consequences, including activation of NF-kB, AP-1, and Stat-3. Notably, activation of NF-kB up-regulates RAGE expression itself, thus generating a positive feed-forward loop, causing more inflammation [27].
Since the pioneering work of Taguchi et al. [28], who demonstrated that blockage of RAGE decreased tumor growth and metastases, the RAGE axis has emerged as a new actor in tumor biology, and its contribution to tumor growth and development has been widely documented in various types of cancer.
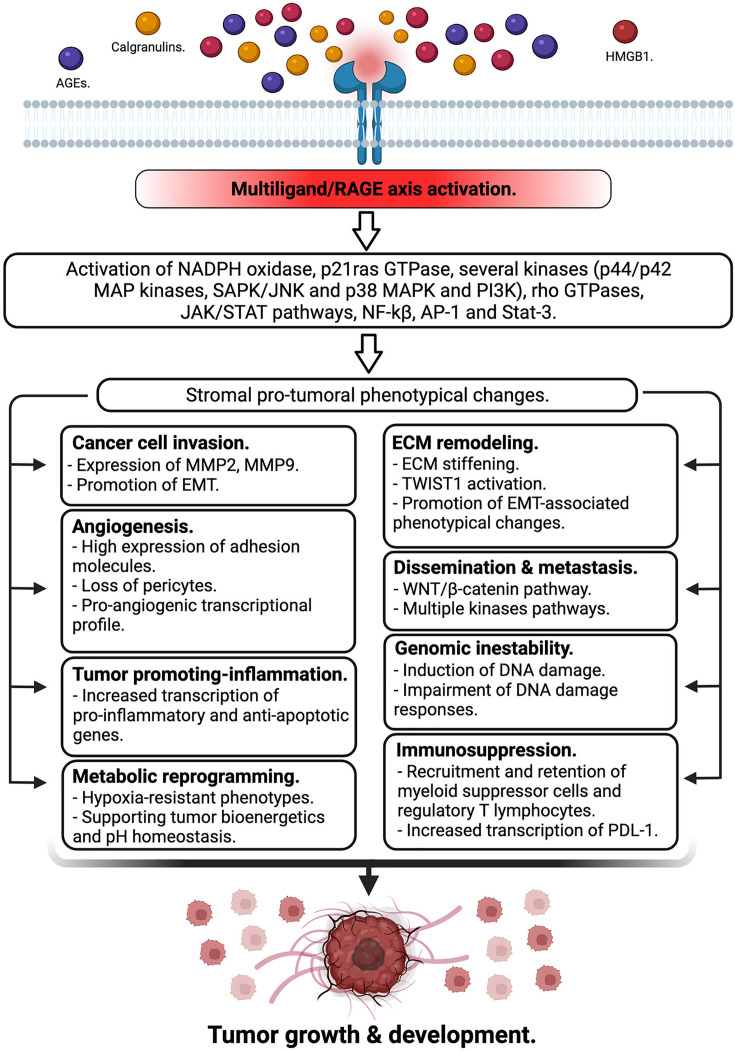
In the present review, we highlight how the RAGE/multiligand axis has emerged as a relevant actor in tumor biology, based not only on the high diversity of signaling cascades triggered upon its activation, but also on the wide repertoire of ligands of this axis, as well as their relative abundance in the TME. We discuss how activation of the axis can promote numerous crucial steps during tumorigenesis, from genetic instability, fueling chronic inflammation and supporting many phenotypic changes in tumor cells favoring their growth and dissemination, to the onset of an immunosuppressive environment that restricts host immune responses, and thus support tumor growth and development (Figure 2).
Figure 2. RAGE axis activation in the tumor microenvironment.
RAGE is either expressed by tumor cells or infiltrating immune cells in the tumor microenvironment. Many of its ligands are also abundantly present in the same tissue niche. The activation of this multilgand receptor triggers different signaling cascades promoting stromal pro-tumoral phenotypical changes mediated by a myriad of mechanisms supporting tumor growth and development.
Cancer cell invasion
Epithelial–mesenchymal transition (EMT) is a form of trans-differentiation of epithelial cells into mesenchymal phenotypes, which plays a key role in many physiological processes, such as embryogenesis and tissue morphogenesis, wound healing, and regulation of stem cell behavior [29]; however, the onset of malignancy of many types of cancer cell, and quite possibly all of them, also depends on EMT activation [30]. This switch in cell phenotype and behavior is mediated by fine regulation of growth and transcription factors, as well as specific signaling pathways that respond to extracellular stimuli [31].
Activation of the RAGE/multiligand axis can strongly influence cell invasion through a wide variety of molecular signaling pathways, which converge in promoting cancer growth, invasion, and metastasis through enhancement of EMT [32–34]. Further, emerging studies have demonstrated effective repression of EMT in experimental RAGE knockout models, highlighting the central role of both AGEs and multiligand/RAGE axis activation in promoting enhanced EMT and contributing to highly invasive malignancies through multiple phenotype changes induced in the ECM, TME, and stromal cells [35,36]. Tumor-associated immune cells, as well as cancer cells themselves, can release high levels of the alarmin, HMGB1, within the TME of a wide variety of malignancies [37,38]. HMGB1 and its subsequent binding to RAGE can trigger the production of proinflammatory cytokines through NF-kB activation [39–42], as well as the expression of pro-invasive and proteolytic matrix metalloproteinases (MMPs), such as MMP2 and MMP9, leading to tumor invasion and metastasis [43].
The central role of the RAGE/HMGB1 axis in contributing to EMT in a PI3K/AKT-dependent manner widely documented in prostate cancer [44–46], and colorectal cancer [33].
In addition, overexpression of the RAGE ligands, S100A7, S100B, S100A4, and S100A8/A9, occurs in both triple-negative breast cancer and colon cancer. In each of these neoplasias, over-expression of these alarmins can increase RAGE expression and fuel an inflammatory milieu at the TME through NF-kB activation-dependent mechanisms, thus favoring invasive properties of these cancer cells [47,48].
Dissemination and metastasis
The metastatic spread of malignant cells from a primary tumor to distant organs is considered the principal cause of cancer morbidity and lethality [49]. Cancer cell dissemination is supported by various cellular mechanisms, including neoplastic cell growth, local invasion of surrounding tissue, entry of circulating tumor cells within the microvasculature of the lymph and blood systems, escape from immune surveillance, and adaption to foreign microenvironments of distant niches, to facilitate cell proliferation and the formation of secondary macroscopic malignant tumors [50,51]. There are convincing data supporting a crucial role for RAGE and its ligands in facilitating tumor growth, progression, and metastatic spread of several types of malignant tumor [44–48,52].
RAGE engagement by HMGB1, S100A8/A9, or S100P proteins can activate different oncogenic pathways, supporting the metastatic spread of colorectal cancer cells. Hence, activation of RAGE by all of these ligands can trigger multiple signaling pathways, including K-RAS, ERK1/2 MAP kinases, p38 and SAPK/JNK MAP kinases, the WNT/β-catenin pathway, NF-κB, AP-1, and mTOR, causing downstream secretion of important inflammatory and apoptotic markers, as well as the upregulation of oncogenic miRNAs, such as miR-21 and miR-155 [53–57].
Interestingly, in vitro essays have revealed that activation of the RAGE/multiligand axis also significantly increases the metastatic potential of breast cancer cells, throughout activation of the RAGE/TLR4/MyD88/NF-kB signaling pathway, which results in elevated expression of MMP9, thus contributing to cancer migration and invasion by degrading ECM in patients with breast cancer [58].
The role of the RAGE axis on lung cancer remains controversial when compared with other cancers. Although RAGE is highly expressed in normal alveolar epithelium, it is surprisingly reduced in lung carcinomas. Some reports show that lung cancer progression may be enhanced by the RAGE downregulation in human lung carcinomas [59]. Novel experiments have demonstrated that RAGE activation promotes lung cancer metastasis in vivo, by supporting cell migration and EMT through ERK1/2-mediated activation of Snail, Slug, and Twist in lung adenocarcinoma cells [60]. This report has shed light on this controversy, based on the identification of a dual role of RAGE, which might cause growth inhibition in the early tumor formation while promoting EMT and providing a beneficial TME for tumorigenesis in lung adenocarcinoma.
ECM remodeling in the TME
The ECM is a heterogeneous and well-orchestrated network of cell-secreted molecules that offer biochemical and structural support to cells, tissues, and organs [61]. In humans, the ECM is composed mainly of water, fibrous proteins, and various polysaccharides, providing both mechanical and biochemical support to tissues, and regulating diverse cellular functions, such as proliferation, survival, growth, differentiation, and maintenance of tissue homeostasis [62].
This complex network is considered highly dynamic, and undergoes continuous remodeling driven by close interplay among ECM components and surrounding cell and non-cellular elements [63]. During those different ECM interactions, several bidirectional signaling pathways are activated through mechanotransduction receptors, which can regulate cell behavior at local and systemic levels in response to modifications detected in the composition and stiffness of the ECM [64,65]. There is increasing evidence that ECM remodeling and stiffening act as key modulators of cell fate and function [66]. The role of tumor-associated ECM modifications in cancer cell biology has been extensively studied, particularly in most solid tumors where desmoplasia is a very common feature that leads to fibrosis through abnormal extracellular matrix (ECM) deposition, remodeling, and post-translational modifications. All these changes are crucial in the progression of the disease toward an increasingly aggressive and invasive tumor phenotype, mainly by forming a scaffold in the TME and contributing to all cancer hallmarks [67,68].
Strikingly, most ECM fibrous proteins are long-lived potential targets of AGE formation, resulting in ECM stiffening which generates mechanical cues that act on many stromal cellular components, including cancer cells, and thus stimulating cell transdifferentiation, EMT, and cell migration and invasion [69–71].
AGEs and AGEs precursors such as dicarbonyls are highly abundant in TME because of the ‘Warburg effect’ in cancer cells [19]. Glycation of ECM proteins mainly occurs at functionally significant arginine residues of arg-gly-asp (RGD) and gly-phe-hyp-gly-glu-arg (GFOGER) motifs generating marked structural distortion and loss of charge, and thus increasing the stiffness of the fibrous components of ECM, such as collagens [72].
The accumulation of highly reactive AGE precursors, such as MGO, and the increased rate of AGE formation not only mediates structural and mechanical changes in the ECM, but can also generate a reservoir of AGEs with the potential to trigger a multitude of pro-tumorigenic RAGE-dependent mechanisms, including altered cell adhesion, increased invasion, and enhanced migration, thus favoring cancer metastasis [73].
Recently, tumor ECM post-translational modifications derived from the accumulation of endogenous intermediates of AGEs, such as MGO, were reported to act as key players involved in cancer onset and progression [19], as well as migratory signaling pathway activation, thus favoring metastatic dissemination of breast and colorectal cancer cells [74,75].
In solid tumors, fibronectin and type I collagen are the most common and abundant fibrillar proteins found in cancer-associated ECM [76,77]. Their accumulation in tumor stroma creates a very dense network of ECM fibrillar proteins, which results from excessive fibrotic remodeling, largely mediated by myofibroblasts expressing alpha-smooth muscle actin. This remodeling leads to extensive modification of surrounding tumor cells and contributes to fibrotic changes in cancer cells, which enhances tumor cell aggression, promoting invasion and migration into stromal tissue [78,79]. Additionally, an extensive transformation of fibroblast-type cells to a myofibroblastic phenotype is also associated with increased tumor interstitial fluid pressure [80]. This biophysical alteration of the TME interferes with the transcapillary transport of therapeutic agents, decreasing tumor uptake of drugs, and consequently decreasing the efficacy of chemotherapy [81], particularly in cancer types with extensive desmoplastic reactions, such as breast and pancreatic cancer [82–84].
Furthermore, AGE-mediated cross-linking of load-bearing proteins leads to ECM stiffening, which promotes cancer cell survival as well as high rates of invasion, proliferation, and metastatic tumor cell interaction with the endothelium, and favors pro-angiogenic tumor phenotypes [73,85,86]. This AGE-induced ECM stiffness is associated with several phenotypic changes linked to the EMT, such as increased expression of vimentin and reduced expression of E-cadherin, which can contribute to the development of pre-metastatic niches in surrounding tissues, supporting the migration and invasion of tumor cells into future sites of metastasis, even before their arrival [79,86–88].
In addition, ECM stiffening can induce integrin-dependent phosphorylation events, resulting in the release of essential regulators of matrix-stiffness-induced EMT, such as the TWIST1 transcription factor, which is untethered from its cytoplasmic anchor, G3BP2, thus favoring its entrance to the nucleus to drive a transcriptional profile supporting cancer cell invasion in epithelial neoplasias [89]. Moreover, a novel cross-talk mechanism occurs, in which ECM stiffening mechano-sensitizes human malignant cells to drive invasiveness and migration in an EGFR-dependent manner. Squamous carcinoma cancer cells can respond to matrix stiffening, by increasing EGFR expression, thus sensitizing carcinoma cells to EGFR phosphorylation, resulting in increased actomyosin contractility and collective invasion [90].
Inflammation in the TME
There is a growing body of evidence supporting a contribution of inflammation to the development of different malignancies through a complex network of mechanisms that represent an active landscape for tumor initiation, progression, and invasion [91–97]. Most cancer cells not only overexpress RAGE but also release high concentrations of its ligands [44,98–100]. These factors lead to increased survival of disseminated tumor cells and consequently increase the metastatic burden in a RAGE-dependent manner [101–103]. As mentioned above, activation of the AGE/RAGE axis triggers a robust pro-inflammatory response, and consequent increased leukocyte activation and apoptosis, enhanced desmoplastic reactions, and recruitment of stromal cells into the TME [104–109]. In this context, the contribution of NF-kB-dependent pathways to induction of pro-inflammatory genes is well-documented, and is key in fueling a pro-inflammatory milieu, in both tumor and tumor-associated cells, as well as surrounding host tissues [110–113]. Notably, a key consequence of RAGE-induced NF-kB activation is transactivation of genes encoding several pro-inflammatory factors, such as TNF-a, COX-s, iNOS, IL-1, and IL-6, as well as proangiogenic factors (vascular endothelial growth factor (VEGF)) and anti-apoptotic signals (BcL-X, BcL-2, XIAP) [114–119].
The increased rate of release of many RAGE ligands by cancer cells can act in both autocrine and paracrine dependent-manners on RAGE-positive cells at the tumor-host interface, to promote cancer cell survival [29,120,121].
A convincing body of evidence shows both increased expression of HMGB1 in several solid tumors and its critical role as an emerging prognostic factor in prostate cancer, breast cancer, and gastric cancer [122–125]. Biological responses downstream of HMGB1 are implicated in promoting tumor proliferation, migration, and invasion by stimulating production of pro-inflammatory cytokines through RAGE-dependent pathways [37]; however, HMGB1 can signal through TLRs (TLR2 and TLR4), as well as RAGE, thereby triggering NF-kB, STAT-3, and MyD88-dependent pathways and promoting inflammation and tumorigenesis [126–128]. Given the similarities between TLRs and RAGE and their signaling cascades, RAGE has been proposed as an emerging non-canonical Toll receptor [129]. Consequently, it is reasonable to consider that HMGB1-activation of both RAGE and TLRs can enhance the recruitment and assembly of homo- and hetero-oligomers, to strengthen pro-inflammatory responses in the TME and stimulate acquisition of a hypoxia-resistant phenotype in hepatocellular carcinoma and breast cancer cells [130,131]. The S100 protein family members, S100A8A/S100A9 [132], which form an S100A8/A9 heterodimeric complex able to interact with both TLR4 and RAGE on tumor cells, are also key in promoting RAGE-mediated inflammatory responses [133,134]. S100A8/A9/RAGE axis activation act as a novel pro-inflammatory signaling cascade in prostate, breast, and pancreatic cancers, triggering multiple downstream pro-inflammatory signaling pathways within the TME, thus promoting tumor cell survival, progression, and metastasis [135,136]. In addition, emerging clinical evidence suggests that tumor-associated S100 protein levels have potential as a new prognostic biomarker and therapeutic target in patients with breast and pancreatic cancers [136,137]. In addition, several studies support an association between NF-kB and hypoxia-inducible factor 1 (HIF-1), where NF-kB is a key transcriptional activator of constitutive HIF-1α, requiring basal NF-kB activity for HIF-1 protein accumulation under hypoxic conditions [138,139].
Angiogenesis
The formation of new blood vessels from pre-existing vasculature is a major mechanism of vascularization during embryonic development and some physiological processes, such as wound healing; however, angiogenesis is also crucial in the delivery of oxygen and nutrients to cancer cells, which produce several proangiogenic factors to overstimulate angiogenesis and thereby support tumor survival, growth, and metastasis [140].
There is a compelling body of evidence supporting that activation of the multiligand/RAGE axis is important in tumor associated-angiogenesis modulation, by triggering upregulation of VEGF and MMP2, as well as disruption of VE-cadherin-catenin complexes, thereby favoring capillary tube formation [141,142]. Additionally, the excessive capillary tube formation induced through AGE-mediated RAGE signaling may also involve increased expression of several scavenger receptors, such as CD36, CD136, and LOX1, and their subsequent activation by AGEs [143]. The crucial role of RAGE signaling in tumor angiogenesis has been highlighted by studies using experimental methodologies aimed at both gene silencing and receptor blockade in different cancer types [144,145].
Strikingly, the pro-inflammatory signaling cascades induced by RAGE activation also directly reduce pericyte numbers, which in turn relieves the restriction of endothelial cell (EC) replication, and thus facilitates new blood vessel formation [146].
Tumor overexpression of the alarmin, HMGB1, and subsequent activation of RAGE signaling induces secretion of pro-inflammatory cytokines, such as TNF-α and IL-8, as well the expression of leukocyte adhesion molecules (ICAM1, VCAM1, and E-selectin) through NF-kB activation in ECs, thus stimulating EC proliferation and sprouting in vitro and neovascularization in vivo [147]. Notably, HMGB1 may also favor endothelial progenitor cell homing and increase their neovascularization capacity in a RAGE/TLR4-dependent manner, thus promoting tumor angiogenesis [148]. Recently, internalization of HMGB1 has been reported as a novel mechanism by which this alarmin induces angiogenesis in ECs through a RAGE-mediated pathway [149]. Hence, HMGB1/RAGE axis activation in ECs not only triggers both positive autocrine and paracrine mechanisms to promote a pro-angiogenic gene expression profile, [148–150] but also stimulates VEGF production by some pro-tumoral stromal cells, such M2 macrophages [151]. Furthermore, in vivo and in vitro data suggest that HMGB1 is crucial for the activation of a positive loop promoting the tumoral pro-angiogenic response by increasing the expression of both RAGE and TLR4, and consequently their pro-inflammatory signaling cascades, which perpetuate the transcription of pro-angiogenic and pro-inflammatory genes [150,152,153]. Activation of the multiligand/RAGE axis by members of the S100/calgranulin protein family contributes to fueling the pro-angiogenic tumoral phenotype [154]. S100A7-mediated RAGE activation is crucial in modulating pro-inflammatory and angiogenic pathways in many cancer types, particularly in cervical [101], breast [47,155,156], and esophageal squamous cell carcinoma [157]. During mammary tumorigenesis S100A7 increases the expression of reactive oxygen species (ROS) and VEGF by RAGE-dependent mechanisms, thus enhancing cancer cell progression by promoting oxidative stress responses and angiogenesis [47,158].
Metabolic disorders, such as obesity and insulin resistance, are associated with poor prognosis and development of highly aggressive breast cancer cells, and are thus critical factors mediating survival prognosis across all stages of breast cancer [159]. Furthermore, patients with these comorbidities show an altered expression profile of both RAGE and the IGF-1/IGF-1R axis, favoring STAT3-dependent transcriptional activation of the S100A7 gene, thus enhancing many mammary cell S100A7/RAGE-dependent pathways, such EC proliferation and angiogenesis [156].
The S100 protein family member, S100A4, is a key factor in several biological functions mediated by RAGE activation, which trigger pro-inflammatory and pro-angiogenic responses to stimulate tumor progression and invasion [27,154]. Interestingly, several growth factors, such as FGF2, may induce upregulation and release of S100A4 through FGFR1, thus favoring pro-angiogenic and pro-tumoral transduction pathways triggered through the S100A4/RAGE axis in triple-negative breast cancer cells [160].
The role of nitric oxide (NO) in tumor angiogenesis remains controversial. NO can mediate angiogenesis by direct and indirect mechanisms [161], while anti-angiogenic effects of NO have also been also reported [162]. This apparent incongruity may be attributable to differences in concentration or cellular compartment, as well as duration of exposure [163,164].
Due to the pro-inflammatory nature of the TME, many stromal cell types, including cancer cells, express inducible nitric oxide synthase (NOS-II), which is upregulated by activation of the RAGE axis [165,166]. Recent reports support a role for NOS-II-derived nitric oxide as an important mediator of tumor growth and vessel maturation [167,168]. Furthermore, NO can induce the synthesis and activation of HIF-1α, which in turn up-regulates VEGF [169].
Recent studies have demonstrated, using in vitro and in vivo assays, that variations in NO flux into the TME induced by NOS-II or TLR/RAGE agonists contribute to HIF-1α stabilization, thus leading to transcription of numerous pro-angiogenic target genes, including VEGF [170,171].
Metabolic reprogramming
Cell proliferation is a crucial hallmark in cancer development and progression, and tumors are forced to reprogram metabolic pathways involved in nutrient uptake and metabolism, to fulfill their high-energy requirements, produce biomolecules on demand, and maintain the redox balance [172–175]. The RAGE axis has emerged as a crucial actor in reprogramming different metabolic pathways, which are essential to ensure cancer cell progress.
A distinctive feature of many cancer types is the presence of a hypoxic TME. During tumor development and progression, both tumor and stromal cells have restricted access to nutrients and oxygen, either permanently or transiently, mainly due to aberrant vascularization and poor blood supply. This hypoxic microenvironment can stimulate HIF-driven transcriptional responses, which are involved in numerous adaptive metabolic changes, such as the switch from oxygen-dependent mitochondrial oxidative phosphorylation to oxygen-independent glycolysis, thus increasing glucose consumption and pyruvate, lactate, and H+ production, to meet their energy requirements [175–177].
The RAGE axis has emerged as a relevant actor in the hypoxic microenvironment. In a hypoxic milieu, the abundance of RAGE ligands is increased, particularly HMGB1, which is released by either infiltrating leukocytes or cancer cells themselves, under hypoxic conditions [178].
Disturbances in intracellular calcium homeostasis are a hallmark of hypoxia, linked to HIF-1α expression and stabilization [179]. Hypoxia-mediated cell damage, as well as the activation of immune cells by inflammatory signals, allows the release of S100 proteins to the extracellular space as damage-associated molecular pattern (DAMP) molecules, also known as alarmins, and then they can be recognized by and activating RAGE-mediated signaling [22,23,180].
Interestingly, the RAGE ligand, S100A10, has been reported to accelerate aerobic glycolysis and tumor growth by activating mTOR signaling through a RAGE-dependent mechanism [181].
Cancer cells exhibit a particular glucose metabolism characterized by increased glucose uptake, accompanied by the overexpression of glucose transporters, which are hypoxia-responsive elements [182,183].
Furthermore, hypoxic tumor cells shows a metabolic shift from mitochondrial aerobic respiration to anaerobic glycolysis process, where, di-carbonyl compounds, a major precursor of AGE formation, are generated [184,185]. Consequently, hypoxia-driven AGE accumulation, favoring activation of RAGE-dependent signaling, has been extensively reported in cancer cells [186].
Furthermore, pyruvate kinase muscle isozyme M2 (PKM2) is a rate-limiting glycolytic enzyme that catalyzes the final step in glycolysis [187]. PKM2 interacts with HIF-1α [188] and activates transcription of glycolysis-related genes, such as glucose transporter 1, which increases glucose uptake, and lactate dehydrogenase A, which increases lactate production, leading to excessive lactate production and HMGB1 hyperacetylation and its subsequent release in the TME [189].
In addition to its contribution by increasing the bioavailability of RAGE ligands, hypoxia itself may increase RAGE expression, because the promoter region of the RAGE gene contains at least one functional hypoxia response element [190–192]. Consequently, different groups have reported increased RAGE expression in the hypoxic TME [193,194]. Furthermore, increased RAGE expression and signaling in a hypoxic environment can also result in RAGE-dependent activation of HIF-1α [193], thus generating a potent amplifying loop.
Monocarboxylate transporters (MCTs) are crucial cellular regulators of cancer pH homeostasis, particularly within tumor cells with high glycolysis rates [195,196], and their plasma expression and activities, particularly those of MCT1 and MCT4, require the presence of the chaperone, CD147 [197,198]. Notably, CD147 expression appears to be regulated by RAGE-mediated signaling, since blocking RAGE suppressed the induction of CD147 expression [199]. Considering the high activation levels of the RAGE axis in the TME, this may favor the bioavailability of this chaperone in this niche, thus supporting the efficient functioning of these crucial pH regulators in cancer cells.
Interestingly, Kang et al. proposed that inflammatory signals within the TME, such as HMGB1, are crucial for the promotion of tumor bioenergetics, and thus support tumor progression. In support of this, HMGB1 is reported to promote RAGE translocation to mitochondria, leading to enhanced complex I activity and increased ATP production, through an ERK1/2 phosphorylation-mediated mechanism [200].
Adipose tissue regulates physiological energy balance [201], acting as a complex organ that stores lipids in adipocytes as an energy source and can release them by responding to physiological demands [202].
Tumors, either locally or during metastatic dissemination, are related closely with adipose tissue. For many cancer types, and particularly for breast, prostate, and ovarian carcinomas, adipocytes from adipose tissue establish a cooperative cross-talk with cancer cells, where adipocytes provide adipokines and lipids to cancer cells, while stromal and immune cells from AT also secrete paracrine factors within the tumor microenvironment to promote cancer survival, proliferation, metastasis, and treatment resistance [202–206].
Emerging data support that an intense reprogramming of many metabolic activities occurs when cancer cells are exposed to hypoxic conditions [207]. One of these metabolic changes involves the increased utilization of lipids, and particularly the use of fatty acids (FAs) as a source of energy through β-oxidation [208–211]. Even with sufficient dietary lipid supply, cancer cells can synthesize most FAs de novo [212]; however, these capacities are sometimes insufficient, as in some aggressive cancers, where tumor cells take up extracellular FAs from surrounding adipocytes [213–217].
CD36, an FA translocase, is a crucial molecule in FA transport from adipocytes to cancer cells [218], and overexpression of CD36 is associated with tumor progression and metastasis [219,220]; crucially, CD36 is up-regulated by RAGE-mediated signaling [220,221].
Immunosuppression in the TME
There is both experimental and clinical evidence that, while tumors grow, the stroma becomes in an immunosuppressive niche [221]. In this context, the contribution of the RAGE axis to immunosuppression in the TME has been extensively documented in recent years.
RAGE activation by members of the calgranulins family (S100A8 and S100A9) contributes to the recruitment and retention of myeloid suppressor cells, as well as increasing the expression of both the alarmins, S100A8/A9, and the activity of inducible nitric oxide synthase and arginase-1, and thus favors tumor growth and metastasis [222–224].
HMGB1 is present in high concentrations in the TME mainly due to its production or release by both tumor cells and infiltrating inflammatory cells, and thus favors the establishment of a highly immunosuppressive TME [37]. HMGB1 can promote the influx of myeloid suppressor cells into the TME as well as the recruitment and activation of regulatory T lymphocytes by RAGE-dependent mechanisms [225–227].
Tumor-associated macrophages, and particularly M2-type macrophages, play a significant immunosuppressive role by secreting immunosuppressive molecules, such as IL-10, TGF-β, and human leukocyte antigen G [228]. Interestingly, hypoxia and the concomitant presence of HMGB1, are reported to promote M2-macrophage retention in the tumor hypoxic core of the TME, through a RAGE-dependent mechanism, by decreasing both the expression of CCR2 and the migration capacity of M2 macrophages [107].
PD-L1 is an immune checkpoint protein that helps cancer cells to escape from immunosurveillance [229]. RAGE activation by HMGB1 promotes NF-κB- and IRF3-dependent transcription of PD-L1 [230].
Cancer genomic instability
The genomic instability in cancer cells is crucial in generating intratumoral genetic heterogeneity, thus supporting the extensive phenotypic diversity observed in many cancer types [231]. The ROS produced by inflammatory cells in the TME represents a crucial source of DNA damage in both tumor and stromal cells. These TME changes caused by oxidative stress may contribute to tumor development and even tumor spreading [232]. Decades of both clinical and experimental research have demonstrated that ROS are essential mediators in tumor biology, by various mechanisms, including induction of mutations in tumor suppressor genes and oncogenes and the activation of various oncogenic signaling pathways, as well as oxidative inactivation of several DNA repair enzymes, among many others [233–235].
Since the pioneering work of Wautier et al., demonstrating that the engagement of RAGE by AGEs triggers ROS generation by NADPH oxidase activation [236], several reports have supported that NADPH oxidase activation is a crucial event in RAGE signaling. As AGE formation is increased under high ROS levels, and the subsequent activation of RAGE by AGEs also leads to ROS production, the onset of a vicious cycle is highly potentiated [27,237,238].
The capacity of ROS to induce DNA damage and affect the DNA damage responses, in particular, the formation of 8-oxo-7,8-dihydroguanine, the most prevalent purine base oxidation, with highly mutagenic potential [239], has been widely documented. Furthermore, increased ROS can also trigger genomic instability, leading to DNA double-strand breaks and altered repair capacity, due to the generation of dysfunctional DNA repair enzymes [240].
As the RAGE cytoplasmatic (ctRAGE) domain lacks kinase activity in all mammals cells, including malignant cells, the mammalian diaphanous-1 (mDia1) protein is used to integrate oxidative and signal transduction pathways when RAGE is engaged [241–243]. Hence, many ctRAGE dependent signaling pathways are regulated through mDia1 [244].
Very recently, a nuclear isoform of RAGE (approximately 64 kDa) was identified as a positive regulator of DNA double-strand break repair via the mechanism of homologous recombination [245]. As the membrane-bound isoform of RAGE is over-expressed in multiple human cancers, it is tempting to speculate that changes in the expression/trafficking of this isoform may occur during malignant transformation, leading to decreased DNA repair capacity and increased genomic instability.
Concluding remarks
There are currently lines of evidence drawn from clinical and experimental data supporting the role of the RAGE/multiligand axis in remodeling the TME to support tumor growth and development, based on the positive impacts of activation of the axis on relevant processes in tumor growth and development. Most cancer types overexpress RAGE, and some of its ligands are highly abundant in the TME. Hence, the potential use of RAGE as a biomarker of prognosis, as well as the therapeutic use of blocking RAGE/multiligand signaling has emerged as an area of intense research, based on data raised from many approaches such as gene-silencing technologies, in vivo RAGE knockout models, and the use of aptamers, and small synthetic molecules.
Additionally, many efforts are further required to understand the clinical significance of different soluble variants of the receptor. A widely held view is that soluble RAGE fulfills a protective anti-inflammatory role by acting as a decoy receptor, binding RAGE ligands and thus blocking their interaction with the full-length receptor. Some promising results indicate that decreased sRAGE levels in patients may contribute to the progression of the disease. However, these findings require much research because many conflicting findings between different studies that have been reported could be related to the demographic, genetic, and health characteristics of the populations under investigation.
However, some questions remain without answering before the whole comprehension of the role of the RAGE/multiligand axis on tumor biology can be achieved. One crucial and intriguing issue is the cross-talk of tumor cells with infiltrating immune cells and other stromal cells, and the characterization of all regulation mechanisms of the RAGE axis requires further investigation. Finally, intense research efforts are required to achieve an integrative comprehension of the consequences of proteome glycation in both cancer and stromal cells.
Abbreviations
- EC
endothelial cell
- FA
fatty acid
- HMGB1
high mobility group box 1
- MCT
monocarboxylate transporter
- NO
nitric oxide
- PKM2
pyruvate kinase muscle isozyme M2
- RAGE
receptor for advanced glycation end-products
- ROS
reactive oxygen species
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
CRediT Author Contribution
Armando Rojas: Conceptualization, Writing—original draft, Writing—review & editing. Ivan Schneider: Conceptualization, Data curation, Writing—review & editing. Cristain Lindner: Conceptualization, Artwork, Data curation, Writing—review & editing. Ileana González: Conceptualization, Data curation, Writing—review & editing. Miguel A. Morales: Conceptualization, Data curation, Writing—review & editing.
References
- 1.Baghban R., Roshangar L., Jahanban-Esfahlan R., Seidi K., Ebrahimi-Kalan A., Jaymand M.et al. (2020) Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 18, 59 10.1186/s12964-020-0530-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meizlish M.L., Franklin R.A., Zhou X. and Medzhitov R. (2021) Tissue homeostasis and inflammation. Annu. Rev. Immunol. 39, 557–581 10.1146/annurev-immunol-061020-053734 [DOI] [PubMed] [Google Scholar]
- 3.Multhoff G., Molls M. and Radons J. (2012) Chronic inflammation in cancer development. Front. Immunol. 2, 98 10.3389/fimmu.2011.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greten F.R. and Grivennikov S.I. (2019) Inflammation and cancer: triggers, mechanisms, and consequences. Immunity 51, 27–41 10.1016/j.immuni.2019.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H., Wu L., Yan G., Chen Y., Zhou M., Wu Y.et al. (2021) Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct. Targeted Therapy 6, 263 10.1038/s41392-021-00658-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramasamy R., Yan S.F. and Schmidt A.M. (2011) Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann. N. Y. Acad. Sci. 1243, 88–102 10.1111/j.1749-6632.2011.06320.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldin A., Beckman J.A., Schmidt A.M. and Creager M.A. (2006) Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114, 597–605 10.1161/CIRCULATIONAHA.106.621854 [DOI] [PubMed] [Google Scholar]
- 8.Singh R., Barden A., Mori T. and Beilin L. (2001) Advanced glycation end-products: a review. Diabetologia 44, 129–146 10.1007/s001250051591 [DOI] [PubMed] [Google Scholar]
- 9.Wolff S.P. and Dean R.T. (1987) Glucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetes. Biochem. J. 245, 243–250 10.1042/bj2450243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhuri J., Bains Y., Guha S., Kahn A., Hall D., Bose N.et al. (2018) The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab. 28, 337–352 10.1016/j.cmet.2018.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uribarri J., del Castillo M.D., de la Maza M.P., Filip R., Gugliucci A., Luevano-Contreras C.et al. (2015) Dietary advanced glycation end products and their role in health and disease. Adv. Nutr. 6, 461–473 10.3945/an.115.008433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmad S., Moinuddin S.A., Dixit K., Shahab U., Alam K. and Ali A. (2011) Genotoxicity and immunogenicity of DNA-advanced glycation end products formed by methylglyoxal and lysine in presence of Cu2+. Biochem. Biophys. Res. Commun. 407, 568–574 10.1016/j.bbrc.2011.03.064 [DOI] [PubMed] [Google Scholar]
- 13.Kang J.H. (2003) Oxidative damage of DNA by the reaction of amino acid with methylglyoxal in the presence of Fe(III). Int. J. Biol. Macromol. 33, 43–48 10.1016/S0141-8130(03)00064-3 [DOI] [PubMed] [Google Scholar]
- 14.van Heijst J.W., Niessen H.W., Musters R.J., van Hinsbergh V.W., Hoekman K. and Schalkwijk C.G. (2006) Argpyrimidine-modified Heat shock protein 27 in human non-small cell lung cancer: a possible mechanism for evasion of apoptosis. Cancer Lett. 241, 309–319 10.1016/j.canlet.2005.10.042 [DOI] [PubMed] [Google Scholar]
- 15.Oya-Ito T., Naito Y., Takagi T., Handa O., Matsui H., Yamada M.et al. (2011) Heat-shock protein 27 (Hsp27) as a target of methylglyoxal in gastrointestinal cancer. Biochim. Biophys. Acta 1812, 769–781 10.1016/j.bbadis.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 16.Chiavarina B., Nokin M.J., Bellier J., Durieux F., Bletard N., Sherer F.et al. (2017) Methylglyoxal-mediated stress correlates with high metabolic activity and promotes tumor growth in colorectal cancer. Int. J. Mol. Sci. 18, 213 10.3390/ijms18010213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garay-Sevilla M.E., Rojas A., Portero-Otin M. and Uribarri J. (2021) Dietary AGEs as exogenous boosters of inflammation. Nutrients 13, 2802 10.3390/nu13082802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayén A.L., Aglago E.K., Knaze V., Cordova R., Schalkwijk C.G., Wagner K.H.et al. (2021) Dietary intake of advanced glycation endproducts and risk of hepatobiliary cancers: a multinational cohort study. Int. J. Cancer 149, 854–864 10.1002/ijc.33612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellahcène A., Nokin M.J., Castronovo V. and Schalkwijk C. (2018) Methylglyoxal-derived stress: an emerging biological factor involved in the onset and progression of cancer. Semin. Cancer Biol. 49, 64–74 10.1016/j.semcancer.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 20.Leone A., Nigro C., Nicolò A., Prevenzano I., Formisano P., Beguinot F.et al. (2021) The dual-role of methylglyoxal in tumor progression - novel therapeutic approaches. Front. Oncol. 11, 645686 10.3389/fonc.2021.645686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sruthi C.R. and Raghu K.G. (2022) Methylglyoxal induces ambience for cancer promotion in HepG2 cells via the Warburg effect and promotes glycation. J. Cell. Biochem. In press 10.1002/jcb.30215 [DOI] [PubMed] [Google Scholar]
- 22.Taneja S., Vetter S.W. and Leclerc E. (2021) Hypoxia and the receptor for advanced glycation end products (RAGE) signaling in cancer. Int. J. Mol. Sci. 22, 8153 10.3390/ijms22158153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palanissami G. and Paul S.F.D. (2018) RAGE and its ligands: molecular interplay between glycation, inflammation, and hallmarks of cancer-a review. Hormones Cancer 9, 295–325 10.1007/s12672-018-0342-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rojas A., Araya P., Romero J., Delgado-López F., Gonzalez I., Añazco C.et al. (2018) Skewed signaling through the receptor for advanced glycation end-products alters the proinflammatory profile of tumor-associated macrophages. Cancer Microenviron. 11, 97–105 10.1007/s12307-018-0214-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojas A., Figueroa H. and Morales E. (2010) Fueling inflammation at tumor microenvironment: the role of multiligand/RAGE axis. Carcinogenesis 31, 334–341 10.1093/carcin/bgp322 [DOI] [PubMed] [Google Scholar]
- 26.El-Far A.H., Sroga G., Jaouni S.K.A. and Mousa S.A. (2020) Role and mechanisms of RAGE-ligand complexes and RAGE-inhibitors in cancer progression. Int. J. Mol. Sci. 21, 3613 10.3390/ijms21103613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rojas A., Delgado-López F., González I., Pérez-Castro R., Romero J. and Rojas I. (2013) The receptor for advanced glycation end-products: a complex signaling scenario for a promiscuous receptor. Cell. Signal. 25, 609–614 10.1016/j.cellsig.2012.11.022 [DOI] [PubMed] [Google Scholar]
- 28.Taguchi A., Blood D.C., del Toro G., Canet A., Lee D.C., Qu W.et al. (2000) Blockade of RAGE-amphoterin signaling suppresses tumour growth and metastases. Nature 405, 354–360 10.1038/35012626 [DOI] [PubMed] [Google Scholar]
- 29.Jayachandran J., Srinivasan H. and Mani K.P. (2021) Molecular mechanism involved in epithelial to mesenchymal transition. Arch. Biochem. Biophys. 710, 108984 10.1016/j.abb.2021.108984 [DOI] [PubMed] [Google Scholar]
- 30.Dongre A. and Weinberg R.A. (2019) New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84 10.1038/s41580-018-0080-4 [DOI] [PubMed] [Google Scholar]
- 31.Lu W. and Kang Y. (2019) Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev. Cell 49, 361–374 10.1016/j.devcel.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin C., Li H., Zhang B., Liu Y., Lu G., Lu S.et al. (2013) RAGE-binding S100A8/A9 promotes the migration and invasion of human breast cancer cells through actin polymerization and epithelial-mesenchymal transition. Breast Cancer Res. Treat. 142, 297–309 10.1007/s10549-013-2737-1 [DOI] [PubMed] [Google Scholar]
- 33.Zhu L., Li X., Chen Y., Fang J. and Ge Z. (2015) High-mobility group box 1: a novel inducer of the epithelial-mesenchymal transition in colorectal carcinoma. Cancer Lett. 357, 527–534 10.1016/j.canlet.2014.12.012 [DOI] [PubMed] [Google Scholar]
- 34.Kwak T., Drews-Elger K., Ergonul A., Miller P.C., Braley A., Hwang G.H.et al. (2017) Targeting of RAGE-ligand signaling impairs breast cancer cell invasion and metastasis. Oncogene 36, 1559–1572 10.1038/onc.2016.324 [DOI] [PubMed] [Google Scholar]
- 35.Zhang L., He J., Wang J., Liu J., Chen Z., Deng B.et al. (2021) Knockout RAGE alleviates cardiac fibrosis through repressing endothelial-to-mesenchymal transition (EndMT) mediated by autophagy. Cell Death Dis. 12, 470 10.1038/s41419-021-03750-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nam M.H., Pantcheva M.B., Rankenberg J. and Nagaraj R.H. (2021) Transforming growth factor-β2-mediated mesenchymal transition in lens epithelial cells is repressed in the absence of RAGE. Biochem. J. 478, 2285–2296 10.1042/BCJ20210069 [DOI] [PubMed] [Google Scholar]
- 37.Rapoport B.L., Steel H.C., Theron A.J., Heyman L., Smit T., Ramdas Y.et al. (2020) High Mobility Group Box 1 in human cancer. Cells 9, 1664 10.3390/cells9071664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan S., Liu Z., Xu Z., Liu J. and Zhang J. (2020) High mobility group box 1 (HMGB1): a pivotal regulator of hematopoietic malignancies. J. Hematol. Oncol. 13, 91 10.1186/s13045-020-00920-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kokkola R., Andersson A., Mullins G., Ostberg T., Treutiger C.J., Arnold B.et al. (2005) RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scand. J. Immunol. 61, 1–9 10.1111/j.0300-9475.2005.01534.x [DOI] [PubMed] [Google Scholar]
- 40.Scaffidi P., Misteli T. and Bianchi M.E. (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418, 191–195 10.1038/nature00858 [DOI] [PubMed] [Google Scholar]
- 41.Liu P.L., Tsai J.R., Hwang J.J., Chou S.H., Cheng Y.J., Lin F.Y.et al. (2009) High-mobility group box 1-mediated matrix metalloproteinase-9 expression in non-small cell lung cancer contributes to tumor cell invasiveness. Am. J. Respir. Cell Mol. Biol. 43, 530–538 10.1165/rcmb.2009-0269OC [DOI] [PubMed] [Google Scholar]
- 42.Dong Y.D., Cui L., Peng C.H., Cheng D.F., Han B.S. and Huang F. (2013) Expression and clinical significance of HMGB1 in human liver cancer: Knockdown inhibits tumor growth and metastasis in vitro and in vivo. Oncol. Rep. 29, 87–94 10.3892/or.2012.2070 [DOI] [PubMed] [Google Scholar]
- 43.Kang R., Zhang Q., Zeh H.J. 3rd, Lotze M.T. and Tang D. (2013) HMGB1 in cancer: good, bad, or both? Clin. Cancer Res. 19, 4046–4057 10.1158/1078-0432.CCR-13-0495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao C.B., Bao J.M., Lu Y.J., Zhao T., Zhou X.H., Zheng D.Y.et al. (2014) Co-expression of RAGE and HMGB1 is associated with cancer progression and poor patient outcome of prostate cancer. Am. J. Cancer Res. 4, 369–377 [PMC free article] [PubMed] [Google Scholar]
- 45.Jung A.R., Kim G.E., Kim M.Y., Ha U.S., Hong S.H., Lee J.Y.et al. (2018) HMGB1 promotes tumor progression and invasion through HMGB1/TNFR1/NF-κB axis in castration-resistant prostate cancer. Am. J. Cancer Res. 11, 2215–2227 [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang J., Shao S., Han D., Xu Y., Jiao D., Wu J.et al. (2018) High mobility group box 1 promotes the epithelial-to-mesenchymal transition in prostate cancer PC3 cells via the RAGE/NF-κB signaling pathway. Int. J. Oncol. 53, 659–671 10.3892/ijo.2018.4420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nasser M.W., Wani N.A., Ahirwar D.K., Powell C.A., Ravi J., Elbaz M.et al. (2015) RAGE mediates S100A7-induced breast cancer growth and metastasis by modulating the tumor microenvironment. Cancer Res. 75, 974–985 10.1158/0008-5472.CAN-14-2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azizian-Farsani F., Abedpoor N., Sheikhha M.H., Gure A.O., Nasr-Esfahani M.H. and Ghaedi K. (2020) Receptor for advanced glycation end products acts as a fuel to colorectal cancer development. Front. Oncol. 10, 552283 10.3389/fonc.2020.552283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanahan D. and Weinberg R.A. (2020) The hallmarks of cancer. Cell 100, 57–70 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 50.Massagué J. and Obenauf A.C. (2016) Metastatic colonization by circulating tumour cells. Nature 529, 298–306 10.1038/nature17038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suhail Y., Cain M.P., Vanaja K., Kurywchak P.A., Levchenko A., Kalluri R.et al. (2019) Systems biology of cancer metastasis. Cell Systems 9, 109–127 10.1016/j.cels.2019.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ko S.Y., Ko H.A., Shieh T.M., Chang W.C., Chen H.I., Chang S.S.et al. (2014) Cell migration is regulated by AGE-RAGE interaction in human oral cancer cells in vitro. PloS ONE 9, e110542 10.1371/journal.pone.0110542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zill H., Günther R., Erbersdobler H.F., Fölsch U.R. and Faist V. (2001) RAGE expression and AGE-induced MAP kinase activation in Caco-2 cells. Biochem. Biophys. Res. Commun. 288, 1108–1111 10.1006/bbrc.2001.5901 [DOI] [PubMed] [Google Scholar]
- 54.Mercado-Pimentel M.E., Onyeagucha B.C., Li Q., Pimentel A.C., Jandova J. and Nelson M.A. (2015) The S100P/RAGE signaling pathway regulates expression of microRNA-21 in colon cancer cells. FEBS Lett. 589, 2388–2393 10.1016/j.febslet.2015.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Onyeagucha B.C., Mercado-Pimentel M.E., Hutchison J., Flemington E.K. and Nelson M.A. (2013) S100P/RAGE signaling regulates microRNA-155 expression via AP-1 activation in colon cancer. Exp. Cell Res. 319, 2081–2090 10.1016/j.yexcr.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qian F., Xiao J., Gai L. and Zhu J. (2019) HMGB1-RAGE signaling facilitates Ras-dependent Yap1 expression to drive colorectal cancer stemness and development. Mol. Carcinog. 58, 500–510 10.1002/mc.22944 [DOI] [PubMed] [Google Scholar]
- 57.Zhu L., Ren L., Chen Y., Fang J., Ge Z. and Li X. (2015) Redox status of high-mobility group box 1 performs a dual role in angiogenesis of colorectal carcinoma. J. Cell. Mol. Med. 19, 2128–2135 10.1111/jcmm.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pan S., Guan Y., Ma Y., Cui Q., Tang Z., Li J.et al. (2022) Advanced glycation end products correlate with breast cancer metastasis by activating RAGE/TLR4 signaling. BMJ Open Diab. Res. Care 10, e002697 10.1136/bmjdrc-2021-002697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bartling B., Hofmann H.S., Weigle B., Silber R.E. and Simm A. (2005) Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma. Carcinogenesis 26, 293–301 10.1093/carcin/bgh333 [DOI] [PubMed] [Google Scholar]
- 60.Chen M.C., Chen K.C., Chang G.C., Lin H., Wu C.C., Kao W.H.et al. (2020) RAGE acts as an oncogenic role and promotes the metastasis of human lung cancer. Cell Death Dis. 11, 265 10.1038/s41419-020-2432-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKee T.J., Perlman G., Morris M. and Komarova S.V. (2019) Extracellular matrix composition of connective tissues: a systematic review and meta-analysis. Sci. Rep. 9, 10542 10.1038/s41598-019-46896-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biteau B., Hochmuth C.E. and Jasper H. (2011) Maintaining tissue homeostasis: dynamic control of somatic stem cell activity. Cell Stem Cell 9, 402–411 10.1016/j.stem.2011.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karamanos N.K., Theocharis A.D., Piperigkou Z., Manou D., Passi A., Skandalis S.S.et al. (2021) A guide to the composition and functions of the extracellular matrix. FEBS J. 288, 6850–6912 10.1111/febs.15776 [DOI] [PubMed] [Google Scholar]
- 64.Romani P., Valcarcel-Jimenez L., Frezza C. and Dupont S. (2021) Crosstalk between mechanotransduction and metabolism. Nat. Rev. Mol. Cell Biol. 22, 22–38 10.1038/s41580-020-00306-w [DOI] [PubMed] [Google Scholar]
- 65.Chaudhuri O., Cooper-White J., Janmey P.A., Mooney D.J. and Shenoy V.B. (2020) Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 10.1038/s41586-020-2612-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Najafi M., Farhood B. and Mortezaee K. (2019) Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J. Cell. Biochem. 120, 2782–2790 10.1002/jcb.27681 [DOI] [PubMed] [Google Scholar]
- 67.Marozzi M., Parnigoni A., Negri A., Viola M., Vigetti D., Passi A.et al. (2021) Inflammation, extracellular matrix remodeling, and proteostasis in tumor microenvironment. Int. J. Mol. Sci. 22, 8102 10.3390/ijms22158102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eble J.A. and Niland S. (2019) The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 36, 171–198 10.1007/s10585-019-09966-1 [DOI] [PubMed] [Google Scholar]
- 69.Dong Y., Zheng Q., Wang Z., Lin X., You Y., Wu S.et al. (2019) Higher matrix stiffness as an independent initiator triggers epithelial-mesenchymal transition and facilitates HCC metastasis. J. Hematol. Oncol. 12, 112 10.1186/s13045-019-0795-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pankova D., Chen Y., Terajima M., Schliekelman M.J., Baird B.N., Fahrenholtz M.et al. (2016) Cancer-associated fibroblasts induce a collagen cross-link switch in tumor stroma. Mol. Cancer Res. 14, 287–295 10.1158/1541-7786.MCR-15-0307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suh Y.J., Hall M.S., Huang Y.L., Moon S.Y., Song W., Ma M.et al. (2019) Glycation of collagen matrices promotes breast tumor cell invasion. Integr. Biol. 11, 109–117 10.1093/intbio/zyz011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vicens-Zygmunt V., Estany S., Colom A., Montes-Worboys A., Machahua C., Sanabria A.J.et al. (2015) Fibroblast viability and phenotypic changes within glycated stiffened three-dimensional collagen matrices. Respir. Res. 16, 82 10.1186/s12931-015-0237-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rojas A., Añazco C., González I. and Araya P. (2018) Extracellular matrix glycation and receptor for advanced glycation end-products activation: a missing piece in the puzzle of the association between diabetes and cancer. Carcinogenesis 39, 515–521 10.1093/carcin/bgy012 [DOI] [PubMed] [Google Scholar]
- 74.Nokin M.J., Bellier J., Durieux F., Peulen O., Rademaker G., Gabriel M.et al. (2019) Methylglyoxal, a glycolysis metabolite, triggers metastasis through MEK/ERK/SMAD1 pathway activation in breast cancer. Breast Cancer Res. 21, 11 10.1186/s13058-018-1095-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chou C.K., Yang P.C., Tsai P.Y., Yang H.Y., Tsai K.F., Chen T.H.et al. (2021) Methylglyoxal levels in human colorectal precancer and cancer: analysis of tumor and peritumor tissue. Life 11, 1319 10.3390/life11121319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Spada S., Tocci A., Di Modugno F. and Nisticò P. (2021) Fibronectin as a multiregulatory molecule crucial in tumor matrisome: from structural and functional features to clinical practice in oncology. J. Exp. Clin. Cancer Res. 40, 102 10.1186/s13046-021-01908-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bansode S., Bashtanova U., Li R., Clark J., Müller K.H., Puszkarska A.et al. (2020) Glycation changes molecular organization and charge distribution in type I collagen fibrils. Sci. Rep. 10, 3397 10.1038/s41598-020-60250-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nissen N.I., Karsdal M. and Willumsen N. (2019) Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J. Exp. Clin. Cancer Res. 38, 115 10.1186/s13046-019-1110-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohan V., Das A. and Sagi I. (2020) Emerging roles of ECM remodeling processes in cancer. Semin. Cancer Biol. 62, 192–200 10.1016/j.semcancer.2019.09.004 [DOI] [PubMed] [Google Scholar]
- 80.Heldin C.H., Rubin K., Pietras K. and Ostman A. (2004) High interstitial fluid pressure - an obstacle in cancer therapy. Nat. Rev. Cancer 4, 806–813 10.1038/nrc1456 [DOI] [PubMed] [Google Scholar]
- 81.Libutti S.K., Tamarkin L. and Nilubol N. (2018) Targeting the invincible barrier for drug delivery in solid cancers: interstitial fluid pressure. Oncotarget 9, 35723–35725 10.18632/oncotarget.26267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pratt S.J.P., Lee R.M. and Martin S.S. (2020) The mechanical microenvironment in breast cancer. Cancers (Basel) 12, 1452 10.3390/cancers12061452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hosein A.N., Brekken R.A. and Maitra A. (2020) Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat. Rev. Gastroenterol. Hepatol. 17, 487–505 10.1038/s41575-020-0300-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Provenzano P.P. and Hingorani S.R. (2013) Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. Br. J. Cancer 108, 1–8 10.1038/bjc.2012.569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bordeleau F., Mason B.N., Lollis E.M., Mazzola M., Zanotelli M.R., Somasegar S.et al. (2017) Matrix stiffening promotes a tumor vasculature phenotype. Proc. Natl. Acad. Sci. 114, 492–497 10.1073/pnas.1613855114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ahmad S., Khan M.Y., Rafi Z., Khan H., Siddiqui Z., Rehman S.et al. (2018) Oxidation, glycation and glycoxidation-The vicious cycle and lung cancer. Semin. Cancer Biol. 49, 29–36 10.1016/j.semcancer.2017.10.005 [DOI] [PubMed] [Google Scholar]
- 87.Houg D.S. and Bijlsma M.F. (2018) The hepatic pre-metastatic niche in pancreatic ductal adenocarcinoma. Mol. Cancer 17, 95 10.1186/s12943-018-0842-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rice A.J., Cortes E., Lachowski D., Cheung B.C.H., Karim S.A., Morton J.P.et al. (2017) Matrix stiffness induces epithelial-mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 6, e352 10.1038/oncsis.2017.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei S.C., Fattet L., Tsai J.H., Guo Y., Pai V.H., Majeski H.E.et al. (2015) Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 17, 678–688 10.1038/ncb3157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grasset E.M., Bertero T., Bozec A., Friard J., Bourget I., Pisano S.et al. (2018) Matrix stiffening and EGFR cooperate to promote the collective invasion of cancer cells. Cancer Res. 78, 5229–5242 10.1158/0008-5472.CAN-18-0601 [DOI] [PubMed] [Google Scholar]
- 91.Negus R.P., Stamp G.W., Hadley J. and Balkwill F.R. (1997) Quantitative assessment of the leukocyte infiltrate in ovarian cancer and its relationship to the expression of C-C chemokines. Am. J. Pathol. 150, 1723–1734 [PMC free article] [PubMed] [Google Scholar]
- 92.Burke F., Relf M., Negus R. and Balkwill F. (1996) A cytokine profile of normal and malignant ovary. Cytokine 8, 578–585 10.1006/cyto.1996.0077 [DOI] [PubMed] [Google Scholar]
- 93.Sica A., Saccani A., Bottazzi B., Bernasconi S., Allavena P., Gaetano B.et al. (2000) Defective expression of the monocyte chemotactic protein-1 receptor CCR2 in macrophages associated with human ovarian carcinoma. J. Immunol. 164, 733–738 10.4049/jimmunol.164.2.733 [DOI] [PubMed] [Google Scholar]
- 94.Hudson J.D., Shoaibi M.A., Maestro R., Carnero A., Hannon G.J. and Beach D.H. (1999) A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 190, 1375–1382 10.1084/jem.190.10.1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.El-Omar E.M., Carrington M., Chow W.H., McColl K.E., Bream J.H., Young H.A.et al. (2000) Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404, 398–402 10.1038/35006081 [DOI] [PubMed] [Google Scholar]
- 96.Langowski J.L., Zhang X., Wu L., Mattson J.D., Chen T., Smith K.et al. (2006) IL-23 promotes tumour incidence and growth. Nature 442, 461–465 10.1038/nature04808 [DOI] [PubMed] [Google Scholar]
- 97.Greten F.R., Eckmann L., Greten T.F., Park J.M., Li Z.W., Egan L.J.et al. (2004) IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 118, 285–296 10.1016/j.cell.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 98.Kostova N., Zlateva S., Ugrinova I. and Pasheva E. (2010) The expression of HMGB1 protein and its receptor RAGE in human malignant tumors. Mol. Cell. Biochem. 337, 251–258 10.1007/s11010-009-0305-0 [DOI] [PubMed] [Google Scholar]
- 99.Ishiguro H., Nakaigawa N., Miyoshi Y., Fujinami K., Kubota Y. and Uemura H. (2005) Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate 64, 92–100 10.1002/pros.20219 [DOI] [PubMed] [Google Scholar]
- 100.Völp K., Brezniceanu M.L., Bösser S., Brabletz T., Kirchner T., Göttel D.et al. (2006) Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut 55, 234–242 10.1136/gut.2004.062729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tian T., Li X., Hua Z., Ma J., Wu X., Liu Z.et al. (2017) S100A7 promotes the migration, invasion and metastasis of human cervical cancer cells through epithelial-mesenchymal transition. Oncotarget 8, 24964–24977 10.18632/oncotarget.15329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pang X., Zhang Y. and Zhang S. (2017) High-mobility group box 1 is overexpressed in cervical carcinoma and promotes cell invasion and migration in vitro. Oncol. Rep. 37, 831–840 10.3892/or.2016.5317 [DOI] [PubMed] [Google Scholar]
- 103.Deng R., Mo F., Chang B., Zhang Q., Ran H., Yang S.et al. (2017) Glucose-derived AGEs enhance human gastric cancer metastasis through RAGE/ERK/Sp1/MMP2 cascade. Oncotarget 8, 104216–104226 10.18632/oncotarget.22185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chavakis T., Bierhaus A., Al-Fakhri N., Schneider D., Witte S., Linn T.et al. (2003) The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J. Exp. Med. 198, 1507–1515 10.1084/jem.20030800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Boulanger E., Wautier M.P., Wautier J.L., Boval B., Panis Y., Wernert N.et al. (2002) AGEs bind to mesothelial cells via RAGE and stimulate VCAM-1 expression. Kidney Int. 61, 148–156 10.1046/j.1523-1755.2002.00115.x [DOI] [PubMed] [Google Scholar]
- 106.Schmidt A.M., Hori O., Chen J.X., Li J.F., Crandall J., Zhang J.et al. (1995) Advanced glycation endproducts interacting with their endothelial receptor induce expression of vascular cell adhesion molecule-1 (VCAM-1) in cultured human endothelial cells and in mice. A potential mechanism for the accelerated vasculopathy of diabetes. J. Clin. Invest. 96, 1395–1403 10.1172/JCI118175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Araya P., Romero J., Delgado-López F., Gonzalez I., Añazco C., Perez R.et al. (2019) HMGB1 decreases CCR-2 expression and migration of M2 macrophages under hypoxia. Inflamm. Res. 68, 639–642 10.1007/s00011-019-01249-5 [DOI] [PubMed] [Google Scholar]
- 108.Orlova V.V., Choi E.Y., Xie C., Chavakis E., Bierhaus A., Ihanus E.et al. (2007) A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J. 26, 1129–1139 10.1038/sj.emboj.7601552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ge X., Arriazu E., Magdaleno F., Antoine D.J., De la Cruz R., Theise N.et al. (2018) High mobility group box-1 drives fibrosis progression signaling via the receptor for advanced glycation end products in mice. Hepatology 68, 2380–2404 10.1002/hep.30093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pikarsky E., Porat R.M., Stein I., Abramovitch R., Amit S., Kasem S.et al. (2004) NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 431, 461–466 10.1038/nature02924 [DOI] [PubMed] [Google Scholar]
- 111.Kojima M., Morisaki T., Sasaki N., Nakano K., Mibu R., Tanaka M.et al. (2004) Increased nuclear factor-kB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 24, 675–681 [PubMed] [Google Scholar]
- 112.Lindholm P.F., Bub J., Kaul S., Shidham V.B. and Kajdacsy-Balla A. (2000) The role of constitutive NF-kappaB activity in PC-3 human prostate cancer cell invasive behavior. Clin. Exp. Metastasis 18, 471–479 10.1023/A:1011845725394 [DOI] [PubMed] [Google Scholar]
- 113.McCall P., Bennett L., Ahmad I., Mackenzie L.M., Forbes I.W., Leung H.Y.et al. (2012) NFκB signalling is upregulated in a subset of castrate-resistant prostate cancer patients and correlates with disease progression. Br. J. Cancer 107, 1554–1563 10.1038/bjc.2012.372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shou Y., Li N., Li L., Borowitz J.L. and Isom G.E. (2002) NF-kappaB-mediated up-regulation of Bcl-X(S) and Bax contributes to cytochrome c release in cyanide-induced apoptosis. J. Neurochem. 81, 842–852 10.1046/j.1471-4159.2002.00880.x [DOI] [PubMed] [Google Scholar]
- 115.Hofer-Warbinek R., Schmid J.A., Stehlik C., Binder B.R., Lipp J. and de Martin R. (2000) Activation of NF-kappa B by XIAP, the X chromosome-linked inhibitor of apoptosis, in endothelial cells involves TAK1. J. Biol. Chem. 275, 22064–22068 10.1074/jbc.M910346199 [DOI] [PubMed] [Google Scholar]
- 116.Liu J.Y., Zeng Q.H., Cao P.G., Xie D., Chen X., Yang F.et al. (2018) RIPK4 promotes bladder urothelial carcinoma cell aggressiveness by upregulating VEGF-A through the NF-κB pathway. Br. J. Cancer 118, 1617–1627 10.1038/s41416-018-0116-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang J. and Peng B. (2009) NF-kappaB promotes iNOS and VEGF expression in salivary gland adenoid cystic carcinoma cells and enhances endothelial cell motility in vitro. Cell Prolif. 42, 150–161 10.1111/j.1365-2184.2009.00588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bianchi R., Adami C., Giambanco I. and Donato R. (2007) S100B binding to RAGE in microglia stimulates COX-2 expression. J. Leukoc. Biol. 81, 108–118 10.1189/jlb.0306198 [DOI] [PubMed] [Google Scholar]
- 119.Shanmugam N., Kim Y.S., Lanting L. and Natarajan R. (2003) Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J. Biol. Chem. 278, 34834–34844 10.1074/jbc.M302828200 [DOI] [PubMed] [Google Scholar]
- 120.Dumitriu I.E., Baruah P., Valentinis B., Voll R.E., Herrmann M., Nawroth P.P.et al. (2005) Release of high mobility group box 1 by dendritic cells controls T cell activation via the receptor for advanced glycation end products. J. Immunol. 174, 7506–7515 10.4049/jimmunol.174.12.7506 [DOI] [PubMed] [Google Scholar]
- 121.Dumitriu I.E., Bianchi M.E., Bacci M., Manfredi A.A. and Rovere-Querini P. (2007) The secretion of HMGB1 is required for the migration of maturing dendritic cells. J. Leukoc. Biol. 81, 84–91 10.1189/jlb.0306171 [DOI] [PubMed] [Google Scholar]
- 122.Kishi S., Nishiguchi Y., Honoki K., Mori S., Fujiwara-Tani R., Sasaki T.et al. (2021) Role of glycated high mobility group box-1 in gastric cancer. Int. J. Mol. Sci. 22, 5185 10.3390/ijms22105185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang H., Wang J., Li J., Zhou X., Yin L., Wang Y.et al. (2021) HMGB1 is a key factor for tamoxifen resistance and has the potential to predict the efficacy of CDK4/6 inhibitors in breast cancer. Cancer Sci. 112, 1603–1613 10.1111/cas.14813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yue Y., Zhou T., Gao Y., Zhang Z., Li L., Liu L.et al. (2017) High mobility group box 1/toll-like receptor 4/myeloid differentiation factor 88 signaling promotes progression of gastric cancer. Tumour Biol. 39, 1010428317694312 10.1177/1010428317694312 [DOI] [PubMed] [Google Scholar]
- 125.Lei X., Hu X., Zhang T., Zhang J., Wu C., Hong W.et al. (2020) HMGB1 release promotes paclitaxel resistance in castration-resistant prostate cancer cells via activating c-Myc expression. Cell. Signal. 72, 109631 10.1016/j.cellsig.2020.109631 [DOI] [PubMed] [Google Scholar]
- 126.Ibrahim Z.A., Armour C.L., Phipps S. and Sukkar M.B. (2013) RAGE and TLRs: relatives, friends or neighbours? Mol. Immunol. 56, 739–744 10.1016/j.molimm.2013.07.008 [DOI] [PubMed] [Google Scholar]
- 127.Furlani D., Donndorf P., Westien I., Ugurlucan M., Pittermann E., Wang W.et al. (2012) HMGB-1 induces c-kit+ cell microvascular rolling and adhesion via both toll-like receptor-2 and toll-like receptor-4 of endothelial cells. J. Cell. Mol. Med. 16, 1094–1105 10.1111/j.1582-4934.2011.01381.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van Zoelen M.A., Yang H., Florquin S., Meijers J.C., Akira S., Arnold B.et al. (2009) Role of toll-like receptors 2 and 4, and the receptor for advanced glycation end products in high-mobility group box 1-induced inflammation in vivo. Shock 31, 280–284 10.1097/SHK.0b013e318186262d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lin L. (2006) RAGE on the Toll Road? Cell. Mol. Immunol. 3, 351–358 [PubMed] [Google Scholar]
- 130.Liu Y., Yan W., Tohme S., Chen M., Fu Y., Tian D.et al. (2015) Hypoxia induced HMGB1 and mitochondrial DNA interactions mediate tumor growth in hepatocellular carcinoma through Toll-like receptor 9. J. Hepatol. 63, 114–121 10.1016/j.jhep.2015.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sun S., Ma J., Xie P., Wu Z. and Tian X. (2020) Hypoxia-responsive miR-141-3p is involved in the progression of breast cancer via mediating the HMGB1/HIF-1α signaling pathway. J. Gene Med. 22, e3230 10.1002/jgm.3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Allgöwer C., Kretz A.L., von Karstedt S., Wittau M., Henne-Bruns D. and Lemke J. (2020) Friend or foe: S100 proteins in cancer. Cancers 12, 2037 10.3390/cancers12082037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ichikawa M., Williams R., Wang L., Vogl T. and Srikrishna G. (2011) S100A8/A9 activate key genes and pathways in colon tumor progression. Mol. Cancer Res. 9, 133–148 10.1158/1541-7786.MCR-10-0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wang S., Song R., Wang Z., Jing Z., Wang S. and Ma J. (2018) S100A8/A9 in inflammation. Front. Immunol. 9, 1298 10.3389/fimmu.2018.01298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kim B., Jung S., Kim H., Kwon J.O., Song M.K., Kim M.K.et al. (2021) The role of S100A4 for bone metastasis in prostate cancer cells. BMC Cancer 21, 137 10.1186/s12885-021-07850-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu Y., Zhou Q., Guo F., Chen M., Tao X. and Dong D. (2021) S100 proteins in pancreatic cancer: current knowledge and future perspectives. Front. Oncol. 11, 711180 10.3389/fonc.2021.711180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Taub C.J., Lippman M.E., Hudson B.I., Blomberg B.B., Diaz A., Fisher H.M.et al. (2019) The effects of a randomized trial of brief forms of stress management on RAGE-associated S100A8/A9 in patients with breast cancer undergoing primary treatment. Cancer 125, 1717–1725 10.1002/cncr.31965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rius J., Guma M., Schachtrup C., Akassoglou K., Zinkernagel A.S., Nizet V.et al. (2008) NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453, 807–811 10.1038/nature06905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Simińska D., Gąssowska-Dobrowolska M., Listos J., Gutowska I., Chlubek D. and Baranowska-Bosiacka I. (2021) Chronic and cycling hypoxia: drivers of cancer chronic inflammation through HIF-1 and NF-κB activation: a review of the molecular mechanisms. Int. J. Mol. Sci. 22, 10701 10.3390/ijms221910701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lugano R., Ramachandran M. and Dimberg A. (2020) Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 77, 1745–1770 10.1007/s00018-019-03351-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chandler K.B., Costello C.E. and Rahimi N. (2019) Glycosylation in the tumor microenvironment: implications for tumor angiogenesis and metastasis. Cells 8, 544 10.3390/cells8060544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhou W., Yang L., Nie L. and Lin H. (2021) Unraveling the molecular mechanisms between inflammation and tumor angiogenesis. Am. J. Cancer Res. 11, 301–317 [PMC free article] [PubMed] [Google Scholar]
- 143.Yamazaki Y., Wake H., Nishinaka T., Hatipoglu O.F., Liu K., Watanabe M.et al. (2021) Involvement of multiple scavenger receptors in advanced glycation end product-induced vessel tube formation in endothelial cells. Exp. Cell Res. 408, 112857 10.1016/j.yexcr.2021.112857 [DOI] [PubMed] [Google Scholar]
- 144.Nakamura N., Matsui T., Nishino Y., Sotokawauchi A., Higashimoto Y. and Yamagishi S.I. (2019) Long-term local injection of RAGE-aptamer suppresses the growth of malignant melanoma in nude mice. J. Oncol. 7387601 10.1155/2019/7387601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zheng J., Zhu W., He F., Li Z., Cai N. and Wang H.H. (2021) An aptamer-based antagonist against the receptor for advanced glycation end-products (RAGE) blocks development of colorectal cancer. Mediators Inflamm. 9958051 10.1155/2021/9958051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rojas A. and Morales M.A. (2004) Advanced glycation and endothelial functions: a link towards vascular complications in diabetes. Life Sci. 76, 715–730 10.1016/j.lfs.2004.09.011 [DOI] [PubMed] [Google Scholar]
- 147.Sims G.P., Rowe D.C., Rietdijk S.T., Herbst R. and Coyle A.J. (2010) HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28, 367–388 10.1146/annurev.immunol.021908.132603 [DOI] [PubMed] [Google Scholar]
- 148.Yang S., Xu L., Yang T. and Wang F. (2014) High-mobility group box-1 and its role in angiogenesis. J. Leukoc. Biol. 95, 563–574 10.1189/jlb.0713412 [DOI] [PubMed] [Google Scholar]
- 149.Lan J., Luo H., Wu R., Wang J., Zhou B., Zhang Y.et al. (2020) Internalization of HMGB1 (High Mobility Group Box 1) promotes angiogenesis in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 40, 2922–2940 10.1161/ATVBAHA.120.315151 [DOI] [PubMed] [Google Scholar]
- 150.van Beijnum J.R., Nowak-Sliwinska P., van den Boezem E., Hautvast P., Buurman W.A. and Griffioen A.W. (2013) Tumor angiogenesis is enforced by autocrine regulation of high-mobility group box 1. Oncogene 32, 363–374 10.1038/onc.2012.49 [DOI] [PubMed] [Google Scholar]
- 151.Rojas A., Delgado-López F., Perez-Castro R., Gonzalez I., Romero J., Rojas I.et al. (2016) HMGB1 enhances the protumoral activities of M2 macrophages by a RAGE-dependent mechanism. Tumor Biol. 37, 3321–3329 10.1007/s13277-015-3940-y [DOI] [PubMed] [Google Scholar]
- 152.Chung H.W. and Lim J.B. (2017) High-mobility group box-1 contributes tumor angiogenesis under interleukin-8 mediation during gastric cancer progression. Cancer Sci. 108, 1594–1601 10.1111/cas.13288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van Beijnum J.R., Buurman W.A. and Griffioen A.W. (2008) Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 11, 91–99 10.1007/s10456-008-9093-5 [DOI] [PubMed] [Google Scholar]
- 154.Bresnick A.R., Weber D.J. and Zimmer D.B. (2015) S100 proteins in cancer. Nat. Rev. Cancer 15, 96–109 10.1038/nrc3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nasser M.W., Qamri Z., Deol Y.S., Ravi J., Powell C.A., Trikha P.et al. (2012) S100A7 enhances mammary tumorigenesis through upregulation of inflammatory pathways. Cancer Res. 72, 604–615 10.1158/0008-5472.CAN-11-0669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Muoio M.G., Talia M., Lappano R., Sims A.H., Vella V., Cirillo F.et al. (2021) Activation of the S100A7/RAGE pathway by IGF-1 contributes to angiogenesis in breast cancer. Cancers 13, 621 10.3390/cancers13040621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu Z., Zheng S., Liu C., Wang X., Zhang G., Wang F.et al. (2021) S100A7 as a potential diagnostic and prognostic biomarker of esophageal squamous cell carcinoma promotes M2 macrophage infiltration and angiogenesis. Clin. Transl. Med. 11, e459 10.1002/ctm2.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shubbar E., Vegfors J., Carlström M., Petersson S. and Enerbäck C. (2011) Psoriasin (S100A7) increases the expression of ROS and VEGF and acts through RAGE to promote endothelial cell proliferation. Breast Cancer Res. Treat. 134, 71–80 10.1007/s10549-011-1920-5 [DOI] [PubMed] [Google Scholar]
- 159.Gallagher E.J., Fei K., Feldman S.M., Port E., Friedman N.B., Boolbol S.K.et al. (2020) Insulin resistance contributes to racial disparities in breast cancer prognosis in US women. Breast Cancer Res. 22, 40 10.1186/s13058-020-01281-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Santolla M.F., Talia M. and Maggiolini M. (2021) S100A4 is involved in stimulatory effects elicited by the FGF2/FGFR1 signaling pathway in triple-negative breast cancer (TNBC) cells. Int. J. Mol. Sci. 22, 4720 10.3390/ijms22094720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ziche M. and Morbidelli L. (2000) Nitric oxide and angiogenesis. J. Neurooncol. 50, 39–148 10.1023/A:1006431309841 [DOI] [PubMed] [Google Scholar]
- 162.Roberts D.D., Isenberg J.S., Ridnour L.A. and Wink D.A. (2007) Nitric oxide and its gatekeeper thrombospondin-1 in tumor angiogenesis. Clin. Cancer Res. 13, 795–798 10.1158/1078-0432.CCR-06-1758 [DOI] [PubMed] [Google Scholar]
- 163.Ridnour L.A., Thomas D.D., Donzelli S., Espey M.G., Roberts D.D., Wink D.A.et al. (2006) The biphasic nature of nitric oxide responses in tumor biology. Antioxid. Redox Signal. 8, 1329–1337 10.1089/ars.2006.8.1329 [DOI] [PubMed] [Google Scholar]
- 164.Somasundaram V., Basudhar D., Bharadwaj G., No J.H., Ridnour L.A., Cheng R.Y.S.et al. (2018) Molecular mechanisms of nitric oxide in cancer progression, signal transduction, and metabolism. Antioxid. Redox Signal. 30, 1124–1143 10.1089/ars.2018.7527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sumi D. and Ignarro L.J. (2004) Regulation of inducible nitric oxide synthase expression in advanced glycation end product-stimulated raw 264.7 cells: the role of heme oxygenase-1 and endogenous nitric oxide. Diabetes 53, 1841–1850 10.2337/diabetes.53.7.1841 [DOI] [PubMed] [Google Scholar]
- 166.Rojas A., Caveda L., Romay C., López E., Valdés S., Padrón J.et al. (1996) Effect of advanced glycosylation end products on the induction of nitric oxide synthase in murine macrophages. Biochem. Biophys. Res. Commun. 225, 358–362 10.1006/bbrc.1996.1180 [DOI] [PubMed] [Google Scholar]
- 167.Papaevangelou E., Whitley G.S., Johnstone A.P., Robinson S.P. and Howe F.A. (2016) Investigating the role of tumour cell derived iNOS on tumour growth and vasculature in vivo using a tetracycline regulated expression system. Mol. Cancer Biol. 138, 2678–2687 [DOI] [PubMed] [Google Scholar]
- 168.Kostourou V., Cartwright J.E., Johnstone A.P., Boult J.K., Cullis E.R., Whitley G.et al. (2011) The role of tumour-derived iNOS in tumour progression and angiogenesis. Br. J. Cancer 104, 83–90 10.1038/sj.bjc.6606034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kimura H. and Esumi H. (2003) Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim. Pol. 50, 49–59 10.18388/abp.2003_3713 [DOI] [PubMed] [Google Scholar]
- 170.Somasundaram V., Gilmore A.C., Basudhar D., Palmieri E.M., Scheiblin D.A., Heinz W.F.et al. (2019) Inducible nitric oxide synthase-derived extracellular nitric oxide flux regulates proinflammatory responses at the single cell level. Redox Biol. 28, 101354 10.1016/j.redox.2019.101354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Heinecke J.L., Ridnour L.A., Cheng R.Y., Switzer C.H., Lizardo M.M., Khanna C.et al. (2014) Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. Proc. Natl. Acad. Sci. 111, 6323–6328 10.1073/pnas.1401799111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Levine A.J. and Puzio-Kuter A.M. (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330, 1340–1344 10.1126/science.1193494 [DOI] [PubMed] [Google Scholar]
- 173.DeBerardinis R.J. and Chandel N.S. (2016) Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 10.1126/sciadv.1600200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Pavlova N.N. and Thompson C.B. (2016) The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 10.1016/j.cmet.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sun L., Suo C., Li S.T., Zhang H. and Gao P. (2018) Metabolic reprogramming for cancer cells and their microenvironment: Beyond the Warburg Effect. Biochim. Biophys. Acta 1870, 51–66 10.1016/j.bbcan.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 176.Sormendi S. and Wielockx B. (2018) Hypoxia pathway proteins as central mediators of metabolism in the tumor cells and their microenvironment. Front. Immunol. 9, 40 10.3389/fimmu.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Parks S.K., Cormerais Y. and Pouysségur J. (2017) Hypoxia and cellular metabolism in tumour pathophysiology. J. Physiol. 595, 2439–2450 10.1113/JP273309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jube S., Rivera Z.S., Bianchi M.E., Powers A., Wang E., Pagano I.et al. (2012) Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 72, 3290–3301 10.1158/0008-5472.CAN-11-3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lee H.J., Jung Y.H., Choi G.E., Kim J.S., Chae C.W. and Han H.J. (2019) Role of HIF1α regulatory factors in stem cells. Int. J. Stem Cells 12, 8–20 10.15283/ijsc18109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bertheloot D. and Latz E. (2017) HMGB1, IL-1α, IL-33 and S100 proteins: dual-function alarmins. Cell. Mol. Immunol. 14, 43–64 10.1038/cmi.2016.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li Y., Li X.Y., Li L.X., Zhou R.C., Sikong Y., Gu X.et al. (2020) S100A10 accelerates aerobic glycolysis and malignant growth by activating mTOR-signaling pathway in gastric cancer. Front. Cell Development. Biol. 8, 559486 10.3389/fcell.2020.559486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Airely R.E. and Mobasheri A. (2012) Hypoxic regulation of glucose transport, anaerobic metabolism and angiogenesis in cancer: Novel pathway and targets for anticancer therapeutics. Chemotherapy 53, 233–256 10.1159/000104457 [DOI] [PubMed] [Google Scholar]
- 183.Adekola K., Rosen S.T. and Shanmugam M. (2012) Glucose transporters in cancer metabolism. Curr. Opin. Oncol. 24, 650–654 10.1097/CCO.0b013e328356da72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bellier J., Nokin M.J., Lardé E., Karoyan P., Peulen O., Castronovo V.et al. (2019) Methylglyoxal, a potent inducer of AGEs, connects between diabetes and cancer. Diabetes Res. Clin. Pract. 148, 200–211 10.1016/j.diabres.2019.01.002 [DOI] [PubMed] [Google Scholar]
- 185.Jahan H. and Choudhary M.I. (2015) Glycation, carbonyl stress and AGEs inhibitors: a patent review. Expert Opin. Ther. Pat. 25, 1267–1284 [DOI] [PubMed] [Google Scholar]
- 186.Khan M.I., Rath S., Adhami V.M. and Mukhtar H. (2018) Hypoxia driven glycation: mechanisms and therapeutic opportunities. Semin. Cancer Biol. 49, 75–82 10.1016/j.semcancer.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dong G., Mao Q., Xia W., Xu Y., Wang J., Xu L.et al. (2016) PKM2 and cancer: the function of PKM2 beyond glycolysis. Oncol. Lett. 11, 1980–1986 10.3892/ol.2016.4168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Luo W. and Semenza G.L. (2011) Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget 2, 551–556 10.18632/oncotarget.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yang L., Xie M., Yang M., Yu Y., Zhu S., Hou W.et al. (2014) PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 5, 4436 10.1038/ncomms5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pichiule P., Chavez J.C., Schmidt A.M. and Vannucci S.J. (2007) Hypoxia-inducible factor-1 mediates neuronal expression of the receptor for advanced glycation end products following hypoxia/ischemia. J. Biol. Chem. 282, 36330–36340 10.1074/jbc.M706407200 [DOI] [PubMed] [Google Scholar]
- 191.Zeng S., Feirt N., Goldstein M., Guarrera J., Ippagunta N., Ekong U.et al. (2004) Blockade of receptor for advanced glycation end product (RAGE) attenuates ischemia and reperfusion injury to the liver in mice. Hepatology 39, 422–432 10.1002/hep.20045 [DOI] [PubMed] [Google Scholar]
- 192.Zhai D.X., Kong Q.F., Xu W.S., Bai S.S., Peng H.S., Zhao K.et al. (2008) RAGE expression is up-regulated in human cerebral ischemia and pMCAO rats. Neurosci. Lett. 445, 117–121 10.1016/j.neulet.2008.08.077 [DOI] [PubMed] [Google Scholar]
- 193.Tafani M., Schito L., Pellegrini L., Villanova L., Marfe G., Anwar T.et al. (2011) Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-κB. Carcinogenesis 32, 1167–1175 10.1093/carcin/bgr101 [DOI] [PubMed] [Google Scholar]
- 194.Kang R., Hou W., Zhang Q., Chen R., Lee Y.J., Bartlett D.L.et al. (2014) RAGE is essential for oncogenic KRAS-mediated hypoxic signaling in pancreatic cancer. Cell Death Dis. 5, e1480 10.1038/cddis.2014.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chandel V., Maru S., Kumar A., Kumar A., Sharma A., Rathi B.et al. (2021) Role of monocarboxylate transporters in head and neck squamous cell carcinoma. Life Sci. 279, 119709 10.1016/j.lfs.2021.119709 [DOI] [PubMed] [Google Scholar]
- 196.Payen V.L., Mina E., Van Hée V.F., Porporato P.E. and Sonveaux P. (2020) Monocarboxylate transporters in cancer. Mol. Metab. 33, 48–66 10.1016/j.molmet.2019.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pinheiro C., Reis R.M., Ricardo S., Longatto-Filho A., Schmitt F. and Baltazar F. (2010) Expression of monocarboxylate transporters 1, 2, and 4 in human tumours and their association with CD147 and CD44. BioMed. Res. Int. 2010, 427694 10.1155/2010/427694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Castorino J.J., Deborde S., Deora A., Schreiner R., Gallagher-Colombo S.M., Rodriguez-Boulan E.et al. (2011) Basolateral sorting signals regulating tissue-specific polarity of heteromeric monocarboxylate transporters in epithelia. Traffic 12, 483–498 10.1111/j.1600-0854.2010.01155.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mahmoud A.M. and Ali M.M. (2021) High glucose and advanced glycation end products induce CD147-mediated MMP activity in human adipocytes. Cells 10, 2098 10.3390/cells10082098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kang R., Tang D., Schapiro N.E., Loux T., Livesey K.M., Billiar T.R.et al. (2014) The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene 33, 567–577 10.1038/onc.2012.631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vegiopoulos A., Rohm M. and Herzig S. (2017) Adipose tissue: between the extremes. EMBO J. 36, 1999–2017 10.15252/embj.201696206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Choe S.S., Huh J.Y., Hwang I.J., Kim J.I. and Kim J.B. (2016) Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front. Endocrinol. (Lausanne) 7, 30 10.3389/fendo.2016.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nieman K.M., Romero I.L., Van Houten B. and Lengyel E. (2013) Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim. Biophys. Acta 1831, 1533–1541 10.1016/j.bbalip.2013.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Duong M.N., Geneste A., Fallone F., Li X., Dumontet C. and Muller C. (2017) The fat and the bad: mature adipocytes, key actors in tumor progression and resistance. Oncotarget 8, 57622–57641 10.18632/oncotarget.18038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wang Y.Y., Attané C., Milhas D., Dirat B., Dauvillier S., Guerard A.et al. (2017) Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight 2, e87489 10.1172/jci.insight.87489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Laurent V., Guérard A., Mazerolles C., Le Gonidec S., Toulet A., Nieto L.et al. (2016) Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat. Commun. 7, 10230 10.1038/ncomms10230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bertout J.A., Patel S.A. and Simon M.C. (2008) The impact of O2 availability on human cancer. Nat. Rev. Cancer 8, 967–975 10.1038/nrc2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Beloribi-Djefaflia S., Vasseur S. and Guillaumond F. (2016) Lipid metabolic reprogramming in cancer cells. Oncogenesis 5, e189 10.1038/oncsis.2015.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hao Y., Li D., Xu Y., Ouyang J., Wang Y., Zhang Y.et al. (2019) Investigation of lipid metabolism dysregulation and the effects on immune microenvironments in pan-cancer using multiple omics data. BMC Bioinform. 20, 195 10.1186/s12859-019-2734-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lengyel E., Makowski L., DiGiovanni J. and Kolonin M.G. (2018) Cancer as a matter of fat: the crosstalk between adipose tissue and tumors. Trends Cancer 4, 374–384 10.1016/j.trecan.2018.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Mukherjee A., Kenny H.A. and Lengyel E. (2017) Unsaturated fatty acids maintain cancer cell stemness. Cell Stem Cell 20, 291–292 10.1016/j.stem.2017.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Balaban S., Shearer R.F., Lee L., van Geldermalsen M., Schreuder M., Shtein H.C.et al. (2017) Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab. 5, 1 10.1186/s40170-016-0163-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang A. and Mottillo E.P. (2020) Adipocyte lipolysis: from molecular mechanisms of regulation to disease and therapeutics. Biochem. J. 477, 985–1008 10.1042/BCJ20190468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Yang D., Li Y., Xing L., Tan Y., Sun J., Zeng B.et al. (2018) Utilization of adipocyte-derived lipids and enhanced intracellular trafficking of fatty acids contribute to breast cancer progression. Cell Commun. Signal. 16, 32 10.1186/s12964-018-0221-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wen Y.A., Xing X., Harris J.W., Zaytseva Y.Y., Mitov M.I., Napier D.L.et al. (2017) Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 8, e2593 10.1038/cddis.2017.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Daquinag A.C., Gao Z., Fussell C., Immaraj L., Pasqualini R., Arap W.et al. (2021) Fatty acid mobilization from adipose tissue is mediated by CD36 posttranslational modifications and intracellular trafficking. JCI Insight 6, e147057 10.1172/jci.insight.147057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Luo X., Zheng E., Wei L., Zeng H., Qin H., Zhang X.et al. (2021) The fatty acid receptor CD36 promotes HCC progression through activating Src/PI3K/AKT axis-dependent aerobic glycolysis. Cell Death Dis. 12, 328 10.1038/s41419-021-03596-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Ladanyi A., Mukherjee A., Kenny H.A., Johnson A., Mitra A.K., Sundaresan S.et al. (2018) Adipocyte-induced CD36 expression drives ovarian cancer progression and metastasis. Oncogene 37, 2285–2301 10.1038/s41388-017-0093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.de Oliveira Silva C., Delbosc S., Araïs C., Monnier L., Cristol J.P. and Pares-Herbute N. (2008) Modulation of CD36 protein expression by AGEs and insulin in aortic VSMCs from diabetic and non-diabetic rats. Nutr. Metab. Cardiovasc. Dis. 18, 23–30 10.1016/j.numecd.2006.07.008 [DOI] [PubMed] [Google Scholar]
- 220.Xanthis A., Hatzitolios A., Fidani S., Befani C., Giannakoulas G. and Koliakos G. (2009) Receptor of advanced glycation end products (RAGE) positively regulates CD36 expression and reactive oxygen species production in human monocytes in diabetes. Angiology 60, 772–779 10.1177/0003319708328569 [DOI] [PubMed] [Google Scholar]
- 221.Labani-Motlagh A., Ashja-Mahdavi M. and Loskog A. (2020) The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front. Immunol. 11, 940 10.3389/fimmu.2020.00940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sinha P., Okoro C., Foell D., Freeze H.H., Ostrand-Rosenberg S. and Srikrishna G. (2008) Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol. 181, 4666–4675 10.4049/jimmunol.181.7.4666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wuren T., Huecksteadt T., Beck E., Warren K., Hoidal J., Ostrand-Rosenberg S.et al. (2021) The receptor for advanced glycation endproducts (RAGE) decreases survival of tumor-bearing mice by enhancing the generation of lung metastasis-associated myeloid-derived suppressor cells. Cell. Immunol. 365, 104379 10.1016/j.cellimm.2021.104379 [DOI] [PubMed] [Google Scholar]
- 224.Huang M., Wu R., Chen L., Peng Q., Li S., Zhang Y.et al. (2019) S100A9 regulates MDSCs-mediated immune suppression via the RAGE and TLR4 signaling pathways in colorectal carcinoma. Front. Immunol. 10, 2243 10.3389/fimmu.2019.02243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Vernon P.J., Zeh H.J. III and Lotze M.T. (2013) The myeloid response to pancreatic carcinogenesis is regulated by the receptor for advanced glycation end-products. Oncoimmunology 2, e24184 10.4161/onci.24184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Vernon P.J., Loux T.J., Schapiro N.E., Kang R., Muthuswamy R., Kalinski P.et al. (2013) The receptor for advanced glycation end products promotes pancreatic carcinogenesis and accumulation of myeloid-derived suppressor cells. J. Immunol. 190, 1372–1379 10.4049/jimmunol.1201151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wild C.A., Bergmann C., Fritz G., Schuler P., Hoffmann T.K., Lotfi R.et al. (2012) HMGB1 conveys immunosuppressive characteristics on regulatory and conventional T cells. Int. Immunol. 24, 485–494 10.1093/intimm/dxs051 [DOI] [PubMed] [Google Scholar]
- 228.Boutilier A.J. and Elsawa S.F. (2021) Macrophage polarization states in the tumor microenvironment. Int. J. Mol. Sci. 22, 6995 10.3390/ijms22136995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jiang X., Wang J., Deng X., Xiong F., Ge J., Xiang B.et al. (2019) Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 18, 10 10.1186/s12943-018-0928-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wang W., Chapman N.M., Zhang B., Li M., Fan M., Laribee R.N.et al. (2019) Upregulation of PD-L1 via HMGB1-activated IRF3 and NF-κB contributes to UV radiation-induced immune suppression. Cancer Res. 79, 2909–2922 10.1158/0008-5472.CAN-18-3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yao Y. and Dai W. (2014) Genomic instability and cancer. J. Carcinogen. Mutagen. 5, 1000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Radisky D.C., Levy D.D., Littlepage L.E., Liu H., Nelson C.M., Fata J.E.et al. (2005) Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature 436, 123–127 10.1038/nature03688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Rapoport B.L., Steel H.C., Theron A.J., Smit T. and Anderson R. (2020) Role of the neutrophil in the pathogenesis of advanced cancer and impaired responsiveness to therapy. Molecules 25, 1618 10.3390/molecules25071618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ameziane El Hassani R., Buffet C., Leboulleux S. and Dupuy C. (2019) Oxidative stress in thyroid carcinomas: biological and clinical significance. Endocrine Related Cancer 26, R131–R143 10.1530/ERC-18-0476 [DOI] [PubMed] [Google Scholar]
- 235.Weyemi U. and Dupuy C. (2012) The emerging role of ROS-generating NADPH oxidase NOX4 in DNA-damage responses. Mutation Res./Rev. Mutation Res. 751, 77–81 10.1016/j.mrrev.2012.04.002 [DOI] [PubMed] [Google Scholar]
- 236.Wautier M.P., Chappey O., Corda S., Stern D.M., Schmidt A.M. and Wautier J.L. (2001) Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am. J. Physiol. Endocrinol. Metab. E685–E694 10.1152/ajpendo.2001.280.5.E685 [DOI] [PubMed] [Google Scholar]
- 237.Shen C.Y., Lu C.H., Wu C.H., Li K.J., Kuo Y.M., Hsieh S.C.et al. (2020) The development of maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules 25, 5591 10.3390/molecules25235591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ott C., Jacobs K., Haucke E., Navarrete-Santos A., Grune T. and Simm A. (2014) Role of advanced glycation end products in cellular signaling. Redox Biol. 2, 411–429 10.1016/j.redox.2013.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Srinivas U.S., Tan B.W.Q., Vellayappan B.A. and Jeyasekharan A.D. (2019) ROS and the DNA damage response in cancer. Redox Biol. 25, 101084 10.1016/j.redox.2018.101084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Sallmyr A., Fan J. and Rassool F.V. (2008) Genomic instability in myeloid malignancies: increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Lett. 270, 1–9 10.1016/j.canlet.2008.03.036 [DOI] [PubMed] [Google Scholar]
- 241.Hudson B.I., Kalea A.Z., Del Mar Arriero M., Harja E., Boulanger E., D'Agati V.et al. (2008) Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J. Biol. Chem. 283, 34457–34468 10.1074/jbc.M801465200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Manigrasso M.B., Pan J., Rai V., Zhang J., Reverdatto S., Quadri N.et al. (2016) Small molecule inhibition of ligand-stimulated RAGE-DIAPH1 signal transduction 2016. Sci. Rep. 6, 22450 10.1038/srep22450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Zhu Q. and Smith E.A. (2019) Diaphanous-1 affects the nanoscale clustering and lateral diffusion of receptor for advanced glycation endproducts (RAGE). Biochim. Biophys. Acta Biomembr. 1861, 43–49 10.1016/j.bbamem.2018.10.015 [DOI] [PubMed] [Google Scholar]
- 244.Rai V., Maldonado A.Y., Burz D.S., Reverdatto S., Yan S.F., Schmidt A.M.et al. (2012) Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. J. Biological Chem. 287, 5133–5144 10.1074/jbc.M111.277731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kumar V., Fleming T., Terjung S., Gorzelanny C., Gebhardt C., Agrawal R.et al. (2017) Homeostatic nuclear RAGE-ATM interaction is essential for efficient DNA repair. Nucleic Acids Res. 45, 10595–10613 10.1093/nar/gkx705 [DOI] [PMC free article] [PubMed] [Google Scholar]