Abstract
Microscopic vascular invasion (MVI) is a strong risk factor associated with tumor recurrence and poor overall survival (OS) among hepatocellular carcinoma (HCC) patients after resection. Two types of MVI are identified: portal vein and capsular vein invasion. However, little is known about the impact of different types of MVI on HCC recurrence. The present study aimed to compare HCC recurrence and OS between the portal vein and capsule vein MVI. Patients with Barcelona Clinic Liver Cancer (BCLC) stage 0 or A HCC who underwent primary resection between January 2001 and June 2016 were consecutively recruited. Factors that influenced OS and recurrence-free survival (RFS) were analyzed using Cox proportional hazards models. Of the 857 eligible patients, 327 (38.2%) had MVI, and 530 (61.8%) were without MVI. Of the 327 patients with MVI, 85 (26.0%) were with portal vein, 178 (54.4%) with capsular vein, and 64 (19.6%) with both-MVI type. Patients with both-MVI type suffered from a higher proportion of BCLC stage A (P < 0.001), capsular invasion (P = 0.002), and satellite nodules (P < 0.001). Both-MVI type is an independent risk factor for HCC recurrence (hazard ratio [HR]: 1.69; 95% CI, 1.22-2.36, P = 0.002) and mortality (HR: 2.29; 95% CI, 1.59-3.29, P < 0.001) compared with non-MVI. We further found that both-MVI type was significantly associated with a higher risk of extrahepatic recurrence (EHR) (HR: 8.74; 95% CI, 2.38-32.03, P = 0.001). Among HCC patients after curative resection, concurrent portal and capsular MVI is a risk factor for HCC recurrence, especially for EHR, in comparison with non-MVI or only portal or capsular MVI alone.
Keywords: Hepatocellular carcinoma, microscopic vascular invasion, overall survival
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer occurrence and the third leading cause of cancer-related death in the world [1,2]. Several international associations have proposed guidelines about the diagnosis, staging, and treatment of HCC [3-5]. Most of the HCC therapeutic strategies are based on the Barcelona Clinic Liver Cancer (BCLC) stage, which considers the tumor number, tumor size, patients’ performance, and Child-Pugh grade. The primary liver resection has been applied as one of the curative treatments for early-stage (BCLC stage 0 or A) HCC [3-7]. However, the 5-year tumor recurrence of approximately 54.8% remains a clinical problem [8]. Previous studies have proposed several risk factors and predictors of poor prognosis and tumor recurrence [9,10], which include pathologic features.
Microscopic vascular invasion (MVI) is one of the pathologic features and has been demonstrated as a strong risk factor for tumor recurrence and poor overall survival (OS) among patients after HCC resection [11-17]. Previous studies have proposed numerous classifications of MVI [18-20], which include those based on the number of vessels with MVI, the number of cancer cells within a vascular lumen, and the distance of MVI from the primary tumor. However, these classifications seem too complicated for daily practice, and no universal consensus about the MVI classification is currently put in place. Recently, a novel and simple MVI classification was reported that divided MVI into microscopic portal vein invasion and microvessel invasion [20]. This classification strengthened the importance of the microscopic portal vein invasion. Kuo et al. validated this MVI classification, in which the patients with microscopic portal vein invasion had worse 5-year OS than patients with microvessel invasion only or patients without MVI [13].
In our daily practice, two types of MVI are identified: portal vein and capsule vein. However, little is known about the impact of the different types of MVI on HCC recurrence. This study aimed to compare the HCC recurrence and OS between the portal vein and capsule vein MVI and provide the implication for further active surveillance or adjuvant therapies in MVI patients.
Patients and methods
Patient enrollment and methods
A total of 2103 patients who received HCC resection between 2001 and 2016 at Kaohsiung Chang Gung Memorial Hospital (KCGMH) were recruited. After excluding patients with (1) BCLC stage B or C, (2) patients who received radiofrequency ablation (RFA) or transarterial chemoembolization (TACE) before resection, and (3) patients who underwent liver transplantation after resection, 857 patients with BCLC stage 0 or A HCC who underwent primary resection were enrolled in this study (Figure 1).
Figure 1.
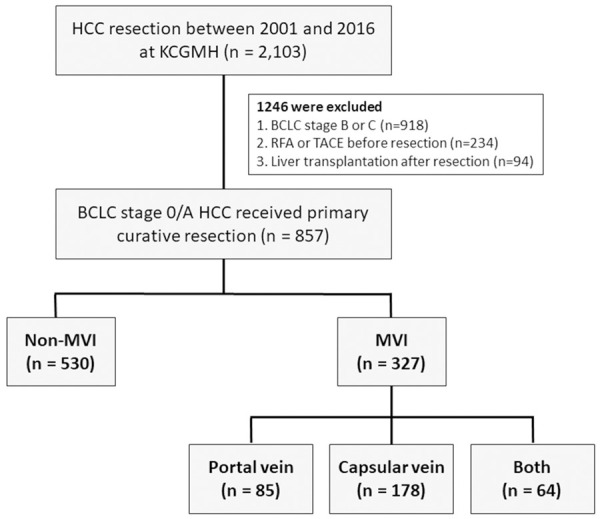
Flowchart of the study population.
The pathologic slides were reviewed by the experienced pathologist (Ting-Ting Liu, M.D.) and diagnosed as different types of MVI (portal vein and capsular vein invasion types) (Figure 2). The patients with MVI were stratified into portal vein type, capsule vein type, or both type (concurrent portal and capsule vein) MVI. The capsule vein type MVI is defined as an MVI in a tumor capsule or peri-tumoral areas. The portal vein type MVI is defined as MVI in the surrounding portal vein.
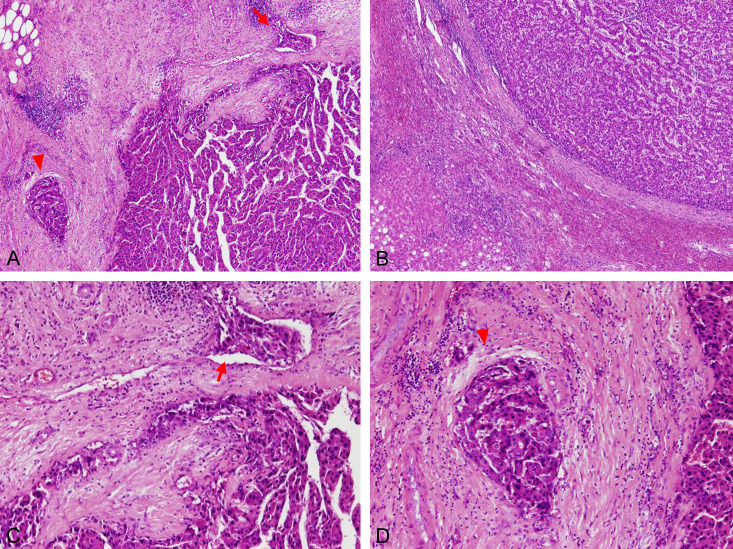
Figure 2.
Pathological features of the portal and capsular MVI. Sections after tumor resection were reviewed under microscopy by experienced pathologists. (A) Both capsule vein MVI (arrow) and portal vein MVI (arrowhead) under 40× magnification, (B) No capsular vein or portal vein MVI surrounding the tumor bed under 40× magnification, (C) Capsular vein MVI under 100× magnification: MVI (arrow) in surrounding tumor capsule (D) Portal vein MVI under 100× magnification: MVI in the portal vein with surrounding bile duct and hepatic arteriole.
Baseline characteristic data including demographic, serum biochemistry, and tumor characteristics were retrieved from the medical record. Histopathological diagnosis and the type of MVI were confirmed by the pathologists. Child-Pugh grade and BCLC stage were documented following current guidelines and international consensus. The diagnosis of HCC was based on the American Association for the Study of Liver Disease and the European Association for the Study of the Liver (EASL) guidelines [7,21]. The diagnosis of liver cirrhosis was based on pathological reports and Ishak score 5-6 [22]. We adopted a new definition of metabolic-associated fatty liver disease (MAFLD) rather than nonalcoholic fatty liver disease based on our acknowledgment of the pathogenesis and its impact on HBV-related early-stage HCC after curative liver resection [23,24]. The diagnosis of MAFLD following the 2020 international consensus of EASL is based on the presence of liver steatosis in addition to either overweight/obesity, presence of type 2 diabetes mellitus (DM), or presence of at least two risk factors of metabolic dysfunction [25].
The diagnosis of HCC recurrence was based on the typical imaging findings or continual elevation of serum alpha-fetoprotein (AFP) level. Biopsies were performed and histopathology evidence for the recurrence was assessed in the patients with controversial image or lab findings. Furthermore, recurrences were divided into either early recurrence (< 2 years) or late recurrence (≥ 2 years), which were from different pathogenesis [26]. Extrahepatic recurrence (EHR) was defined as situation that the patients who have EHR with or without concurrent intrahepatic recurrence as their first recurrence. Patients with previous local recurrence were excluded. The diagnosis of EHR was confirmed by contrast-induced computed tomography (CT) or magnetic resonance imaging (MRI). Bone scintigraphy, positron emission tomography-CT, brain CT, MRI, or pathological examination were applied as part of the investigation in some patients. Recurrence-free survival (RFS) was defined as the period from tumor resection to the detection of recurrence. The OS was defined as the period from tumor removal by resection to the death, to the last contact, or by December 31, 2020.
Statistical analysis
We performed statistical analysis using SPSS 23.0 statistical package (SPSS, Inc., Chicago, IL, USA). Data were expressed as mean (± standard deviation (SD)) for quantitative variables with normal distribution or as the median for variables without normal distribution. The differences in continuous and categorical variables across the four groups were assessed using ANOVA and Chi-square. The RFS and OS in different types of MVI were analyzed using Kaplan-Meier survival curves and the log-rank test. Chi-square test and Fisher’s exact test were applied in the analysis for categorical variables. A p-value < 0.05 was considered a statistical significance level.
Analysis of predictor factors for RFS and OS was performed by the Cox proportional hazards model. To reduce the probability of false positive in multivariate analysis, all potential risk factors (P < 0.1 in the univariate analysis) were required to be included in the multivariate analysis for the HCC recurrence, all-cause mortality, and HCC EHR. In this study, we demonstrated two models in multivariate analysis. In the multivariate model 1, all potential risk factors (P < 0.1 in the univariate analysis) were forced into the model. For the multivariate model 2, it used forward stepwise conditional LR method with P < 0.05 as selection criterion. As demonstrated in the separated tables for different prognosis, the multivariate model 2 was better for the interpretation. All the statistical results in this article referred to the multivariate model 2.
Results
Characteristics of the study population
Patients’ baseline characteristics are shown in Table 1. A total of 670 (78.2%) men and 187 (21.8%) women were recruited, with a mean age of 58-year-old at enrollment. A total of 222 (25.9%) patients had DM; 484 patients (56.5%) had chronic hepatitis B (CHB), and 300 (35.0%) had chronic hepatitis C (CHC), similar to the contributions of CHB and CHC in Taiwan HCC cohort [27]. A total of 400 patients (46.7%) suffered from liver cirrhosis, and 446 patients (52.5%) had high preoperative AFP levels (> 10 ng/mL). The majority in this population consisted of Child-Pugh grade A (91.7%) and BCLC stage A (85.5%). The mean tumor size was 2.9 ± 1.0 cm, and 780 patients (91.0%) had a single tumor. Most of the patients (84.3%) had a tumor in moderate differentiation and (86.3%) with capsular invasion. The mean follow-up duration was 87.2 ± 44.5 months.
Table 1.
Patient Characteristics of the study population
| Total (n = 857) | MVI (n = 327) | Non-MVI (n = 530) | P value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 58.6 ± 11.5 | 58.9 ± 11.4 | 58.5 ± 11.6 | 0.645 |
| Male, n (%) | 670 (78.2) | 259 (79.2) | 411 (77.5) | 0.568 |
| Diabetes mellitus, n (%) | 222 (25.9) | 89 (27.2) | 133 (25.1) | 0.491 |
| HBV, n (%) | 484 (56.5) | 185 (56.5) | 299 (56.4) | 0.963 |
| HCV, n (%) | 300 (35.0) | 129 (39.4) | 171 (32.3) | 0.032 |
| AST > 40 U/L, n (%) | 321 (37.5) | 119 (36.4) | 202 (38.1) | 0.613 |
| ALT > 40 U/L, n (%) | 374 (43.6) | 139 (42.5) | 235 (44.3) | 0.599 |
| Platelets < 150 103/µL, n (%) | 411 (48.0) | 171 (52.3) | 240 (45.3) | 0.046 |
| Albumin (g/dL); mean ± SD | 3.6 ± 0.6 | 3.6 ± 0.6 | 3.7 ± 0.6 | 0.129 |
| Liver cirrhosis, n (%) | 400 (46.7) | 157 (48.0) | 243 (45.8) | 0.537 |
| Child-Pugh grade, n (%) | 0.216 | |||
| A | 786 (91.7) | 295 (90.2) | 491 (92.6) | |
| B | 71 (8.3) | 32 (9.8) | 39 (7.4) | |
| BCLC stage, n (%) | < 0.001 | |||
| 0 | 124 (14.5) | 6 (1.8) | 118 (22.3) | |
| A | 733 (85.5) | 321 (98.2) | 412 (77.7) | |
| AFP > 10 ng/mL, n (%) | 446 (52.5) | 198 (60.6) | 248 (46.8) | < 0.001 |
| Tumor size (cm)a; mean ± SD | 2.9 ± 1.0 | 3.0 ± 1.0 | 2.8 ± 1.0 | < 0.001 |
| Tumor number, n (%) | 0.186 | |||
| Single | 780 (91.0) | 303 (92.7) | 477 (90.0) | |
| Multiple | 77 (9.0) | 24 (7.3) | 53 (10.0) | |
| Histological grade n (%) | < 0.001 | |||
| Well | 111 (13.1) | 13 (4.0) | 98 (18.8) | |
| Moderate | 715 (84.3) | 302 (92.6) | 413 (79.1) | |
| Poor | 22 (2.6) | 11 (3.4) | 11 (2.1) | |
| Capsule invasionb, n (%) | 738 (86.3) | 283 (86.5) | 455 (86.2) | 0.878 |
| Satellite nodule, n (%) | 21 (2.5) | 19 (5.8) | 2 (0.4) | < 0.001 |
| MAFLD, n (%) | 105 (23.3) | 43 (22.1) | 62 (24.2) | 0.589 |
Data are expressed as mean ± standard deviation or n (%). Abbreviations: MVI, microvascular invasion; CHB, chronic hepatitis B; CHC, chronic hepatitis C; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha fetoprotein; BCLC, Barcelona clinical liver cancer; MAFLD, metabolic-associated fatty liver disease.
Diameter of the largest tumor nodule.
Including partial and totally capsule invasion, and no well capsule.
Patients with MVI were 327 (38.2%) in this population. In comparison with non-MVI group, MVI group had higher proportion of BCLC stage A (P < 0.001), higher serum AFP level (P < 0.001), larger tumor size (P < 0.001), more satellite nodule (P < 0.001), and less well differentiated tumors (P < 0.001). However, no significant difference was observed in the ChildPugh grade. The presence of liver cirrhosis between the two groups did not differ significantly. The demographics (age and sex category), serum biochemistry (AST, ALT, platelet, and albumin), underlying liver disease (CHB, CHC, and MAFLD), and the proportion of DM comorbid were comparable between the two groups.
Clinicopathologic characteristics of HCC patients according to different MVI subgroups
In the MVI group, 327 patients were further divided into three subgroups. A total of 85 (26.0%) patients were classified into the MVI-portal vein group, 178 (54.4%) patients into the MVI-capsule vein group, and 64 (19.6%) patients into the MVI-both type group based on the pathologic diagnosis. The patient characteristics in different MVI subgroups are shown in Table 2.
Table 2.
Clinicopathological characteristics of HCC patients with different types of MVI
| MVI (n = 327) | MVI-portal vein (n = 85) | MVI-capsular vein (n = 178) | MVI-both (n = 64) | P value | |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 58.9 ± 11.4 | 59.4 ± 11.5 | 58.7 ± 10.9 | 58.7 ± 11.6 | 0.889 |
| Male, n (%) | 259 (79.2) | 61 (71.8) | 143 (80.3) | 55 (85.9) | 0.093 |
| Diabetes mellitus, n (%) | 89 (27.2) | 26 (30.6) | 39 (21.9) | 24 (37.5) | 0.040 |
| HBV, n (%) | 185 (56.6) | 47 (55.3) | 101 (56.7) | 37 (57.8) | 0.952 |
| HCV, n (%) | 129 (39.4) | 40 (47.1) | 61 (34.3) | 28 (43.8) | 0.103 |
| AST > 40 U/L, n (%) | 119 (36.4) | 31 (36.5) | 65 (36.5) | 23 (35.9) | 0.996 |
| ALT > 40 U/L, n (%) | 139 (42.5) | 29 (34.1) | 80 (44.9) | 30 (46.9) | 0.185 |
| Platelets < 150 103/µL, n (%) | 171 (52.3) | 47 (55.3) | 92 (51.7) | 32 (50.0) | 0.791 |
| Albumin (g/dL); mean ± SD | 3.6 ± 0.6 | 3.6 ± 0.6 | 3.6 ± 0.6 | 3.4 ± 0.7 | 0.225 |
| Liver cirrhosis, n (%) | 157 (48.0) | 43 (50.6) | 82 (46.1) | 32 (50.0) | 0.742 |
| Child-Pugh grade, n (%) | 0.678 | ||||
| A | 295 (90.2) | 78 (91.8) | 161 (90.4) | 56 (87.5) | |
| B | 32 (9.8) | 7 (8.2) | 17 (9.6) | 8 (12.5) | |
| BCLC stage, n (%) | < 0.001 | ||||
| 0 | 6 (1.8) | 4 (4.7) | 2 (1.1) | 0 (0) | |
| A | 321 (98.2) | 81 (95.3) | 176 (98.9) | 64 (100) | |
| AFP > 10 ng/mL, n (%) | 198 (60.6) | 52 (61.2) | 102 (57.3) | 44 (68.8) | 0.272 |
| Tumor size (cm)a; mean ± SD | 3.0 ± 1.0 | 2.9 ± 0.9 | 3.0 ± 1.1 | 3.2 ± 0.9 | 0.320 |
| Tumor number, n (%) | 0.065 | ||||
| Single | 303 (92.7) | 81 (95.3) | 167 (93.8) | 55 (95.9) | |
| Multiple | 24 (7.3) | 4 (4.7) | 11 (6.2) | 9 (4.1) | |
| Histological grade n (%) | 0.939 | ||||
| Well | 13 (4.0) | 3 (3.6) | 7 (3.9) | 3 (4.7) | |
| Moderate | 302 (92.6) | 77 (91.7) | 166 (93.3) | 59 (92.2) | |
| Poor | 11 (3.4) | 4 (4.8) | 5 (2.8) | 2 (3.1) | |
| Capsule invasionb, n (%) | 283 (86.5) | 65 (76.5) | 164 (92.1) | 54 (84.4) | 0.002 |
| Satellite nodule, n (%) | 19 (5.8) | 11 (2.9) | 1 (0.6) | 7 (10.9) | < 0.001 |
| MAFLD, n (%) | 43 (22.1) | 14 (26.9) | 19 (19.0) | 10 (23.3) | 0.523 |
Data are expressed as mean ± standard deviation or n (%). Abbreviations: MVI, microvascular invasion; CHB, chronic hepatitis B; CHC, chronic hepatitis C; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha fetoprotein; BCLC, Barcelona clinical liver cancer; MAFLD, metabolic-associated fatty liver disease.
Diameter of the largest tumor nodule.
Including partial and totally capsule invasion, and no well capsule.
MVI-both type group had a higher proportion of BCLC stage A (P < 0.001) and satellite nodules (P < 0.001) than the others. The demography, underlying liver disease, biochemistry data, Child-Pugh grade, AFP level, tumor size, number, or differentiation were comparable among groups.
Recurrence and survival analysis in HCC patients with and without MVI
A total of 471 (55.0%) patients experienced an HCC recurrence after 83 month-mean follow-ups, in which 202 (61.8%) were in the MVI group and 269 (50.8%) in the non-MVI group. In the MVI group, the 1-, 3-, 5-year RFS rates were 71.5%, 51.4%, and 41.5%, respectively. However, RFS rates were 84%, 63.5%, and 54.6%, respectively, in the non-MVI group (P < 0.001, Figure 3A). A total of 321 (37.5%) patients died during the follow-up period: 141 (43.1%) in the MVI group and 180 (34%) in the non-MVI group. The 1-, 3-, 5-year OS rates were 94.8%, 85.3%, and 70.9%, respectively in the MVI group. However, it was 98.1%, 92.4%, and 83.4%, respectively in the non-MVI group (P < 0.001, Figure 3B).
Figure 3.
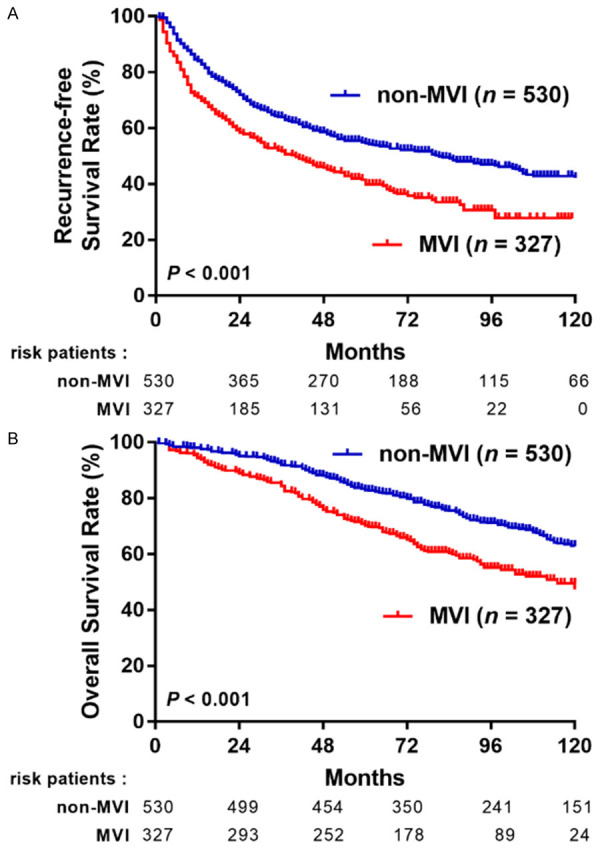
RFS (A) and OS (B) of HCC patients with or without MVI.
Recurrence and survival analysis stratified by different types of MVI
Of the 327 HCC patients with MVI, 85 (26.0%) were portal vein; 178 (54.4%) were capsular vein, and 64 (19.6%) were with MVI-both types. In the subgroup analysis, no significant differences between each group were observed (Figure 4A). However, in the OS analysis, patients with MVI-capsule type were significantly associated with higher survival outcomes than those with MVI-portal type (P = 0.024) and those with MVI-both type (P = 0.001) (Figure 4B).
Figure 4.
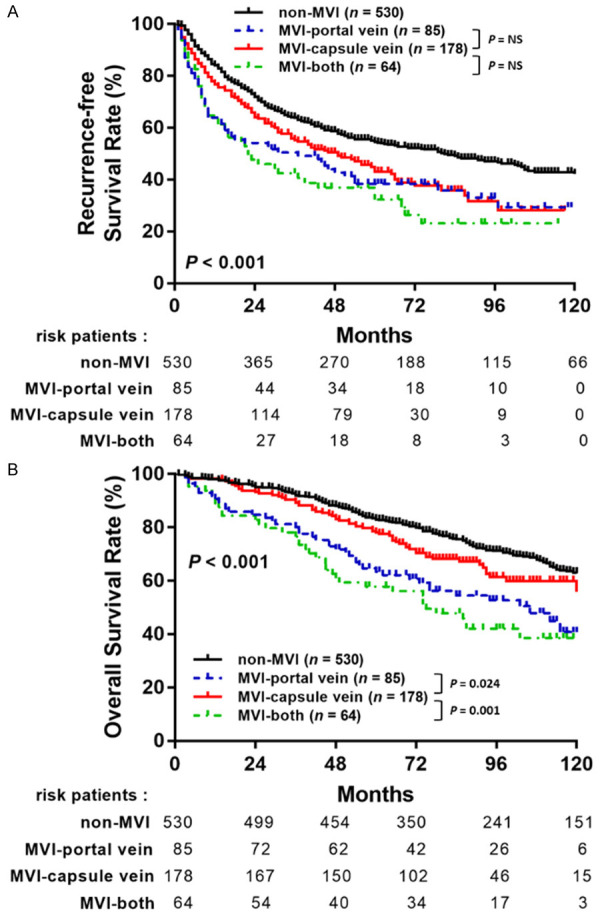
The comparison of RFS (A) and OS (B) stratified by different types of MVI.
In the multivariate analysis for HCC recurrence, MVI-both type is one of the strongest predictive factors for HCC recurrence (hazard ratio [HR]: 1.69; 95% CI, 1.22-2.36, P = 0.002). MVI-portal vein (HR: 1.55; 95% CI, 1.15-2.09, P = 0.004) and MVI-capsular vein (HR: 1.43; 95% CI, 1.14-1.80, P = 0.002) are associated with HCC recurrence. In addition to MVI, comorbidity related to DM (HR: 1.27; 95% CI, 1.04-1.56, P = 0.021), CHC (HR: 1.33; 95% CI, 1.10-1.62, P = 0.003), presence of liver cirrhosis (HR: 1.67; 95% CI, 1.39-2.01, P < 0.001), multiple tumor numbers (HR: 1.83; 95% CI, 1.38-2.44, P < 0.001), larger tumor size (> 2 cm) (HR: 1.48; 95% CI, 1.18-1.86, P = 0.001) and poor differentiated histological grade (HR: 2.37; 95% CI, 1.47-3.82, P < 0.001) are also predictors of HCC recurrence (Table 3).
Table 3.
Predict factors associated with HCC recurrence
| Variable | Comparison | Univariate | Multivariate 1a | Multivariate 2b | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age (years) | > 60 vs. ≤ 60 | 1.33 (1.11-1.60) | 0.002 | 1.21 (1.00-1.47) | 0.052 | ||
| Sex | Male vs. Female | 1.01 (0.81-1.26) | 0.913 | ||||
| Diabetes mellitus | Yes vs. No | 1.45 (1.19-1.77) | < 0.001 | 1.25 (1.02-1.54) | 0.031 | 1.27 (1.04-1.56) | 0.021 |
| CHB | Yes vs. No | 0.87 (0.73-1.05) | 0.136 | ||||
| CHC | Yes vs. No | 1.42 (1.18-1.71) | < 0.001 | 1.26 (1.03-1.54) | 0.022 | 1.33 (1.10-1.62) | 0.003 |
| MAFLD | Yes vs. No | 1.11 (0.83-1.47) | 0.482 | ||||
| AFP (ng/mL) | > 10 vs. ≤ 10 | 1.28 (1.07-1.54) | 0.007 | 1.18 (0.98-1.43) | 0.084 | ||
| Liver cirrhosis | Yes vs. No | 1.67 (1.40-2.01) | < 0.001 | 1.63 (1.35-1.96) | < 0.001 | 1.67 (1.39-2.01) | < 0.001 |
| Child-Pugh grade | B vs. A | 1.41 (1.02-1.94) | 0.036 | 1.15 (0.83-1.59) | 0.411 | ||
| BCLC stage | A vs. 0 | 1.71 (1.28-2.29) | < 0.001 | 1.17 (0.76-1.79) | 0.486 | ||
| Tumor number | Multiple vs. Single | 1.73 (1.31-2.28) | < 0.001 | 1.80 (1.35-2.41) | < 0.001 | 1.83 (1.38-2.44) | < 0.001 |
| Tumor size (cm) | > 2 vs. ≤ 2 | 1.45 (1.16-1.81) | 0.001 | 1.36 (0.99-1.86) | 0.059 | 1.48 (1.18-1.86) | 0.001 |
| Histological grade | Poor vs. well + moderate | 2.56 (1.60-4.10) | < 0.001 | 2.30 (1.43-3.72) | 0.001 | 2.37 (1.47-3.82) | < 0.001 |
| Capsule invasion | Yes vs. No | 1.10 (0.84-1.44) | 0.508 | ||||
| Satellite nodule | Yes vs. No | 1.53 (0.88-2.66) | 0.130 | ||||
| Microvascular invasion | None | Reference | |||||
| Capsule vein | 1.39 (1.11-1.74) | 0.004 | 1.38 (1.08-1.75) | 0.01 | 1.43 (1.14-1.80) | 0.002 | |
| Portal vein | 1.63 (1.21-2.19) | 0.001 | 1.49 (1.09-2.02) | 0.012 | 1.55 (1.15-2.09) | 0.004 | |
| Both | 1.97 (1.43-2.73) | < 0.001 | 1.59 (1.13-2.23) | 0.007 | 1.69 (1.22-2.36) | 0.002 | |
Abbreviations: HR, hazard ratio; CI, confidence interval; MVI, microvascular invasion; CHB, chronic hepatitis B; CHC, chronic hepatitis C; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha fetoprotein; BCLC, Barcelona clinical liver cancer; MAFLD, metabolic-associated fatty liver disease.
Multivariate 1: multivariable analysis based on all potential risk factors (P < 0.1 in the univariate Cox model) forced into the model.
Multivariate 2: multivariable analysis with variables (P < 0.1 in the univariate Cox model) using forward stepwise conditional LR method with P < 0.05 as selection criterion.
In the multivariate analysis for all-cause mortality, most of the associated predictors are similar for HCC recurrence, which include DM (HR, 1.72; 95% CI, 1.36-2.18, P < 0.001), Liver cirrhosis (HR: 1.81; 95% CI 1.44-2.27, P < 0.001), larger tumor size (> 2 cm) (HR: 1.51; 95% CI, 1.14-2.00, P = 0.004), MVI-portal vein type (HR: 1.85; 95% CI 1.31-2.62, P < 0.001) and MVI-both type (HR: 2.29; 95% CI 1.59-3.29, P < 0.001). Other predictors for all-cause mortality include old age (> 60-year-old) (HR: 1.34; 95% CI, 1.06-1.69, P = 0.013), Child-Pugh grade B vs. A (HR 1.78; 95% CI, 1.27-2.52, P = 0.001). However, MVI-capsular vein type failed to reach the statistically significant level for all causes of mortality (Table 4).
Table 4.
Predict factors associated with all-caused mortality
| Variable | Comparison | Univariate | Multivariate 1a | Multivariate 2b | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age (years) | > 60 vs. ≤ 60 | 1.46 (1.17-1.82) | 0.001 | 1.32 (1.04-1.69) | 0.024 | 1.34 (1.06-1.69) | 0.013 |
| Sex | Male vs. Female | 0.97 (0.74-1.26) | 0.807 | ||||
| Diabetes mellitus | Yes vs. No | 1.98 (1.57-2.50) | < 0.001 | 1.75 (1.37-2.22) | < 0.001 | 1.72 (1.36-2.18) | < 0.001 |
| CHB | Yes vs. No | 0.77 (0.62-0.96) | 0.021 | 0.83 (0.61-1.11) | 0.209 | ||
| CHC | Yes vs. No | 1.29 (1.03-1.61) | 0.027 | 0.92 (0.68-1.25) | 0.586 | ||
| MAFLD | Yes vs. No | 1.01 (0.72-1.42) | 0.956 | ||||
| AFP (ng/mL) | > 10 vs. ≤ 10 | 1.29 (1.03-1.60) | 0.025 | 1.24 (0.98-1.56) | 0.071 | ||
| Liver cirrhosis | Yes vs. No | 1.84 (1.47-2.30) | < 0.001 | 1.77 (1.41-2.22) | < 0.001 | 1.81 (1.44-2.27) | < 0.001 |
| Child-Pugh grade | B vs. A | 2.06 (1.47-2.88) | < 0.001 | 1.76 (1.25-2.50) | 0.001 | 1.78 (1.27-2.52) | 0.001 |
| BCLC stage | A vs. 0 | 1.57 (1.11-2.22) | 0.011 | 0.85 (0.49-1.46) | 0.544 | ||
| Tumor number | Multiple vs. Single | 1.41 (1.00-2.00) | 0.052 | 1.44 (1.00-2.08) | 0.05 | ||
| Tumor size (cm) | > 2 vs. ≤ 2 | 1.50 (1.14-1.98) | 0.004 | 1.66 (1.10-2.53) | 0.017 | 1.51 (1.14-2.00) | 0.004 |
| Histological grade | Poor vs. well + moderate | 2.34 (1.31-4.17) | 0.004 | 1.85 (1.02-3.34) | 0.041 | ||
| Capsule invasion | Yes vs. No | 1.20 (0.86-1.69) | 0.289 | ||||
| Satellite nodule | Yes vs. No | 2.37 (1.36-4.12) | 0.002 | 1.51 (0.82-2.81) | 0.189 | ||
| Microvascular invasion | None | Reference | |||||
| Capsule vein | 1.34 (1.00-1.80) | 0.05 | 1.27 (0.94-1.73) | 0.126 | |||
| Portal vein | 2.07 (1.47-2.91) | < 0.001 | 1.83 (1.27-2.63) | 0.001 | 1.85 (1.31-2.62) | < 0.001 | |
| Both | 2.59 (1.81-3.70) | < 0.001 | 2.11 (1.45-3.09) | < 0.001 | 2.29 (1.59-3.29) | < 0.001 | |
Abbreviations: HR, hazard ratio; CI, confidence interval; MVI, microvascular invasion; CHB, chronic hepatitis B; CHC, chronic hepatitis C; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha fetoprotein; BCLC, Barcelona clinical liver cancer; MAFLD, metabolic-associated fatty liver disease.
Multivariate 1: multivariable analysis based on all potential risk factors (P < 0.1 in the univariate Cox model) forced into the model.
Multivariate 2: multivariable analysis with variables (P < 0.1 in the univariate Cox model) using forward stepwise conditional LR method with P < 0.05 as selection criterion.
Early and late recurrence analysis stratified by different types of MVI
Among the total of 327 patients with MVI, 133 (40.7%) had early (< 2-year follow-up period), and 69 (59.3%) patients had late (≥ 2 years) HCC recurrence. As shown in Figure 5A, the Kaplan-Meier method showed that the MVI-both type group was significantly associated with a higher risk of early recurrence than the MVI-capsule vein group (P = 0.017). Moreover, the MVI-portal vein group had a borderline significant trend than the MVI-capsule vein group (P = 0.068). In late recurrence, no significant difference was observed between each group (Figure 5B). Similarly, the multivariate analysis revealed that MVI-both type (HR, 1.81; 95% CI, 1.22-2.70, P = 0.003) and MVI-portal vein type (HR, 1.79; 95% CI, 1.24-2.58, P = 0.002) were independent risk factors of early recurrence when compared with a non-MVI group (Table 5). Other factors that were significantly associated with a higher risk of early recurrence were comorbidity related to CHC (HR, 1.34; 95% CI, 1.05-1.71, P = 0.019), higher AFP level (> 10 ng/mL) (HR, 1.39; 95% CI, 1.08-1.79, P = 0.010), presence of liver cirrhosis (HR: 1.89; 95% CI, 1.47-2.42, P < 0.001), multiple tumor number (HR, 1.79; 95% CI, 1.24-2.57, P = 0.002), larger tumor size (> 2 cm) (HR: 1.61; 95% CI, 1.18-2.19, P = 0.003) and poor differentiated histological grade (HR, 2.63; 95% CI, 1.53-4.52, P < 0.001).
Figure 5.
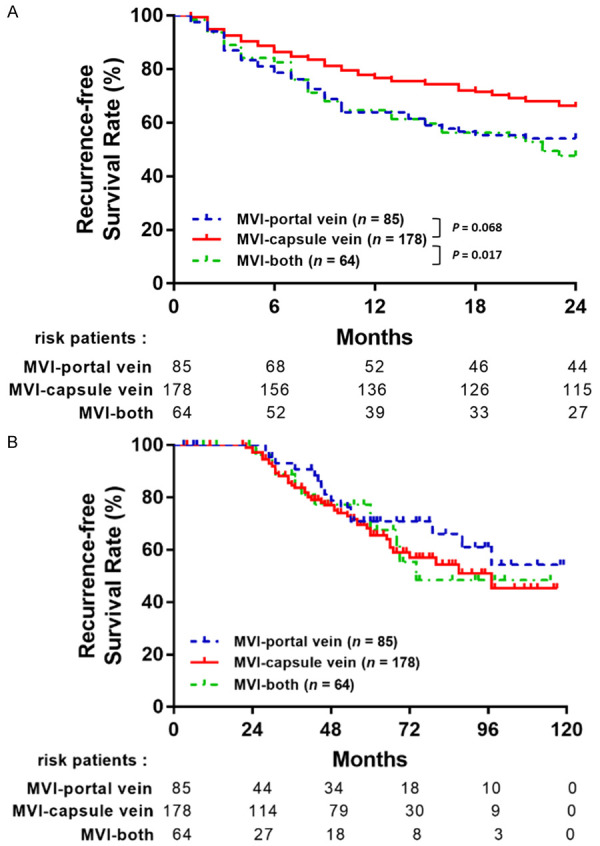
The comparison of (A) early RFS and (B) late RFS stratified by different types of MVI.
Table 5.
Predict factors associated with HCC early recurrence
| Variable | Comparison | Univariate | Multivariate 1a | Multivariate 2b | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age (years) | > 60 vs. ≤ 60 | 1.29 (1.01-1.63) | 0.04 | 1.15 (0.89-1.48) | 0.303 | ||
| Sex | Male vs. Female | 0.89 (0.67-1.18) | 0.414 | ||||
| Diabetes mellitus | Yes vs. No | 1.31 (1.01-1.69) | 0.043 | 1.16 (0.89-1.52) | 0.263 | ||
| CHB | Yes vs. No | 0.88 (0.69-1.12) | 0.292 | ||||
| CHC | Yes vs. No | 1.42 (1.12-1.80) | 0.004 | 1.26 (0.97-1.63) | 0.086 | 1.34 (1.05-1.71) | 0.019 |
| MAFLD | Yes vs. No | 1.16 (0.82-1.64) | 0.409 | ||||
| AFP (ng/mL) | > 10 vs. ≤ 10 | 1.62 (1.27-2.07) | < 0.001 | 1.42 (1.10-1.82) | 0.007 | 1.39 (1.08-1.79) | 0.01 |
| Liver cirrhosis | Yes vs. No | 1.93 (1.52-2.46) | < 0.001 | 1.87 (1.46-2.39) | < 0.001 | 1.89 (1.47-2.42) | < 0.001 |
| Child-Pugh grade | B vs. A | 1.49 (1.01-2.21) | 0.044 | 1.14 (0.76-1.71) | 0.526 | ||
| BCLC stage | A vs. 0 | 2.04 (1.33-3.13) | 0.001 | 1.29 (0.71-2.33) | 0.402 | ||
| Tumor number | Multiple vs. Single | 1.67 (1.17-2.38) | 0.005 | 1.72 (1.19-2.49) | 0.004 | 1.79 (1.24-2.57) | 0.002 |
| Tumor size (cm) | > 2 vs. ≤ 2 | 1.59 (1.17-2.15) | 0.003 | 1.40 (0.93-2.10) | 0.112 | 1.61 (1.18-2.19) | 0.003 |
| Histological grade | Poor vs. well + moderate | 2.94 (1.71-5.03) | < 0.001 | 2.46 (1.42-4.26) | 0.001 | 2.63 (1.53-4.52) | < 0.001 |
| Capsule invasion | Yes vs. No | 0.83 (0.60-1.14) | 0.25 | ||||
| Satellite nodule | Yes vs. No | 2.17 (1.19-3.97) | 0.012 | 1.15 (0.59-2.24) | 0.686 | ||
| Microvascular invasion | None | Reference | |||||
| Capsule vein | 1.33 (0.98-1.79) | 0.064 | 1.25 (0.91-1.72) | 0.161 | |||
| Portal vein | 2.02 (1.41-2.88) | < 0.001 | 1.71 (1.17-2.51) | 0.006 | 1.79 (1.24-2.58) | 0.002 | |
| Both | 2.31 (1.57-3.39) | < 0.001 | 1.72 (1.114-2.59) | 0.009 | 1.81 (1.22-2.70) | 0.003 | |
Abbreviations: HR, hazard ratio; CI, confidence interval; MVI, microvascular invasion; CHB, chronic hepatitis B; CHC, chronic hepatitis C; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha fetoprotein; BCLC, Barcelona clinical liver cancer; MAFLD, metabolic-associated fatty liver disease.
Multivariate 1: multivariable analysis based on all potential risk factors (P < 0.1 in the univariate Cox model) forced into the model.
Multivariate 2: multivariable analysis with variables (p < 0.1 in the univariate Cox model) using forward stepwise conditional LR method with P < 0.05 as selection criterion.
Extrahepatic recurrence analysis stratified by different types of MVI
A higher prevalence of EHR in MVI patients had been demonstrated in a previous study [28]. However, less is known about the effect of MVI types on EHR. We analyzed stratified EHR based on different MVI types to explore the effect of MVI on EHR in HCC. Among 857 patients, only 15 patients (1.75%) developed EHR during the follow-up period, 5 patients (0.94%) in the non-MVI group, 2 patients (2.35%) in the MVI-portal vein group, 3 patients (1.69%) in MVI-capsule vein group, and 5 patients (7.81%) in MVI-both type groups. The MVI-both type was significantly associated with a higher risk of EHR than other groups (P = 0.001, Figure 6). When considering the advanced analysis of EHR, only MVI-both type (HR, 8.74; 95% CI, 2.38-32.03, P = 0.001) and poor tumor differentiation (HR, 18.29; 95% CI, 3.56-93.88, P < 0.001) are strong predictors for EHR (Table 6).
Figure 6.
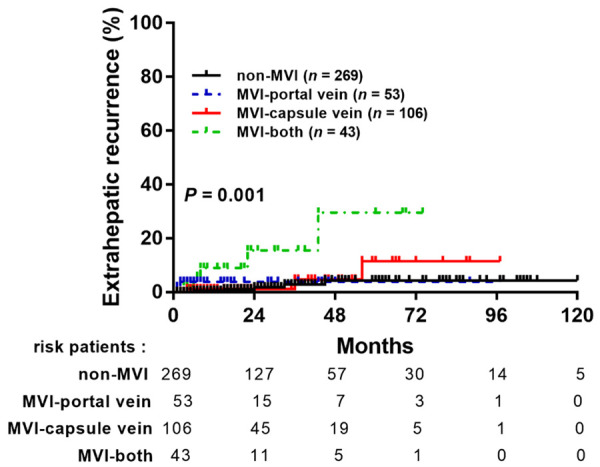
The comparison of extrahepatic recurrence of HCC stratified by different types of MVI.
Table 6.
Predict factors associated with HCC extrahepatic recurrence
| Variable | Comparison | Univariate | Multivariate 1a | Multivariate 2b | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age (years) | > 60 vs. ≤ 60 | 0.36 (0.12-1.14) | 0.083 | 0.55 (0.14-2.16) | 0.387 | ||
| Sex | Male vs. Female | 0.54 (0.19-1.59) | 0.265 | ||||
| Diabetes mellitus | Yes vs. No | 1.21 (0.38-3.80) | 0.747 | ||||
| CHB | Yes vs. No | 2.01 (0.64-6.32) | 0.232 | ||||
| CHC | Yes vs. No | 0.76 (0.24-2.39) | 0.638 | ||||
| MAFLD | Yes vs. No | 0.43 (0.05-3.46) | 0.429 | ||||
| AFP (ng/mL) | > 10 vs. ≤ 10 | 14.89 (1.96-113.24) | 0.009 | 6.69 (0.81-55.12) | 0.077 | ||
| Liver cirrhosis | Yes vs. No | 2.05 (0.73-5.78) | 0.173 | ||||
| Child-Pugh grade | B vs. A | 0.96 (0.13-7.32) | 0.969 | ||||
| Tumor number | Multiple vs. Single | 0.85 (0.11-6.49) | 0.877 | ||||
| Tumor size (cm) | > 2 vs. ≤ 2 | 1.53 (0.43-5.41) | 0.514 | ||||
| Histological grade | Poor vs. well + moderate | 15.01 (4.20-53.68) | < 0.001 | 16.279 (3.09-85.75) | 0.001 | 18.29 (3.56-93.88) | < 0.001 |
| Capsule invasion | Yes vs. No | 2.03 (0.27-15.53) | 0.495 | ||||
| Microvascular invasion | None | Reference | |||||
| Capsule vein | 1.92 (0.46-8.04) | 0.371 | |||||
| Portal vein | 3.01 (0.58-15.55) | 0.188 | |||||
| Both | 10.94 (3.16-37.96) | < 0.001 | 8.54 (2.34-31.22) | 0.001 | 8.74 (2.38-32.03) | 0.001 | |
Abbreviations: HR, hazard ratio; CI, confidence interval; MVI, microvascular invasion; CHB, chronic hepatitis B; CHC, chronic hepatitis C; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha fetoprotein; BCLC, Barcelona clinical liver cancer; MAFLD, metabolic-associated fatty liver disease.
Multivariate 1: multivariable analysis based on all potential risk factors (P < 0.1 in the univariate Cox model) forced into the model.
Multivariate 2: multivariable analysis with variables (P < 0.1 in the univariate Cox model) using forward stepwise conditional LR method with P < 0.05 as selection criterion.
Discussion
HCC represents one of the most common cancers in the world, especially in the high hepatitis B prevalent area, such as Taiwan and other East Asia countries. Although surveillance of HCC in high-risk populations may improve early tumor diagnosis, improve treatment, and reduce mortality rates, the clinical outcomes of patients receiving curative treatments remain unsatisfactory owing to the high rate of recurrence. MVI is a recent proposed pathological feature that has a strong association and predicted value for the recurrence and the poor prognosis in HCC patients receiving curative liver resection. A thorough search of the literature reveals that this is the first large-scale cohort study that focuses on RFS and OS of HCC after liver resection in different MVI subtypes. The results of this study showed the importance of MVI on the HCC recurrence after curative liver resection and OS. The results also reveal that the concurrent portal and capsular MVI is the most risk factor for HCC recurrence compared with the only portal or capsular MVI, which is a novel finding without previously published data.
Previous studies proposed that the patients with MVI had a worse 5-year disease-free survival (DFS) rate. Shuji Sumie et al. reported 5-year DFS in their cohort, revealing 20.8% and 52.6% in the MVI group and non-MVI group, respectively [11]. Kuo et al. demonstrated the importance of microscopic portal vein invasion. The 5-year cumulative recurrence rate was 26.6% in the microscopic portal vein invasion group. However, 5-year cumulative recurrence rate was 13.8% in non-vascular invasion group [13]. In the present study, the RFS and OS in MVI group were worse than non-MVI groups (5-year RFS: MVI: 41.5% vs. non-MVI: 54.6%, P < 0.05; 5-year OS: MVI: 70.9% vs. non-MVI: 83.4%, P < 0.05). The findings are consistent with that of previous studies [11,13].
It is agreed that the blood flowing into the HCC is mainly fed via neovascularization from arterial vessels and drained mostly via portal vein around the tumor. The spread of cancer cells via a portal vein in HCC is the main mechanism of intrahepatic metastasis [29]. Some studies mentioned that the interaction between HCC and the microenvironment may precede the vascular invasion of MVI [14]. Based on this mechanism, we assume that the progression of MVI commonly starts from the capsular vein to the surrounding small portal vein. MVI-both type is considered to have rapid growing and vascular invasive features. These characteristics represent the worst prognostic factors for the recurrence, early recurrence, OS, and EHR, which is compatible with the results of our present study.
EHR has a worse prognosis, most likely from their presentation at multiple sites with aggressive features. Patients with EHR are not amenable to resection, even for liver transplantation, revealing that the patient with a higher risk of EHR should consider more aggressive treatment. Thus, we focus on the first recurrence in EHR. In our analysis, patients with MVI-both type have the highest EHR, and MVI-both type is the only MVI subtype to predict EHR. This finding indicates that patients with MVI-both type should be monitored closely and consider further treatment, such as liver transplantation or adjuvant immune or target therapy before the development of EHR.
Unlike macroscopic vascular invasion, MVI is a pathological finding after curative HCC resection, which shows a lack of detection of MVI before operation. Therefore, we could only discuss further treatment based on the pathology diagnosis after the operation. This study revealed that the effect of MVI and related subtypes on outcome could be important evidence to make share decisions with the patient regarding the further plan. Unfortunately, histological information about the presence of MVI is not always available for HCC patients who receive non-surgical approaches, such as RFA, TACE, or radiotherapy. Hence, there is a pressing demand for an accurate and non-invasive method to detect MVI in preoperative riskstratify patients. To date, many studies evaluate clinical risk factors that could be used to predict MVI in HCC, which include AFP, des-γ-carboxy prothrombin, tumor number, tumor size, and thrombocytopenia [30,31], as shown in the presentation of the present study (Table 1). However, the performance evaluation of these clinical markers is not yet part of current clinical practice owing to poor sensitivity and specificity. Recently, Krishnan et al. identified that fibronectin was a promising predictor of MVI in HCC [32]. They found that combining AFP and fibronectin improved Area under the Curve by up to 0.93, revealing a better prediction effect of MVI than either fibronectin or AFP alone. In the future, a combination of clinical and molecular serological markers for early and accurate prediction of different types of MVI can improve patients’ outcomes.
In further clinical practice, getting precision medicine into the mainstream of clinical practice can promote patient management, since precision medicine can be customized to the whole person instead of the disease stage [33]. This approach considers many elements, both prognosis and the therapeutic decision are unfeasible to be included in the present practice guidelines. Currently, there is no established treatment in the adjuvant setting post HCC resection. Many neoadjuvant and adjuvant treatments have been tested unsuccessfully, including STORM trial [34]. Recently, systemic therapies, such as immune checkpoint inhibitors, tyrosine kinase inhibitors, and monoclonal antibodies offer encouraging results in the treatment of advanced HCC. The combination of Atezolizumab (anti-PDL1 antibody) and Bevacizumab (anti-VEGF antibody) has been demonstrated to offer better OS than Sorafenib [35,36]. Thus, the combination of atezolizumab and bevacizumab has become the standard of care in first-line therapies for advanced HCC. Consequently, several clinical trials incorporate such agents in reducing the risk of recurrence. For the adjuvant trial design, identifying higher risk characteristics is crucial for the appropriate selection of target populations since complementary treatment strategies are beneficial. The results of the present study provide more information on MVI extensively, demonstrating it as an independent risk factor for adverse outcomes in HCC patients.
Several limitations are identified in our study design. First, this is a single-center, retrospective study, in which different surgeons performed the operations, and different pathologists reported the pathological findings. Second, the number of different MVI subtype groups is not even, which is based on the pathological findings and is inevitable in the study design. Third, future studies should validate the classification of MVI subtypes adopted, especially the underestimation of MVI-both type group. Our HCC registry only records etiologies that include hepatitis B virus, hepatitis C virus, and MAFLD. Data regarding postoperative treatment, other severe comorbidities, anti-viral therapy, and the abstinence from alcohol, which might affect prognosis, were not available.
Conclusions
Among early-stage HCC patients after curative resection, concurrent portal and capsular MVI is a risk factor for HCC recurrence (HR: 1.69; P = 0.002), especially the EHR (HR: 8.74; P = 0.001), in comparison with non-MVI, only portal or capsular MVI. This finding discloses the different risks associated with the MVI subtype. Thus, an individualized strategy for the MVI subtype should be con-sidered. A further large-scale cohort study is needed for further validation.
Acknowledgements
We thank the service provided by the Cancer Registration of Cancer Center Department, Kaohsiung Chang Gung Memorial Hospital. We also appreciated the Biostatistics Center, Kaohsiung Chang Gung Memorial Hospital for statistics work. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, Jafri W, Payawal DA, Ohki T, Ogasawara S, Chen PJ, Lesmana CRA, Lesmana LA, Gani RA, Obi S, Dokmeci AK, Sarin SK. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–370. doi: 10.1007/s12072-017-9799-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the study of liver diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Orcutt ST, Anaya DA. Liver resection and surgical strategies for management of primary liver cancer. Cancer Control. 2018;25:1073274817744621. doi: 10.1177/1073274817744621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruix J, Sherman M Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 8.Gelli M, Sebagh M, Porcher R, Romanelli E, Vibert E, Sa Cunha A, Castaing D, Rosmorduc O, Samuel D, Adam R, Cherqui D. Liver resection for early hepatocellular carcinoma: preoperative predictors of non transplantable recurrence and implications for treatment allocation. Ann Surg. 2020;272:820–826. doi: 10.1097/SLA.0000000000004259. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Wang Z, Cao J, Han B, Zou H, Zang Y, Wu L. Risk factors and prognosis of patients with recurrent hepatocellular carcinoma who undergo liver re-resections. Eur J Surg Oncol. 2019;45:1684–1690. doi: 10.1016/j.ejso.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Xie QS, Chen ZX, Zhao YJ, Gu H, Geng XP, Liu FB. Systematic review of outcomes and meta-analysis of risk factors for prognosis after liver resection for hepatocellular carcinoma without cirrhosis. Asian J Surg. 2021;44:36–45. doi: 10.1016/j.asjsur.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, Watanabe Y, Kojiro M, Sata M. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Guo Y, Zhong J, Wang Q, Wang X, Wei H, Li J, Xiu P. The clinical significance of microvascular invasion in the surgical planning and postoperative sequential treatment in hepatocellular carcinoma. Sci Rep. 2021;11:2415. doi: 10.1038/s41598-021-82058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo FY, Liu YW, Lin CC, Yong CC, Wang CC, Chen CL, Cheng YF, Wang JH, Yen YH. Microscopic portal vein invasion is a powerful predictor of prognosis in patients with hepatocellular carcinoma who have undergone liver resection. J Surg Oncol. 2021;123:222–235. doi: 10.1002/jso.26260. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Li J, Shen F, Lau WY. Significance of presence of microvascular invasion in specimens obtained after surgical treatment of hepatocellular carcinoma. J Gastroenterol Hepatol. 2018;33:347–354. doi: 10.1111/jgh.13843. [DOI] [PubMed] [Google Scholar]
- 15.Zhang EL, Cheng Q, Huang ZY, Dong W. Revisiting surgical strategies for hepatocellular carcinoma with microvascular invasion. Front Oncol. 2021;11:691354. doi: 10.3389/fonc.2021.691354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, Kim SY, Sinn DH, Kim JM, Kim K, Ha SY. Effect of microvascular invasion risk on early recurrence of hepatocellular carcinoma after surgery and radiofrequency ablation. Ann Surg. 2021;273:564–571. doi: 10.1097/SLA.0000000000003268. [DOI] [PubMed] [Google Scholar]
- 17.Erstad DJ, Tanabe KK. Prognostic and therapeutic implications of microvascular invasion in hepatocellular carcinoma. Ann Surg Oncol. 2019;26:1474–1493. doi: 10.1245/s10434-019-07227-9. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez-Peralvarez M, Luong TV, Andreana L, Meyer T, Dhillon AP, Burroughs AK. A systematic review of microvascular invasion in hepatocellular carcinoma: diagnostic and prognostic variability. Ann Surg Oncol. 2013;20:325–339. doi: 10.1245/s10434-012-2513-1. [DOI] [PubMed] [Google Scholar]
- 19.Roayaie S, Blume IN, Thung SN, Guido M, Fiel MI, Hiotis S, Labow DM, Llovet JM, Schwartz ME. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009;137:850–855. doi: 10.1053/j.gastro.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang I, Jang M, Lee JG, Han DH, Joo DJ, Kim KS, Kim MS, Choi JS, Kim SI, Park YN, Choi GH. Subclassification of microscopic vascular invasion in hepatocellular carcinoma. Ann Surg. 2021;274:e1170–e1178. doi: 10.1097/SLA.0000000000003781. [DOI] [PubMed] [Google Scholar]
- 21.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Westin J, Lagging LM, Wejstål R, Norkrans G, Dhillon AP. Interobserver study of liver histopathology using the Ishak score in patients with chronic hepatitis C virus infection. Liver. 1999;19:183–187. doi: 10.1111/j.1478-3231.1999.tb00033.x. [DOI] [PubMed] [Google Scholar]
- 23.Eslam M, Sanyal AJ, George J International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158:1999–2014. e1991. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 24.Lin YP, Lin SH, Wang CC, Lin CC, Chen DW, Chuang CH, Huang PY, Hung CH, Yang SY, Cho WR, Chen YS, Tsai MC. Impact of MAFLD on HBV-related stage 0/A hepatocellular carcinoma after curative resection. J Pers Med. 2021;11:684. doi: 10.3390/jpm11080684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, Zelber-Sagi S, Wai-Sun Wong V, Dufour JF, Schattenberg JM, Kawaguchi T, Arrese M, Valenti L, Shiha G, Tiribelli C, Yki-Jarvinen H, Fan JG, Gronbaek H, Yilmaz Y, Cortez-Pinto H, Oliveira CP, Bedossa P, Adams LA, Zheng MH, Fouad Y, Chan WK, Mendez-Sanchez N, Ahn SH, Castera L, Bugianesi E, Ratziu V, George J. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 26.Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S, Makuuchi M. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200–207. doi: 10.1016/s0168-8278(02)00360-4. [DOI] [PubMed] [Google Scholar]
- 27.Liao SH, Chen CL, Hsu CY, Chien KL, Kao JH, Chen PJ, Chen TH, Chen CH. Long-term effectiveness of population-wide multifaceted interventions for hepatocellular carcinoma in Taiwan. J Hepatol. 2021;75:132–141. doi: 10.1016/j.jhep.2021.02.029. [DOI] [PubMed] [Google Scholar]
- 28.Yoon JH, Lee WJ, Kim SM, Kim KT, Cho SB, Kim HJ, Ko YS, Kook HY, Jun CH, Choi SK, Kim BS, Cho SY, You HS, Lee Y, Son S. Simple parameters predicting extrahepatic recurrence after curative hepatectomy for hepatocellular carcinoma. Sci Rep. 2021;11:12984. doi: 10.1038/s41598-021-92503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi M, Zhang CQ, Zhang YQ, Liang XM, Li JQ. Micrometastases of solitary hepatocellular carcinoma and appropriate resection margin. World J Surg. 2004;28:376–381. doi: 10.1007/s00268-003-7308-x. [DOI] [PubMed] [Google Scholar]
- 30.Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A, Wang K, Wan X, Lau WY, Wu M, Shen F. Nomogram for preoperative estimation of microvascular invasion risk in hepatitis b virus-related hepatocellular carcinoma within the Milan criteria. JAMA Surg. 2016;151:356–363. doi: 10.1001/jamasurg.2015.4257. [DOI] [PubMed] [Google Scholar]
- 31.Ryu T, Takami Y, Wada Y, Tateishi M, Hara T, Yoshitomi M, Momosaki S, Yasumori K, Saitsu H, Okuda K. A clinical scoring system for predicting microvascular invasion in patients with hepatocellular carcinoma within the Milan criteria. J Gastrointest Surg. 2019;23:779–787. doi: 10.1007/s11605-019-04134-y. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan MS, Rajan Kd A, Park J, Arjunan V, Garcia Marques FJ, Bermudez A, Girvan OA, Hoang NS, Yin J, Nguyen MH, Kothary N, Pitteri S, Felsher DW, Dhanasekaran R. Genomic analysis of vascular invasion in HCC reveals molecular drivers and predictive biomarkers. Hepatology. 2021;73:2342–2360. doi: 10.1002/hep.31614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–795. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruix J, Takayama T, Mazzaferro V, Chau GY, Yang J, Kudo M, Cai J, Poon RT, Han KH, Tak WY, Lee HC, Song T, Roayaie S, Bolondi L, Lee KS, Makuuchi M, Souza F, Berre MA, Meinhardt G, Llovet JM STORM investigators. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16:1344–1354. doi: 10.1016/S1470-2045(15)00198-9. [DOI] [PubMed] [Google Scholar]
- 35.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL IMbrave150 Investigators. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 36.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL. IMbrave150: updated overall survival (OS) data from a global, randomized, open-label phase III study of atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in patients (pts) with unresectable hepatocellular carcinoma (HCC) J. Clin. Oncol. 2021;39:267–267. [Google Scholar]