Abstract
An elderly man with refractory Mycobacterium abscessus lung disease previously developed anti-phage neutralizing antibodies while receiving intravenous phage therapy. Subsequent phage nebulization resulted in transient weight gain, decreased C-reactive protein, and reduced Mycobacterium burden. Weak sputum neutralization may have limited the outcomes, but phage resistance was not a contributing factor.
Keywords: Mycobacterium abscessus, bacteriophage, mycobacteriophage, phage therapy
Mycobacterium abscessus is an emerging opportunistic pathogen of increasing clinical significance [1]. The medical management of M abscessus lung disease is often complicated due to limited numbers of effective antibiotics, prolonged duration of therapy, and frequent treatment-related adverse events [2]. Bacteriophage therapy, in which viruses that infect bacteria are utilized as targeted antimicrobial agents, is a therapeutic strategy that has been applied to treat challenging infections [3–5]. Poor treatment outcomes in M abscessus infections have prompted exploration of mycobacteriophage therapy for these organisms, although the optimal patient selection, dosing, and route of phage administration for nontuberculous mycobacteria (NTM) remain unclear [5, 6].
Two recent case reports [7, 8] described divergent outcomes with the use of an intravenous (IV) mycobacteriophage cocktail against M abscessus. The first patient was a 15-year-old girl with cystic fibrosis (CF) whose post–lung transplant course was complicated by treatment-refractory disseminated M abscessus infection, which was successfully controlled with the use of an IV mycobacteriophage cocktail [7]. A second patient, an immunocompetent elderly man with non-CF bronchiectasis and refractory M abscessus subspecies massiliense lung disease [8], was treated with the identical phage cocktail, dose, and route (IV) as the first patient [7]; however, he developed a robust phage neutralizing antibody-mediated response that temporally associated with M abscessus treatment failure and led to its discontinuation. Here we report the outcomes of this patient after switching the route of phage administration to aerosolized delivery.
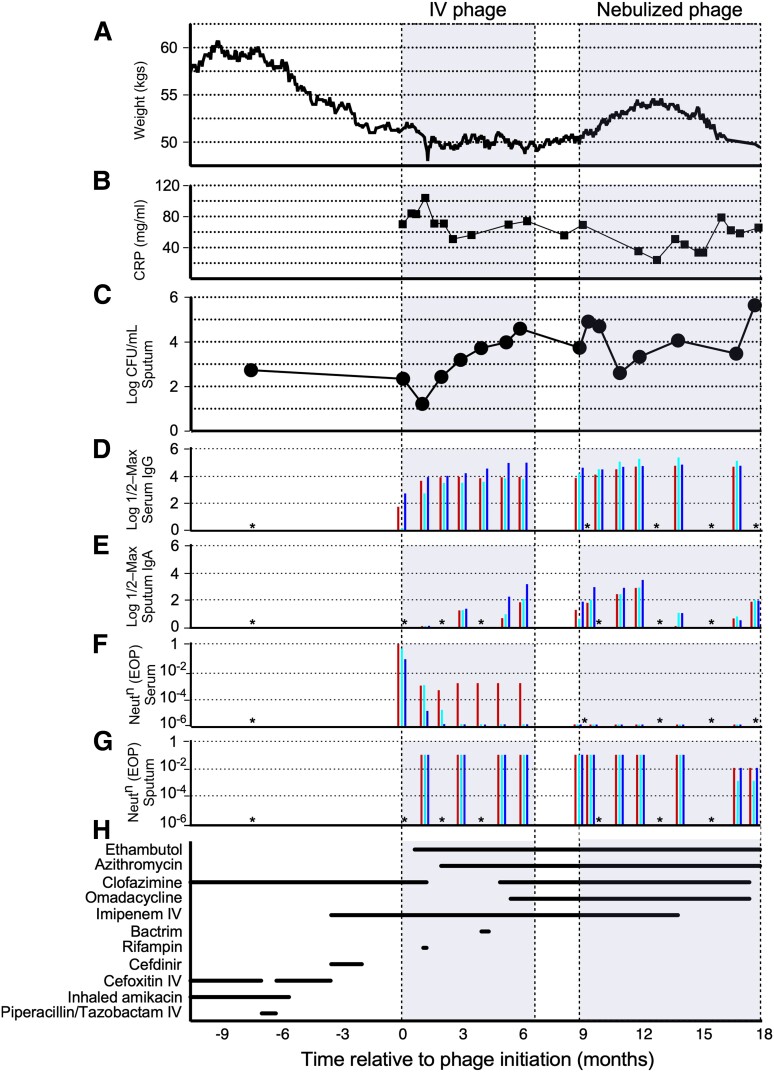
An 81-year-old man with non-CF bronchiectasis and refractory macrolide-resistant M abscessus lung disease was treated for 6 months with an IV cocktail of 3 mycobacteriophages (Muddy, BPsΔ33HTH_HRM10, and ZoeJΔ45) in addition to a multidrug antibiotic regimen, as previously described [8]. An IV route of phage administration initially was selected due to concerns that anatomic abnormalities from lung cavitation could limit phage access to infected lung tissues. IV phage therapy resulted in a transient decline in M abscessus burden in sputum at month 1 (Figure 1C), with subsequent rebound in mycobacterial colony-forming units (CFUs) that temporally correlated with the emergence of a robust anti-phage immunoglobulin M and immunoglobulin G (IgG) neutralizing antibody response, specific to the phages used therapeutically [8]. No alternative phages that infect this strain were available that might have circumvented the immune response [9]. After a continuous 6 months of twice-daily IV treatment, phage therapy was discontinued due to lack of sustained clinical effectiveness.
Figure 1.
Clinical response to intravenous and nebulized mycobacteriophage therapy for refractory Mycobacterium abscessus lung disease. A, Patient weight. B, Serum C-reactive protein level. C, Mycobacterial burden as measured in M abscessus log colony-forming units (CFUs)/ mL−1 in sputum. D and E, Serum immunoglobulin G (IgG) (D) and sputum immunoglobulin A (panel E) log half-maximal antibody levels against phages Muddy, BPsΔ33HTH_HRM10, and ZoeJΔ45 (blue, aqua, and red, respectively) determined by enzyme-linked immunosorbent assay. F and G, Serum (F) and sputum (G) phage neutralization after 24 hours of incubation with phage in vitro expressed as efficiency of plating (EOP) relative to a control with no addition. An EOP of 1 reflects no neutralization; reduction in EOP reflects phage neutralization. H, Timeline of companion antibiotic exposure. Details provided in Supplementary Materials. Sputum CFU−1 counts, serum IgG, and serum neutralization during intravenous administration are as reported previously [8]. *Samples not available or not tested. Abbreviations: CFU, colony-forming units; CRP, C-reactive protein; EOP, efficiency of plating; IgA, immunoglobulin A; IgG, immunoglobulin G; IV, intravenous; Neutn, neautralization.
To overcome serum neutralization and to increase phage delivery to the site of infection, the patient was started on nebulized administration of the same triple phage cocktail. Regulatory and institutional approval was obtained from the United States Food and Drug Administration and the Johns Hopkins Institutional Review Board and written informed consent was obtained prior to initiation. While continuing multidrug antibiotic therapy for NTM lung disease (Figure 1H), the phage cocktail was administered twice daily via nebulization using a Philips Respironics InnoSpire Essence Compressor Nebulizer System at a dose of 1 × 109 plaque-forming units in 3 mL of 0.9% normal saline. Safety and efficacy monitoring during nebulized therapy included serial clinical and microbiological assessments, in-home spirometry with the Microlife PF 100 digital peak flow and forced expiratory volume in 1 second (FEV1) meter, and serial chest computed tomography (CT). Standing weight was recorded regularly by the patient (Figure 1A) and quantitative assessment of M abscessus counts in expectorated sputum was calculated approximately monthly as the mean CFUs/mL−1 (Figure 1C). Serial M abscessus isolates were assessed for changes in antibiotic susceptibility and for acquisition of phage resistance.
Nebulized phage delivery was relatively safe and well tolerated. Prior to initiation of nebulized phage, baseline lung function was measured with an FEV1 of 1.71 L (Supplementary Figure 1A). Following initiation of nebulized phage therapy, FEV1 transiently increased to 2.13 L on day 3, and later remained relatively stable over time, with a 0.12 L decline during the 9-month nebulized treatment period (Supplementary Figure 1B). Changes in companion antibiotics during phage therapy were motivated by adverse events suspected to be antibiotic-related. These events included development of eosinophilia, prompting discontinuation of IV imipenem, and nausea and mild liver function test abnormalities, leading to discontinuation of omadacycline and clofazimine (Figure 1H and Supplementary Figure 2). There were no other clinically significant differences in blood counts or serum chemistries (Supplementary Figure 2A). Parenchymal lung abnormalities on chest CT appeared relatively unchanged over time, with only minor interval changes (Supplementary Figure 3). A brief (5-day) interruption in the use of nebulized phage therapy occurred in month 7 of nebulized phage due to development of self-limited small volume hemoptysis, a not uncommon event in patients with bronchiectasis. Hemoptysis was not temporally related to phage administration, resolved spontaneously without intervention, and did not recur after resuming nebulized phage.
With initiation of nebulized phage therapy, there was a reduction in subjective sputum production. By approximately 3.5 months into nebulized phage therapy, the patient’s body weight increased by 8.7% for a total weight gain of 4.3 kg (9.5 lbs) from a prenebulized phage baseline weight of 49.7 kg (109.5 lb) (Figure 1A). C-reactive protein (CRP) decreased from 69.3 mg/L prenebulized phage to a nadir of 23.9 mg/L 4 months into nebulized phage (Figure 1B). While M abscessus burden in sputum fluctuated (Figure 1C), weight gain during nebulized phage therapy correlated temporally with a relative quantitative reduction in M abscessus in sputum and a decrease in CRP—consistent with an improved systemic response to infection with the use of nebulized phage.
Approximately 4 months after initiation of nebulized phage delivery, these apparent benefits from phage administration dissipated, and the subsequent 7 weeks of treatment were accompanied by weight loss, increase in CRP, and rising sputum bacterial counts (Figure 1A–1C). Because of concerns for potential physical destruction and phage loss with the jet compression-type nebulizer [10, 11], administration was switched after 7.5 months to a vibrating mesh-type nebulizer (Philips Respironics InnoSpire Go), although this change did not improve clinical outcomes (Figure 1).
To determine if treatment failure was caused by the emergence of phage resistance, M abscessus isolates recovered 7 and 8 months after the start of phage nebulization were tested for phage susceptibility; these specimens had similar profiles to earlier isolates and remained fully sensitive to all 3 phages in the regimen (Supplementary Figure 4A). The emergence of bacterial resistance to phage therapy was not observed, nor were there changes in antibiotic susceptibilities (Supplementary Table 1). Sputum samples were tested for an immunoglobulin A (IgA)–mediated response to the phages (Figure 1E, Supplementary Figure 4C), and a weak but notable response to all 3 phages prior to nebulization was observed, which fluctuated after the start of nebulization but did not increase consistently or substantially; sputum IgA titers remained about 100-fold lower than serum IgG levels to the same phages (Figure 1D). This sputum IgA reactivity may have occurred, in part, as a response to the 9 months of prior IV phage administration. However, sputum samples showed only weak phage neutralization (Figure 1G, Supplementary Figure 4B), which was about 4 logs lower than observed with serum samples 8 months post–phage nebulization (Figure 1F).
The clinical progression following the switch to aerosolized phage administration mirrors what was observed after the start of IV administration: some benefits initially, followed by subsequent dissipation of those clinical gains. For IV administration, the emergence of strong neutralizing antibody response to the phages was temporally correlated with increases in mycobacterial burden and rises in CRP (Figure 1B and 1C). After the start of aerosolized delivery, weight gain, fall in CRP, and decline in mycobacterial load were initially encouraging. These clinical improvements lasted longer (3–4 months) than those observed after start of IV therapy (1–2 months). However, these gains with nebulized phage were again only transient, and the mechanisms underlying the limited duration of the benefits of phage aerosolization in this patient are unclear. We anticipated that emergence of phage resistance might occur, but after 8 months of nebulization—and 15 months of total phage therapy—the strain remained fully sensitive to phages Muddy and BPsΔ33HTH_HRM10, and with only a slight reduction in sensitivity to ZoeJΔ45 (Supplementary Figure 4A), which was also observed after IV treatment alone [8]. The failure to encounter phage resistance in M abscessus clinical isolates is consistent with in vitro studies [9] and is an encouraging indicator of the clinical utility of phage therapy against Mycobacterium infections. Aerosolized treatment failure did not result from antibody-mediated phage neutralization either, as only relatively weak sputum IgA responses to the phages were observed, and only mild neutralization (Figure 1E and 1G). However, neutralization did increase at later times (7 and 8 months) after the start of nebulization, and it is plausible that this contributed to limitation of treatment effect.
This case study highlights the challenges with using aerosolized bacteriophages for controlling Mycobacterium infections. Some patients may see clinical improvement and long-term benefits [7] whereas others may see little or only transitory health gains. It is likely that pathological details such as the specific sites of infection within the lung, access of the phages to infected foci, and host responses are all important factors that warrant further investigation.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
The authors thank Andrew Wu for assistance with laboratory processing, Lisa Ruppel for assistance with pharmacy management and dispensing of phage, and Rebecca Garlena for technical assistance.
Disclaimer. The funders had no role in the study design; data collection and analysis; preparation of the manuscript; or the decision to submit the manuscript for publication.
Financial support. This work was funded by the Cystic Fibrosis Foundation (grant number HATFUL19GO); the National Institutes of Health (NIH) (grant number GM131729); the Howard Hughes Medical Institute (grant number GT12053); and the Fowler Fund for Phage Research (to G. F. H.), as well as grants from the National Heart, Lung, and Blood Institute, NIH (grant number K08 HL139994) and the Burroughs Wellcome Fund Career Award for Medical Scientists to K. A. C.
Potential conflicts of interest. J. A. N. has received consulting fees from AstraZeneca. G. F. H. is a compensated consultant for Janssen. K. A. C. has received consulting fees from Insmed, Hillrom, Paratek, Microbion, and AN2. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Rebekah M Dedrick, Biological Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Krista G Freeman, Biological Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Jan A Nguyen, Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Asli Bahadirli-Talbott, Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Mitchell E Cardin, Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Madison Cristinziano, Biological Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Bailey E Smith, Biological Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
Soowan Jeong, Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Elisa H Ignatius, Division of Clinical Pharmacology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA; Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Cheng Ting Lin, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Keira A Cohen, Division of Pulmonary and Critical Care Medicine, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Graham F Hatfull, Biological Sciences, University of Pittsburgh, Pittsburgh, Pennsylvania, USA.
References
- 1. Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol 2020; 18:392–407. [DOI] [PubMed] [Google Scholar]
- 2. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J 2020; 56:2000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kortright KE, Chan BK, Koff JL, Turner PE. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 2019; 25:219–32. [DOI] [PubMed] [Google Scholar]
- 4. Salmond GP, Fineran PC. A century of the phage: past, present and future. Nat Rev Microbiol 2015; 13:777–86. [DOI] [PubMed] [Google Scholar]
- 5. Hatfull GF, Dedrick RM, Schooley RT. Phage therapy for antibiotic-resistant bacterial infections. Annu Rev Med 2021; 73:197–211. [DOI] [PubMed] [Google Scholar]
- 6. Aslam S, Lampley E, Wooten D, et al. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. Open Forum Infect Dis 2020; 7:ofaa389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dedrick R, Guerrero Bustamante C, Garlena RA, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat Med 2019; 25:730–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dedrick RM, Freeman KG, Nguyen JA, et al. Potent antibody-mediated neutralization limits bacteriophage treatment of a pulmonary Mycobacterium abscessus infection. Nat Med 2021; 27:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dedrick RM, Smith BE, Garlena RA, et al. Mycobacterium abscessus strain morphotype determines phage susceptibility, the repertoire of therapeutically useful phages, and phage resistance. mBio 2021; 12:e03431-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carrigy NB, Chang RY, Leung SSY, et al. Anti-tuberculosis bacteriophage D29 delivery with a vibrating mesh nebulizer, jet nebulizer, and soft mist inhaler. Pharm Res 2017; 34:2084–96. [DOI] [PubMed] [Google Scholar]
- 11. Leung SSY, Carrigy NB, Vehring R, et al. Jet nebulization of bacteriophages with different tail morphologies—structural effects. Int J Pharm 2019; 554:322–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.