Abstract
Background
In hospitalized patients with skin and soft tissue infections (SSTIs), intravenous (IV) empiric antibiotic treatment is initiated. The best time point for switching from IV to oral treatment is unknown. We used an algorithm-based decision tree for the switch from IV to oral antibiotics within 48 hours and aimed to investigate the treatment outcome of this concept.
Methods
In a nonrandomized trial, we prospectively enrolled 128 patients hospitalized with SSTI from July 2019 to May 2021 at 3 institutions. Clinical and biochemical response data during the first week and at follow-up after 30 days were analyzed. Patients fulfilling criteria for the switch from IV to oral antibiotics were assigned to the intervention group. The primary outcome was a composite definition consisting of the proportion of patients with clinical failure or death of any cause.
Results
Ninety-seven (75.8%) patients were assigned to the intervention group. All of them showed signs of clinical improvement (ie, absence of fever or reduction of pain) within 48 hours of IV treatment, irrespective of erythema finding or biochemical response. The median total antibiotic treatment duration was 11 (interquartile range [IQR], 9–13) days in the invention group and 15 (IQR, 11–24) days in the nonintervention group (P < .001). The median duration of hospitalization was 5 (IQR, 4–6) days in the intervention group and 8 (IQR, 6–12) days in the nonintervention group (P < .001). There were 5 (5.2%) failures in the intervention group and 1 (3.2%) in the nonintervention group after a median follow-up of 37 days.
Conclusions
In this pilot trial, the proposed decision algorithm for early switch from IV to oral antibiotics for SSTI treatment was successful in 95% of cases.
Clinical Trials Registration. ISRCTN15245496
Keywords: cellulitis, erysipelas, oral antibiotics, skin and soft tissue infection
Skin and soft tissue infections (SSTIs) (ie, erysipelas and cellulitis without abscess formation or necrosis) rank among the most common community-acquired bacterial infections. The incidence of SSTI in the United States is approximately 50 per 1000 patient-years [1, 2]. The infection is typically caused by β-hemolytic streptococci (approximately 75%) and Staphylococcus aureus [1–3]. A causative microorganism is identified in only 20%–30% of cases [1, 3]. Even when no microorganisms are identified, clinical response to β-lactam antibiotics occurs in 95% of cases [3]. The empiric treatment recommendations for SSTIs in our institutions include amoxicillin-clavulanate as the first choice. In the case of penicillin allergy, oral clindamycin is a possible alternative. Depending on the severity of the disease, a decision for hospitalization or outpatient treatment is made. In hospitalized patients, intravenous (IV) empiric antibiotic treatment is commonly initiated. The optimal time point for switching from IV to oral antibiotic treatment is unknown. In this study, we aimed to investigate the treatment outcome for uncomplicated SSTIs in hospitalized patients by using an algorithm-based decision tree for the switch from IV to oral antibiotics within a maximum of 48 hours after IV treatment initiation.
METHODS
The prospective, nonrandomized, multicenter pilot trial was performed in 3 institutions (1 primary care–level centers and 2 secondary care–level center) in the Canton Bern, Switzerland. Eligible participants with SSTIs were 18 years or older and required hospitalization because of the severity of the disease. Exclusion criteria were antibiotic treatment in the 14 days before enrollment, surgical site infections, impetigo without erysipelas/cellulitis, mastitis, nonbacterial infection or sterile skin inflammation (eg, Sweet syndrome, hypersensitivity reaction), and criteria consistent with a “complicated” SSTI (ie, bacteremia with S aureus or Pseudomonas aeruginosa, necrotizing fasciitis, skin abscess, a septic shock or infection requiring intensive medical care, septic arthritis, osteomyelitis, tendosynovitis, bursitis, or foreign body infection). Other exclusion criteria included Gram-negative bacteria as the causative organism for an SSTI or ecthyma gangrenosum. If these latter criteria were fulfilled after enrollment, study participants were excluded after being included in the first 48 hours of treatment.
Demographics, comorbidities, clinical characteristics, microbiology findings, and laboratory values (designated as “lab1” to “lab3”) were prospectively collected. Variables specifically obtained for SSTI included visual analogue scale (VAS) for pain, the anatomic body site of the infection, and the size of the skin lesion (cm2) over time. Erythema in each enrolled patient was photographed.
Empiric IV antibiotics consisted of amoxicillin-clavulanate 2.2 g every 8 hours. In the case of penicillin allergy, cefuroxime 1.5 g (every 8 hours in case of delayed type allergy) or vancomycin (15 mg per kg body weight every 12 hours in case of immediate type allergy) was administered. In case of renal function impairment, doses were adapted accordingly. Oral antibiotics consisted of amoxicillin-clavulanate 1 g (3 times per day) or clindamycin (600 mg 3 times per day). The decision about total treatment duration was at the discretion of the responsible physician.
The study protocol flowchart is illustrated in Supplementary Figure 1.
The criteria for the switch from IV (maximum 48 hours, irrespective of time point of study inclusion) to oral treatment are shown in Supplementary Figure 2. Study participants who did not fulfill these criteria or declined to be included in the intervention group were assigned to the nonintervention group. In addition, prior to the switch to oral treatment, the local principal investigator (PI) evaluated clinical and laboratory values and was allowed to overrule the study intervention at his discretion because of insufficient clinical response to treatment at day 2. Study participants who remained on IV treatment because of the PI's decision were assigned to the nonintervention group.
For follow-up examination, patients were contacted via telephone on day 30 after initiation of antibiotic treatment and interviewed with a predefined questionnaire (Supplementary Figure 3). If available, clinical data (derived from telephone interviews) were complemented with laboratory examination (designated as “lab 4”) results performed by the patient's general practitioner.
The primary outcome was the proportion of “clinical failure,” a composite outcome according to definition. Clinical failure was defined as (i) a new increase in symptoms during antibiotic treatment or after switch to oral therapy; (ii) a second course of antibiotic therapy after discontinuing the first course; (iii) readmission within 30 days after making the diagnosis of SSTI because of persistent SSTI; or (iv) death. Cure was defined as absence of clinical failure.
Because this was a pilot study, we aimed for 100 patients in the intervention group and did not define the patient numbers in the nonintervention group. The number in the intervention group was arbitrarily chosen in the light of a previously reported study with a clinical response to β-lactam antibiotics in 95% of the SSTI cases [3].
Categorical parameters were compared with Fisher exact test, and continuous variables were compared with the Mann-Whitney U test. A P value of <.05 was considered statistically significant. The analysis and graphs were made with Stata/IC15.1 and GraphPad Prism (version 9.2.0) software.
The study was registered at ISRCTN (International Standard Randomized Controlled Trial Number 15245496).
Patient Consent Statement
Written consent was obtained from all study participants. The design of the work was approved by the ethics committee of the Canton Bern, Switzerland (KEK 2019-00558).
RESULTS
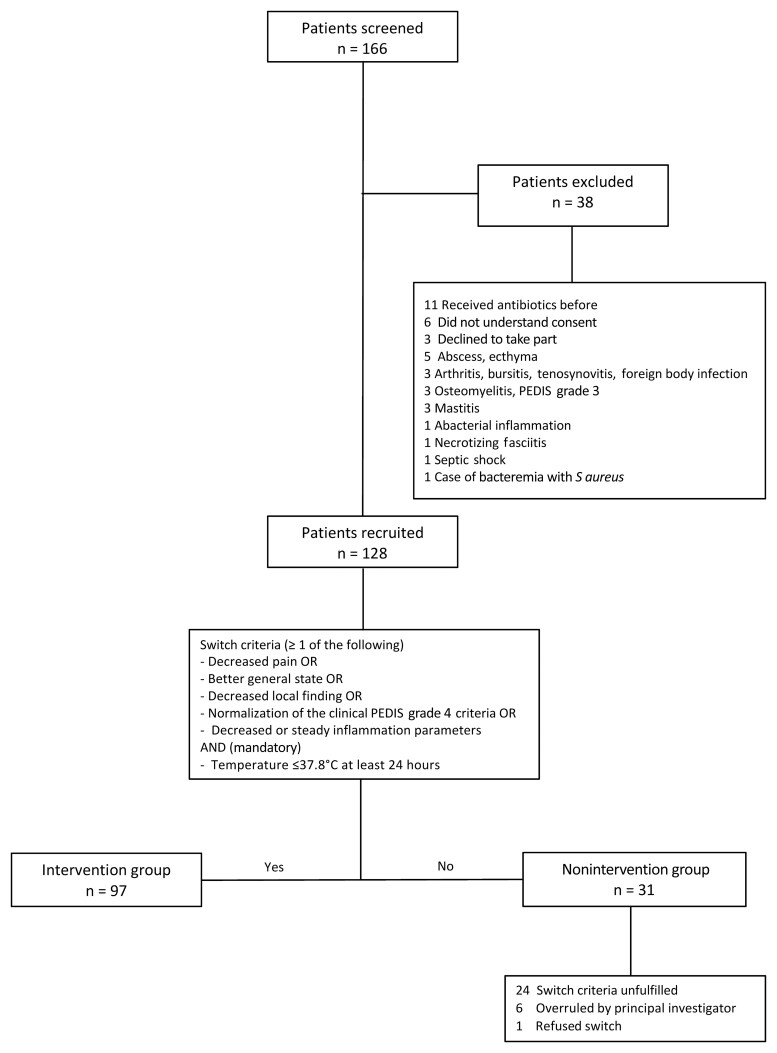
We screened 166 eligible patients and recruited 128 participants across the 3 sites between July 2019 and May 2021. Thirty-one (24.2%) study participants were assigned to the nonintervention group and 97 (75.8%) to the intervention group (Figure 1). Patient characteristics are shown in Table 1. The lower limb was the most commonly affected body site (79%), followed by the head (9%), upper limb (7%), and trunk/buttocks (5%) (Table 2). A causative microorganism was identified in 12 of 128 (9.4%) individuals; in 9 (7.0%) individuals blood cultures were positive, and in 3 patients samples from a skin blister showed bacterial growth. Streptococcus dysgalactiae subspecies equisimilis was the most frequent microorganism (8 cases), followed by Streptococcus agalactiae (3 cases) and Staphylococcus aureus (2 cases). In both infections due to S aureus, a second microorganism was co-isolated (1 sample each with S dysgalactiae subspecies equisimilis and Enterococcus faecalis).
Figure 1.
Flowchart of patient screening, exclusion, and enrollment. Abbreviation: PEDIS, Perfusion, Extent, Depth, Infection, and Sensation.
Table 1.
Patient Characteristics and Comorbidities of Patients Included in the Study
| Patient Characteristics | Study Population | Intervention | No Intervention | P Value |
|---|---|---|---|---|
| (n= 128) | (n = 97) | (n = 31) | ||
| Sex | ||||
| Male | 84 (65.6) | 62 (63.9) | 22 (70.9) | .1331 |
| Female | 44 (34.4) | 35 (36.1) | 9 (29.0) | |
| Age, y, median (IQR) | 62 (52–74.75) | 62 (50–76) | 60 (53–73) | .7339 |
| BMI, kg/m2, median (IQR) | 30.7 (26.3–36.2) | 29.5 (25.5–34.1) | 35.9 (29.1–43.3) | .0010 |
| DM type 2 | 23 (17.9) | 13 (13.4) | 10 (32.3) | .0292 |
| DM type 2 with insulin therapy | 9 (7.0) | 4 (4.1) | 5 (16.1) | |
| DM type 1 | 1 (0.8) | 0 (0) | 1 (3.2) | |
| DM type not recorded | 1 (0.8) | 0 (0) | 1 (3.2) | |
| Renal insufficiency | ||||
| None or grade 1 | 63 (49.2) | 53 (54.64) | 10 (32.3) | .1660 |
| Grade 2 | 51 (39.8) | 33 (34.02) | 18 (58.1) | |
| Grade 3a | 5 (3.9) | 4 (4.12) | 1 (3.2) | |
| Grade 3b | 9 (7.0) | 7 (7.22) | 2 (6.5) | |
| Grade 4/5 | 0 (0) | 0 (0) | 0 (0) | |
| Total patients with renal insufficiency | 65 (50.8) | 44 (45.4) | 21 (67.7) | |
| Cardiovascular disease | ||||
| PAOD | 7 (5.5) | 3 (3.1) | 4 (12.9) | .4026 |
| CHD | 18 (14.1) | 10 (10.3) | 8 (25.8) | |
| Hypertonia | 70 (54.7) | 48 (49.5) | 22 (70.9) | |
| 2 of the above | 18 (14.1) | 10 (10.3) | 8 (25.8) | |
| All 3 of the above | 3 (2.3) | 1 (1.0) | 2 (6.5) | |
| Total (≥1 of the listed) | 60 (46.9) | 38 (39.2) | 22 (70.9) | |
| Immunodeficiency | .5286 | |||
| Exogenous (ie, drugs) | 4 (3.1) | 3 (3.1) | 1 (3.2) | |
| Endogenous (ie, disease) | 3 (2.3) | 2 (2.1) | 1 (3.2) | |
| Neoplasia | 5 (3.9) | 2 (2.1) | 3 (9.7) | |
| Immunocompetent | 116 (90.6) | 90 (92.8) | 26 (83.8) | |
| Risk factors for SSTI | .2123 | |||
| Radiotherapy | 10 (7.8) | 5 (5.2) | 5 (16.1) | |
| Previous SSTI | 38 (29.7) | 26 (26.8) | 12 (38.7) | |
| Edema | 44 (34.5) | 26 (26.8) | 18 (58.2) | |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: BMI, body mass index; CHD, coronary heart disease; DM, diabetes mellitus; IQR, interquartile range; PAOD, peripheral artery occlusive disease; SSTI, skin and soft tissue infection.
Table 2.
Localization and Affected Site of Skin and Soft Tissue Infections
| Patient Characteristics | Study Population | Intervention | No Intervention | P Value |
|---|---|---|---|---|
| (n = 128) | (n = 97) | (n = 31) | ||
| Fever >38°C | 69 (53.91) | 48 (49.48) | 21 (67.74) | .1145 |
| Localization of SSTI | .0338 | |||
| Lower limb right | 52 (40.6) | 37 (38.1) | 15 (48.4) | |
| Lower limb left | 49 (38.3) | 36 (37.1) | 13 (41.9) | |
| Buttocks | 4 (3.1) | 4 (4.1) | 0 (0) | |
| Trunk | 3 (2.3) | 2 (2.1) | 1 (3.2) | |
| Upper limb right | 5 (3.9) | 5 (5.2) | 0 (0) | |
| Upper limb left | 4 (3.1) | 4 (4.1) | 0 (0) | |
| Head | 11 (8.6) | 9 (9.3) | 2 (6.5) |
Data are presented as No. (%) unless otherwise indicated. Ten patients (11.1%) had 2 localizations and 1 patient had 3 localizations of SSTI.
Abbreviation: SSTI, skin and soft tissue infection.
All individuals in the intervention group showed clinical improvement as defined in the study protocol after 48 hours of IV antibiotic treatment and thus qualified for a switch to oral antibiotic treatment. In the nonintervention group, 77% (24/31) did not fulfill these switch criteria. Seven participants fulfilled these criteria but either refused to switch (1 individual) or the switch decision was overruled by the local PI (6 individuals) (Figure 1).
The proportions of the primary outcomes (failures) were 5.2% (n = 5) in the intervention group and 3.2% (n = 1) the nonintervention group. The 5 failures in the intervention group consisted of 2 individuals with increase in symptoms or findings (abscess) after switch to oral therapy and 3 individuals with a second course of antibiotic therapy after discontinuing the first course and readmission within 30 days (ie, relapse). In the nonintervention group, there was 1 relapse. There were no deaths in either group.
In the intervention group, the median size of the erythema was 486 (interquartile range [IQR], 147–7380) cm2 on the day of hospital admission. During treatment, the size of the erythema diminished, and had reduced to a median of 100 (IQR, 9–531) cm2 on the day of discharge. On hospital admission, the mean reported VAS for pain was 3/10 and 2/10 when participants moved or rested, respectively, the extremity. During the course of hospitalization, these values improved to 1 and 0, respectively, in patients belonging to the intervention group (Supplementary Figure 4).
In the nonintervention group, the median size on the day of admission was 924 (IQR, 512–1750) cm2, which had reduced to a median of 256 (IQR, 42–910) cm2 on the day of discharge. In the nonintervention group, higher VAS pain scores than ones in the intervention group were reported (Supplementary Figure 4).
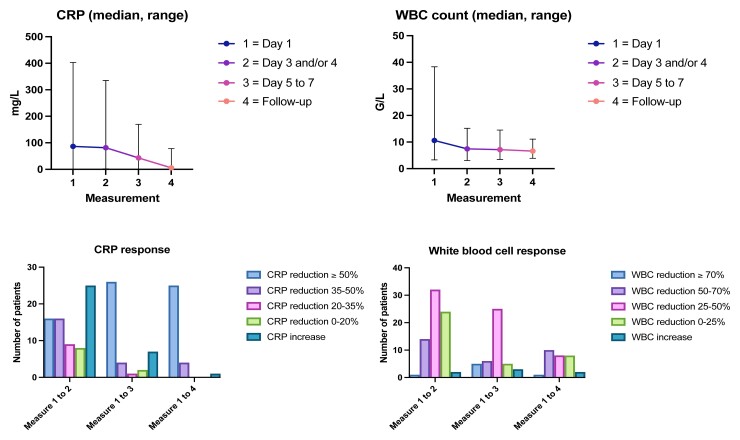
The dynamics of laboratory results obtained from study participants in the intervention group are illustrated in Figure 2.
Figure 2.
Biomedical response compared with the first measurement at hospital admission of the intervention group. Abbreviations: CRP, C-reactive protein; WBC, white blood cell.
The median total antibiotic treatment duration was 11 (IQR, 9–15) days in the entire study population, 11 (IQR, 9–13) days in the intervention group, and 15 (IQR, 11–24) days in the nonintervention group (P < .001). The median duration of hospitalization was 5 (IQR, 4–8) days in the study population, 5 (IQR, 4–6) days in the intervention group, and 8 (IQR, 6–12) days in the nonintervention group (P = .001).
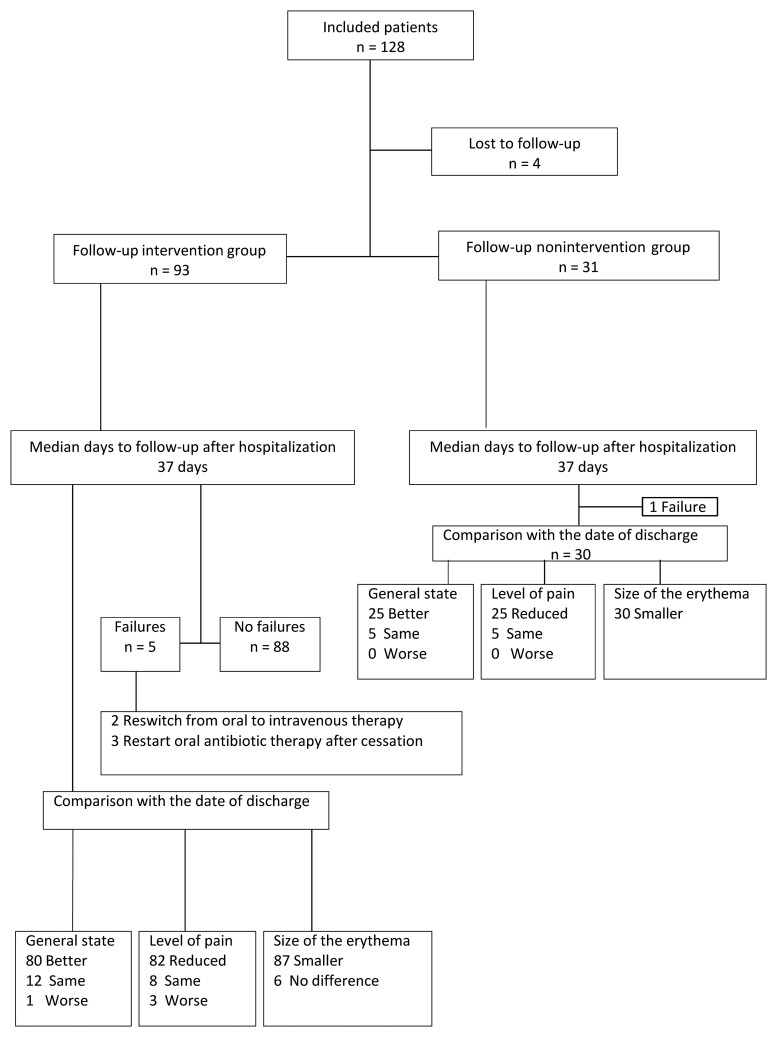
The follow-up phone call of the study population was performed on a median of day 37 (IQR, 32–50). Four individuals (3.13%) were lost to follow-up. The results from the follow-up questionnaire in the cured cases are illustrated in Figure 3.
Figure 3.
Responses of study participants to follow-up questionnaire.
DISCUSSION
In this prospective pilot study, we aimed to investigate whether or not the switch from IV to oral antibiotic treatment within 48 hours is safe and effective for a selected group of hospitalized patients with uncomplicated SSTIs. We used predefined criteria to assess the clinical response to antibiotic treatment. A biochemical response was not a prerequisite for switching, if clinical criteria indicated a response. As an intended consequence of the selection process, the comparison groups are uneven with more severe SSTI cases (larger size of erythema, higher inflammation values) in the nonintervention group. We proposed an algorithm to identify patients who are too ill to be managed in an outpatient setting but still qualify for an early switch from IV to oral treatment. The proportion of cure was 95% in 97 patients with a median follow-up of 37 days. With our approach, we observed a median length of stay of 5 days in the intervention group and 8 days in the nonintervention group (P = .001). This pilot trial demonstrates the potential reduction in hospitalization days for a frequent infectious disease entity with a good prognosis.
In our experience, IV antibiotic treatment is continued in hospitalized patients despite qualifying for an early switch to oral treatment. The reasons for prolonged IV treatment in these patients have not been explored and may include inadvertence, fear of unfavorable outcomes, or reliance on erythema or laboratory values for decision making.
Variable criteria for switching from IV to oral antibiotic treatment have been previously published. Ahkee et al [4] applied improvement in local signs and symptoms of an infection, guarantee of adequate gastrointestinal absorption, absence of fever (<37.8°C) for at least 8 hours, and decreasing leukocytosis as decision arguments. The authors did not restrict this concept to SSTIs. They included respiratory and urinary tract infections as well as intra-abdominal infections in their study [4]. A study from the Netherlands deemed a switch after 48 to 72 hours from IV to oral therapy as possible if the patient was hemodynamically stable, showed a trend toward normalization of body temperature, and exhibited improvement in leukocytosis for several infection entities [5]. Mertz et al [6] used similar switch criteria for different types of infections after 48–72 hours of IV treatment. In their switch criteria, a body temperature of <38.0°C for at least 24 hours was mandatory. A study from Norway defined switch criteria for cellulitis for days 1 and 3 [7]. On day 1, there had to be an improvement in clinical presentation (cessation of lesion spread and local inflammation defined by the intensity of erythema, warmth and tenderness). On day 3, there had to be an improvement in clinical presentation and a reduction of ≥20% in C-reactive protein (CRP) levels compared to day 1 or 2 [7]. An improvement in local findings, a body temperature of <37.8°C for at least 24 hours, and a reduction in the white blood cell count and CRP values were useful criteria for an early switch, provided that there was no impairment of gastrointestinal absorption. The strongest concordance between biomedical and clinical response occurred on days 2 and 3 [7]. In our study, nearly 35% of patients in the intervention group showed an increase in the CRP value while being on IV treatment but were still switched to an oral antibiotic compound. Similar to reports of others [7], our study indicates that CRP dynamics may be delayed. In our view, the CRP value is not a useful criterion for the switch decision. In addition, the local findings are frequently difficult to interpret and correlate poorly with the time-point bacterial killing, as erythema may persist for a prolonged time. Thus, in retrospect, CRP and erythema were overestimated as predefined switch criteria and may be removed from the algorithm in the future.
All patients included in our study had a level of severity of infection (not of comorbidity) that required hospitalization. The algorithm led to uneven distribution of the groups, with more severe cases in the nonintervention group. Similarly, the median body mass index and the frequency of diabetes mellitus was significantly higher in this group (Table 1); both comorbid conditions are risk factors for SSTI and poor outcome [8, 9]. In addition, obesity is an independent risk factor for recurrent skin infection and failure of antibiotic treatment in patients with cellulitis or cutaneous abscesses [10]. Thus, the proposed algorithm may also be helpful in identifying patients who do not qualify for an early switch for IV to orals, and who are at risk for failure if switched too early. However, this hypothesis was not the scope of the study. Our study demonstrates that the proposed criteria can be applied to a large proportion of patients hospitalized for SSTI, taking into account the regional epidemiology of comorbidities and low rates of methicillin-resistant bacteria.
Patients were assessed by a senior infectious disease physician after 48 hours to confirm or overrule the switch decision. Under these conditions, the cure rate for SSTIs was 95% in the intervention group, and similar to reports of other studies [3]. However, the cure rate according to oral or IV antibiotic treatment stratification is less known, because most studies report the overall cure rate of SSTI. The cure rate in the nonintervention group was 97%. We cannot exclude that a prolonged IV treatment would have led to an even higher cure rate in the intervention group. However, IV antibiotics should be switched to orals when clinical improvement is apparent [11]. In an antibiotic stewardship program, Gibbons et al [12] diminished the median number of days of IV antibiotic therapy to oral conversion in SSTI infections by 2 days (ie, from 5 to 3 days). These data illustrate that the time point of switch is reasonable between 2 and 3 days in most SSTI cases.
Our study has limitations. The enrollment of patients was delayed because of the coronavirus disease 2019 pandemic. The algorithm led to an intended uneven distribution of cases, with more severe cases in the nonintervention group. As this was a pilot study, there was no randomization, and the noninferiority statement would clearly require a larger sample size. We enrolled 97 patients in the intervention group. Both the targeted sample size of 100 patients in the intervention group and the switch time point of 48 hours were scientifically arbitrary, but in our view clinically reasonable. To demonstrate the number of hospital days saved on a larger scale, a further study is necessary. The group—designated as intervention group in this study—must be further randomized in an oral and IV treatment arm in a future trial. Considering a cure rate of 95% in this study, a sample size of 902 patients (451 in each group) would be necessary to confirm the noninferiority hypothesis. The measurement of the size of the erythema is subject to an interexaminer bias and the measurement of the pain intensity is subjective, adding bias in an open study setting. These variables did not play a major role in the decision-making process for the switch to orals. Similarly, the decision to overrule despite fulfilling criteria for switch to orals is biased by the perspective of the treating physicians. As this was a pilot study, we did not investigate whether there was an examiner bias when the switch criteria were assessed. In the follow-up phone call, there is potential recall bias. The proportion lost to follow-up was <5%. Finally, the high cure rate in both groups together with the small sample size in the nonintervention group did not allow any firm statistical conclusions.
In summary, in this prospective pilot trial on uncomplicated SSTIs in hospitalized patients, an algorithm-based switch from IV to oral antibiotic treatment after a maximum of 48 hours was successful in 95% of cases. Approximately 75% of the study population was switched from IV to oral treatment according to the algorithm. We observed a significantly shorter median duration hospitalization by 5 (IQR, 4–6) days in the intervention group compared to the nonintervention group with 8 (IQR, 6–12) days. This pilot study proposes a method to identify SSTI patients who do not require a prolonged hospitalization for IV treatment. A prospective randomized noninferiority multicenter trial will be required to confirm these results on level 1a evidence.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Sandra Dellsperger, Clinic of Internal Medicine, Emmental Hospital, Langnau, Switzerland.
Simea Kramer, Clinic of Internal Medicine, Emmental Hospital, Burgdorf, Switzerland.
Michael Stoller, Clinic of Internal Medicine, Emmental Hospital, Burgdorf, Switzerland.
Annika Burger, Clinic of Internal Medicine, Emmental Hospital, Langnau, Switzerland.
Elio Geissbühler, Clinic of Internal Medicine, Emmental Hospital, Langnau, Switzerland.
Isabel Amsler, Clinic of Internal Medicine, Emmental Hospital, Langnau, Switzerland.
Anna Hirsig, Clinic of Internal Medicine, Emmental Hospital, Langnau, Switzerland.
Linda Weyer, Clinic of Internal Medicine, Emmental Hospital, Langnau, Switzerland.
Ursula Hebeisen, Clinic of Internal Medicine, Emmental Hospital, Burgdorf, Switzerland.
Philipp Aebi, Clinic of Internal Medicine, Emmental Hospital, Langnau, Switzerland.
Nicolas Burgherr, Clinic of Internal Medicine, Emmental Hospital, Langnau, Switzerland.
Fabienne Brügger, Clinic of Internal Medicine, Emmental Hospital, Burgdorf, Switzerland.
Edouard Chaix, Department of Internal Medicine, Spitalzentrum Biel, Biel/Bienne, Switzerland.
Jérôme Salamoni, Department of Internal Medicine, Spitalzentrum Biel, Biel/Bienne, Switzerland.
Sandra Glauser, Clinic of Internal Medicine, Emmental Hospital, Langnau, Switzerland.
Annina Elisabeth Büchi, Department of General Internal Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland; Department of Emergency Medicine, Inselspital, Bern University Hospital, University of Bern, Bern, Switzerland.
Charles Béguelin, Department of Internal Medicine, Spitalzentrum Biel, Biel/Bienne, Switzerland.
Gabriel Waldegg, Clinic of Internal Medicine, Emmental Hospital, Burgdorf, Switzerland.
Bernhard Kessler, Clinic of Internal Medicine, Emmental Hospital, Burgdorf, Switzerland.
Martin Egger, Clinic of Internal Medicine, Emmental Hospital, Langnau, Switzerland.
Parham Sendi, Institute for Infectious Diseases, University of Bern, Bern, Switzerland.
Notes
Author contributions. Study design, conceptualization, clinical responsibility, writing, and critical revision: M. E., B. K., G. W., C. B., S. D., and P. S. Conducting the study, clinical examination of patients, and data entry: M. E., B. K., G. W., C. B., S. D., A. H., A. B., E. C., E. G., F. B., I. A., J. S., L. W., M. S., N. B., P. A., S. G., S. K., and U. H. Follow-up and data entry: S. D., S. G., and S. K. Data monitoring: A. E. B.
Acknowledgments. The generation and obtainment of data that led to this manuscript was possible because of volunteer work of the co-authors and allowance of research time for the co-authors within their clinical employment. Part of this work was included in the dissertation of Sandra Dellsperger at the Medical Faculty of the University of Bern, Switzerland. The thesis is available at BORIS Bern Open Repository and Information System of the University of Bern (https://boristheses.unibe.ch/3145). Barbara Every, ELS, BioMedical Editor (St Albert, Canada), provided English-language editing for the dissertation.
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Kaye KS, Petty LA, Shorr AF, Zilberberg MD. Current epidemiology, etiology, and burden of acute skin infections in the United States. Clin Infect Dis 2019; 68:S193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller LG, Eisenberg DF, Liu H, et al. Incidence of skin and soft tissue infections in ambulatory and inpatient settings, 2005–2010. BMC Infect Dis 2015; 15:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeng A, Beheshti M, Li J, Nathan R. The role of beta-hemolytic streptococci in causing diffuse, nonculturable cellulitis: a prospective investigation. Medicine (Baltimore) 2010; 89:217–26. [DOI] [PubMed] [Google Scholar]
- 4. Ahkee S, Smith S, Newman D, Ritter W, Burke J, Ramirez JA. Early switch from intravenous to oral antibiotics in hospitalized patients with infections: a 6-month prospective study. Pharmacotherapy 1997; 17:569–75. [PubMed] [Google Scholar]
- 5. Sevinç F, Prins JM, Koopmans RP, et al. Early switch from intravenous to oral antibiotics: guidelines and implementation in a large teaching hospital. J Antimicrob Chemother 1999; 43:601–6. [DOI] [PubMed] [Google Scholar]
- 6. Mertz D, Koller M, Haller P, et al. Outcomes of early switching from intravenous to oral antibiotics on medical wards. J Antimicrob Chemother 2009; 64:188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bruun T, Oppegaard O, Hufthammer KO, Langeland N, Skrede S. Early response in cellulitis: a prospective study of dynamics and predictors. Clin Infect Dis 2016; 63:1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ray GT, Suaya JA, Baxter R. Incidence, microbiology, and patient characteristics of skin and soft-tissue infections in a U.S. population: a retrospective population-based study. BMC Infect Dis 2013; 13:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ki V, Rotstein C. Bacterial skin and soft tissue infections in adults: a review of their epidemiology, pathogenesis, diagnosis, treatment and site of care. Can J Infect Dis Med Microbiol 2008; 19:173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Halilovic J, Heintz BH, Brown J. Risk factors for clinical failure in patients hospitalized with cellulitis and cutaneous abscess. J Infect 2012; 65:128–34. [DOI] [PubMed] [Google Scholar]
- 11. Esposito S, Bassetti M, Bonnet E, et al. Hot topics in the diagnosis and management of skin and soft-tissue infections. Int J Antimicrob Agents 2016; 48:19–26. [DOI] [PubMed] [Google Scholar]
- 12. Gibbons JA, Smith HL, Kumar SC, et al. Antimicrobial stewardship in the treatment of skin and soft tissue infections. Am J Infect Control 2017; 45:1203–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.