Abstract
Background
Enterococcal bacteremia carries significant mortality. While multiple studies have evaluated the impact of infectious disease consultation (IDC) on this condition, these studies were limited by the low numbers of patients enrolled. This systemic literature review and meta-analysis was conducted to determine whether IDC is associated with a mortality benefit among patients with enterococcal bacteremia.
Methods
We performed a systematic literature search using 5 databases for studies evaluating IDC among patients with enterococcal bacteremia. We conducted a meta-analysis to assess whether IDC was associated with reduced mortality. Random-effects models were used to calculate pooled odds ratios (pORs). Heterogeneity was evaluated using I2 estimation and the Cochran's Q statistic test.
Results
The systematic literature review revealed 6496 reports, from which 18 studies were evaluated in the literature review and 16 studies in the meta-analysis. When all studies were pooled, the association between IDC and mortality was not statistically significant with a pOR of 0.81 (95% CI, 0.61–1.08) and substantial heterogeneity (I2 = 58%). When the studies were limited to those reporting multivariate analysis including IDC, there was a significant protective effect of IDC (pOR, 0.40; 95% CI, 0.24–0.68) without heterogeneity (I2 = 0%). Some studies also showed additional benefits to IDC, including appropriate antibiotic therapy and improved diagnostic use.
Conclusions
IDC was associated with 60% lower odds of mortality when patients were well-matched, potentially through improvement in the care of patients with enterococcal bacteremia. IDC should be considered part of routine care for patients with enterococcal bacteremia.
Keywords: enterococcal bacteremia, Enterococcus, infectious diseases consultation
Enterococcus is a bacterial genus that is part of the commensal gastrointestinal microbiota [1] that can translocate across the intestine [2], causing bacteremia and other nosocomial infections [3]. Enterococcal bacteremia is associated with >20% mortality rates, especially with inappropriate antimicrobial therapy [4]. Most enterococcal infections are caused by Enterococcus faecalis or Enterococcus faecium. E. faecalis is typically ampicillin-susceptible and vancomycin-susceptible and is more frequently observed in community-acquired infections. E. faecium is highly associated with nosocomial infections and can often be a vancomycin-resistant Enterococcus (VRE); it is typically associated with worse outcomes [5, 6]. According to the 2015–2017 National Healthcare Safety Network data from the Centers for Disease Control and Prevention, Enterococcus species are the second most common antimicrobial-resistant pathogen implicated in all types of health care–associated infections, and the single most common cause of catheter-related bloodstream infections (CLABSIs) in patients at long-term acute care hospitals (LTACHs) with most of these representing E. faecalis infections [7]. Longitudinal surveillance has shown stable rates of VRE bacteremia, accounting for 16% of bloodstream isolates since 2012 [8]. When compared with vancomycin-susceptible strains, VRE has been associated with a 2-fold increase in morbidity and mortality, likely related to differences in virulence [9, 10].
Enterococcal species have also been implicated as the causative pathogen in cases of infective endocarditis (IE); these infections are more frequently caused by E. faecalis, with an incidence of 13%–25% of patients presenting with E. faecalis bacteremia [9, 11]. One study found a 12% prevalence of IE in patients with enterococcal bacteremia, with in-hospital mortality of 15% and a 1-year mortality rate of 35% [11], while another study found a relapse rate of about 5% [12].
Infectious disease consultation (IDC) is often helpful for managing aspects of complicated infections including bacteremia and IE, such as identifying the primary source of infection and choosing an appropriate antimicrobial therapy. Studies have previously shown that IDC has been associated with improved rates of mortality in patients with bloodstream infections such as Staphylococcus aureus bacteremia [13–18] and candidemia [19], as well as other types of infections including cryptococcosis, compared with patients who did not receive IDC. There have been several studies evaluating the effect of IDC for patients with enterococcal bacteremia, but fewer studies compared with S. aureus bacteremia. These studies were also limited by the low numbers of patients enrolled. Whether there is a role for mandatory IDC in enterococcal bacteremia remains somewhat unclear. We conducted a systematic literature review and meta-analysis to determine whether IDC is associated with improved mortality among patients with enterococcal bacteremia.
METHODS
Search Strategy and Selection Criteria for the Systematic Literature Review
A systematic literature review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement [20] and the Meta-Analysis of Observational Studies in Epidemiology (MOOSE) guidelines [21]. The study protocol had been approved by the International Prospective Register for Systematic Reviews (PROSPERO) database (CRD42021266399). The systematic literature search was developed and performed by a health sciences librarian (R.J.S.). Search strategies using subject headings and keywords were created for PubMed (United States NIH), Embase (Elsevier), Web of Science – Core Collection (Clarivate), CINAHL (EBSCO), and Cochrane CENTRAL (Wiley). The searches were conducted on August 5, 2021, and updated on December 30, 2021, without any date limitations; the studies were not limited to only those published in English. All database results were exported to EndNote and de-duplicated (Supplementary Table 1) [22]. Inclusion criteria were as follows: (1) original research manuscripts (ie, randomized control trials, cross-sectional, case–control, and cohort studies) and (2) assessed the effect of IDC on mortality in patients with enterococcal bacteremia. Exclusion criteria included the following: (1) editorials and commentaries and (2) animal studies. All potentially relevant studies were divided and screened independently by 2 of the reviewers (J.T. and H.S.).
Data Abstraction and Quality Assessment
Three independent reviewers (J.T., T.K., and A.R.M.) abstracted data from the studies using a standardized abstraction form; 2 of these 3 reviewers analyzed each article, with 1 reviewer (J.T.) reviewing all articles. The following data were collected as available: study design, study period, population characteristics, source of enterococci, percentage of VRE, the proportion of echocardiograms performed, and mortality. Nine authors were contacted for additional information, and 3 were able to provide additional information regarding the number of patients who died with and without IDC [23–25]. Any discordance was mediated by a fourth independent reviewer (H.S.) who also reviewed all articles; after discussion, the consensus response was reported.
Each study was assessed with the Downs and Black scale to evaluate the risk of bias and quality (Supplementary Table 2) [26]. All questions were answered as intended except for question #27 regarding Power, which was changed to a yes/no answer with associated points. The reviewers performed the component quality analysis independently, reviewed any discrepancies, and came to a consensus after discussion as previously described.
Statistical Analysis
We used random-effects models with inverse variance weighting to calculate the pooled odds ratio (pOR) and 95% CI. We performed stratified analyses by study location, presence/absence of adjustment for confounders, type of outcome (30-day mortality, 90-day mortality, or in-hospital mortality), and quality of study. Heterogeneity was assessed using I2 estimation and the Cochran's Q statistic test. Publication bias was evaluated by visual inspection of funnel plots. All meta-analyses were conducted using the Cochrane Review Manager (Revman), version 5.4 (The Cochrane Collaboration, Copenhagen, Denmark).
RESULTS
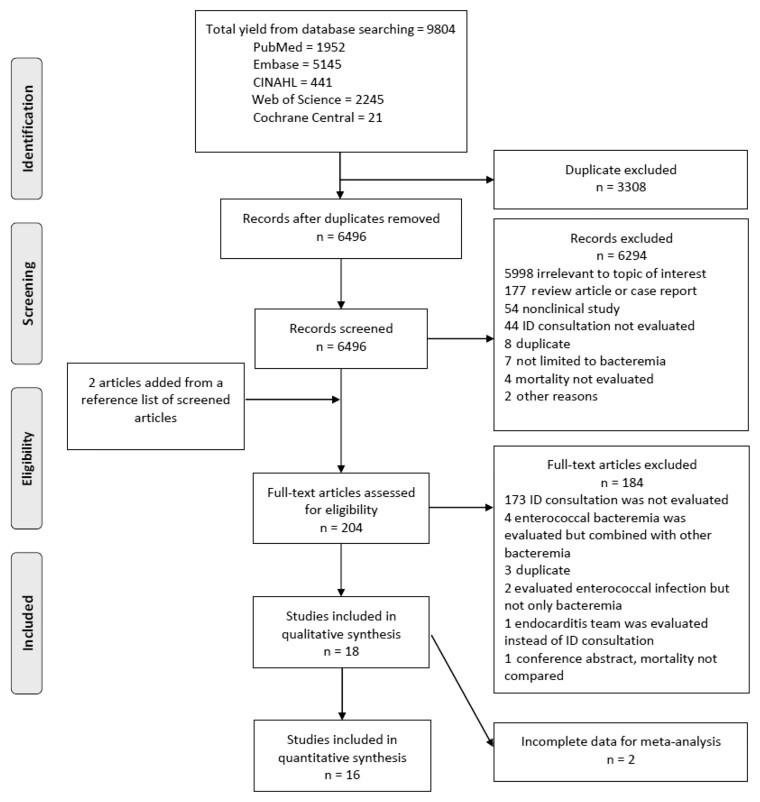
A flowchart summarizing the article selection process is shown in Figure 1. We initially identified 9804 articles from the database search; after the removal of duplicate articles, 6496 articles were identified. After screening by title and abstracts, 204 full-text articles were evaluated, including an additional 2 studies that were added after screening the references of the articles. A total of 18 articles were included for our systemic literature review [23–25, 27–41] (Table 1). Two articles [31, 38] were later excluded due to incomplete data for the meta-analysis, leaving 16 studies for the meta-analysis [23–25, 27–30, 32–37, 39–41].
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 flow diagram. Abbreviation: ID, infectious diseases.
Table 1.
Summary of the 18 Studies Included in the Systematic Review
| First Author/Publication Year/Location | Setting | Study Design | Adjustment for Confounders | Study Period | Characteristic of Included Patients | Source of Bacteremia | Enterococcus Species | Antimicrobials Studied | Outcome | D&B Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Bartoletti/2019/Bologna, Italy [27] | Single academic center | Quasi-experimental study | Cox proportional hazard regression (result of multivariate analysis not available) | 4 y | Adult, mean age 70 y 20.9% community-acquired 10.3% ICU admission 20.1% DM 24.7% CKD 12.8% cirrhosis 23.1% immunocompromised 23.6% malignancy 7.3% solid organ transplant Excluded polymicrobial bacteremia | UTI 17.7% IAI 36.4% Line-associated 14.4% Endocarditis 9.8% | E. faecalis 56% E. faecium 35.1% Others 8.7% VRE 1.4% | Evaluated appropriateness of therapy and use of combination therapy, with suggested dosages included | Pre–post study of alert system and structured IDC for enterococcal bacteremia; 30-d mortality was the primary outcome. Bivariate analysis showed IDC was protective (HR, 0.42; 95% CI, 0.28–0.62). During the postimplementation period, more patients got appropriate therapy, follow-up blood cultures, echocardiography, and source control. | 20 |
| Britt/2017/USA [28] | 99 Veterans Affairs hospitals | Retrospective cohort study | None | 11 y | Adult patients who had VRE bacteremia treated with either linezolid or daptomycin 32% ICU admission 51.8% CKD 9.5% cirrhosis 3.2% HIV 37.3% malignancy 3.2% transplant Enterococcus with resistance to both linezolid and daptomycin was excluded | UTI 11.6% IAI 4.4% Line-associated 10.8% SSTI 3.3% Endocarditis 8% Unknown 47% Multiple sites 14.9% | E. faecium 100% VRE 100% | Linezolid and daptomycin | IDC was associated with decreased 30-d mortality (370/1335, 27.7%, for IDC vs 549/1444, 38.0%, for NIDC: RR, 0.86; 95% CI, 0.82–0.90; P ≤ .001). The trend is the same among the linezolid therapy group and daptomycin therapy group, but not in the linezolid-to-daptomycin group. | 21 |
| Cattaneo/2021/Freiburg, Germany [23] | Single academic center | Prospective cohort study | Cox regression analysis | 3 y 1 mo | Adult, median age 68 y 26% DM 30% CKD 10% chronic liver disease 3% IVDU 43% malignancy 13% severe immunosuppression 40% polymicrobial bacteremia 42% ICU admission | UTI 14% IAI 33% Line-associated 13% Endocarditis 20% Osteomyelitis 2% Unknown 15% | E. faecalis 100% VRE 0% | Ampicillin as definitive therapy | In multivariate Cox regression analysis, ID was not significantly protective for mortality or recurrence within 90 d (HR, 0.87; 95% CI, 0.45–1.66). IDC was significantly associated with follow-up blood culture (91% vs 56%; P < .001), early source control (89% vs 65%; P = .001), echocardiography (84% vs 25%; P < .001), use of ampicillin as definitive therapy (78% vs 30%; P < .001), and adequate treatment duration (76% vs 43%; P = .001). | 21 |
| Erlandson/2008/Nebraska, USA [29] | 2 academic centers | Retrospective cohort study | None | 12 y 2 mo | 32.7% CKD 61.9% liver dysfunction 40.7% malignancy 55.8% transplant | Not reported | E. faecalis 0.9% E. faecium 99.1% VRE 100% | Linezolid and quinupristin-dalfopristin | Outcome was in-hospital mortality. IDC was associated with higher in-hospital mortality (OR, 2.96; 95% CI, 1.02–8.61; P < .05) but not selected in the multivariate model. | 15 |
| Furuichi/2018/Tokyo, Japan [30] | Single academic center | Retrospective cohort study | Multiple logistic regression | 14 y 9 mo | Pediatric, median age 9.3 mo 31% polymicrobial bacteremia 15% transplant | UTI 9% IAI 19% Line-associated 59% Endocarditis 2% SSI 1% Unknown 10% | E. faecalis 62% E. faecium 30% E. casseliflavus or E. gallinarum 5% Others 3% VRE 2.7% | Penicillins, glycopeptides (ie, vancomycin), and aminoglycosides | 30-d mortality was 8% (8/100) among those who got IDC vs 17% (9/52) among those who did not (OR, 0.42; 95% CI, 0.15–1.15; P = .11). Multivariate analysis showed an OR of 0.55 (95% CI, 0.16–1.71; P = .28). IDC was associated with appropriate empiric therapy and definitive therapy. Source of bacteremia was less likely unknown with IDC (5% IDC vs 19% NIDC). | 20 |
| Gray/2018/Virginia, USA [31] | Single academic center | Quasi-experimental study | None | 3 y 11 mo | Adult, mean age 60 y 73.6% central venous catheter 38.2% community-acquired 38.2% renal failure 15.5% cirrhosis 33.6% immunocompromised 28.2% malignancy 20% transplant Excluded polymicrobial bacteremia | UTI 5.5% IAI 26.4% Line-associated 30.9% SSTI 3.6% Endocarditis 11.8% SSI 5.5% Unknown 16.4% | E. faecalis 30.9% E. faecium 69.1% VRE 59.1% | Penicillins, vancomycin, daptomycin, and linezolid | Relationship between IDC and mortality was not available. | 21 |
| Jindai/2014/Wisconsin, USA [32] | Single academic center | Retrospective cohort study | None | 2 y 10 mo | Adult, mean age 57.1 y 57.1% central venous catheter 13.2% community-acquired 24.3% polymicrobial bacteremia 31.6% DM 12.8% cirrhosis 42.9% malignancy 31.8% transplant | UTI 9.5% IAI 27.5% Line-associated 18.5% | E. faecalis 39.7% E. faecium 56.6% Others 3.7% VRE 39.7% | Not reported | Outcome was in-hospital mortality. IDC was similarly made among those who survived (86/153, 56.2%) vs those who died (20/35, 57.1%; P = .92). As a secondary outcome, IDC was significantly associated with elimination of bacteremia in multivariate analysis (OR, 2.50; 95% CI, 1.32–4.72; P = .005). | 19 |
| Jumah/2018/Singapore [33] | Single academic center | Retrospective cohort study | Multiple logistic regression (result of multivariate analysis not available) | 6 y 5 mo | Adult, median age 75 y 14% ICU admission 36.8% DM 10.5% cirrhosis 7% immunocompromised 31.6% malignancy Excluded polymicrobial bacteremia | UTI 22.8% IAI 31.6% Line-associated 3.5% SSTI 5.3% Endocarditis 5.3% Unknown 24.6% | E. faecalis 36.8% E. faecium 56.1% Others 7% VRE 0% | Vancomycin with and without aminoglycosides | IDC was similarly done for patients who survived after 30 d (28/47, 59.6%) vs those who died within 30 d (5/10, 50%; P = .72). | 22 |
| Lee/2020/Alabama, USA [24] | Single academic center | Retrospective cohort study | Multiple logistic regression | 1 y 6 mo | Adult, median age 59 y 16% community-acquired 53% ICU admission 36% DM 28% CKD 9% cirrhosis 5% IVDU 25% immunocompromised 4% connective tissue disease 2% HIV 8% hematological malignancy 12% transplant | UTI 10% IAI 14% Line-associated 21% SSTI 3% Endocarditis 8% Bone & joint 8% Others 7% Unknown 32% | E. faecalis 65% E. faecium 33% Others 2% VRE 33% | Evaluated appropriateness of therapy; regimen not discussed | 30-d mortality was 12% (16/131) among those who got IDC vs 27% (20/74) among NIDC (P = .007). IDC was a significant protective factor for 30-d mortality (aOR, 0.35; 95% CI, 0.16–0.76; P = .07). IDC was associated with increased likelihood of repeat blood cultures (aOR, 12.83), echocardiography (aOR, 2.45), appropriate antibiotic duration (aOR, 6.65), and decreased likelihood of unknown source of bacteremia (aOR, 0.31). | 21 |
| MacVane/2016/South Carolina, USA [34] | Single academic center | Quasi-experimental study | None | 5 y | Adult, 33.8% community-acquired 20.6% polymicrobial bacteremia 38.2% ICU admission 35.3% DM 19.1% renal replacement therapy 5.9% liver disease 4.4% HIV 47.1% hematological malignancy | UTI 10.3% IAI 26.5% Line-associated 19.1% SSTI 1.5% Unknown 42% | E. faecalis 7% E. faecium 93% VRE 100% | Vancomycin, daptomycin, and linezolid | Outcome was in-hospital mortality; 36.2% (17/47) of patients who got IDC died, and 23.8% (5/21) of patients who did not died (OR, 1.81; 95% CI, 0.31–6.47). | 20 |
| Malone/1986/Ohio, USA [35] | 2 community hospitals | Retrospective cohort study | None | 4 y | Adult, 36.4% community-acquired 38.2% polymicrobial bacteremia | UTI 23.6% IAI 10.9% Line-associated 5.5% SSTI 10.9% | Not reported | Evaluated appropriateness of therapy and use of combination therapy, with regimens included | Unclear what time frame was used for mortality; 54.5% (12/22) of patients who got IDC died, and 36.4% (12/33) of patients who did not died (OR, 2.1; 95% CI, 0.32–5.94). | 15 |
| McKinnell/2011/Alabama, USA [36] | Single academic center | Retrospective cohort study | Multiple logistic regression | 3 y 7 mo | Adult patients with nosocomial VRE bacteremia Mean age 53.7 y 51% polymicrobial bacteremia 39% ICU admission 36% DM 49% CKD 23% liver disease 22% immunocompromised 3% HIV 30% hematological malignancy 16% transplant | Not reported | E. faecalis 3% E. faecium 97% VRE 100% | Linezolid and daptomycin | IDC was done for patients who survived after 30 d (27/153, 18%) vs who died within 30 d (7/82, 9%; P = .06). Multivariate analysis showed that IDC had a nonsignificant trend toward being protective (OR, 0.4; 95% CI, 0.2–1.2; P = .06). | 21 |
| Mercuro/2020/Michigan, USA [37] | Single academic center | Retrospective cohort study | None | 6 y 2 mo | Adult patients with history of solid organ transplant Median age 61 y 75% central venous catheter 36.5% community-acquired 50% polymicrobial bacteremia | UTI 8% IAI 83% Unknown 8% | E. faecium 100% VRE 79% | Daptomycin with or without a beta-lactam, and linezolid | 30-d mortality was 23% (11/48) among those who got transplant IDC. No information about N IDC. Transplant IDC was not associated with mortality in bivariate analysis (OR, 0.3; 95% CI, 0.2–5.2). | 18 |
| Nakagawa/2018/Washington, USA [38] | Single academic center | Retrospective cohort study | None | 3 y 11 mo | Adult, 17.2% DM 10.9% CKD 21.9% cirrhosis 81.3% immunocompromised 1.6% HIV 71.9% malignancy 10.9% solid organ transplant | Not reported | VRE 100% | Daptomycin and linezolid | Relationship between IDC and mortality was not available. | 19 |
| Nakakura/2019/Osaka, Japan [25] | Single academic center | Retrospective cohort study | None | 5 y 11 mo | Adult with Enterococcus faecium bacteremia treated with vancomycin Median age 73 y 26.7% central venous catheter Excluded if received dialysis | UTI 11.1% IAI 11.1% Line-associated 8.9% Biliary tract 48.9% Unknown 20% | E. faecium 100% VRE 0% | Vancomycin | IDC was done for 63.6% of patients who survived after 30 d (21/33) vs 50% of those who died within 30 d (6/12; P = .50). | 20 |
| Narayanan/2019/New Jersey, USA [39] | Single academic center | Retrospective cohort study | None | 4 y 8 mo | Patients with VRE bacteremia treated with either linezolid or daptomycin 59.1% central venous catheter 16.1% community-acquired 28% ICU admission | IAI 17.2% Line-associated 26.9% Endocarditis 5.4% Unknown 45.2% | E. faecium 100% VRE 100% | Linezolid and daptomycin | Outcome was 14-d in-hospital mortality. Mortality was 22.1% (19/86) among those who got IDC vs 14.3% (1/7) among those who did not (P = .629). | 20 |
| Valentin/2021/Graz, Austria [40] | Single academic center | Prospective cohort study | None | 1 y | Not well described Patients after RAST followed by ID consultation were compared with a historical cohort | Not reported | E. faecalis or E. faecium 100% VRE 0% | Evaluated changes in therapy after IDC; specific regimens not discussed | 30-d mortality was 50% (5/10) among those who got RAST and IDC vs 27.7% (13/47) among those who did not. | 14 |
| Zasowski/2016/Michigan, USA [41] | Single academic center | Retrospective cohort study | Multiple logistic regression (result of multivariate analysis for mortality was not available) | 5 y | Adult with hospital-onset enterococcal bacteremia Mean age 63.4 y 32.1% polymicrobial bacteremia 36.3% ICU admission 45.3% DM 53.2% CKD 17.4% cirrhosis 6.8% IVDU 11.6% immunocompromised 4.7% HIV 16.8% malignancy 1.1% transplant | UTI 10.5% IAI 15.8% Line-associated 49.4% SSTI 9.5% Endocarditis 3.7% Unknown 10.5% | E. faecalis 53.2% E. faecium 46.8% VRE 62.6% | Evaluated appropriateness of empiric and definitive therapies, including vancomycin, ampicillin, piperacillin-tazobactam, linezolid, and daptomycin | IDC within 24 h after blood culture was done for 50% of patients who survived after 30 d (22/44) vs 52.1% of those who died within 30 d (76/146; P = .81). IDC was an independent factor for less delayed therapy (adjusted risk ratio, 0.41; 95% CI, 0.26–0.64; P < .001). IDC was also associated with shorter time to appropriate therapy (HR, 0.593; 95% CI, 0.436–0.805; P = .001). | 21 |
Abbreviations: aOR, adjusted odds ratio; CKD, chronic kidney disease; DM, diabetes mellitus; HR, hazard ratio; IAI, intra-abdominal infection; ICU, intensive care unit; ID, infectious diseases; IDC, infectious disease consultation; IVDU, intravenous drug use; NIDC, no infectious disease consultation; OR, odds ratio; RAST, rapid antimicrobial susceptibility testing; RR, relative risk; UTI, urinary tract infection; SSTI, skin and soft tissue infection; VRE, vancomycin-resistant Enterococci.
Study Characteristics
Of the 18 included studies, 14 (78%) were retrospective cohort studies [24, 25, 27–30, 32, 33, 35–39, 41], 2 (11%) were prospective cohort studies [23, 40], and 2 (11%) were quasi-experimental studies [31, 34]. Thirteen (72%) were performed at a single academic center [23–25, 27, 30–34, 36–41], 1 study was performed at 2 academic centers [29], another study was performed at 99 Veteran Affairs hospitals [28], and the other study was performed at 2 community hospitals [35]. Two were performed at the same academic center [24, 36]. Most of the studies were conducted in the United States (12/18, 67%) [24, 28, 29, 31, 32, 34–39, 41], followed by Japan (2/18, 11%) [25, 30], Germany (1/18, 6%) [23], Italy (1/18, 6%) [27], Singapore (1/18, 6%) [33], and Austria (1/18, 6%) [40]. Most of the studies (94%) evaluated adult patients [23–25, 27–29, 31–41], with median ages ranging from 53.7 to 75 years, though 1 (6%) evaluated pediatric patients, with a median age of 9.3 months [30]. Enterococcus species identification was available in 15 of the studies (83%) [23–25, 27–34, 36–41]. VRE was identified in 13 of the studies (72%) [24, 27–32, 34, 36–39, 41], and was solely studied in 6 (33%) of the studies [28, 29, 34, 36, 38, 39]; most of the VRE identified was E. faecium. The source of enterococcal bacteremia was described in 14 studies (78%) [23–25, 27, 28, 30–35, 37, 39, 41]. Primary line–associated bloodstream infection was the main source in 3 studies (17%) [30, 31, 41], intra-abdominal infections were the main source in 6 studies (33%) [23, 25, 27, 32, 33, 37], and urinary tract infections were the main source in 1 study (6%) [35]. Patients with identified IE due to Enterococcus species were noted in 9 of the 18 studies (50%) [23, 24, 27, 28, 30, 31, 33, 39, 41]. Ten studies evaluated 30-day mortality (56%) [24, 25, 27, 28, 30, 33, 36, 37, 41], and 3 evaluated in-hospital mortality (17%) [29, 32, 34]. One study evaluated 14-day mortality (6%) [39], 2 evaluated 90-day mortality (6%) [23, 30], and another did not specify the time frame (6%) [35]. Eight of the studies (44%) used multivariate analysis [23, 24, 27, 29, 30, 33, 36, 41], with 3 of these reporting the multivariate analysis results for mortality alone (17%) [24, 30, 36]. When assessing the quality of the 18 included studies, 15 (83%) were classified as high quality with a score of ≥19 on the Downs and Black scale [23–25, 27, 28, 30–34, 36, 38–41]. Five studies (28%) evaluated outcomes other than mortality [23, 24, 30, 32, 41]. Among these studies, 4 [23, 24, 30, 41] reported more appropriate treatment in patients receiving IDC, such as appropriate empiric and definitive therapy [23, 30], appropriate treatment duration [23, 24], and shorter time to appropriate therapy [41]. Two studies [24, 30] reported more frequent identification of the primary source of bacteremia. The studies also reported increased use of diagnostics such as follow-up blood cultures and echocardiography [23, 24], earlier source control [23], and increased elimination of bacteremia [32].
Meta-analysis
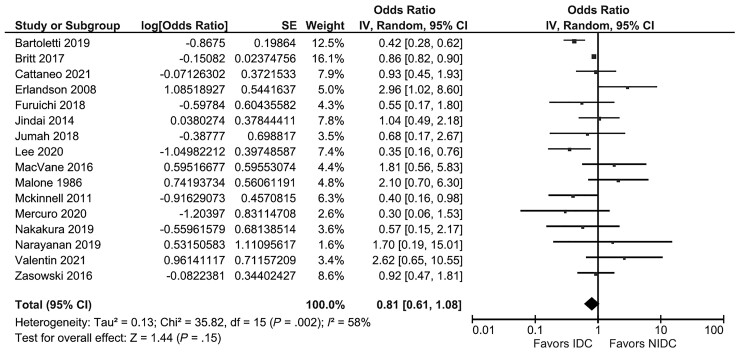
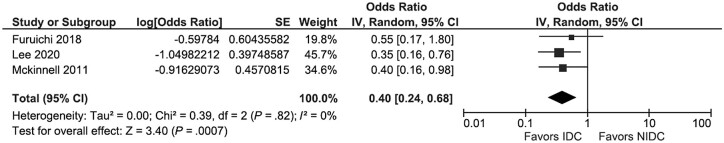
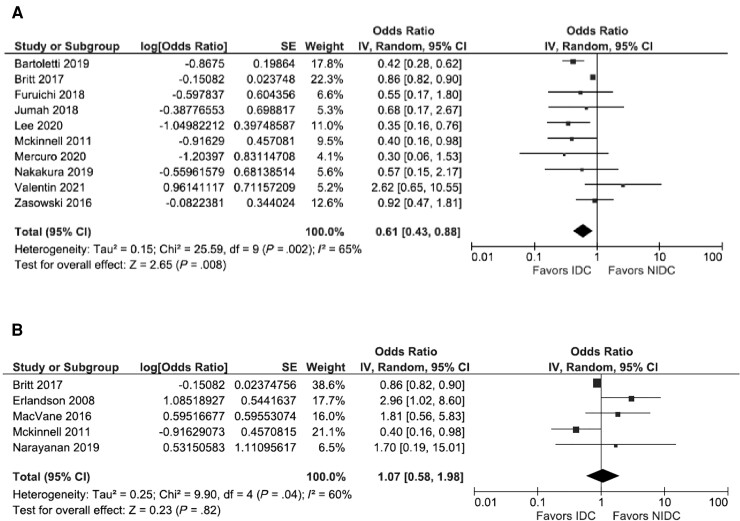
Sixteen studies were included in the meta-analysis [23–25, 27–30, 32–37, 39–41]. The between-study heterogeneity differed depending on the outcome assessed. When all 16 studies were included, studies were substantially heterogeneous, and there was not a statistically significant association between IDC and overall mortality (pOR, 0.81; 95% CI, 0.61–1.08; I2 = 58%) (Figure 2). When the 3 studies that reported the multivariate analysis results were pooled, studies were homogenous, and there was a significant protective effect of IDC (pOR, 0.40; 95% CI, 0.24–0.68; I2 = 0%) (Figure 3). For the 10 studies that used 30-day mortality as an outcome, IDC was significantly protective, but there remained substantial heterogeneity (pOR, 0.61; 95% CI, 0.43–0.88; I2 = 65%) (Figure 4A). For the 5 studies that exclusively evaluated VRE bacteremia, there was no association between IDC and mortality (pOR, 1.07; 95% CI, 0.58–1.98; I2 = 60%) (Figure 4B). For the 12 retrospective cohort studies included in the meta-analysis, there was also no association between IDC and mortality (pOR, 0.81; 95% CI, 0.59–1.10; I2 = 42%) (Supplementary Figure 1A). For the 12 studies with a Downs and Black score ≥19, there was a significant protective association between IDC and mortality, with significant heterogeneity (pOR, 0.70; 95% CI, 0.52–0.93; I2 = 54%) (Supplementary Figure 1B).
Figure 2.
All 16 studies included in the meta-analysis. Abbreviations: IDC, infectious disease consultation; IV, inverse variance.
Figure 3.
Studies using multivariate analysis with results available. Abbreviations: IDC, infectious disease consultation; IV, inverse variance.
Figure 4.
Stratified analysis with studies using 30-day mortality as an outcome (A) and studies exclusively evaluating VRE bacteremia (B). Abbreviations: IDC, infectious disease consultation; IV, inverse variance; NIDC, no infectious disease consultation.
Publication Bias
The funnel plot (Supplementary Figure 2) revealed that the studies were reasonably balanced around the pORs. Thus, there was little evidence of publication bias.
DISCUSSION
When we pooled all 16 studies together from our systematic literature review and meta-analysis, we did not identify a statistically significant effect of IDC on overall mortality. However, when the studies were limited to only those that reported the result of multivariate analysis, there was a statistically significant protective associated between IDC and mortality. Stratified analyses suggested a protective effect of IDC although some heterogeneity remained. Some studies also showed additional benefits to IDC, including a shorter time to appropriate antibiotic therapy, appropriate duration of antibiotic use, increased diagnostic use such as repeat blood cultures or echocardiography, and better source identification.
There was noted to be marked heterogeneity in the included studies. An explanation for the lack of statistical significance and presence of heterogeneity when all 16 studies were included in the meta-analysis could be the retrospective nature of these studies, as many of them were observational studies or pre–post studies. It may have been that these patients were not well-matched, such as more severely ill patients having received IDC vs those who were not as ill. When limited to studies that evaluated 30-day mortality, there was a statistically significant impact on mortality; however, there remained a degree of heterogeneity among the studies included.
When the meta-analysis was limited to studies that performed multivariate analysis including adjustment for patients receiving IDC compared with patients who did not receive IDC [24, 30, 36], the association between IDC and mortality became significantly more protective with a 60% reduction in mortality. There was also greater homogeneity noted among these 3 studies. This result aligns with the benefit of IDC in patients with S. aureus bacteremia [13–18]. These studies have suggested increases in quality of care and decreases in treatment failure, mortality, and hospital length of stay. Like S. aureus bacteremia, enterococcal bacteremia is highly associated with IE and significant mortality [11]. ID physicians can assist in choosing appropriate antimicrobial therapy in a timely manner, which is associated with improved outcomes [41]. Patients with IDC have more blood cultures drawn, leading to a more accurate assessment of bacteremia clearance [23, 32]. ID physicians are often able to elucidate the likely primary source of infection and recommend appropriate workup for potential complications, including higher rates of central line removal [24] and other early source control [23]. IDC is also associated with higher rates of obtaining echocardiography, an increase in appropriate antibiotic duration based on the primary source of infection, and increased surgical interventions [23, 24]. Identification of IE in patients with enterococcal bacteremia is very important, as prolonged combination antibiotic therapy, rather than monotherapy, is recommended by the guidelines for the treatment of enterococcal IE to achieve a better outcome [42, 43]. We believe that such improvements in the quality of care for patients with enterococcal bacteremia may lead to improved outcomes for those patients.
When assessing the association between IDC and mortality in patients with VRE bacteremia, there does not appear to be an improvement in patients who receive IDC. The exact reason for not seeing the benefits of IDC in patients with VRE bacteremia is not clear. Among the 6 studies included in the systematic review that exclusively evaluated patients with VRE bacteremia, 2 studies reported improved outcomes with IDC [28, 36], 1 study reported worse outcomes with IDC [29], and 3 did not find any significant difference [34, 38, 39]. Britt et al. reported in their study involving 2779 patients in 99 Veterans Affairs health care systems that IDC was associated with a significantly lower 30-day mortality (27.7% in IDC vs 38.0% in no IDC; P < .001). The protective effect was observed among patients treated with daptomycin, linezolid, or sequential treatment with linezolid to daptomycin [28]. Unfortunately, detailed information was not available in the other studies. It is possible that a protective effect was not observed due to the heterogeneity among studies. Perhaps unfamiliarity with therapeutic options beyond vancomycin in patients with VRE is more likely to prompt IDC, but any potential benefit is limited by the smaller number of available effective antimicrobials. It is likely that a diagnosis of VRE bacteremia is more likely to prompt an IDC compared with a diagnosis of E. faecalis bacteremia, especially if there is a lack of knowledge regarding the association of E. faecalis bacteremia with IE and without routine echocardiography which may not occur without IDC [42]. Around 25% of patients with enterococcal bacteremia undergo routine echocardiography [43], likely leading to underdiagnosis of IE in these patients. A prospective study showed that routine echocardiography done on consecutive patients diagnosed with E. faecalis bacteremia was associated with a concurrent diagnosis of IE in 26% of patients after discussion with an endocarditis team [44]. Patients with enterococcal IE may also be more prone to poorer outcomes compared with patients with enterococcal bacteremia without IE, regardless of IDC.
Our systematic literature review and meta-analysis have several limitations. First, all the studies included in our meta-analysis were either cohort studies or quasi-experimental pre–post studies, and 2 were performed at the same academic center. In addition, most of the included studies did not aim to compare patients who received IDC with those who did not. Although our stratified analysis which used the 3 studies with multivariate analysis results suggested a protective effect of IDC after confounders were adjusted, there is still a risk for residual confounders which could not have been adjusted. Furthermore, most of the included studies did not evaluate IDC as a time-dependent variable; therefore, there may be an implicit survival bias in patients who lived long enough to receive IDC (immortal bias). Second, we did not include 4 studies that conducted multivariate analysis but did not report their results in our stratified analysis. It is possible that the presence of IDC was not selected in their final model due to the lack of a strong association with mortality, and therefore was not reported. In that case, it is possible that the estimate of a 60% reduction in the association between IDC and mortality was overestimated. Third, some of the included studies did not have well-matched data. When we used meta-analysis with inverse variance weighting, the results of these studies were modified to a normal distribution. Nevertheless, we believe this effect was minimal and did not affect the overall interpretation of our results. Finally, our meta-analysis did not evaluate the rates of empiric appropriate antibiotic use, nor obtain repeat blood cultures or echocardiograms in patients who received IDC compared with those who did not due to the limited number of studies. We also did not evaluate the effect of IDC on hospital length of stay or other costbenefit analyses. Such subanalyses might further elicit a benefit of IDC and would likely merit further research.
In conclusion, our meta-analysis did not observe a significant decrease in mortality in patients with enterococcal bacteremia who received IDC. However, the association between IDC and improved mortality outcomes was more apparent when analyzing studies adjusting for important confounders. There also did not appear to be a significant association between IDC and improved mortality in patients with VRE bacteremia. IDC confers additional benefits in improving quality of care, such as shorter time to appropriate antibiotic therapy, appropriate duration of antibiotic use, and increased diagnostic use such as repeat blood cultures or echocardiography, which results in better source identification, suggesting a role for IDC even without an apparent mortality benefit. Although this meta-analysis’s results need to be validated by a well-designed large-scale study, we believe our study highlights another condition in which IDC should be considered a part of the standard of care.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank Drs. Mark Rupp, Shawn MacVane, and Navaneeth Narayanan for providing additional information about their studies.
Financial support. None.
Potential conflicts of interest. All authors: no reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Disclosures. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans’ Affairs or the United States government.
Patient consent. As a systematic literature review and meta-analysis, our study did not include factors necessitating patient consent.
Contributor Information
Joseph Tholany, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Takaaki Kobayashi, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA.
Alexandre R Marra, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA; Center for Access & Delivery Research & Evaluation (CADRE), Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA; Instituto Israelita de Ensino e Pesquisa Albert Einstein, Hospital Israelita Albert Einstein, São Paulo, Brazil.
Marin L Schweizer, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA; Center for Access & Delivery Research & Evaluation (CADRE), Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA.
Riley J Samuelson, Hardin Library for the Health Sciences, University of Iowa Libraries, Iowa City, Iowa, USA.
Hiroyuki Suzuki, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, Iowa, USA; Center for Access & Delivery Research & Evaluation (CADRE), Iowa City Veterans Affairs Health Care System, Iowa City, Iowa, USA.
References
- 1. Murray BE. Vancomycin-resistant enterococcal infections. N Engl J Med 2000; 342:710–21. [DOI] [PubMed] [Google Scholar]
- 2. Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology (Reading) 2009; 155:1749–57. [DOI] [PubMed] [Google Scholar]
- 3. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012; 10:266–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Suppli M, Aabenhus R, Harboe ZB, et al. Mortality in enterococcal bloodstream infections increases with inappropriate antimicrobial therapy. Clin Microbiol Infect 2011; 17:1078–83. [DOI] [PubMed] [Google Scholar]
- 5. Billington EO, Phang SH, Gregson DB, et al. Incidence, risk factors, and outcomes for Enterococcus spp. blood stream infections: a population-based study. Int J Infect Dis 2014; 26:76–82. [DOI] [PubMed] [Google Scholar]
- 6. Prematunge C, MacDougall C, Johnstone J, et al. VRE and VSE bacteremia outcomes in the era of effective VRE therapy: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2016; 37:26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weiner-Lastinger LM, Abner S, Edwards JR, et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect Control Hosp Epidemiol 2020; 41:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diekema DJ, Hsueh PR, Mendes RE, et al. The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother 2019; 63:e00355-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinholt M, Ostergaard C, Arpi M, et al. Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006–2009: a population-based cohort study. Clin Microbiol Infect 2014; 20:145–51. [DOI] [PubMed] [Google Scholar]
- 10. Reyes K, Bardossy AC, Zervos M. Vancomycin-resistant enterococci: epidemiology, infection prevention, and control. Infect Dis Clin North Am 2016; 30:953–65. [DOI] [PubMed] [Google Scholar]
- 11. Dahl A, Lauridsen TK, Arpi M, et al. Risk factors of endocarditis in patients with Enterococcus faecalis bacteremia: external validation of the NOVA score. Clin Infect Dis 2016; 63:771–75. [DOI] [PubMed] [Google Scholar]
- 12. Fernandez-Hidalgo N, Almirante B, Gavalda J, et al. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating Enterococcus faecalis infective endocarditis. Clin Infect Dis 2013; 56:1261–68. [DOI] [PubMed] [Google Scholar]
- 13. Bai AD, Showler A, Burry L, et al. Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis 2015; 60:1451–61. [DOI] [PubMed] [Google Scholar]
- 14. Jenkins TC, Price CS, Sabel AL, et al. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:1000–8. [DOI] [PubMed] [Google Scholar]
- 15. Pragman AA, Kuskowski MA, Abraham JM, Filice GA. Infectious disease consultation for Staphylococcus aureus bacteremia improves patient management and outcomes. Infect Dis Clin Pract 2012; 20:261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paulsen J, Solligård E, Damås JK, et al. The impact of infectious disease specialist consultation for Staphylococcus aureus bloodstream infections: a systematic review. Open Forum Infect Dis 2016; 3:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vogel M, Schmitz RP, Hagel S, et al. Infectious disease consultation for Staphylococcus aureus bacteremia - a systematic review and meta-analysis. J Infect 2016; 72:19–28. [DOI] [PubMed] [Google Scholar]
- 18. Lopez-Cortes LE, Del Toro MD, Galvez-Acebal J, et al. Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis 2013; 57:1225–33. [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi T, Marra AR, Schweizer ML, et al. Impact of infectious disease consultation in patients with candidemia: a retrospective study, systematic literature review, and meta-analysis. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med 2009; 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000; 283:2008–12. [DOI] [PubMed] [Google Scholar]
- 22. Bramer WM, Giustini D, de Jonge GB, et al. De-duplication of database search results for systematic reviews in EndNote. J Med Libr Assoc 2016; 104:240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cattaneo C, Rieg S, Schwarzer G, et al. Enterococcus faecalis bloodstream infection: does infectious disease specialist consultation make a difference? Infection 2021; 49:1289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee RA, Vo DT, Zurko JC, et al. Infectious diseases consultation is associated with decreased mortality in enterococcal bloodstream infections. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakakura I, Sakakura K, Imanishi K, et al. Association between vancomycin pharmacokinetic/pharmacodynamic parameters, patient characteristics, and mortality in patients with bacteremia caused by vancomycin-susceptible Enterococcus faecium: a single-center retrospective study. J Pharm Health Care 2019; 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998; 52:377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bartoletti M, Tedeschi S, Scudeller L, et al. Impact on mortality of a bundle for the management of enterococcal bloodstream infection. Open Forum Infect Dis 2019; 6:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Britt NS, Potter EM, Patel N, Steed ME. Effect of continuous and sequential therapy among veterans receiving daptomycin or linezolid for vancomycin-resistant Enterococcus faecium bacteremia. Antimicrob Agents Chemother 2017; 61:e02216-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Erlandson KM, Sun J, Iwen PC, Rupp ME. Impact of the more-potent antibiotics quinupristin-dalfopristin and linezolid on outcome measure of patients with vancomycin-resistant Enterococcus bacteremia. Clin Infect Dis 2008; 46:30–6. [DOI] [PubMed] [Google Scholar]
- 30. Furuichi M, Furuichi M, Horikoshi Y, Miyairi I. Infectious diseases consultation improves treatment and decreases mortality by enterococcal bacteremia in children. Pediatr Infect Dis J 2018; 37:856–60. [DOI] [PubMed] [Google Scholar]
- 31. Gray ME, Cox HL, Donohue LE, et al. The effect of rapid diagnostic testing with Infectious Diseases fellow consultative intervention on the management of enterococcal bloodstream infection. Diagn Microbiol Infect Dis 2018; 92:319–24. [DOI] [PubMed] [Google Scholar]
- 32. Jindai K, Strerath MS, Hess T, Safdar N. Is a single positive blood culture for Enterococcus species representative of infection or contamination? Eur J Clin Microbiol 2014; 33:1995–2003. [DOI] [PubMed] [Google Scholar]
- 33. Jumah MTB, Vasoo S, Menon SR, et al. Pharmacokinetic/pharmacodynamic determinants of vancomycin efficacy in enterococcal bacteremia. Antimicrob Agents Chemother 2018; 62:e01602-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. MacVane SH, Hurst JM, Boger MS, Gnann JW Jr. Impact of a rapid multiplex polymerase chain reaction blood culture identification technology on outcomes in patients with vancomycin-resistant enterococcal bacteremia. Infect Dis (Lond) 2016; 48:732–7. [DOI] [PubMed] [Google Scholar]
- 35. Malone DA, Wagner RA, Myers JP, Watanakunakorn C. Enterococcal bacteremia in two large community teaching hospitals. Am J Med 1986; 81:601–6. [DOI] [PubMed] [Google Scholar]
- 36. McKinnell JA, Patel M, Shirley RM, et al. Observational study of the epidemiology and outcomes of vancomycin-resistant Enterococcus bacteraemia treated with newer antimicrobial agents. Epidemiol Infect 2011; 139:1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mercuro NJ, Gill CM, Kenney RM, et al. Treatment and outcomes of Enterococcus faecium bloodstream infections in solid organ transplant recipients. Transpl Infect Dis 2020; 22:e13251. [DOI] [PubMed] [Google Scholar]
- 38. Nakagawa R, Jain R, Bryan AB, Chan JD. Optimization of antimicrobial therapy in vancomycin-resistant enterococcal bacteraemia using a rapid detection gram-positive blood culture assay. J Hosp Infect 2018; 99:153–7. [DOI] [PubMed] [Google Scholar]
- 39. Narayanan N, Rai R, Vaidya P, et al. Comparison of linezolid and daptomycin for the treatment of vancomycin-resistant enterococcal bacteremia. Ther Adv Infect Dis 2019; 6:2049936119828964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Valentin T, Koenig E, Prattes J, et al. Implementation of rapid antimicrobial susceptibility testing combined with routine infectious disease bedside consultation in clinical practice (RAST-ID): a prospective single-centre study. J Antimicrob Chemother 2021; 76:233–8. [DOI] [PubMed] [Google Scholar]
- 41. Zasowski EJ, Claeys KC, Lagnf AM, et al. Time is of the essence: the impact of delayed antibiotic therapy on patient outcomes in hospital-onset enterococcal bloodstream infections. Clin Infect Dis 2016; 62:1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 43. Habib G, Lancellotti P, Iung B. 2015 ESC guidelines on the management of infective endocarditis: a big step forward for an old disease. Heart 2016; 102:992–4. [DOI] [PubMed] [Google Scholar]
- 44. Dahl A, Iversen K, Tonder N, et al. Prevalence of infective endocarditis in Enterococcus faecalis bacteremia. J Am Coll Cardiol 2019; 74:193–201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.