Abstract
Sorafenib is an oral multikinase inhibitor approved by the US Food and Drug Administration for treatment of the patients with surgically unresectable hepatocellular carcinoma (HCC). Sorafenib mitigates angiogenesis by targeting vascular endothelial growth factor receptors and platelet-derived growth factor receptors in endothelial cells and pericytes. Moreover, it suppresses cell proliferation via blockage of B-RAF and RAF1 of the mitogen-activated protein kinase pathway in tumor cells. Sorafenib has been the standard molecular targeted medication in the treatment of advanced-stage HCC patients ineligible for potentially curative interventional (radiofrequency or microwave ablation) or palliative trans-arterial chemoembolization (TACE) therapies for over a decade. However, it only increases overall survival by less than 3 months, and systemic exposure to sorafenib causes clinically significant toxicities (about 50% of patients). Given the high frequency and severity of these toxicities, sorafenib dose must be often reduced or discontinued altogether. In this review, we discussed the mechanism of sorafenib-associated adverse events and their management during HCC treatment.
Keywords: Adverse events, hepatocellular carcinoma, sorafenib, toxicity
Introduction
Hepatocellular carcinoma (HCC) remains the third-leading cause of cancer-related mortality worldwide, with the percentage of new deaths being 8.3% in 2020, following lung cancer (18.0%) and colorectal cancer (9.4%) [1-3]. Tyrosine kinase inhibitors are the standard molecular targeted drugs for the treatment of patients who are not suitable for surgical resection, interventional curative (radiofrequency or microwave ablation), or palliative trans-arterial chemoembolization (TACE) therapies [4,5]. Sorafenib is a tyrosine kinase inhibitor approved by the US Food and Drug Administration in 2007 for the treatment of advanced HCC patients [6,7]. It can inhibit angiogenesis by targeting vascular endothelial growth factor receptors (VEGFR) and platelet-derived growth factor receptors (PDGFR) in endothelial cells and pericytes, and also suppress cell proliferation via blockage of B-RAF and RAF1 of the mitogen-activated protein kinase (MAPK) pathway in tumor cells [8,9]. As a result, sorafenib is capable of increasing overall survival (OS) by about 3 months compared with supportive care [10]. However, for the pharmaceutical form of oral administration, larger portion of sorafenib dosage undergoes either oxidation or glucuronidation, thus reducing efficacy and potency in the treatment of HCC patients [11]. Furthermore, clinical studies have shown that approximately 50% of an orally administered sorafenib dose is excreted without any alteration [12]. Low serum levels of sorafenib in some patients suggest that oral administration may not be adequate to elicit consistent and potent therapeutic responses [13]. Besides, similar to other tyrosine kinase inhibitors, systemically administered sorafenib leads to clinically significant adverse events (AEs) e.g., diarrhea, hypertension, hand-foot skin reaction, and fatigue, for lacking tumor specificity [14-16]. Given the frequency and severity of these toxicities, dosage of sorafenib must be decreased frequently or discontinued altogether (>30% of patients) [17,18], which diminishes therapeutic response and OS. Efforts have been taken to improve the safety of sorafenib administration in HCC therapy. Chen et al. and Poursaid et al. respectively used poly (D,L-lactide-co-glycolide) microsphere and silk-elastinlike protein polymer for localized release of sorafenib to reduce systemic exposures [19,20]. Some researchers found that several derivatives of sorafenib got through structure modifications displayed higher bioavailability and fewer side effects [21]. Cumulative studies indicate that combination therapy with sorafenib may produce a better result in HCC treatment [22-25]. This review will briefly describe sorafenib’s function, sorafenib-associated AEs, probable mechanism of toxicity, and methods for reducing toxicity-related AEs.
Hepatocellular carcinoma
Etiology
HCC, comprising 75%-85% of primary liver cancer cases, is a major health problem and accounted for more than 670,000 new cases and 620,000 deaths in 2020 worldwide [3]. The main risk factors for HCC include chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV), aflatoxin-contaminated foods, excess body weight, type 2 diabetes, heavy alcohol intake, and smoking [26,27]. A different spectrum of vital risk factors is observed depending on environmental and cultural behaviors. Vaccination against HBV, which has dramatically decreased the prevalence of HBV infection, incidence and mortality rates of HCC in high-risk countries in Eastern Asia, is proved to be a major public health success [28]. However, incidence rates in countries across Europe and America have increased or stabilized at a higher level than before, probably associated with the prevalence of excess body weight and diabetes [29,30].
HCC therapeutic options
Early diagnosis of HCC through surveillance prompts the potential benefits of several types of treatment approaches [31]. Staging systems play a key role in predicting the prognosis of HCC patients, and Barcelona Clinic Liver Cancer (BCLC) staging system has been validated as capable of providing the best prognostic information with the highest discriminatory ability, and best suited for treatment guidance among many systems [32,33]. According to this guideline, early-stage HCC patients are treated with surgical resection, transplantation, or image-guided loco-regional ablation. However, only 10%-15% of patients are suitable candidates for these potentially curative treatments [34-36]. Intermediate-stage HCC patients tend to be treated with TACE, yet OS benefits from current transcatheter approaches directed to the liver remain relatively modest [26,37-40]. Systemic therapies can be utilized for patients with advanced HCC, but this type of drug offers little survival benefit, with the critical exception of multi-kinase inhibitor sorafenib [7,41-43]. Notably, sorafenib has been available for advanced HCC patients for over a decade and is still used as first-line systemic therapy (Figure 1) [41,44,45].
Figure 1.
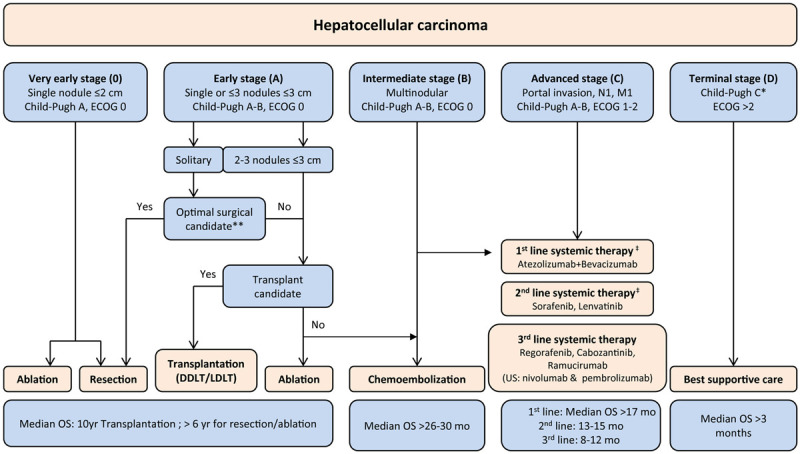
Modified BCLC staging system considering effective therapies in advanced stages (modified and updated from European Association for the Study of the Liver (EASL) guidelines). Management of patients with HCC is guided by the BCLC staging system, which takes into account both tumor extent and the severity of the underlying liver disease and defines 5 prognostic subgroups with respective treatments. Treatment for early-stage tumors is with curative intent, and options include RFA, hepatic resection, and liver transplantation. Patients with intermediate or advanced HCC are candidates for chemoembolization or systemic therapies, respectively. *Patients with end-stage liver disease if Child-Pugh class C should first be considered for liver transplantation. **Patients with preserved hepatic function Child-Pugh class A with normal bilirubin and no portal hypertension are optimal candidates for hepatic resection. ‡Atezolizumab plus bevacizumab has been approved as a new first-line treatment for advanced HCC. Nonetheless, sorafenib and lenvatinib are still considered first-line options when there is a contraindication for the combination treatment. Abbreviations: DDLT, deceased donor liver transplantation; LDLT, living donor liver transplantation; M1, distant metastasis; N1, lymph node metastasis.
Sorafenib already has a global assurance for HCC treatment [31]. In the western population, where the majority of cases are related to HCV infection or alcohol consumption, the use of sorafenib was validated by the sorafenib hepatocellular carcinoma assessment randomized protocol (SHARP) trial [10]. While most cases are related to HBV infection or alcohol consumption in the eastern population, the safety and efficacy of sorafenib were assessed by a multinational phase III trial in patients from the Asia-Pacific region with advanced HCC [46].
Sorafenib in HCC treatment
Sorafenib’s tosylate is a solid nonchiral molecule with a carbamido in its structure [47]. In the molecular pathogenesis of HCC, both extracellular signaling pathway mediated by epidermal growth factor receptor (EGFR), VEGFR, PDGFR, and intracellular signaling pathway mediated by RAS/RAF/mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (ERK), phosphatidylinositol-3-kinase (PI3K)/phosphatase and tensin homolog deleted on chromosome 10 (PTEN)/AKT/mammalian target of rapamycin (mTOR) has been implicated [48-51]. Sorafenib exerts antitumor function by targeting various protein kinases and signal transduction pathways. It can mitigate angiogenesis by blocking the autophosphorylation of several tyrosine kinases receptors, e.g., VEGFR1/2/3 and PDGFRs in endothelial cells and pericytes [52]. Also, it can suppress cell survival and proliferation by directly inhibiting RAF/MEK/ERK signaling pathway in tumor cells. More specifically, as a unique electron-withdrawing chemical group in sorafenib, carbamido forms hydrogen bonds with B-RAF and RAF1 protein, thus interrupting signal transmission [21]. Additionally, sorafenib can regulate PI3K/AKT/PTEN signaling pathway, which might activate an escape pathway from the MAPK cascade and result in resistance to sorafenib [53,54]. Furthermore, sorafenib can down-regulate the expression of myeloid cell leukemia sequence-1 (Mcl-1) and cellular inhibitor of apoptosis 2 (cIAP2), two anti-apoptotic proteins, by preventing nuclear factor-κB (NF-κB) from binding at the promoters of them, during which RAF signaling pathway is involved [55,56]. Thus, sorafenib can inhibit the proliferation and angiogenesis of HCC and increase its apoptosis rate (Figure 2).
Figure 2.
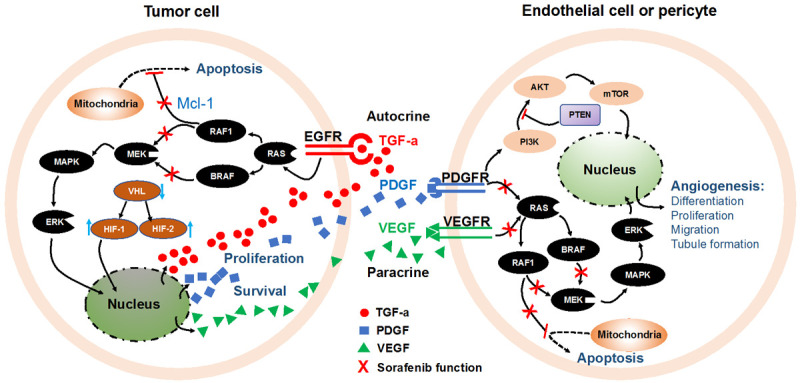
Mechanism of sorafenib functions by targeting tumor proliferation, apoptosis, and angiogenesis. AKT, serine/threonine-protein kinase; BRAF, v-Raf murine sarcoma viral oncogene homolog B1; EGFR, epidermal-growth-factor receptor; ERK, extracellular signal-regulated kinase; HIF, hypoxia-inducible factor; MAPK, mitogen-activated protein kinase; MEK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; Mcl-1, myeloid cell leukemia sequence 1; PDGF, platelet-derived growth factor; PDGFR, PDGF receptor; PI3K, phosphoinositol 3-kinase; PTEN, phosphatase and tensin homology deleted on chromosome 10; RAF, rapidly accelerated fibrosarcoma; RAF1, v-Raf-1 murine leukemia viral oncogene homolog 1; RAS, rat sarcoma virus; TGFα, tumor growth factor α; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; VHL, von Hippel-Lindau tumor suppressor gene.
After oral administration, plasma level of sorafenib reaches a peak value in 3 hours, and its mean half-life varies from 25 hours to 48 hours. In vitro binding experiments indicated that human plasma proteins could bind 99.5% of sorafenib, and high-fat meal might decrease its bioavailability [57]. In the liver, sorafenib undergoes oxidative metabolism mediated by cytochrome P450 (CYP)3A4. At the same time, sorafenib also undergoes glucuronidation, which is mediated by uridine diphosphate (UDP) glucuronosyltransferase family 1 member A9 (UGT1A9). In plasma, sorafenib accounts for 70%-85% of the circulating analytes at the steady-state in which 5 metabolites of sorafenib have been detected. The main metabolite of sorafenib in blood circulation is pyridine N-oxide M2, which possesses similar potency to that of sorafenib in vitro. After being taken orally, 96% of a sorafenib dose can be detected in blood circulation for up to 14 days, with 77% (of which 51% in the form of unchanged sorafenib) and 19% was excreted in feces and urine respectively as glucuronidated metabolites [12].
Toxicity
During the treatment of HCC with sorafenib, some drug-related AEs would occur [57]. Diarrhea, hand-foot skin reaction (HFSR), hypertension, fatigue, bilirubin elevation, thrombocytopenia, aspartate aminotransferase (AST) elevation, rash, anorexia, and alopecia are most frequently reported as dose-limiting toxicities (Table 1) [10,58-60].
Table 1.
Common adverse events occurred in HCC treatment with sorafenib
| Adverse Event | Reference | All grades [% (x/n)] | Grade ¾ [% (x/n)] |
|---|---|---|---|
| Hand-foot skin reaction | [10] | 20.87% (62/297) | 8.08% (24/297) |
| [46] | 44.97% (67/149) | 10.74% (16/149) | |
| [58] | 65.71% (46/70) | 15.71% (11/70) | |
| [59] | 52.42% (249/475) | 11.37% (54/475) | |
| [60] | 48.08% (75/156) | 8.33% (13/156) | |
| Average | 43.51% (499/1147) | 10.29% (118/1147) | |
| Rash | [10] | 16.16% (48/297) | 1.01% (3/297) |
| [46] | 20.13 (30/149) | 0.67% (1/149) | |
| [58] | 30.00% (21/70) | 0% (0/70) | |
| [59] | 16.00% (76/475) | 0.42% (2/475) | |
| [60] | 17.31% (27/156) | 2.56% (4/156) | |
| Average | 17.62% (202/1147) | 0.87% (10/1147) | |
| Hypertension | [10] | 4.71% (14/297) | 2.02% (6/297) |
| [46] | 18.79% (28/149) | 2.01% (3/149) | |
| [58] | 47.14% (33/70) | 15.71% (11/70) | |
| [59] | 30.32% (144/475) | 14.32% (68/475) | |
| [60] | 24.36% (38/156) | 12.18% (19/156) | |
| Average | 22.41% (257/1147) | 9.33% (107/1147) | |
| Diarrhea | [10] | 39.06% (116/297) | 8.08% (24/297) |
| [46] | 25.50% (38/149) | 6.04% (9/149) | |
| [58] | 47.14% (33/70) | 7.14% (5/70) | |
| [59] | 46.32% (220/475) | 4.21% (20/475) | |
| [60] | 49.36% (77/156) | 5.13% (8/156) | |
| Average | 42.20% (484/1147) | 5.75% (66/1147) | |
| Fatigue | [10] | 21.89% (65/297) | 4.04% (12/297) |
| [46] | 20.13% (30/149) | 3.36% (5/149) | |
| [58] | 28.57% (20/70) | 1.43% (1/70) | |
| [59] | 25.05% (119/475) | 3.58% (17/475) | |
| [60] | 18.59% (29/156) | 3.21% (5/156) | |
| Average | 22.93% (263/1147) | 3.49% (40/1147) | |
| Anorexia (Decreased appetite) | [10] | 14.14% (42/297) | 1.01% (3/297) |
| [46] | 12.75% (19/149) | 0% (0/149) | |
| [58] | 20.00% (14/70) | 2.86% (2/70) | |
| [59] | 26.74% (127/475) | 1.26% (6/475) | |
| [60] | 24.36% (38/156) | 3.85% (6/156) | |
| Average | 20.92% (240/1147) | 1.48% (17/1147) | |
| Alopecia | [10] | 14.14% (42/297) | 0% (0/297) |
| [46] | 24.83% (37/149) | NA | |
| [58] | 20.00% (14/70) | 0% (0/70) | |
| [59] | 25.05% (119/475) | 0% (0/475) | |
| [60] | 14.10% (22/156) | 0% (0/156) | |
| Average | 20.40% (234/1147) | 0% (0/1147) | |
| AST elevation (Aspartate aminotransferase increase) | [10] | NA | NA |
| [46] | NA | NA | |
| [58] | 27.14% (19/70) | 8.57% (6/70) | |
| [59] | 16.84% (80/475) | 8.00% (38/475) | |
| [60] | 16.67% (26/156) | 5.13% (8/156) | |
| Average | 17.83% (125/701) | 7.42% (52/701) | |
| Bilirubin elevation (Blood bilirubin increase) | [10] | NA | NA |
| [46] | NA | NA | |
| [58] | 54.29% (38/70) | 20.00% (14/70) | |
| [59] | 13.26% (63/475) | 4.84% (23/475) | |
| [60] | 14.10% (22/156) | 6.41% (10/156) | |
| Average | 17.54% (123/701) | 6.71% (47/701) | |
| Thrombocytopenia (Decreased platelet count) | [10] | NA | NA |
| [46] | NA | NA | |
| [58] | 51.43% (36/70) | 15.71% (11/70) | |
| [59] | 12.21% (58/475) | 3.37% (16/475) | |
| [60] | 11.54% (18/156) | 1.28% (2/156) | |
| Average | 15.98% (112/701) | 4.14% (29/701) |
HFSR is frequently observed in treatments with sorafenib, which functions by targeting VEGFR. VEGFR is expressed not only on dermal endothelial cells but on hair follicles and keratinocytes [61]. In 2009, Kong et al. reported that blocking the VEGFR pathway in the vascular endothelium with sorafenib might result in HFSR development [62]. HFSR would exacerbate in frequency and severity if sorafenib is administrated in combination with bevacizumab, which is a humanized antibody against VEGF [63]. Furthermore, the incidence of HFSR is directly associated with the cumulative bevacizumab dose, which supports the deduction that inhibition of VEGF may be a critical factor in HFSR development. Inhibiting VEGFR can hamper the mechanisms of vascular repair from functioning properly, thus causing HFSR in such high-pressure areas as the palms and soles [64]. However, it is important to note that receptors besides VEGFR may also be associated with the induction of HFSR, and more studies are needed to elucidate the biological mechanisms of sorafenib-associated HFSR.
Hypertension is another side effect caused by sorafenib in HCC treatment. It is suggested that blocking the VEGF/EGFR signaling pathway with sorafenib would result in downregulation of vasodilatation, in that VEGFR is expressed on endothelial cells in blood vessels, and sorafenib functions via targeting VEGFR [65]. Also, the investigators of “VEGF in ischemia for vascular angiogenesis (VIVA)” found that giving VEGF to humans could induce dose-dependent vasodilation, which would result in hypotension, tachycardia, and a decreased cardiac output [66,67]. Vasodilation is mainly mediated by nitric oxide and prostacyclin (also called prostaglandin I2, PGI2), which are produced by endothelial cells predominantly through a signal pathway involving the receptor of VEGFR2. Technically, nitric oxide is produced through endothelial-type nitric oxide synthase via the calcium-independent PI3K/AKT pathway under the condition of VEGF binding to VEGFR2 [67-70]. Additionally, VEGF is an inhibitor for the secretion of endothelin 1, which is a potent vascular constrictor that plays a crucial part in vascular remodeling [71]. These findings indicated that hypertension induced in treatment with sorafenib might have a relationship to VEGFR2 signaling pathways inhibition. Blocking of the VEGF/EGFR signaling pathway leads to decreased production of nitric oxide, PGI2, and endothelin 1, and finally elevation of blood pressure as a result.
Symptoms and management of AEs
Although these sorafenib-associated AEs are considered to be positively correlated with the survival rate of patients, and therefore can be used as clinical biomarkers for efficacy appraisal [72], efforts should be made to prevent and manage AEs with a final aim to increase patient compliance and improve clinical benefit.
Dose modification is the first consideration of management for any kind of sorafenib-related AEs. According to BCLC recommendations, dose modification should be made conforming to AEs severity. For mild AEs (grade 1), no dose modification is needed but only symptomatic treatment; for moderate AEs (grade 2), dose reduction should be made; for severe AEs (grade 3/4), dose interruption must be carried out [73]. Yet for each kind of AEs, there should be more specific management strategies. Here, we describe the symptoms and related management methods of the following types of common AEs.
Diarrhea
Diarrhea is one of the most frequently observed AEs associated with sorafenib therapy, and the incidence rates of it in all grades range from 39% to 58% [10,74]. Because the severity of diarrhea ranges from mild to severe, a thorough assessment of severity should be undertaken before making recommendations for diarrhea management. Patients should be reminded to report symptoms of diarrhea to their healthcare team immediately since early intervention can reduce the severity and improve both therapy efficacy and life quality. The primary aims of diarrhea management are to alleviate symptoms, prevent complications and restore normal bowel movements.
Dehydration may be caused by under-estimated or inadequately managed diarrhea. An imbalance in electrolytes can result in fatigue. In this case, maintaining hydration by orally intaking extra electrolyte-containing fluids is critical. Patient education is also very helpful in handling diarrhea symptoms, such as advising them to prefer a low-fiber diet, avoid foods with high insoluble fiber, and take some anti-diarrheal medicines e.g., loperamide if necessary [75].
HFSR
HFSR is another kind of widely reported AEs associated with sorafenib. According to our analysis of several milestone trials involving sorafenib administration (Table 2), the incidence of HFSR for all grades is about 21%-66% (with the mean value being 43.5%) (Table 1). HFSR poses the most significant impact on a patient’s life quality, especially for individuals with grades 3/4, who frequently need dose reduction or treatment withdrawal. Also, HFSR is a dose-dependent side effect and it will regress rapidly if sorafenib is discontinued.
Table 2.
Information on the trials involving sorafenib included in Table 1
| Reference | [10] | [46] | [58] | [59] | [60] |
|---|---|---|---|---|---|
| Trial keyword | SHARP | Asia-Pacific | Japanese | REFLECT | IMbrave150 |
| Trial arms | Sorafenib VS placebo | Sorafenib VS placebo | Safety profile of sorafenib, HCC VS RCC | Lenvatinib VS sorafenib | Atezolizumab plus bevacizumab VS sorafenib |
| Number of patients in sorafenib group | 297 | 149 | 70 | 475 | 156 |
| Year of data publish | 2008 | 2009 | 2014 | 2018 | 2020 |
| Trial phase | III | III | Real-life conditions | III | III |
| Trial number | NCT00105443 | NCT00492752 | - | NCT01761266 | NCT03434379 |
| Patients enrolled | 121 centers, 21 countries in Europe, North America, South America, Australasia | 23 centers in China, South Korea, Taiwan | Japanese HCC patients | 154 sites in 20 countries throughout the Asia-Pacific region, Europe, North America | Asia excluding Japanese v.s. the rest of the world |
For different grade HFSR, management strategies are mainly empirical. For grade 1, the clinical goal is to provide supportive measures, continue treatment with sorafenib, control hyperkeratotic areas, maintain skin moisture, and educate patients with skincare and protection knowledge. For grade 2, the clinical goal is to control hyperkeratosis, cushion callused areas, moisturize the skin, control symptoms and relieve discomfort. For grades 3/4, the clinical goal is to reduce symptoms and prevent further progression. Specific approaches may include administering some reagents, like salicylic acid, urea-based products, nonsteroidal anti-inflammatory drugs (NSAID), and corticosteroids [76].
Rash
A rash is frequently reported during many studies, with the incidence of all grades being 16%-40% [10]. The severity of the rash should be appraised together with clinical features such as fever, mucosal injury, enlargement of organs, and biological abnormalities to detect hypersensitivity reaction. For grade 1/2 rash, treatment can be continued under the monitoring of related clinical and biological markers to verify the favorable outcomes.
Management of the general rash is similar to that of HFSR in which anti-inflammatory therapies such as corticosteroid ointments and creams can be used topically for symptom relief [77,78]. For minimization of skin irritation, thick and alcohol-free moisturizers can be used regularly. Moreover, antihistamines e.g., diphenhydramine or hydroxyzine can be taken orally for some relief if necessary [79].
Hypertension
Hypertension with an incidence of all grades ranging from 5% to 47% is observed during HCC treatment with sorafenib (Table 1). For early detection of hypertension, frequent monitoring of blood pressure by medical personnel is essential. Also, patients can be trained to monitor and record their blood pressure for medical personnel.
For the management of hypertensive patients, we can follow Joint National Committee (JNC) guidelines for prevention, diagnosis, appraisal, and treatment of high blood pressure. Hypertension can be easily managed with 5 classes of drugs validated in blood pressure control (calcium channel blockers, angiotensin-converting enzyme (ACE) inhibitors, angiotensin 2 receptor blockers (ARBs), thiazide diuretics, and beta-blockers) alone or combination. If preexistent hypertension can be well controlled with a certain agent, patients should remain on that treatment. However, it should be noted that sorafenib can compete with calcium channel blockers such as diltiazem or 1,4-dihydropyridine in the CYP3A4 pathway, which may increase the plasma exposition of these medications [80].
Fatigue
Fatigue is the most common side effect with a frequency of between 18.6% and 29% for all grades and 1%-4% for grades 3/4 (Table 1). For the management of fatigue, multifactorial causes should be investigated carefully. At first, patients should be informed regarding multifactorial causes of fatigue such as hypohydration, depression, anorexia, diarrhea, and pain. Second, patients need to be advised to better management of their routine movements by working effectively around occurrences of fatigue. Third, encouraging patients to report fatigue symptoms and keep in contact with their medical team is crucial. Specifically, patients should be reminded to remain hydrated in case of fatigue resulting from dehydration. For patients with diarrhea, increasing hydration is especially important. At the same time, energy management techniques, such as adding resting periods as needed in the daytime, lowering body movements, and beginning a strength-training exercise program before sorafenib treatment, can also be employed for fatigue management [81,82].
Future
Currently, sorafenib remains the first-line drug for advanced HCC treatment according to BCLC guidelines, especially in case of contraindication for atezolizumab plus bevacizumab treatment strategy. Therefore, awareness of the AEs induced in HCC treatments is essential.
There are several recommendations and suggestions for the administration of sorafenib at a higher safety level, including modification of sorafenib’s molecular structure, transformation of pharmaceutical dosage forms, and optimization of drug delivery systems. Among these options, dose modification is the first consideration for the management of any kind of sorafenib-related AEs. Many reports have revealed that the efficacy of sorafenib under a modified dosage could be maintained. Two Japanese studies found comparable progression-free survival (PFS) and OS in HCC patients who started on full or half-dose sorafenib [83,84]. Also, a multivariate analysis made by Korean scientists indicated that a decreased dosage of sorafenib provided significantly better PFS and OS than another dosing [85]. Similarly, a research group from Canada revealed that starting sorafenib at a full and lower dose did not affect OS, and patients receiving half-dose sorafenib for 70% of their treatment had a better OS than those who maintained full dose for 70% of the treatment period [15]. Based on feasibly administrating sorafenib at a lower dosage level, combination therapy is gaining the attention of the research community. Because the tumor microenvironment of HCC is complicated by various kinds of immune cells, such as immune-stimulating cells like dendritic cells (DCs), CD8+ T, natural killer (NK), gamma delta T, and immune-suppressive cells like regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSC), it is reasonable to make a consideration of administrating sorafenib in combination with adoptive cell therapy [86,87]. Indeed, sorafenib can both decrease immune-suppressive cell populations and enhance immune-stimulating cell groups in the tumor microenvironment of HCC. Sorafenib can improve NK cell function in a low-dose manner without leading to exhaustion, thus paving the path to developing a rationale for using it in combination with NK cell-based adoptive immune therapy [88,89]. In conclusion, combination therapy with a consideration of orchestrating diverse types of treatment strategies to produce a synergistic antitumor effect and lower related AEs of each type of monotherapy method is emerging as the best possible option for the treatment of advanced HCC.
Acknowledgements
This study was supported by National Cancer Institute (grants R01CA209886 and R01CA241532), 2019 Harold E. Eisenberg Foundation Scholar Award, SIR Foundation Pilot Grant (PR-0000000012), and University of California Irvine Anti-Cancer Challenge Pilot Program.
Disclosure of conflict of interest
None.
References
- 1.Kaczynski J, Odén A. The rising incidence of hepatocellular carcinoma. N Engl J Med. 1999;341:451. doi: 10.1056/NEJM199908053410613. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Knox JJ, Kortmansky J, Leaf A, Remak WM, Shroff RT, Sohal DPS, Taddei TH, Venepalli NK, Wilson A, Zhu AX, Rose MG. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J. Clin. Oncol. 2020;38:4317–4345. doi: 10.1200/JCO.20.02672. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, De Baere T, Kulik L, Haber PK, Greten TF, Meyer T, Lencioni R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:293–313. doi: 10.1038/s41575-020-00395-0. [DOI] [PubMed] [Google Scholar]
- 6.Abou-Alfa GK. Selection of patients with hepatocellular carcinoma for sorafenib. J Natl Compr Canc Netw. 2009;7:397–403. doi: 10.6004/jnccn.2009.0028. [DOI] [PubMed] [Google Scholar]
- 7.Ray EM, Sanoff HK. Optimal therapy for patients with hepatocellular carcinoma and resistance or intolerance to sorafenib: challenges and solutions. J Hepatocell Carcinoma. 2017;4:131–138. doi: 10.2147/JHC.S124366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer R, Fetterly G, Lugade A, Thanavala Y. Sorafenib: a clinical and pharmacologic review. Expert Opin Pharmacother. 2010;11:1943–1955. doi: 10.1517/14656566.2010.496453. [DOI] [PubMed] [Google Scholar]
- 9.Takezawa K, Okamoto I, Yonesaka K, Hatashita E, Yamada Y, Fukuoka M, Nakagawa K. Sorafenib inhibits non-small cell lung cancer cell growth by targeting B-RAF in KRAS wild-type cells and C-RAF in KRAS mutant cells. Cancer Res. 2009;69:6515–6521. doi: 10.1158/0008-5472.CAN-09-1076. [DOI] [PubMed] [Google Scholar]
- 10.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 11.Hussaarts K, van Doorn L, Eechoute K, Damman J, Fu Q, van Doorn N, Eisenmann ED, Gibson AA, Oomen-de Hoop E, de Bruijn P, Baker SD, Koolen SLW, van Gelder T, van Leeuwen RWF, Mathijssen RHJ, Sparreboom A, Bins S. Influence of probenecid on the pharmacokinetics and pharmacodynamics of sorafenib. Pharmaceutics. 2020;12:788. doi: 10.3390/pharmaceutics12090788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lathia C, Lettieri J, Cihon F, Gallentine M, Radtke M, Sundaresan P. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol. 2006;57:685–692. doi: 10.1007/s00280-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Sheu AY, Li W, Zhang Z, Kim DH, Lewandowski RJ, Omary RA, Shea LD, Larson AC. Poly(lactide-co-glycolide) microspheres for MRI-monitored transcatheter delivery of sorafenib to liver tumors. J Control Release. 2014;184:10–17. doi: 10.1016/j.jconrel.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gong L, Giacomini MM, Giacomini C, Maitland ML, Altman RB, Klein TE. PharmGKB summary: sorafenib pathways. Pharmacogenet Genomics. 2017;27:240–246. doi: 10.1097/FPC.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alghamdi MA, Amaro CP, Lee-Ying R, Sim HW, Samwi H, Chan KK, Knox JJ, Ko YJ, Swiha M, Batuyong E, Romagnino A, Cheung WY, Tam VC. Effect of sorafenib starting dose and dose intensity on survival in patients with hepatocellular carcinoma: results from a Canadian Multicenter Database. Cancer Med. 2020;9:4918–4928. doi: 10.1002/cam4.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brose MS, Frenette CT, Keefe SM, Stein SM. Management of sorafenib-related adverse events: a clinician’s perspective. Semin Oncol. 2014;41(Suppl 2):S1–S16. doi: 10.1053/j.seminoncol.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Granito A, Marinelli S, Negrini G, Menetti S, Benevento F, Bolondi L. Prognostic significance of adverse events in patients with hepatocellular carcinoma treated with sorafenib. Therap Adv Gastroenterol. 2016;9:240–249. doi: 10.1177/1756283X15618129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin S, Bi F, Gu S, Bai Y, Chen Z, Wang Z, Ying J, Lu Y, Meng Z, Pan H, Yang P, Zhang H, Chen X, Xu A, Cui C, Zhu B, Wu J, Xin X, Wang J, Shan J, Chen J, Zheng Z, Xu L, Wen X, You Z, Ren Z, Liu X, Qiu M, Wu L, Chen F. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled phase II-III trial. J. Clin. Oncol. 2021;39:3002–3011. doi: 10.1200/JCO.21.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, White SB, Harris KR, Li W, Yap JW, Kim DH, Lewandowski RJ, Shea LD, Larson AC. Poly(lactide-co-glycolide) microspheres for MRI-monitored delivery of sorafenib in a rabbit VX2 model. Biomaterials. 2015;61:299–306. doi: 10.1016/j.biomaterials.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poursaid A, Jensen MM, Nourbakhsh I, Weisenberger M, Hellgeth JW, Sampath S, Cappello J, Ghandehari H. Silk-elastinlike protein polymer liquid chemoembolic for localized release of doxorubicin and sorafenib. Mol Pharm. 2016;13:2736–2748. doi: 10.1021/acs.molpharmaceut.6b00325. [DOI] [PubMed] [Google Scholar]
- 21.Chen F, Fang Y, Zhao R, Le J, Zhang B, Huang R, Chen Z, Shao J. Evolution in medicinal chemistry of sorafenib derivatives for hepatocellular carcinoma. Eur J Med Chem. 2019;179:916–935. doi: 10.1016/j.ejmech.2019.06.070. [DOI] [PubMed] [Google Scholar]
- 22.Dal Lago L, D’Hondt V, Awada A. Selected combination therapy with sorafenib: a review of clinical data and perspectives in advanced solid tumors. Oncologist. 2008;13:845–858. doi: 10.1634/theoncologist.2007-0233. [DOI] [PubMed] [Google Scholar]
- 23.Lachenmayer A, Toffanin S, Cabellos L, Alsinet C, Hoshida Y, Villanueva A, Minguez B, Tsai HW, Ward SC, Thung S, Friedman SL, Llovet JM. Combination therapy for hepatocellular carcinoma: additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J Hepatol. 2012;56:1343–1350. doi: 10.1016/j.jhep.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wada Y, Takami Y, Matsushima H, Tateishi M, Ryu T, Yoshitomi M, Matsumura T, Saitsu H. The safety and efficacy of combination therapy of sorafenib and radiotherapy for advanced hepatocellular carcinoma: a retrospective study. Intern Med. 2018;57:1345–1353. doi: 10.2169/internalmedicine.9826-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raybould AL, Sanoff H. Combination antiangiogenic and immunotherapy for advanced hepatocellular carcinoma: evidence to date. J Hepatocell Carcinoma. 2020;7:133–142. doi: 10.2147/JHC.S224938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kulik L, El-Serag HB. Epidemiology and management of hepatocellular carcinoma. Gastroenterology. 2019;156:477–491. doi: 10.1053/j.gastro.2018.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pattyn J, Hendrickx G, Vorsters A, Van Damme P. Hepatitis B vaccines. J Infect Dis. 2021;224:S343–S351. doi: 10.1093/infdis/jiaa668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrick JL, Florio AA, Znaor A, Ruggieri D, Laversanne M, Alvarez CS, Ferlay J, Valery PC, Bray F, McGlynn KA. International trends in hepatocellular carcinoma incidence, 1978-2012. Int J Cancer. 2020;147:317–330. doi: 10.1002/ijc.32723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M, Bray F, McGlynn KA, Petrick JL. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020;126:2666–2678. doi: 10.1002/cncr.32803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reig M, Bruix J. Sorafenib for hepatocellular carcinoma: global validation. Gastroenterology. 2009;137:1171–1173. doi: 10.1053/j.gastro.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Pons F, Varela M, Llovet JM. Staging systems in hepatocellular carcinoma. HPB (Oxford) 2005;7:35–41. doi: 10.1080/13651820410024058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adhoute X, Pénaranda G, Raoul JL, Edeline J, Blanc JF, Pol B, Campanile M, Perrier H, Bayle O, Monnet O, Beaurain P, Muller C, Castellani P, Le Treut YP, Bronowicki JP, Bourlière M. Barcelona clinic liver cancer nomogram and others staging/scoring systems in a French hepatocellular carcinoma cohort. World J Gastroenterol. 2017;23:2545–2555. doi: 10.3748/wjg.v23.i14.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wahl DR, Stenmark MH, Tao Y, Pollom EL, Caoili EM, Lawrence TS, Schipper MJ, Feng M. Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J. Clin. Oncol. 2016;34:452–459. doi: 10.1200/JCO.2015.61.4925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lencioni R, Crocetti L. Image-guided ablation for hepatocellular carcinoma. Recent Results Cancer Res. 2013;190:181–194. doi: 10.1007/978-3-642-16037-0_12. [DOI] [PubMed] [Google Scholar]
- 36.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 37.Gnutzmann D, Kortes N, Sumkauskaite M, Schmitz A, Weiss KH, Radeleff B. Transvascular therapy of Hepatocellular Carcinoma (HCC), status and developments. Minim Invasive Ther Allied Technol. 2018;27:69–80. doi: 10.1080/13645706.2018.1432489. [DOI] [PubMed] [Google Scholar]
- 38.Gbolahan OB, Schacht MA, Beckley EW, LaRoche TP, O’Neil BH, Pyko M. Locoregional and systemic therapy for hepatocellular carcinoma. J Gastrointest Oncol. 2017;8:215–228. doi: 10.21037/jgo.2017.03.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fidelman N, Kerlan RK Jr. Transarterial chemoembolization and (90)Y radioembolization for hepatocellular carcinoma: review of current applications beyond intermediate-stage disease. AJR Am J Roentgenol. 2015;205:742–752. doi: 10.2214/AJR.15.14802. [DOI] [PubMed] [Google Scholar]
- 40.Rognoni C, Ciani O, Sommariva S, Facciorusso A, Tarricone R, Bhoori S, Mazzaferro V. Trans-arterial radioembolization in intermediate-advanced hepatocellular carcinoma: systematic review and meta-analyses. Oncotarget. 2016;7:72343–72355. doi: 10.18632/oncotarget.11644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:6. doi: 10.1038/s41572-020-00240-3. [DOI] [PubMed] [Google Scholar]
- 42.Sangro B, Sarobe P, Hervás-Stubbs S, Melero I. Advances in immunotherapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2021;18:525–543. doi: 10.1038/s41575-021-00438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Grazie M, Biagini MR, Tarocchi M, Polvani S, Galli A. Chemotherapy for hepatocellular carcinoma: the present and the future. World J Hepatol. 2017;9:907–920. doi: 10.4254/wjh.v9.i21.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale A, Farinati F, Finotti M, Di Renzo C, Brancaccio G, Piscaglia F, Cabibbo G, Caturelli E, Missale G, Marra F, Sacco R, Giannini EG, Trevisani F, Cillo U Associazione Italiana Per Lo Studio Del Fegato Aisf Hcc Special Interest Group, Italian Liver Cancer Ita Li Ca Study Group. Overview of prognostic systems for hepatocellular carcinoma and ITA.LI.CA external validation of MESH and CNLC classifications. Cancers (Basel) 2021;13:1673. doi: 10.3390/cancers13071673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie DY, Ren ZG, Zhou J, Fan J, Gao Q. 2019 Chinese clinical guidelines for the management of hepatocellular carcinoma: updates and insights. Hepatobiliary Surg Nutr. 2020;9:452–463. doi: 10.21037/hbsn-20-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 47.Zustovich F, Lombardi G, Pastorelli D, Farina P, Bianco MD, De Zorzi L, Palma MD, Nicoletto O, Zagonel V. Clinical experience and critical evaluation of the role of sorafenib in renal cell carcinoma. Open Access J Urol. 2011;3:69–82. doi: 10.2147/OAJU.S7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Villanueva A, Newell P, Chiang DY, Friedman SL, Llovet JM. Genomics and signaling pathways in hepatocellular carcinoma. Semin Liver Dis. 2007;27:55–76. doi: 10.1055/s-2006-960171. [DOI] [PubMed] [Google Scholar]
- 49.Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, Factor VM, Thorgeirsson SS. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–1128. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 50.Semela D, Dufour JF. Angiogenesis and hepatocellular carcinoma. J Hepatol. 2004;41:864–880. doi: 10.1016/j.jhep.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 52.Cervello M, McCubrey JA, Cusimano A, Lampiasi N, Azzolina A, Montalto G. Targeted therapy for hepatocellular carcinoma: novel agents on the horizon. Oncotarget. 2012;3:236–260. doi: 10.18632/oncotarget.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tang S, Tan G, Jiang X, Han P, Zhai B, Dong X, Qiao H, Jiang H, Sun X. An artificial lncRNA targeting multiple miRNAs overcomes sorafenib resistance in hepatocellular carcinoma cells. Oncotarget. 2016;7:73257–73269. doi: 10.18632/oncotarget.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jindal A, Thadi A, Shailubhai K. Hepatocellular carcinoma: etiology and current and future drugs. J Clin Exp Hepatol. 2019;9:221–232. doi: 10.1016/j.jceh.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ricci MS, Kim SH, Ogi K, Plastaras JP, Ling J, Wang W, Jin Z, Liu YY, Dicker DT, Chiao PJ, Flaherty KT, Smith CD, El-Deiry WS. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 56.Mahoney DJ, Cheung HH, Mrad RL, Plenchette S, Simard C, Enwere E, Arora V, Mak TW, Lacasse EC, Waring J, Korneluk RG. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc Natl Acad Sci U S A. 2008;105:11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keating GM, Santoro A. Sorafenib: a review of its use in advanced hepatocellular carcinoma. Drugs. 2009;69:223–240. doi: 10.2165/00003495-200969020-00006. [DOI] [PubMed] [Google Scholar]
- 58.Fukudo M, Ito T, Mizuno T, Shinsako K, Hatano E, Uemoto S, Kamba T, Yamasaki T, Ogawa O, Seno H, Chiba T, Matsubara K. Exposure-toxicity relationship of sorafenib in Japanese patients with renal cell carcinoma and hepatocellular carcinoma. Clin Pharmacokinet. 2014;53:185–196. doi: 10.1007/s40262-013-0108-z. [DOI] [PubMed] [Google Scholar]
- 59.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 60.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 61.Man XY, Yang XH, Cai SQ, Yao YG, Zheng M. Immunolocalization and expression of vascular endothelial growth factor receptors (VEGFRs) and neuropilins (NRPs) on keratinocytes in human epidermis. Mol Med. 2006;12:127–136. doi: 10.2119/2006-00024.Man. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Azad NS, Aragon-Ching JB, Dahut WL, Gutierrez M, Figg WD, Jain L, Steinberg SM, Turner ML, Kohn EC, Kong HH. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:1411–1416. doi: 10.1158/1078-0432.CCR-08-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kazazi-Hyseni F, Beijnen JH, Schellens JH. Bevacizumab. Oncologist. 2010;15:819–825. doi: 10.1634/theoncologist.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Robert C, Soria JC, Spatz A, Le Cesne A, Malka D, Pautier P, Wechsler J, Lhomme C, Escudier B, Boige V, Armand JP, Le Chevalier T. Cutaneous side-effects of kinase inhibitors and blocking antibodies. Lancet Oncol. 2005;6:491–500. doi: 10.1016/S1470-2045(05)70243-6. [DOI] [PubMed] [Google Scholar]
- 65.Wu S, Chen JJ, Kudelka A, Lu J, Zhu X. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 66.Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, Shah PK, Willerson JT, Benza RL, Berman DS, Gibson CM, Bajamonde A, Rundle AC, Fine J, McCluskey ER. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- 67.Sane DC, Anton L, Brosnihan KB. Angiogenic growth factors and hypertension. Angiogenesis. 2004;7:193–201. doi: 10.1007/s10456-004-2699-3. [DOI] [PubMed] [Google Scholar]
- 68.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 69.He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J Biol Chem. 1999;274:25130–25135. doi: 10.1074/jbc.274.35.25130. [DOI] [PubMed] [Google Scholar]
- 70.Blanchet B, Billemont B, Barete S, Garrigue H, Cabanes L, Coriat R, Francès C, Knebelmann B, Goldwasser F. Toxicity of sorafenib: clinical and molecular aspects. Expert Opin Drug Saf. 2010;9:275–287. doi: 10.1517/14740330903510608. [DOI] [PubMed] [Google Scholar]
- 71.Stachon A, Schlüter T, Junker K, Knopf HJ, Neuser RD, Krieg M. The secretion of endothelin-1 by microvascular endothelial cells from human benign prostatic hyperplasia is inhibited by vascular endothelial growth factor. Growth Factors. 2004;22:281–289. doi: 10.1080/08977190400004835. [DOI] [PubMed] [Google Scholar]
- 72.Abdel-Rahman O, Lamarca A. Development of sorafenib-related side effects in patients diagnosed with advanced hepatocellular carcinoma treated with sorafenib: a systematic-review and meta-analysis of the impact on survival. Expert Rev Gastroenterol Hepatol. 2017;11:75–83. doi: 10.1080/17474124.2017.1264874. [DOI] [PubMed] [Google Scholar]
- 73.Reig M, Gazzola A, Di Donato R, Bruix J. Systemic treatment. Best Pract Res Clin Gastroenterol. 2014;28:921–935. doi: 10.1016/j.bpg.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B, Taylor I, Moscovici M, Saltz LB. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J. Clin. Oncol. 2006;24:4293–4300. doi: 10.1200/JCO.2005.01.3441. [DOI] [PubMed] [Google Scholar]
- 75.Benson AB 3rd, Ajani JA, Catalano RB, Engelking C, Kornblau SM, Martenson JA Jr, McCallum R, Mitchell EP, O’Dorisio TM, Vokes EE, Wadler S. Recommended guidelines for the treatment of cancer treatment-induced diarrhea. J. Clin. Oncol. 2004;22:2918–2926. doi: 10.1200/JCO.2004.04.132. [DOI] [PubMed] [Google Scholar]
- 76.Anderson R, Jatoi A, Robert C, Wood LS, Keating KN, Lacouture ME. Search for evidence-based approaches for the prevention and palliation of hand-foot skin reaction (HFSR) caused by the multikinase inhibitors (MKIs) Oncologist. 2009;14:291–302. doi: 10.1634/theoncologist.2008-0237. [DOI] [PubMed] [Google Scholar]
- 77.Seruga B, Gan HK, Knox JJ. Managing toxicities and optimal dosing of targeted drugs in advanced kidney cancer. Curr Oncol. 2009;16(Suppl 1):S52–59. doi: 10.3747/co.v16i0.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robert C, Mateus C, Spatz A, Wechsler J, Escudier B. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60:299–305. doi: 10.1016/j.jaad.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 79.Pérez-Soler R, Delord JP, Halpern A, Kelly K, Krueger J, Sureda BM, von Pawel J, Temel J, Siena S, Soulières D, Saltz L, Leyden J. HER1/EGFR inhibitor-associated rash: future directions for management and investigation outcomes from the HER1/EGFR inhibitor rash management forum. Oncologist. 2005;10:345–356. doi: 10.1634/theoncologist.10-5-345. [DOI] [PubMed] [Google Scholar]
- 80.National High Blood Pressure Education Program. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Bethesda (MD): National Heart, Lung, and Blood Institute (US); 2004. [PubMed] [Google Scholar]
- 81.Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E, Eisenberger MA, Escalante CP, Jacobsen PB, Jankowski C, LeBlanc T, Ligibel JA, Loggers ET, Mandrell B, Murphy BA, Palesh O, Pirl WF, Plaxe SC, Riba MB, Rugo HS, Salvador C, Wagner LI, Wagner-Johnston ND, Zachariah FJ, Bergman MA, Smith C. Cancer-related fatigue, version 2.2015. J Natl Compr Canc Netw. 2015;13:1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.PDQ Cancer Information Summaries, editor. PDQ Supportive and Palliative Care Editorial Board. Gastrointestinal complications (PDQ®): patient version. Bethesda (MD): National Cancer Institute (US); 2002. [PubMed] [Google Scholar]
- 83.Nishikawa H, Osaki Y, Endo M, Takeda H, Tsuchiya K, Joko K, Ogawa C, Taniguchi H, Orito E, Uchida Y, Izumi N. Comparison of standard-dose and halfdose sorafenib therapy on clinical outcome in patients with unresectable hepatocellular carcinoma in field practice: a propensity score matching analysis. Int J Oncol. 2014;45:2295–2302. doi: 10.3892/ijo.2014.2654. [DOI] [PubMed] [Google Scholar]
- 84.Morimoto M, Numata K, Kondo M, Kobayashi S, Ohkawa S, Hidaka H, Nakazawa T, Okuwaki Y, Okuse C, Matsunaga K, Suzuki M, Morita S, Taguri M, Tanaka K. Field practice study of half-dose sorafenib treatment on safety and efficacy for hepatocellular carcinoma: a propensity score analysis. Hepatol Res. 2015;45:279–287. doi: 10.1111/hepr.12354. [DOI] [PubMed] [Google Scholar]
- 85.Tak KY, Nam HC, Choi JY, Yoon SK, Kim CW, Kim HY, Lee SW, Lee HL, Chang UI, Song DS, Yang JM, Kwon JH, Yoo SH, Sung PS, Choi SW, Song MJ, Kim SH, Jang JW. Effectiveness of sorafenib dose modifications on treatment outcome of hepatocellular carcinoma: analysis in real-life settings. Int J Cancer. 2020;147:1970–1978. doi: 10.1002/ijc.32964. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y, Li H, Liang Q, Liu B, Mei X, Ma Y. Combinatorial immunotherapy of sorafenib and blockade of programmed death-ligand 1 induces effective natural killer cell responses against hepatocellular carcinoma. Tumour Biol. 2015;36:1561–1566. doi: 10.1007/s13277-014-2722-2. [DOI] [PubMed] [Google Scholar]
- 87.Liu ZL, Liu JH, Staiculescu D, Chen J. Combination of molecularly targeted therapies and immune checkpoint inhibitors in the new era of unresectable hepatocellular carcinoma treatment. Ther Adv Med Oncol. 2021;13:17588359211018026. doi: 10.1177/17588359211018026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sprinzl MF, Reisinger F, Puschnik A, Ringelhan M, Ackermann K, Hartmann D, Schiemann M, Weinmann A, Galle PR, Schuchmann M, Friess H, Otto G, Heikenwalder M, Protzer U. Sorafenib perpetuates cellular anticancer effector functions by modulating the crosstalk between macrophages and natural killer cells. Hepatology. 2013;57:2358–2368. doi: 10.1002/hep.26328. [DOI] [PubMed] [Google Scholar]
- 89.Lohmeyer J, Nerreter T, Dotterweich J, Einsele H, Seggewiss-Bernhardt R. Sorafenib paradoxically activates the RAS/RAF/ERK pathway in polyclonal human NK cells during expansion and thereby enhances effector functions in a dose- and time-dependent manner. Clin Exp Immunol. 2018;193:64–72. doi: 10.1111/cei.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]


