Abstract
Background
Population-based studies of Staphylococcus aureus bacteremia (SAB) in the United States are limited. We provide a contemporary evaluation of SAB incidence in Olmsted County, Minnesota, from 2006 to 2020.
Methods
This was a retrospective population-based study of all adult patients with SAB residing in Olmsted County from 1 January 2006 through 31 December 2020. Initial episodes of SAB were identified using the microbiology laboratory databases at both Olmsted Medical Center and Mayo Clinic Rochester.
Results
Overall, 541 incident SAB cases were identified with a median age of 66.8 (interquartile range, 54.4–78.5) years, and 60.4% were male. Among these cases, 298 (56.2%) were due to methicillin-susceptible S aureus (MSSA) and 232 (43.8%) cases of methicillin-resistant S aureus (MRSA). The overall age- and sex-adjusted SAB incidence rate (IR) was 33.9 (95% confidence interval [CI], 31.0–36.8) cases/100 000 person-years (PY). Males had a higher age-adjusted IR of 46.0 (95% CI, 41.0–51.0) cases/100 000 PY compared to females (IR, 24.4 [95% CI, 21.1–27.7] cases/100 000 PY). Age- and sex-adjusted SAB IRs due to MSSA and MRSA were 18.7 and 14.6 cases/100 000 PY, respectively, and the percentage of incident SAB cases due to MRSA fluctuated across the study period. There was no apparent temporal trend in SAB incidence over the study period (P = .093).
Conclusions
Our investigation represents the only contemporary population-based study in the United States. Despite the impression that SAB incidence may have increased based on Centers for Disease Control and Prevention surveillance data, our finding of no change in SAB incidence was somewhat unanticipated.
Keywords: bacteremia, incidence, population-based, Staphylococcus aureus, United States
Key points
To our knowledge, this is the only contemporary population-based survey of Staphylococcus aureus bacteremia (SAB) incidence in the United States. Overall, SAB incidence did not change significantly over the 15-year study period in Olmsted County, Minnesota.
INTRODUCTION
Staphylococcus aureus is a frequent cause of both community-acquired (CA) and nosocomial bacteremias. Prior investigations revealed that it was the second most common pathogen causing bacteremia in 49 United States (US) hospitals participating in the Surveillance and Control of Pathogens of Epidemiologic Importance (SCOPE) project from 1995 to 2002 as well as in the Olmsted County population from 2003 to 2005 [1, 2]. Staphylococcus aureus bacteremia (SAB) has a mortality rate up to 30%, warranting continued surveillance [3]. Methicillin-resistant S aureus (MRSA) is an emerging threat and was recently classified as a high-priority pathogen by the World Health Organization [4]. Length of hospital stay and mortality tend to be higher with MRSA compared to methicillin-susceptible S aureus (MSSA) bacteremia [5, 6].
Determining the burden of SAB in a population is crucial when considering, for example, allocations of healthcare resources. A multinational population-based study conducted in Finland, Sweden, Denmark, Australia, and Canada from 2000 to 2008, with nationwide surveillance systems, reported an annual incidence for SAB of 26.1 per 100 000 population, more specifically, 24.2 for MSSA and 1.9 for MRSA [7]. During this 8-year period, the mortality rate was 3.4 per 100 000 per year, with 3.1 for MSSA and 0.3 MRSA bacteremias [8]. Findings from US population-based studies regarding the incidence of SAB are scarce and dated. The National Nosocomial Infections Surveillance System first reported an increase in SAB incidence by 287% in the US from 1980 to 1989 in nonteaching hospitals and 176% in large teaching hospitals [9]. In 1998, the first US population-based study conducted in 4 metropolitan areas in Connecticut described an incidence of CA-SAB of 17 per 100 000 person-years (PY) [10]. Consequently, our group published a population-based study conducted in Olmsted County, Minnesota, from 1998 to 2005 [11]. There was stable incidence of SAB in adults at an age- and sex-adjusted rate of 38.2 per 100 000 PY; however, the MRSA to MSSA ratio notably increased over this period [11]. Our group also conducted a separate study that examined trends in bacteremia in the Olmsted County’s population and reported a slightly lower SAB incidence rate of 32 per 100 000 PY from 2003 to 2005 [1]. More recent data reported by the Centers for Disease Control and Prevention from select counties in 6 states found that rates of healthcare-associated (HCA) MSSA bacteremia have remained stable since 2005, whereas CA-MSSA bacteremia has moderately increased (3.9% per year) from 2012 to 2017; MRSA bacteremia has decreased from 2005 to 2016 with a slower decline during the last 4 years of the investigation [12].
The aim of our study was to examine the clinical profile of incident cases and to characterize the incidence and temporal trends of SAB in Olmsted County, Minnesota, from 2006 to 2020.
METHODS
Population
Patient health records have been collected in Olmsted County since 1966 in a large unified medical care system entitled the Rochester Epidemiological Project (REP). The database has remained unique in the US and has been used to profile clinical characteristics and outcomes of an array of disease syndromes [13]. All residents of Olmsted County who have been seen by a healthcare provider in an inpatient, outpatient, or emergent setting as well as in long-term care facilities, regardless of provider, have a single dossier in the database. All medical diagnoses, surgical interventions, and results of laboratory tests and imaging are frequently abstracted from patients’ health records and entered currently using the International Classification of Diseases, Tenth Revision [13].
Olmsted County, located approximately 90 miles southeast of Minneapolis/Saint Paul, had a population of 164 365, including 125 132 adults (67 077 [53.6%] women and 58 055 [46.4%] men), as estimated by the REP in 2020 [14]. As per the US Census Bureau estimates of 2020, 84% of Olmsted County’s population is White [15].
Case Identification
The institutional review boards (IRBs) at both the Mayo Clinic (MC) (IRB number 20-012295) and Olmsted Medical Center (OMC) (IRB number 061-OMC-20) in Rochester, Minnesota, approved our study. Cases were identified by using the REP browser from the only 2 microbiology laboratories in Olmsted County, MC and OMC. All adults (18 years and older) residing in Olmsted County, Minnesota, with a positive S aureus blood culture between 1 January 2006 and 31 December 2020 were included in this study. Only the first SAB episode per person during the study period was included in the study. Cases where S aureus was identified along with only 1 set of positive blood cultures yielding coagulase-negative Staphylococcus, Corynebacterium species, or Cutibacterium acnes were also included [16]. Exclusion criteria were polymicrobial bacteremias (any other pathogen associated with S aureus), residency outside of Olmsted County at the time of SAB, and absence of research authorization.
SAB was classified in the following 3 categories as per the criteria from Friedman et al [17]:
-
Healthcare-associated bacteremia was defined as a positive blood culture obtained at the time of hospitalization or within 48 hours of admission in addition to 1 of the following criteria:
Received intravenous (IV) therapy, wound care, or specialized nursing care within the 30 days prior to the bacteremia.
Received hemodialysis or IV chemotherapy in the 30 days prior to the bacteremia.
Was admitted in an acute care hospital for 48 hours or more in the 90 days prior to the bacteremia.
Lived in a long-term care facility or a nursing home.
Community-acquired bacteremia was defined as a positive blood culture obtained at the time of hospitalization or within 48 hours after admission with the absence of HCA bacteremia criteria.
Nosocomial bacteremia was defined as a positive blood culture obtained after 2 days of hospitalization. Patients transferred from other hospitals had their duration of hospitalization calculated from the date of the first hospitalization.
Complicated SAB was defined by the following criteria: (1) presence of infective endocarditis (IE) on echocardiography; (2) presence of indwelling catheters or implanted prosthesis; (3) positive blood cultures within 2–4 days after IV antibiotherapy initiation and control of any infection focus; (4) presence of fever within 2–3 days after IV antibiotic therapy initiation and control of any infection focus; and (5) presence of signs of metastatic infection [18]. Source control was defined by catheter removal, drainage procedure, or debridement of abscesses or necrotic tissue.
Bacteremia relapse was defined as a positive blood culture after negative follow-up blood cultures within 12 weeks of the first positive blood culture [19].
Standard blood cultures at MC and OMC include 2 sets. Each set consists of 2 BD BACTEC Plus Aerobic/F bottles and 1 BD BACTEC Lytic Anaerobic/F bottle (Becton Dickinson, Heidelberg, Germany). Each bottle is inoculated with 10 mL of whole blood and these are then incubated for 5 days with the BD BACTEC FX platform.
Electronic health records of all SAB cases were manually reviewed and information on site of acquisition and complicated SAB was obtained. The Charlson Comorbidity Index (CCI) was calculated for each patient [20]. The source of bacteremia and metastatic infections were defined based on findings from history, physical examination, laboratory, and imaging results from patients’ electronic health records. Staphylococcus aureus isolation from a potential site of infection was not a prerequisite.
Immunocompromising conditions included neutropenia, long-term corticosteroid use, chemotherapy, immunosuppressive drugs for bone marrow or solid organ transplantation, and disease-modifying antirheumatic drugs for autoimmune conditions. Malignancies included solid tumors, lymphomas, and leukemias.
Data Analysis
Baseline characteristics of incident SAB cases are summarized on a per-patient basis. Descriptive statistics included median and interquartile range (IQR) for continuous variables, and count and percentage for categorical variables. These data were then rearranged and aggregated across the population to facilitate incidence calculations. Specifically, data were stratified by calendar time (per year from 2006 to 2020), patient age (per year from 18 to 100), and sex, and in each stratum both the SAB case and population counts were aggregated. SAB incidence rates were calculated for men and women in age strata by dividing the number of SAB cases by person-time in the population, expressed per 100 000 PY. When describing SAB incidence across all ages, sex-specific rates were standardized to the age distribution of the US White population in 2010, and the overall incidence rate was standardized to the age and sex distribution of the same background population. The 95% confidence intervals (CIs) were calculated based on a Poisson distributional assumption.
A multivariable Poisson regression model with overdispersion correction was applied to the aggregated data to detect patterns in SAB incidence. The crude incidence counts entered the model as the dependent variable, while age, sex, and calendar year were included as independent variables, with the counts across the population added in an offset variable. By accounting for the stratum-specific population counts as offsets (log-scale), the corresponding incidence counts are normalized for the denominator and thereby converted into rates. Age and calendar time were modeled flexibly with the use of regression splines to relax linearity assumptions. Partial effect plots were generated from the model to display how the predicted incidence rates change as a function of age, sex, and year. Evidence for a temporal trend in SAB incidence was based on the overall test of the year effect in the model, which essentially tests for any “nonflatness” in the estimated trend. Statistical significance was defined by an α level of .05. All analyses were performed using the statistical programming language R version 4.0.3 (R Foundation, Vienna, Austria).
RESULTS
Demographics and Clinical Characteristics
During our 15-year study period, 629 adults residing in Olmsted County had at least 1 episode of SAB. A total of 43 patients were excluded due to polymicrobial bacteremia, and 45 denied research authorization. Therefore, the remaining 541 incident cases of monomicrobial SAB comprised our study population and were included in the analysis. Antimicrobial susceptibilities were missing in 11 cases, and 2 patients’ files lacked information about the SAB site of acquisition.
The median age of these patients was 66.8 (IQR, 54.4–78.5) years and 60.4% were male (Table 1). Most patients (86.0%) were White, reflecting the demographics in southeastern Minnesota. The median CCI of the cohort was 2 (IQR, 1–4).
Table 1.
Baseline Characteristics of Staphylococcus aureus Bacteremia Cases
| Characteristic | No.a | Overall (N = 541) |
|---|---|---|
| Age, y, median (IQR) | 541 | 66.8 (54.4–78.5) |
| Male sex | 541 | 327 (60.4) |
| Race | 541 | |
| White | 465 (86.0) | |
| Black | 25 (4.6) | |
| Asian | 15 (2.8) | |
| AI/AN or NH/PI | 6 (1.1) | |
| Other/multiracial | 30 (5.5) | |
| Hispanic ethnicity | 530 | 20 (3.8) |
| BMI, kg/m2, median (IQR) | 534 | 28.0 (23.8–33.8) |
| IE based on modified Duke criteria | 539 | 40 (7.4) |
| Type of SAB | 530 | |
| MRSA | 232 (43.8) | |
| MSSA | 298 (56.2) | |
| Site of infection onset | 539 | |
| Nosocomial | 57 (10.6) | |
| Healthcare-associated | 265 (49.2) | |
| Community-acquired | 217 (40.3) | |
| Diabetes mellitus | 541 | 415 (76.7) |
| Chronic kidney disease | 541 | 236 (43.6) |
| Peritoneal dialysis | 236 | 40 (16.9) |
| Hemodialysis | 236 | 54 (22.9) |
| Catheter | 54 | 27 (50.0) |
| Graft | 54 | 4 (7.4) |
| Fistula | 54 | 26 (48.1) |
| HIV infection | 541 | 1 (0.2) |
| COPD | 541 | 88 (16.3) |
| Peripheral artery disease | 541 | 211 (39.0) |
| Cerebrovascular disease | 541 | 107 (19.8) |
| Heart failure | 541 | 243 (44.9) |
| Myocardial infarction | 541 | 171 (31.6) |
| Immunocompromising conditions or malignancy | 541 | 333 (61.6) |
| Injection drug use | 541 | 11 (2.0) |
| Charlson Comorbidity Index, median (IQR) | 541 | 2.0 (1.0–4.0) |
| Complicated bacteremia | 540 | 202 (37.4) |
| Bacteremia relapse | 536 | 7 (1.3) |
| Possible source of SABb | 541 | |
| SSTI | 203 (37.5) | |
| Pneumonia | 70 (12.9) | |
| Catheter related | 67 (12.4) | |
| Septic arthritis | 52 (9.6) | |
| Foley catheter–associated UTI | 43 (7.9) | |
| Osteomyelitis | 42 (7.8) | |
| Endocarditis | 27 (5.0) | |
| Graft infection | 4 (0.7) | |
| Other | 14 (2.6) | |
| Unknown | 108 (20.0) |
Data are presented as No. (%) unless otherwise indicated.
Abbreviations: AI/AN, American Indian/Alaska Native; BMI, body mass index; COPD, chronic obstructive pulmonary disease; HIV, human immunodeficiency virus; IE, infective endocarditis; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; NH/PI, Native Hawaiian/Pacific Islander; SAB, Staphylococcus aureus bacteremia; SSTI, skin and soft tissue infection; UTI, urinary tract infection.
No. is the number of patients with available information for each variable.
Because some patients had >1 possible source of SAB, the sum of source-specific percentages is >100%.
Among the 541 incident SAB cases, 298 (56.2%) and 232 (43.8%) were due to MSSA and MRSA, respectively; 57 (10.6%) episodes were nosocomial, 265 (49.2%) HCA, and 217 (40.3%) CA. More specifically, the majority of MRSA cases were either HCA (47.4%) or CA bacteremias (43.5%) with nosocomial bacteremias representing a small minority (9.1%).
The most common source of SAB was skin and soft tissue infection (SSTI) (37.5%), followed by unknown etiology (20.0%). Two hundred seven patients (38.3%) had complicated SAB. Relapsing bacteremia occurred in 7 (1.3%) patients.
Incidence Rates and Trends of SAB, 2006–2020
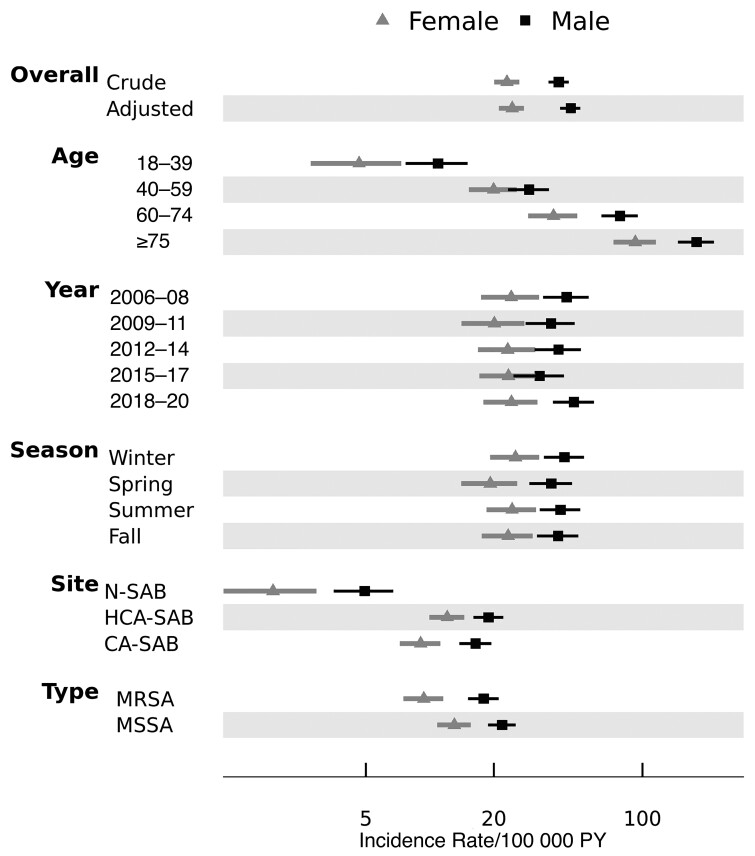
During the 15-year period, the overall age- and sex-adjusted SAB incidence rate (IR) was 33.9 (95% CI, 31.0–36.8) per 100 000 PY (Supplementary Table 1). Men had a higher age-adjusted IR (46.0 [95% CI, 41.0–51.0] cases per 100 000 PY) compared to that of women (IR, 24.4 [95% CI, 21.1–27.7]), and in both sexes the crude IR increased progressively with each advancing age group (range, 7.6–128.3 per 100 000 PY). In contrast, there was no discernible temporal or seasonal variation in rates suggested in Figure 1.
Figure 1.
Sex-specific incidence rates of Staphylococcus aureus bacteremia stratified by age group, year, seasonality, and types of infection. Error bars represent 95% confidence intervals. Men had higher incidence than women across all groups, and rates varied the most according to age. Abbreviations: CA, community-acquired; HCA, healthcare-associated; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; N, nosocomial; PY, person-years; SAB, Staphylococcus aureus bacteremia.
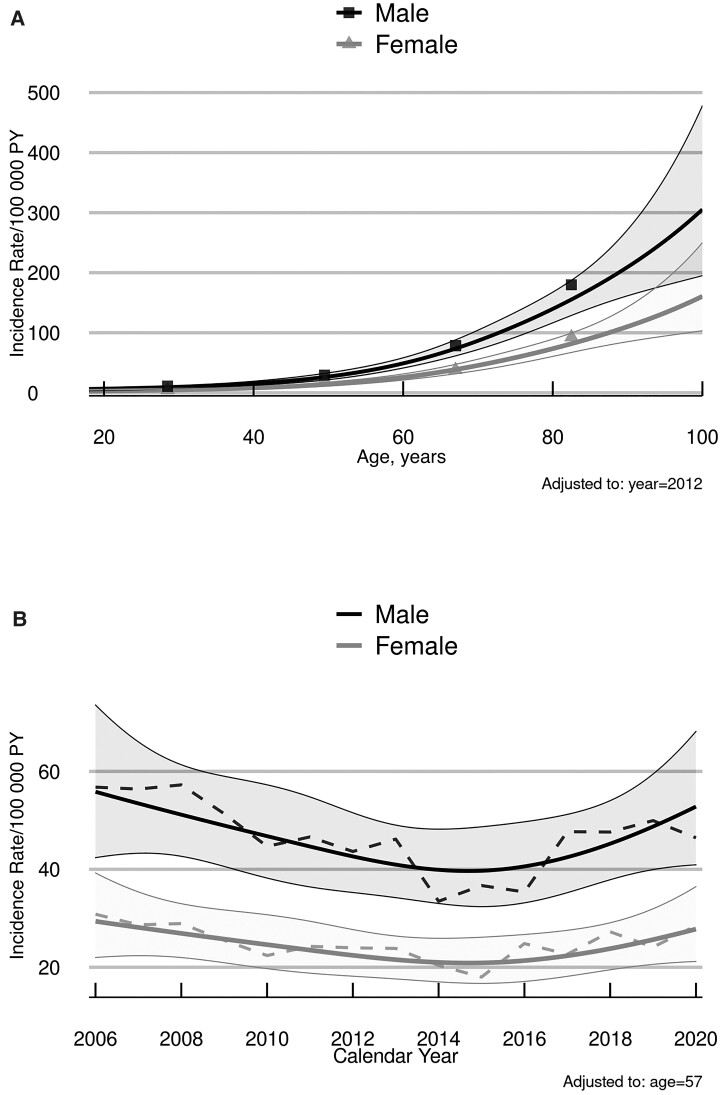
These patterns of SAB incidence were then formally tested in a multivariable Poisson regression model, with age and calendar year analyzed as continuous variables. In the model, the partial effects of age (χ2 = 459.52, P < .001) and sex (χ2 = 51.80, P < .001) on IR were both highly significant. Specifically, SAB incidence increased exponentially with age in both sexes (Figure 2A), and the rates in men were nearly 2-fold higher (IR ratio, 1.90 [95% CI, 1.60–2.26]) than in women. Based on model-estimated trends in SAB incidence over time, as illustrated in Figure 2B for men and women adjusted for age, the evidence for a nonflat time effect was not statistically significant (χ2 = 6.42, P = .093).
Figure 2.
Predicted incidence rates of Staphylococcus aureus bacteremia according to patient age (A) and calendar year (B), by sex. Solid lines are model-estimated incidence rates illustrating the Poisson analyses, with shaded areas representing 95% confidence bands of the predicted values. “Observed” rates based on age-subgrouped estimates (symbols in panel A) or 3-year-centered moving averages (dashed lines in panel B) correspond closely to the modeled trends. Abbreviation: PY, person-years.
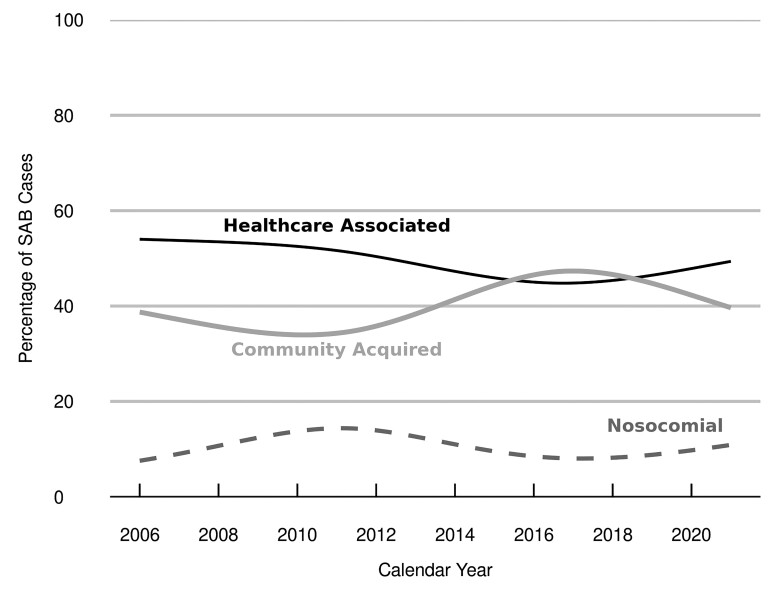
We repeated the incidence calculations for relevant subgroups of SAB according to site of acquisition and methicillin resistance/susceptibility. During the period of 2006–2020, the age- and sex-adjusted IRs of CA-SAB, HCA-SAB, and nosocomial SAB were 13.5 (95% CI, 11.7–15.3), 16.7 (95% CI, 14.7–18.7), and 3.6 (95% CI, 2.7–4.6) per 100 000 PY, respectively (Supplementary Table 2). In all of these settings, IRs rose with age in both sexes, with higher IRs in men compared to that of women. The temporal distribution of SAB by site of acquisition remained relatively constant over the 15-year period (Figure 3).
Figure 3.
Trends in Staphylococcus aureus bacteremia (SAB) cases, by site of onset. Trend lines depict the percentage of SAB cases for each site of acquisition over time, as estimated by a nonparametric loess smoother.
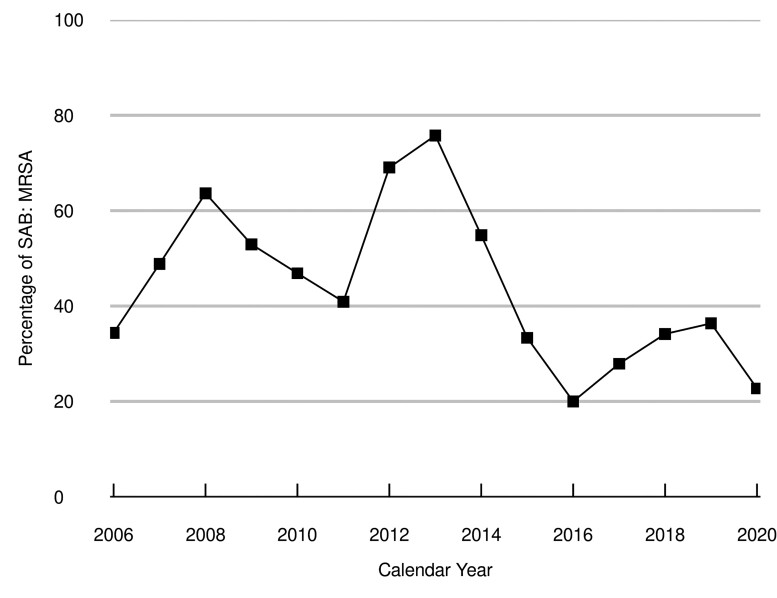
During the study period, the age- and sex-adjusted IRs for MSSA and MRSA bacteremias were 18.7 (95% CI, 16.5–20.8) and 14.6 (95% CI, 12.7–16.4) per 100 000 PY, respectively (Supplementary Table 3). IRs of MSSA and MRSA bacteremias showed similar patterns by age and sex as the rates of SAB overall and by site of acquisition. Changes in the percentage of incident SAB cases due to MRSA fluctuated over the study period (Figure 4).
Figure 4.
Trends in Staphylococcus aureus bacteremia (SAB) cases due to methicillin-resistant Staphylococcus aureus (MRSA). The squares represent the observed percentage of SAB cases that were from MRSA for each year of the study, which during the first half of the period ranged from 34.4% in 2006 to 75.8% in 2013, before decreasing to 20.0% in 2016 and 22.7% in 2020.
DISCUSSION
To our knowledge, this is the only contemporary US population–based investigation of SAB incidence over a 15-year period. The overall age- and sex-adjusted SAB incidence in adults residing in Olmsted County, Minnesota, was 33.9 (95% CI, 31.0–36.8) per 100 000 PY, which did not change significantly over the study period. In addition, the percentage of SAB cases due to MRSA fluctuated during the first half of the study (range, 34.4% [2006] to 75.8% [2013]), before leveling off to less than one-third of cases over the second half (average, 2014–2020 = 32.0%). Robust methodology of our study based on the use of REP resources for case identification and data collection on a population level makes it ideal for incidence calculations due to reduced referral bias [21].
The percentage of nosocomial MRSA bacteremia cases was low (9.1%), which is in concordance with a decline described in the US from 2012 to 2017 and in other countries such as Australia [12, 22]. These observations may be attributable to several factors including shorter hospital stays, increased number of ambulatory-care procedures, and implementation of stricter infection prevention and control practices as pertain to catheter-related bacteremias, particularly mandatory utilization review and discontinuation of temporary intravascular catheters and use of newer materials and devices [23–25]. In addition, numerous efforts have been made to reduce MRSA transmission as well as to implement robust antimicrobial stewardship programs to diminish the selection of multidrug resistance [26, 27]. However, the high observed percentage (47.4%) of HCA cases among those with MRSA bacteremia highlights the need to strictly reinforce and standardized prevention practices.
During the period 1998–2005, the age- and sex-adjusted SAB incidence was higher as compared to that of the current study period, 2006–2020 [11]. However, higher IRs and percentages of MRSA were observed in the current study as compared to our previous one [11]. The latter may be attributed to the high percentage of patients with immunocompromising conditions and malignancies (61.6%) and cardiovascular devices (8.9%) in addition to patients undergoing hemodialysis (22.9%). Another potential explanation might be the high percentage of SSTI as a possible source of SAB in our current cohort (37.5%) combined with the emergence of CA-MRSA causing SSTI in the US [28]. However, despite the nationwide spread of CA-MRSA largely due to the USA300 clone, the incidence of this pathogen in nonpurulent lower extremity cellulitis in residents of Olmsted County was lower in 2013 compared to 1999 [29].
The most common possible source of SAB in our previous study was line-associated or endovascular including IE (28.3%) [11], whereas the most common source in our current cohort was SSTI (37.5%). The prevalence of indwelling catheters and IE as potential sources of SAB was 12.4% and 5.0%, respectively, which is lower than that of previous single center–based studies [30, 31]. Only 2% of our cohort reported IV drug use, which could account for the low percentage of IE observed.
When our results are compared to non-US population–based investigations of SAB, several findings deserve mention. First, although 4 contemporary studies conducted in Denmark, Finland, Australia, and Spain reported an increase in national SAB rates, our IRs of SAB, especially when stratified by sex, were above their highest levels [32–35]. Second, similar to our findings, the Danish and Spanish studies reported a significant increase in SAB IRs with age and, more specifically, in older men [32, 34]. Third, the national Finnish IRs of MRSA bacteremia are quasi-inexistent compared to our results [33], highlighting the need to institute more aggressive practices in MRSA detection and eradication. Fourth, our IRs of HCA MRSA bacteremia were comparable to the ones reported in the Finnish study [33].
There were several limitations to this study. First, our calculated SAB incidence in Olmsted County could be underestimated due to the exclusion of 7.2% of patients with SAB who denied review of their health records. Moreover, several cases could have been missed if Olmsted County residents had SAB while outside the county, did not undergo blood cultures, or received appropriate antibiotherapy prior to blood cultures being drawn. However, these scenarios do not likely constitute a sizable portion of SAB cases. In addition, molecular typing techniques were not performed on MRSA isolates, preventing us from recognizing the predominant clonal types of MRSA. Some S aureus strains causing bacteremias may be either associated with better clinical outcomes or more virulent of recent [36, 37]; performing molecular-based studies to further evaluate SAB pathogenesis is warranted. Last, given the limited diversity in race and ethnicity of the Olmsted County population, our study findings are likely applicable to only White adults from a similar socioeconomic background.
CONCLUSIONS
To our knowledge, this is the only contemporary population-based investigation of SAB incidence in the US, and SAB incidence did not change significantly in adults residing in Olmsted County, Minnesota, from 2006 to 2020. The percentage of incident SAB cases due to MRSA fluctuated during the first half of the study period, before leveling off to about one-third of cases over the second half. Subsequent risk factor and outcomes analyses are warranted to profile clinical features of SAB in the current era.
Supplementary Material
Contributor Information
Joya Rita Hindy, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Juan A Quintero-Martinez, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Brian D Lahr, Division of Clinical Trials and Biostatistics, Department of Quantitative Health Sciences, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Raj Palraj, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
John R Go, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Madiha Fida, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Omar M Abu Saleh, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Verda Arshad, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Khawaja M Talha, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Daniel C DeSimone, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA; Department of Cardiovascular Disease, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
M Rizwan Sohail, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA; Section of Infectious Diseases, Department of Medicine, Baylor College of Medicine, Houston, Texas, USA.
Larry M Baddour, Division of Infectious Diseases, Department of Medicine, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA; Department of Cardiovascular Disease, College of Medicine, Mayo Clinic, Rochester, Minnesota, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The authors are extremely grateful for the philanthropic support provided by a gift from Eva and Gene Lane (to L. M. B.), which was paramount in our work to advance the science of cardiovascular infections, which has been an ongoing focus of investigation at Mayo Clinic for over 60 years. We also recognize the unique expertise of Barbara A. Abbott for data retrieval from the Rochester Epidemiology Project (REP).
Disclaimer. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or the Mayo Clinic.
Financial support. This study used the resources of the REP medical records linkage system, which is supported by the National Institute on Aging (grant number AG 058738), by the Mayo Clinic Research Committee, and by fees paid annually by REP users.
Potential conflicts of interest. L. M. B. has been a consultant to Roivant Sciences and Boston Scientific. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Uslan DZ, Crane SJ, Steckelberg JM, et al. Age- and sex-associated trends in bloodstream infection: a population-based study in Olmsted County, Minnesota. Arch Intern Med 2007; 167:834–9. [DOI] [PubMed] [Google Scholar]
- 2. Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 2004; 39:309–17. [DOI] [PubMed] [Google Scholar]
- 3. Chang FY, MacDonald BB, Peacock JE, et al. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore) 2003; 82:322–32. [DOI] [PubMed] [Google Scholar]
- 4. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18:318–27. [DOI] [PubMed] [Google Scholar]
- 5. Kaasch AJ, Barlow G, Edgeworth JD, et al. Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 2014; 68:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsuzuki S, Yu J, Matsunaga N, Ohmagari N. Length of stay, hospitalisation costs and in-hospital mortality of methicillin-susceptible and methicillin-resistant Staphylococcus aureus bacteremia in Japan. Public Health 2021; 198:292–6. [DOI] [PubMed] [Google Scholar]
- 7. Laupland KB, Lyytikäinen O, Søgaard M, et al. The changing epidemiology of Staphylococcus aureus bloodstream infection: a multinational population-based surveillance study. Clin Microbiol Infect 2013; 19:465–71. [DOI] [PubMed] [Google Scholar]
- 8. Tom S, Galbraith JC, Valiquette L, et al. Case fatality ratio and mortality rate trends of community-onset Staphylococcus aureus bacteraemia. Clin Microbiol Infect 2014; 20:O630–2. [DOI] [PubMed] [Google Scholar]
- 9. Banerjee SN, Emori TG, Culver DH, et al. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. National Nosocomial Infections Surveillance System. Am J Med 1991; 91:86S–9S. [DOI] [PubMed] [Google Scholar]
- 10. Morin CA, Hadler JL. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J Infect Dis 2001; 184:1029–34. [DOI] [PubMed] [Google Scholar]
- 11. El Atrouni WI, Knoll BM, Lahr BD, et al. Temporal trends in incidence of Staphylococcus aureus bacteremia in Olmsted County, Minnesota, 1998 to 2005: a population-based study. Clin Infect Dis 2009; 49:e130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kourtis AP, Hatfield K, Baggs J, et al. Vital signs: epidemiology and recent trends in methicillin-resistant and in methicillin-susceptible Staphylococcus aureus bloodstream infections—United States. MMWR Morb Mortal Wkly Rep 2019; 68:214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Melton LJ. History of the Rochester Epidemiology Project. Mayo Clin Proc 1996; 71:266–74. [DOI] [PubMed] [Google Scholar]
- 14. St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011; 173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. US Census Bureau . QuickFacts: Olmsted County, Minnesota. https://www.census.gov/quickfacts/fact/faq/olmstedcountyminnesota/RHI125219. Accessed 14 December 2021.
- 16. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev 2006; 19:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 18. Chong YP, Moon SM, Bang KM, et al. Treatment duration for uncomplicated Staphylococcus aureus bacteremia to prevent relapse: analysis of a prospective observational cohort study. Antimicrob Agents Chemother 2013; 57:1150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–72. [DOI] [PubMed] [Google Scholar]
- 20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 21. Steckelberg JM, Rouse MS, Wilson WR, et al. Influence of referral bias on the apparent clinical spectrum of infective endocarditis. Am J Med 1990; 88:582–8. [DOI] [PubMed] [Google Scholar]
- 22. Mitchell BG, Collignon PJ, McCann R, et al. A major reduction in hospital-onset Staphylococcus aureus bacteremia in Australia—12 years of progress: an observational study. Clin Infect Dis 2014; 59:969–75. [DOI] [PubMed] [Google Scholar]
- 23. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006; 355:2725–32. [DOI] [PubMed] [Google Scholar]
- 24. Timsit JF, Dubois Y, Minet C, et al. New materials and devices for preventing catheter-related infections. Ann Intensive Care 2011; 1:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walz JM, Ellison RTI, Mack DA, et al. The bundle “plus”: the effect of a multidisciplinary team approach to eradicate central line-associated bloodstream infections. Anesth Analg 2015; 120:868–76. [DOI] [PubMed] [Google Scholar]
- 26. Perlin JB, Hickok JD, Septimus EJ, et al. A bundled approach to reduce methicillin-resistant Staphylococcus aureus infections in a system of community hospitals. J Healthc Qual 2013; 35:57–68; quiz 68–9. [DOI] [PubMed] [Google Scholar]
- 27. Zimmermann N, Allen R, Fink G, et al. Antimicrobial stewardship with and without infectious diseases specialist services to improve quality-of-care in secondary and tertiary care hospitals in Germany: study protocol of the ID ROLL OUT study. Infect Dis Ther 2021; 9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med 2005; 352:1436–44. [DOI] [PubMed] [Google Scholar]
- 29. Marcelin JR, Challener DW, Tan EM, et al. Incidence of and effects of seasonality on non-purulent lower extremity cellulitis after the emergence of community-acquired methicillin-resistant Staphylococcus aureus. Mayo Clin Proc 2017; 92:1227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nickerson EK, Hongsuwan M, Limmathurotsakul D, et al. Staphylococcus aureus bacteraemia in a tropical setting: patient outcome and impact of antibiotic resistance. PLoS One 2009; 4:e4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hewagama S, Spelman T, Einsiedel LJ. Staphylococcus aureus bacteraemia at Alice Springs Hospital, Central Australia, 2003–2006. Intern Med J 2012; 42:505–12. [DOI] [PubMed] [Google Scholar]
- 32. Thorlacius-Ussing L, Sandholdt H, Larsen AR, et al. Age-dependent increase in incidence of Staphylococcus aureus bacteremia, Denmark, 2008–2015. Emerg Infect Dis 2019; 25:875–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jokinen E, Laine J, Huttunen R, et al. Trends in incidence and resistance patterns of Staphylococcus aureus bacteremia. Infect Dis 2018; 50:52–8. [DOI] [PubMed] [Google Scholar]
- 34. Imam N, Tempone S, Armstrong PK, et al. Increased incidence of community-associated Staphylococcus aureus bloodstream infections in Victoria and Western Australia, 2011–2016. Med J Aust 2019; 210:87–8. [DOI] [PubMed] [Google Scholar]
- 35. Ruiz-Azcona L, Santibañez M, Gimeno A, et al. Etiology of bloodstream infections at a population level during 2013–2017 in the Autonomous Community of Valencia, Spain. Rev Esp Quimioter 2020; 33:200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Souli M, Ruffin F, Choi SH, et al. Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, longitudinal study. Clin Infect Dis 2019; 69:1868–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lalani T, Federspiel JJ, Boucher HW, et al. Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J Clin Microbiol 2008; 46:2890–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.