Highlights
-
•
Children with active Rolandic epilepsy have increasing thalamocortical functional connectivity in the motor circuit with age.
-
•
Children with resolved Rolandic epilepsy have increasing thalamocortical structural connectivity in the motor circuit with age.
-
•
Children with Rolandic epilepsy have no differences in thalamocortical connectivity in the sensory circuit compared to controls.
-
•
Rolandic thalamocortical structural connectivity does not predict functional connectivity in Rolandic epilepsy or controls.
Keywords: fMRI, DTI, Network, BECTS, CECTS, SeLECTS
Abstract
Rolandic epilepsy (RE) is the most common focal, idiopathic, developmental epilepsy, characterized by a transient period of sleep-potentiated seizures and epileptiform discharges in the inferior Rolandic cortex during childhood. The cause of RE remains unknown but converging evidence has identified abnormalities in the Rolandic thalamocortical circuit. To better localize this transient disease, we evaluated Rolandic thalamocortical functional and structural connectivity in the sensory and motor circuits separately during the symptomatic and asymptomatic phases of this disease. We collected high resolution structural, diffusion, and resting state functional MRI data in a prospective cohort of children with active RE (n = 17), resolved RE (n = 21), and controls (n = 33). We then computed the functional and structural connectivity between the inferior Rolandic cortex and the ventrolateral (VL) nucleus of the thalamus (efferent pathway) and the ventroposterolateral (VPL) nucleus of the thalamus (afferent pathway) across development in children with active, resolved RE and controls. We compared connectivity with age in each group using linear mixed-effects models. We found that children with active RE have increasing thalamocortical functional connectivity between the VL thalamus and inferior motor cortex with age (p = 0.022) that is not observed in controls or resolved RE. In contrast, children with resolved RE have increasing thalamocortical structural connectivity between the VL nucleus and the inferior motor cortex with age (p = 0.025) that is not observed in controls or active RE. No relationships were identified between VPL nuclei and the inferior sensory cortex with age in any group. These findings localize the functional and structural thalamocortical circuit disruption in RE to the efferent thalamocortical motor pathway. Further work is required to determine how these circuit abnormalities contribute to the emergence and resolution of symptoms in this developmental disease.
1. Introduction
Rolandic epilepsy (RE, also called self-limited epilepsy with centrotemporal spikes; SeLECTS (Specchio et al., 2022)) is the most common focal developmental epilepsy, accounting for 8–23% of childhood epilepsy (Astradsson et al., 1998, Berg and Rychlik, 2015, Camfield and Camfield, 2014, Guerrini, 2006, Ross et al., 2020), and characterized by a transient period of sleep-potentiated seizures and epileptiform discharges in the inferior Rolandic cortex during childhood (Lin et al., 2003). RE usually presents during school-age years (Ross et al., 2020), and seizures always remit by late adolescence (Berg et al., 2008). In addition to seizures, children with RE exhibit subtle to severe cognitive deficits that also resolve with disease resolution (Ross et al., 2020).
The pathophysiology behind symptom onset and resolution in RE is still not fully understood, but several studies have identified both functional and structural disruptions within the Rolandic thalamocortical circuit. Using resting-state functional magnetic resonance imaging (fMRI), abnormal functional activity has been reported in the inferior postcentral gyrus in RE (Wu et al., 2015). Diffusion imaging studies have identified that children with RE have white matter microstructural abnormalities in the Rolandic gyri using both voxel (Ciumas et al., 2014, Kim et al., 2014, Xiao et al., 2014) or ROI-based (Ostrowski et al., 2019, Xiao et al., 2014) comparison methods. In addition, children with RE have abnormal thalamocortical structural connectivity to the Rolandic cortex over development (Thorn et al., 2020). Children with unilateral continuous spike wave of sleep, a sleep-potentiated developmental epilepsy syndrome with genetic, electrographic, and clinical overlap with RE have ipsilateral thalamic lesions that are identified beyond chance (Leal et al., 2018, Sanchez Fernandez et al., 2012). Likewise, children with neonatal thalamic injury are at high risk of developing similar sleep-potentiated discharges (Guzzetta et al., 2005, Kersbergen et al., 2013).
We have previously shown that children with RE have transiently decreased Rolandic sleep spindles, characteristic sleep oscillations that are generated in the thalamus and elaborated by efferent and afferent thalamocortical circuits (Kramer et al., 2021). Decreased sleep spindles correlate with the cognitive deficits observed in RE (Kramer et al., 2021, Stoyell et al., 2021), suggesting that thalamocortical circuit disruption may contribute to symptoms in this disease. In contrast, children with resolved RE no longer exhibit a spindle deficit, indicating this thalamocortical disruption is transient. Prior studies evaluating functional and structural connectivity in RE have not evaluated whether the observed differences are present during the active or resolved state of RE, or both. Further, prior studies have not localized whether the circuit dysfunction impacts connectivity in the motor efferent or sensory afferent thalamocortical Rolandic pathways, or both.
Given these background studies, we used combined advanced imaging techniques to localize the abnormalities in functional and structural connectivity to the efferent or afferent Rolandic thalamocortical circuits during the symptomatic and asymptomatic phases of disease. Identification and localization of these developmental abnormalities could provide insight into the network imbalances that underlie epilepsy onset and support epilepsy resolution in this common age-specific, self-limited disease.
2. Materials and methods
2.1. Subjects
We prospectively recruited 38 children with RE, including 17 with active disease (defined as seizure within 12 months) and 21 in disease resolution (seizure free for > 12 months) (Ross et al., 2020). Children with RE were diagnosed by a child neurologist following International League Against Epilepsy criteria, including history of at least one focal motor or generalized seizure and EEG-confirmed sleep-activated centrotemporal spikes (1989, Fisher et al., 2014). We chose to use one year of seizure freedom among RE subjects to signify a low risk of seizure recurrence because most children with RE who are seizure-free for 1 year have a sustained remission (Berg et al., 2004, Ross et al., 2020). Thirty-three control children (14F, ages 11.91 ± 2.84 years) without history of seizure or known neurologic disorder were also recruited. All children underwent sleep EEG at the time of enrollment. Control children were excluded if sleep-potentiated spikes were observed (n = 1). Children with attention disorders and mild learning difficulties were included, as these profiles are consistent with known RE comorbidities (Wickens et al., 2017). Informed consent was obtained from all participants and this study was approved by from the Massachusetts General Hospital Institutional Review Board. Clinical characteristics for all subjects in the study are summarized in Table 1.
Table 1.
Subject characteristics.
| Clinical and electrographic features | Active, n = 17 | Resolved, n = 21 | Controls, n = 33 |
|---|---|---|---|
| Age, years (mean ± std, range) | 10.8 ± 1.8, 8.4–14.7 | 12.9 ± 2.2, 9.0–17.2 | 11.9 ± 2.8, 7.4–18.3 |
| Gender | 6 Female | 6 Female | 14 Female |
| Duration seizure-free, month (mean ± std, range) | 3.2 ± 3.8, 0.1–10.9 | 32.7 ± 14.4, 13.8–67.6 | |
| Epilepsy duration, month (mean ± std, range) | 32.4 ± 30.2, 1.0–102.0 | 24.9 ± 23.4, 0–72.0 | |
| Laterality of epileptiform discharges |
8B, 1 R, 1 L, 3 N, 4 N/A |
8B, 1 R, 4 L, 6 N, 2 N/A |
Abbreviations: B, bilateral independent discharges; R, right hemisphere only; L, left hemisphere only; N, neither; N/A, no NREM EEG.
2.2. MRI data acquisition
All subjects underwent high-resolution structural, functional and diffusion MRI in a 3 T Magnetom Prisma Simens MRI scanner with a 64-channel head coil at the Martinos Center for Biomedical Imaging. Multiecho MEMPRAGE (TE = 1.74 ms, TR = 2530 ms, flip angle = 7°, voxel size = 1 × 1 × 1 mm3), T2 FLAIR (TE = 3 ms, TR = 5000 ms, flip angle = 7°, voxel size = 0.9 × 0.9 × 0.9 mm3), functional MRI (TE = 21 ms, TR = 568 ms, voxel size = 3 × 3 × 3 mm3, 44 slices, 634 volumes, eyes open) and diffusion MRI (64 diffusion-encoding directions, TE = 82 ms, TR = 8080 ms, flip angle = 90°, voxel size = 2.0 × 2.0 × 2.0 mm3, diffusion sensitivity of b = 2000 s/mm2, number of slices = 74, skip 0) were acquired in a relaxed state with eyes opened.
2.3. Regions of interest
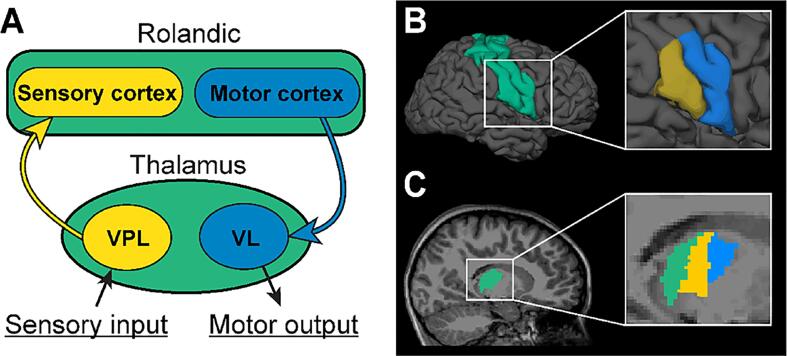
The thalamic and Rolandic cortex regions of interest (ROIs) were defined in each subject using the MEMPRAGE data and FreeSurfer 7.1.1 (https://surfer.nmr.mgh.harvard.edu/). For this, the cortical surfaces were reconstructed and registered to a common surface space with a nonrigid curvature alignment procedure. The thalami in both hemispheres were identified using the subcortical structure segmentation tool in volume space. Within the thalamus, we targeted the ventrolateral (VL) nucleus, which receives inputs from the primary motor cortex, and the ventroposteriolateral (VPL) nucleus, which projects to the primary sensory cortex (Fang et al., 2006, Toulmin et al., 2015). These thalamic nuclei were defined using thalamic segmentation tools from FreeSurfer which estimates thalamic nuclei based on a human probabilistic thalamic atlas (Iglesias et al., 2018). Available T2-FLAIR images were used to improve the thalamic segmentation performance. The Rolandic cortex in each hemisphere was defined by combining pre- and post-central gyri labels in the Desikan-Killiany atlas (Desikan et al., 2006) which uses a surface-based approach. The inferior Rolandic cortex was targeted here to approximate the seizure onset zone in RE, based on the stereotyped seizure semiology and spike localization observed in this disease (Boor et al., 2007, Pataraia et al., 2008). The inferior Rolandic cortex was defined using a sphere from the most inferior vertex in Rolandic cortex with a radius equal to half of the distance between the most superior and inferior vertices in Rolandic cortex (Ostrowski et al., 2019, Song et al., 2019, Spencer et al., 2022). All ROIs were inspected visually for accuracy. The cortical and thalamic ROIs are shown in Fig. 1.
Fig. 1.
Regions of interest within the Rolandic thalamocortical circuit. (A) Illustration of the inferior Rolandic thalamocortical circuit. The ventrolateral nucleus (VL) nucleus receives inputs from the primary motor cortex (blue arrow), and the ventroposteriolateral (VPL) projects to the primary sensory cortex (yellow arrow). (B) Cortical regions of interest (ROIs) include the Rolandic cortex (green), the inferior primary motor cortex (blue), and the inferior primary sensory cortex (yellow). (C) Thalamic ROIs include the thalamus (green), the ventrolateral nucleus (VL, blue), and the ventroposteriolateral nucleus (VPL, yellow).
2.4. fMRI preprocessing and connectivity
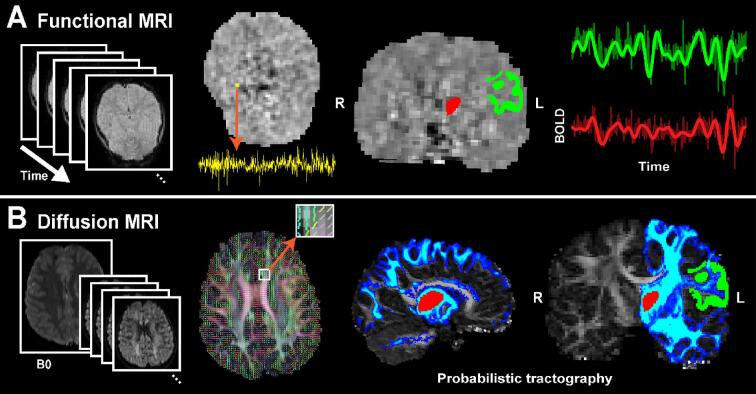
Resting-state functional connectivity MRI (rsfcMRI) preprocessing was performed using FSFAST (part of FreeSurfer’s package, https://surfer.nmr.mgh.harvard.edu/fswiki/FsFast). The first four volumes of functional MRI data were excluded to avoid data with unsaturated T1 signals. We applied slice time correction for multiple interleaved acquisitions, motion correction to the middle volume, and regressed out artifacts from head motion, CSF, white matter, and global signals. Data were spatially smoothed using a 5 mm FWHM Gaussian filter. To define ROIs in functional space, the T1-weighted image was co-registered to the fMRI using the middle point of both volumes using boundary-based registration (FreeSurfer’s bbregister tool). Functional signals from each voxel were averaged within each ROI. After visualization of the fMRI power spectra, signals were temporally band-pass filtered from 0.01 to 0.08 Hz (filter order 100, FIR) using the filtfilt function in MATLAB (R2021b, Signal Processing Toolbox). Volumes were conservatively identified as having motion artifact if any of the following deviations were identified: if the mean global signal was > 3 STD from the mean of temporal change, if there was framewise displacement > 0.5 mm from the reference volume, or rotation or translational values > 1 mm between contiguous volumes (Power et al., 2014). Volumes with motion artifact were then excluded and the rsfcMRI was computed as the cross-correlation of the final time-series signal averaged across voxels within the ROIs of interest, for each hemisphere for each subject separately. The subjects with >50% of volumes contaminated by motion artifact were excluded from the analysis (2 active RE subjects, 3% of total population), however the uncontaminated volumes from these subjects were included in post-hoc analysis to test the consistency of our findings. The functional MRI processing pipeline and ROIs are shown in Fig. 2A.
Fig. 2.
Thalamocortical functional and structural connectivity processing pipeline. (A) Thalamocortical functional connectivity processing pipeline. High temporal resolution functional images are obtained during a resting state with eyes open. ROIs are defined through co-registration with structural data. Functional data is spatially smoothed, averaged, and band-pass filtered within each ROI. The cross-correlation of the signals between two ROIs is computed. (B) Thalamocortical structural connectivity processing pipeline. High-resolution, multidirectional diffusion images are obtained. Diffusion tensor models are estimated at each voxel. ROIs are defined through co-registration with structural data. Streamlines are executed from each ROI seed. The connectivity index (CI) is calculated as the proportion of streamlines that reach the target ROI.
2.5. DTI preprocessing and structural connectivity
DTI preprocessing was done with eddy current distortion correction and diffusion tensor modeling at each voxel using the eddy and DTIFIT programs (part of FSL’s package, https://fsl.fmrib.ox.ac.uk/fsl/fslwiki, version 6.0.5.1). To calculate thalamocortical structural connectivity, probabilistic tractography was executed using Probtrackx2 (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FDT/UserGuide) (Behrens et al., 2007). The local posterior distribution was estimated using a Bayesian approach with a 0.5 mm step and 500 streamlines initiated from each voxel within the see ROI using Bedpostx. Streamlines were terminated when they reached the target ROI or they surpassed a 0.2 curvature threshold or 2000 maximum steps within a hemisphere. Streamlines with recursive looping or a volume fraction <0.01 of the parent fiber volume were ignored. This process was performed bidirectionally for each seed and target ROI in each hemisphere in each subject. To account for volume differences between ROIs and across subjects, the structural connectivity index (CI) was then computed as the total number of streamlines that reached the target divided by the total number of streamlines initialized from the seed ROI (Chu et al., 2015, Thorn et al., 2020). The structural connectivity processing pipeline is shown in Fig. 2B.
2.6. Statistical analyses
To reduce the influence of extreme observations, we applied a logarithmic transformation to all connectivity measures. We also repeated each analysis after excluding extreme outliers (beyond the 95% CI of values) to confirm stability of results. The developmental trajectory of thalamocortical connectivity was evaluated for each group (active, resolved, and control) using a linear mixed-effects model with the connectivity measure as the dependent variable, age as the predictor, and random subject-specific intercept to account for two observations per subject (connectivity measures from the left and right hemispheres for each subject).
| (1) |
The b0 indicates the intercept and b1 the regression coefficient (beta) of age. After identifying relationships in the a priori motor and sensory thalamocortical ROIs, to confirm that the methods replicated known observations in the literature, we also evaluated the relationship between age and functional and structural connectivity between the thalamus and the entire Rolandic cortex for each group. We tested whether duration seizure free, the laterality of epileptiform spikes, or the presence of antiseizure medication at the time of MRI predicted connectivity features. To do so, we used linear mixed-effects models with the connectivity measure as the dependent variable, age and either a) duration seizure-free; b) the presence of ipsilateral epileptiform spikes; or c) antiseizure medication, as fixed effects and a random subject-specific intercept. In each model, we tested sex as a fixed effect and included it in the model if p < 0.1.
To test for a relationship between functional and structural thalamocortical connectivity between the same ROIs in each group, we used a linear mixed-effects model with functional thalamocortical connectivity as the dependent variable, structural thalamocortical connectivity as the predictor, age as a fixed effect, and random subject-specific intercept.
| (2) |
Here, b0 indicates the intercept, b1 the regression coefficient of age, and b2 the regression coefficient of the structural connectivity measure. To reduce the impact of false discovery across the two thalamic circuits evaluated (VL to inferior motor cortex and inferior sensory cortex to VPL) we used the Holm-Bonferroni procedure, where P-values < 0.05 after correction (Holm, 1979) were considered significant.
2.7. Data availability
Raw data were generated at Massachusetts General Hospital and the Athinoula A. Martinos Center for Biomedical Imaging. Derived data supporting the findings of this study are available from the corresponding author on request. All code for the analysis used in this study as described in the Methods are available at https://github.com/Hunki-Kwon/Thalamocortical-Connectivity.
3. Results
3.1. Functional connectivity in the thalamic motor circuit increases in active Rolandic epilepsy
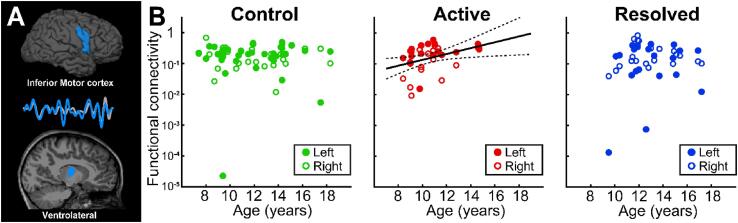
Within the Rolandic thalamocortical circuit, the ventrolateral (VL) nuclei receive inputs from the primary motor and premotor cortices, including the inferior Rolandic cortex where epileptiform discharges are most prominent in RE (Lin et al., 2003). Evaluation of this pathway revealed that functional connectivity between the inferior Rolandic motor cortex and the VL nucleus increases with age in children with active RE (p = 0.022 uncorrected, p = 0.044 corrected, beta = 0.095, 95% CI [0.015, 0.175], Fig. 3). The results were consistent when the uncontaminated volumes from the two children with active RE who were excluded due to excessive motion artifact were included (p = 0.03 uncorrected, beta = 0.087, 95% CI [0.007, 0.167]). This relationship was not present in controls (p = 0.9 uncorrected) or children with resolved RE (p = 0.4, uncorrected). These results remained qualitatively consistent after excluding the extreme outliers (n = 1 control, p = 0.4, uncorrected; n = 2 resolved RE, p = 0.3 uncorrected). We found no relationship between sex, duration seizure free, the presence of ipsilateral epileptiform spikes, or concurrent antiseizure medication and inferior Rolandic thalamocortical functional connectivity (p > 0.4, all combinations), with the exception of a positive relationship between male sex and connectivity in the remission cohort (p = 0.02). This finding identifies a focal developmental abnormality within the inferior Rolandic motor thalamocortical circuit in children with Rolandic epilepsy during the symptomatic phase of the disease.
Fig. 3.
Abnormal functional connectivity from ventrolateral thalamus to the inferior motor cortex in Rolandic epilepsy. (A) The inferior Rolandic motor cortex and ventrolateral (VL) thalamus (blue) were used as seed and target ROIs in each analysis. (B) Control children have no evidence of a relationship between functional connectivity from the VL th alamus to the inferior Rolandic motor cortex with age (p = 0.9). In contrast, children with active Rolandic epilepsy have increased functional connectivity between the VL thalamus and the inferior motor cortex with age (p = 0.022, effect size = 0.095). Similar to controls, children with resolved Rolandic epilepsy have no evidence of a relationship between functional connectivity from the VL thalamus to the inferior Rolandic motor cortex with age (p = 0.4).
3.2. Structural connectivity in the thalamic motor circuit increases in resolved Rolandic epilepsy
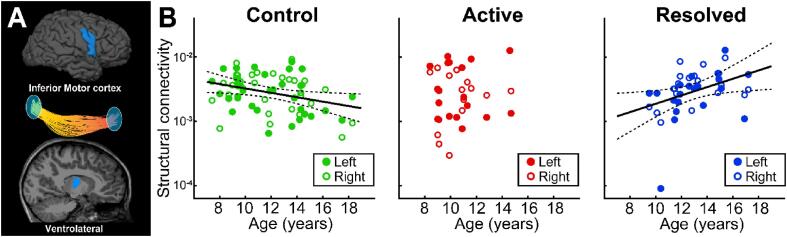
Evaluation of the structural connectivity of the inferior motor Rolandic thalamocortical circuit revealed that children with resolved RE have increased thalamocortical structural connectivity between inferior motor Rolandic cortex and the VL thalamic nuclei with age (p = 0.025 uncorrected, p = 0.05 corrected, effect size = 0.065, 95% CI [0.009, 0.121], Fig. 4). This relationship was not observed in children with active RE (p = 0.4, uncorrected). In contrast, control children have decreased thalamocortical structural connectivity from to the inferior motor Rolandic cortex to the VL thalamic nuclei with age (p = 0.022 uncorrected, p = 0.044 corrected, effect size = -0.034, 95% CI [-0.063, −0.005]). We found no relationship between sex, duration seizure free, the presence of ipsilateral epileptiform spikes, or concurrent antiseizure medication and inferior Rolandic thalamocortical structural connectivity (p > 0.7, all combinations). This finding reveals a focal abnormality in the developmental trajectory of the structural Rolandic thalamocortical motor circuit after symptom resolution that is an opposite pattern in children with resolved RE compared to control children.
Fig. 4.
Abnormal structural connectivity from ventrolateral thalamus to the inferior motor cortex in Rolandic epilepsy. (A) The inferior Rolandic motor cortex and ventrolateral (VL) thalamus (blue) were used as seed and target ROIs in each analysis. (B) Control children have decreased structural connectivity from the VL thalamus to the inferior Rolandic motor cortex with age (p = 0.022, β = -0.034). In contrast, children with active Rolandic epilepsy (red) have no relationship between structural connectivity from the inferior motor cortex to the VL thalamus and with age (p = 0.4). Opposite to controls, children with resolve Rolandic epilepsy (blue) have increase structural connectivity from the inferior Rolandic motor cortex to the VL thalamus with age (p = 0.025, β = 0.065).
3.3. No functional or structural connectivity relationships in the thalamic sensory circuit in any group
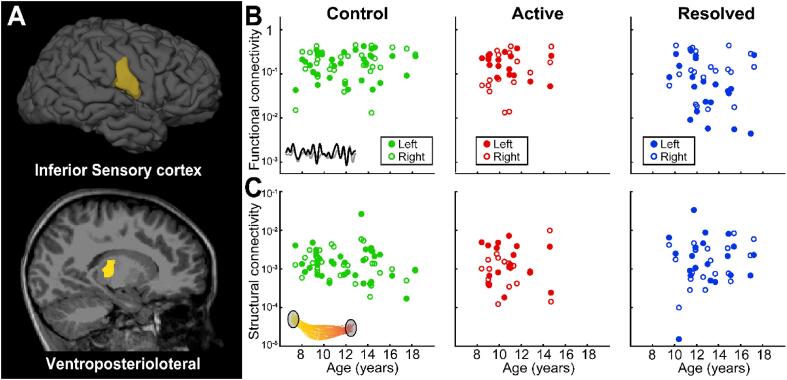
Within the Rolandic thalamocortical circuit, the ventroposteriolateral (VPL) thalamic nuclei project sensory information to the primary sensory cortex, including the inferior Rolandic cortex. Investigation of this component of the thalamocortical Rolandic circuit revealed no evidence of a relationship between age and functional connectivity (p > 0.25 uncorrected, all groups, Fig. 5A, B) or structural connectivity in any group (p > 0.12 uncorrected, all groups, Fig. 5A, C). These findings suggest that the developmental abnormalities in thalamocortical connectivity observed in this disease are confined to the Rolandic motor circuit.
Fig. 5.
Functional and structural connectivity from ventroposteriolateral thalamus to the inferior sensory cortex. (A) The inferior Rolandic sensory cortex and ventroposteriolateral (VPL) thalamus (yellow) were used as seed and target ROIs in each analysis. (B) We found no evidence of a relationship between age and functional connectivity from the VPL thalamus to the inferior Rolandic sensory cortex in any group (controls, p = 0.3; active Rolandic epilepsy, p = 0.7; resolved Rolandic epilepsy, p = 0.3) (C) We found no evidence of a relationship between age and structural connectivity from the VPL thalamus to the inferior Rolandic sensory cortex in any group (controls, p = 0.12; active Rolandic epilepsy, p = 0.9; resolved Rolandic epilepsy, p = 0.3).
3.4. No relationship between Rolandic structural and functional connectivity
Within the Rolandic thalamocortical motor circuit to the ventrolateral (VL) nuclei, we found no evidence of a relationship between functional and structural connectivity in any group (p > 0.47 uncorrected, all groups, Fig. S1A). This result persisted after excluding extreme outliers (n = 1 control, p = 0.5 uncorrected; n = 2 resolved RE, p = 0.3 uncorrected). Within the Rolandic thalamocortical sensory circuit to the ventroposteriolateral (VPL) thalamic nuclei, there was a trend toward a negative relationship between functional and structural connectivity in the VPL sensory circuit in control children that did not persist after correction for multiple comparisons (p = 0.09 uncorrected, p = 0.18 corrected, effect size = -0.19, 95% CI [-0.416, 0.028]). We found no evidence of a relationship between functional or structural connectivity in the VPL sensory circuit in children with active or resolved RE (p > 0.22 for both groups, uncorrected) (Fig. S1B).
3.5. Thalamocortical functional and structural connectivity to the Rolandic cortex typically increases with age
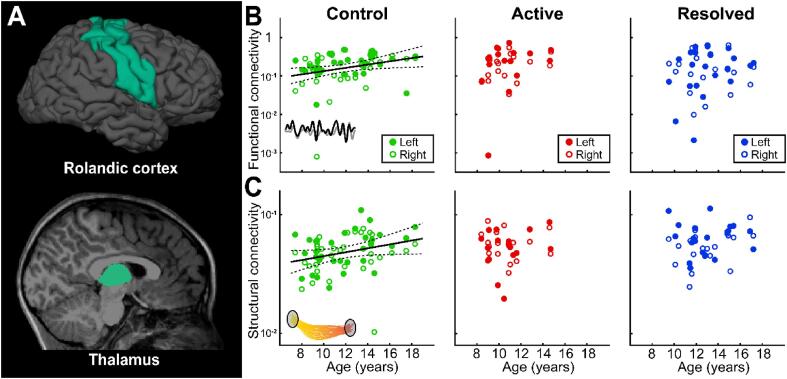
Consistent with several prior reports (Fair et al., 2010, Giedd et al., 1999, Paus, 2005, Thorn et al., 2020), we found that thalamocortical functional connectivity to the Rolandic cortex increases with age in control children (p = 0.02 uncorrected, p = 0.04 corrected, Beta = 0.042, 95% CI [0.007, 0.078], Fig. 6A, B). This result remained qualitatively consistent after excluding the 1 extreme outlier in the control group (p = 0.025 uncorrected, p = 0.05 corrected, Beta = 0.03, 95% CI [0.004, 0.057]). Similarly, we found that thalamocortical structural connectivity to the Rolandic cortex trended to increase with age in control children (p = 0.057 uncorrected, p = 0.114 corrected), Beta = 0.016, 95% CI [-0.0001, 0.033], Fig. 6A, C). This finding was significant after excluding the 1 extreme outlier in the control group (p = 0.003 uncorrected, p = 0.006 corrected, Beta = 0.022, 95% CI [0.008, 0.035]). In contrast, we found no evidence of a relationship between thalamocortical functional connectivity to the Rolandic cortex and age in active or resolved RE (p > 0.17, uncorrected, Fig. 6B; p = 0.3 after excluding the 1 extreme outlier in the active RE group). Similarly, no evidence of a relationship between thalamocortical structural connectivity and age in active or resolved RE (p > 0.21 both groups, uncorrected, Fig. 6C). Thus, we detect the expected structural and functional connectivity relationships with age in controls and identify a broad disruption of this typical thalamocortical developmental trajectory in both active and resolved RE. We note however, that the abnormalities observed in the inferior Rolandic cortex in children with RE (e.g. Fig. 3, Fig. 4) were not detected when the entire Rolandic cortex was evaluated, confirming that these developmental abnormalities are focal to the inferior Rolandic cortex.
Fig. 6.
Abnormal thalamocortical connectivity to the Rolandic cortex in Rolandic epilepsy (A) The Rolandic cortex and thalamus (green) were used as seed and target ROIs in each analysis. (B) Control children have increased functional connectivity from the thalamus to the Rolandic cortex with age (p = 0.02). In contrast, there is no evidence of a relationship between age and functional connectivity from the thalamus to the Rolandic cortex in children with active (p = 0.2) or resolved Rolandic epilepsy (blue, p = 0.5). (C) Control children have a trend toward increased structural connectivity from the thalamus to the Rolandic cortex with age (p = 0.052). In contrast, there is no evidence of a relationship between age and structural connectivity from the thalamus to the Rolandic cortex in children with active (p = 0.6) or resolved Rolandic epilepsy (p = 0.2).
4. Discussion
Rolandic epilepsy is the most common self-limited focal developmental epilepsy, but the pathophysiology behind the emergence and resolution of symptoms in this disease is not yet understood. Several lines of converging evidence have identified disruption of the Rolandic thalamocortical circuit (Ciumas et al., 2014, Fuentealba and Steriade, 2005, Grigg-Damberger et al., 2007, Kim et al., 2014, Ostrowski et al., 2019, Sanchez Fernandez et al., 2017, Steriade et al., 1993, Wu et al., 2015, Xiao et al., 2014), however the timing of these disruptions relative to symptoms and the precise localization of these abnormalities within the thalamocortical circuit are not known. Here, we show that children with RE have increasing functional connectivity along the thalamocortical motor circuit with age during the symptomatic phase of the disease. In contrast, children with RE have increased structural connectivity along this same component of the thalamocortical circuit after symptom resolution. We found no disease-specific abnormalities along the Rolandic sensory circuit, localizing the thalamocortical functional and structural lesions to the thalamocortical motor circuit in this disease. Identification of these circuit disruptions provides new insights into the network imbalances that coincide with symptom onset and resolution in this common developmental epilepsy.
We found increased functional connectivity using resting state fMRI with age in the Rolandic thalamocortical motor circuit during the period of active epilepsy. Consistent with our finding, previous work has found that thalamocortical functional connectivity increased with disease duration in both temporal lobe epilepsy (Peng and Hsin, 2017) and juvenile myoclonic epilepsy (O'Muircheartaigh et al., 2012). In this self-limited disease, we also found that the increased functional connectivity with age observed during the active period of disease resolved during disease resolution. Taken together, these findings suggest that increased functional connectivity between the thalamus and the seizure onset zone may reflect or contribute to increased seizure risk.
We found increased structural connectivity with age in the Rolandic thalamocortical motor circuit during epilepsy resolution using probabilistic tractography. Prior work in temporal lobe epilepsy has identified white matter microstructural abnormalities in humans (Owen et al., 2021) and rodent models (Wang et al., 2017). In these models, the degree of white matter disruption correlated with epilepsy duration (Owen et al., 2021) and seizure burden (Wang et al., 2017), suggesting a direct impact of seizures on white matter integrity. In contrast, we did not see structural abnormalities in the Rolandic thalamocortical motor circuit during the active phase of disease in RE. Rather, the structural changes coincided with symptom resolution, raising the possibility that the atypical developmental white matter trajectory observed in RE may play a compensatory role in this disease. We note that the connectivity abnormalities are evident when children are grouped into “active” and “resolved” states and were not simply a function of duration seizure free, the presence of ipsilateral epileptiform discharges, or antiseizure medication use. This suggests that there are transient abnormalities that only occur proximal to the period of seizure susceptibility in this self-limited epilepsy, and other abnormalities that are only detectable at least one year into recovery.
We did not find a direct relationship between thalamocortical structural and functional connectivity in any of the circuits we evaluated. These findings are consistent with prior work evaluating structural and functional brain networks simultaneously which has not identified a strong relationship between the two (Honey et al., 2009). Rather, brain functional activity is thought to be constrained by brain structure, but within these constraints can demonstrate diverse dynamics, as observed here (Chu et al., 2015, Park and Friston, 2013).
We found no relationship between the VPL and inferior Rolandic connectivity in any groups, suggesting that thalamocortical motor circuits, not thalamocortical sensory circuits, drive the pathology and symptoms in RE. Thus, we were able to localize the thalamocortical circuit abnormality to the efferent inferior Rolandic VL thalamus motor circuit. This improved localization may provide potential treatment targets for drug discovery or neuromodulation (Baumer et al., 2020) to dampen thalamocortical functional connectivity in the setting of medication refractory symptomatic disease (Tovia et al., 2011).
Although we used robust methods applied to high quality combined functional and structural MRI imaging in this targeted multimodal study, there are still several limitations in this study. Here, we evaluated a moderately sized sample of children and controls, where larger sample sizes would increase the statistical power to detect subtle differences in other components of the Rolandic thalamocortical circuit. We do note that using our methods and sample size, we were able to replicate the expected linear increase in Rolandic thalamocortical functional and structural connectivity with age reported in children in several prior studies (Fair et al., 2010, Giedd et al., 1999, Paus, 2005). Our study was limited to cross-sectional data so these developmental findings would be strengthened by longitudinal data over the course of the disease. Last, to reduce the risk of false discovery due to multiple comparisons, we targeted specific ROIs in our study. While this approach improved our power to detect differences, it may have missed network abnormalities outside the Rolandic thalamocortical circuit.
Using combined structural and functional imaging, we identify disrupted development of the structural and functional Rolandic thalamocortical circuits in RE, the most common idiopathic, focal, developmental epilepsy. These data reveal abnormal ventrolateral thalamocortical functional circuits during the symptomatic phase of this disease and identify that thalamocortical structural changes coincide with symptom resolution. This work validates and localizes Rolandic thalamocortical circuit disruption in Rolandic epilepsy and illustrates the dynamic motor network alterations present in this transient developmental disease.
CRediT authorship contribution statement
Hunki Kwon: Writing – original draft, Data curation, Investigation, Formal analysis. Dhinakaran M. Chinappen: Data curation. Jonathan F. Huang: Data curation. Erin D. Berja: Data curation. Katherine G. Walsh: Data curation. Shi Wen: Data curation. Mark A. Kramer: Methodology, Writing – review & editing. Catherine J. Chu: Conceptualization, Methodology, Resources, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: CJC and MAK have served as paid consultants for Biogen Inc. CJC has served as a paid consultant for Ovid Pharmaceuticals. None of the other authors have any conflicts of interest.
Acknowledgments
Acknowledgments
The authors would like to thank Anvitha Sathya, Dan Y. Song, Emily L. Thorn, and Sally M. Stoyell for their assistance in MRI data collection for this project.
Ethics statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Author contributions
HK planned the study, collected data, analyzed the data, and wrote the manuscript. DMC, JFH, EDB, KGW and SW collected data and provided feedback on the manuscript. MAK contributed to developing analysis methods and provided feedback on the manuscript. CJC planned the study, helped develop analysis methods, and contributed to writing the manuscript.
Funding
This work was supported by NINDS R01NS115868.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103102.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1989.1989. Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 30, 389-399. [DOI] [PubMed]
- Astradsson A., Olafsson E., Ludvigsson P., Bjorgvinsson H., Hauser W.A. Rolandic epilepsy: an incidence study in Iceland. Epilepsia. 1998;39:884–886. doi: 10.1111/j.1528-1157.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- Baumer F.M., Pfeifer K., Fogarty A., Pena-Solorzano D., Rolle C.E., Wallace J.L., Rotenberg A., Fisher R.S. Cortical Excitability, Synaptic Plasticity, and Cognition in Benign Epilepsy With Centrotemporal Spikes: A Pilot TMS-EMG-EEG Study. J. Clin. Neurophysiol. 2020;37:170–180. doi: 10.1097/WNP.0000000000000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens T.E., Berg H.J., Jbabdi S., Rushworth M.F., Woolrich M.W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? NeuroImage. 2007;34:144–155. doi: 10.1016/j.neuroimage.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A.T., Langfitt J.T., Testa F.M., Levy S.R., DiMario F., Westerveld M., Kulas J. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49:608–614. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Berg A.T., Lin J., Ebrahimi N., Testa F.M., Levy S.R., Shinnar S. Modeling remission and relapse in pediatric epilepsy: application of a Markov process. Epilepsy Res. 2004;60:31–40. doi: 10.1016/j.eplepsyres.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Berg A.T., Rychlik K. The course of childhood-onset epilepsy over the first two decades: a prospective, longitudinal study. Epilepsia. 2015;56:40–48. doi: 10.1111/epi.12862. [DOI] [PubMed] [Google Scholar]
- Boor R., Jacobs J., Hinzmann A., Bauermann T., Scherg M., Boor S., Vucurevic G., Pfleiderer C., Kutschke G., Stoeter P. Combined spike-related functional MRI and multiple source analysis in the non-invasive spike localization of benign rolandic epilepsy. Clin. Neurophysiol. 2007;118:901–909. doi: 10.1016/j.clinph.2006.11.272. [DOI] [PubMed] [Google Scholar]
- Camfield C.S., Camfield P.R. Rolandic epilepsy has little effect on adult life 30 years later: a population-based study. Neurology. 2014;82:1162–1166. doi: 10.1212/WNL.0000000000000267. [DOI] [PubMed] [Google Scholar]
- Chu C.J., Tanaka N., Diaz J., Edlow B.L., Wu O., Hamalainen M., Stufflebeam S., Cash S.S., Kramer M.A. EEG functional connectivity is partially predicted by underlying white matter connectivity. NeuroImage. 2015;108:23–33. doi: 10.1016/j.neuroimage.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciumas C., Saignavongs M., Ilski F., Herbillon V., Laurent A., Lothe A., Heckemann R.A., de Bellescize J., Panagiotakaki E., Hannoun S., Marinier D.S., Montavont A., Ostrowsky-Coste K., Bedoin N., Ryvlin P. White matter development in children with benign childhood epilepsy with centro-temporal spikes. Brain. 2014;137:1095–1106. doi: 10.1093/brain/awu039. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Segonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fair D.A., Bathula D., Mills K.L., Dias T.G., Blythe M.S., Zhang D., Snyder A.Z., Raichle M.E., Stevens A.A., Nigg J.T., Nagel B.J. Maturing thalamocortical functional connectivity across development. Front. Syst. Neurosci. 2010;4:10. doi: 10.3389/fnsys.2010.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang P.C., Stepniewska I., Kaas J.H. The thalamic connections of motor, premotor, and prefrontal areas of cortex in a prosimian primate (Otolemur garnetti) Neuroscience. 2006;143:987–1020. doi: 10.1016/j.neuroscience.2006.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R.S., Acevedo C., Arzimanoglou A., Bogacz A., Cross J.H., Elger C.E., Engel J., Jr., Forsgren L., French J.A., Glynn M., Hesdorffer D.C., Lee B.I., Mathern G.W., Moshe S.L., Perucca E., Scheffer I.E., Tomson T., Watanabe M., Wiebe S. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55:475–482. doi: 10.1111/epi.12550. [DOI] [PubMed] [Google Scholar]
- Fuentealba P., Steriade M. The reticular nucleus revisited: intrinsic and network properties of a thalamic pacemaker. Prog. Neurobiol. 2005;75:125–141. doi: 10.1016/j.pneurobio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., Castellanos F.X., Liu H., Zijdenbos A., Paus T., Evans A.C., Rapoport J.L. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Grigg-Damberger M., Gozal D., Marcus C.L., Quan S.F., Rosen C.L., Chervin R.D., Wise M., Picchietti D.L., Sheldon S.H., Iber C. The visual scoring of sleep and arousal in infants and children. J. Clin. Sleep Med. 2007;3:201–240. [PubMed] [Google Scholar]
- Guerrini R. Epilepsy in children. Lancet. 2006;367:499–524. doi: 10.1016/S0140-6736(06)68182-8. [DOI] [PubMed] [Google Scholar]
- Guzzetta F., Battaglia D., Veredice C., Donvito V., Pane M., Lettori D., Chiricozzi F., Chieffo D., Tartaglione T., Dravet C. Early thalamic injury associated with epilepsy and continuous spike-wave during slow sleep. Epilepsia. 2005;46:889–900. doi: 10.1111/j.1528-1167.2005.64504.x. [DOI] [PubMed] [Google Scholar]
- Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scand. J. Stat. 1979;6:65–70. [Google Scholar]
- Honey C.J., Sporns O., Cammoun L., Gigandet X., Thiran J.P., Meuli R., Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias, J.E., Insausti, R., Lerma-Usabiaga, G., Bocchetta, M., Van Leemput, K., Greve, D.N., van der Kouwe, A., Alzheimer's Disease Neuroimaging, I., Fischl, B., Caballero-Gaudes, C., Paz-Alonso, P.M., 2018. A probabilistic atlas of the human thalamic nuclei combining ex vivo MRI and histology. NeuroImage 183, 314-326. [DOI] [PMC free article] [PubMed]
- Kersbergen K.J., de Vries L.S., Leijten F.S., Braun K.P., Nievelstein R.A., Groenendaal F., Benders M.J., Jansen F.E. Neonatal thalamic hemorrhage is strongly associated with electrical status epilepticus in slow wave sleep. Epilepsia. 2013;54:733–740. doi: 10.1111/epi.12131. [DOI] [PubMed] [Google Scholar]
- Kim S.E., Lee J.H., Chung H.K., Lim S.M., Lee H.W. Alterations in white matter microstructures and cognitive dysfunctions in benign childhood epilepsy with centrotemporal spikes. Eur. J. Neurol. 2014;21:708–717. doi: 10.1111/ene.12301. [DOI] [PubMed] [Google Scholar]
- Kramer M.A., Stoyell S.M., Chinappen D., Ostrowski L.M., Spencer E.R., Morgan A.K., Emerton B.C., Jing J., Westover M.B., Eden U.T., Stickgold R., Manoach D.S., Chu C.J. Focal Sleep Spindle Deficits Reveal Focal Thalamocortical Dysfunction and Predict Cognitive Deficits in Sleep Activated Developmental Epilepsy. J. Neurosci. 2021;41:1816–1829. doi: 10.1523/JNEUROSCI.2009-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal A., Calado E., Vieira J.P., Mendonca C., Ferreira J.C., Ferreira H., Carvalho D., Furtado F., Gomes R., Monteiro J.P. Anatomical and physiological basis of continuous spike-wave of sleep syndrome after early thalamic lesions. Epilepsy Behav. 2018;78:243–255. doi: 10.1016/j.yebeh.2017.08.027. [DOI] [PubMed] [Google Scholar]
- Lin Y.Y., Shih Y.H., Chang K.P., Lee W.T., Yu H.Y., Hsieh J.C., Yeh T.C., Wu Z.A., Ho L.T. MEG localization of rolandic spikes with respect to SI and SII cortices in benign rolandic epilepsy. NeuroImage. 2003;20:2051–2061. doi: 10.1016/j.neuroimage.2003.08.019. [DOI] [PubMed] [Google Scholar]
- O'Muircheartaigh J., Vollmar C., Barker G.J., Kumari V., Symms M.R., Thompson P., Duncan J.S., Koepp M.J., Richardson M.P. Abnormal thalamocortical structural and functional connectivity in juvenile myoclonic epilepsy. Brain. 2012;135:3635–3644. doi: 10.1093/brain/aws296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski L.M., Song D.Y., Thorn E.L., Ross E.E., Stoyell S.M., Chinappen D.M., Eden U.T., Kramer M.A., Emerton B.C., Morgan A.K., Stufflebeam S.M., Chu C.J. Dysmature superficial white matter microstructure in developmental focal epilepsy. Brain Commun. 2019;1:fcz002. doi: 10.1093/braincomms/fcz002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen T.W., de Tisi J., Vos S.B., Winston G.P., Duncan J.S., Wang Y., Taylor P.N. Multivariate white matter alterations are associated with epilepsy duration. Eur. J. Neurosci. 2021;53:2788–2803. doi: 10.1111/ejn.15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.J., Friston K. Structural and functional brain networks: from connections to cognition. Science. 2013;342:1238411. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Pataraia E., Feucht M., Lindinger G., Aull-Watschinger S., Baumgartner C. Combined electroencephalography and magnetoencephalography of interictal spikes in benign rolandic epilepsy of childhood. Clin. Neurophysiol. 2008;119:635–641. doi: 10.1016/j.clinph.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends Cogn. Sci. 2005;9:60–68. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Peng S.J., Hsin Y.L. Altered structural and functional thalamocortical networks in secondarily generalized extratemporal lobe seizures. Neuroimage Clin. 2017;13:55–61. doi: 10.1016/j.nicl.2016.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross E.E., Stoyell S.M., Kramer M.A., Berg A.T., Chu C.J. The natural history of seizures and neuropsychiatric symptoms in childhood epilepsy with centrotemporal spikes (CECTS) Epilepsy Behav. 2020;103:106437. doi: 10.1016/j.yebeh.2019.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Fernandez I., Peters J.M., Akhondi-Asl A., Klehm J., Warfield S.K., Loddenkemper T. Reduced thalamic volume in patients with Electrical Status Epilepticus in Sleep. Epilepsy Res. 2017;130:74–80. doi: 10.1016/j.eplepsyres.2017.01.010. [DOI] [PubMed] [Google Scholar]
- Sanchez Fernandez I., Takeoka M., Tas E., Peters J.M., Prabhu S.P., Stannard K.M., Gregas M., Eksioglu Y., Rotenberg A., Riviello J.J., Jr., Kothare S.V., Loddenkemper T. Early thalamic lesions in patients with sleep-potentiated epileptiform activity. Neurology. 2012;78:1721–1727. doi: 10.1212/WNL.0b013e3182582ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D.Y., Stoyell S.M., Ross E.E., Ostrowski L.M., Thorn E.L., Stufflebeam S.M., Morgan A.K., Emerton B.C., Kramer M.A., Chu C.J. Beta oscillations in the sensorimotor cortex correlate with disease and remission in benign epilepsy with centrotemporal spikes. Brain Behav. 2019;9:e01237. doi: 10.1002/brb3.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specchio N., Wirrell E.C., Scheffer I.E., Nabbout R., Riney K., Samia P., Guerreiro M., Gwer S., Zuberi S.M., Wilmshurst J.M., Yozawitz E., Pressler R., Hirsch E., Wiebe S., Cross H.J., Perucca E., Moshé S.L., Tinuper P., Auvin S. International League Against Epilepsy classification and definition of epilepsy syndromes with onset in childhood: Position paper by the ILAE Task Force on Nosology and Definitions. Epilepsia. 2022;63:1398–1442. doi: 10.1111/epi.17241. [DOI] [PubMed] [Google Scholar]
- Spencer E.R., Chinappen D., Emerton B.C., Morgan A.K., Hämäläinen M.S., Manoach D.S., Eden U.T., Kramer M.A., Chu C.J. Source EEG reveals that Rolandic epilepsy is a regional epileptic encephalopathy. Neuroimage Clin. 2022;33:102956. doi: 10.1016/j.nicl.2022.102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M., McCormick D.A., Sejnowski T.J. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Stoyell S.M., Baxter B.S., McLaren J., Kwon H., Chinappen D.M., Ostrowski L., Zhu L., Grieco J.A., Kramer M.A., Morgan A.K., Emerton B.C., Manoach D.S., Chu C.J. Diazepam induced sleep spindle increase correlates with cognitive recovery in a child with epileptic encephalopathy. BMC Neurol. 2021;21:355. doi: 10.1186/s12883-021-02376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn E.L., Ostrowski L.M., Chinappen D.M., Jing J., Westover M.B., Stufflebeam S.M., Kramer M.A., Chu C.J. Persistent abnormalities in Rolandic thalamocortical white matter circuits in childhood epilepsy with centrotemporal spikes. Epilepsia. 2020;61:2500–2508. doi: 10.1111/epi.16681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toulmin H., Beckmann C.F., O'Muircheartaigh J., Ball G., Nongena P., Makropoulos A., Ederies A., Counsell S.J., Kennea N., Arichi T., Tusor N., Rutherford M.A., Azzopardi D., Gonzalez-Cinca N., Hajnal J.V., Edwards A.D. Specialization and integration of functional thalamocortical connectivity in the human infant. Proc. Natl. Acad. Sci. U. S. A. 2015;112:6485–6490. doi: 10.1073/pnas.1422638112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovia E., Goldberg-Stern H., Ben Zeev B., Heyman E., Watemberg N., Fattal-Valevski A., Kramer U. The prevalence of atypical presentations and comorbidities of benign childhood epilepsy with centrotemporal spikes. Epilepsia. 2011;52:1483–1488. doi: 10.1111/j.1528-1167.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- Wang H., Huang Y., Coman D., Munbodh R., Dhaher R., Zaveri H.P., Hyder F., Eid T. Network evolution in mesial temporal lobe epilepsy revealed by diffusion tensor imaging. Epilepsia. 2017;58:824–834. doi: 10.1111/epi.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens S., Bowden S.C., D'Souza W. Cognitive functioning in children with self-limited epilepsy with centrotemporal spikes: A systematic review and meta-analysis. Epilepsia. 2017;58:1673–1685. doi: 10.1111/epi.13865. [DOI] [PubMed] [Google Scholar]
- Wu Y., Ji G.J., Zang Y.F., Liao W., Jin Z., Liu Y.L., Li K., Zeng Y.W., Fang F. Local Activity and Causal Connectivity in Children with Benign Epilepsy with Centrotemporal Spikes. PLoS ONE. 2015;10:e0134361. doi: 10.1371/journal.pone.0134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Chen Q., Yu X., Tang Y., Luo C., Fang J., Liu L., Huang X., Gong Q., Zhou D. Hemispheric lateralization of microstructural white matter abnormalities in children with active benign childhood epilepsy with centrotemporal spikes (BECTS): a preliminary DTI study. J. Neurol. Sci. 2014;336:171–179. doi: 10.1016/j.jns.2013.10.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data were generated at Massachusetts General Hospital and the Athinoula A. Martinos Center for Biomedical Imaging. Derived data supporting the findings of this study are available from the corresponding author on request. All code for the analysis used in this study as described in the Methods are available at https://github.com/Hunki-Kwon/Thalamocortical-Connectivity.