Abstract
Objective
To assess the benefits and harms of different types and doses of anticoagulant drugs for the prevention of venous thromboembolism in patients who are acutely ill and admitted to hospital.
Design
Systematic review and network meta-analysis.
Data sources
Cochrane CENTRAL, PubMed/Medline, Embase, Web of Science, clinical trial registries, and national health authority databases. The search was last updated on 16 November 2021.
Eligibility criteria for selecting studies
Published and unpublished randomised controlled trials that evaluated low or intermediate dose low-molecular-weight heparin, low or intermediate dose unfractionated heparin, direct oral anticoagulants, pentasaccharides, placebo, or no intervention for the prevention of venous thromboembolism in acutely ill adult patients in hospital.
Main outcome measures
Random effects, bayesian network meta-analyses used four co-primary outcomes: all cause mortality, symptomatic venous thromboembolism, major bleeding, and serious adverse events at or closest timing to 90 days. Risk of bias was also assessed using the Cochrane risk-of-bias 2.0 tool. The quality of evidence was graded using the Confidence in Network Meta-Analysis framework.
Results
44 randomised controlled trials that randomly assigned 90 095 participants were included in the main analysis. Evidence of low to moderate quality suggested none of the interventions reduced all cause mortality compared with placebo. Pentasaccharides (odds ratio 0.32, 95% credible interval 0.08 to 1.07), intermediate dose low-molecular-weight heparin (0.66, 0.46 to 0.93), direct oral anticoagulants (0.68, 0.33 to 1.34), and intermediate dose unfractionated heparin (0.71, 0.43 to 1.19) were most likely to reduce symptomatic venous thromboembolism (very low to low quality evidence). Intermediate dose unfractionated heparin (2.63, 1.00 to 6.21) and direct oral anticoagulants (2.31, 0.82 to 6.47) were most likely to increase major bleeding (low to moderate quality evidence). No conclusive differences were noted between interventions regarding serious adverse events (very low to low quality evidence). When compared with no intervention instead of placebo, all active interventions did more favourably with regard to risk of venous thromboembolism and mortality, and less favourably with regard to risk of major bleeding. The results were robust in prespecified sensitivity and subgroup analyses.
Conclusions
Low-molecular-weight heparin in an intermediate dose appears to confer the best balance of benefits and harms for prevention of venous thromboembolism. Unfractionated heparin, in particular the intermediate dose, and direct oral anticoagulants had the least favourable profile. A systematic discrepancy was noted in intervention effects that depended on whether placebo or no intervention was the reference treatment. Main limitations of this study include the quality of the evidence, which was generally low to moderate due to imprecision and within-study bias, and statistical inconsistency, which was addressed post hoc.
Systematic review registration
PROSPERO CRD42020173088.
Introduction
Venous thromboembolism is a multifactorial disease frequently complicating the course of acute illness.1 Up to half of all thrombotic events have been estimated to be attributable to a current or recent hospitalisation, making prevention of in-hospital venous thromboembolism a worthwhile aim.2 Any anticoagulant intervention such as coumarin, unfractionated heparin, low-molecular-weight heparin, pentasaccharides, or direct oral anticoagulants can be used for this purpose. However, the balance of benefits and harms is different for each drug. Major European and North American guidelines recommend the use of low-molecular-weight heparin or pentasaccharides for thrombosis prophylaxis in acutely ill patients at risk of venous thromboembolism, but they differ with regard to unfractionated heparin.3 4 Other anticoagulants are not routinely recommended.3 4
Pentasaccharides have been studied and registered for venous thromboembolism prevention in a single dose, but low-molecular-weight heparin and unfractionated heparin can be administered in several doses depending on the country and setting.5 6 7 8 9 We previously distinguished between low, intermediate, and high dose low-molecular-weight heparin based on the summary of product characteristics and randomised controlled trial dosing regimens.10 11 A network meta-analysis on prophylaxis in surgical patients found different intervention effects for different prophylactic low-molecular-weight heparin doses.12Additionally, a Cochrane meta-analysis in patients with covid-19 found no conclusive benefits of high dose low-molecular-weight heparin or unfractionated heparin over lower doses. 13 Apart from trials in patients with covid-19, head-to-head comparisons of different anticoagulant doses in acutely ill patients admitted to hospital have been scarce.14 Given the widespread use of thrombosis prophylaxis in this patient category, the question remains as to which anticoagulant type and dose are optimal in terms of benefits and harms.
We conducted a systematic review with network meta-analysis to combine all available direct and indirect evidence. Our objective was to assess the benefits and harms of different types and doses of anticoagulant drugs for prevention of venous thromboembolism in acutely ill adults who have been admitted to hospital.
Methods
This systematic review was conducted according to a prospectively registered protocol (PROSPERO CRD42020173088) and is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for reporting network meta-analyses (supplementary table 1).15
Information sources and search
We searched Cochrane CENTRAL, PubMed/Medline, Embase, and Web of Science using a search strategy developed with the help of a medical information specialist (supplementary table 2). The search consisted of four domains (intervention, outcome, methodology, and some exclusion terms) and medical subject headings were used for searching PubMed/Medline. We also searched Google Scholar, clinicaltrials.gov, the World Health Organization International Clinical Trials Registry Platform, the European Medicines Agency registry, and the US Federal Drug Agency registry. The search was first conducted on 3 March 2020 and was last updated on 16 November 2021. We did not restrict on date, language, publication status, or year.
Eligibility criteria
We included all randomised controlled trials that evaluated pharmacological thrombosis prophylaxis for prevention of venous thromboembolism in acutely ill adults admitted to hospital. Quasi-randomised studies and observational studies were excluded. We included trials regardless of the type of acute illness but excluded randomised controlled trials that focused on elective surgical patients. We adhered to the illness definitions of the individual trials, which generally meant that critical illness was defined as admittance to an intensive care unit and acutely ill patients as admitted either to general medical wards, cardiology, or stroke units. We excluded studies in which patients were treated with intravenous anticoagulation or non-pharmacological methods. Mechanical prophylaxis and anticoagulant co-interventions were allowed in our inclusion criteria provided they were either balanced or both intervention groups were equally likely to have received them.
Interventions and comparators
We assessed low dose low-molecular-weight heparin, intermediate dose low-molecular-weight heparin, low dose unfractionated heparin, intermediate dose unfractionated heparin, standard dose direct oral anticoagulants, pentasaccharides, coumarins, heparinoids, platelet inhibitors, placebo, and no intervention. The interventions low-molecular-weight heparin and unfractionated heparin were categorised as low or intermediate dose according to a priori defined cut-offs that were based on summary of product characteristics and clinical trials dosing regimens (table 1). We assumed that intervention effects within either of the dose strata were interchangeable with respect to benefits and harms. We originally planned to distinguish between different doses of direct oral anticoagulants and different intensities of coumarins, but no such trials were identified. Additionally, placebo and no intervention were assessed as separate nodes in the network. The remaining interventions were assessed on a class level, regardless of dose. In two small randomised controlled trials where weight adjusted doses were used, we classified the trial according to the dose that was judged to be used most frequently.75 85
Table 1.
Classification of low and intermediate dose low-molecular-weight heparin and unfractionated heparin. Classification is based on the total daily dose regardless of dosing frequency. IU=international units
| Intervention and type | Classification | Dose applied by included randomised controlled trials | |
|---|---|---|---|
| Low dose | Intermediate dose | ||
| Low-molecular-weight heparin: | |||
| Nadroparin | <5700 IU | ≥5700 IU | 2850 to 8200 IU |
| Dalteparin | <5000 IU | ≥5000 IU | 2500 to 5000 IU |
| Enoxaparin | <40 mg | ≥40 mg | 20 to 60 mg |
| Certoparin | <5000 IU | ≥5000 IU | 3000 to 6000 IU |
| Unfractionated heparin | ≤10 000 IU | >10 000 IU | 10 000 to 25 000 IU |
Outcomes
We collected data for four co-primary outcomes: all cause mortality, symptomatic venous thromboembolism, major bleeding, and serious adverse events. Symptomatic venous thromboembolism was a composite of deep vein thrombosis and pulmonary embolism. A diagnosis of deep vein thrombosis or pulmonary embolism was accepted when objectified by radiological imaging or autopsy, regardless of anatomical location. If only deep vein thrombosis or pulmonary embolism was reported, we used that value for the venous thromboembolism estimate. In randomised controlled trials that screened for deep vein thrombosis but did not specify which events were symptomatic, the deep vein thrombosis count was excluded from the venous thromboembolism outcome. Major bleeding and serious adverse events were defined according to the criteria used in the individual trials. The timing of all outcome assessments was 90 days or any reported timing closest to 90 days.
Data collection
Two authors (RE and TE) independently screened titles, abstracts, and full texts, where applicable, using the web based application Rayyan.16 Data extraction was then conducted independently by the same authors using a structured form. We extracted information about study characteristics (publication year, country, participating sites, and number of participants enroled); participant characteristics (age, sex, and other baseline characteristics); intervention characteristics (dose and duration of thrombosis prophylaxis, venous thromboembolism screening methods, and co-interventions); potential effect modifiers (defined in the statistical analysis); and outcomes. We extracted arm based data from the trial reports and constructed two by two tables for each trial to calculate unadjusted odds ratios. For the IST 1997 trial, we used publicly available individual patient data to merge the original six groups into three, with aspirin as an equally balanced co-intervention.17 No trials with cluster or crossover designs were found. If we had doubt as to whether two trial reports shared the same participants, we contacted the authors of the trials to clarify whether the trial report had been duplicated. We also sought contact with the authors in case of missing outcome data for a particular trial. Any differences in judgment regarding trial inclusion or data collection were resolved through discussion with a third and fourth author (FK and KM).
Risk of bias
Two authors (RE and TE) independently assessed the risk of bias of included trials, using the Cochrane risk-of-bias tool (RoB 2), in the following five domains: bias arising from the randomisation process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and finally bias in selection of the reported result.18 Only trials at low risk of bias in all five domains were classified at overall low risk of bias, whereas trials with one or more domains assessed as some concerns or high risk were classified at high risk of bias. We did a separate risk-of-bias assessment for each outcome of each trial because some domains could be judged differently depending on the outcome assessed. We did not construct funnel plots adjusted by comparison for detecting reporting bias because of the low number of trials (<10) that directly informed each comparison. We used the Confidence in Network Meta-Analysis (known as CINeMA) framework and web application to rate the quality of the treatment effect estimates in comparison with placebo.19 20 This framework distinguishes six domains (bias within studies, reporting bias, indirectness, imprecision, heterogeneity, and incoherence). For the assessment, we used the average risk of bias for judging bias within studies and indirectness of each comparison. The reporting bias domain was not assessed, as discussed previously. We defined a 15% relative risk reduction or increase as a clinically relevant effect size for judging the imprecision, heterogeneity, and incoherence domains.
Statistical analysis
Assessment of transitivity
Transitivity is the key underlying assumption of a network meta-analysis. From a theoretical perspective, we assumed that patients who fulfilled the inclusion were equally eligible to be randomly assigned to any of the interventions that we compared.21 We reviewed this assumption before conducting any analyses, exploring the dataset of eligible studies for differences in a priori specified effect modifiers: average patient age, sex, severity of illness, duration of intervention, length of follow-up, and risk of bias. In case the distribution of effect modifiers was judged to be sufficiently comparable, studies were eligible for data synthesis.
Pairwise meta-analysis and trial sequential analysis
We conducted conventional, random effects, pairwise meta-analyses of all the comparisons that included placebo for each outcome. To assess the risk of random error, we used trial sequential analysis to evaluate the direct comparisons with placebo. This analysis is a sequential meta-analysis method that combines required information size estimation (ie, the number of participants needed to detect an a priori specified relative risk reduction with an adjusted threshold for statistical significance).22 23 We did the trial sequential analysis using the control event proportion from the actual meta-analyses, an α of 2%, a β of 10%, an anticipated relative risk reduction of 15%, and the diversity (D2) suggested in the trial results in the meta-analysis. We present results as odds ratio with 95% confidence interval and odds ratio with trial sequential analysis-adjusted confidence intervals.
Network meta-analysis
We generated network diagrams for each outcome to visually inspect which interventions had been compared within the randomised controlled trials (direct comparisons). We then performed random effects network meta-analyses of all outcomes in a bayesian framework.24 25 Trials with zero events in both groups were omitted. Multiarm trials were included, accounting for the inherent correlation between their treatment arms. We set a vague prior distribution for all means and assumed a common variance between trials (τ) with a uniform prior distribution for all comparisons (supplementary table 3). To interpret the level of heterogeneity, we calculated prediction intervals, as these intervals could reflect the variation in treatment effects over different settings, including what effect is to be expected in patients in the future.26 We assessed statistical heterogeneity based on the magnitude of τ2 and the width of the prediction intervals. All analyses were run using four Markov chains with 250 000 iterations after an initial burn-in of 50 000 and a thinning of 10. Convergence was monitored using trace plots and Brooks-Gelman-Rubin statistics (supplementary figure 1-4).
We used node splitting models to assess local inconsistency and obtain indirect effect estimates. Because tests for inconsistency can be underpowered, we also visually inspected direct and indirect effect estimates for obvious differences. The initial node splitting analysis of the venous thromboembolism outcome was hampered by numerical instability due to sparse events. This issue was solved by a continuity adjustment, adding 0.5 to all cells in the two by two table of randomised controlled trials with events in at least one group but zero events in another group. No continuity correction was applied in the main models or in the other node splitting models.
We assessed the relative probability of an intervention being among the best options by calculating mean treatment rankings and surface under the cumulative ranking scores. We presented results from the network meta-analysis as odds ratio with 95% credible intervals; as ranking curves that display the cumulative probability that a given intervention ranks first, second, or third, etc, among all tested interventions for reducing (or increasing) the outcome of interest; and as posterior probabilities of benefit or harm for the highest ranked interventions. We used trial sequential analysis software, version 0.9.5.10; MetaInsight, version 1.1; and R software, version 4.0.4 (gemtc, RJAGS, BUGSnet, and meta packages) for all analyses.27 28 29 30 31
Subgroup and sensitivity analyses
We conducted several subgroup analyses according to the risk of bias (low compared with any risk), risk of vested interests (low compared with any risk), patient population (three analyses respectively excluding participants with stroke, critical illness, or acute medical illness), and publication year (split by median). We conducted five sensitivity network meta-analyses: including all identified studies irrespective of the distribution of effect modifiers or the intervention used; including all anticoagulant interventions on a group level irrespective of the dose; limiting follow-up to 30 days; using two alternative priors for the variance between trials; and including placebo and no treatment as one common control intervention in the network. All subgroup and sensitivity analyses were either preplanned or conducted per reviewer requests.
Deviations from protocol
In contrast with our protocol, we decided to use odds ratios instead of relative risks given their independence of the outcome incidence.32 In addition to the search strategy specified in the protocol, we searched for patients who have had a stroke because they were eligible for inclusion. We did not perform sensitivity analyses of the serious adverse events outcome to assess the impact of attrition bias or underreporting because this process was unfeasible in the network meta-analytical context of indirect comparisons. We limited the trial sequential analysis to the direct comparisons with placebo and did not conduct sensitivity analyses with more strict conditions because none of the main trial sequential analysis results was conclusive. We did not conduct a subgroup analysis according to intervention duration because, in almost all randomised controlled trials, the participants were treated in fewer than 30 days.
Patient and public involvement
No patients or members of the public were directly involved in this study as no primary data were collected. During clinical routine, several of the authors have daily (or regular) contacts with acutely ill or critically ill patients where benefits and harms of thrombosis prophylaxis are discussed. The experiences from these interactions have been taken into consideration during the planning, conduct, and reporting of this systematic review.
Results
Search results
After screening and selection, we identified 78 randomised controlled trials that were eligible for inclusion in the systematic review (supplementary figure 5). Data from two trials were published in registries, and we received additional data for one trial.33 34 35 Eighteen trials were excluded from analyses because they either compared interventions that we had grouped in the same treatment node; compared interventions that we could not classify; or compared a mix of interventions (supplementary table 4).34 36 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52
Transitivity
After review of collected data but before analyses, we decided to exclude 10 randomised controlled trials from the main analysis, judging that differences in some of the predefined effect modifiers (baseline disease status, median participant age, and mean treatment duration) would threaten transitivity of the network.35 53 54 55 56 57 58 59 60 61 62 Nevertheless, we noted evidence of inconsistency in the node-splitting models of the venous thromboembolism and bleeding outcome. We found that six additional trials on interventions that are generally not used anymore in daily practice (platelet inhibitors, heparinoids, and coumarins) increased statistical inconsistency without contributing significant information to the network.63 64 65 66 67 68 Therefore we excluded these trials from the main analysis. We also excluded two randomised controlled trials from the venous thromboembolism outcome analysis (but not from the other outcomes) because of unusually high pulmonary embolism rates.69 70 Afterwards, node splitting still showed statistical inconsistency in the bleeding outcome, but this appeared to be driven by sparse events and sampling variation (supplementary figure 6-9). All 16 randomised controlled trials that were excluded from the main analysis to preserve transitivity are displayed in supplementary table 5. In sensitivity analysis 1, we included these trials to assess the effect of the previously mentioned considerations on the results (supplementary table 9).
Characteristics of included trials
Forty four trials that included 90 095 participants were included in the main analysis (supplementary table 6).14 17 33 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 104 105 106 107 108 109 Participants were admitted to hospital for critical illness (seven trials, n=7330), acute medical illness (23 trials, n=59 428), ischaemic stroke (11 trials, n=23 013), or myocardial infarction (three trials, n=324). A total of 5612 (6.2%) participants had received low dose low-molecular-weight heparin, 22 482 (25.0%) intermediate dose low-molecular-weight heparin, 15 235 (16.9%) low dose unfractionated heparin, 9240 (10.3%) intermediate dose unfractionated heparin, 429 (0.5%) pentasaccharides, 11 064 (12.3%) direct oral anticoagulants, 10 095 (11.2%) placebo, and 15 938 (17.7%) no intervention. The publication year ranged from 1973 to 2016. The median reported age across trials was 68.5 years (interquartile range 65.5-74.7), the median percentage of women was 46.5% (40-55%), and the median (planned or observed) duration of intervention was 10 days (7-14). The included randomised controlled trials reported 118 outcomes that were assessed for risk of bias: 70 (59%) outcomes were at low risk of bias in the randomisation process; 67 (57%) at low risk of deviations from intended interventions; 84 (71%) at low risk of missing outcome data, 93 (79%) at low risk of bias in measurement of the outcome; and 87 (74%) at low risk of bias in selection of the reported results. The overall risk of bias was low in 13 (36%) of 36 trials assessing mortality, in nine (28%) of 32 trials assessing venous thromboembolism, in 16 (55%) of 29 trials assessing major bleeding, and in three (38%) of eight trials assessing serious adverse events (supplementary table 7). A summary of bias within studies, per comparison, is displayed in supplementary figures 10-13. In addition, 33 (75%) of 44 trials were at unclear or high risk of vested interests (supplementary table 7).
All cause mortality
Thirty six randomised controlled trials including 70 404 participants reported 5608 (8.0%) deaths (fig 1). Low to moderate quality evidence suggested none of the interventions was associated with a difference in all cause mortality compared with placebo (fig 2; supplementary table 8). The amount of statistical heterogeneity was low (τ2 median 0.003; 95% credible intervals 0.00 to 0.05) and prediction intervals were similar (supplementary table 12). By contrast, when compared with no intervention, all cause mortality was reduced by intermediate dose low-molecular-weight heparin (odds ratio 0.79; 95% credible intervals 0.61 to 0.96) and pentasaccharides (0.44; 0.20 to 0.92; fig 2, fig 3). Pentasaccharides and intermediate dose low-molecular-weight heparin ranked highest for reduction of mortality (fig 4 ; supplementary table 13).Trial sequential analysis showed risk of random errors beyond the chosen maximum type 1 and 2 errors in the comparison of low dose low-molecular-weight heparin versus placebo, whereas intermediate dose low-molecular-weight heparin crossed the boundary of futility (supplementary table 8).
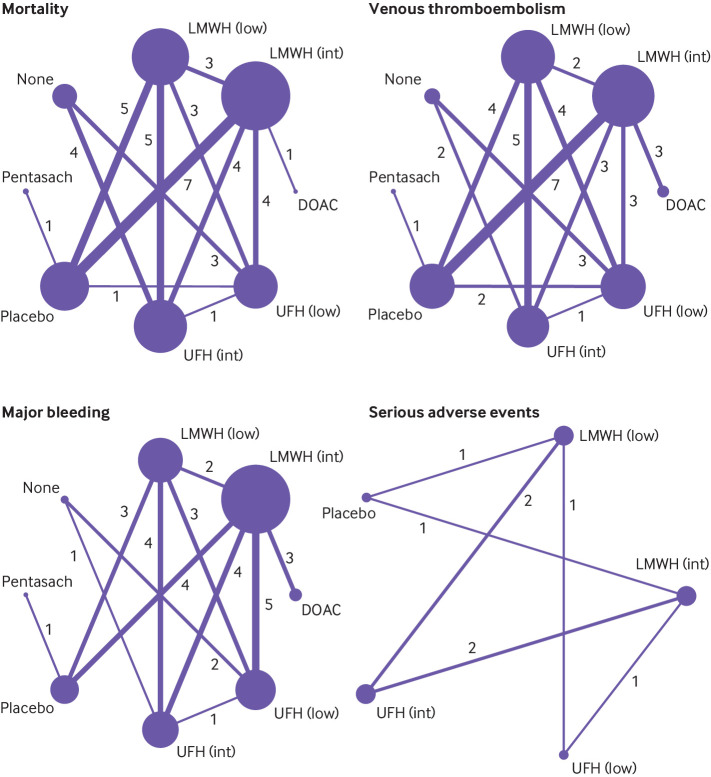
Fig 1.
Network geometry for each outcome. Network plot is the overview of direct comparisons between interventions. Each circle represents an intervention and is referred to as node. Node size correlates with the number of studies that included the intervention. Lines or edges between nodes represent direct comparisons, and their thickness is proportional to the number of trials contributing to each comparison. The number of trials is also included on the edges. DOAC=direct oral anticoagulant; Int=intermediate dose; low=low dose; LMWH=low-molecular-weight heparin; Pentasach=pentasaccharides; UFH=unfractionated heparin
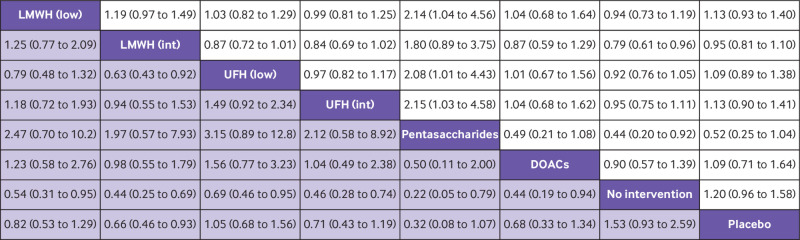
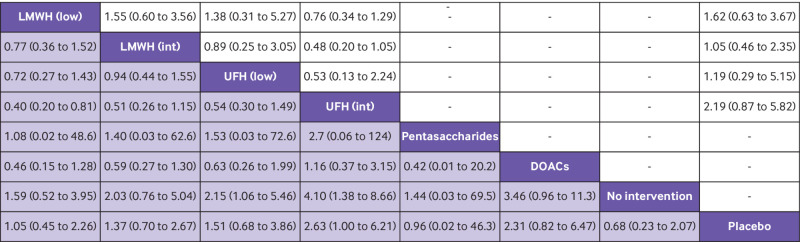
Fig 2.
Head-to-head comparisons of interventions for risk of mortality (upper white fields) and venous thromboembolism (lower shaded fields). Data are odds ratio with 95% credible interval. Figure should be read from left to right: for comparisons of the mortality outcome (upper white fields) odds ratios <1 favour the row defining treatment, whereas for venous thromboembolism (lower shaded fields), odds ratios <1 favour the column defining treatment. For example, LMWH intermediate dose conclusively reduced venous thromboembolism compared with no intervention (odds ratio 0.44; 95% credible interval 0.25 to 0.69), placebo (0.66; 0.46 to 0.93), and UFH low dose (0.63; 0.43 to 0.92), but not to other anticoagulants. To obtain odds ratios for comparisons in the opposite direction, reciprocals should be taken. DOAC=direct oral anticoagulant; int=intermediate dose; LMWH=low-molecular-weight heparin; low=low dose; UFH=unfractionated heparin
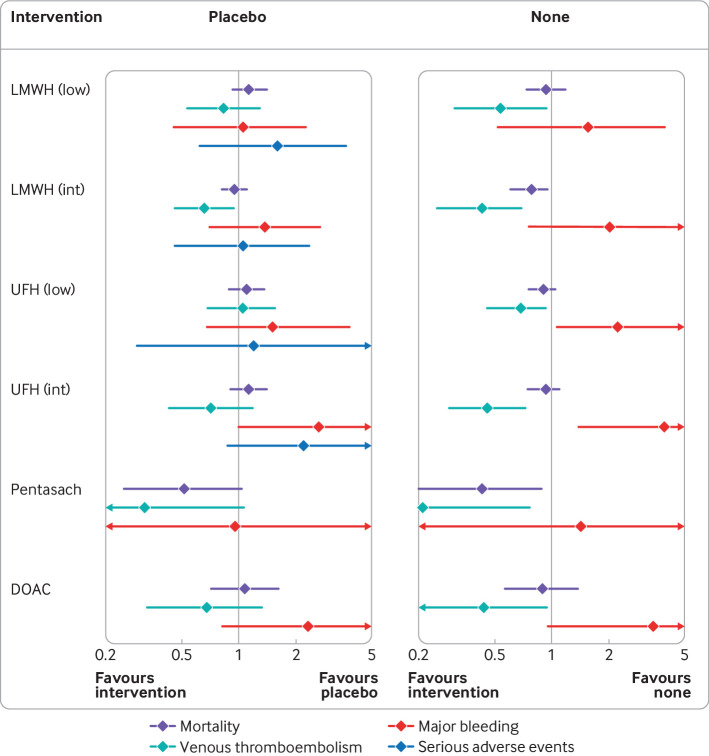
Fig 3.
Effect estimates of anticoagulants versus placebo or no intervention. Forest plots show effect estimates of each anticoagulant compared with placebo and with no intervention on all four outcomes. Results <1.0 favour the anticoagulant and results >1.0 favour placebo or no intervention. DOAC=direct oral anticoagulant; int=intermediate dose; low=low dose; LMWH=low-molecular-weight heparin; Pentasach=pentasaccharides; UFH=unfractionated heparin
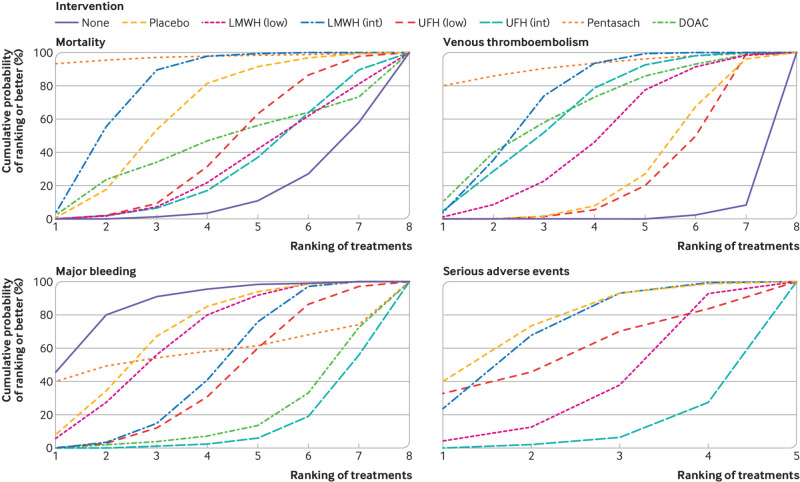
Fig 4.
Cumulative ranking curves for all four outcomes. Graphs show the cumulative probability of each intervention ranking, from best (rank 1) to worst (rank 8 or 5 depending on the number of treatments) for each outcome. A rank indicates the probability that an intervention is best, second best, etc. For example, pentasaccharides and intermediate dose LMWH ranked best for preventing venous thromboembolism, whereas no intervention ranked worst. DOAC=direct oral anticoagulant; int=intermediate dose; low=low dose; LMWH=low-molecular-weight heparin; Pentasach=pentasaccharides; UFH=unfractionated heparin. An interactive version of this graphic is available at: https://bit.ly/3nmPgNs
Symptomatic venous thromboembolism
Thirty two randomised controlled trials including 84 903 participants reported 749 (0.9%) people with symptomatic venous thromboembolism (fig 1). The most likely treatments to reduce symptomatic venous thromboembolism compared with placebo were pentasaccharides (odds ratio 0.32, 95% credible interval 0.08 to 1.07; posterior probability of benefit 97%), intermediate dose low-molecular-weight heparin (0.66, 0.46 to 0.93; 99%), direct oral anticoagulants (0.68, 0.33 to 1.34; 88%), and intermediate dose unfractionated heparin (0.71, 0.43 to 1.19; 91%), although the only conclusive estimate was of intermediate dose low-molecular-weight heparin (very low to low quality evidence; fig 2, fig 3, fig 4; supplementary tables 8 and 13). The amount of statistical heterogeneity was low (τ2 median 0.011; 95% credible interval 0.00 to 0.18) and prediction intervals were mostly compatible with benefit (supplementary table 12). When compared with no intervention, all interventions were associated with a reduced risk of venous thromboembolism (fig 2, fig 3). A dose-response effect was noted for both low-molecular-weight heparin and unfractionated heparin (ie, the higher the dose, the larger the effect), but with an overlap in credible intervals (fig 3). The trial sequential analysis showed risk of random errors beyond the chosen maximum type 1 and 2 errors in all direct comparisons with placebo (supplementary table 8).
Major bleeding
Twenty nine randomised controlled trials including 84 483 participants reported 793 (0.9%) major bleeding events (fig 1). Intermediate dose unfractionated heparin (odds ratio 2.63; 95% credible interval 1.00 to 6.21) and direct oral anticoagulants (2.31; 0.82 to 6.47) were most likely to increase major bleeding compared with placebo, with posterior probabilities of a harmful effect of 98% and 95%, respectively (low to moderate quality evidence; fig 3, fig 4, fig 5; supplementary tables 8 and 13). Statistical heterogeneity was high (τ2 median 0.16; 95% credible interval 0.00 to 0.96) and prediction intervals included potential for both major benefit and harm (supplementary table 12). When compared with no intervention, estimates of unfractionated heparin in any dose, direct oral anticoagulants, and intermediate dose low-molecular-weight heparin were mostly compatible with increased risk of major bleeding (fig 3, fig 5). A dose-response effect was noted for both low-molecular-weight heparin and unfractionated heparin, but with an overlap in credible intervals (fig 3). The trial sequential analysis showed risk of random errors beyond the chosen maximum type 1 and 2 errors in all direct comparisons with placebo (supplementary table 8).
Fig 5.
Head-to-head comparisons of interventions for risk of serious adverse events (upper white fields) and major bleeding (lower shaded fields). Data are odds ratio with 95% credible interval. Figure should be read from left to right: for comparisons of the serious adverse event outcome (upper white fields) odds ratios <1 favour the row defining treatment, whereas for major bleeding (lower shaded fields), odds ratios <1 favour the column defining treatment. For example, UFH low dose conclusively increased risk of major bleeding compared with no intervention (odds ratio 2.15; 95% credible interval 1.06 to 5.46), but not to placebo (1.51; 0.68 to 3.86) or other anticoagulants. To obtain odds ratio for comparisons in the opposite direction, reciprocals should be taken. DOAC=direct oral anticoagulant; int=intermediate dose; LMWH=low-molecular-weight heparin; low=low dose; UFH=unfractionated heparin
Serious adverse events
Eight randomised controlled trials including 17 002 participants reported 1039 (6.1%) serious adverse events (fig 1). All estimates suffered from imprecision, but the effect estimate of intermediate dose unfractionated heparin was mostly compatible with an increased risk of serious adverse events (96% posterior probability of a harmful effect; odds ratio 2.19; 95% credible interval 0.87 to 5.82; very low quality evidence; fig 3, fig 4, fig 5; supplementary table 8). Statistical heterogeneity was moderate (τ2 median 0.09; 95% credible interval 0.00 to 0.90) and prediction intervals included potential for both major benefit and harm (supplementary table 12). The trial sequential analysis showed risk of random errors beyond the chosen maximum type 1 and 2 errors in all direct comparisons with placebo (supplementary table 8).
Sensitivity and subgroup analyses
The discrepancy between intervention effects of placebo and no intervention regarding the risk of major bleeding was partly attenuated by excluding patients who have had a stroke (subgroup analysis 4.1, supplementary table 10). All other sensitivity and subgroup analysis results were either in line with the main results or suffered from imprecision (supplementary tables 9-11).
Discussion
Principal findings
This systematic review with network meta-analysis provides a comprehensive estimation of the intervention effects of different anticoagulants for the prevention of venous thromboembolism in acutely ill patients who have been admitted to hospital. We found that none of the interventions reduced all cause mortality compared with a placebo. Pentasaccharides, intermediate dose low-molecular-weight heparin, direct oral anticoagulants, and intermediate dose unfractionated heparin were most likely to reduce symptomatic venous thromboembolism, but only the effect estimate of intermediate dose low-molecular-weight heparin was conclusive. Additionally, unfractionated heparin in any dose and direct oral anticoagulants were most likely to increase the risk of major bleeding. We observed a systematic discrepancy in intervention effects depending on whether placebo or no intervention was the reference treatment. When compared with no intervention instead of placebo, all active interventions performed more favourable with regard to risk of venous thromboembolism and mortality, and less favourable with regard to risk of major bleeding. Overall, low-molecular-weight heparin in an intermediate dose appears to confer the best balance of benefits and harms for venous thromboembolism prevention. Although pentasaccharides seemed at least as effective and safe in ranking curves, their relative benefit over other anticoagulants should be interpreted with caution because these estimates were based on a single randomised clinical trial, possibly underestimating the risk of bleeding.91 110 Unfractionated heparin, in particular the intermediate dose, and direct oral anticoagulants had the least favourable profiles. The quality of evidence within comparisons was generally low to moderate, mainly due to bias within trials and imprecision . The intervention effect estimates were robust across several preplanned sensitivity and subgroup analyses.
Strengths and limitations of this study
Our systematic review has several strengths. We used a prespecified protocol and were transparent in reporting any deviations. Our search was comprehensive and included regulatory agency databases and clinical trial registries, which allowed us to include some previously unpublished data. We conducted a duplicate independent review of trial eligibility, data extraction, and risk of bias. Head-to-head comparisons of different prophylactic low-molecular-weight heparin doses were very few, and conventional meta-analyses generally did not stratify according to dose. Our network meta-analytical approach provides the first estimation of different low-molecular-weight heparin dose intervention effects in patients who are acutely ill.
We should acknowledge several limitations as well. Most importantly, transitivity is key to the validity of network meta-analysis: it assumes that the randomised clinical trials that inform different comparisons do not differ in effect modifiers, such as participant age or publication year.21 111 Despite a priori efforts to address this assumption, we did encounter statistical inconsistency in the initial node-splitting models. To solve this inconsistency, we used a systematic approach that included re-assessment of extracted data, exclusion of obsolete interventions, specific assessment of randomised controlled trials with high residual deviance, and exclusion of two randomised controlled trials with aberrant venous thromboembolism data. This process mostly eliminated statistical inconsistency, but we could also have merely lowered the power to detect the inconsistency. Some of these decisions were inevitably made on a post hoc basis. We assessed the influence of our approach under different conditions in a variety of prespecified sensitivity and subgroup analyses and found that estimates were generally robust. This suggests that the influence of (our post hoc) decisions on the results was probably limited.
Secondly, we grouped several patient categories with varying baseline risks. Patient type might be an important proxy for effect modification (ie, patients with stroke are generally older than are trauma patients), but where to set a limit with respect to eligibility remains a challenge. For example, the latest guidelines combined patients with stroke and acute medical illness but evaluated patients who are critically ill as a separate category, whereas previous meta-analyses focused only on single patient categories.3 112 113 Nevertheless, our subgroup analyses according to population types were generally in line with our main analysis. Thirdly, not all randomised controlled trials reported whether venous thromboembolism were symptomatic, and definitions of major bleeding were quite heterogeneous across trials, necessitating some judgment in data extraction.
Furthermore, many randomised controlled trials screened venous thromboembolism and initiated anticoagulant treatment after detection of an event, which deviates from the natural course of disease and makes assessment as to whether, and which, events would have become symptomatic or even deadly impossible. Therefore, the absolute risk of symptomatic venous thromboembolism in our research is an underestimation and we cannot exclude that screening influenced the intervention effect on mortality as well. However, this limitation applies to all meta-analyses in this field that focus on symptomatic events. Additionally, although we undertook efforts to substantiate the dose classification of unfractionated heparin and low-molecular-weight heparin based on summary of product characteristics and trial dosing regimens, such a distinction ultimately remains arbitrary. Trials also had different lengths of follow-up and the summary odds ratios represent an average over different time points; however, this limitation is for all meta-analyses, and results were similar in a sensitivity analysis restricting follow-up to 30 days.
Finally, partitioning interventions according to dose and distinguishing between placebo and no intervention decreased the number of trials that directly informed each comparison. This limited our power to detect smaller effect estimates, and the absence of a conclusive estimate of benefit or harm over placebo should not be interpreted as true absence of an intervention effect. The trial sequential analysis results of the direct comparisons further support this statement by suggesting risks of random errors.
Implications
Our results support the National Institute for Health and Care Excellence and the American Society of Hematology guidelines on thrombosis prophylaxis in their recommendations on the use of low-molecular-weight heparins or pentasaccharides.3 4 Our conclusion to prefer low-molecular-weight heparin over unfractionated heparin is in line with a previous network meta-analysis that focused on patients who are critically ill.114 Additionally, our analyses contribute new information on the low-molecular-weight heparin dose by showing that an intermediate dose is probably preferable over a low dose for prophylaxis. Current guidelines do not provide any dose recommendations, even though no unambiguous definition of standard dose prophylaxis is available. Recent randomised controlled trials in patients with covid-19 compared higher doses of low-molecular-weight heparin with various standard prophylactic regimens that included doses we defined as both low and intermediate.38 39 40 42 115
Importantly, our definition of an intermediate dose is lower than the one used by the INSPIRATION trial.42 Compared with another network meta-analysis on prophylaxis with different low-molecular-weight heparin doses and direct oral anticoagulants in surgical patients, the directions of the effect estimates for reducing venous thromboembolism seem similar, while the effect sizes appear smaller in acutely ill patients.12 Additionally, the estimates of bleeding risks were similar for direct oral anticoagulants but differed for low-molecular-weight heparins.12 These differences can be explained by clinical and methodological factors such as patient population, quality of the evidence base, and dose cut-off points for low-molecular-weight heparin. No similar analysis has focused on the dose-response relation of low-molecular-weight heparins in the low to intermediate dose range in acutely ill patients who have been admitted to hospital. Previous pairwise meta-analyses could not answer this question because of the small number of direct comparisons between different low-molecular-weight heparins.112 113 Judging from the summary of product characteristics, the low-molecular-weight heparin dose range that we defined as intermediate is most commonly used in the United States (eg, enoxaparin 40-60 mg), whereas in Europe, Canada, and Israel, the low dose (eg, enoxaparin 20 mg) appears more often registered.5 6 7 8 9 116
The systematic difference in intervention effects according to the use of placebo or no intervention as reference treatment was unanticipated. This difference is best illustrated by examining the indirect comparisons between placebo and no intervention that favoured placebo with respect to mortality and venous thromboembolism, and no intervention with respect to bleeding risk. Of course, both interventions are biologically inactive, and therefore, this favourability is driven by other effects. We consider three potential explanations, involving sampling variation, bias in our network meta-analysis, or bias in the underlying evidence base. Firstly, given the wide credible intervals, the finding could be by chance, based on sampling variation. Secondly, some comparisons could have been associated with specific patient populations or periods of time.21 117 The network geometry illustrates that most randomised controlled trials either compared unfractionated heparin versus no intervention (older trials), low-molecular-weight heparin versus placebo (newer trials), or low-molecular-weight heparin versus unfractionated heparin (newer trials). In subgroup analysis 4.1, we removed patients with stroke and found that the difference between placebo and no intervention regarding major bleeding risk was partly attenuated, suggesting clinical characteristics accounted for the observed difference. However, we found no such explanation for venous thromboembolism or all cause mortality.
Thirdly, the difference could reflect performance bias caused by a propensity towards imbalanced detection of outcomes in unblinded randomised controlled trials. This bias is not implausible, given that a previous meta-epidemiological study estimated lack of blinding was associated with an average of 13% exaggeration of interventions effects.118 Another recent network meta-analysis on anticoagulation in surgical patients encountered an even larger difference between the placebo and no intervention groups.12 Not only semi-objective outcomes, such as venous thromboembolism, but also objective outcomes, such as mortality, can be affected by lack of blinding, due to various mechanisms such as imbalanced use of co-interventions or selective outcome assessment.118 Our findings require further exploration, and future network meta-analyses should consider to assess no intervention and placebo as distinct treatments. We have emphasised comparisons with a placebo as reference treatment throughout our review, as these were mainly informed by direct evidence from more recent, blinded randomised controlled trials at lower risks of bias.
Conclusions
Low-molecular-weight heparin in an intermediate dose appears to confer the best balance between benefits and harms for prevention of venous thromboembolism. Unfractionated heparin, and particularly the intermediate dose, and direct oral anticoagulants had the least favourable profile. A systematic discrepancy was noted in intervention effects that depended on whether a placebo or no intervention was the reference treatment. The quality of evidence was generally low to moderate due to imprecision and within-trial bias.
What is already known on this topic
The optimal anticoagulant type and dose for the prevention of venous thromboembolism in acutely ill adults in hospital is unknown
Previous pairwise meta-analyses have had a limited number of direct comparisons between different anticoagulant drugs and doses
What this study adds
Low-molecular-weight heparin in an intermediate dose might confer the best balance between benefits and harms for prevention of venous thromboembolism; other anticoagulant types and doses had smaller thromboembolism reductions or an increased bleeding risk
A systematic discrepancy was noted in intervention effects that depended on whether placebo or no intervention was the reference treatment; future network meta-analyses should consider separating placebo and no intervention arms
The main limitations of this study were the quality of the evidence, which was generally low to moderate, and statistical inconsistency, which was dealt with post hoc
Acknowledgments
This study is supported by the National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands and the NIHR Complex Reviews Support Unit. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. We thank medical information specialist Sjoukje van der Werf for her expert advice and assistance with the study search strategy.
Web extra.
Extra material supplied by authors
Web appendix: Online appendix
Contributors: RE, AS, JW, CG, IvdH, RG, KM, and FK conceived and designed the study. RE and TE collected, screened, and extracted the data. RE, TE, FK, and AS analysed the data and conducted the statistical analyses. All authors critically revised the manuscript for important intellectual content. RE and FK had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis. FK is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: RE is supported by a personal grant from the Groninger AGIKO programme, funded by the University Medical Center Groningen. The funder was not involved in the design, conduct, or report of this project.
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: RE declares a personal grant from the Groninger AGIKO programme, funded by the University Medical Center Groningen, no support from any organisation for the submitted work; AJS has been a paid consultant by Janssen-Cilag and GlaxoSmithKline. KM reports consulting fees for discussion of gene therapy study results in haemophilia B (fees paid to institution); speaker fees for presentations on haemophilia treatment and DOAC antidotes (fees paid to institution); steering committee for trial of factor VIII concentrate for haemophilia for Bayer; data safety and monitoring board for trial of prothrombin complex concentrate for Octapharma (fees paid to institution); the other authors declare no competing interests.
FK (the guarantor) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as originally planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: We will share the results within our professional societies and networks, to inform guideline committees. We will disseminate our work through our own social media and the University of Groningen and University Medical Centre Groningen channels. Moreover, we will disseminate our work through the social media of all coauthors in the UK and Denmark. We will construct a plain language summary for publication on the relevant websites and for wider dissemination where possible.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
Not required.
Data availability statement
Statistical code and the datasets are available from the corresponding author (r.j.eck@umcg.nl) and will be published online.
References
- 1. Heit JA. Epidemiology of venous thromboembolism. Nat Rev Cardiol 2015;12:464-74. 10.1038/nrcardio.2015.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Heit JA, O’Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med 2002;162:1245-8. 10.1001/archinte.162.11.1245 [DOI] [PubMed] [Google Scholar]
- 3. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized medical patients. Blood Adv 2018;2:3198-225. 10.1182/bloodadvances.2018022954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Institute for Health and Care Excellence. Venous thromboembolism in over 16s: reducing the risk of hospital-acquired deep vein thrombosis or pulmonary embolism. Published Online First: 2018.https://www.nice.org.uk/guidance/ng89 [PubMed]
- 5.Food and Drug Administration (United States). Drug approval reports. www.accessdata.fda.gov/scripts/cder/daf/ (accessed 7 Feb 2019).
- 6.Medicines Evaluation Board (The Netherlands). Summary of product characteristics. www.geneesmiddeleninformatiebank.nl/nl/ (accessed 7 Feb 2019).
- 7.Medicines and Healthcare products Regulatory Agency. United Kingdom. Summary of product characteristics. http://www.mhra.gov.uk/spc-pil/ (accessed 31 Jul 2017).
- 8.The Israeli Drug Registry (Israel). Summary of product characteristics. https://data.health.gov.il/drugs/index.html#/byDrug (accessed 7 Feb 2019).
- 9.Health Canada (Canada). Summary of Product Characteristics. https://health-products.canada.ca/dpd-bdpp/info.do?lang=en&code=60205 (accessed 7 Feb 2019).
- 10. Eck RJ, Bult W, Wetterslev J, et al. Low dose low-molecular-weight heparin for thrombosis prophylaxis: systematic review with meta-analysis and trial sequential analysis. J Clin Med 2019;8:E2039. 10.3390/jcm8122039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eck RJ, Bult W, Wetterslev J, et al. Intermediate dose low-molecular-weight heparin for thrombosis prophylaxis: systematic review with meta-analysis and trial sequential analysis. Semin Thromb Hemost 2019;45:810-24. 10.1055/s-0039-1696965 [DOI] [PubMed] [Google Scholar]
- 12. Marcucci M, Etxeandia-Ikobaltzeta I, Yang S, et al. Benefits and harms of direct oral anticoagulation and low molecular weight heparin for thromboprophylaxis in patients undergoing non-cardiac surgery: systematic review and network meta-analysis of randomised trials. BMJ 2022;376:e066785. 10.1136/bmj-2021-066785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flumignan RLG, Civile VT, Tinôco JDS, et al. Prophylactic anticoagulants for people hospitalised with COVID-19. Cochrane Database Syst Rev 2022;CD013739. 10.1002/14651858.CD013739.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samama MM, Cohen AT, Darmon J-Y, et al. Prophylaxis in Medical Patients with Enoxaparin Study Group . A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. N Engl J Med 1999;341:793-800. 10.1056/NEJM199909093411103 [DOI] [PubMed] [Google Scholar]
- 15. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777-84. 10.7326/M14-2385 [DOI] [PubMed] [Google Scholar]
- 16. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. International Stroke Trial Collaborative Group . The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet 1997;349:1569-81. 10.1016/S0140-6736(97)04011-7 [DOI] [PubMed] [Google Scholar]
- 18. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19. Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med 2020;17:e1003082. 10.1371/journal.pmed.1003082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papakonstantinou T, Nikolakopoulou A, Higgins JPT, Egger M, Salanti G. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev 2020;16:1-15. 10.1002/cl2.1080 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Chapter 11: Undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. www.training.cochrane.org/handbook
- 22. Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta-analysis. Control Clin Trials 1997;18:580-93, discussion 661-6. 10.1016/S0197-2456(97)00051-2 [DOI] [PubMed] [Google Scholar]
- 23. Pogue J, Yusuf S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet 1998;351:47-52. 10.1016/S0140-6736(97)08461-4 [DOI] [PubMed] [Google Scholar]
- 24.Dias S, Welton NJ, Sutton AJ, Ades A. NICE Decision Support Unit. Evidence synthesis technical support document series. http://nicedsu.org.uk/technical-support-documents/evidence-synthesis-tsd-series/
- 25. Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Network Meta-Analysis for Decision-Making. Wiley, 2018. 10.1002/9781118951651 [DOI] [Google Scholar]
- 26. IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6:e010247. 10.1136/bmjopen-2015-010247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Owen RK, Bradbury N, Xin Y, Cooper N, Sutton A. MetaInsight: An interactive web-based tool for analyzing, interrogating, and visualizing network meta-analyses using R-shiny and netmeta. Res Synth Methods 2019;10:569-81. 10.1002/jrsm.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorlund K, Engstrøm J, Wetterslev J, et al. Software for trial sequential analysis (TSA) version 0.9.5.10 Beta. Copenhagen Trial Unit, Centre for Clinical Intervention Research. www.ctu.dk/tsa
- 29. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods 2012;3:285-99. 10.1002/jrsm.1054 [DOI] [PubMed] [Google Scholar]
- 30. Béliveau A, Boyne DJ, Slater J, Brenner D, Arora P. BUGSnet: an R package to facilitate the conduct and reporting of bayesian network meta-analyses. BMC Med Res Methodol 2019;19:196. 10.1186/s12874-019-0829-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019;22:153-60. 10.1136/ebmental-2019-300117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Doi SA, Furuya-Kanamori L, Xu C, et al. Questionable utility of the relative risk in clinical research: a call for change to practice. J Clin Epidemiol 2020. 10.1016/j.jclinepi.2020.08.019 [DOI] [PubMed] [Google Scholar]
- 33.Clinicaltrials.gov. NCT00445328. Dalteparin vs unfractionated heparin for the prevention of venous thromboembolism (vte) in hospitalized acutely ill medical patients. https://clinicaltrials.gov/show/nct00445328 2009.
- 34.Clinicaltrials.gov. NCT00714597. A multinational, multicenter, randomized, double-blind study comparing the efficacy and safety of AVE5026 with Enoxaparin for the primary prevention of venous thromboembolism in acutely ill medical patients with restricted mobility (SAVE-VEMED). https://clinicaltrials.gov/ct2/show/NCT00714597 2012.
- 35. Paciaroni M, Agnelli G, Alberti A, et al. Prevention of venous thromboembolism in hemorrhagic stroke patients - PREVENTIHS study: A randomized controlled trial and a systematic review and meta-analysis. Eur Neurol 2020;83:566-75. 10.1159/000511574 [DOI] [PubMed] [Google Scholar]
- 36. Takkuner O, Alaspaa A, Heinonen P, Huotilainen H, Hynninen M. Comparison of two low-molecular weight heparins (dalteparin and enoxaparin) in the prevention of thromboembolism in intensive care patients. Eur J Anaesthesiol 1996;13:192 10.1097/00003643-199603000-00074 . [DOI] [Google Scholar]
- 37. Lemos ACB, do Espírito Santo DA, Salvetti MC, et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: A randomized phase II clinical trial (HESACOVID). Thromb Res 2020;196:359-66. 10.1016/j.thromres.2020.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Goligher EC, Bradbury CA, McVerry BJ, et al. REMAP-CAP Investigators. ACTIV-4a Investigators. ATTACC Investigators . Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med 2021;385:777-89. 10.1056/NEJMoa2103417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lawler PR, Goligher EC, Berger JS, et al. ATTACC Investigators. ACTIV-4a Investigators. REMAP-CAP Investigators . Therapeutic anticoagulation with heparin in noncritically ill patients with covid-19. N Engl J Med 2021;385:790-802. 10.1056/NEJMoa2105911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. ACTION Coalition COVID-19 Brazil IV Investigators . Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet 2021;397:2253-63. 10.1016/S0140-6736(21)01203-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Perepu US, Chambers I, Wahab A, et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J Thromb Haemost 2021;19:2225-34. 10.1111/jth.15450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sadeghipour P, Talasaz AH, Rashidi F, et al. INSPIRATION Investigators . Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with covid-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA 2021;325:1620-30. 10.1001/jama.2021.4152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sholzberg M, Tang GH, Rahhal H, et al. RAPID trial investigators . Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ 2021;375:n2400. 10.1136/bmj.n2400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Spyropoulos AC, Goldin M, Giannis D, et al. HEP-COVID Investigators . Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with covid-19: the HEP-COVID randomized clinical trial. JAMA Intern Med 2021;181:1612-20. 10.1001/jamainternmed.2021.6203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chiou-Tan FY, Garza H, Chan KT, et al. Comparison of dalteparin and enoxaparin for deep venous thrombosis prophylaxis in patients with spinal cord injury. Am J Phys Med Rehabil 2003;82:678-85. 10.1097/01.PHM.0000083671.27501.47 [DOI] [PubMed] [Google Scholar]
- 46. Robinson S, Zincuk A, Larsen UL, et al. A comparative study of varying doses of enoxaparin for thromboprophylaxis in critically ill patients: a double-blinded, randomised controlled trial. Crit Care 2013;17:R75. 10.1186/cc12684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Abbas MS. Bemiparin versus enoxaparin in the prevention of venous thromboembolism among intensive care unit patients. Indian J Crit Care Med 2017;21:419-23. 10.4103/ijccm.IJCCM_23_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chamoun N, Ghanem H, Hachem A, et al. Evaluation of prophylactic dosages of Enoxaparin in non-surgical elderly patients with renal impairment. BMC Pharmacol Toxicol 2019;20:27. 10.1186/s40360-019-0308-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zwicker JI, Roopkumar J, Puligandla M, et al. Dose-adjusted enoxaparin thromboprophylaxis in hospitalized cancer patients: a randomized, double-blinded multicenter phase 2 trial. Blood Adv 2020;4:2254-60. 10.1182/bloodadvances.2020001804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Elias A, Milandre L, Lagrange G, et al. [Prevention of deep venous thrombosis of the leg by a very low molecular weight heparin fraction (CY 222) in patients with hemiplegia following cerebral infarction: a randomized pilot study (30 patients)]. Rev Med Interne 1990;11:95-8. 10.1016/S0248-8663(05)80622-8 [DOI] [PubMed] [Google Scholar]
- 51. Cohn SM, Moller BA, Feinstein AJ, et al. Prospective trial of low-molecular-weight heparin versus unfractionated heparin in moderately injured patients. Vasc Endovascular Surg 1999;33:219-23. 10.1177/153857449903300219. [DOI] [Google Scholar]
- 52. Li B, Wang K, Zhao X, Lin C, Sun H. Comparison of fondaparinux sodium and low molecular weight heparin in the treatment of hypercoagulability secondary to traumatic infection. Chin J Traumatol 2015;18:147-9. <jrn[REMOVED IF= FIELD]><jrn[REMOVED IF= FIELD]> 10.1016/j.cjtee.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 53. Green D, Lee MY, Lim AC, et al. Prevention of thromboembolism after spinal cord injury using low-molecular-weight heparin. Ann Intern Med 1990;113:571-4. 10.7326/0003-4819-113-8-571 [DOI] [PubMed] [Google Scholar]
- 54. Bergqvist D, Flordal PA, Friberg B, et al. Thromboprophylaxis with a low molecular weight heparin (Tinzaparin) in emergency abdominal surgery. A double-blind multicenter trial. Vasc Dis 1996;25:156-60. [PubMed] [Google Scholar]
- 55. Geerts WH, Jay RM, Code KI, et al. A comparison of low-dose heparin with low-molecular-weight heparin as prophylaxis against venous thromboembolism after major trauma. N Engl J Med 1996;335:701-7. 10.1056/NEJM199609053351003 [DOI] [PubMed] [Google Scholar]
- 56. Lohmann U, Gläser E, Braun BE, Bötel U. Thromboembolieprophylaxe bei Wirbelsäulenfrakturen mit Rückenmarkverletzungen. Standard-Heparin versus niedermolekulares Heparin bei frischen Querschnittsyndromen. Zentralbl Chir 2001;126:385-90. 10.1055/s-2001-14757 [DOI] [PubMed] [Google Scholar]
- 57. Agarwal NK, Mathur N. Deep vein thrombosis in acute spinal cord injury. Spinal Cord 2009;47:769-72. 10.1038/sc.2009.37 [DOI] [PubMed] [Google Scholar]
- 58. Halim TA, Chhabra HS, Arora M, Kumar S. Pharmacological prophylaxis for deep vein thrombosis in acute spinal cord injury: an Indian perspective. Spinal Cord 2014;52:547-50. 10.1038/sc.2014.71 [DOI] [PubMed] [Google Scholar]
- 59. Olson EJ, Bandle J, Calvo RY, et al. Heparin versus enoxaparin for prevention of venous thromboembolism after trauma: A randomized noninferiority trial. J Trauma Acute Care Surg 2015;79:961-9. 10.1097/TA.0000000000000750 [DOI] [PubMed] [Google Scholar]
- 60. Piran S, Zondag M, Bednar D, et al. Apixaban Versus Dalteparin for Thromboprophylaxis in Patients with Acute Spinal Cord Injury: A Pilot Study. Blood 2019;134:2434-2434. 10.1182/blood-2019-124601 . [DOI] [Google Scholar]
- 61. Ahuja RB, Bansal P, Pradhan GS, Subberwal M. An analysis of deep vein thrombosis in burn patients (Part 1): Comparison of D-dimer and Doppler ultrasound as screening tools. Burns 2016;42:1686-92. 10.1016/j.burns.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 62. Ahuja RB, Bansal P, Pradhan GS, Subberwal M. An analysis of deep vein thrombosis in burn patients (part II): A randomized and controlled study of thrombo-prophylaxis with low molecular weight heparin. Burns 2016;42:1693-8. 10.1016/j.burns.2016.08.007 [DOI] [PubMed] [Google Scholar]
- 63. Turpie AGG, Levine MN, Hirsh J, et al. Double-blind randomised trial of Org 10172 low-molecular-weight heparinoid in prevention of deep-vein thrombosis in thrombotic stroke. Lancet 1987;329:523-6. 10.1016/S0140-6736(87)90173-5 [DOI] [PubMed] [Google Scholar]
- 64. Turpie AGG, Gent M, Côte R, et al. A low-molecular-weight heparinoid compared with unfractionated heparin in the prevention of deep vein thrombosis in patients with acute ischemic stroke. A randomized, double-blind study. Ann Intern Med 1992;117:353-7. 10.7326/0003-4819-117-5-353 [DOI] [PubMed] [Google Scholar]
- 65. Dumas R, Woitinas F, Kutnowski M, et al. A multicentre, double-blind, randomized study to compare the safety and efficacy of once-daily ORG 10172 and twice-daily low-dose heparin in preventing deep-vein thrombosis in patients with acute ischaemic stroke. Age Ageing 1994;23:512-6. 10.1093/ageing/23.6.512 [DOI] [PubMed] [Google Scholar]
- 66. Peters SHA, Jonker JJC, de Boer AC, den Ottolander GJH. The incidence of deep venous thrombosis in patients with an acute myocardial infarction treated with acenocoumarol or indobufen. Thromb Haemost 1982;48:222-5. 10.1055/s-0038-1657261 [DOI] [PubMed] [Google Scholar]
- 67. Chen ZM, CAST (Chinese Acute Stroke Trial) Collaborative Group . CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. Lancet 1997;349:1641-9. 10.1016/S0140-6736(97)04010-5 [DOI] [PubMed] [Google Scholar]
- 68. Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet 2001;358:702-10. 10.1016/S0140-6736(01)05837-8 [DOI] [PubMed] [Google Scholar]
- 69. Kapoor M, Kupfer Y, Tessler S. Subcutaneous heparin prophylaxis significantly reduces the incidence of venous thromboembolic events in the critically ill. Crit Care Med 1999;27:A69 10.1097/00003246-199912001-00165 . [DOI] [Google Scholar]
- 70. McCarthy ST, Turner J. Low-dose subcutaneous heparin in the prevention of deep-vein thrombosis and pulmonary emboli following acute stroke. Age Ageing 1986;15:84-8. 10.1093/ageing/15.2.84 [DOI] [PubMed] [Google Scholar]
- 71. Poniewierski M, Barthels M, Poliwoda H. The safety and efficacy of a low molecular weight heparin (fragmin) in the prevention of deep vein thrombosis in medical patients: a randomized double-blind trial. Thromb Haemost 1987;58:119-119. 10.1046/j.1365-2796.1996.494834000.x. [DOI] [Google Scholar]
- 72. Dickmann U, Voth E, Schicha H, Henze T, Prange H, Emrich D. Heparin therapy, deep-vein thrombosis and pulmonary embolism after intracerebral hemorrhage. Klin Wochenschr 1988;66:1182-3. 10.1007/BF01727666 [DOI] [PubMed] [Google Scholar]
- 73. Prins MH, Gelsema R, Sing AK, van Heerde LR, den Ottolander GJ. Prophylaxis of deep venous thrombosis with a low-molecular-weight heparin (Kabi 2165/Fragmin) in stroke patients. Haemostasis 1989;19:245-50. 10.1159/000215979. [DOI] [PubMed] [Google Scholar]
- 74. Zawilska K, Psuja P, Lewandowski K, Wróz M. Low-dose heparin in the prevention of thrombotic complications following acute myocardial infarction. Cor Vasa 1989;31:179-85. [PubMed] [Google Scholar]
- 75.Aquino J, Gambier A, Ducros J. Prevention of Thromboembolic Accidents in Elderly Subjects with Frapxine. In: Fraxiaparine. 2nd International Symposium. Recent pharmacological and clinical data . 1990. 51-4. [Google Scholar]
- 76. Harenberg J, Kallenbach B, Martin U, et al. Randomized controlled study of heparin and low molecular weight heparin for prevention of deep-vein thrombosis in medical patients. Thromb Res 1990;59:639-50. 10.1016/0049-3848(90)90422-9 [DOI] [PubMed] [Google Scholar]
- 77. Sandset PM, Dahl T, Stiris M, Rostad B, Scheel B, Abildgaard U. A double-blind and randomized placebo-controlled trial of low molecular weight heparin once daily to prevent deep-vein thrombosis in acute ischemic stroke. Semin Thromb Hemost 1990;16(Suppl):25-33. [PubMed] [Google Scholar]
- 78. Forette B, Wolmark Y. Nadroparine calcique dans la prévention de la maladie thrombo-embolique chez le sujet âgé. Etude de la tolérance. Presse Med 1995;24:567-71. [PubMed] [Google Scholar]
- 79. Kay R, Wong KS, Yu YL, et al. Low-molecular-weight heparin for the treatment of acute ischemic stroke. N Engl J Med 1995;333:1588-93. 10.1056/NEJM199512143332402 [DOI] [PubMed] [Google Scholar]
- 80. Bergmann JF, Neuhart E, The Enoxaparin in Medicine Study Group . A multicenter randomized double-blind study of enoxaparin compared with unfractionated heparin in the prevention of venous thromboembolic disease in elderly in-patients bedridden for an acute medical illness. Thromb Haemost 1996;76:529-34. 10.1055/s-0038-1650617 [DOI] [PubMed] [Google Scholar]
- 81. Gårdlund B, The Heparin Prophylaxis Study Group . Randomised, controlled trial of low-dose heparin for prevention of fatal pulmonary embolism in patients with infectious diseases. Lancet 1996;347:1357-61. 10.1016/S0140-6736(96)91009-0 [DOI] [PubMed] [Google Scholar]
- 82. Harenberg J, Roebruck P, Heene DL, The Heparin Study in Internal Medicine Group . Subcutaneous low-molecular-weight heparin versus standard heparin and the prevention of thromboembolism in medical inpatients. Haemostasis 1996;26:127-39. [DOI] [PubMed] [Google Scholar]
- 83. Lechler E, Schramm W, Flosbach CW, The Prime Study Group . The venous thrombotic risk in non-surgical patients: epidemiological data and efficacy/safety profile of a low-molecular-weight heparin (enoxaparin). Haemostasis 1996;26(Suppl 2):49-56. [DOI] [PubMed] [Google Scholar]
- 84. Goldhaber S, Kett D, Cusumano C, Kuroki C. Low molecular weight heparin versus minidose unfractionated heparin for prophylaxis against venous thromboembolism in medical intensive care unit patients: a randomized controlled trial. In: J Am Coll Cardiol 2000. 325a. [Google Scholar]
- 85. Fraisse F, Holzapfel L, Couland JM, et al. The Association of Non-University Affiliated Intensive Care Specialist Physicians of France . Nadroparin in the prevention of deep vein thrombosis in acute decompensated COPD. Am J Respir Crit Care Med 2000;161:1109-14. 10.1164/ajrccm.161.4.9807025 [DOI] [PubMed] [Google Scholar]
- 86. Diener HC, Ringelstein EB, von Kummer R, et al. Therapy of Patients With Acute Stroke (TOPAS) Investigators . Treatment of acute ischemic stroke with the low-molecular-weight heparin certoparin: results of the TOPAS trial. Stroke 2001;32:22-9. 10.1161/01.STR.32.1.22 [DOI] [PubMed] [Google Scholar]
- 87. Hillbom M, Erilä T, Sotaniemi K, Tatlisumak T, Sarna S, Kaste M. Enoxaparin vs heparin for prevention of deep-vein thrombosis in acute ischaemic stroke: a randomized, double-blind study. Acta Neurol Scand 2002;106:84-92. 10.1034/j.1600-0404.2002.01215.x [DOI] [PubMed] [Google Scholar]
- 88. Kleber FX, Witt C, Vogel G, Koppenhagen K, Schomaker U, Flosbach CW, THE-PRINCE Study Group . Randomized comparison of enoxaparin with unfractionated heparin for the prevention of venous thromboembolism in medical patients with heart failure or severe respiratory disease. Am Heart J 2003;145:614-21. 10.1067/mhj.2003.189 [DOI] [PubMed] [Google Scholar]
- 89. Leizorovicz A, Cohen AT, Turpie AGGG, Olsson CG, Vaitkus PT, Goldhaber SZ, PREVENT Medical Thromboprophylaxis Study Group . Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004;110:874-9. 10.1161/01.CIR.0000138928.83266.24 [DOI] [PubMed] [Google Scholar]
- 90. Mahé I, Bergmann JF, d’Azémar P, Vaissie JJ, Caulin C. Lack of effect of a low-molecular-weight heparin (nadroparin) on mortality in bedridden medical in-patients: a prospective randomised double-blind study. Eur J Clin Pharmacol 2005;61:347-51. 10.1007/s00228-005-0944-3 [DOI] [PubMed] [Google Scholar]
- 91. Cohen AT, Davidson BL, Gallus AS, et al. ARTEMIS Investigators . Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ 2006;332:325-9. 10.1136/bmj.38733.466748.7C [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Diener HC, Ringelstein EB, von Kummer R, et al. PROTECT Trial Group . Prophylaxis of thrombotic and embolic events in acute ischemic stroke with the low-molecular-weight heparin certoparin: results of the PROTECT Trial. Stroke 2006;37:139-44. 10.1161/01.STR.0000195182.67656.ee [DOI] [PubMed] [Google Scholar]
- 93. Lederle FA, Sacks JM, Fiore L, et al. The prophylaxis of medical patients for thromboembolism pilot study. Am J Med 2006;119:54-9. 10.1016/j.amjmed.2005.03.049 [DOI] [PubMed] [Google Scholar]
- 94. Sherman DG, Albers GW, Bladin C, et al. PREVAIL Investigators . The efficacy and safety of enoxaparin versus unfractionated heparin for the prevention of venous thromboembolism after acute ischaemic stroke (PREVAIL Study): an open-label randomised comparison. Lancet 2007;369:1347-55. 10.1016/S0140-6736(07)60633-3 [DOI] [PubMed] [Google Scholar]
- 95. Levi M, Levy M, Williams MD, et al. Xigris and Prophylactic HepaRin Evaluation in Severe Sepsis (XPRESS) Study Group . Prophylactic heparin in patients with severe sepsis treated with drotrecogin alfa (activated). Am J Respir Crit Care Med 2007;176:483-90. 10.1164/rccm.200612-1803OC [DOI] [PubMed] [Google Scholar]
- 96. De A, Roy P, Garg VK, Pandey NK. Low-molecular-weight heparin and unfractionated heparin in prophylaxis against deep vein thrombosis in critically ill patients undergoing major surgery. Blood Coagul Fibrinolysis 2010;21:57-61. 10.1097/MBC.0b013e3283333505 [DOI] [PubMed] [Google Scholar]
- 97. Riess H, Haas S, Tebbe U, et al. A randomized, double-blind study of certoparin vs. unfractionated heparin to prevent venous thromboembolic events in acutely ill, non-surgical patients: CERTIFY Study. J Thromb Haemost 2010;8:1209-15. 10.1111/j.1538-7836.2010.03848.x [DOI] [PubMed] [Google Scholar]
- 98. Schellong SM, Haas S, Greinacher A, et al. An open-label comparison of the efficacy and safety of certoparin versus unfractionated heparin for the prevention of thromboembolic complications in acutely ill medical patients: CERTAIN. Expert Opin Pharmacother 2010;11:2953-61. 10.1517/14656566.2010.521498 [DOI] [PubMed] [Google Scholar]
- 99. Cook D, Meade M, Guyatt G, et al. PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group . Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med 2011;364:1305-14. 10.1056/NEJMoa1014475 [DOI] [PubMed] [Google Scholar]
- 100. Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. ADOPT Trial Investigators . Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med 2011;365:2167-77. 10.1056/NEJMoa1110899 [DOI] [PubMed] [Google Scholar]
- 101. Kakkar AK, Cimminiello C, Goldhaber SZ, Parakh R, Wang C, Bergmann JF, LIFENOX Investigators . Low-molecular-weight heparin and mortality in acutely ill medical patients. N Engl J Med 2011;365:2463-72. 10.1056/NEJMoa1111288 [DOI] [PubMed] [Google Scholar]
- 102.Ishi SV, Lakshmi M, Kakde ST, et al. Randomised controlled trial for efficacy of unfractionated heparin (ufh) versus low molecular weight heparin (LMWH) in thrombo-prophylaxis. J Assoc Physicians India 2013;61:882-6. https://www.japi.org/v274/randomised-controlled-trial-for-efficacy-of-unfractionated-heparin-ufh-versus-low-molecular-weight-heparin-lmwh-in-thrombo-prophylaxis [PubMed]
- 103. Cohen AT, Spiro TE, Büller HR, et al. MAGELLAN Investigators . Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med 2013;368:513-23. 10.1056/NEJMoa1111096 [DOI] [PubMed] [Google Scholar]
- 104. Cohen AT, Harrington RA, Goldhaber SZ, et al. APEX Investigators . Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med 2016;375:534-44. 10.1056/NEJMoa1601747 [DOI] [PubMed] [Google Scholar]
- 105. Warlow C, Terry G, Kenmure ACF, Beattie AG, Ogston D, Douglas AS. A double-blind trial of low doses of subcutaneous heparin in the prevention of deep-vein thrombosis after myocardial infarction. Lancet 1973;302:934-6. 10.1016/S0140-6736(73)92597-X [DOI] [PubMed] [Google Scholar]
- 106. Mccarthy ST, Turner JJ, Robertson D, Hawkey CJ, Macey DJ. Low-dose heparin as a prophylaxis against deep-vein thrombosis after acute stroke. Lancet 1977;310:800-1. 10.1016/S0140-6736(77)90728-0 [DOI] [PubMed] [Google Scholar]
- 107. Belch JJ, Lowe GD, Ward AG, Forbes CD, Prentice CR. Prevention of deep vein thrombosis in medical patients by low-dose heparin. Scott Med J 1981;26:115-7. 10.1177/003693308102600205 [DOI] [PubMed] [Google Scholar]
- 108. Prandoni P, Russo R, Pengo V, et al. Prevenzione della trombosi venosa profonda con eparina a basse dosi in pazienti con infarto miocardico acuto non complicato. Studio in doppio cieco controllato. Minerva Cardioangiol 1986;34:653-7. [PubMed] [Google Scholar]
- 109. Dahan R, Houlbert D, Caulin C, et al. Prevention of deep vein thrombosis in elderly medical in-patients by a low molecular weight heparin: a randomized double-blind trial. Haemostasis 1986;16:159-64. [DOI] [PubMed] [Google Scholar]
- 110. Dong K, Song Y, Li X, et al. Pentasaccharides for the prevention of venous thromboembolism. Cochrane Database Syst Rev 2016;10:CD005134. 10.1002/14651858.CD005134.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med 2013;11:159. 10.1186/1741-7015-11-159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Beitland S, Sandven I, Kjærvik LK, Sandset PM, Sunde K, Eken T. Thromboprophylaxis with low molecular weight heparin versus unfractionated heparin in intensive care patients: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 2015;41:1209-19. 10.1007/s00134-015-3840-z [DOI] [PubMed] [Google Scholar]
- 113. Alikhan R, Bedenis R, Cohen AT. Heparin for the prevention of venous thromboembolism in acutely ill medical patients (excluding stroke and myocardial infarction). Cochrane Database Syst Rev 2014;5:CD003747. 10.1002/14651858.CD003747.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fernando SM, Tran A, Cheng W, et al. VTE prophylaxis in critically ill adults: a systematic review and network meta-analysis. Chest 2022;161:418-28. 10.1016/j.chest.2021.08.050. 10.1016/j.chest.2021.08.050 [DOI] [PubMed] [Google Scholar]
- 115. ten Cate H. Surviving Covid-19 with heparin? N Engl J Med 2021;385:845-6. 10.1056/NEJMe2111151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.National Agency for the Safety of Medicine and Health Products (France). Summary of product characteristics. www.ansm.sante.fr/Produits-de-sante/Medicaments (accessed 7 Feb 2019).
- 117. Chaimani A, Caldwell DM, Li T, Higgins JPT, Salanti G. Additional considerations are required when preparing a protocol for a systematic review with multiple interventions. J Clin Epidemiol 2017;83:65-74. 10.1016/j.jclinepi.2016.11.015 [DOI] [PubMed] [Google Scholar]
- 118. Savović J, Turner RM, Mawdsley D, et al. Association between risk-of-bias assessments and results of randomized trials in cochrane reviews: the ROBES meta-epidemiologic study. Am J Epidemiol 2018;187:1113-22. 10.1093/aje/kwx344 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Online appendix
Data Availability Statement
Statistical code and the datasets are available from the corresponding author (r.j.eck@umcg.nl) and will be published online.