Abstract
Background
Human metapneumovirus (hMPV) and parainfluenza virus type 3 (PIV3) cause respiratory tract illness in children and the elderly. No licensed vaccines are available.
Methods
In this phase 1, randomized, dose-ranging, first-in-human study, the safety, reactogenicity, and humoral immunogenicity of an investigational mRNA-based hMPV and PIV3 combination vaccine, mRNA-1653, were evaluated in healthy adults aged 18–49 years. Sentinel participants (n = 20) received 2 doses of mRNA-1653 (25, 75, 150, or 300 μg) in the dose escalation phase, and participants (n = 104) received 2 doses of mRNA-1653 (75, 150, or 300 μg) or placebo in the dose selection phase; injections were 28 days apart.
Results
The most common solicited reactogenicity events were injection site pain, headache, fatigue, and myalgia, the majority of which were grade 1 or 2. A single mRNA-1653 dose increased neutralization titers against hMPV and PIV3 1 month after vaccination compared with baseline. No notable increases in neutralizing antibody titers were observed with escalating dose levels after mRNA-1653, although no statistical inferences were made; a second mRNA-1653 dose had little observable impact on antibody titers. Neutralizing titers through 1 year remained above baseline for hMPV and returned to baseline for PIV3.
Conclusions
mRNA-1653 was well tolerated, with an acceptable safety profile and increased hMPV and PIV3 neutralization titers in healthy adults.
Keywords: human metapneumovirus, human parainfluenza virus, parainfluenza virus type 3, mRNA vaccine, safety and immunogenicity, adult
Human metapneumovirus (hMPV) of the Pneumoviridae family consists of 2 major genotypes (A and B) and is an important cause of upper and lower respiratory tract infections [1–3]. Clinical features of hMPV infection range from mild respiratory symptoms to severe respiratory disease, including bronchitis and pneumonia [1]. Infants and young children are particularly at risk for severe disease [4]. Globally in 2018, hMPV was attributed to an estimated 11.1 million cases of acute lower respiratory tract infections, 502 000 hospital admissions, and 11 300 deaths among children ≤5 years of age [4]. hMPV infection often requires medical attention and is associated with a substantial health care burden in children ≤5 years of age, particularly infants, accounting for 6%–7% of all acute respiratory illness or fever in inpatient and outpatient settings [5]. Children hospitalized with hMPV are more likely to be diagnosed with pneumonia and to require supplemental oxygen [5]. Among the elderly, outbreaks of hMPV in long-term care and skilled nursing facilities are associated with high morbidity and mortality rates [6, 7].
Human parainfluenza viruses (PIVs) belong to the Paramyxoviridae family and are another important cause of acute respiratory illnesses [8]. Four serotypes have been identified; serotype 3 (PIV3) is the most frequent among PIV isolates and is associated with pneumonia, bronchiolitis, and bronchitis [8, 9]. PIVs are a major cause of inpatient hospital burden among young children and are responsible for a reported 6.8% of all hospitalizations for acute respiratory illness or fever [10, 11]. Although more common among infants and children, PIV and hMPV infections can occur at any age; older adults and individuals with chronic or immunocompromising conditions are at particular risk [12–19].
Currently, no licensed vaccines or antiviral therapies are available for the treatment or prevention of hMPV and PIV3 infections [1, 8, 20]. Messenger RNA (mRNA) vaccines present an ideal platform to effectively combat these pathogens.
mRNA vaccines have advantages over other vaccine platforms, including rapid, simple, and scalable manufacturing; versatile antigen design; and inclusion of multiple antigens within a single vaccine, potentially across different pathogens [21, 22]. Several clinical trials have evaluated the safety, immunogenicity, and efficacy of mRNA-based vaccines, most notably mRNA-1273, which targets severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [23–25]. A phase 3 trial of mRNA-1273 reported 94.1% efficacy in preventing symptomatic SARS-CoV-2 infection and 100% efficacy against severe infection, with an acceptable safety profile, supporting emergency use authorization or regulatory approvals in numerous countries during the coronavirus disease 2019 pandemic [25–28].
An investigational combination mRNA-based vaccine, mRNA-1653, is in development for the prevention of respiratory disease associated with hMPV and PIV3 infections. This bivalent vaccine consists of 2 distinct nucleoside-modified mRNA sequences that encode for the full-length membrane-bound fusion proteins of hMPV and PIV3 co-formulated in a lipid nanoparticle. mRNA-1653 is the first combination mRNA vaccine simultaneously targeting these 2 respiratory viruses. The current phase 1 dose-ranging, first-in-human study evaluated the safety, reactogenicity, and immunogenicity of mRNA-1653 in healthy adults 18–49 years of age.
METHODS
Study Design
This phase 1 randomized, observer-blind, placebo-controlled, dose-ranging, first-in-human study evaluated the safety, reactogenicity, and immunogenicity of mRNA-1653 in healthy adults across 3 sites in the United States from November 2017 to July 2019 (Protocol number mRNA-1653-P101; ClinicalTrials.gov NCT03392389).
The study design included dose escalation and dose selection phases. Before dosing, vaccine administration personnel carried out randomization using an interactive response technology (IRT) system. In the dose escalation phase, 20 sentinel participants (5 per dose level) were sequentially recruited to receive 1 of 4 escalating dose levels of mRNA-1653 (25, 75, 150, or 300 μg) or placebo via intramuscular injection. These 5 participants per dose level cohort were randomized in a 4:1 ratio to receive mRNA-1653 or placebo at day 1 (dose 1) and the same dose level at month 1 (dose 2; 28 days after dose 1). Blinded safety reviews were conducted by an internal safety team at prespecified time points, and favorable outcomes permitted dose continuation within each cohort and dose escalation to the next dose level cohort. Following review of the dose escalation phase (up to day 35) by the safety monitoring committee, the 3 highest dose levels with acceptable safety and reactogenicity profiles were evaluated in the dose selection phase.
In the dose selection phase, 104 participants were randomized (1:1:1:1) to receive 1 of 3 dose levels of mRNA-1653 (75, 150, or 300 μg) or placebo at day 1 (dose 1). At month 1 (dose 2; 28 days after dose 1), participants within each mRNA-1653 dose level were randomized (1:1) to receive a second dose of mRNA-1653 (2-dose mRNA-1653 groups) or placebo (1-dose mRNA-1653 groups). All participants who received placebo on day 1 (dose 1) received placebo at month 1 (dose 2). The unblinded safety monitoring committee reviewed safety and reactogenicity data at prespecified time points.
Study Objectives
The primary objective was to evaluate the safety, reactogenicity, and humoral immunogenicity of mRNA-1653 administered according to a 1-dose vs 2-dose schedule through 28 days following the last vaccination. An additional primary objective was to select an optimal dose range and schedule of mRNA-1653 for further clinical development. Secondary objectives were to evaluate the safety and humoral immunogenicity of a 1-dose vs 2-dose schedule through 12 months following dose 2.
Participants
Participants included generally healthy males and nonpregnant females 18–49 years of age with a body mass index (BMI) of 18–35 kg/m2. Full eligibility requirements are described in the Supplementary Data.
Patient Consent
All patients provided written informed consent before study procedures. The design of the study, protocol, amendments, and informed consent form were approved by the investigators’ institutional review boards. The study was conducted according to the principles of the International Council for Harmonisation Good Clinical Practice guidelines. All aspects of the study were performed in accordance with the ethical principles originating in the Declaration of Helsinki, the protocol, and all national, state, and local laws or regulations.
Vaccine
The mRNA-1653 vaccine is bivalent, containing 2 distinct nucleoside-modified mRNA sequences encoding the native, membrane-anchored fusion glycoproteins of hMPV and PIV3 encapsulated in a lipid nanoparticle, as described previously [29, 30]. The encoded antigens are not engineered with prefusion conformation stabilizing mutations, yet cell-based in vitro expression studies using prefusion-specific monoclonal antibodies demonstrate that a large portion of the expressed hMPV and PIV3 fusion proteins are in the prefusion conformation (data not shown). The sources of the encoded antigens were the hMPV A strain, TN/92-4, and the PIV3 strain, PER/FLA4815/2008. LNPs have become a preferred method for the delivery of mRNAs and typically consist of 4 components: (1) ionizable lipids that promote the assembly of LNPs into delivery vehicles; (2) phospholipids that form the lipid bilayer structures in LNPs; (3) polyethylene glycol (PEG) lipids; and (4) sterols that improve the stability of mRNA formulations within the LNPs and the LNP vehicle, respectively [21, 31]. The vaccine (25, 75, 150, or 300 μg total mRNA [1:1 mass ratio of hMPV and PIV3 fusion glycoprotein mRNA sequences]) or placebo (0.9% sodium chloride) was administered as 0.5-mL intramuscular injections into the deltoid muscle on a 2-dose vaccination schedule at day 1 (preferably the nondominant arm) and month 1 (either arm).
Safety Assessments
Safety assessments in the dose escalation and dose selection phases included monitoring and recording of solicited local and systemic adverse events (AEs), unsolicited AEs, medically attended AEs (MAEs), serious AEs (SAEs), AEs of special interest (AESIs), clinical laboratory test results, vital sign measurements, and physical examinations. Solicited (local and systemic) and unsolicited AEs were collected within 7 and 28 days of vaccination, respectively. Reports of MAEs, SAEs, and AESIs were collected during the entirety of the study (day 1 through month 13). AE intensity was graded according to the Center for Biologics Evaluation and Research Guidance Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials [32]. Blood for safety laboratory testing was collected at days 1, 7, and 35 and at months 1 and 2.
Immunogenicity Assessments
Blood for immunogenicity analysis was collected before injection on day 1 and month 1 and at months 2, 7, and 13. Assessments of anti-hMPV and anti-PIV3 antibodies were performed using qualified virus neutralization assays (HMPV A strain NL/1/00, HMPV B strain NL/1/99, and HPIV3 strain C243), similar to the assessments previously described for quantification of respiratory syncytial virus serum neutralizing antibodies [33]. Assay method details are described in the Supplementary Methods.
Data Analysis
As this is a descriptive study, there was no formal hypothesis testing or formal statistical comparisons across dose levels and vaccination schedules. The sample size was based on practical considerations to provide a sufficient descriptive summary of the safety and immunogenicity of mRNA-1653. All analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC, USA).
Data from the participants receiving mRNA-1653 in the dose escalation phase were pooled for analysis with the data from the participants assigned to the 2-dose schedule of the corresponding dose level in the dose selection phase. Data from placebo recipients across cohorts in the dose escalation phase were pooled for analysis with the data from placebo recipients in the dose selection phase. Arms were grouped into the following categories: placebo, 25-μg mRNA-1653 2-dose, 75 μg-mRNA-1653 2-dose, 75-μg mRNA-1653 1-dose, 150-μg mRNA-1653 2-dose, 150-μg mRNA-1653 1-dose, 300-μg mRNA-1653 2-dose, and 300-μg mRNA-1653 1-dose. Data analysis sets are defined in the Supplementary Methods.
Geometric mean titers (GMTs) of the serum anti-hMPV and anti-PIV3 neutralizing antibodies with 95% CIs and geometric mean ratios (GMRs) of neutralizing antibody titers at each individual postvaccination time point over baseline with 95% CIs were tabulated. The 95% CIs of mean difference in natural log-transformed values were calculated using t-distribution and back-transformed to the original scale. The percentages of participants with a seroresponse (defined as ≥4× baseline value) in serum anti-hMPV and anti-PIV3 neutralizing antibody titers from visit day 1 (baseline) to postvaccination time points were tabulated with 2-sided 95% Clopper-Pearson CIs.
RESULTS
Participants
A total of 124 participants were enrolled, including 20 participants in the dose escalation phase and 104 participants in the dose selection phase (Supplementary Figure 1). All participants received dose 1, and 120 participants (96.8%) received dose 2. A total of 120 participants (96.8%) completed the study, 20 (100%) in the dose escalation phase and 100 (96.2%) in the dose selection phase. Of the 4 participants (3.2%) who discontinued, 2 (1.6%) were lost to follow-up and 2 (1.6%) discontinued for other reasons (1 moved to a new location, and 1 was unavailable for clinic visits).
Most participants were female (62.9%), White (65.3%), and not of Hispanic, Latino, or Spanish ethnicity (88.7%), with a mean age (range) of 36.7 (19–49) years and a mean BMI of 27.3 kg/m2 at baseline (Table 1). Mean baseline values for age and BMI were generally similar across the mRNA-1653 and placebo groups.
Table 1.
Participant Baseline Characteristics (Exposed Set a )
| Placebo (n = 30)b | mRNA-1653 | Combined (n = 94)b | All Participants (n = 124)b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 25 μg | 75 μg | 150 μg | 300 μg | |||||||
| 2-Dose (n = 4)b | 1-Dose (n = 13)b | 2-Dose (n = 17)b | 1-Dose (n = 13)b | 2-Dose (n = 17)b | 1-Dose (n = 13)b | 2-Dose (n = 17)b | ||||
| Age | ||||||||||
| Mean ± SD, y | 39.7 ± 7.7 | 28.0 ± 9.4 | 36.4 ± 6.2 | 39.6 ± 6.9 | 34.3 ± 9.1 | 35.6 ± 8.0 | 34.9 ± 8.3 | 35.1 ± 9.5 | 35.7 ± 8.2 | 36.7 ± 8.3 |
| Range, y | 19–48 | 20–38 | 25–45 | 29–49 | 20–47 | 22–48 | 19–47 | 19–49 | 19–49 | 19–49 |
| Sex, No. (%)c | ||||||||||
| Male | 12 (40.0) | 1 (25.0) | 3 (23.1) | 4 (23.5) | 4 (30.8) | 7 (41.2) | 4 (30.8) | 11 (64.7) | 34 (36.2) | 46 (37.1) |
| Female | 18 (60.0) | 3 (75.0) | 10 (76.9) | 13 (76.5) | 9 (69.2) | 10 (58.8) | 9 (69.2) | 6 (35.3) | 60 (63.8) | 78 (62.9) |
| Race, No. (%)c | ||||||||||
| American Indian or Alaskan Native | 1 (3.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.8) |
| Asian | 1 (3.3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.8) |
| Black or African American | 8 (26.7) | 2 (50.0) | 6 (46.2) | 3 (17.6) | 5 (38.5) | 6 (35.3) | 2 (15.4) | 8 (47.1) | 32 (34.0) | 40 (32.3) |
| White | 20 (66.7) | 2 (50.0) | 7 (53.8) | 14 (82.4) | 8 (61.5) | 10 (58.8) | 11 (84.6) | 9 (52.9) | 61 (64.9) | 81 (65.3) |
| Multiracial | 0 | 0 | 0 | 0 | 0 | 1 (5.9) | 0 | 0 | 1 (1.1) | 1 (0.8) |
| Ethnicity, No. (%)c | ||||||||||
| Hispanic, Latino, or Spanish | 3 (10.0) | 0 | 3 (23.1) | 1 (5.9) | 2 (15.4) | 1 (5.9) | 2 (15.4) | 2 (11.8) | 11 (11.7) | 14 (11.3) |
| Not Hispanic, Latino, or Spanish | 27 (90.0) | 4 (100.0) | 10 (76.9) | 16 (94.1) | 11 (84.6) | 16 (94.1) | 11 (84.6) | 15 (88.2) | 83 (88.3) | 110 (88.7) |
| BMI | ||||||||||
| Mean ± SD, kg/m2 | 27.0 ± 3.9 | 26.4 ± 5.5 | 26.8 ± 3.3 | 27.7 ± 4.0 | 26.2 ± 5.2 | 28.1 ± 3.8 | 26.9 ± 3.7 | 28.5 ± 4.3 | 27.4 ± 4.1 | 27.3 ± 4.0 |
| Range, kg/m2 | 20.4–34.7 | 19.4–32.5 | 21.1–32.6 | 20.5–34.6 | 18.5–34.5 | 22.7–34.8 | 20.6–35.0 | 20.1–34.7 | 18.5–35.0 | 18.5–35.0 |
Abbreviation: BMI, body mass index.
All participants in the randomized set who received any study vaccination.
Number of participants randomized and exposed to study vaccination.
Number of participants in each treatment group who have nonmissing data for the corresponding baseline characteristic.
Concomitant use of medication within 28 days of vaccination was recorded; analgesic or antipyretic medications were taken by 35.1% and 18.7% of the mRNA-1653 recipients and by 3.3% and 0.0% of placebo recipients after the first and second vaccinations, respectively. No prophylactic use of analgesics or antipyretics was reported.
Solicited Injection Site Adverse Events
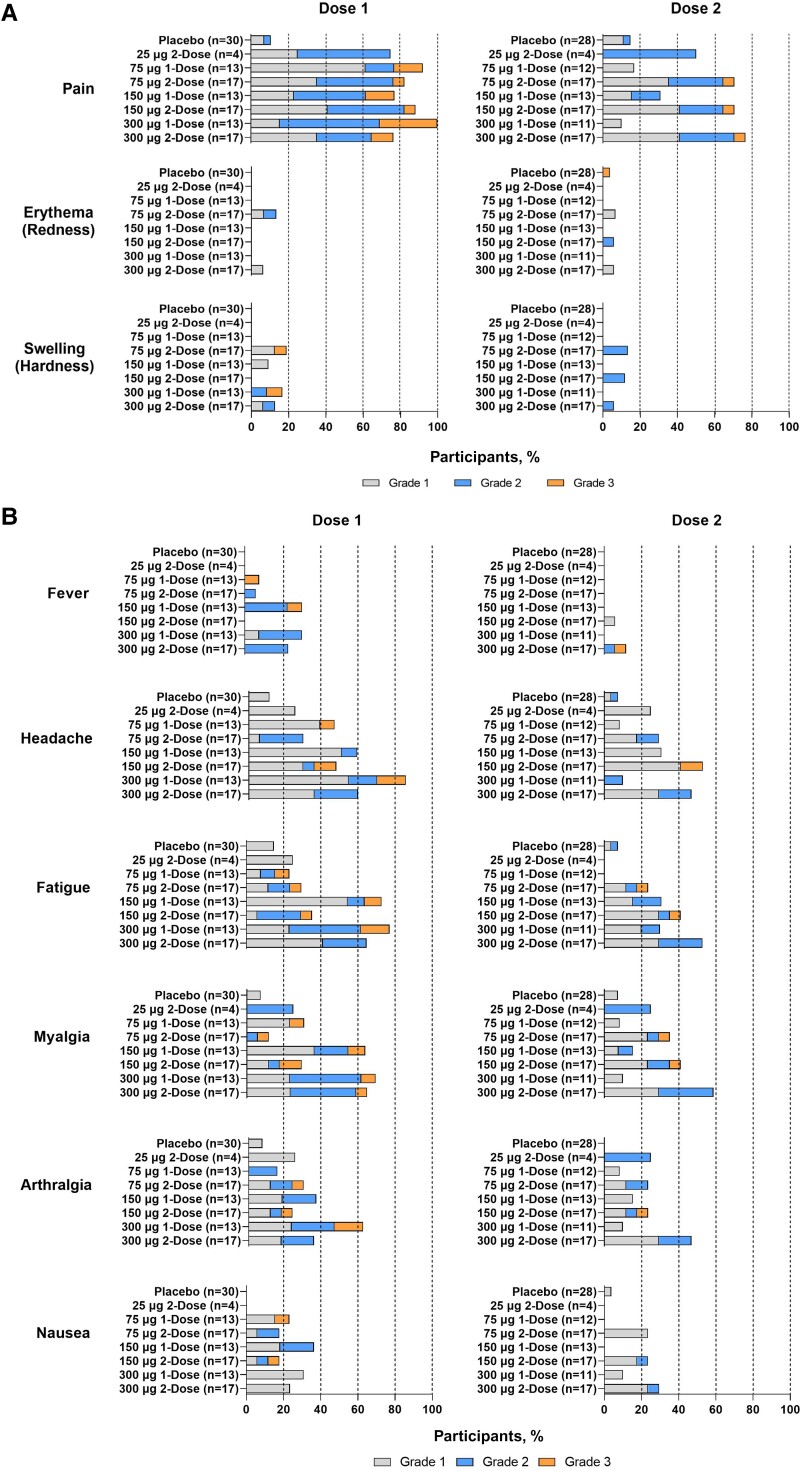
All 124 subjects were included in the solicited safety set and the overall safety set. The majority of solicited local reactogenicity events were grades 1 and 2. Injection site pain was the most common solicited local reactogenicity event, reported by 80 (85.1%) and 39 participants (70.9%) in the mRNA-1653 treatment groups after doses 1 and 2 (2-dose groups only), respectively (Figure 1). In the placebo group, 3 (10.7%) and 4 participants (14.8%) reported injection pain after doses 1 and 2, respectively. Rates of injection site pain after dose 1 were lowest after the 25-μg mRNA-1653 dose (3 [75.0%]); however, a dose–response trend was not observed at the higher doses (75 μg: 26 [86.7%]; 150 μg: 25 [83.3%]; 300 μg: 26 [86.7%]). A higher percentage of participants reported pain at the injection site after dose 2 in the 75-, 150-, and 300-μg mRNA-1653 2-dose groups than in the 1-dose groups that received placebo for the second injection. A lower or similar percentage of participants reported grade 3 injection site pain after the second mRNA-1653 vaccination (2-dose groups only; 3 participants [5.45%]) compared with the first mRNA-1653 vaccination (both 1-dose and 2-dose groups; 12 participants [12.8%]). A grade 3 solicited AE (erythema) was reported by 1 participant (3.8%) in the placebo group. Grade 3 erythema and swelling were reported by 1 (1.2%) and 2 participants (2.4%), respectively, in the mRNA-1653 treatment groups after dose 1.
Figure 1.
Solicited local and systemic adverse events within 7 days of doses 1 and 2 (first vaccination and second vaccination solicited safety seta). Solicited local (A) and systemic (B) adverse events presented by grade and treatment group following each dose. Dose 1, n = number of participants in the first vaccination solicited safety set; Dose 2, n = number of participants in the second vaccination solicited safety set. aAll participants in the exposed set with any solicited local or systemic reactogenicity events data after dose 1 or 2.
The mean duration ranges for injection site pain, erythema, and swelling in the mRNA-1653 treatment groups were 2.3–3.4, 0–4.0, and 0–2.5 days, respectively, after dose 1 and 2.2–3.0, 0–4.0, and 0–3.0 days, respectively, after dose 2 (2-dose groups only). After dose 2, the mean durations of injection site pain in the mRNA-1653 2-dose groups were similar to the mRNA-1653 1-dose groups that received placebo for the second injection (2.2–3.0 vs 1.0–2.8 days, respectively).
Solicited Systemic Adverse Events
Headache, fatigue, and myalgia were the most common solicited systemic reactogenicity events, reported by 48 (51.6%), 44 (47.8%), and 39 (42.4%) mRNA-1653 recipients after dose 1 and by 23 (41.8%), 20 (36.4%), and 24 (43.6%) mRNA-1653 recipients after dose 2 (2-dose groups only) (Figure 1). After dose 2, a higher percentage of participants reported headache, fatigue, and myalgia in the 75-, 150-, and 300-μg mRNA-1653 2-dose groups compared with the 1-dose groups that received placebo for the second injection. One participant in the 300-μg mRNA-1653 1-dose group reported a nonurticarial thoracic rash beginning 3 days after vaccination that lasted for 2 days; the rash did not recur following dose 2. After dose 2, the frequency of solicited systemic events generally increased with dose level in the 2-dose groups.
The majority of solicited systemic reactogenicity events were grades 1 and 2. Grade 3 solicited systemic reactogenicity events were not reported by placebo or 25-μg mRNA-1653 recipients after dose 1 or 2. Grade 3 fever, headache, fatigue, myalgia, arthralgia, and nausea were reported by 2 (2.1%), 5 (5.4%), 6 (6.5%), 7 (7.6%), 4 (4.3%), and 2 (2.2%) mRNA-1653 recipients, respectively, after dose 1 and 1 (1.8%), 2 (3.6%), 2 (3.6%), 2 (3.6%), 1 (1.8%), and 0 (0%) mRNA-1653 recipients, respectively, after dose 2 (Supplementary Table 2).
The mean durations for systemic reactogenicity events in the mRNA-1653 treatment groups ranged from 1.3 to 2.2 days after dose 1 and from 0 to 3.0 days after dose 2 (2-dose groups only). After dose 2, the mRNA-1653 2-dose groups were similar to the mRNA-1653 1-dose groups that received placebo for the second injection in the mean durations of systemic reactogenicity events (1.0–3.0 vs 1.0–5.5 days, respectively).
Unsolicited Adverse Events
Unsolicited AEs were reported by 48 mRNA-1653 recipients (51.1%) and 10 placebo recipients (33.3%) (Supplementary Table 1). The most common unsolicited AEs among mRNA-1653 recipients were upper respiratory tract infection, chills, and headache, which were reported by 5 participants (5.3%) each. One participant (1.1%) reported an SAE (breast cancer) ∼1 month after receiving the mRNA-1653 75-μg 1-dose vaccination; the event was not considered related to treatment by the investigator. Unsolicited MAEs were reported by 33 mRNA-1653 recipients (35.1%) and 9 placebo recipients (30.0%) after vaccination. The most common unsolicited MAEs reported by mRNA-1653 recipients included influenza, sinusitis, and nausea (3 participants each). No participant withdrew from the study due to an unsolicited AE, and there were no deaths or AESIs reported. Grade 3 or 4 unsolicited AEs were reported by 6 mRNA-1653 recipients (6.4%; injection site pain [n = 2], increased AST [n = 1], diverticulitis [n = 1], myalgia [n = 1], decreased hemoglobin [n = 1], and prolonged prothrombin time [n = 1], which was the only grade 3 unsolicited AE that was considered treatment related) and 1 placebo recipient (3.3%; grade 3 decreased glucose) after vaccination.
Treatment-related unsolicited AEs were reported by 11 participants (11.7%) who received mRNA-1653 (Table 2); no treatment-related unsolicited AEs were reported for participants in the 25-μg mRNA-1653 2-dose and 75-μg mRNA-1653 1-dose groups. Chills were the most common treatment-related unsolicited AE and were reported by 5 participants (5.3%).
Table 2.
Unsolicited Treatment-Related Adverse Events (Exposed Set a )
| Placebo (n = 30)b | mRNA-1653 | Combined (n = 94)b | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 μg | 75 μg | 150 μg | 300 μg | ||||||
| 2-Dose (n = 4)b | 1-Dose (n = 13)b | 2-Dose (n = 17)b | 1-Dose (n = 13)b | 2-Dose (n = 17)b | 1-Dose (n = 13)b | 2-Dose (n = 17)b | |||
| No. (%)c | |||||||||
| All unsolicited AEs | 0 | 0 | 0 | 1 (5.9) | 1 (7.7) | 3 (17.6) | 3 (23.1) | 3 (17.6) | 11 (11.7) |
| SAEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Fatal AEs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AEs leading to withdrawal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| AEs grade 3+ | 0 | 0 | 0 | 0 | 0 | 0 | 1 (7.7) | 2 (11.8) | 3 (3.2) |
| AESIs | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MAEs | 0 | 0 | 0 | 0 | 0 | 0 | 2 (15.4) | 3 (17.6) | 5 (5.3) |
Abbreviations: AE, adverse event; AESI, adverse event of special interest; MAE, medically attended adverse event; SAE, serious adverse event.
All participants in the randomized set who received any study vaccination.
Number of participants randomized and exposed to study vaccination.
Number of participants in each treatment group reporting the event.
There were no treatment-related, dose-related, or clinically relevant trends for the hematology, coagulation, or serum chemistry results. Means above the reference ranges were observed for AST and erythrocyte distribution width; these discrepancies were largely explained by high values for individual participants. No trends were observed in the changes from baseline in vital sign measurements. Most vital sign measurement results were grade 0; there were no reports of toxicity higher than grade 2 for any vital sign measurement. Physical examination analyses were not performed.
Human Metapneumovirus and Parainfluenza Virus 3 Serum Neutralization
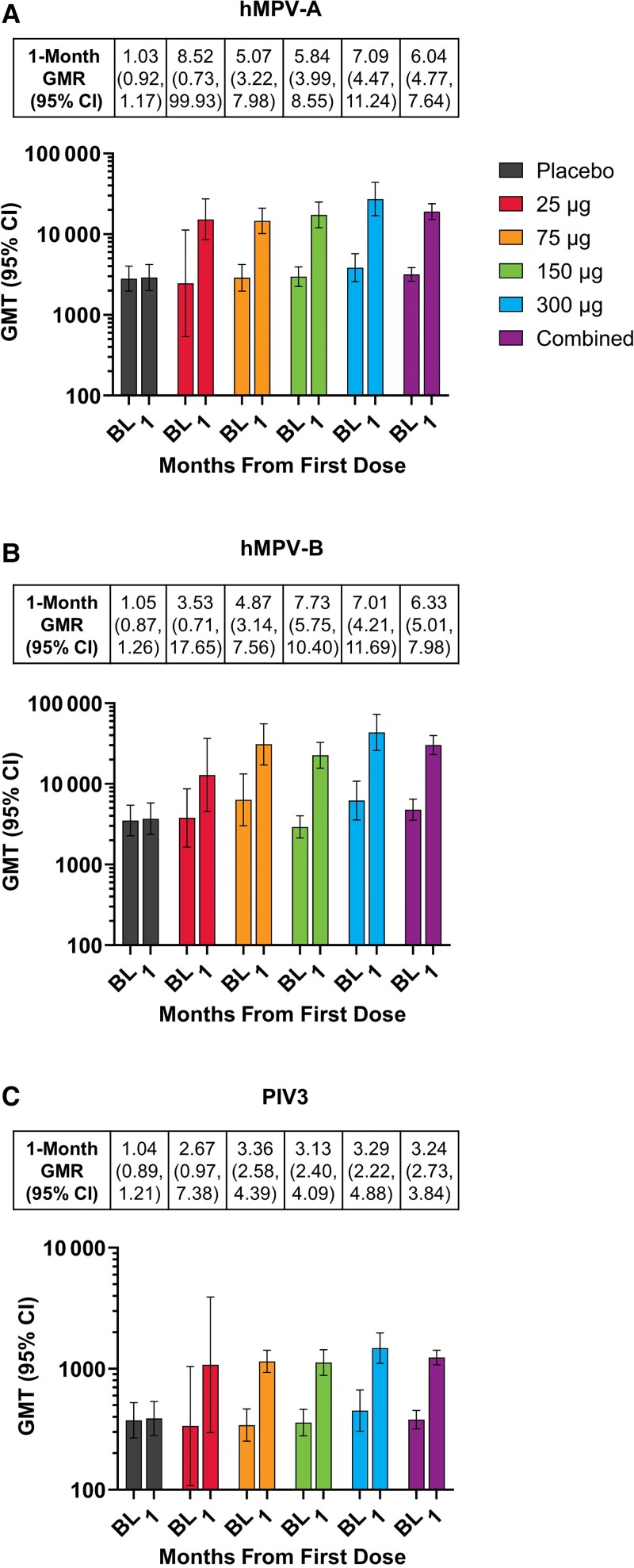
hMPV and PIV3 neutralizing antibody titers were present at baseline (day 1) in all participants; baseline GMTs were similar across treatment groups (Figure 2).
Figure 2.
GMRs and GMTs of serum neutralizing antibodies by dose levela at baseline and month 1 for (A) hMPV-A, (B) hMPV-B, and (C) PIV3 (per-protocol immunogenicity setb). aData are combined for the 1-dose and 2-dose mRNA-1653 groups at the 75-, 150-, and 300-μg dose levels. bAll evaluable participants from the full analysis set who met all eligibility criteria, received study vaccination per protocol, did not receive a concomitant medication or vaccine leading to exclusion from analysis, did not present with a medical condition that might impact immunogenicity assessment, complied with the vaccination schedule and timing of the postvaccination blood sampling, and had ≥1 assay component for immune response evaluation at the respective time point (visit month 1). Abbreviations: BL, baseline; GMR, geometric mean ratio; GMT, geometric mean titer; hMPV, human metapneumovirus; PIV3, human parainfluenza virus type 3.
After dose 1, mRNA-1653 increased neutralizing antibody titers against hMPV-A, hMPV-B, and PIV3 for all dose levels (25, 75, 150, and 300 μg), and although formal statistical comparisons were not conducted, no notable increase was observed in titers with escalating dose levels (Figure 2). The GMRs (postbaseline/baseline titers) for the combined mRNA-1653 groups at month 1 were 6.04, 6.33, and 3.24 for hMPV-A, hMPV-B, and PIV3, respectively. The seroresponses (defined as ≥4× baseline value) for the combined mRNA-1653 groups were 60.2%, 68.2%, and 39.8% for hMPV-A, hMPV-B, and PIV3, respectively. An exploratory analysis showed an inverse relationship between baseline neutralizing antibody titer and the response to the first mRNA-1653 vaccination (month 1/day 1 titer ratio) (Supplementary Figure 2).
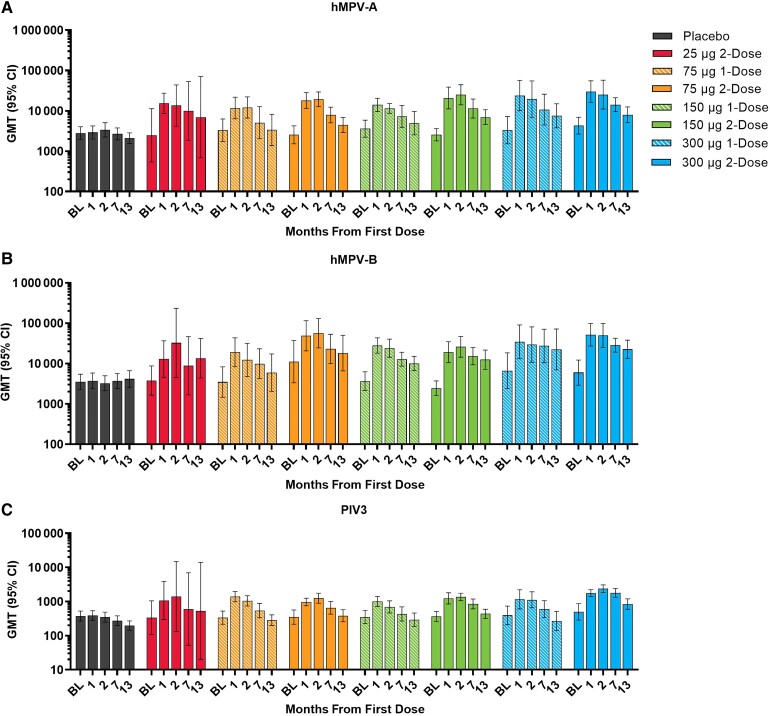
Dose 2 (month 1) had little observable impact on the hMPV and PIV3 neutralizing antibody titers when measured at month 2 (Figure 3). At month 7, the GMTs for all mRNA-1653 dose levels remained above baseline. At month 13, the GMTs for all mRNA-1653 dose levels remained above baseline for hMPV-A and hMPV-B and returned approximately to baseline for PIV3. Across the mRNA-1653 vaccination groups, the month 7 GMR ranges were 1.82–5.54 for hMPV-A, 2.08–6.22 for hMPV-B, and 1.26–3.52 for PIV3 (Supplementary Table 3); the month 13 GMR ranges were 1.21–3.88 for hMPV-A, 1.76–5.38 for hMPV-B, and 0.73–1.65 for PIV3.
Figure 3.
GMTs of serum neutralizing antibodies by vaccination group at baseline and months 1, 2, 7, and 13 for (A) hMPV-A, (B) hMPV-B, and (C) PIV3 (per-protocol immunogenicity seta). aAll evaluable participants from the full analysis set who met all eligibility criteria, received study vaccination per protocol, did not receive a concomitant medication or vaccine leading to exclusion from analysis, did not present with a medical condition that might impact immunogenicity assessment, complied with the vaccination schedule and timing of the postvaccination blood sampling, and had ≥1 assay component for immune response evaluation at the respective time point (visit month 1, 2, 7, or 13). Abbreviations: BL, baseline; GMT, geometric mean titer; hMPV, human metapneumovirus; PIV3, human parainfluenza virus type 3.
DISCUSSION
This first-in-human study of mRNA-1653 in healthy adult participants indicated that mRNA-1653 administered intramuscularly had an acceptable safety profile and was well tolerated at the 25-, 75-, 150-, and 300-μg dose levels, with no increase in the overall number or severity of adverse events after dose 2. A single dose of mRNA-1653 increased serum neutralization titers against hMPV-A, hMPV-B, and PIV3 ∼1 month after vaccination. While formal statistical analyses were not conducted, increases in serum neutralization titers appeared similar across dose levels. Furthermore, a second dose of mRNA-1653 had little observable impact on the magnitude of hMPV or PIV3 neutralization titers measured ∼1 month after dose 2 compared with 1 month after dose 1. While the hMPV neutralizing antibody titers remained above baseline at all mRNA-1653 dose levels through month 13, PIV3 neutralizing antibody titers were above baseline through month 7 and returned to baseline at month 13.
Neutralizing antibodies against hMPV-A, hMPV-B, and PIV3 were present at baseline in all participants, indicating previous exposure to both viruses. Most individuals are initially infected with hMPV and PIV3 in early childhood, with 100% seroprevalence of hMPV antibodies in older children through the elderly [34, 35]. Reinfection in young and elderly adults is well documented [13, 14, 35].
An increase in serum neutralizing antibody titers was not observed across escalating dose levels following 1 or 2 doses of mRNA-1653, suggesting that a low dose is sufficient to induce the maximum increase in neutralizing antibody titers observed. Results from this trial indicate the potential for mRNA-1653 to induce humoral immune responses against hMPV and PIV3 in a previously exposed population.
The safety and immunogenicity results of this phase 1 trial informed the dose selection of mRNA-1653 for further clinical development. A phase 1b trial in healthy adults aged 18–49 years and children aged 12–59 months with serologic evidence of prior hMPV and PIV3 exposure is expected to provide additional safety and immunogenicity data for mRNA-1653 (NCT04144348).
mRNA-1653 is the first combination mRNA-based vaccine evaluated clinically for hMPV and PIV3 and the only vaccine against hMPV and PIV3 that has successfully demonstrated a boost in neutralizing antibody titers to both viruses. Differences in the potency of neutralizing antibodies elicited by prefusion and postfusion stabilized hMPV F and PIV3 F have been observed [36–38]. As such, engineering of the mRNA constructs to exclusively encode prefusion stabilized hMPV F and PIV3 F may further boost neutralizing antibody titers and serves as a potential avenue for future optimization of the mRNA-1653 vaccine. Importantly, this is the first clinical report of an mRNA vaccine simultaneously targeting ≥2 pathogens by combining multiple mRNAs in a single injection. This approach is novel, cost-effective, and may improve vaccination uptake due to fewer injections and easier integration into existing vaccination schedules [39].
The limitations of this study include the small number of participants enrolled. mRNA-1653 was evaluated in a healthy adult population aged 18–49 years, potentially limiting the generalizability of the results to children and older adults; despite the absence of an increase in titers with escalating dose levels in the adult naturally primed population, the selected dose may not be optimal for children and the elderly. Additionally, the overall trial population was predominantly female and White non-Hispanic, and the 300-µg 2-dose group was predominantly male, limiting the generalizability of these results to the US population. The relevance to both seropositive and seronegative children is yet to be demonstrated, and a 1-dose series may be insufficient for the latter. Protective antibody levels for both hMPV and PIV3 are unknown; therefore, the clinical significance of increased neutralizing antibody titers requires further evaluation. As no formal statistical inferences were made regarding immunogenicity across dose levels and vaccine schedules, these comparisons are observational. A study strength is the randomized, observer-blind, placebo-controlled design and the robust assay performance that allowed observance of clear immunogenicity results.
Overall, this study indicated that mRNA-1653 increased hMPV and PIV3 neutralization titers in healthy adults aged 18–49 years and was well tolerated, with an acceptable safety profile. These findings support the continued clinical development of mRNA-1653 for the prevention of respiratory illness associated with hMPV and PIV3 infections.
Supplementary Data
Supplementary materials are available at the Journal of The Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Acknowledgments
Medical writing and editorial assistance were provided by Kate Russin, PhD, and Jessica Nepomuceno, PhD, of MEDiSTRAVA in accordance with Good Publication Practice (GPP3) guidelines, funded by Moderna, Inc., and under the direction of the authors.
Financial support. This work was supported by Moderna, Inc.
Potential conflicts of interest. A.A., C.A.S., H.L., C.K., S.K., T.Z., I.S., and L.P. are or were employees of Moderna, Inc., and hold stock/stock options in the company. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data sharing statement. Upon request, and subject to review, Moderna will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Moderna may also provide access to the related individual anonymized participant data.
Protocol number. mRNA-1653-P101.
ClinicalTrials.gov identifier. NCT03392389.
Contributor Information
Allison August, Moderna, Inc., Cambridge, Massachusetts, USA.
Christine A Shaw, Moderna, Inc., Cambridge, Massachusetts, USA.
Heather Lee, Moderna, Inc., Cambridge, Massachusetts, USA.
Conor Knightly, Moderna, Inc., Cambridge, Massachusetts, USA.
Shiva Kalidindia, Moderna, Inc., Cambridge, Massachusetts, USA.
Laurence Chu, Benchmark Research, Austin, Texas, USA.
Brandon J Essink, Meridian Clinical Research, Omaha, Nebraska, USA.
William Seger, Benchmark Research, Fort Worth, Texas, USA.
Tal Zaks, Moderna, Inc., Cambridge, Massachusetts, USA.
Igor Smolenov, Moderna, Inc., Cambridge, Massachusetts, USA.
Lori Panther, Moderna, Inc., Cambridge, Massachusetts, USA.
References
- 1. Centers for Disease Control and Prevention . Human metapneumovirus (HMPV) clinical features. Updated 5 September 2019. Available at: https://www.cdc.gov/surveillance/nrevss/hmpv/clinical.html. Accessed 21 April 2021.
- 2. van den Hoogen BG, Herfst S, Sprong L, et al. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis 2003; 10:658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rima B, Collins P, Easton A, et al. ICTV virus taxonomy profile: pneumoviridae. J Gen Virol 2017; 98:2912–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang X, Li Y, Deloria-Knoll M, et al. Global burden of acute lower respiratory infection associated with human metapneumovirus in children under 5 years in 2018: a systematic review and modelling study. Lancet Glob Health 2021; 9:e33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edwards KM, Zhu Y, Griffin MR, et al. Burden of human metapneumovirus infection in young children. N Engl J Med 2013; 368:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liao RS, Appelgate DM, Pelz RK. An outbreak of severe respiratory tract infection due to human metapneumovirus in a long-term care facility for the elderly in Oregon. J Clin Virol 2012; 53:171–3. [DOI] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention . Outbreaks of human metapneumovirus in two skilled nursing facilities – West Virginia and Idaho, 2011–2012. MMWR Morb Mortal Wkly Rep 2013; 62:909–13. [PMC free article] [PubMed] [Google Scholar]
- 8. Centers for Disease Control and Prevention . Clinical overview of human parainfluenza viruses (HPIVs). Updated 31 October 2019. Available at: https://www.cdc.gov/parainfluenza/hcp/clinical.html. Accessed 21 April 2021.
- 9. Reed G, Jewett PH, Thompson J, Tollefson S, Wright PF. Epidemiology and clinical impact of parainfluenza virus infections in otherwise healthy infants and young children <5 years old. J Infect Dis 1996; 175:807–13. [DOI] [PubMed] [Google Scholar]
- 10. Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics 2004; 113:1758–64. [DOI] [PubMed] [Google Scholar]
- 11. Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr 2009; 154:694–9. [DOI] [PubMed] [Google Scholar]
- 12. Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 2004; 350:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med 2008; 168:2489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis 2003; 187:785–90. [DOI] [PubMed] [Google Scholar]
- 15. Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med 2006; 144:344–9. [DOI] [PubMed] [Google Scholar]
- 16. Martinello RA, Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Infect 2006; 53:248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu W-K, Liu Q, Chen D-H, et al. Epidemiology and clinical presentation of the four human parainfluenza virus types. BMC Infect Dis 2013; 13:28. 10.1186/1471-2334-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hodson A, Kasliwal M, Streetly M, MacMahon E, Raj K. A parainfluenza-3 outbreak in a SCT unit: sepsis with multi-organ failure and multiple co-pathogens are associated with increased mortality. Bone Marrow Transplant 2011; 46:1545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah DP, Shah PK, Azzi JM, El Chaer F, Chemaly RF. Human metapneumovirus infections in hematopoietic cell transplant recipients and hematologic malignancy patients: a systematic review. Cancer Lett 2016; 379:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ren J, Phan T, Bao X. Recent vaccine development for human metapneumovirus. J Gen Virol 2015; 96:1515–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines – a new era in vaccinology. Nat Rev Drug Discov 2018; 17:261–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. John S, Yuzhakov O, Woods A, et al. Multi-antigenic human cytomegalovirus mRNA vaccines that elicit potent humoral and cell-mediated immunity. Vaccine 2018; 36:1689–99. [DOI] [PubMed] [Google Scholar]
- 23. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 – preliminary report. N Engl J Med 2020; 383:1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020; 383:2427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Food and Drug Administration . Moderna COVID-19 vaccine. Updated 1 April 2021. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/Moderna-covid-19-vaccine. Accessed 19 May 2021.
- 27. European Medicines Agency . EMA recommends COVID-19 vaccine Moderna for authorisation in the EU. Updated 6 January 2021. Available at: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-moderna-authorisation-eu#:~:text=COVID%2D19%20Vaccine%20Moderna%20is,Commission%20on%206%20January%202021.&text=A%20very%20large%20clinical%20trial,from%2018%20years%20of%20age. Accessed 20 May 2021. [Google Scholar]
- 28. Government of Canada . Moderna COVID-19 vaccine: authorized with conditions. Government of Canada. Updated 23 March 2021. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/vaccines/moderna/authorization.html. Accessed 20 May 2021. [Google Scholar]
- 29. Hassett KJ, Benenato KE, Jacquinet E, et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Ther Nucleic Acids 2019; 15:1–11. 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson J, Sorensen EW, Mintri S, et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci Adv 2020; 6:eaaz6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cao Y, Gao GF. mRNA vaccines: a matter of delivery. EClinicalMedicine 2021; 32:100746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research . Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. Updated September 2007. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical. Accessed 19 May 2022.
- 33. Zielinska E, Liu D, Wu H-Y, Quiroz J, Rappaport R, Yang D-P. Development of an improved microneutralization assay for respiratory syncytial virus by automated plaque counting using imaging analysis. Virol J 2005; 2:84. 10.1186/1743-422X-2-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med 2001; 7:719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Branche AR, Falsey AR. Parainfluenza virus infection. Semin Respir Crit Care Med 2016; 37:538–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Más V, Rodriguez L, Olmedillas E, et al. Engineering, structure and immunogenicity of the human metapneumovirus F protein in the postfusion conformation. PLoS Pathog 2016; 12:e1005859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stewart-Jones GBE, Chuang G-Y, Xu K, et al. Structure-based design of a quadrivalent fusion glycoprotein vaccine for human parainfluenza virus types 1-4. Proc Natl Acad Sci U S A 2018; 115:12265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stewart-Jones GBE, Gorman J, Ou L, et al. Interprotomer disulfide-stabilized variants of the human metapneumovirus fusion glycoprotein induce high titer-neutralizing responses. Proc Natl Acad Sci U S A 2021; 118:e2106196118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maman K, Zollner Y, Greco D, Duru G, Sendyona S, Remy V. The value of childhood combination vaccines: from beliefs to evidence. Hum Vaccin Immunother 2015; 11:2132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.