Abstract
Ecology and biodiversity studies of Agrobacterium spp. require tools such as selective media and DNA probes. Tellurite was tested as a selective agent and a supplement of previously described media for agrobacteria. The known biodiversity within the genus was taken into account when the selectivity of K2TeO3 was analyzed and its potential for isolating Agrobacterium spp. directly from soil was evaluated. A K2TeO3 concentration of 60 ppm was found to favor the growth of agrobacteria and restrict the development of other bacteria. Morphotypic analyses were used to define agrobacterial colony types, which were readily distinguished from other colonies. The typical agrobacterial morphotype allowed direct determination of the densities of agrobacterial populations from various environments on K2TeO3-amended medium. The bona fide agrobacterium colonies growing on media amended with K2TeO3 were confirmed to be Agrobacterium colonies by using 16S ribosomal DNA (rDNA) probes. Specific 16S rDNA probes were designed for Agrobacterium biovar 1 and related species (Agrobacterium rubi and Agrobacterium fici) and for Agrobacterium biovar 2. Specific pathogenic probes from different Ti plasmid regions were used to determine the pathogenic status of agrobacterial colonies. Various morphotype colonies from bulk soil suspensions were characterized by colony blot hybridization with 16S rDNA and pathogenic probes. All the Agrobacterium-like colonies obtained from soil suspensions on amended media were found to be bona fide agrobacteria. Direct colony counting of agrobacterial populations could be done. We found 103 to 104 agrobacteria · g of dry soil−1 in a silt loam bulk soil cultivated with maize. All of the strains isolated were nonpathogenic bona fide Agrobacterium biovar 1 strains.
The ecology and biodiversity of Agrobacterium have been studied mainly by using collections of isolates from crown gall tumors. However, soil agrobacteria are usually nonpathogenic, and a better understanding of agrobacteria in soil habitats is necessary. Media suitable for studying low concentrations of agrobacteria in soil are still needed in spite of earlier attempts to produce them (for a review see reference 18). The percentage of cells recovered with some media depends upon the agrobacterial genotype (15), and such media should not be used to study the biodiversity of agrobacteria. Other media, such as those described by Brisbane and Kerr (6), do not result in significant differences in the percentage of cells recovered and can be used for biodiversity studies. Most of these media have been developed for isolating Agrobacterium from rich soils or tumors, and they are not selective enough to inhibit the growth of undesired microorganisms from biotopes containing relatively low concentrations of agrobacteria. Agrobacterium-like colonies selected by visual inspection also require additional tests to ensure that they are bona fide Agrobacterium colonies. As a result, agrobacterial density cannot be determined by direct counting.
Kinkle et al. (14) showed that several Rhizobium species were resistant to selenite and tellurite. Incorporation of selenite and tellurite into growth media has allowed direct isolation of Rhizobium meliloti from soil (14). The genera Agrobacterium and Rhizobium are close relatives. Thus, incorporation of selenite, which is present at a low concentration in the media of Brisbane and Kerr, or tellurite might improve the selectivity of media used to isolate Agrobacterium spp. However, such media could be used to study biodiversity only if the added oxidative metalloids did not significantly alter recovery of any of the agrobacterial genotypes.
Several bona fide species of the genus Agrobacterium and some putative new species not completely described yet have been identified by conventional morphological and biochemical analysis and by DNA-DNA hybridization studies. A relationship has been established between the classic assignments of agrobacteria in biovars (12) and the species designations, as follows: Agrobacterium vitis for biovar 3 (25), Agrobacterium rhizogenes for biovar 2, and Agrobacterium radiobacter for biovar 1 (35). This latter name was contested by Bouzar (3), who proposed Agrobacterium tumefaciens instead. Notwithstanding this, exhaustive studies have shown that there are at least nine genomic species within biovar 1 alone (31). Thus, the general term biovar 1 as defined by Keane et al. (12) is used in this paper to designate a cluster of closely related genomic species that includes, but is not restricted to, A. tumefaciens sensu Bouzar (3). Two other putative species of Agrobacterium still remain to be completely described. One putative species includes agrobacteria related to strain NCPPB1650 (13, 35). The other consists of agrobacteria isolated from weeping fig trees (5), which have been named Agrobacterium fici in the Biolog catalog. The species and biovar designations have been corroborated by 16S rRNA (rrs) analysis (5, 35, 41). All bona fide and putative Agrobacterium species contain specific rrs sequences. As a result, the rrs gene is now routinely used to identify the main species or biovars of newly isolated Agrobacterium strains, for instance, restriction fragment length polymorphism analysis by PCR (24, 30). Specific oligonucleotide probes based on variable parts of the rrs gene can thus be designed for rapid, accurate species identification on colony blots.
Pathogenic agrobacteria also occur in soils (3). As pathogenicity requires a large plasmid designated the Ti plasmid, some of the regions of this plasmid are routinely targeted by PCR amplification in order to identify pathogenic strains (29, 30). DNA probes based on the same Ti plasmid regions used in these PCR screening analyses can also be used to detect Ti plasmids on colony blots.
Here, we investigated whether media amended with selenite or tellurite are suitable for both direct counting and isolation of bona fide agrobacteria from soil. Most of the presently known biodiversity of Agrobacterium spp. was considered in order to evaluate the resistance of individual strains to selenite and tellurite and the effects of the two additives on cell recovery. Chromosome and Ti plasmid probes were used to establish the agrobacterium and pathogenicity status of the agrobacterium-like colonies isolated from soil by using amended or unamended media.
MATERIALS AND METHODS
Bacterial strains and media.
The strains of Agrobacterium spp. listed in Table 1 include representatives of all bona fide species plus representatives of putative new species and members of heterogeneous biovar 1, as described by Popoff et al. (31). Most strains used in this study that were isolated from the same host were confirmed to be genotypically different by using molecular methods described by Ponsonnet and Nesme (30), and these strains reflected the wide diversity of pathogenic populations involved in crown gall outbreaks (4, 29). Pathogenicity was tested by inoculating standard host plants as previously described (30). Bacteria were grown at 28°C for 48 h on nonselective MG agar medium and for 5 days on 1A medium (selective for all biovar 1 strains) and 2E medium (selective for A. rhizogenes) (6, 12). Strains were also grown overnight in LPG broth (30).
TABLE 1.
Agrobacterium strains used in this study
| Straina | Opine type produced in tumorb | Host plant or other properties | Primary designation and/or origin | MIC (mg · liter−1) of:
|
16S rDNA probee | Hybridization with plasmid Ti DNA probesf
|
||||
|---|---|---|---|---|---|---|---|---|---|---|
| tmr | nos |
vir
|
||||||||
| Na2SeO3c | K2TeO3d | Plasmid Ti nopaline type | Plasmid Ti octopine type | |||||||
| Biovar 1 | ||||||||||
| Pathogenic strains | ||||||||||
| CFBP2413T | O/M | Apple tree | B6, United States | 8,000 | 320 | 1 | + | − | + | + |
| Ach5 | O/M | Prunus sp. | United States (Y. Dessaux, France)h | 8,000 | 320 | 1 | + | − | + | + |
| CFPB2407 | C/O | Vitis vinifera cv. Danom | France | 8,000 | 320 | 1 | + | − | + | − |
| CFBP1903 | N | Prunus cerasus | C58, New York | 8,000 | 320 | 1 | + | + | + | + |
| CFBP1901 | N | C58 with pTiT37 | (Y. Dessaux, France)h | 8,000 | 320 | 1 | + | + | + | + |
| CFBP354 | N | ND | B10, France | 8,000 | 160 | 1 | + | + | + | + |
| CFBP1904 | N | Vitis vinifera | AG20, Greece | 8,000 | 320 | 1 | + | + | + | + |
| CFBP2410 | N | Populus sp. | M22, ND | 8,000 | 160 | 1 | + | + | + | + |
| CFBP2411 | N | Salix purpurea | CG4, New Zealand | 8,000 | 160 | 1 | + | + | + | + |
| CFBP2177 (6) | N | Populus section Leuce | France | 8,000 | 160 | 1 | + | + | + | + |
| CFBP2516 (5) | N | Populus section Leuce | France | 8,000 | 320 | 1 | + | + | + | + |
| A96.11 | N | Populus section Leuce | France | ND | ND | 1 | + | + | + | + |
| A134.2 (5) | N | Populus section Leuce | France | ND | 160 | 1 | + | + | + | + |
| M10 (4) | N | Populus section Leuce | France | ND | 320 | 1 | + | + | + | + |
| S56 | M | NDg | France (G1)i | ND | 160 | 1 | − | − | − | − |
| S377 | ND | ND | France (G1)i | ND | 160 | 1 | − | − | − | − |
| S4 | N | Black raspberry | France (G1)i | ND | 160 | 1 | + | + | + | + |
| ATCC 4720 | ND | ND | United States (G1)i | ND | 160 | 1 | + | − | − | − |
| NCPPB925 | ND | Dahlia sp. | South Africa (G6)i | ND | 160 | 1 | − | − | − | − |
| F/1Zutra | ND | Dahlia sp. | Israel (G6)i | ND | 160 | 1 | + | − | − | − |
| 3/1Zutra | ND | Apple tree | Israel (G7)i | ND | 160 | 1 | − | − | − | − |
| NCPPB1641 | ND | Flacourtia ramontchi | United Kingdom (G7)i | ND | 160 | 1 | − | − | − | − |
| T37 | N | Walnut | United States (G8)i | ND | 160 | 1 | + | + | + | + |
| ICPB TT9 | ND | Hop | United States (G8)i | ND | 160 | 1 | + | + | + | + |
| 6 Mushin | ND | Hop | Australia (G8)i | ND | 160 | 1 | + | + | + | + |
| O362 | ND | Soil | South Australia (G9)i | ND | 160 | 1 | + | + | + | + |
| 2T3Pb (3) | ND | Walnut | Spain (M. Lopez, Spain)h | ND | 160 | 1 | + | + | + | + |
| 6MS3 | ND | Walnut | Spain (M. Lopez, Spain)h | ND | 320 | 1 | + | + | + | + |
| 436.3SA | M | Prunus hybrid cv. GF677 | Spain (M. Lopez, Spain)h | ND | 320 | 1 | + | − | + | + |
| CFBP296 | N | Lycopersicon esculentum | 111, France | 8,000 | 160 | 1 | − | − | − | − |
| M15 | N | Populus section Leuce | France | 8,000 | 160 | 1 | − | − | − | − |
| Nonpathogenic strains | ||||||||||
| CFBP2414T | NAj | Unknown | 3-24-2, The Netherlands | 8,000 | 160 | 1 | − | − | − | − |
| C58C1 | NA | C58 cured of pTiC58 | 8,000 | 160 | 1 | − | − | − | − | |
| GMI9023 | NA | C58 cured of pTiC58 and pAtC58 | (Y. Dessaux, France)h | 8,000 | 160 | 1 | − | − | − | − |
| CFBP2518 | NA | Populus section Leuce | France | 8,000 | 160 | 1 | − | − | − | − |
| O363 | NA | Soil | Australia (G9)i | ND | 160 | 1 | − | − | − | − |
| CFBP2456 | NA | ND | CDC B6016, United States | ND | ND | 1 | − | − | − | − |
| CFBP2457 | NA | ND | CDC B3771, United States | ND | ND | 1 | − | − | − | − |
| RV3 | NA | ND | ND (G7)i | ND | 80 | 1 | − | − | − | − |
| CIP28-75 (3) | NA | Human | France (G2)i | ND | ND | 1 | − | − | − | − |
| CIP111-78 | NA | Human | France (G3)i | ND | ND | 1 | − | − | − | − |
| CFBP2454 | NA | Human | France | ND | ND | 1 | − | − | − | − |
| CFBP2458 | NA | ND | M2/1, ND | 8,000 | 160 | 1 | − | − | − | − |
| CFBP2241 | NA | Human | CDC 7258, ND | ND | ND | 1 | − | − | − | − |
| CFBP2243 | NA | Human | CDC A6597, United States | ND | ND | 1 | − | − | − | − |
| Biovar 2 | ||||||||||
| Pathogenic strains | ||||||||||
| CFBP2408T | A/M | ND | ND | ND | ND | 2 | − | − | + | + |
| CFBP450 | ND | Malus pumila cv. M IX | France | 2,000 | 1,280 | 2 | − | − | − | − |
| CFBP1804 | N | Prunus persicae cv. GF305 | France | 4,000 | 1,280 | 2 | + | + | + | + |
| CFBP1905 | N | Vitis vinifera | AG28, Greece | 4,000 | 1,280 | 2 | + | + | + | + |
| CFBP1936 | N | Rosa sp. | Tahiti | 2,000 | 1,280 | 2 | + | + | + | + |
| CFBP1961 | N | Populus bolleana | France | 4,000 | 1,280 | 2 | + | + | + | + |
| CFBP1962 | N | Prunus cerasus cv. Mahaleb | Spain | 4,000 | 1,280 | 2 | + | + | + | + |
| CFBP2178 | N | Prunus aviumcv. F12-1 | France | 12,000 | 1,280 | 2 | + | + | + | + |
| CFBP2417 (3) | N | Cherry hybrid cv. Colt | France | 6,000 | 1,280 | 2 | + | + | + | + |
| CFBP2519 (3) | N | Populus section Leuce | France | 4,000 | 1,280 | 2 | + | + | + | + |
| C104.12 | N | Populus section Leuce | France | 6,000 | 1,280 | 2 | + | + | + | + |
| C104.22 (8) | N | Populus section Leuce | France | ND | 1,280 | 2 | + | + | + | + |
| M120 | N | Populus section Leuce | France | ND | 640 | 2 | + | + | + | + |
| Nonpathogenic strains | ||||||||||
| CFBP1937 | NA | Soil | K84, Australia | ND | ND | 2 | − | − | − | − |
| CFBP2520 | NA | Populus section Leuce | France | 4,000 | 640 | 2 | − | − | − | − |
| Biovar 3 | ||||||||||
| Pathogenic strains | ||||||||||
| CFBP2512 | N | Vitis vinifera | 565-5, Spain | 10,000 | 1,280 | − | + | + | + | (+) |
| CFBP2620 | N | Vitis vinifera | K374, Australia | 14,000 | 1,280 | − | + | + | + | (+) |
| CFBP2618 | C/O | Vitis vinifera cv. Cabernet | 85-255, France | 8,000 | 1,280 | − | + | − | + | − |
| CFBP2622 | C/O | Vitis vinifera cv. Sultana | Ag63, Greece | 14,000 | 1,280 | − | + | − | + | (+) |
| CFBP2621 | C/O | Vitis vinifera cv. Sultana | Ag57, Greece | 14,000 | 1,280 | − | − | − | − | − |
| A. rubi | ||||||||||
| Pathogenic strain | ||||||||||
| CFBP999T | N | Rubus sp. | TR2, United States | 8,000 | 160 | 1 | + | + | + | + |
| A. fici | ||||||||||
| AF3.44 | Ficus benjamina | United States (H. Bouzar, United States) | 8,000 | 320 | 1 | ND | ND | ND | ND | |
CFBP, Collection Française de Bactéries Phytopathogènes, Institut National de la Recherche Agronomique, Angers, France; NCPPB, National Collection of Plant Pathogenic Bacteria, Harpenden, United Kingdom; ICPB, International Colleciton of Phytopathogenic Bacteria, Davis, Calif.; CIP, Collection de l'Institut Pasteur, Paris, France; ATCC, American Type Culture Collection, Manassas, Va. The numbers in parentheses indicate the numbers of strains with the same origin and the same relevant characteristics that gave the same MIC and hybridization results. The groups of strains were as follows: CFBP2177, B100.11, M9, 85.2, 85.6, and 85.104; CFBP2516, A134.6, M292, X88.283, and 85.66; A134.2, A134.3, M214, X88.299, and 85.49; M10, X88.293, X88.303, and 85.52; 2T3Pb, 1C3Pb, and 2T3Sa; CIP28-75, CIP43-76, and CIP127-76; CFBP2417, CFBP2418, and CFBP2419; CFBP2519, 85.100, and 85.186; and C104.22, M3, M32, M84, M111, 85.30, 85.120, and 85.123.
O/M, octopine/mannopine; N, nopaline; M, mannopine; C/O, cucumopine/octopine; A/M, agropine/mannopine.
All Se MICs were determined on MG medium.
Te MICs were determined by using 1A medium for biovar 1, 2E medium for biovar 2, and MG medium for biovar 3, A. rubi, and A. fici.
1, hybridization with the biovar 1 cluster chromosomal probes; 2, hybridization with the biovar 2 chromosomal probe for 16S DNA analysis.
+, hybridization; −, no hybridization; (+), weak hybridization.
ND, not determined.
The information in parentheses indicates the person who provided the strain.
G1 to G3 and G6 to G9 correspond to genomic species defined by Popoff et al. (31).
NA, not applicable.
MICs of potassium selenite and potassium tellurite.
The MICs of tellurite for pure cultures were determined on MG medium, as well as 1A and 2E media (selective for Agrobacterium biovars 1 and 2, respectively). Twofold serial dilutions were prepared with 0.9% (wt/vol) NaCl, and dilutions were plated onto MG, 1A, and 2E media with or without K2TeO3. A stock solution (100 μg ml−1) of K2TeO3 was prepared in ultrapure water and sterilized by filtration. Each strain was tested with different concentrations of metal. The experiment was performed by using three plates per dilution, and the plates were incubated for 7 days.
Soil.
The 10- to 30-cm superficial layer of a standard silt loam soil from a maize field close to Lyon (La-Côte-Saint-André, France) was used as a source of soil. Two soil samples, one collected in May 1998 and the other collected in July 1998, were sieved through a <2-mm mesh. The soil properties were as follows: 17% clay, 35.3% loam, 47.7% sand, and 2% organic matter; pH (water) 7; and water-holding capacity, 25.8 g of H2O 100 g (dry weight)−1 (32).
Counting the Agrobacterium population in soil.
The microorganisms were extracted from 5-g portions of soil by blending samples with 50 ml of sterile distilled water for 90 s in a blender (Waring Commercial, New Hartford, Conn.). The resulting soil suspensions were serially diluted in sterile distilled water, and 100 -μl aliquots of appropriate dilutions were spread on agar plates. Three plates were inoculated per dilution.
Total viable heterotrophic bacteria were counted on Trypticase soy agar (TSA) (Gibco BRL, Rockville, Md.) diluted 1/10. Agrobacteria were counted on 1A and 2E media with or without 80 μg of K2TeO3 per ml. Cycloheximide (200 mg · liter−1) was used as an antifungal agent. All counting was done after incubation for 3 and 5 days at 28°C. Data were expressed as means and standard errors of the means based on three independent replicate determinations. Agrobacterium-like colonies were purified by suspending individual colonies for at least 30 min in sterile distilled water and then streaking them on LPG agar. The process was repeated until all colonies appeared to be homogeneous. Production of 3-ketolactose (2) and production of acid from erythritol were used to separate the strains into biovars 1 and 2 (11).
DNA probes, PCR, and hybridization conditions.
DNA oligonucleotide probes were designed by comparing nine 16S rRNA sequences (from four biovar 1 strains, one strain of Agrobacterium rubi, one strain isolated from Ficus benjamina [A. fici], and three biovar 2 strains). The sequences were compared by using the multiple-alignment ClustalW algorithm (40). Consensus probes F639rrsAT41 (AAACCCCGAATGTCAAGAGC) and F640rrsAT42 (ATACCCCGAATGTCAAGAGC) were designed to detect the cluster containing A. tumefaciens, A. rubi, and Agrobacterium isolated from F. benjamina. F641rrsAR5 (CCATATCTCTACGGGTAACA) was designed to detect A. rhizogenes. DNA probes were defined by using OLIGO software (33), and their specificities for the targets were confirmed by a BLASTn analysis performed with the GenBank database (1). The specificities of the DNA probes were tested with collection strains by using a slot blot technique with 16S rDNA PCR products obtained with primers FGPS6 and FGPS1509′, exactly as described by Ponsonnet and Nesme (30). The oligonucleotide probes were synthesized by Eurogentec (Seraing, Belgium). Synthetic DNA oligonucleotide probes were 3′ end labelled by using a DNA tailing kit (Boehringer Mannheim, Meylan, France) with [α-32P]dATP (NEN Life Science Products, Boston, Mass.) at a specific activity of 6,000 Ci/mmol according to the manufacturer's recommendations. Unincorporated nucleotides were removed with a Qiaquick column, as recommended by the manufacturer (Qiagen S.A., Courtaboeuf, France).
The PCR DNA pathogenicity probes consisted of three regions of the Ti plasmid. These probes were amplified from genomic DNA of strain C58 by using primers FGPtmr530 and FGPtmr701′ (for the tumor morphology root [tmr] probe) and primers FGPnos1236′ and FGPnos975 (for the nopaline synthase [nos] probe, specific for nopaline pTi20). These two DNA probes corresponded to genes on transferred DNA T-DNA. The virulence (inter-vir) DNA probe was obtained with a pair of primers designed to amplify the virB-virG intergene using F749 (GCTAGCTTGGAAGATCGCAC) (this study) and FGPvirG15′ by using the sequence of a conserved region of virB11 in order to amplify the virB-virG intergene of all of the Ti and Ri plasmids sequenced (data not shown). Two genomic DNAs were used to amplify this probe: the genomic DNA of C58 (pTiC58, nopaline type of Ti plasmid) and the genomic DNA of B6 (pTiB6, octopine type of Ti plasmid). The PCR conditions used were those described by Nesme et al. (21) and Picard et al. (27, 28). The PCR DIG probes were obtained by incorporating digoxigenin-11-dUTP (Roche Diagnostic, Basel, Switzerland) during PCR. Labelling was performed by using the reaction conditions recommended by the manufacturer. The specificities of the probes were tested with a collection of strains.
Colony hybridization.
Pure colonies were transferred directly onto nylon membranes (GeneScreen Plus; NEN Research Products, Boston, Mass.). Colonies were lysed as described by Sambrook et al. (34), with some modifications. The filters were first wetted with 0.6% (wt/vol) lysozyme in 10 mM Tris-HCl–1 mM EDTA (dissodium salt dihydrate), (pH 8) for 15 min, and lysis was performed for 10 min in 10% (wt/vol) sodium dodecyl sulfate (SDS). The preparations were denatured for 10 min in denaturation solution (0.5 N NaOH, 1.5 M NaCl) and neutralized for 10 min in neutralizing solution (1 M Tris [pH 7.5], 1.5 M NaCl). Finally, the nylon membranes were soaked for 10 min in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate, pH 7.0). The transferred DNA was cross-linked by irradiation with UV light for 3 min, and the membranes were treated with proteinase K (2 mg ml−1 in 2× SSC) for 1 h at 37°C. For oligonucleotide hybridization, baked membrane filters were placed in 50 ml of prehybridization solution (1% [wt/vol] SDS, 10% [wt/vol] dextran sulfate, 2× SSC, 5 μg of denatured herring sperm DNA per ml, 2 μg of tRNA per ml and incubated for 2 h at the hybridization temperature (47°C). The prehybridization solution was discarded, and the membranes were incubated in the hybridization solution (prehybridization solution plus probe) overnight at the hybridization temperature. The membranes were washed twice in 2× SSC for 10 min at room temperature, once in 2× SSC–1% SDS for 10 min at the hybridization temperature, twice in 1× SSC–0.1% SDS at the hybridization temperature, and once in 0.1× SSC–0.1% SDS at the hybridization temperature. They were then exposed to X-ray film for autoradiography for 4 h to locate individual positive colony signals. Hybridization with PCR DIG probes was performed by using the reaction conditions recommended by the manufacturer (Roche Diagnostic).
RESULTS AND DISCUSSION
Direct counting of agrobacterial populations and studies of the genetic structure of isolated strains require new selective media that ensure recovery of sparse populations without producing any significant differences between agrobacterial genotypes. We investigated the suitability of selenite and tellurite for this purpose by testing the responses of strains chosen to obtain the greatest possible biodiversity of Agrobacterium spp.
Resistance of agrobacteria to Na2SeO3.
As indicated by Lippincott et al. (15), agrobacterial colonies growing on 1A and 2E media develop an orange-brown to red-brown pigmentation. This red coloration is produced by all of the agrobacteria tested and is presumably due to reduction of the added Se compound to its elemental form. Selenite reduction was thus used to improve medium selectivity. However, the original concentration of Na2SeO3 in 1A and 2E media (0.1 g · liter−1) was not high enough to effectively control the competing microflora when the density of agrobacteria in the soil was low (less than 10−4 CFU · g of soil−1). The selenite concentration in the medium could not be increased because MIC studies have shown that the resistance of agrobacterial strains to Na2SeO3 varies considerably (the MICs range from 2 to 14 g · liter−1) (Table 1), while complete control of the competing microflora requires at least 10 g · liter−1 (data not shown). As reduction of the Se compound is generally associated with reduction of other metal salts that are much more toxic, such as K2TeO3 (14), the latter compound was used to improve the selectivity of the 1A and 2E media used to isolate agrobacteria directly from soils.
Resistance of agrobacteria to K2TeO3.
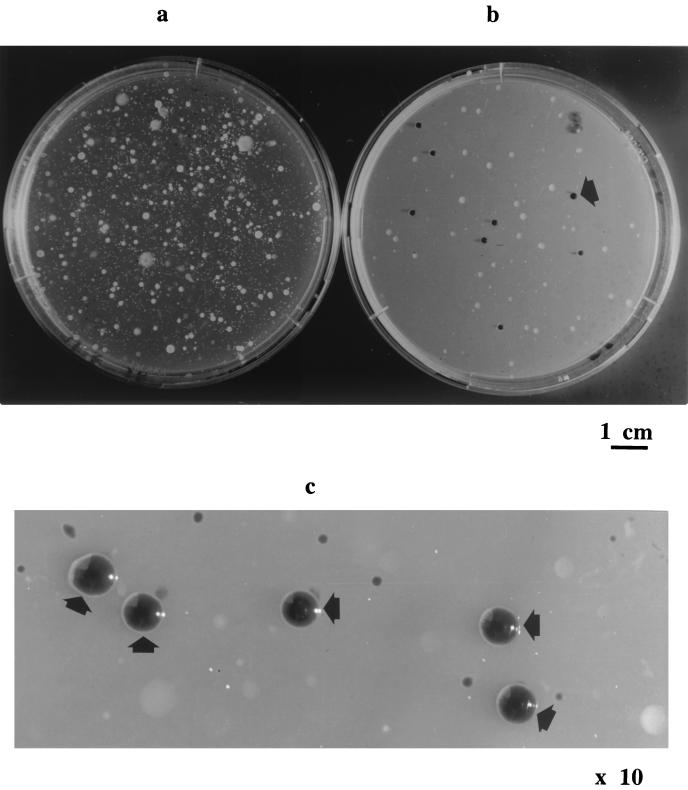
Here, we show for the first time that agrobacteria are resistant to K2TeO3 (Table 1). Agrobacteria growing on amended media had the typical colony morphology (convex, glistening, circular with entire edges) but a typical black color (Fig. 1), probably due to intracellular accumulation of black crystals of metallic tellurium (16, 38). Other members of the alpha subdivision of the Proteobacteria, such as Rhodobacter spp. Rhodopseudomonas palustris, Bradyrhizobium spp., and Rhizobium spp., were also found to be resistant to tellurite (19).
FIG. 1.
Plating of a 10−1 dilution suspension of soil on 1A medium (a) or 1A medium amended with 60 ppm of K2TeO3 (b). (c) Enlarged (magnification, × 10) typical black colonies of A. tumefaciens on the amended medium. Some agrobacterial colonies are indicated by arrows.
Resistance to tellurite was studied by determining the MICs of K2TeO3 for 76 strains selected to represent most of the diversity presently known in the genus Agrobacterium. Special emphasis was placed on representatives of biovar 1 and biovar 2 because these agrobacteria are the organisms most frequently isolated from crown gall tumors in fruit and forest tree nurseries. The MICs of K2TeO3 varied from 640 to 1,280 μg ml−1 for biovar 2 and from 80 to 320 μg ml−1 for biovar1, A. rubi, A vitis, and A. fici (Table 1) in all of the amended media (MG, 1A, and 2E media). Thus, the closely related organisms A. tumefaciens, A. rubi and A. fici could be selectively isolated by using the same K2TeO3 concentration with the same medium (1A medium). Hence, K2TeO3 concentrations of 60 to 80 μg · ml−1 should allow growth of almost all agrobacteria.
We estimated the sampling bias caused by adding tellurite to the medium by determining the percentage of cells recovered in amended medium to see if this value depended upon the agrobacterial genotype. The recoveries of 38 biovar 1 and 2 strains with amended and unamended media were compared. Two concentrations of K2TeO3 were tested with 1A medium containing K2TeO3 (60 and 80 μg · ml−1), and two concentrations were tested with 2E medium containing K2TeO3 (80 and 160 μg · ml−1). The percentage of cell recovery was determined by dividing the average number of CFU obtained with amended medium by the number obtained with unamended medium, based on three independent experiments. The average levels of cell recovery were 95% ± 3% (mean ± standard error of the mea and 85% ± 8% for 1A medium containing 60 and 80 μg of K2TeO3 per ml, respectively, and 75% ± 16% and 61% ± 12% for 2E medium containing 80 and 160 μg of K2TeO3 per ml, respectively. No significant interactions between medium and genotype and no genotype or medium effects were detected (P > 0.05, as determined by analysis of variance). This suggests that almost all strains of agrobacteria can be isolated by using media amended with these concentrations of K2TeO3 without any significant bias for individual genotypes. The relative levels of the various agrobacterial genotypes isolated from a given population should be identical in amended and unamended media. However, the amended media should facilitate sampling of agrobacteria in heavily contaminated environments.
There are several determinants of resistance to tellurite, and there is little or no link among them (39). Nothing is known about resistance to tellurite in agrobacteria. However, the characteristic garlicky odor of the volatile dimethyl telluride resulting from tellurite reduction by a thiopurine methyltransferase found, for instance, in Pseudomonas syringae pathovar pisi (8) has never been reported for agrobacteria, suggesting that the mechanism of resistance is different. However, Summers and Jacoby (37) showed that resistance to tellurite (Ter) is plasmid mediated in several bacterial species, while the tellurite resistance of Rhodobacter sphaeroides is borne on the chromosome (23). Tellurite resistance is probably chromosomal in agrobacteria, since the resistance of C58 and the resistance of its derivatives C58C1 (cured of Ti plasmid pTiC58) and GMI9023 (cured of both pTiC58 and cryptic plasmid pAtC58) were identical (Table 1).
Design of chromosomal DNA probes.
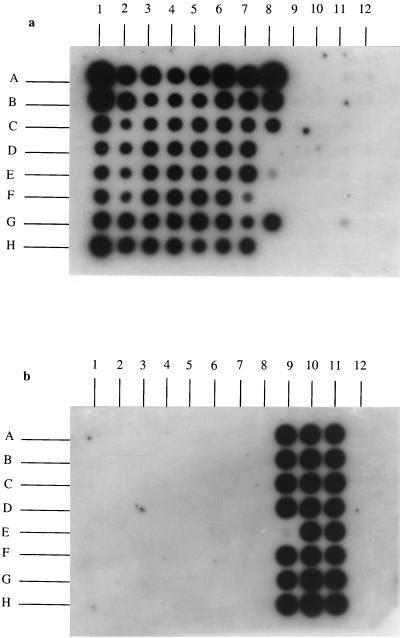
Molecular probes were developed to hybridize colony DNA blots and therefore verify that Agrobacterium-like colonies growing on selective media were bona fide agrobacterial colonies. Specific regions of the rrs (16S rRNA) gene were identified from previously published sequences and used to define three 20-mer oligonucleotides, F639rrsAT41 and F640rrsAT42 specific for biovar 1, A. rubi, and A. fici, and F641rrsAR5 specific for biovar 2. When pooled F639rrsAT41 and F640rrsAT42 were used as radioactive DNA probes, they hybridized with colony DNA blots of all of the strains of biovar 1, A. rubi, and A. fici tested but did not hybridize with the strains of biovar 2 and A. vitis used in this study (Fig. 2 and Table 1). The 20-mer oligonucleotide F641rrsAR5 hybridized with colony DNA blots of only A. rhizogenes.
FIG. 2.
16S rDNA slot blot analyses of PCR products of agrobacterium collection with the biovar 1 cluster DNA probes F639rrsAT41 and F640rrsAT42 (a) and the biovar 2 DNA probe F641rrsAR5 (b). See Table 1 for a description of the strains. Biovar 1 DNAs were obtained from strains CFBP2413T (Spot A1), Ach5 (B1), CFBP1903 (C1), GMI9023 (D1), CFBP1901 (E1), CFBP296 (F1), CFBP354 (G1), CFBP1904 (H1), CFBP2241 (A2), CFBP2243 (B2), CFPB2407 (C2), CFBP2410 (D2), CFBP2411 (E2), CFBP2414T (F2), CFBP2454 (G2), CFBP2456 (H2), CFBP2457 (A3), CFBP2458 (B3), CFBP2177 (C3), CFBP2516 (D3), CFBP2518 (E3), S56 (F3), S377 (G3), S4 (H3), ATCC 4720 (A4), CIP28-75 (B4), CIP43-76 (C4), CIP127-76 (D4), CIP111-78 (E4), NCPPB925 (F4), F/1Zutra (G4), RV3 (H4), 3/1Zutra (A5), NCPPB1641 (B5), T37 (C5), ICPB TT9 (D5), 6 Mushin (E5), O362 (F5), O363 (G5), A96.11 (H5), A134.2 (A6), A134.3 (B6), A134.6 (C6), B100.11 (D6), M9 (E6), M10 (F6), M15 (G6), M214 (H6), M292 (A7), X88.283 (B7), X88.293 (C7), X88.299 (D7), X88.303 (E7), 85.2 (F7), 85.6 (G7), 85.49 (H7), 85.52 (A8), 85.66 (B8), and 85.104 (C8). A. fici DNA was obtained from strain AF3.44; (E8). A. rubi DNA was obtained from strain CFBP999T (G8). Biovar 2 DNAs were obtained from strains CFBP450 (A9), CFBP1804 (B9), CFBP1905 (C9), CFBP1936 (D9), CFBP1937 (E9), CFBP1961 (F9), CFBP1962 (G9), CFBP2178 (H9), CFBP2408T (A10), CFBP2417 (B10), CFBP2418 (C10), CFBP2419 (D10), CFBP2519 (E10), CFBP2520 (F10), C104.12 (G10), C104.22 (H10), M3 (A11), M32 (B11), M84 (C11), M111 (D11), M120 (E11), 85.30 (F11), 85.110 (G11), and 85.120 (H11). Biovar 3 DNAs were obtained from strains CFBP2512 (A12), CFBP2618 (B12), CFBP2620 (C12), CFBP2621 (D12), and CFBP2622 (E12).
Design of Ti plasmid probes.
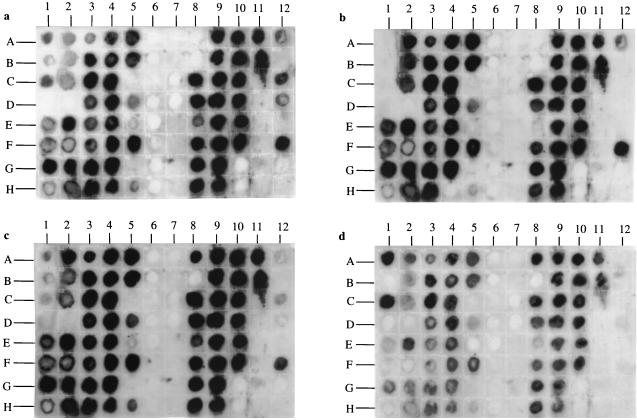
Molecular probes were also designed to detect wild Agrobacterium strains harboring a Ti plasmid. Four nonradioactive DNA probes were prepared from PCR products corresponding to the following conserved regions of Ti plasmids: the tmr region, two inter-vir regions, and the nos regions. The results of a hybridization analysis confirmed the predicted specificities of the probes based on the Ti plasmid contents of control strains. There was no hybridization with nonpathogenic (i.e., Ti plasmid-free) agrobacteria, whatever probe was used (Fig. 3 and Table 1), confirming the results obtained by PCR (data not shown).
FIG. 3.
Pathogenicity status as determined by cell blot analyses of Agrobacterium spp. (see Materials and Methods). The DNA probes used were the tmr probe (a), the nos probe (b), and vir probes obtained with pTiC58 (c) and with pTiB6 (d). The biovar 1 pathogenic strains with the octopine type of Ti plasmid used were strains CFBP2413T (Spot A1), CFBP2407 (B1), and Ach5 (C1). The biovar 1 pathogenic strains with the nopaline type of Ti plasmid used were strains CFBP296 (D1), CFBP354 (E1), CFBP1904 (F1), CFBP2410 (G1), CFBP2177 (H1), CFBP2516 (A2), A134.6 (B2), A134.2 (C2), M15 (D2), B100.11 (E2), M292 (F2), 85.104 (G2), 85.66 (H2), 85.52 (A3), X88.283 (B3), A134.3 (C3), 85.2 (D3), 85.49 (E3), X88.293 (F3), M9 (G3), M10 (H3), 85.6 (A4), X88.299 (B4), M214 (C4), X88.303 (D4), A99.11 (E4), CFBP1903 (D5), CFBP1901 (F5), and CFBP999T (H5). The biovar 1 pathogenic strains with an unknown opine type of Ti plasmid used were strains 2T3Pb (F4), 6MS3 (G4), 436.3SA (H4), 1C3Pb (A5), and 2T3Sa (B5). The biovar 1 nonpathogenic strains used were strains CFBP2414 (A6), GMI9023 (B6 and E5), CFBP2241 (C6), CFBP2243 (D6), CFBP2454 (E6), CFBP2456 (F6), CFBP2457 (G6), CFBP2458 (H6), and CFBP2518 (A7). The biovar 2 pathogenic strains with the nopaline type of Ti plasmid used were strains CFBP1804 (C8), CFBP1905 (D8), CFBP1936 (E8), CFBP1961 (F8), CFBP1962 (G8), CFBP2178 (H8), CFBP2417 (A9), CFBP2418 (B9), 85.186 (C9), C104.12 (D9), C104.22 (E9), 85.110 (F9), 85.30 (G9), 85.123 (H9), M3 (A10), M32 (B10), M120 (C10), M111 (D10), M84 (E10), 85.120 (F10), CFBP2419 (A11), and CFBP2519 (B11). The biovar 2 pathogenic strain with the agropine/mannopine type of Ti plasmid used was strain CFBP2408T (A8). The biovar 2 pathogenic strain with an unknown opine type of Ti plasmid used was strain CFBP450 (B8). The biovar 2 nonpathogenic strains used were strains CFBP1937 (C7 and G10) and CFBP2520 (D7 and H10). The biovar 3 pathogenic strains with the nopaline type of Ti plasmid used were strains CFBP2512 (A12) and CFBP2620 (G12). The biovar 3 pathogenic strains with the cucumopine/octopine type of Ti plasmid used were strains CFBP2618 (C12), CFBP2622 (D12), and CFBP2621 (G12).
The tmr probe is 173 bp long and is found in nopaline and octopine types of Ti plasmids (20). Positive hybridization signals were obtained with this probe only with DNAs of agrobacteria known to harbor a Ti plasmid (69 strains) (Table 1), which confirmed the results obtained by PCR and suggested that the tmr region is a good indicator of the presence of a Ti plasmid (9, 21, 30). However, the tmr probe did not give positive results with 10 strains described as pathogenic. In three instances (CFBP296, M15, CFBP450), the strains had probably lost the Ti plasmids, since they were not amplified in the present study although they had been amplified in previous studies (data not shown). Ti plasmids are generally stable in agrobacteria, but incubation at a high temperature can result in loss of these plasmids (10). A lack of tmr hybridization was expected in two instances because A. vitis CFBP2621 (= Ag57) and strain CFBP2408 are known to have no tmr gene (7, 26). Two strains (S56 and 3/1Zutra) showed no DNA hybridization, while PCR products of the expected size were obtained, suggesting that the tmr sequences of these strains differ significantly enough to hinder probe hybridization. The cause of the lack of hybridization with the three remaining strains (NCPPB1641, NCPPB925, S377) is not well understood, but these strains belong to rare genomic groups of agrobacteria (31) and could harbor unusual Ti or Ri plasmids with no or divergent tmr sequences.
The nos probe corresponds to a region of the T-DNA encoding nopaline synthase. This probe hybridized with the DNAs of strains harboring a Ti plasmid known to produce tumors containing nopaline. There was no hybridization with strains that did not form nopaline in tumors. Strain CFBP2618, which uses nopaline but does not synthesize nopaline in tumors, did not hybridize with the nopaline synthetase probe. This probe is thus adequate for identifying the nopaline type of Ti plasmid.
Two inter-vir probes, one amplified from pTiC58 (nopaline type of Ti plasmid) and the other amplified from pTiB6 (octopine type of Ti plasmid), were generated. These two probes hybridized with DNAs of all typical nopaline and octopine types of Ti plasmids. The intensities of the hybridization signals varied according to the similarity to the Ti plasmid and also to the mannopine-agropine type of Ri plasmid of CFBP2408 (Fig. 3 and Table 1). The octopine cucumopine type of Ti plasmids hybridized only with the PCR-amplified probe from pTiC58. The other hybridization patterns were obtained with strains having an undetermined opine type.
Densities of Agrobacterium spp. populations in bulk soil and necrosed tumors.
Amended media were tested for the ability to isolate bona fide agrobacteria from plant tumors. Pathogenic isolates were recovered from old or necrosed plant material (data not shown), for which unamended media are inappropriate because of the high density of competing bacteria (mainly fluorescent Pseudomonas sp.). The tellurite-amended media were developed under a program funded by the European Community (Integrated Control of Crown Gall in Mediterranean Countries). Our findings represented such a marked improvement that the other partners in this program rapidly adopted the method for isolation of agrobacteria from various materials.
The other reservoirs of agrobacteria are the soil and rhizospheres. It is laborious to determine agrobacterial densities with unamended media, because delineation of bona fide agrobacteria from Agrobacterium-like colonies always requires additional tests to determine identities. These tests include biochemical or molecular assays but are generally limited to pathogenicity trials. Consequently, very few data on the ecology of agrobacteria in soils and rhizospheres are available.
The assay described below was designed to check whether amended media could be used for direct plate counting of soil agrobacteria by visual inspection alone. Samples of the La-Côte-Saint-André soil taken at 2-month intervals were used to isolate heterotrophic culturable bacteria (TSA), biovar 1 and related agrobacteria (medium 1A), and biovar 2 agrobacteria (medium 2E), with or without K2TeO3. The efficacies of the amended media were tested by directly plating soil suspensions and by verifying the bona fide Agrobacterium status of the isolates by colony DNA blot hybridization with chromosomal oligonucleotide probes. Significantly fewer bacterial colonies were recovered with all tellurite-amended media. However, this was not true when Agrobacterium-like colonies alone were considered, at least with 1A medium since no Agrobacterium-like colonies were detected with 2E medium (Table 2). Plating soil suspensions on K2TeO3-amended media also resulted in the typical black color of Agrobacterium-like colonies together with strong inhibition of the competing microflora, which facilited both visualization and isolation of Agrobacterium candidates (Fig. 1).
TABLE 2.
Soil bacterial densities determined with media supplemented or not supplemented with tellurite
| Bacteria | Medium | Density (log CFU · g [dry wt] of soil−1) ina:
|
|
|---|---|---|---|
| June | August | ||
| Total cultivable bacteria | TSA | 6.89 ± 0.07 | 6.99 ± 0.04 |
| TSA + K2TeO3 | 6.21 ± 0.04 | 6.43 ± 0.06 | |
| 1A | 5.89 ± 0.23 | 5.76 ± 0.11 | |
| 1A + K2TeO3 | 5.00 ± 0.15 | 4.79 ± 0.03 | |
| 2E | 4.63 ± 0.07 | 5.47 ± 0.05 | |
| 2E + K2TeO3 | 3.00 ± 0.11 | 4.42 ± 0.04 | |
| Agrobacterium like colonies | 1A | 2.90 ± 0.31 | 4.23 ± 0.11 |
| 1A + K2TeO3 | 2.85 ± 0.42 | 3.80 ± 0.16 | |
| 2E | <2.3b | <2.3 | |
| 2E + K2TeO3 | <2.3 | <2.3 | |
| Bona fide Agrobacterium | 1A | 2.81 ± 0.31 (0.08)c | 4.10 ± 0.11 (2.2) |
| 1A + K2TeO3 | 2.85 ± 0.42 (0.7) | 3.80 ± 0.16 (10) | |
Values are means ± standard errors of the means based on three independent counts.
No Agrobacterium-like colonies were recovered at the threshold indicated.
Bona fide Agrobacterium values were determined after colony blot hybridization of Agrobacterium-like colonies with chromosomal probes specific for Agrobacterium biovar 1 (see text). The values in parentheses are the percentages of bona fide Agrobacterium colonies among all of the colonies growing on the same medium.
Agrobacterium-like colonies were recovered from 1A medium (40 isolates) and from 1A medium containing K2TeO3 (180 isolates). Isolates obtained from 1A medium were characterized by hybridization with 16S ribosomal DNA (rDNA) probes, and nine of them were shown to be fluorescent pseudomonads. All 180 isolates obtained from 1A medium containing K2TeO3 were identified as bona fide Agrobacterium isolates. The densities of agrobacteria in bulk soil could thus be determined directly by visual inspection of plates containing Te-amended medium. The percentages of bona fide Agrobacterium isolates in Agrobacterium-like colonies (78% with unamended medium and 100% with amended 1A medium) were used to estimate the densities of bona fide agrobacteria in bulk La-Côte-Saint-André soil (Table 2). Analysis of variance showed that there was no significant effect of added tellurite on determination of agrobacterial density in soil (Table 3), confirming the usefulness of this amended medium for direct counting of soil populations of Agrobacterium.
TABLE 3.
Variance analysis of the densities of bona fide Agrobacterium soil isolates in 1A medium amended or not amended with tellurite on two arbitrary soil sampling dates
| Source of variation | df | Square sum | Mean square | F value | P value |
|---|---|---|---|---|---|
| Medium | 1 | 0.050 | 0.050 | 0.543 | 1 |
| Sampling date | 1 | 3.776 | 3.776 | 16.44 | 0.0037 |
| Medium × date | 1 | 0.092 | 0.092 | 0.402 | 1 |
| Error | 8 | 1.838 | 0.230 |
Ecology of Agrobacterium spp. in the La-Côte-Saint-André soil.
In this work, we discovered three significant ecological features about agrobacteria from La-Côte-Saint-André soil. One was the lack of culturable agrobacteria when was used 2E medium that was amended or not amended with K2TeO3 (Table 2). This indicated that the density of culturable biovar 2 organisms in the bulk La-Côte-Saint-André soil was less than 200 CFU · g−1. However, members of this taxon were present, since 2E medium containing K2TeO3 allowed direct counting of many bona fide biovar 2 isolates from the rhizospheres of plants grown in the same soil (unpublished results). The question of the predominance of Agrobacterium biovars has been studied in several instances (17), but the data never applied to nonpathogenic agrobacteria in bulk soil. The low density of biovar 2 in the La-Côte-Saint-André bulk soil is an interesting ecological trait of this taxon and is probably related to a property of the local La-Côte-Saint-André environment. The density of biovar 1 agrobacteria reported in the present study, 103 to 104 CFU · g (dry weight) of soil−1, is similar to the values reported for other soils (22, 36). This value is lower than the 107 agrobacterium-like colonies per g of soil reported by Bouzar et al. (4) under favorable conditions. Thus, even if agrobacteria are present in the sandy loam soil which we studied, environmental factors, especially the low organic matter content, were probably not optimal for growth of agrobacteria in the La-Côte-Saint-André bulk soil.
The second important ecological feature was the effect of sampling on the soil density of biovar 1 (Table 3). This could have been due to a temporal effect. Temporal variations in soil agrobacteria can occur, and the amended media used in the present work should facilitate study of seasonal variations in agrobacterial populations.
The third ecological feature was the lack of a Ti plasmid in any of the agrobacteria isolated from the bulk soil, as determined by DNA hybridization with the Ti plasmid probes described above and confirmed by the lack of tumor formation in Kalanchoe daigremontiana plants (data not shown). Similar results were obtained with rhizosphere agrobacteria from the same soil (unpublished results). The frequency of Agrobacterium harboring a Ti plasmid in the La-Côte-Saint-André soil was less than 1/1,300. This agrees with previous reports showing that the population of tumor-inducing agrobacteria in natural or cultivated soil is low to undetectable except in the vicinity of infected plants (22, 36). The ratio was highest (1/13) in soils in which host plants had been growing and lowest (1/500) in soils that had never been cultivated or supported host plants other than dicotyledonous weeds (17). This was the case for La-Côte-Saint-André soil, which had been cultivated with maize for many years.
In conclusion, the selectivity and sensitivity of the 1A and 2E media were increased by taking advantage of the intrinsic resistance of agrobacteria to tellurite. Agrobacteria could be reliably counted directly on plates by using media amended with tellurite. Isolation of agrobacterium strains from soil and characterization of isolates by using biovar-specific oligonucleotide probes designed by using the 16S rDNA allowed direct study of natural populations in a bulk soil. The amended media and the PCR DNA probes for pathogenicity were also used to determine the density of pathogenic agrobacteria in contaminated soil. This procedure should be useful for sanitary inspection of soils before planting.
ACKNOWLEDGMENTS
We thank M. A. Poirier for technical assistance.
This research was supported by project ERB1C18CT970198 (Intergrated Control of Crown Gall in Mediterranean Countries) funded by the European Union INCO-DC Programme to X.N.
REFERENCES
- 1.Altshul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bernaerts M J, De Ley J. A biochemical test for crown gall bacteria. Nature (London) 1963;197:406–407. [Google Scholar]
- 3.Bouzar H. Request for a judicial opinion concerning the type species of Agrobacterium. Int J Syst Bateriol. 1994;44:373–374. [Google Scholar]
- 4.Bouzar H, Ouadah D, Krimi Z, Jones J B, Trovato M, Petit A, Dessaux Y. Correlative association between resident plasmids and the host chromosome in a diverse Agrobacterium soil population. Appl Environ Microbiol. 1993;59:1310–1317. doi: 10.1128/aem.59.5.1310-1317.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bouzar H, Chilton W S, Nesme X, Dessaux Y, Vaudequin V, Petit A, Jones J B, Hodge N C. A new Agrobacterium strain isolated from aerial tumors on Ficus benjamina L. Appl Environ Microbiol. 1995;61:65–73. doi: 10.1128/aem.61.1.65-73.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brisbane P G, Kerr A. Selective media for the three biovars of Agrobacterium. J Appl Bacteriol. 1983;54:425–431. [Google Scholar]
- 7.Camilleri C, Jouanin L. The TR-DNA region carrying the auxin synthesis genes of the Agrobacterium rhizogenes agropine-type plasmid pRiA4: nucleotide sequence analysis and introduction into tobacco plants. Mol Plant-Microbe Interact. 1991;4:155–162. doi: 10.1094/mpmi-4-155. [DOI] [PubMed] [Google Scholar]
- 8.Cournoyer B, Watanabe S, Vivian A. A tellurite-resistance genetic determinant from phytopathogenic pseudomonads encodes a thiopurine methyltransferase: evidence of a widely-conserved family of methyltransferases. Biochim Biophys Acta. 1998;1397:161–168. doi: 10.1016/s0167-4781(98)00020-7. [DOI] [PubMed] [Google Scholar]
- 9.Cubero J, Martinez M C, Llop P, Lopez M M. A simple and efficient PCR method for the detection of Agrobacterium tumefaciens in plant tumor. J Appl Microbiol. 1999;86:591–602. doi: 10.1046/j.1365-2672.1999.00700.x. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton R H, Fall M Z. The loss of tumor-initiating ability in Agrobacterium tumefaciens by incubation at high temperature. Experientia. 1971;27:229–230. doi: 10.1007/BF02145913. [DOI] [PubMed] [Google Scholar]
- 11.Hayward A C. Characteristics of Pseudomonas solanacearum. J Appl Bacteriol. 1964;27:265–277. [Google Scholar]
- 12.Keane P J, Kerr A, New P B. Crown gall of stone fruit. II. Identification and nomenclature of Agrobacterium isolates. Aust J Biol Sci. 1970;23:585–595. [Google Scholar]
- 13.Kersters K, De Ley J, Sneath P H A, Sackin M. Numerical taxonomic analysis of Agrobacterium. J Gen Microbiol. 1973;78:227–239. [Google Scholar]
- 14.Kinkle B K, Sadowsky M J, Johnstone K, Koskinen W C. Tellurium and selenium resistance in rhizobia and its potential use for direct isolation of Rhizobium meliloti from soil. Appl Environ Microbiol. 1994;60:1674–1677. doi: 10.1128/aem.60.5.1674-1677.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lippincott J A, Lippincott B B, Starr M P. The genus Agrobacterium. In: Starr M P, editor. The procaryotes: a handbook of habitats, isolation and identification of bacteria. New York, N.Y: Springer-Verlag; 1983. pp. 842–845. [Google Scholar]
- 16.Llyod-Jones G, Osborn A M, Rithie D A, Strike P, Hobman J L, Brown N L, Duncan A R. Accumulation and intracellular fate of tellurite in tellurite-resistant Escherichia coli: a model for the mechanism of resistance. FEMS Microbiol Lett. 1994;118:113–120. doi: 10.1111/j.1574-6968.1994.tb06812.x. [DOI] [PubMed] [Google Scholar]
- 17.Moore L W, Cooksey D A. Biology of Agrobacterium tumefaciens: plant interactions. In: Giles K L, Atherly A G, editors. Biology of the Rhizobiaceae. Orlando, Fla: Academic Press, Inc.; 1981. pp. 15–42. [Google Scholar]
- 18.Moore L W, Kado C I, Bouzar H. Gram-negative bacteria. A. Agrobacterium. In: Schaad N W, editor. Laboratory guide for identification of plant pathogenic bacteria. 2nd ed. St. Paul, Minn: The American Phytopathological Society; 1988. pp. 16–36. [Google Scholar]
- 19.Moore M K, Kaplan S. Identification of intrinsic high-level resistance to rare-earth oxides and oxyanions in members of the class Proteobacteria: characterization of tellurite, selenite, and rhodium sesquioxide reduction in Rhodobacter sphaeroides. J Bacteriol. 1992;174:1505–1514. doi: 10.1128/jb.174.5.1505-1514.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nesme X, Leclerc M C, Bardin R. PCR detection of an original endosymbiont: the Ti plasmid of Agrobacterium tumefaciens. In: Nardon P, Gianinazzi-Peason V, Greines A M, Margulis L, Smith D C, editors. Endocytobiology IV. Paris, France: Institut National de la Recherche Agronomique; 1989. pp. 47–50. [Google Scholar]
- 21.Nesme X, Picard C, Simonet P. Specific DNA sequences for detection of soil bacteria. In: Trevor J T, Van Elsas J D, editors. Nucleic acids in the environment. Berlin, Germany: Springer-Verlag; 1995. pp. 111–139. [Google Scholar]
- 22.New P B, Kerr A. Biological control of crown gall: field measurements and glasshouse experiments. J Appl Bacteriol. 1972;35:279–287. [Google Scholar]
- 23.O'Gara J P, Gomelsky M, Kaplan S. Identification and molecular analysis of multiple loci contributing to high-level tellurite resistance in Rhodobacter sphaeroides 2.4.1. Appl Environ Microbiol. 1997;63:4713–4720. doi: 10.1128/aem.63.12.4713-4720.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oger P, Dessaux Y, Petit A, Gardan L, Manceau C, Chomel C, Nesme X. Validity, sensitivity and resolution limit of the PCR-RFLP analysis of the rrs (16S rRNA gene) as a tool to identify soil-borne and plant-associated bacterial populations. Genet Sel Evol. 1998;30:S311–S321. [Google Scholar]
- 25.Ophel K, Kerr A. Agrobacterium vitis—new species for strains of Agrobacterium biovar 3 from grapevine. Int J Syst Bacteriol. 1990;40:236–241. [Google Scholar]
- 26.Otten L, Canaday J, Gérard J C, Fournier P, Crouzet P, Paulus F. Evolution of agrobacteria and their Ti plasmids—a review. Mol Plant-Microbe Interact. 1992;5:279–287. doi: 10.1094/mpmi-5-279. [DOI] [PubMed] [Google Scholar]
- 27.Picard C, Ponsonnet C, Recorbet G, Antonelli F, Simonet P, Nesme X. Detection of pathogenic Agrobacterium in soils by PCR. In: Lematrre M, Freigoun S, Rudolph K, Swings J G, editors. Plant pathogenic bacteria. Paris, France: Les Colloques, Editions INRA; 1994. pp. 729–734. [Google Scholar]
- 28.Picard C, Nesme X, Simonet P. Detection and enumeration of soil bacteria by using the MPN-PCR technique. 1995. p. 2.7.3. :1–9. In A. D. L. Akkermans, J. D. van Elsas, and F. J. de Bruijn (ed.), Molecular microbial ecology. Kluwer Academic Publishers, Dordrecht, Holland. [Google Scholar]
- 29.Pionnat S, Keller H, Héricher D, Bettachini A, Dessaux Y, Nesme X, Poncet C. Ti plasmids from Agrobacterium characterize rootstock clones that initiated a spread of crown gall disease in Mediterranean countries. Appl Environ Microbiol. 1999;65:4197–4206. doi: 10.1128/aem.65.9.4197-4206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponsonnet C, Nesme X. Identification of Agrobacterium strains by PCR-RFLP analysis of pTi and chromosomal regions. Arch Microbiol. 1994;161:300–309. doi: 10.1007/BF00303584. [DOI] [PubMed] [Google Scholar]
- 31.Popoff M Y, Kerster K, Kiredjian M, Miras I, Coynault C. Position taxonomique de souches de Agrobacterium d'origine hospitalière. Ann Microbiol (Paris) 1984;135:427–442. [PubMed] [Google Scholar]
- 32.Ranjard L, Richaume A, Jocteur-Monrozier L, Nazaret S. Response of soil bacteria to Hg(II) in relation to soil characteristics and cell location. FEMS Microbiol Ecol. 1997;24:312–331. [Google Scholar]
- 33.Rychlik W, Rhoads R E. A computer program for choosing optimal oligonucleotides for filter hybridization, sequencing and in vitro amplification of DNA. Nucleic Acids Res. 1989;17:8543–8551. doi: 10.1093/nar/17.21.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsh E F, Maniatis T E. Molecular cloning: a laboratory manual 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 35.Sawada H, Ieki H, Oyaizu H, Matsumoto S. Proposol for rejection of Agrobacterium tumefaciens and revised descriptions for the genus Agrobacterium and for Agrobacterium radiobacter and Agrobacterium rhizogenes. Int J Syst Bateriol. 1993;43:694–702. doi: 10.1099/00207713-43-4-694. [DOI] [PubMed] [Google Scholar]
- 36.Schroth M N, Weinhold A R, McCain A H, Hildebrand D C, Ross N. Biology and control of Agrobacterium tumefaciens. Hilgardia. 1971;40:537–552. [Google Scholar]
- 37.Summers A O, Jacoby G A. Plasmid-determined resistance to tellurium compounds. J Bacteriol. 1977;129:276–281. doi: 10.1128/jb.129.1.276-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor D E, Walter E G, Sherburne R, Bazett-Jones D P. Structure and location of tellurium deposited in Escherichia coli cells harbouring tellurite resistance plasmids. J Ultrastruct Mol Struct Res. 1988;99:18–26. doi: 10.1016/0889-1605(88)90029-8. [DOI] [PubMed] [Google Scholar]
- 39.Taylor D E. Bacterial tellurite resistance. Trends Microbiol. 1999;7:111–115. doi: 10.1016/s0966-842x(99)01454-7. [DOI] [PubMed] [Google Scholar]
- 40.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choise. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yanagi M, Yamasato K. Phylogenetic analysis of the family Rhizobiaceae and related bacteria by sequencing of 16S rRNA gene using PCR and DNA sequencer. FEMS Microbiol Lett. 1993;107:115–120. doi: 10.1111/j.1574-6968.1993.tb06014.x. [DOI] [PubMed] [Google Scholar]