Abstract
Background
As a result of the essential role of oestrogens in epiphyseal closure, aromatase inhibitors have been trialled as an intervention to improve height outcomes in male children and adolescents by inhibiting the conversion of testosterone to oestradiol.
Objectives
To assess the effects of aromatase inhibitors in male children and adolescents with short stature.
Search methods
To identify relevant trials, we searched the Cochrane Library (2014, Issue 7), MEDLINE, EMBASE, and the World Health Organization (WHO) ICTRP trial register from their inception until August 2014. In addition, we conducted citation searches and screened reference lists of included trials.
Selection criteria
We included randomised controlled trials (RCTs) if they compared use of an aromatase inhibitor with placebo in male children and adolescents with short stature.
Data collection and analysis
Two authors independently screened titles and abstracts for relevance. Both authors carried out screening for inclusion, data extraction, and risk of bias assessment, with any disagreements resolved following discussion. We assessed trials for quality of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) instrument. We contacted study authors regarding missing information. Primary outcomes were final or near‐final height, adverse events, and health‐related quality of life. Secondary outcomes included all‐cause mortality, cognitive outcomes, socioeconomic effects, laboratory measures, short‐term growth parameters, and assessment of effects on bone health. Meta‐analysis was not appropriate due to the substantial clinical heterogeneity between trials; we presented the findings of the review in narrative format.
Main results
We included four RCTs involving 207 participants (84 on interventions) in the review. Trials included males with constitutional delay of growth and puberty (CDGP), idiopathic short stature (ISS), and growth hormone (GH) deficiency. Three of the trials had an overall low or unclear risk of bias for primary outcomes. Short‐term growth outcomes, such as predicted adult height, improved in all trials. Just one trial reported the primary outcome of final and near‐final height as an extension under non‐randomised conditions. None of the trials assessed health‐related quality of life. One publication provided detailed information regarding the incidence of adverse events. A significant proportion (45%) of prepubertal boys with ISS treated with letrozole developed mild morphological abnormalities of their vertebrae, compared with none in the placebo group.
Authors' conclusions
Available evidence suggested that aromatase inhibitors improved short‐term growth outcomes. There was no evidence to support an increase in final adult height, based on limited data, with only one of four trials publishing final height data under non‐randomised conditions.
Plain language summary
Aromatase inhibitors for short stature in male children and adolescents
Review question
This review considered whether a class of medications called aromatase inhibitors could increase adult height in male children and adolescents.
Background
Aromatase inhibitors are an orally available medicine used to prevent conversion of the male hormone, testosterone, to oestrogen. In both sexes, oestrogen causes closure of the growth plates (areas produce new bone growth and so lengthen bones) in long bones (e.g. thigh bone) when growth is complete at the end of puberty. Blocking this conversion in boys reduces oestrogen levels and can prolong the period of growth, and theoretically increase adult height. Oestrogen is important for female pubertal development, and thus, aromatase inhibitors are not suitable for use in teenage girls. Aromatase inhibitors are currently not approved to treat short stature but are rather used as an 'off‐label' medicine (i.e. they are licensed to treat other illnesses).
Study characteristics
We searched scientific databases for clinical trials comparing an aromatase inhibitor with placebo or no treatment. We identified four trials (involving 207 male participants) that met inclusion criteria. In one trial, participants received co‐treatment with growth hormone (a hormone that causes growth) and, in another trial, participants had six months' co‐treatment with testosterone. Underlying diagnoses of study participants included short stature of unknown cause, delayed puberty, or lack of growth hormone. Due to differing study designs, only descriptions of individual trials were available and there were no opportunities for combining the trial results.
Key results
Short‐term growth outcomes such as predicted adult height (there is currently no proven way to predict a child's adult height accurately, several formulas are used to provide a reasonable guess for child growth) improved in all trials. However, only one trial reported final adult height data, and demonstrated no relevant difference. There was concern that final adult height data were not published for the other studies. Aromatase inhibitors were generally well tolerated and no participants withdrew from the trials because of side effects. However, only one publication reported detailed information regarding side effects. There were concerns regarding the rate of abnormalities in the spine of 45% of young boys who were treated before entering puberty. None of the trials provided information on overall health‐related quality of life or costs.
Quality of the evidence
We considered the overall quality of the evidence of the included trials as low or moderate, mainly because of the small number of trials and participants. There remain concerns regarding non‐publication of final height data.
Status of the evidence
This evidence is up to date as of August 2014.
Summary of findings
Summary of findings for the main comparison. Aromatase inhibitors for short stature in male children and adolescents.
| Aromatase inhibitors for short stature in male children and adolescents | ||||||
|
Patient or population: male children and adolescents with short stature Settings: paediatric endocrinology outpatient clinic Intervention: aromatase inhibitor Comparison: placebo or no treatment | ||||||
| Outcomes | Placebo | Aromatase inhibitor | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Final height | See comments | See comments | Not estimable | See comments | See comments | Not reported (only data from an extension period under non‐randomised conditions) |
| Near‐final height | See comments | See comments | Not estimable | See comments | See comments | Not reported (only data from an extension period under non‐randomised conditions) |
|
Cognitive outcomes WISC‐III test (verbal performance similarities, comprehension, block design); visuospatial performance; Rey‐Osterrieth test (complex figure copying task) Follow‐up: 2 years |
See comments | See comments | Not estimable | 28 (1) | ⊕⊕⊝⊝ Low | Intervention and placebo groups cognitive performance different at baseline. Comparable improvement in task performances of both groups over time (analysis of age‐adjusted cognitive test data produced similar results; no statistically significant group‐treatment‐time interactions were observed) |
| All‐cause mortality | See comments | See comments | Not estimable | See comments | See comments | No recorded deaths |
| Health‐related quality of life | See comments | See comments | Not estimable | See comments | See comments | Not assessed |
|
Adverse events Follow‐up: 12‐36 months |
See comments | See comments | See comments | 174 (3) | ⊕⊕⊕⊝ Moderateb |
4/26 anastrozole‐treated versus 0/26 placebo‐treated participants had a serious adverse event (Mauras 2008); 1/15 placebo‐treated versus 0/16 letrozole‐treated participants had a serious adverse event (Hero 2005) 5/11 (45%) of prepubertal males with ISS treated with letrozole developed mild morphological abnormalities of their vertebrae, compared to 0 in the placebo group (Hero 2005) |
| Socioeconomic effects | See comments | See comments | Not estimable | See comments | See comments | Not assessed |
| CI: confidence interval; ISS: idiopathic short stature; WISC‐III: Wechsler Intelligence Scale for Children version III. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aDowngraded by two levels because of one study only and very small numbers of participants. bDowngraded by one level because of small numbers of participants and only three of four studies providing detailed information about adverse events.
Background
Description of the condition
Concerns regarding short stature in childhood and adolescence are a frequent reason for consultation with a paediatric endocrinologist. Short stature is defined as height below the third percentile or approximately two standard deviations (SDs) or more below the mean height for children of a given age, sex, and population group. The Utah growth study assessed height and growth velocity in approximately 115,000 American children (Lindsay 1994). A total of 555 children had short stature as defined previously and diminished growth rate (defined as growth velocity less than 5 cm annually); however, only 5% had an endocrine disorder. The most frequent causes of short stature were constitutional delay of growth and pubertal (CDGP) development in addition to familial short stature. Idiopathic short stature (ISS) is a clinical description for a heterogeneous group of short children in whom no chronic disease, nutritional deficiency, psychosocial adversity, endocrinopathy, or other process is responsible for their height outcome (Cohen 2008; Ranke 1996). Children with ISS fall into two main groups: familial short stature and CDGP. The characteristics of familial short stature include bone age appropriate for chronological age, normal growth velocity, and predicted adult height appropriate to the familial pattern. In contrast, constitutional growth delay is characterised by delayed bone age, normal growth velocity, and predicted adult height appropriate to the familial pattern.
A number of interventions are currently available to increase adult height in an attempt to reduce the psychological burden attributable to short stature in childhood and adult life. Growth hormone (GH) therapy is approved for the treatment of short children with reduced adult height expectation. For individuals with constitutional delay, the use of physiological doses of sex steroids accelerates linear growth and the onset of pubertal changes but does not improve predicted adult height (Richmond 2007).
Available evidence suggests only modest efficacy for GH treatment in children and adolescents with ISS (Bryant 2007). Treatment costs are substantial and represent an important consideration regarding allocation of medical resources. In the context of current costs of GH treatment in the US, this corresponds to more than USD 20,000 (approximately EUR 14,774, November 2013 conversion rate) per centimetre in adult height gained (Lee 2006). Studies indicated that final adult height is increased with early therapy, thus the earlier the diagnosis, the better the prognosis for height. Nonetheless, most children treated with GH remain short when compared to their peers of normal stature. In GH deficiency, the effect of the GH dose on total pubertal growth appears to be smaller than the relative effect on growth during prepubertal years (Ranke 2003). Consequently, it is anticipated that GH treatment started during puberty has only limited effect on final height, a fact compounded by continuing skeletal maturation and progression towards epiphyseal closure.
Gonadotropin‐releasing hormone analogues (GnRHa) are the standard of care for treatment of central precocious puberty (CPP) and their efficacy in increasing adult height is undisputed in early‐onset CPP. Given the aforementioned limitations of GH therapy started during puberty, interruption of normal puberty in short children with GnRHa has been considered in an attempt to improve adult height. A significant number of non‐randomised trials suggested that GnRHas are ineffective for this purpose (Balducci 1995; Carel 1996; Job 1994; Lanes 1998; Lindner 1993). Conversely, other non‐randomised trials (Mul 2001; Pasquino 2000), and two randomised trials (Kamp 2001; Mericq 2000), suggested treatment was beneficial, although a follow‐up report of the Kamp study at final height suggested that approximately 50% of the increase in predicted adult height obtained during the trial was lost with discontinuation of treatment, such that final height was not significantly different (Van Gool 2007). One consensus statement proposed that routine use of GnRHa, an expensive and invasive treatment, to improve final height in short children with normally timed puberty, could not be recommended (Carel 2009). Bone mineral density (BMD) may decrease during GnRHa therapy, although bone mass accrual returns to normal when treatment is discontinued (Pasquino 2008). Furthermore, one of the principal indications to offer treatment is because of the adverse psychosocial impact of short stature. One might argue that the psychosocial consequences of delaying puberty do not represent an acceptable trade‐off in this regard.
Description of the intervention
Aromatase, encoded by the CYP19A1 gene, catalyses the rate‐limiting step in the conversion of testosterone to oestradiol and androstenedione to oestrone. Use of aromatase inhibitors has been reported for an assortment of conditions in children and adolescents, including peripheral precocious puberty (Feuillan 2007; Reiter 2010), congenital adrenal hyperplasia (Merke 2000), pubertal gynaecomastia (Mauras 2009), and short stature (Mauras 2004).
The fundamental role of oestrogen in skeletal maturation was initially recognised in the mid‐1990s following reports of two young adult males in their 20s, one with an oestrogen receptor defect and another with an inactivating mutation of the aromatase gene (Morishima 1995; Smith 1994). Both were described as having tall stature (adult height up to 204 cm) and unfused epiphyses despite normal pubertal development. These observations led to use of therapies specifically targeting oestrogen to counteract its effects at the growth plate, thus prolonging growth potential. Third‐generation aromatase inhibitors including anastrozole and letrozole and selective oestrogen receptor modulators (SERMs) such as tamoxifen have consequently been used as an intervention to improve height outcomes in males as outlined in a number of reports (Kreher 2005; Kumar 2009; Zhou 2005). Duration of therapy appears to influence final height, with one report that five‐year letrozole monotherapy resulted in improvements up to 15 cm on pre‐treatment predicted adult height (Krebs 2012).
Oral administration and the ability to allow puberty to continue at a physiologically appropriate time make aromatase inhibitors an attractive intervention to delay skeletal maturation while augmenting height potential.
Adverse effects of the intervention
The clinical phenotype of aromatase deficiency in males, in addition to tall stature, includes osteoporosis, dyslipidaemia (elevated low‐density lipoprotein (LDL), reduced high‐density lipoprotein (HDL)), and insulin resistance (Carani 1997; Maffei 2004; Morishima 1995). These effects appear reversible following oestrogen supplementation. The possible association of aromatase deficiency with abnormal testicular size, low sperm count, and low motility has not yet been conclusively resolved. To date, seven adult males with aromatase deficiency have been described (De Ronde 2011), with varying testicular sizes ranging from micro‐ to macro‐orchidism. Four men were infertile, but in one younger male, fertility was not described. Two aromatase‐deficient men had a brother who also had infertility despite a normal aromatase genotype, suggesting an unrelated cause may also have contributed. Conversely, aromatase inhibitors have been used with success in adults to treat male infertility (Raman 2002; Ramasamy 2009).
Other reported adverse effects of aromatase inhibitors include nausea, vomiting, abdominal pain, diarrhoea, headache, erythrocytosis, arthralgia, bone pain, and skin rashes. They are recommended to be avoided in people with hepatic and renal impairment.
How the intervention might work
The rate of linear growth accelerates during puberty, followed by deceleration and cessation of growth. Clinical findings in people with oestrogen insensitivity or aromatase deficiency suggest that oestrogen is essential for this change in height at puberty. Prolonging the period of growth by diminishing the biological action of oestrogen and, ultimately, by delaying the senescence of the growth plate can theoretically increase adult height. Aromatase inhibitors have been used to achieve this effect.
Why it is important to do this review
Although there have been some reviews regarding the use of aromatase inhibitors in paediatrics (Cernich 2004; Shulman 2008; Wit 2011), none has used detailed systematic methodology. Current strategies for increasing adult height are expensive and require parenteral administration. Several years of therapy are required to obtain modest height increases and are of limited benefit to children who are already pubertal. Furthermore, interruption of normal puberty using GnRHa substitutes the psychological impact of short stature with that of delayed puberty. The emergence of aromatase inhibitors as a possible alternative means of delaying epiphyseal fusion and prolonging linear growth has therefore generated immense interest. Aromatase inhibitors are possibly more effective than GnRHa in promoting increased adult height in children with short stature and, unlike GnRHa, they do not delay pubertal development in males.
Objectives
To assess the effects of aromatase inhibitors in male children and adolescents with short stature.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) or quasi‐RCTs that compared an aromatase inhibitor with placebo or no treatment.
Types of participants
Males with a chronological age of 16 years or younger with idiopathic short stature (ISS), growth hormone (GH) deficiency, or constitutional delay of growth and pubertal development (CDGP) were eligible for inclusion.
Diagnostic criteria
GH deficiency defined as evidence of peak GH less than 10 ng/mL following provocative testing using insulin, glucagon, arginine, clonidine, growth hormone releasing hormone (GHRH) or L‐dopa, and propranolol.
ISS defined as a height two SDs or more below the mean for age, sex, and population group, in whom no chronic disease, nutritional deficiency, psychosocial adversity, endocrinopathy, or other condition was responsible for their height outcome.
CDGP defined as testicular volume less than 4 mL at age 14 years or two SDs older than the mean age of pubertal onset in the reference population, where no chronic disease, nutritional deficiency, psychosocial adversity, endocrinopathy, or other condition was responsible.
Types of interventions
Trials where an aromatase inhibitor was used as an adjunctive treatment to existing growth‐promoting therapies, including, but not limited to, GH therapy.
We investigated the following comparisons of intervention versus control/comparator where the same letters indicate direct comparisons.
Intervention
(a) Aromatase inhibitor.
(b) GH plus aromatase inhibitor.
(c) Androgen therapy plus aromatase inhibitor.
Comparator
Placebo compared with (a).
No treatment compared with (a).
GH plus placebo compared with (b).
Androgen therapy plus placebo compared with (c).
Concomitant therapies had to be the same in the intervention and comparator groups.
Minimum duration of intervention
Trials were eligible for inclusion provided the duration of aromatase inhibitor therapy was a minimum of 12 months.
Types of outcome measures
Primary outcomes
Final or near‐final height.
Health‐related quality of life.
Adverse events.
Secondary outcomes
All‐cause mortality.
Cognitive outcomes.
Height measures other than final or near‐final height.
Bone density and morphology.
Testosterone levels.
Lipid abnormalities.
Insulin sensitivity.
Socioeconomic effects.
Method and timing of outcome measurement
Final height: defined as height attained at a bone age greater than 16 years or when height velocity less than 1 cm per year, or both.
Near‐final height: defined as height attained when bone age greater than 15 years and height velocity less than 2 cm per year.
Height measures other than final or near‐final height: change in height or height standard deviation score (SDS) (or both), change in predicted adult height, final height SDS minus predicted adult height SDS, final height SDS minus target (mid‐parental) height SDS, change in height velocity (cm/year), difference in height velocity, and measured at the end of treatment.
Health‐related quality of life: measured by a validated instrument such as 36‐item Short Form (SF‐36) or EQ‐5D and measured before the study and at the end of treatment or when final height was achieved.
All‐cause mortality: defined as death from any cause during the study and measured at the end of treatment.
Cognitive outcomes: measured by a validated instrument such as the 'Wechsler Intelligence Scale for Children' (WISC) and measured at baseline, during the study, at the end of treatment, or a combination of these.
Adverse events: including headache, myalgia, arthralgia, or erythrocytosis and measured at any point during the study or at the end of treatment.
Bone density, bone turnover, and morphology: change in BMD z‐score, markers of bone turnover such as urinary collagen type 1 cross‐linked N‐telopeptide (NTX), morphological assessment using magnetic resonance imaging (MRI), and measured at baseline and the end of treatment.
Testosterone levels or testosterone: oestradiol ratio and measured at baseline, during treatment, or at study completion.
Lipid abnormalities: measurement of LDL‐cholesterol, HDL‐cholesterol, and triglycerides and measured at baseline, during treatment, or at study completion.
Insulin sensitivity: fasting insulin, homeostasis model of assessment‐insulin resistance (HOMA‐IR), and euglycaemic clamp technique and measured at baseline, during treatment, at study completion, or a combination of these.
Socioeconomic effects: including cost of treatment per centimetre of height gained measured at final or near‐final height.
Summary of findings
We presented a 'Summary of findings' table reporting the following outcomes listed according to priority.
Final or near‐final height.
Cognitive outcomes.
All‐cause mortality.
Health‐related quality of life.
Adverse events.
Socioeconomic effects.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to the specified date.
-
Cochrane Library (2014, Issue 7).
Cochrane Database of Systematic Reviews (CDSR).
Cochrane Central Register of Controlled Trials (CENTRAL).
Database of Abstracts of Reviews of Effects (DARE).
Health Technology Assessment (HTA).
MEDLINE (to 11 August 2014).
EMBASE (to 11 August 2014).
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch/), which is a meta‐register of studies including several trial registers:
Australian New Zealand Clinical Trials Registry (to 5 August 2014).
ClinicalTrials.gov (to 4 August 2014).
EU Clinical Trials Register (EU‐CTR) (to 5 August 2014).
ISRCTN (to 5 August 2014).
Brazilian Clinical Trials Registry (ReBec) (to 21 July 2014).
Chinese Clinical Trial Registry (to 29 June 2014).
Clinical Trials Registry ‐ India (to 20 July 2014).
Clinical Research Information Service ‐ Republic of Korea (to 21 July 2014).
Cuban Public Registry of Clinical Trials (to 20 July 2014).
German Clinical Trials Register (to 21 July 2014).
Iranian Registry of Clinical Trials (to 21 July 2014).
Japan Primary Registries Network (to 20 July 2014).
Pan African Clinical Trial Registry (to 30 June 2014).
Sri Lanka Clinical Trials Registry (to 29 June 2014).
The Netherlands National Trial Register (to 5 August 2014).
Thai Clinical Trials Register (TCTR) (to 21 July 2014).
We continuously applied a MEDLINE (via Ovid SP) email alert service for identification of newly published trials using the MEDLINE search strategy (for details on search strategies, see Appendix 1). After supplying the final review draft for editorial approval, the Cochrane Metabolic and Endocrine Disorders (CMED) Group performed a complete updated search on all databases available at the editorial office and sent the results to the review authors.
Had we detected additional relevant key words during any of the electronic or other searches we would have modified the electronic search strategies to incorporate these terms and documented the changes. We placed no restrictions on the language of publication when searching the electronic databases or reviewing reference lists in identified trials.
Searching other resources
We endeavoured to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, (systematic) reviews, meta‐analyses, and health technology assessment reports. In addition, we searched pharmaceutical company trial databases and subsequently contacted pharmaceutical companies manufacturing aromatase inhibitors to identify data not obtained by other methods. We also searched sources of grey literature including SIGLE (System for Information on Grey Literature in Europe) (www.opengrey.eu/).
Data collection and analysis
Selection of studies
Two review authors (NMG, MOG) independently scanned the abstract or title, or both, of every record retrieved, to determine which trials should be assessed further. We examined the full‐text articles of all potentially relevant articles. Where differences in opinion existed, we resolved them by discussion. We present a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram to show the process of trial selection (Liberati 2009).
Data extraction and management
For trials that fulfilled inclusion criteria, two review authors (NMG, MOG) independently extracted key participant and intervention characteristics. We reported data on efficacy outcomes and adverse events using standard data extraction sheets from the CMED Group. Where differences in opinion existed, we resolved them by discussion (for details see Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11).
1. Overview of study populations.
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible [N] | Randomised [N] | Safety [N] | ITT [N] | Finishing study [N] | Randomised finishing study [%] | Follow‐up timeb | |
| Hero 2005 | I: letrozole | 31 (80% power to detect a difference in PAH between groups of 5 cm and allow for up to a 10% drop‐out rate) | 40 | 16 | 16 | 16 | 16 | 100 | 2 years |
| C: placebo | 15 | 15 | 15 | 14 | 93 | ||||
| total: | 31 | 31 | 31 | 30 | 97 | ||||
| Mauras 2008 | I: GH plus anastrozole 1 mg | ‐ | ‐ | 26 | 26 | 26 | N/A | N/A | 12‐36 months |
| C: GH plus placebo | 26 | 26 | 26 | N/A | N/A | ||||
| total: | 52 | 52 | 52 | ||||||
| Salehpour 2010 | I1: letrozole 2.5 mg | 15 in each group (90% power, based on the data of Wickman 2001) | 91 | 31 | 31 | 31 | 31 | 100 | 2 years |
| I2: oxandrolone 2.5 mg | 30 | 30 | 30 | 30 | 100 | ||||
| C: placebo | 30 | 30 | 22 | 22 | 73 | ||||
| total: | 91 | 91 | 83 | 83 | 91 | ||||
| Wickman 2001 | I: testosterone plus letrozole 2.5 mg | ‐ | 33 | 11 | 11 | 9 | 9 | 82 | 18 months |
| C1: testosterone plus placebo | 12 | 12 | 10 | 10 | 83 | ||||
| C2: untreated (non‐randomised) | 10 | 10 | 7 | 7 | 70 | ||||
| total: | 33 | 33 | 26 | 26 | 79 | ||||
| Grand total | All interventions | 84 | Not estimablec | ||||||
| All comparators | 123 (113 randomised) | ||||||||
| All interventions and comparators | 207 | ||||||||
aAccording to power calculation in study publication or report. bDuration of intervention or follow‐up, or both under randomised conditions until end of study. cNot estimable as allocations of withdrawals and exclusions not provided by Mauras 2008.
C: comparator; GH: growth hormone; I: intervention; ITT: intention‐to‐treat; N: number of participants; N/A: not applicable; PAH: predicted adult height.
We provide information, including trial identifier regarding potentially relevant ongoing trials in the 'Characteristics of ongoing studies' table and Appendix 6. We endeavoured to find the protocol of each included trial, in databases of ongoing trials, publications of trial designs, or both, and specified data in Appendix 6.
We sent an email to authors of included trials to enquire whether they were willing to answer questions regarding their trials. Appendix 12 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the primary author(s) of the article, if required.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary trial, we maximised the yield of information by collating all available data. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (NMG, MOG) assessed the risk of bias of each included trial independently. We resolved disagreements by consensus, or by consultation with a third party (the CMED Group).
We used Cochrane's 'Risk of bias' assessment tool (Higgins 2011a; Higgins 2011b), and evaluated the following criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding (performance bias and detection bias), separated for blinding of participants and personnel and blinding of outcome assessment.
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other potential sources of bias.
We assessed outcome reporting bias (Kirkham 2010) by integrating the results of 'Examination of outcome reporting bias' (Appendix 7), 'Matrix of study endpoints (trial documents)' (Appendix 6), and section 'Outcomes (outcomes reported in abstract of publication)' of the Characteristics of included studies table. This analysis formed the basis for the judgement of selective reporting (reporting bias).
We judged the above 'Risk of bias' criteria as 'low risk', 'high risk', or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented a risk of bias graph and a risk of bias summary. We assessed the impact of individual bias domains on trial results at the endpoint and trial levels. In case of high risk of selection bias, we marked all endpoints investigated in the associated trial as high risk.
For performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessors), and attrition bias (incomplete outcome data), we evaluated risk of bias separately for subjective and objective outcomes (Hróbjartsson 2013). We considered the implications of missing outcome data from individual participants.
We defined the following endpoints as subjective outcomes.
Health‐related quality of life.
Adverse events.
We defined the following endpoints as objective outcomes.
Height measures.
All‐cause mortality.
Cognitive outcomes.
Bone density, turnover, and morphology.
Testosterone levels.
Lipid abnormalities.
Insulin sensitivity.
Measures of treatment effect
We planned to express dichotomous data as odds ratios (ORs) or risk ratios (RRs) with 95% confidence intervals (CIs). We planned to express continuous data as mean differences (MDs) with 95% CIs where available.
Unit of analysis issues
We planned to take into account the level at which randomisation occurred, such as cluster‐randomised trials and multiple observations for the same outcome.
Dealing with missing data
If possible, we obtained relevant missing data from trial authors and carefully evaluated important numerical data such as screened, randomly assigned participants as well as intention‐to‐treat (ITT), as‐treated, and per‐protocol populations. We investigated attrition rates (e.g. drop‐outs, losses to follow‐up, withdrawals), and critically appraised issues of missing data and imputation methods (e.g. last observation carried forward (LOCF)).
Where SDs for outcomes were not reported, we would have imputed these values by assuming the SD of the missing outcome to be the mean of the SDs from those trials where this information was reported.
Assessment of heterogeneity
As a result of substantial clinical and methodological heterogeneity, namely diversity in diagnosis, pubertal stage, and co‐treatments, we did not report trial results as the pooled effect estimate in a meta‐analysis.
We planned to identify heterogeneity (inconsistency) through visual inspection of the forest plots and by using a standard Chi2 test with a significance level of α = 0.1. In view of the low power of this test, we would have also considered the I2 statistic, which quantifies inconsistency across trials to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003); where an I2 statistic of 75% or more indicates a considerable level of heterogeneity (Higgins 2011a).
In case of heterogeneity, we would have determined possible reasons for it by examining individual trial and subgroup characteristics.
Assessment of reporting biases
In order to assess for reporting bias, we cross‐referenced the outcomes reported with those pre‐specified in the methods section of the paper or the trial protocol if available. Where the trial protocol was available, we compared the outcomes in the published paper and protocol with clarification sought from authors where discrepancies existed.
If we had included 10 or more trials investigating a particular outcome, we would have used funnel plots to assess small‐trial effects. Several explanations may account for funnel plot asymmetry, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials), and publication bias. Therefore, we planned to interpret results carefully (Sterne 2011).
Data synthesis
Unless there was good evidence for homogeneous effects across trials, we planned to summarise primarily low risk of bias data using a random‐effects model (Wood 2008). We planned to interpret random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). We also planned to perform statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Quality of evidence
We present the overall quality of the evidence for each outcome according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results. Two review authors (NMG, MOG) independently rated the quality for each outcome. We presented a summary of the evidence in Table 1. This provided key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences, for each relevant comparison of alternative management strategies, numbers of participants, and studies addressing each important outcome and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented results for the outcomes as described in Types of outcome measures section. If meta‐analysis was not possible, we presented results in a narrative 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity, and planned to carry out the following subgroup analyses with investigation of interactions.
Age at start of treatment.
Pubertal status at start of treatment.
Underlying diagnosis (ISS, GH deficiency, CDGP).
Duration of intervention.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of the following factors (when applicable) on effect sizes by restricting the analysis to:
published trials;
taking into account risk of bias, as specified in the 'Assessment of risk of bias in included studies' section;
very long or large trials to establish the extent to which they dominate the results;
trials using the following filters: diagnostic criteria, imputation, language of publication, source of funding (industry versus other), or country.
We also wanted to test the robustness of the results by repeating the analyses using different measures of effect size (RR, OR, etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
For a detailed description of trials, see the 'Characteristics of included studies', 'Characteristics of excluded studies, and 'Characteristics of ongoing studies' tables.
Results of the search
Our comprehensive literature searches identified 747 records; from these, we identified 13 full‐text papers for further examination. We excluded the other trials on the basis of their titles or abstracts because they did not meet the inclusion criteria or were not relevant to the question under trial (see Figure 1 for the amended PRISMA (preferred reporting items for systematic reviews and meta‐analyses) flow diagram). After screening the full text of the selected publications, four trials (13 publications) met the inclusion criteria. All trials were published in English. We contacted trial authors of all included trials and received a reply from trial authors of three trials (Hero 2005; Mauras 2008; Wickman 2001). We sought additional information from the trial authors of three trials (Hero 2005; Mauras 2008; Wickman 2001) who responded to these requests and provided further data.
1.
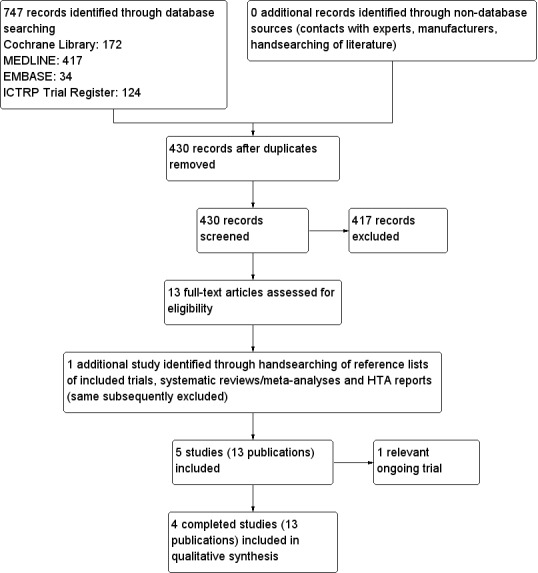
Study flow diagram.
Included studies
A detailed description of the characteristics of included trials is presented elsewhere (see Characteristics of included studies table). The following is a succinct overview.
Overview of study populations
The four trials included 207 participants, 84 participants were randomised to intervention and 123 to comparator groups. One trial had 10 participants, which had an untreated control group but were not randomly selected (Wickman 2001). One hundred and seventy randomised participants completed the trials (86%); however, separate percentages were not estimable for the intervention and comparator groups as the breakdown of numbers withdrawing according to allocated treatment was not provided in one trial (Mauras 2008). The individual sample size ranged from 31 to 91 participants.
Study design
Included trials were all RCTs. All four trials adopted a parallel group superiority design and all trials used a placebo comparator (Hero 2005; Mauras 2008; Salehpour 2010; Wickman 2001). One trial was a seven‐site multicentre study (Mauras 2008). In terms of blinding, all four trials were reported as double‐blinded for participants and personnel. Outcome assessors were categorically stated as being blinded in three trials (Hero 2005; Salehpour 2010; Wickman 2001). Trials were performed between 1998 and 2009. The duration of interventions ranged from 12 to 36 months, with a mean duration of 24 months. The duration of follow‐up ranged from the end of the intervention in two trials (Mauras 2008; Salehpour 2010), to near‐final and final height in two trials (Hero 2005; Wickman 2001); however, the follow‐up to these endpoints was by extension under non‐randomised conditions.
No trials had a run‐in period; however, one trial required that participants be on a stable dose of GH for at least six months prior to study entry (Mauras 2008). No trials were terminated before their scheduled end.
Settings
All included trials were performed in the setting of a paediatric endocrinology outpatient clinic.
Participants
The study populations consisted of males with short stature, constitutional delay of growth and puberty (CDGP), or both (Hero 2005; Salehpour 2010; Wickman 2001), or GH‐deficient males (Mauras 2008), and included participants from Finland, Iran, and the US; all are economically developed countries. In one trial, all participants were prepubertal at enrolment (Salehpour 2010). In another trial, although participants were included on the basis of pubertal delay, some had already entered puberty (Wickman 2001). The trial of GH‐deficient males specified participants had to have entered puberty prior to enrolment (Mauras 2008). The RCT of males with ISS did not exclude participants based on pubertal status; however, 87% of participants were prepubertal at baseline (Hero 2005). The mean age of participants in the four trials ranged from 11 to 15 years. The mean bone age at baseline ranged from nine to 13.5 years. Three trials reported co‐morbidities and co‐medications used by participants (Hero 2005; Mauras 2008; Wickman 2001). Two trials reported co‐interventions in participants, namely GH subcutaneously (Mauras 2008), and testosterone intramuscularly (Wickman 2001). Criteria for entry into the individual trials are outlined in the Characteristics of included studies table. Major exclusion criteria were participation in other trials involving GH therapy within six months or previous sex‐hormone treatment.
Diagnosis
Participants were diagnosed with CDGP in two trials (Salehpour 2010; Wickman 2001). Both used prepubertal Tanner pubic hair or genital stage at an age plus two SDs from the mean of the reference population. Another criterion was a prepubertal testicular volume at age 12.5 years (Salehpour 2010) or 13.5 years (Wickman 2001); reflecting differences in the age of pubertal onset in the populations studied. One trial included GH‐deficient boys whose height was minus two SDs from the mean, had diminished growth velocity, and had peak GH to two pharmacological stimuli of less than 10 ng/mL (Mauras 2008). One trial included boys with ISS, which was defined as height minus two SDs from the mean or midparental target height (Hero 2005). GH deficiency was out ruled by a GH stimulation test if suspected based on slow growth velocity, low insulin‐like growth factor‐1 (IGF‐1), or IGF binding protein‐3 concentration.
Interventions
One trial reported treatment with GH before start of the trial (Mauras 2008), the dose of which had to be stable and of at least six months' duration. The intervention, a third‐generation aromatase inhibitor, was administered orally once daily in a dose of 2.5 mg for letrozole (Hero 2005; Salehpour 2010; Wickman 2001) and 1 mg for anastrozole (Mauras 2008). All trials used placebo as a comparator intervention. The duration of treatment ranged from 12 to 36 months, with a mean treatment duration of 24 months.
Outcomes
Included trials explicitly stated at least one primary endpoint in the publication, with predicted adult height (PAH) reported in all four trials (197 participants randomised, minimum 184 completed 12 months). Numerous secondary endpoints were also reported.
Reporting of endpoints
Included trials collected a median of two (range one to four) primary and eight (range four to 10) secondary outcomes.
All four trials (197 participants randomised), reported height velocity, pubertal stage, bone age, BMD, sex hormones, and IGF‐1. Lipid profiles were measured in all four trials; however, for two of these trials, the results were reported in follow‐up papers (Hero 2005; Wickman 2001). Two of these trials reported on changes in body composition (84 participants randomised, 69 assessed for this outcome) (Mauras 2008; Wickman 2001). Two trials reported changes in vertebral morphology, again in follow‐up papers (54 randomised, 35 assessed for this outcome) (Hero 2005; Wickman 2001). Final and near‐final height were measured in one trial and again in subsequent publications (23 randomised, 17 assessed at near‐final height, 12 assessed at final height) (Wickman 2001). One trial outlined the effects of aromatase inhibitors on cognition (31 randomised, 28 assessed), once more in a separate report (Hero 2005).
One paper (52 randomised) reported detailed adverse events (Mauras 2008). A further trial (31 randomised) reported one serious adverse event in a person allocated to placebo (Hero 2005), and a further trial (91 randomised) stated that no adverse events occurred (Salehpour 2010). No trials reported death from any cause, health‐related quality of life, or socioeconomic effects.
For a summary of all outcomes assessed in each trial, see Appendix 5.
Excluded studies
We excluded one trial after careful evaluation of the full publication as it was an open‐label pilot study rather than a randomised trial (Mauras 2004).
For further details, see Characteristics of excluded studies table.
Risk of bias in included studies
For details on risk of bias of included trials see Characteristics of included studies table.
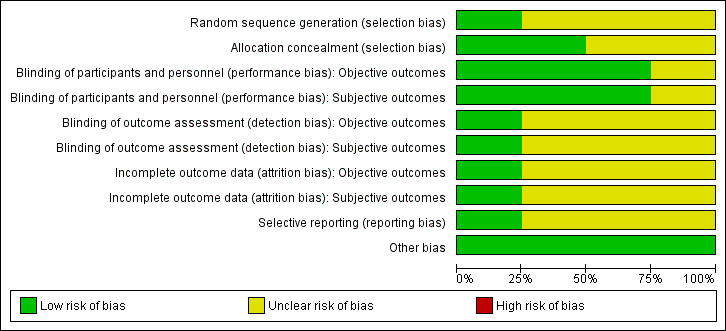
For an overview of review authors' judgements about each risk of bias item for individual trials and across all trials, see Figure 2 and Figure 3.
2.
Risk of bias graph.
3.

Risk of bias summary
Allocation
Only one trial reported the method of random sequence generation whereby participants were randomised using computer‐generated blocks of 10 (Hero 2005). Two trials provided the method of allocation concealment (Hero 2005; Mauras 2008). Two trials did not describe the methods used (Salehpour 2010; Wickman 2001).
Blinding
Three trials explicitly stated that blinding of the participants and personnel was undertaken (Hero 2005; Salehpour 2010; Wickman 2001). In the trial by Hero 2005, randomisation codes were not released to investigators until all data had been entered into a computer. Salehpour 2010 reported that intervention and control medications were identical and explicitly stated that assessment of bone age, a key determinant of PAH, was blinded. In the trial by Mauras 2008, all data were unblinded to one individual and analysed after 24 months, although some participants continued the intervention to 36 months. Three trials did not provide sufficient information about outcome assessor blinding procedures (Mauras 2008; Salehpour 2010; Wickman 2001).
Incomplete outcome data
Withdrawals occurred in all four trials (Hero 2005; Mauras 2008; Salehpour 2010; Wickman 2001). Three trials described numbers of trial withdrawals according to allocated treatment (Hero 2005; Salehpour 2010; Wickman 2001). In the trial by Salehpour 2010, all losses to follow‐up were in the placebo group. Participants withdrew due to the perceived lack of effect of the treatment but the trial remained adequately powered. In the trial by Hero 2005, one participant in the placebo group developed type 1 diabetes and was excluded from the trial by the research team. In the trial by Mauras 2008, treatment duration ranged from 12 to 36 months as the trial continued until completion of linear growth. Consequently, numbers diminished as the trial progressed. In the primary publication, participant numbers in the intervention and control groups were reported as a pooled figure at analysed time points; however, we clarified this during subsequent correspondence with the authors. Nine participants withdrew because of satisfaction with achieved height or psychosocial difficulties and the investigators excluded five; however, the treatment allocations of those withdrawing or excluded were not described. One trial reported analysis as ITT (Mauras 2008). Detailed descriptions of participants' withdrawals and the reasons underpinning them were not provided in trials by Mauras 2008 and Wickman 2001.
Selective reporting
We detected no selective reporting bias in any of the primary publications. However, there was some unclear risk of reporting bias for adverse events (Salehpour 2010; Wickman 2001), and bone density (Wickman 2001). One trial provided observational follow‐up data on final and near‐final height (Wickman 2001).
Other potential sources of bias
We did not detect another potential source of bias.
Subgroup analyses
We did not perform subgroup analyses because there were not enough trials to estimate effects in various subgroups.
Sensitivity analyses
We did not perform sensitivity analyses, as meta‐analysis was not undertaken due to substantial study heterogeneity.
Ongoing studies
We found one ongoing RCT (see Characteristics of ongoing studies table).
Effects of interventions
See: Table 1
Aromatase inhibitors versus comparators
Primary outcomes
Final or near‐final height
These outcomes were only evaluated during an extension period under non‐randomised conditions of one original RCT. Therefore, the following data have to be interpreted with care and should be seen as primarily hypothesis‐generating.
In a follow‐up report to Wickman 2001, vertebral morphology was assessed in 12 of the 23 randomised participants following completion of growth (bone age 19 years). Final height was 177.6 cm in the testosterone plus letrozole group compared to 170.1 cm in the testosterone plus placebo group. The difference between the groups was 7.5 cm. However the participants in the testosterone plus letrozole group were 7.2 cm taller at baseline and, nonetheless, the difference did not reach statistical significance (P value = 0.06), given the small numbers involved.
Another publication of Wickman 2001 reported near‐final height data for 17 of the 23 originally randomised participants. Participants were considered to be at near‐final height if their bone age was 15.75 years or greater. The boys treated with testosterone plus letrozole were older at near‐final height, despite similar bone ages (19.2 years with testosterone plus letrozole versus 18.2 years with testosterone plus placebo). Two boys lost to follow‐up had received testosterone plus placebo; however, their missing data reduced mean PAH in the testosterone plus placebo group by 1.9 cm. Near‐final height of the testosterone plus letrozole group was 6.9 cm more than the testosterone plus placebo group (175.8 cm with testosterone plus letrozole versus 169.1 cm with testosterone plus placebo; P value = 0.04) and only 1.3 cm below mid‐parental target height, a mean increment of 0.6 height SDS. In contrast, boys treated with testosterone plus placebo were 4.8 cm below mid‐parental target height. Near‐final height for five of the original 10 non‐randomised, untreated boys was 173.5 cm (Appendix 12).
Health‐related quality of life
None of the trials assessed health‐related quality of life.
Adverse events
Hero 2005: one participant allocated to placebo developed type 1 diabetes.
Mauras 2008: anastrozole was well tolerated and largely free of adverse effects. There were no differences between the groups on complete blood counts, urinalysis, or liver profiles. The frequency for adverse events was similar in both groups. There were seven serious adverse events; four were in the same participant who had repeated episodes of cyclical vomiting, diagnosed after extensive work‐up in gastroenterology as psychogenic emesis. All reported serious adverse events were in the anastrozole group, none were considered related to the study medication by the investigators, and in all, anastrozole was quickly resumed after hospital discharge. Extensive three‐year safety data in these boys showed no differences in lipid or glucose concentrations, liver function tests, or adverse event rates between the groups. Anastrozole was well tolerated and safe for up to three years. However, long‐term data are still needed and continued surveillance is a must as well as careful monitoring of BMD accrual.
Salehpour 2010: there were no adverse events in the two‐year study period.
Secondary outcomes
All‐cause mortality
There were no deaths reported during the trials.
Cognitive outcomes
In a follow‐up paper on the second Finnish cohort of boys with ISS, treatment with letrozole did not impact on cognitive functioning as assessed using subtests from the Wechsler Intelligence Scale for Children (WISC‐III), Rey‐Osterrieth Complex figure, a developmental Neuropsychological Assessment (NEPSY) and the Wechsler Memory Scale Revised (WMS‐R) (Hero 2005).
Height measures other than final or near‐final height
Change in predicted adult height
All included trials reported a change in PAH as their primary outcome. Three trials were undertaken in boys with various combinations of short stature and delayed puberty (Hero 2005; Salehpour 2010; Wickman 2001), and one trial was undertaken in boys with GH deficiency (Mauras 2008). In a Finnish study, 23 boys with CDGP were randomly assigned to letrozole 2.5 mg once daily or placebo for 12 months (Wickman 2001). For the first six months, both treatment groups also received intramuscular testosterone 1 mg/kg every four weeks and the effect of treatment was evaluated 18 months after initiation of therapy. PAH increased more in the testosterone plus letrozole group than the testosterone plus placebo group in the 19 boys assessed for the primary endpoint (5.1 cm with testosterone plus letrozole versus 0.3 cm with testosterone plus placebo). A third non‐randomised group of 10 boys received no treatment and gained 2 cm in PAH. In a second Finnish study, 30 boys with ISS aged nine to 14.5 years were randomly assigned to letrozole 2.5 mg daily or placebo for two years (Hero 2005). The majority of the participants were pre‐pubertal at the start of treatment (81% in letrozole group and 93% in placebo group). PAH increased by 5.9 cm in the letrozole group, reflecting an increase in height SDS for bone age of 0.7, compared to no improvement in either measure in the placebo group. In a follow‐up to the study in boys with ISS six years after study start, the difference in PAH in 23/30 participants of the original cohort was no longer statistically significant (166.5 cm with letrozole versus 162.4 cm with placebo; P value = 0.57) compared to the end of two years of treatment in the full cohort, favouring letrozole (172.8 with letrozole versus 166.9 cm with placebo) (Hero 2005). An Iranian study enrolled 91 boys with CDGP and randomly assigned them to letrozole 2.5 mg once daily, oxandrolone (a non‐aromatisable androgen) 2.5 mg once daily, or placebo for two years (Salehpour 2010). The increase from pre‐treatment PAH was only significant in the letrozole‐treated boys, with a gain of 6.1 cm, compared to 1.9 cm in the oxandrolone group and 1.4 cm in the placebo group. In a study from the US, 52 GH‐deficient adolescent males were randomised to co‐treatment with anastrozole 1 mg daily or placebo for 12 to 36 months (Mauras 2008). Participants had to be pubertal to be included and were required to have been treated with GH for a minimum of six months prior to entry into the study. PAH increased in the anastrozole‐treated group (at 12 months: +1.3 cm; 24 months: +4.5 cm; 36 months: +6.7 cm), in comparison to 1 cm PAH gain in the placebo‐treated group. This translated to an increase on height SDS adjusted for bone age in anastrozole‐treated compared to placebo‐treated participants of +0.48 after 24 months and +0.87 after 36 months.
Change in height velocity
In the Finnish study, height velocity was more rapid in the first five months of treatment in the testosterone plus placebo group compared to the testosterone plus letrozole group, but was similar at other time points (Wickman 2001). In the second Finnish study, there were no differences in height velocity between treatment groups when stratified according to pubertal status (Hero 2005). In the US study of pubertal GH‐deficient boys, growth velocity slowed in both groups over time but more so in the placebo‐treated compared to the anastrozole‐treated group (Mauras 2008).
Bone age
Four trials reported bone age as an outcome. In the Finnish study, bone age increased by 1.7 years in the testosterone plus placebo group compared to 0.9 years in the testosterone plus letrozole group when assessed 18 months from study entry (six months post end of treatment) (Wickman 2001). In follow‐up of these boys at near‐final height, the difference between chronological age and bone age remained greater in the testosterone plus letrozole group having been similar at the start of treatment (2.2 years with testosterone plus letrozole versus 1.5 years with testosterone plus placebo). In the second Finnish study in boys with ISS, bone age advancement during two years of treatment was less in letrozole‐treated participants than those treated with placebo (1.24 years with letrozole versus 2.05 years with placebo) (Hero 2005). The efficacy of letrozole in increasing PAH was independent of the bone age at study entry. In the Iranian study in boys with CDGP or ISS, or both, bone age advancement during two years of treatment was different between all treatment groups and surprisingly slowest in the placebo, compared to the letrozole and oxandrolone treatment groups (1.1 years with letrozole versus 2.3 years with oxandrolone versus 0.48 years with placebo) (Salehpour 2010). The reason why bone age only advanced by only six months over two years in the placebo group was not clarified and this outcome was contrary to what was reported in placebo groups in all other trials. In the study of GH‐deficient boys, bone age advancement after 24 and 36 months of treatment were different between anastrozole and placebo groups (at 24 months: 1.7 years with anastrozole versus 2.6 years with placebo; at 36 months: 2.2 years with anastrozole versus 3.8 years with placebo) (Mauras 2008).
Bone density and morphology
Bone mineral density
BMD has been an area of concern, particularly as men with aromatase deficiency develop profound osteoporosis. All included trials assessed this as a secondary outcome. During treatment with letrozole for between 12 and 24 months, BMD in the lumbar spine or femoral neck, as evaluated using dual‐energy X‐ray absorptiometry increased in a similar manner to placebo (Hero 2005; Salehpour 2010; Wickman 2001). Similarly, although anastrozole resulted in less increase in BMD compared to placebo at 24 months in GH‐deficient boys, there was no substantial difference in BMD noted when evaluated at 36 months (Mauras 2008).
Bone turnover
Two trials reported changes in serum and urinary biomarkers of bone turnover during treatment with letrozole or placebo in follow‐up reports (Hero 2005; Wickman 2001). In adolescents treated with placebo, there was an increase in markers of bone turnover as puberty progressed, which correlated with growth velocity. Boys treated with letrozole appeared to have reduced bone turnover that did not seem to correlate with any particular pattern of hormonal changes. However, letrozole‐treated boys with ISS had increased cortical bone growth, which is an important predictor of bone strength (Hero 2005).
Vertebral morphology
Both Finnish cohorts were evaluated by post‐treatment magnetic resonance imaging (MRI) to assess vertebral body morphology (Hero 2005; Wickman 2001). In adolescents with ISS, the majority of whom were pre‐pubertal at initiation of treatment, there were mild vertebral body abnormalities in 5/11 (45%) of participants assigned to letrozole compared to none in the placebo group (Hero 2005). In contrast, in boys with CDGP who were pubertal at initiation of treatment, there was no marked difference between the letrozole and placebo cohort, although vertebral abnormalities were prevalent in both groups (Wickman 2001). It was postulated that this may have been because they were older, had a more advanced bone age, and received treatment for a shorter duration.
Testosterone levels
In the Finnish study of boys with CDGP, testosterone levels rose by 33% in the group treated with testosterone plus placebo (from 11.9 to 15.8 nmol/L); however, in boys treated with testosterone plus letrozole, testosterone levels increased by 420% (from 9.5 to 49.1 nmol/L) (Wickman 2001). Despite this increase in testosterone, oestradiol levels remained unchanged from baseline in the testosterone plus letrozole group, in contrast to the testosterone plus placebo group, where they rose by 130% to a mean of 37.9 pmol/L. In the second Finnish study with ISS, pubertal boys treated with letrozole had testosterone levels that were higher at six, 12, 18, and 24 months than those of controls (Hero 2005). Testosterone levels reached a mean peak of 30.9 nmol/L at 24 months, a level more than four‐fold higher than placebo‐treated boys.
Additionally, there was a trend towards higher oestradiol levels in the placebo group that did not reach statistical significance. There were no marked differences between treatment groups in testosterone or oestradiol levels among the subgroup of prepubertal boys. In the Iranian study of boys with CDGP, testosterone levels in the letrozole group increased from prepubertal pre‐study levels of 0.25 mmol/L to 15.9 mmol/L at the end of treatment, when they were statistically no different to levels of those receiving oxandrolone and seven‐fold higher than testosterone levels in the placebo group (Salehpour 2010). The study did not report oestradiol levels (Salehpour 2010). In the study of GH‐deficient boys, testosterone levels were higher in the anastrozole group at 12 months, but not at 24 or 36 months (Mauras 2008). In contrast, oestradiol levels remained stable in the anastrozole group, but rose in the placebo group.
Lipid profile
A follow‐up paper from the initial Finnish cohort reported that HDL‐cholesterol was lower in the testosterone plus letrozole‐treated group after five months, but not at the end of treatment or six months following discontinuation (Wickman 2001). In a follow‐up report on the second Finnish cohort, there was a 0.47 mmol/L reduction in HDL‐cholesterol in pubertal testosterone plus letrozole‐treated boys, compared to no change in the pubertal testosterone plus placebo group (Hero 2005). This effect was not observed in prepubertal boys; however, there was a small increase of 0.5 mmol/L in total cholesterol in prepubertal letrozole‐treated boys. In the Iranian study, HDL‐cholesterol reduced by 0.26 mmol/L from baseline in the letrozole group, compared to a lesser reduction in the placebo group of 0.13 mmol/L (Salehpour 2010). In GH‐deficient boys, mean total cholesterol and HDL‐cholesterol were 0.44 and 0.1 mmol/L lower in the anastrozole‐treated compared to the placebo‐treated group. This difference was only seen after one year of treatment and was not evident at other time points (Mauras 2008).
Insulin sensitivity
In boys with CDGP, treatment with testosterone plus letrozole was associated with an improvement in insulin sensitivity, as assessed using fasting insulin compared to treatment with testosterone plus placebo (Wickman 2001). Insulin sensitivity returned to baseline following discontinuation of treatment. In boys with ISS, insulin sensitivity as evaluated using HOMA did not change during treatment with letrozole (Hero 2005).
Socioeconomic effects
None of the trials assessed socioeconomic effects.
Discussion
Summary of main results
To date, use of aromatase inhibitors for short stature has only been studied under RCT conditions in 84 male children and adolescents (58 letrozole, 26 anastrozole). Results from the randomised trials evaluated in this review suggested that aromatase inhibitors improve short‐term growth outcomes but do not improve final height (Table 1). In a follow‐up to the trial by Wickman 2001, final height was 7.5 cm greater in the testosterone plus letrozole group, compared to testosterone plus placebo, however, the testosterone plus letrozole group were 7.2 cm taller at baseline. There is a suggestion from another study of boys with ISS that PAH fell after the intervention ceased and the gain in PAH with letrozole over placebo was lost four years from study completion, intimating again that there was likely no substantial difference in final height (Hero 2005).
Aromatase inhibitors were generally well tolerated and there were no withdrawals from any of the trials because of adverse effects. BMD did not appear to be adversely affected. However, one area of concern was that a significant proportion (45%) of prepubertal males with ISS treated with letrozole developed mild morphological abnormalities of their vertebrae, compared to none in the placebo group. Treatment with an aromatase inhibitor tended to cause a reduction in HDL‐cholesterol at end of treatment of 0.26 and 0.47 mmol/L in two trials, with the reduction in the latter trial only seen in pubertal participants. There was no evidence for development of insulin resistance or impairment of cognitive performance. Male infertility is another area of concern regarding use of aromatase inhibitors; this was not evaluated in any of the randomised trials.
There were some data regarding a lack of effect on final adult height, which was not explicitly reported for three of the trials, and may reflect publication bias concerning negative findings. There remain unanswered questions regarding the optimum time to initiate this therapy; however, the finding of a significant proportion of prepubertal boys developing vertebral abnormalities is a cautionary tale, suggesting that it may be best to treat males during, rather than before, puberty. The optimum duration of treatment is unknown, as is the safety of longer‐term use and final height data are necessary before aromatase inhibitors can become an approved, rather than off‐label agent for short stature.
Overall completeness and applicability of evidence
This systematic review examined the evidence from four RCTs regarding the use of aromatase inhibitors in 84 males with short stature of varying aetiologies. Due to the variances in trial inclusion criteria, underlying diagnoses, and trial design, our ability to combine data from studies was limited. Despite efforts to obtain additional information from the authors, we could not obtain all relevant data. Our review revealed a dearth of evidence to evaluate our primary outcomes of final or near‐final height, adverse events, and health‐related quality of life.
One paper reported no relevant difference in final height in one cohort (Wickman 2001), and found that gains in PAH that were lost four years post treatment cessation in a second cohort (Hero 2005), resulted in a failure to increase adult height relevantly in two trials. Final height data were lacking for the other two trials, which, considering when the trials were completed, may reflect publication bias (Mauras 2008; Salehpour 2010). There were no reports of health‐related quality of life. Only one trial presented adverse events in detail; however, all included trials presented safety data.
The results of this review are unlikely to influence current practice as aromatase inhibitors remain an off‐label treatment for short stature and this review did not provide evidence for improvement in final height outcomes.
Quality of the evidence
Three trials did not explicitly report final height data leading to concerns regarding publication bias (Hero 2005; Mauras 2008; Salehpour 2010). Only three of four trials systematically evaluated adverse events (Hero 2005; Mauras 2008; Salehpour 2010), which resulted in a downgrading of the evidence by one GRADE level. We downgraded the evidence regarding cognitive outcomes by two GRADE levels because they were evaluated in one trial with a small number of participants only (Hero 2005). Variation in diagnosis, pubertal status, duration of treatment, and co‐treatments limited comparability of trials. Only 84 participants were randomised to the intervention across four trials; three of the trials were single centre.
Potential biases in the review process
This review was completed according to standard Cochrane methodology; however, potential biases in the review process could not be dismissed entirely. Every effort was made to identify all relevant trials and avoid potential omission of important data. We cannot conclusively determine whether publication bias existed, however, three trials did not report important outcomes such as final height.
Agreements and disagreements with other studies or reviews
Although there have been some reviews regarding use of aromatase inhibitors in paediatrics (Cernich 2004; Shulman 2008; Wit 2011), none has used detailed systematic methodology. The most recent review is in accordance with our assessment, that further trials are required to define the safety and efficacy of aromatase inhibitors in increasing final adult height in male children and adolescents (Wit 2011).
Authors' conclusions
Implications for practice.
Use of aromatase inhibitors in an attempt to improve height outcomes has been studied under randomised conditions in just 84 male children and adolescents. There is currently no definite evidence that treatment with aromatase inhibitors improves final adult height and they remain an off‐label treatment for short stature. Safety concerns remain paramount and only one trial provided detailed adverse event data. Furthermore, on the basis of the evidence from one trial included in this review, their use in prepubertal boys carries a risk of vertebral morphological abnormalities. If off‐label treatment is considered, close monitoring of bone density, vertebral morphology, haematocrit, liver function, gonadotropins, testosterone and oestradiol, total cholesterol, and HDL cholesterol are necessary.
Implications for research.
Further randomised trials in males with idiopathic short stature, constitutional delay of growth and puberty, or growth hormone deficiency are necessary that focus on definitive growth outcomes such as final height assessed under randomised conditions rather than controversial predictive surrogate markers such as improvement in predicted adult height (PAH). Multicentre trials may be more appropriate to provide sufficient participant numbers to assess necessary outcomes adequately. There needs to be greater focus in studies regarding monitoring and reporting of adverse events. Outcomes such as treatment costs and psychosocial outcomes such as health‐related quality of life should also be evaluated. Trials should primarily focus on pubertal participants and the duration of treatment should be a minimum of two years. Consideration could also be given to continuation of treatment until growth is nearly complete, since it was observed that the gain in PAH was lost over time following treatment cessation.
Notes
We have based parts of the background, the methods section, appendices, additional tables, and figures 1 to 3 of this review on a standard template established by the CMED Group.
Acknowledgements
We are grateful for the contributions of Gudrun Paletta and Bernd Richter from the Cochrane Metabolic and Endocrine Disorders (CMED) Group. We would also like to acknowledge the assistance provided by Karla Bergerhoff and Maria‐Inti Metzendorf in their role as Trials Search Co‐ordinators.
Appendices
Appendix 1. Search strategies
| Cochrane Library (Wiley) |
| #1 [mh ^"Growth Disorders"] #2 [mh ^"Puberty, Delayed"] #3 [mh ^"Body Height"] #4 (short near/4 (stature* or adolescent* or boys)):ti,ab,kw #5 ((delay* or retard*) near/4 (growth or puberty)):ti,ab,kw #6 ((linear or longitudinal or disorder* or disturb* or anomal*) near/4 growth):ti,ab,kw #7 "adult height":ti,ab,kw #8 {or #1‐#7} #9 [mh ^"aromatase inhibitors"] #10 [mh ^aminoglutethimide] #11 [mh ^fadrozole] #12 [mh ^testolactone] #13 "aromatase inhibit*":ti,ab,kw #14 (aminoglutethimide* or testolactone* or teslac):ti,ab,kw #15 (anastrozol* or arimidex or letrozol* or femara or exemestane* or aromasi*):ti,ab,kw #16 (resveratrol or nicotine or myosmine or zinc or catechin or chalcones or apigenin or eriodictyol or isoliqairitigenin or mangostin):ti,ab,kw #17 {or #9‐#16} #18 #8 and #17 |
| MEDLINE (Ovid SP) |
| 1 Growth Disorders/
2 Puberty, Delayed/
3 Body Height/
4 (short adj3 (stature* or adolescent* or boys)).tw.
5 ((delay* or retard*) adj3 (growth or puberty)).tw.
6 ((linear or longitudinal or disorder* or disturb* or anomal*) adj3 growth).tw.
7 adult height.tw.
8 or/1‐7
9 aromatase inhibitors/
10 aminoglutethimide/
11 fadrozole/
12 testolactone/
13 aromatase inhibit*.tw.
14 (aminoglutethimide* or testolactone* or teslac).tw.
15 (anastrozol* or arimidex or letrozol* or femara or exemestane* or aromasi*).tw.
16 (resveratrol or nicotine or myosmine or zinc or catechin or chalcones or apigenin or eriodictyol or isoliqairitigenin or mangostin).tw.
17 or/9‐16
18 8 and 17 [19‐29: Cochrane Handbook 2008 RCT filter ‐ sensitivity maximizing version] 19 randomized controlled trial.pt. 20 controlled clinical trial.pt. 21 randomi?ed.ab. 22 placebo.ab. 23 drug therapy.fs. 24 randomly.ab. 25 trial.ab. 26 groups.ab. 27 or/19‐26 28 exp animals/ not humans/ 29 27 not 28 30 18 and 29 |
| EMBASE (Ovid SP) |
| 1 exp growth disorder/
2 exp delayed puberty/
3 exp short stature/
4 (short adj3 (stature* or adolescent* or boys)).tw.
5 ((delay* or retard*) adj3 (growth or puberty)).tw.
6 ((linear or longitudinal or disorder* or disturb* or anomal*) adj3 growth).tw.
7 adult height.tw.
8 or/1‐7
9 exp aromatase inhibitor/
10 resveratrol/
11 nicotine/
12 zinc/
13 catechin/
14 chalcone derivative/
15 apigenin/
16 eriodictyol/
17 isoliquiritigenin/
18 aromatase inhibit*.tw.
19 (aminoglutethimide* or testolactone* or teslac).tw.
20 (anastrozol* or arimidex or letrozol* or femara or exemestane* or aromasi*).tw.
21 (resveratrol or nicotine or myosmine or zinc or catechin or chalcones or apigenin or eriodictyol or isoliqairitigenin or mangostin).tw. 22 or/9‐21 23 8 and 22 [24: Wong et al. 2006 "sound treatment studies" filter – BS version] 24 random*.tw. or clinical trial*.mp. or exp health care quality/ 25 23 and 24 26 limit 25 to exclude medline journals |
| ICTRP search platform |
| growth AND aromatase OR growth AND anastrozol* OR growth AND aminoglutethimid* OR growth AND testolacton* OR growth AND teslac* OR growth AND arimidex OR growth AND letrozol* OR growth AND femara OR growth AND exemestan* OR growth AND aromasi* OR growth AND resveratrol OR growth AND nicotine OR growth AND myosmine OR growth AND zinc OR growth AND catechin OR growth AND chalcones OR growth AND apigenin OR growth AND eriodictyol OR growth AND isoliqairitigenin OR growth AND mangostin OR stature AND aromatase OR stature AND anastrozol* OR stature AND aminoglutethimid* OR stature AND testolacton* OR stature AND teslac* OR stature AND arimidex OR stature AND letrozol* OR stature AND femara OR stature AND exemestan* OR stature AND aromasi* OR stature AND resveratrol OR stature AND nicotine OR stature AND myosmine OR stature AND zinc OR stature AND catechin OR stature AND chalcones OR stature AND apigenin OR stature AND eriodictyol OR stature AND isoliqairitigenin OR stature AND mangostin OR height AND aromatase OR height AND anastrozol* OR height AND aminoglutethimid* OR height AND testolacton* OR height AND teslac* OR height AND arimidex OR height AND letrozol* OR height AND femara OR height AND exemestan* OR height AND aromasi* height AND resveratrol OR height AND nicotine OR height AND myosmine OR height AND zinc OR height AND catechin OR height AND chalcones OR height AND apigenin OR height AND eriodictyol OR height AND isoliqairitigenin OR height AND mangostin |
Appendix 2. Description of interventions
| Intervention(s) [route, frequency, total dose/day] |
Adequatea intervention [Yes / No] |
Comparator(s) [route, frequency, total dose/day] |
Adequatea comparator [Yes / No] |
|
| Hero 2005 | Letrozole 2.5 mg orally once daily | Yes | Placebo orally once daily | Yes |
| Mauras 2008 | Anastrozole 1 mg orally once daily plus GH 0.35 mg/kg/week s.c. | Yes | Placebo orally once daily plus GH 0.35 mg/kg/week s.c. | Yes |
| Salehpour 2010 | Letrozole 2.5 mg orally once daily | Yes | Placebo orally once daily | Yes |
| Oxandrolone 2.5 mg orally once daily | Yes | |||
| Wickman 2001 | Letrozole 2.5 mg orally once daily plus testosterone 1 mg/kg i.m. 4 weekly | Yes | Placebo orally once daily plus testosterone 1 mg/kg i.m. 4 weekly | Yes |
| No treatment | N/A | |||
|
aThe term 'adequate' refers to sufficient use of the intervention/comparator with regard to dose, dose escalation, dosing scheme, provision for contraindications, and other features necessary to establish a fair contrast between intervention and comparator GH: growth hormone; i.m.: intramuscularly; N/A: not applicable; s.c.: subcutaneously | ||||
Appendix 3. Baseline characteristics (I)
| Intervention(s) and comparator(s) | Duration of intervention (duration of follow‐up) | Participating population | Study period [year to year] | Country | Setting | Ethnic groups [%] | Duration of condition [mean/range years (SD), or as reported] | |
| Hero 2005 | I: letrozole | 2 years (2 years) | Prepubertal and pubertal males with ISS | 2001‐2004 | Finland | OPD | ‐ | ‐ |
| C: placebo | ||||||||
| Mauras 2008 | I: anastrozole + GH | 12‐36 months (12‐36 months) | Pubertal males with GH deficiency | ‐ | USA | OPD | ‐ | Minimum 6 months on GH |
| C: placebo + GH | ||||||||
| Salehpour 2010 | I1: letrozole | 2 years (2 years) | Males with CDGP and short stature | ‐ | Iran | OPD | ‐ | ‐ |
| I2: oxandrolone | ||||||||
| C: placebo | ||||||||
| Wickman 2001 | I: letrozole + testosterone | 12 months (18 months) | Males with CDGP | 1998‐2000 | Finland | OPD | ‐ | ‐ |
| C1: placebo + testosterone | ||||||||
| C2: no treatment | ||||||||
| "‐" denotes not reported C: control; CDGP: constitutional delay of growth and puberty; GH: growth hormone; I: intervention; ISS: idiopathic short stature; OPD: outpatient department; SD: standard deviation | ||||||||
Appendix 4. Baseline characteristics (II)
| Intervention(s) and comparator(s) | Age [mean years (SD)] | Height [mean cm (SD)] | Comedications/Cointerventions | Comorbidities | |
| Hero 2005 | I: letrozole | 11 (1.7) | 128.5 (6.9) | Nil | 4 on ICS |
| C: placebo | 11 (1.5) | 127.7 (6.6) | Nil | 2 on ICS | |
| Mauras 2008 | I: anastrozole + GH | 13.8 (0.3) | 149.7 (1.6) | GH | ‐ |
| C: placebo + GH | 14.2 (0.2) | 151.6 (1.3) | GH | ‐ | |
| Salehpour 2010 | I1: letrozole | 13.6 (0.8) | ‐ | Nil | ‐ |
| I2: oxandrolone | 13.7 (0.9) | Nil | ‐ | ||
| C: placebo | 13.5 (0.9) | Nil | ‐ | ||
| Wickman 2001 | I: letrozole + testosterone | 15.2 (0.8) | 155.3 (6.7) | Testosterone | ‐ |
| C1: placebo + testosterone | 15 (0.8) | 151.9 (8.3) | Testosterone | ‐ | |
| C2: no treatment | 15 (0.7) | 154.3 (4.4) | Nil | ‐ | |
| "‐" denotes not reported GH: growth hormone; ICS: inhaled corticosteroids; SD: standard deviation | |||||
Appendix 5. Matrix of study endpoints (publications)
| Endpoint | Endpoint reported in publication | Endpoint not reported in publication | Time of measurementa | |
| Hero 2005 | Review's primary outcomes | |||
| Height measures | x | ‐ | 12, 24 months | |
| Health‐related quality of life | ‐ | x | N/A | |
| Adverse events | x | ‐ | 24 months | |
| Review's secondary outcomes | ||||
| All‐cause mortality | ‐ | x | N/A | |
| Cognitive outcomes | x | ‐ | 12, 24 months | |
| Bone density | x | ‐ | 6, 12, 18, 24 months | |
| Testosterone levels | x | ‐ | 6,12,18, 24 months | |
| Lipid abnormalities | x | ‐ | 12, 24 months | |
| Socioeconomic costs | ‐ | x | N/A | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| Body composition (S), insulin sensitivity (S), pubertal progression (S), vertebral morphology (S) | ||||
| Subgroups reported in publication | ||||
| Pubertal, prepubertal | ||||
| Mauras 2008 | Endpoint | Endpoint reported in publication | Endpoint not reported in publication | Time of measurementa |
| Review's primary outcomes | ||||
| Height measures | x | ‐ | 12, 24, 36 months | |
| Health‐related quality of life | ‐ | x | N/A | |
| Adverse events | x | ‐ | 36 months | |
| Review's secondary outcomes | ||||
| All‐cause mortality | ‐ | x | N/A | |
| Cognitive outcomes | ‐ | x | N/A | |
| Bone density | x | ‐ | 24, 36 months | |
| Testosterone levels | x | ‐ | 6, 12, 18, 24, 30, 36 months | |
| Lipid abnormalities | x | ‐ | 6, 12, 18, 24, 30, 36 months | |
| Socioeconomic costs | ‐ | x | N/A | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| Body composition (S) | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| Salehpour 2010 | Endpoint | Endpoint reported in publication | Endpoint not reported in publication | Time of measurementa |
| Review's primary outcomes | ||||
| Height measures | x | ‐ | 24 months | |
| Health‐related quality of life | ‐ | x | N/A | |
| Adverse events | x | ‐ | 24 months | |
| Review's secondary outcomes | ||||
| All‐cause mortality | ‐ | x | N/A | |
| Cognitive outcomes | ‐ | x | N/A | |
| Bone density | x | ‐ | 24 months | |
| Testosterone levels | x | ‐ | 12, 24 months | |
| Lipid abnormalities | x | ‐ | 6, 12, 18, 24 months | |
| Socioeconomic costs | ‐ | x | N/A | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| Pubertal progression (S) | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| Wickman 2001 | Endpoint | Endpoint reported in publication | Endpoint not reported in publication | Time of measurementa |
| Review's primary outcomes | ||||
| Height measures | x | ‐ | 2, 5, 12, 18 months | |
| Health‐related quality of life | ‐ | x | N/A | |
| Adverse events | ‐ | x | N/A | |
| Review's secondary outcomes | ||||
| All‐cause mortality | ‐ | x | N/A | |
| Cognitive outcomes | ‐ | x | N/A | |
| Bone density | x | ‐ | 12, 18 months | |
| Testosterone levels | x | ‐ | 2, 5, 12, 18 months | |
| Lipid abnormalities | x | ‐ | 2, 5, 12, 18 months | |
| Socioeconomic costs | ‐ | x | N/A | |
| Other than review's primary/secondary outcomes reported in publication (classification: P/S/O)b | ||||
| Pubertal progression, gonadotropins, IGF‐1 and IGFBP3 | ||||
| Subgroups reported in publication | ||||
| ‐ | ||||
| "‐" denotes not reported aUnderlined data denote times of measurement for primary and secondary review outcomes, if measured and reported in the results section of the publication (other times represent planned but not reported points in time) b(P) Primary or (S) secondary endpoint(s) refer to verbatim statements in the publication, (O) other endpoints relate to outcomes that were not specified as 'primary' or 'secondary' outcomes in the publication IGF‐1: insulin‐like growth factor‐1; IGFBP3: insulin‐like growth factor binding protein 3; N/A: not applicable | ||||
Appendix 6. Matrix of study endpoints (trial documents)
| Endpointa | Review's primary outcome | Review's secondary outcome | Time of measurement | Sourceb | |
|
NCT00133354 (Mauras 2008) |
Adverse effects (S) | x | ‐ | 24 months | Trial protocol accessed at www.clinicaltrials.gov; no study results were posted in the trial register |
| Body composition (O) | ‐ | ‐ | ‐ | ||
| Bone age (S) | ‐ | ‐ | ‐ | ||
| Bone mineral density (O) | ‐ | x | 24, 36 months | ||
| Growth velocity (S) | ‐ | x | 12, 24, 36 months | ||
| Height SDS (S) | x | ‐ | 12, 24, 36 months | ||
| Hormonal measures (S) | ‐ | x | 12, 18, 24, 30, 36 months | ||
| Insulin sensitivity (S) | ‐ | ‐ | ‐ | ||
| Lipid abnormalities (S) | ‐ | x | 12, 18, 24, 30, 36 months | ||
| Liver function (S) | ‐ | ‐ | ‐ | ||
| PAH (P) | x | ‐ | ‐ | ||
| Puberty (S) | ‐ | ‐ | ‐ | ||
|
a(P) Primary or (S) secondary endpoint(s) refer to verbatim statements in the publication, (O) other endpoints relate to outcomes which were not specified as 'primary' or 'secondary' outcomes in the report bFood and Drug Administration (US) document, European Medicines Agency document, manufacturer's website, design paper, trial protocol document PAH: predicted adult height (based on rate of bone age advancement); SDS: standard deviation score | |||||
Appendix 7. Examination of outcome reporting bias according to ORBIT classification
| Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d | |
| Salehpour 2010 | Adverse events | x | ‐ | ‐ | ‐ |
| Wickman 2001 | Adverse events | ‐ | ‐ | ‐ | x |
| Bone density | x | ‐ | ‐ | ‐ | |
| aClear that outcome was measured and analysed; trial report stated that outcome was analysed but only reported that result was not significant (Classification 'A', table 2, Kirkham 2010) bClear that outcome was measured and analysed; trial report stated that outcome was analysed but no results reported (Classification 'D', table 2, Kirkham 2010) cClear that outcome was measured but not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results (Classification 'E', table 2, Kirkham 2010) dUnclear whether the outcome was measured; not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results (Classification 'G', table 2, Kirkham 2010) | |||||
Appendix 8. Definition of endpoint measurement
| Height measures | Cognitive outcomes | Socioeconomic effects | Bone density | Testosterone levels | Lipid abnormalities | Severe/serious adverse events | |
| Hero 2005 | PAH (Bayley and Pinneau) | WISC‐III, NEPSY, WMS‐R | N/I | BMD, BMAD in LS, NoF | ng/dL and nmol/L | TC, LDL, HDL, Trig (mmol/L) Apo‐A1, Apo‐A2, Apo‐B, Lp‐(a) (mg/dL) |
N/D |
| Mauras 2008 | PAH (Bayley and Pinneau) | N/I | N/I | BMD Z‐score LS | ng/dL | TC, LDL, HDL, Trig (mg/dL) | N/D |
| Salehpour 2010 | PAH (Bayley and Pinneau) | N/I | N/I | BMD, BMAD Z‐ score in LS, NoF | mmol/L | TC, LDL, HDL, Trig (mmol/L) | N/D |
| Wickman 2001 | PAH (Bayley and Pinneau) | N/I | N/I | BMD in LS, NoF | nmol/L | LDL, HDL, Trig (mmol/L) | N/D |
| Apo: apolipoprotein; BMAD: bone mineral apparent volumetric density; BMD: bone mineral density; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein; Lp(a); lipoprotein a; LS: lumbar spine; N/D: not defined; NEPSY: NEuroPSYchological assessment of development; N/I: not investigated; NoF: neck of femur; PAH: predicted adult height; TC: total cholesterol; Trig: triglycerides; WISC‐III: Wechsler Intelligence Scale for Children version III; WMS‐R: Wechsler Memory Scale‐Reading | |||||||
Appendix 9. Adverse events (I)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Deaths [N] | Deaths [%] | Participants with adverse events [N] | Participants with adverse events [%] | Participants with severe/serious adverse events [N] | Participants with severe/serious adverse events [%] | |
| Hero 2005 | I: etrozole | 16 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo | 15 | 0 | 0 | 1 | 7 | 1 | 7 | |
| Mauras 2008 | I: anastrozole + GH | 26 | 0 | 0 | ‐ | ‐ | 4 | 15 |
| C: placebo + GH | 26 | 0 | 0 | ‐ | ‐ | 0 | 0 | |
| Salehpour 2010 | I1: letrozole | 31 | 0 | 0 | 0 | 0 | 0 | 0 |
| I2: oxandrolone | 30 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C: placebo | 30 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Wickman 2001 | I: letrozole + testosterone | 11 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C1: placebo + testosterone | 12 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| C2: no treatment | 10 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| "‐" denotes not reported GH: growth hormone; N: number of participants | ||||||||
Appendix 10. Adverse events (II)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Participants discontinuing study due to adverse events [N] | Participants discontinuing study due to adverse event [%] | Participants hospitalised [N] | Participants hospitalised [%] | Participants with outpatient treatment [N] | Participants with outpatient treatment [%] | |
| Hero 2005 | I: letrozole | 16 | 0 | 0 | 0 | 0 | 0 | 0 |
| C: placebo | 15 | 1 | 7 | 1 | 7 | 0 | 0 | |
| Mauras 2008 | I: anastrozole + GH | 26 | 0 | 0 | 4a | 15 | 0 | 0 |
| C: placebo + GH | 26 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Salehpour 2010 | I1: letrozole | 31 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| I2: oxandrolone | 30 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| C: placebo | 30 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Wickman 2001 | I: letrozole + testosterone | 11 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C1: placebo + testosterone | 12 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| C2: no treatment | 10 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| "‐" denotes not reported a4 participants admitted to hospital, 1 participant had 4 separate admissions GH: growth hormone; N: number of participants | ||||||||
Appendix 11. Adverse events (III)
| Intervention(s) and comparator(s) | Participants included in analysis [N] | Participants with specific adverse events [description] | Participants with specific adverse events [N] | Participants with specific adverse events [%] | |
| Hero 2005 | I: letrozole | 16 | 0 | 0 | 0 |
| C: placebo | 15 | Newly diagnosed type 1 diabetes | 1 | 7 | |
| Mauras 2008 | I: anastrozole + GH | 26 | Psychogenic emesis, fractured arm, forearm bone surgery for previous injury, septic arthritis | 4 | 15 |
| C: placebo + GH | 26 | 0 | 0 | 0 | |
| Salehpour 2010 | I1: letrozole | 31 | ‐ | ‐ | ‐ |
| I2: oxandrolone | 30 | ‐ | ‐ | ‐ | |
| C: placebo | 30 | ‐ | ‐ | ‐ | |
| Wickman 2001 | I: letrozole + testosterone | 11 | ‐ | ‐ | ‐ |
| C1: placebo + testosterone | 12 | ‐ | ‐ | ‐ | |
| C2: no treatment | 10 | ‐ | ‐ | ‐ | |
| "‐" denotes not reported GH: growth hormone; N: number of participants |
|||||
Appendix 12. Survey of authors providing information on included trials
| Study author contacted [DD/MM/YY] | Study author replied [DD/MM/YY] | Study author asked for additional information [short summary] | Study author provided data [short summary] | |
| Hero 2005 | 31/05/2014 | 25/06/2014 | Final height data | Final height data not available |
| Mauras 2008 | 15/09/2013 | 07/08/2014 | Final height data, details on withdrawals and study attrition | Final height data not available. Attrition over time between groups clarified, but treatment allocations of withdrawals and when they withdrew not clarified |
| Salehpour 2010 | 16/09/2013 and 31/05/2014 | No reply received | Final height data | N/A |
| Wickman 2001 | 31/05/2014 | 25/06/2014 | Data on near‐final/final height data in untreated controls | Near‐final height in 5/10 untreated participants was 173.5 cm |
| DD: date; MM: month; N/A: not applicable; YY: year | ||||
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hero 2005.
| Methods | RCT (Finland) Allocation to treatment groups: randomised using computer‐generated blocks of 10 in the hospital pharmacy Randomisation ratio: 1 : 1 Superiority design Blinding: double‐blinded, randomisation codes not released until all data entered into computer at the end of the trial Comparability of treatment groups: no differences of significance at baseline Method of data analysis: not stated ITT. Mean (SD). Within‐group differences analysed by paired T tests, between‐group differences by unpaired T tests or repeated measures ANOVA. Friedman test used for within‐group and Mann‐Whitney U test used for between‐groups changes in Tanner staging Sample size/power calculation: planned sample size of 31 had 80% power to detect a difference in PAH between groups of 5 cm and allow for up to a 10% drop‐out rate Attrition/drop‐out: 1 withdrawal, developed type 1 diabetes after 6 months of treatment with placebo |
|
| Participants | Total number: 31 boys Placebo: 15 Letrozole: 16 Characteristics of target population: boys aged 9‐14.5 years with ISS (height 2 SD below mean for age or 2 SD below mid‐parental height). GH deficiency excluded. 81% of letrozole‐ and 93% of placebo‐treated boys were prepubertal at enrolment. 4 boys in letrozole and 3 boys in placebo group were small for gestational age at birth Exclusion criteria: bone age > 14 years Setting: single tertiary paediatric endocrinology centre |
|
| Interventions | Treatment arms:
Length of treatment: 2 years Other interventions used: none |
|
| Outcomes | Change in PAH Effects on bone mineral density |
|
| Study details | Study continued for pre‐specified duration of 2 years, no criteria used for early cessation | |
| Publication details | Published in English Work was supported by the Foundation for Pediatric Research (Helsinki, Finland) Publication status: full article in peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "The primary end point of the study was the efficacy of Letrozole in improving PAH, as measured by a change in PAH after 24 months of treatment" | |
| Notes | Generalisability: inclusion and exclusion criteria well defined Outcomes: final adult height not reported. Difference in PAH disappeared 4 years from the end of the trial. Inter‐centre variability: single‐centre study Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Randomization was carried out in blocks of 10 at the hospital pharmacy by a computer generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Comment: treatments were centrally allocated by the hospital pharmacy |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote from publication: "Researchers and subjects were blind to treatment assignment until the end of follow‐up, when the randomization code was revealed to the researchers after the data had been entered into a computer" |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote from publication: "Researchers and subjects were blind to treatment assignment until the end of follow‐up, when the randomization code was revealed to the researchers after the data had been entered into a computer" |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote from publication: "Researchers and subjects were blind to treatment assignment until the end of follow‐up, when the randomization code was revealed to the researchers after the data had been entered into a computer" |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk | Quote from publication: "Researchers and subjects were blind to treatment assignment until the end of follow‐up, when the randomization code was revealed to the researchers after the data had been entered into a computer" |
| Incomplete outcome data (attrition bias) Objective outcomes | Low risk | Comment: no stated withdrawals other than 1 boy excluded from placebo arm after 6 months having developed type 1 diabetes. All pre‐specified outcomes reported on |
| Incomplete outcome data (attrition bias) Subjective outcomes | Low risk | Comment: no stated withdrawals other than 1 boy excluded from placebo arm after 6 months having developed type 1 diabetes. All pre‐specified outcomes reported on |
| Selective reporting (reporting bias) | Low risk | Comment: no follow‐up data on final adult height; however, a subsequent paper (Hero 2010) reported no difference in near‐adult height. This was assessed in 23/31 participants initially randomised and attrition was even between groups |
| Other bias | Low risk | Comment: none detected |
Mauras 2008.
| Methods | RCT (USA) Allocation to treatment groups: randomised, details not provided. Allocation sequence determined by AstraZeneca (drug company) Randomisation ratio: 1 : 1 Superiority design Blinding: method not reported. Data unblinded to 1 individual after all participants had completed 24 months and analysed Comparability of treatment groups: no significant differences between study participants at baseline Method of data analysis: by ITT. Mean (SEM). 2‐sided T tests for treatment comparison Sample size/power calculation: none reported Attrition/drop‐out: 9 withdrawals, treatment allocation of those withdrawing not reported, or when they withdrew. 5 participants discontinued by investigators, treatment allocation and time of withdrawal not reported |
|
| Participants | Total number: 52 (100% male) Placebo: 26 Anastrozole: 26 Characteristics of target population: GH deficiency (height > 2 SD below mean or height velocity < 25th centile and peak GH < 10 ng/mL on 2 pharmacological tests), treatment with GH of 0.3 mg/kg per week for 6 months and pubertal (Tanner stage ≥ II or testicular volume > 4 mL) with residual height potential (bone age 11.5‐15 years at study entry) Exclusion criteria: involvement in other trials of hormone treatment, chronic illness requiring long‐term medication that impaired growth Setting: 7 paediatric endocrinology centres |
|
| Interventions | Treatment arms:
Minimum 6‐month treatment with GH prior to entry into study Length of treatment: until completion of linear growth (bone age of 16 years and growth velocity of 2 cm per year), or for up to 36 months Other interventions used: GH (mean dose 0.35 mg/kg per week) |
|
| Outcomes | Change in PAH Change in bone age Oestradiol and oestrone concentrations Effects on glucose and lipid concentrations |
|
| Study details | Study not terminated before scheduled to | |
| Publication details | Published in English "This work was supported by an investigator‐initiated research grant from AstraZeneca and drug supply grants from Genentech and EMD Serono" Full text in peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "Our objective was to investigate whether anastrozole, a potent aromatase inhibitor, could delay bone age acceleration and increase predicted adult height in adolescent boys with GH deficiency" | |
| Notes | Generalisability: clear inclusion and exclusion criteria Outcomes: treatment allocation of withdrawals and exclusions not reported. Final or near‐final height not reported Inter‐centre variability: 7 centres, inter‐centre variability not assessed Conflict of interest: AstraZeneca sponsored study, involved in generating allocation sequence. Biostatistician was an employee of AstraZeneca. First author had consulted for AstraZeneca |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from publication: "Treatment with anastrozole or placebo was determined by a randomization schedule prepared by AstraZeneca who provided the study drug and placebo tablets for the trial" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Investigators, staff and patients were unaware of the randomization codes until after completion of the trial" |
| Blinding of participants and personnel (performance bias) Objective outcomes | Unclear risk | Quote from publication: "Data were unblinded to one single individual and analysed after all available subjects completed 24 months of treatment" Comment: method of blinding not reported |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Unclear risk | Quote from publication: "Data were unblinded to one single individual and analysed after all available subjects completed 24 months of treatment” Comment: method of blinding not reported |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: method of blinding not reported |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: method of blinding not reported |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Quote from publication: "Analysis of endpoints included only patients with measurements available at that timepoint and baseline" Comment: 52 participants recruited (26 intervention: 26 control) 50 completed 12 months (26 intervention: 24 control) 41 completed 24 months (20 intervention: 21 control) 28 completed 36 months (16 intervention: 12 control) 9 participants withdrew (satisfied with achieved height or psychosocial difficulties) and 5 participants discontinued by investigators (compliance or loss to follow‐up). Treatment allocation of participants withdrawing not reported, or when they withdrew |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Quote from publication: "Analysis of endpoints included only patients with measurements available at that timepoint and baseline" |
| Selective reporting (reporting bias) | Unclear risk | Comment: final adult height not reported in this paper or subsequent publications and data not available following consultation with corresponding author (not completed due to lack of funding). Measurement of final height was a reported aim |
| Other bias | Low risk | Comment: none detected |
Salehpour 2010.
| Methods | RCT (Iran) Allocation to treatment groups: randomised, method not reported Randomisation ratio: 1 : 1 : 1 Superiority design Blinding: placebo and letrozole tablets identical in appearance. Radiologist assessing bone ages reported to be blinded to treatment allocation Comparability of treatment groups: no reported differences at baseline Method of data analysis: not stated ITT. Mean (SD). Comparisons using ANOVA, Wilcoxon, or paired T tests Sample size/power calculation: planned 15 participants per treatment group would have 90% power to detect a 5 cm difference in PAH. Number recruited to each group more than twice this Attrition/drop‐out: 8 withdrawals from placebo group due to lack of observed treatment effect |
|
| Participants | Total number: 91 children (all male) Placebo: 30 Oxandrolone: 30 Letrozole: 31 Characteristics of target population: boys aged 12.6‐14.6 years with CDGP (testicular volume ≤ 3 mL after 12.5 years and Tanner genital or pubic hair stage > 2 SD delayed for healthy Indo‐Iranian boys) and unexplained intrinsic short stature (1 SD below mid‐parental target height centile) Exclusion criteria: evidence of an underlying disorder to explain short stature Setting: paediatric endocrinology unit, Mofid Childrens Hospital |
|
| Interventions | Treatment arms:
Length of treatment: 2 years Other interventions used: none reported |
|
| Outcomes | Change in PAH Effects on bone mineral density Effects on lipid profile |
|
| Study details | Study not terminated prior to scheduled end date | |
| Publication details | Published in English Non‐commercial funding from Genomic Research Centre Full text in a peer reviewed journal |
|
| Stated aim for study | Quote from publication: "We compared the effects of letrozole with that of oxandrolone on predicted adult height, puberty, bone mineral density, serum insulin‐like growth factor and blood lipoproteins" | |
| Notes | Generalisability: clear inclusion and exclusion criteria, definitions of pubertal delay and unexplained short stature not the standard definitions used. Specific ethnic subgroup Outcomes: final height not reported Inter‐centre variability: single‐centre study Conflicts of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: method to conceal allocation sequence not described |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Quote from publication: "... double‐blinded random allocation ..." Comment: placebo and letrozole tablets identical in appearance |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Quote from publication: "... double‐blinded random allocation ..." Comment: placebo and letrozole tablets identical in appearance |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: bone age stated to have been reported blindly by 1 experienced paediatric radiologist |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: bone age stated to have been reported blindly by 1 experienced paediatric radiologist |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Quote from publication: "There was no significant difference between the remaining and missing subjects in all the primary and secondary outcomes" Comment: all participants in both the letrozole and oxandrolone groups completed the study, only 22/30 of the placebo group completed the study due to perceived lack of efficacy. Study remained adequately powered with remaining number of participants |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Quote from publication: "There was no significant difference between the remaining and missing subjects in all the primary and secondary outcomes" Comment: all participants in both the letrozole and oxandrolone groups completed the study, only 22/30 of the placebo group completed the study due to perceived lack of efficacy. Study remained adequately powered with remaining number of participants |
| Selective reporting (reporting bias) | Unclear risk | Comment: clear that adverse events were measured, trial report states that outcome was analysed but only reports that results did not differ |
| Other bias | Low risk | Comment: none detected |
Wickman 2001.
| Methods | RCT (Finland) Allocation to treatment groups: randomised, method not reported. 1 self selected untreated group Randomisation ratio: 1 : 1 Superiority design Blinding: 3 authors assessing bone ages and calculating PAH were blinded Comparability of treatment groups: so significant difference at baseline Method of data analysis: not stated as ITT. Data reported as mean (SD). Differences in summary measures assessed using 1‐way analysis of variance, Students unpaired T test, Kruskal‐Wallis or Mann‐Whitney U tests were used as appropriate to compare between groups. Within group comparisons were analysed using Students T test or Wilcoxon signed‐rank sum test Sample size/power calculation: small, no power calculation performed Attrition/drop‐out: 7 participants did not reach primary endpoint. 6 were withdrawals, 1 excluded for non‐compliance. Reasons for withdrawal not specified |
|
| Participants | Total number: 33 children (all male) Untreated controls: 10 Testosterone and placebo: 12 Testosterone and letrozole: 11 Characteristics of target population: children with constitutional delay of puberty, short stature, or both. 76% had a family history of delayed puberty. Mean chronological age 15.1 years, mean bone age 12.8 years Exclusion criteria: mid puberty Setting: tertiary paediatric endocrinology centre |
|
| Interventions | Treatment arms:
Length of treatment: as above Final assessment 18 months from entry Other interventions used: none reported |
|
| Outcomes | Change in PAH Bone age change |
|
| Study details | Study not terminated before regular end | |
| Publication details | Published in English Study was supported by the Foundation of Pediatric Research, Helsinki, Finland Full text in peer‐reviewed journal |
|
| Stated aim for study | Quote from publication: "We did a randomised, double‐blind, placebo controlled study in which we treated boys with constitutional delay of growth and puberty with testosterone and placebo or testosterone and letrozole" | |
| Notes | Generalisability: clear inclusion and exclusion criteria Outcomes: near‐final height reported in subsequent paper (Hero 2006). Final height reported for 50% of original cohort (Hero 2010) Inter‐centre variability: single‐centre study Conflict of interest: none reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information provided regarding the sequence generation process |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information provided regarding the allocation concealment process |
| Blinding of participants and personnel (performance bias) Objective outcomes | Low risk | Comment: stated as a double‐blind, placebo‐controlled study. No information provided on blinding methods used |
| Blinding of participants and personnel (performance bias) Subjective outcomes | Low risk | Comment: stated as a double‐blind, placebo‐controlled study. No information provided on blinding methods used |
| Blinding of outcome assessment (detection bias) Objective outcomes | Unclear risk | Comment: 3 of 5 authors (SW, IS, LD) stated they were unaware of groupings and were involved in assessment of bone ages and calculation of PAH, which was primary outcome. Unclear what the roles of the other 2 authors (CAL, EN) were and whether or not they had any involvement in assessment of other objective outcomes |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk | Comment: unclear whether adverse events were measured; not mentioned but clinical judgement says likely to have been measured |
| Incomplete outcome data (attrition bias) Objective outcomes | Unclear risk | Comment: the primary report of this study did not clearly state primary and secondary outcome measures. 10/12 analysed for primary endpoint in the control group, 9/11 in intervention group. 1 participant in intervention group excluded for suspected non‐compliance. 17/23 assessed for near‐final height, 12/23 assessed for final height. Attrition even, however numbers not stated |
| Incomplete outcome data (attrition bias) Subjective outcomes | Unclear risk | Comment: the primary report of this study did not clearly state primary and secondary outcome measures. 10/12 analysed for primary endpoint in the control group, 9/11 in intervention group. 1 participant in intervention group excluded for suspected non‐compliance. 17/23 assessed for near‐final height, 12/23 assessed for final height. Attrition even, however numbers not stated |
| Selective reporting (reporting bias) | Unclear risk | Comment: this paper reported on change in PAH in 3 groups (untreated, testosterone plus placebo and testosterone plus letrozole groups). Follow‐up reports at near‐final height (Hero 2006) and final height (Hero 2010) only reported on the 2 treated groups, although this is unlikely to introduce bias Comment: unclear whether adverse events were measured, not mentioned but clinical judgement says likely to have been measured; clear that bone density was measured and analysed, trial report stated that outcome was analysed but only reports that result was not significant |
| Other bias | Low risk | Comment: none detected |
CDGP: constitutional delay of growth and pubertal development; GH: growth hormone; IM: intramuscular; ISS: idiopathic short stature; ITT: intention to treat; PAH: predicted adult height; RCT: randomised controlled trial; SD: standard deviation; SEM: standard error of the mean.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Mauras 2004 | Open‐label pilot study rather than randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
NCT01248416.
| Trial name or title | A Randomized Controlled Trial of the Use of Aromatase Inhibitors, Alone and in Combination with Growth Hormone in Adolescent Boys with Idiopathic Short Stature |
| Methods |
Type of study: efficacy Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: idiopathic short stature Enrolment: planned 77 Inclusion criteria:
Exclusion criteria
|
| Interventions |
Interventions
|
| Outcomes |
Primary outcome(s): increasing adult height potential in adolescent boys with idiopathic short stature treated for 2 years (safety and efficacy of AIs alone versus GH alone versus AIs plus GH) Secondary outcome(s):
Other outcome(s): not reported |
| Starting date | November 2010 |
| Contact information | Responsible party/principal investigator: Nelly Mauras, Nemours Childrens Clinic |
| Study identifier | NCT number: NCT01248416 |
| Official title | A Randomized Controlled Trial of the Use of Aromatase Inhibitors, Alone and in Combination with Growth Hormone in Adolescent Boys with Idiopathic Short Stature |
| Stated purpose of study | This study will assess the effect of AIs alone, GH alone, and combination treatment in enhancing height potential in adolescent boys with idiopathic short stature |
| Notes |
AI: aromatase inhibitors; GH: growth hormone; IGF‐1: insulin‐like growth factor‐1; IGFBP3: insulin‐like growth factor binding protein‐3; SD: standard deviation.
Differences between protocol and review
In the Methods section, under the subheading 'Electronic searches', we presented a more detailed breakdown of trial registries searched. A new search strategy was devised by the trials search co‐ordinators and is outlined in Appendix 1. Under the subheading of 'Searching other resources', we added searches to pharmaceutical company trial databases and the grey literature, in an attempt to obtain further trials of relevance. We were unable to perform meta‐analysis as planned due to substantial clinical heterogeneity between the participant's diagnoses, pubertal status, and co‐interventions.
Contributions of authors
Niamh McGrath (NMG): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft, and future update draft.
Michael J O'Grady (MOG): protocol draft, search strategy development, acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review draft, and future update draft.
Declarations of interest
Niamh McGrath: none known.
Michael J O'Grady: none known.
New
References
References to studies included in this review
Hero 2005 {published data only (unpublished sought but not used)}
- Hero M, Ankarberg‐Lindgren C, Taskinen MR, Dunkel L. Blockade of oestrogen biosynthesis in peripubertal boys: effects on lipid metabolism, insulin sensitivity, and body composition. European Journal of Endocrinology 2006;155(3):453‐60. [DOI] [PubMed] [Google Scholar]
- Hero M, Maury S, Luotoniemi E, Service E, Dunkel L. Cognitive effects of aromatase inhibitor therapy in peripubertal boys. European Journal of Endocrinology 2010;163(1):149‐55. [DOI] [PubMed] [Google Scholar]
- Hero M, Mäkitie O, Kröger H, Nousiainen E, Toiviainen‐Salo S, Dunkel L. Impact of aromatase inhibitor therapy on bone turnover, cortical bone growth and vertebral morphology in pre‐ and peripubertal boys with idiopathic short stature. Hormone Research 2009;71(5):290‐7. [DOI] [PubMed] [Google Scholar]
- Hero M, Norjavaara E, Dunkel L. Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial. Journal of Clinical Endocrinology and Metabolism 2005;90(12):6396‐402. [DOI] [PubMed] [Google Scholar]
- Hero M, Toiviainen‐Salo S, Wickman S, Mäkitie O, Dunkel L. Vertebral morphology in aromatase inhibitor‐treated males with idiopathic short stature or constitutional delay of puberty. Journal of Bone Mineral Research 2010;25(7):1536‐43. [DOI] [PubMed] [Google Scholar]
Mauras 2008 {published and unpublished data}
- Mauras N, Gonzalez de Pijem L, Hsiang HY, Desrosiers P, Rapaport R, Schwartz ID, et al. Anastrozole increases predicted adult height of short adolescent males treated with growth hormone: a randomized, placebo‐controlled, multicenter trial for one to three years. Journal of Clinical Endocrinology and Metabolism 2008;93(3):823‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salehpour 2010 {published data only (unpublished sought but not used)}
- Salehpour S, Alipour P, Razzaghy‐Azar, Ardeshipour L, Shamshiri A, Monfared MF, et al. A double‐blind, placebo‐controlled comparison of letrozole to oxandrolone effects upon growth and puberty of children with constitutional delay of puberty and idiopathic short stature. Hormone Research in Paediatrics 2010;74:428‐35. [DOI] [PubMed] [Google Scholar]
Wickman 2001 {published and unpublished data}
- Dunkel L, Wickman S. Novel treatment of delayed male puberty with aromatase inhibitors. Hormone Research 2002;57 Suppl 2:44‐52. [DOI] [PubMed] [Google Scholar]
- Hero M, Toiviainen‐Salo S, Wickman S, Mäkitie O, Dunkel L. Vertebral morphology in aromatase inhibitor‐treated males with idiopathic short stature or constitutional delay of puberty. Journal of Bone Mineral Research 2010;25(7):1536‐43. [DOI] [PubMed] [Google Scholar]
- Hero M, Wickman S, Dunkel L. Treatment with the aromatase inhibitor letrozole during adolescence increases near‐final height in boys with constitutional delay of puberty. Clinical Endocrinology (Oxford) 2006;64(5):510‐3. [DOI] [PubMed] [Google Scholar]
- Hero M, Wickman S, Hanhijärvi R, Siimes MA, Dunkel L. Pubertal upregulation of erythropoiesis in boys is determined primarily by androgen. Journal of Pediatrics 2005;146(2):245‐52. [DOI] [PubMed] [Google Scholar]
- Wickman S, Kajantie E, Dunkel L. Effects of suppression of estrogen action by the p450 aromatase inhibitor letrozole on bone mineral density and bone turnover in pubertal boys. Journal of Clinical Endocrinology & Metabolism 2003;88(8):3785‐93. [DOI] [PubMed] [Google Scholar]
- Wickman S, Saukkonen T, Dunkel L. The role of sex steroids in the regulation of insulin sensitivity and serum lipid concentrations during male puberty: a prospective study with a P450‐aromatase inhibitor. European Journal of Endocrinology 2002;146(3):339‐46. [DOI] [PubMed] [Google Scholar]
- Wickman S, Sipila I, Ankarberg‐Lindgren C, Norjavaara E, Dunkel L. A specific aromatase inhibitor and potential increase in adult height in boys with delayed puberty: a randomised controlled trial. Lancet 2001;357:1743‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Mauras 2004 {published data only}
- Mauras N, Welch S, Rini A, Klein KO. An open label 12‐month pilot trial on the effects of the aromatase inhibitor anastrozole in growth hormone (GH)‐treated GH deficient adolescent boys. Journal of Pediatric Endocrinology and Metabolism 2004;17(12):1597‐606. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01248416 {published and unpublished data}
- A Randomized Controlled Trial of the Use of Aromatase Inhibitors, Alone and in Combination with Growth Hormone in Adolescent Boys with Idiopathic Short Stature. Ongoing study November 2010.
Additional references
Balducci 1995
- Balducci R, Toscano V, Mangiantini A, Municchi G, Vaccaro F, Picone S, et al. Adult height in short normal adolescent girls treated with gonadotropin‐releasing hormone analog and growth hormone. Journal of Clinical Endocrinology and Metabolism 1995;80(12):3596‐600. [DOI] [PubMed] [Google Scholar]
Bryant 2007
- Bryant J, Baxter L, Cave CB, Milne R. Recombinant growth hormone for idiopathic short stature in children and adolescents. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD004440.pub2] [DOI] [PubMed] [Google Scholar]
Carani 1997
- Carani C, Qin K, Simoni M, Faustini‐Fustini M, Serpente S, Boyd J, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. New England Journal of Medicine 1997;337(2):91‐5. [DOI] [PubMed] [Google Scholar]
Carel 1996
- Carel JC, Hay F, Coutant R, Rodrigue D, Chaussain JL. Gonadotropin‐releasing hormone agonist treatment of girls with constitutional short stature and normal pubertal development. Journal of Clinical Endocrinology and Metabolism 1996;81(9):3318‐22. [DOI] [PubMed] [Google Scholar]
Carel 2009
- Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmert MR, ESPE‐LWPES GnRH Analogs Consensus Conference Group, et al. Consensus statement on the use of gonadotropin‐releasing hormone analogs in children. Pediatrics 2009;123(4):e752‐62. [DOI] [PubMed] [Google Scholar]
Cernich 2004
- Cernich J, Jacobson JD, Moore WV, Popovic J. Use of aromatase inhibitors in children with short stature. Pediatric Endocrinology Reviews 2004;2(1):2‐7. [PubMed] [Google Scholar]
Cohen 2008
- Cohen P, Rogol AD, Deal CL, Saenger P, Reiter EO, Ross JL, et al. 2007 ISS Consensus Workshop participants. Consensus statement on the diagnosis and treatment of children with idiopathic short stature: a summary of the Growth Hormone Research Society, the Lawson Wilkins Pediatric Endocrine Society, and the European Society for Paediatric Endocrinology Workshop. Journal of Clinical Endocrinology and Metabolism 2008;93(11):4210‐7. [DOI] [PubMed] [Google Scholar]
De Ronde 2011
- Ronde W, Jong FH. Aromatase inhibitors in men: effects and therapeutic options. Reproductive Biology and Endocrinology 2011;9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feuillan 2007
- Feuillan P, Calis K, Hill S, Shawker T, Robey PG, Collins MT. Letrozole treatment of precocious puberty in girls with the McCune‐Albright syndrome: a pilot study. Journal of Clinical Endocrinology and Metabolism 2007;92(6):2100‐6. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Job 1994
- Job JC, Toublanc JE, Landier F. Growth of short normal children in puberty treated for 3 years with growth hormone alone or in association with gonadotropin‐releasing hormone agonist. Hormone Research 1994;41(5‐6):177‐84. [DOI] [PubMed] [Google Scholar]
Kamp 2001
- Kamp GA, Mul D, Waelkens JJ, Jansen M, Delemarre‐van de Waal HA, Verhoeven‐Wind L, et al. A randomized controlled trial of three years growth hormone and gonadotropin‐releasing hormone agonist treatment in children with idiopathic short stature and intrauterine growth retardation. Journal of Clinical Endocrinology and Metabolism 2001;86(7):2969‐75. [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Krebs 2012
- Krebs A, Moske‐Eick O, Doerfer J, Roemer‐Pergher C, Werf‐Grohmann N, Schwab KO. Marked increase of final height by long‐term aromatase inhibition in a boy with idiopathic short stature. Journal of Pediatric Endocrinology and Metabolism 2012;25(5‐6):581‐5. [DOI] [PubMed] [Google Scholar]
Kreher 2005
- Kreher NC, Eugster EA, Shankar RR. The use of tamoxifen to improve height potential in short pubertal boys. Pediatrics 2005;116(6):1513‐5. [DOI] [PubMed] [Google Scholar]
Kumar 2009
- Kumar KV, Jayaraman M, Verma A, Modi KD. Letrozole as a booster therapy in growth hormone deficiency. Indian Pediatrics 2009;46(7):625‐7. [PubMed] [Google Scholar]
Lanes 1998
- Lanes R, Gunczler P. Final height after combined growth hormone and gonadotrophin‐releasing hormone analogue therapy in short healthy children entering into normally timed puberty. Clinical Endocrinology 1998;49(2):197‐202. [DOI] [PubMed] [Google Scholar]
Lee 2006
- Lee JM, Davis MM, Clark SJ, Hofer TP, Kemper AR. Estimated cost‐effectiveness of growth hormone therapy for idiopathic short stature. Archives of Pediatrics and Adolescent Medicine 2006;160(3):263‐9. [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindner 1993
- Lindner D, Job JC, Chaussain JL. Failure to improve height prediction in short‐stature pubertal adolescents by inhibiting puberty with luteinizing hormone‐releasing hormone analogue. European Journal of Pediatrics 1993;152(5):393‐6. [DOI] [PubMed] [Google Scholar]
Lindsay 1994
- Lindsay R, Feldkamp M, Harris D, Robertson J, Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. Journal of Pediatrics 1994;125(1):29‐35. [DOI] [PubMed] [Google Scholar]
Maffei 2004
- Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. Journal of Clinical Endocrinology and Metabolism 2004;89(1):61‐70. [DOI] [PubMed] [Google Scholar]
Mauras 2009
- Mauras N, Bishop K, Merinbaum D, Emeribe U, Agbo F, Lowe E. Pharmacokinetics and pharmacodynamics of anastrozole in pubertal boys with recent‐onset gynecomastia. Journal of Clinical Endocrinology and Metabolism 2009;94(8):2975‐8. [DOI] [PubMed] [Google Scholar]
Mericq 2000
- Mericq MV, Eggers M, Avila A, Cutler GB Jr, Cassorla F. Near final height in pubertal growth hormone (GH)‐deficient patients treated with GH alone or in combination with luteinizing hormone‐releasing hormone analog: results of a prospective, randomized trial. Journal of Clinical Endocrinology and Metabolism 2000;85(2):569‐73. [DOI] [PubMed] [Google Scholar]
Merke 2000
- Merke DP, Keil MF, Jones JV, Fields J, Hill S, Cutler GB Jr. Flutamide, testolactone, and reduced hydrocortisone dose maintain normal growth velocity and bone maturation despite elevated androgen levels in children with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 2000;85(3):1114‐20. [DOI] [PubMed] [Google Scholar]
Morishima 1995
- Morishima A, Grumbach M, Simpson E, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. Journal of Clinical Endocrinology and Metabolism 1995;80(12):3689‐98. [DOI] [PubMed] [Google Scholar]
Mul 2001
- Mul D, Wit JM, Oostdijk W, Broeck J, Dutch Advisory Group on Growth Hormone. The effect of pubertal delay by GnRH agonist in GH‐deficient children on final height. Journal of Clinical Endocrinology and Metabolism 2001;86(10):4655‐6. [DOI] [PubMed] [Google Scholar]
Pasquino 2000
- Pasquino AM, Pucarelli I, Roggini M, Segni M. Adult height in short normal girls treated with gonadotropin‐releasing hormone analogs and growth hormone. Journal of Clinical Endocrinology and Metabolism 2000;85(2):619‐22. [DOI] [PubMed] [Google Scholar]
Pasquino 2008
- Pasquino AM, Pucarelli I, Accardo F, Demiraj V, Segni M, Nardo R. Long‐term observation of 87 girls with idiopathic central precocious puberty treated with gonadotropin‐releasing hormone analogs: impact on adult height, body mass index, bone mineral content, and reproductive function. Journal of Clinical Endocrinology and Metabolism 2008;93(1):190‐5. [DOI] [PubMed] [Google Scholar]
Raman 2002
- Raman JD, Schlegel PN. Aromatase inhibitors for male infertility. Journal of Urology 2002;167(2 Pt 1):624‐9. [DOI] [PubMed] [Google Scholar]
Ramasamy 2009
- Ramasamy R, Ricci JA, Palermo GD, Gosden LV, Rosenwaks Z, Schlegel PN. Successful fertility treatment for Klinefelter's syndrome. Journal of Urology 2009;182(3):1108‐13. [DOI] [PubMed] [Google Scholar]
Ranke 1996
- Ranke MB. Towards a consensus on the definition of idiopathic short stature. Hormone Research 1996;45 Suppl 2:64. [DOI] [PubMed] [Google Scholar]
Ranke 2003
- Ranke MB, Lindberg A, Martin DD, Bakker B, Wilton P, Albertsson‐Wikland K, et al. The mathematical model for total pubertal growth in idiopathic growth hormone (GH) deficiency suggests a moderate role of GH dose. Journal of Clinical Endocrinology and Metabolism 2003;88(10):8478‐53. [DOI] [PubMed] [Google Scholar]
Reiter 2010
- Reiter EO, Mauras N, McCormick K, Kulshreshtha B, Amrhein J, Luca F, et al. Bicalutamide plus anastrozole for the treatment of gonadotropin‐independent precocious puberty in boys with testotoxicosis: a phase II, open‐label pilot study (BATT). Journal of Pediatric Endocrinology and Metabolism 2010;23(10):999‐1009. [DOI] [PubMed] [Google Scholar]
Richmond 2007
- Richmond EJ, Rogol AD. Male pubertal development and the role of androgen therapy. Nature Clinical Practice, Endocrinology & Metabolism 2007;3(4):338‐44. [DOI] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Shulman 2008
- Shulman DI, Francis GL, Palmert MR, Eugster EA, Lawson Wilkins Pediatric Endocrine Society Drug and Therapeutics Committee. Use of aromatase inhibitors in children and adolescents with disorders of growth and adolescent development. Pediatrics 2008;121(4):e975‐83. [DOI] [PubMed] [Google Scholar]
Smith 1994
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen‐receptor gene in a man. New England Journal of Medicine 1994;331(16):1056‐61. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Van Gool 2007
- Gool SA, Kamp GA, Visser‐van Balen H, Mul D, Waelkens JJ, Jansen M, et al. Final height outcome after three years of growth hormone and gonadotropin‐releasing hormone agonist treatment in short adolescents with relatively early puberty. Journal of Clinical Endocrinology and Metabolism 2007;92(4):1402‐8. [DOI] [PubMed] [Google Scholar]
Wit 2011
- Wit JM, Hero M, Nunez SB. Aromatase inhibitors in pediatrics. Nature Reviews, Endocrinology 2011;8(3):135‐47. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2005
- Zhou P, Shah B, Prasad K, David R. Letrozole significantly improves growth potential in a pubertal boy with growth hormone deficiency. Pediatrics 2005;115(2):e245‐8. [DOI] [PubMed] [Google Scholar]


