Abstract
Background and Purpose Oral anticoagulants (OACs) prevent stroke recurrence and vascular embolism in patients with acute ischemic stroke (AIS) and atrial fibrillation (AF). Based on empirical consensus, current guidance recommends a “1–3–6–12 days” rule to resume OACs after AIS. This study investigated the suitability of guideline-recommended timing for OAC initiation.
Methods Using data of 12,307 AF patients hospitalized for AIS, for the period 2012 to 2016, in Taiwan's National Health Insurance Research Database, we constructed a sequence of cohorts of OAC users and propensity score-matched nonusers, creating one cohort on each day of OAC initiation for 30 days since admission. Composite outcome included effectiveness (cardiovascular death, ischemic stroke, myocardial infarction, transient ischemic attack, systemic embolism, and venous thromboembolism) and safety (intracranial hemorrhage, gastrointestinal bleeding, and hematuria) outcomes. Comparing with nonusers, we examined the risks in the early OAC use (within 1–3–6–12 days) or guideline-recommended delayed use. Indirect comparison between the early and delayed use was conducted using mixed treatment comparison.
Results Across the AIS severity, the risks of composite or effectiveness outcome were lower in OAC users than nonusers, and the risks were similar between the early and delayed use groups. In patients with severe AIS, early OAC use was associated with an increased risk of safety outcome, with a hazard ratio (HR) of 1.67 (confidence interval [CI]: 1·30–2·13) compared with nonusers and a HR of 1.44 (CI: 0·99–2·09) compared with the delayed use.
Conclusion Our study findings support an early OAC initiation in AF patients with mild-to-moderate AIS and a routine delayed use of OACs can be considered in those with severe AIS to avoid a serious bleeding event.
Keywords: atrial fibrillation, acute ischemic stroke, oral anticoagulants, timing, stroke recurrence
Introduction
Stroke is a leading cause of mortality and disability, resulting in substantial economic costs in terms of poststroke care. 1 Cardioembolic strokes, most frequently caused by atrial fibrillation (AF), 2 are found to be related to worse outcomes compared with other non-AF-related strokes.
Lifelong use of oral anticoagulants (OACs) has been recommended for secondary stroke prevention. 3 4 However, the optimal timing to resume OAC in AF patients with acute ischemic stroke (AIS) remains a clinical challenge. Early non-vitamin K antagonist OACs (NOACs) within 2 days of AIS had been shown to be associated with a 5% rate of hemorrhagic transformation, 5 whereas a delayed initiation may leave the patients at an increased risk of recurrent ischemic stroke. The 2018 European Heart Rhythm Association practical guide proposed a “1–3–6–12 days rule” to resume OAC after an AIS in patients with AF, 4 6 based on expert consensus opinion without supporting evidence from large-scale randomized controlled trials (RCTs). Recently, one meta-analysis of individual-level data from seven prospective observational studies, including CROMIS-2, 7 RAF, 8 RAF-NOACs, 9 SAMURAI, 10 NOACISP, 11 Erlangen, 12 and Verona 13 registry, suggested that that early NOAC treatment after AIS, when compared with vitamin K antagonist (VKA), was associated with a reduced risk of intracranial hemorrhage (ICH). 14 Interestingly, Mizoguchi and colleagues compared the early (≤3 days) with the delayed (≥4 days) initiation of NOACs after AIS or transient ischemic attack (TIA) in the SAMURAI study and did not observe a difference in risks of stroke, major bleeding, and death between the groups. 15 The study by Mizoguchi and coauthors, however, did not address the potential immortal-time bias because patients who initiated NOACs in the delayed period, by definition, had to be alive and free of ischemic stroke in the early period (and thus to be “immortal” to outcomes of interest.)
Four ongoing RCTs, including ELAN (NCT03148457, Switzerland), OPTIMAS (EudraCT, 2018003859–38, United Kingdom), TIMING (NCT02961348, Sweden), and START (NCT03021928, United States), are to determine the optimal time for initiating OACs after AIS. However, these RCTs only compare the early and delayed initiation of NOACs with fixed intervals, without stratified randomization based on prespecified AIS severity, except ELAN stratifies patients based on the size of the infarction. Moreover, these RCTs fail to investigate the comparative effectiveness and safety of VKA in patients with AIS secondary to AF.
The present study aimed to examine the benefit and risk of early and delayed use of OACs, including NOACs and VKAs, in AF patients hospitalized for AIS. Immortal-time bias is a challenging issue in comparing different strategies of treatment initiation in observational studies, and we constructed a sequence of stroke severity-specific cohorts with propensity score (PS) matching to reduce immortal-time bias and confounding bias. The study results could provide real-world evidence of the optimal timing to initiate OACs after an AIS event among patients with AF.
Methods
Taiwan National Health Insurance Research Databases
Taiwan initiated its single-payer, universal National Health Insurance program in March 1995. Enrolment is mandatory. As of 2020, membership consisted of approximately 23,622,000 individuals (99·9% of Taiwan's population). The National Health Insurance Research Database (NHIRD) captures all medical claims, including disease diagnoses, procedures, and prescription fills in the records of inpatient, outpatient, and emergency visits since 2000 for research purposes. The consistency, reliability, and disease diagnostic accuracy of the NHIRD for research in cardiovascular, bleeding, and mortality outcomes among patients with AF and/or AIS have been validated. 16 17 18 19 20 21 22 23 The Institutional Review Board of the National Yang-Ming University, Taiwan, approved this research study (YM104104E).
Study Population
The base cohort included 268,715 patients who presented a new AIS (“index stroke event”) from January 1, 2012 to December 31, 2016, who did not have a diagnosis of hemorrhagic stroke or TIA on the admission day, and who did not have any inpatient diagnosis of ischemic stroke within 5 years before the index stroke event ( Fig. 1 ). Using algorithms validated in the NHIRD, AIS was validated by the noncontrast computed tomography (CT) or magnetic resonance imaging (MRI), 21 and AF was defined as having at least one inpatient or outpatient record of the International Classification of Diseases (ICD)-9 or ICD-10 diagnosis code for AF as the primary diagnosis, or having at least two records of AF diagnosis as the secondary diagnosis within 5 years before the index stroke event 23 24 ( Supplementary Table S1 , available in the online version). The final study population consisted of 12,307 AF patients with a new AIS, after excluding patients who lacked AF diagnosis in 2007 to 2011 ( n = 252,607), were of unknown sex ( n = 13), died on admission ( n = 16), or had an ICH diagnosis on admission ( n = 3,772).
Fig. 1.
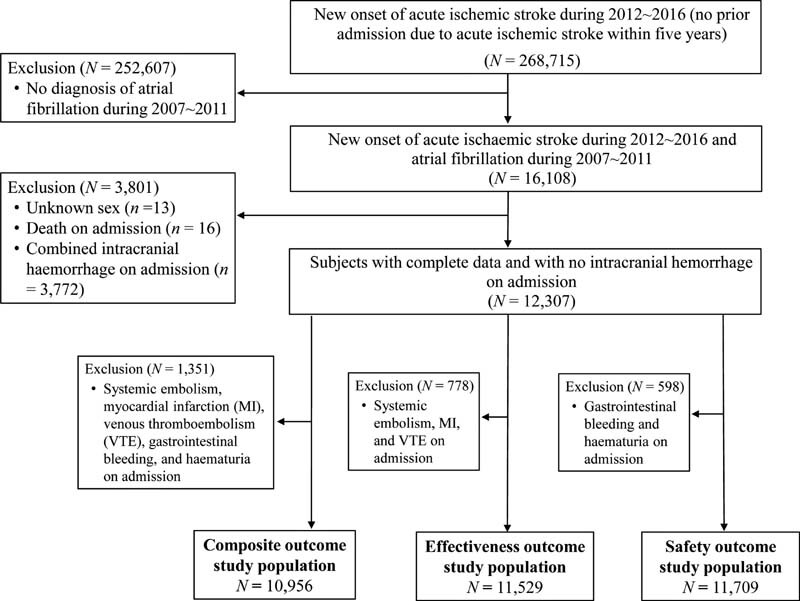
Flowchart of patient selection. AIS, acute ischemic stroke.
Severity of Acute Ischemic Stroke
We calculated a validated stroke severity index (SSI) to categorize the index stroke event into mild (SSI ≤ 5), moderate (5 < SSI ≤ 12), and severe (SSI >12) stroke. 25 26 27 Stroke severity assessed by the National Institutes of Health Stroke Scale (NIHSS) cannot be captured in the administrative claims data, and SSI was closely correlated with NIHSS 27 and performed well in 30-day and 1-year mortality prediction in validation studies. 26 SSI calculation was based on the number of the following tests on admission: airway suctioning, bacterial sensitivity test, general ward stay, intensive care unit stay, nasogastric intubation, osmotherapy (mannitol or glycerol), and urinary catheterization. 27
Guideline-Recommended OAC Use in AF Patients Hospitalized for Stroke
Guidelines recommend initiation of OACs, including NOACs or warfarin, on the 4th day of admission for a minor AIS, 7th day for a moderate AIS, and 13th day for a severe AIS. 4 6 Depending on the stroke severity, “early use” referred to initiation of OACs within 3 (minor AIS), 6 (moderate AIS), or 12 days (severe AIS) of admission; “delayed use” referred to initiation of OACs between the guideline-recommended initiation day and the 30th day of admission. On a specific day, the exposed group included patients who initiated OACs and the unexposed group included patients who did not initiate OACs. Information of NOAC or warfarin initiation was based on prescriptions in inpatient and outpatient settings. Within 30 days of admission, patients who had prescriptions of NOAC only or VKA only were categorized into the “NOAC group” or the “VKA group,” respectively, and patients who had prescriptions of both NOAC and VKA were categorized into the “both group.”
Composite Outcome of Effectiveness and Safety
The primary outcome was the first occurrence of a composite outcome of an effectiveness or a safety event. Effectiveness outcomes included ischemic stroke, myocardial infarction (MI), TIA, systemic embolism, venous thromboembolism (VTE), and cardiovascular death. Safety outcomes included ICH, gastrointestinal (GI) bleeding, and hematuria. These outcome events were identified using ICD-9 or ICD-10 diagnosis codes in inpatient records based on validated algorithms in the NHIRD 16 18 19 20 21 22 23 28 ( Supplementary Table S1 , available in the online version).
Immortal-Time Bias and the Sequence of Cohorts with PS-Matching on Each Day of OAC Initiation
Immortal time refers to a period of cohort follow-up when study subjects cannot have outcome(s) because of exposure definition. For example, patients who initiated an OAC on the sixth day of admission had to be alive and cannot develop any outcome from the first to the sixth day. Additionally, patients may initiate OACs on a specific day based on physicians' decisions, patients' clinical status, and possibly guideline suggestions. 6 Consequently, the early and delayed use groups likely have different baseline risks of outcomes, and a direct comparison between the two groups could introduce immortal-time bias and confounding bias. 29 30 To reduce these biases, we constructed a sequence of PS-matched cohorts of OAC users and nonusers, creating one cohort on each day of OAC initiation for 30 days since admission ( Fig. 2 ). 30 The day of OAC initiation was defined as the index date for each cohort. Across the three categories of stroke severity and the 30 possible days of OAC initiation, we constructed 90 PS-matched cohorts nested within the study population ( n = 12,307) for each of the composite outcome, effectiveness outcome, and safety outcome. The analytic sample of AF patients eligible for PS-matching included 10,956 patients for the composite outcomes, 11,529 patients for the effectiveness outcomes, and 11,709 patients for the safety outcomes ( Fig. 1 ).
Fig. 2.
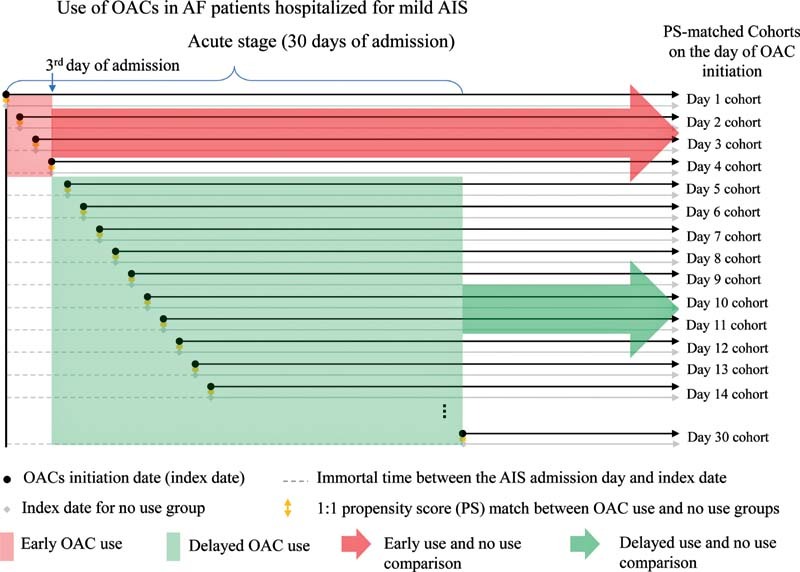
A sequence of propensity-score matched cohorts on each day of OAC initiation from the first to the 30th day of admission, using AF patients with mild AIS as an example. AF, atrial fibrillation; AIS, acute ischemic stroke; OAC, oral anticoagulant.
Follow-up for each PS-matched cohort started from the cohort index date until the first occurrence of a composite outcome event, noncardiovascular death, loss to follow-up, initiation of OACs in an unexposed group, or December 31, 2017.
Statistical Analyses
On each of the cohort index dates, we calculated PS using a logistic regression model that included age, sex, use of medication (antihypertensive drugs, antidiabetic drugs, lipid-lowering drugs, OACs, antiplatelets, nonsteroid anti-inflammatory drugs), and medical history of liver disease, peptic ulcer, hypertension, dyslipidemia, ischemic heart disease, ICH, TIA, alcohol intoxication, GI bleeding, hematuria, VTE, systemic embolism, congestive heart failure (CHF), MI, peripheral vascular disease (PVD), cerebrovascular accident, diabetes mellitus, and chronic kidney disease (CKD). We 1:1 matched OAC users to nonusers using the greedy nearest-neighbor technique within a specified caliper width of 0·25 of the standard deviation of the logit of the PS. 31 A nonuser was allowed to be matched to multiple OAC users who initiated OACs on different days. Nonusers could later initiate OACs and become OAC users.
For each level of AIS severity, we pooled all PS-matched cohorts into one analytic sample. 32 Using nonusers as the reference, we performed Cox proportional-hazards models to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for outcomes in the early use and separately in the delayed use. We stratified Cox models on the index date and adjusted for the PS-matching variables. We used a robust variance estimate to account for within-person correlation. 32
We used the cumulative incidence function 33 to calculate cumulative incidence to account for possible competing risks. 34 Categorical variables were expressed as the number (percentage) and assessed using the Chi-square (χ 2 ) or Fisher's exact test.
Mixed Treatment Comparison
We indirectly compared the risk of outcomes in the early with the delayed use groups using a random-effects model. 35 The indirect comparisons were based on the assumptions of cohort independence and consistency between direct and indirect comparisons. We applied the results comparing the exposed with the unexposed group as direct evidence, and extrapolated the indirect comparison from the direct evidence.
Net Clinical Benefit Analysis
We performed a net clinical benefit (NCB) analysis, proposed by Singer et al 36 , to examine the risk and benefit profile of early or delayed OAC use, compared with the no use group. The NCB was calculated as: (rate of effectiveness outcome in the no use group – rate of effectiveness outcome in the early [or delayed] use group) − weighting factor × (rate of safety outcome in the early [or delayed] use group – rate of safety outcome in the no use group). The 95% CIs were calculated from rate differences and standard errors estimates using Poisson regression. The weighting factor reflects the relative impact of a safety outcome while receiving an early or a delayed OAC, as opposed to experiencing an effectiveness outcome while not using OACs. We selected three weighting factors (1.5, 2.0, and 3.0) based on publications of the risk and benefit of warfarin use in AF patients. 36 37
Results
Of the 10,956 patients with AF in the composite outcome analysis ( Supplementary Table S2 , available in the online version), 41·2% ( n = 4,513) had a mild stroke, 23·6% ( n = 2,582) had a moderate stroke, and 35·2% ( n = 3,861) had a severe stroke. Among patients with AF, the proportion of guideline-recommended use of OACs decreased with an increasing stroke severity (early use: 29·0, 26·8, and 21·8%; delayed use: 25·9, 22·2, and 14·5% in mild, moderate, and severe stroke, respectively). Conversely, the proportion of patients with no OAC use increased (45·1, 51·0, and 63·7% in mild, moderate, and severe stroke, respectively).
Across the stroke severity, AF patients who did not initiate OACs after an AIS tended to be older and have more comorbidities than those who did ( Table 1 , Supplementary Tables S3–S13 , available in the online version). For example, peptic ulcers, hypertension, ICH, GI bleeding, CHF, MI, PVD, diabetes, and CKD were more common in AF patients with mild stroke in the no use group than those in the early or delayed OAC use group (all p -values before PS-matching: <0·05; Table 1 ). Patients in the no use group were more likely to use antiplatelet therapy and less likely to use OAC at baseline than those in the OAC use groups ( p -values before PS-matching: <0·05; Table 1 ). After PS-matching, differences between the OAC use and no use groups disappeared.
Table 1. Baseline characteristics of AF patients with mild stroke before and after PS-matching in the analytic sample for composite outcome.
| Unmatched cohort ( n = 4,513) | PS-matched cohorts | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Early use ( n = 1,307) | Delayed use ( n = 1,171) | No use ( n = 2,035) | p -Value a | p -Value b | Early use ( n = 1,307) | No use ( n = 1,307) | p -Value | Delayed use ( n = 1,171) | No use ( n = 1,171) | p -Value | |
| Gender | 0·308 | 0·224 | 0·694 | 0·706 | |||||||
| Female | 597 (45·7) | 488 (41·7) | 893 (43·9) | 597 (45·7) | 587 (44·9) | 488 (41·7) | 497 (42·4) | ||||
| Male | 710 (54·3) | 683 (58·3) | 1,142 (56·1) | 710 (54·3) | 720 (55·1) | 683 (58·3) | 674 (57·6) | ||||
| Age | <0·001 | <0·001 | <0·001 | 0·991 | |||||||
| ≥80 | 433 (33·1) | 383 (32·7) | 921 (45·3) | 433 (33·1) | 436 (33·4) | 383 (32·7) | 380 (32·5) | ||||
| 70–80 | 470 (36·0) | 426 (36·4) | 654 (32·1) | 470 (36·0) | 482 (36·9) | 426 (36·4) | 428 (36·5) | ||||
| < 70 | 404 (30·9) | 362 (30·9) | 460 (22·6) | 404 (30·9) | 389 (29·8) | 362 (30·9) | 363 (31·0) | ||||
| Medication use | |||||||||||
| Antihypertensive | 1,158 (88·6) | 993 (84·8) | 1767 (86·8) | 0·131 | 0·109 | 1,158 (88·6) | 1,146 (87·7) | 0·468 | 993 (84·8) | 996 (85·1) | 0·862 |
| Antidiabetic | 366 (28·0) | 298 (25·5) | 601 (29·5) | 0·341 | 0·013 | 366 (28·0) | 376 (28·8) | 0·664 | 298 (25·5) | 300 (25·6) | 0·924 |
| Lipid-lowering agents | 306 (23·4) | 277 (23·6) | 425 (20·9) | 0·084 | 0·067 | 306 (23·4) | 300 (22·9) | 0·780 | 277 (23·6) | 274 (23·4) | 0·883 |
| Anticoagulant | 622 (47·6) | 271 (23·1) | 237 (11·6) | <0·001 | <0·001 | 622 (47·6) | 625 (47·8) | 0·906 | 271 (23·1) | 267 (22·8) | 0·844 |
| Antiplatelet | 601 (46·0) | 622 (53·1) | 1197 (58·8) | <0·001 | 0·002 | 601 (46·0) | 620 (47·4) | 0·456 | 622 (53·1) | 587 (50·1) | 0·147 |
| NSAIDs | 1,307 (100) | 1,171 (100) | 2,032 (99·8) | 1 | 1 | 1,307 (100) | 1,307 (100) | 1 | 1,171 (100) | 1,171 (100) | 1 |
| Comorbidities | |||||||||||
| Liver disease | 179 (13·7) | 193 (16·5) | 306 (15·0) | 0·283 | 0·277 | 179 (13·7) | 170 (13·0) | 0·604 | 193 (16·5) | 174 (14·9) | 0·280 |
| Peptic ulcer disease | 353 (27·0) | 344 (29·4) | 706 (34·7) | <0·001 | 0·002 | 353 (27·0) | 357 (27·3) | 0·860 | 344 (29·4) | 314 (26·8) | 0·167 |
| Hypertension | 1028 (78·6) | 924 (78·9) | 1694 (83·2) | <0·001 | 0·002 | 1,028 (78·6) | 1,054 (80·6) | 0·206 | 924 (78·9) | 931 (79·5) | 0·721 |
| Dyslipidemia | 454 (34·7) | 414 (35·3) | 707 (34·7) | 0·997 | 0·726 | 454 (34·7) | 459 (35·1) | 0·837 | 414 (35·3) | 392 (33·5) | 0·338 |
| IHD | 615 (47·1) | 547 (46·7) | 1021 (50·2) | 0·078 | 0·059 | 615 (47·0) | 626 (47·9) | 0·666 | 547 (46·7) | 539 (46·0) | 0·740 |
| ICH | 26 (2·0) | 20 (1·7) | 63 (3·1) | 0·052 | 0·017 | 26 (2·0) | 28 (2·1) | 0·783 | 20 (1·7) | 16 (1·4) | 0·501 |
| TIA | 94 (7·2) | 76 (6·5) | 154 (7·6) | 0·686 | 0·255 | 94 (7·2) | 115 (8·8) | 0·129 | 76 (6·5) | 69 (5·9) | 0·548 |
| Alcohol intoxication | 10 (0·8) | 10 (0·8) | 24 (1·2) | 0·244 | 0·386 | 10 (0·8) | 12 (0·9) | 0·668 | 10 (0·8) | 9 (0·8) | 0·817 |
| GI bleeding | 125 (9·6) | 113 (9·6) | 277 (13·6) | <0·001 | 0·001 | 125 (9·6) | 131 (10·0) | 0·693 | 113 (9·6) | 104 (8·9) | 0·521 |
| Hematuria | 65 (5·0) | 57 (4·9) | 94 (4·6) | 0·638 | 0·749 | 65 (5·0) | 68 (5·2) | 0·789 | 57 (4·9) | 47 (4·0) | 0·316 |
| VTE | 27 (2·1) | 18 (1·5) | 31 (1·5) | 0·241 | 0·975 | 27 (2·1) | 33 (2·5) | 0·433 | 18 (1·5) | 18 (1·5) | 1 |
| Systemic embolism | 29 (2·2) | 28 (2·4) | 63 (3·1) | 0·130 | 0·247 | 29 (2·2) | 34 (2·6) | 0·523 | 28 (2·4) | 22 (1·89) | 0·391 |
| CHF | 602 (46·1) | 448 (38·3) | 907 (44·6) | 0·398 | <0·001 | 602 (46·1) | 576 (44·1) | 0·306 | 448 (38·3) | 431 (36·8) | 0·468 |
| Myocardial infarction | 90 (6·9) | 68 (5·8) | 193 (9·5) | 0·008 | <0·001 | 90 (6·9) | 77 (5·9) | 0·298 | 68 (5·8) | 63 (5·4) | 0·653 |
| PVD | 107 (8·2) | 76 (6·5) | 200 (9·8) | 0·108 | 0·001 | 107 (8·2) | 95 (7·3) | 0·379 | 76 (6·5) | 60 (5·1) | 0·157 |
| CVA | 610 (46·7) | 558 (47·6) | 953 (46·8) | 0·928 | 0·653 | 610 (46·7) | 590 (45·1) | 0·432 | 558 (47·6) | 550 (47·0) | 0·740 |
| Diabetes mellitus | 458 (35·0) | 390 (33·3) | 796 (39·1) | 0·017 | 0·001 | 458 (35·0) | 463 (35·4) | 0·837 | 390 (33·3) | 376 (32·1) | 0·537 |
| CKD | 361 (27·6) | 300 (25·6) | 678 (33·3) | <0·001 | <0·001 | 361 (27·6) | 370 (28·3) | 0·694 | 300 (25·6) | 293 (25·0) | 0·739 |
| Secondary prevention with OAC use | |||||||||||
| NOAC only | 354 (27·1) | 666 (56·9) | 0 | 354 (27·1) | 0 | 666 (56·9) | 0 | ||||
| Warfarin only | 811 (62·0) | 449 (38·3) | 0 | 811 (62·1) | 0 | 449 (38·3) | 0 | ||||
| Both | 142 (10·8) | 56 (4·8) | 0 | 142 (10·9) | 0 | 56 (4·8) | 0 | ||||
| Antiplatelet c | n/a | n/a | n/a | 829 (63·4) | 829 (63·4) | 1 | 987 (84·3) | 979 (83·6) | 0·652 | ||
Abbreviations: AF, atrial fibrillation; AIS, acute ischemic stroke; CHF, congestive heart failure; CKD, chronic kidney disease; CVA, cerebrovascular accident; GI, gastrointestinal; ICH, intracranial hemorrhage; IHD, ischemic heart disease; NOAC, non-vitamin K antagonist oral anticoagulant; NSAIDs, nonsteroidal anti-inflammatory drugs; OACs, oral anticoagulants; PS-matched, propensity score-matched; PVD, peripheral vascular disease; TIA, transient ischemic attack; VTE, venous thromboembolism.
Note: Data are shown in n (%). Statistically significant p -values are denoted in bold.
p -Value: comparing early use versus no use.
p -Value: comparing delayed use versus no use.
Antiplatelet use from the day of AIS admission to the index date (i.e., the day of OAC initiation or matching).
As the stroke severity increased from mild to severe ( Supplementary Table S14 , available in the online version), the incidence of composite outcome increased (444·5 to 928·3 cases per 1,000 person-years), as did the incidence of effectiveness outcome (292·1 to 654·7 cases per 1,000 person-years). The incidence of safety outcome did not vary substantially (149·5 to 196·4 cases per 1,000 person-years).
Use of OACs versus No Use and the Risk of Outcomes
AF Patients with Mild or Moderate Stroke
When compared with no OAC use, the early or delayed use was associated with a decreased risk of composite outcome with HR ranging from 0·73 (95% CI: 0·62–0·85) to 0·82 (95% CI: 0·67–1·00). The OAC use was not associated with an increased risk of safety outcomes ( Table 2 ).
Table 2. Hazard ratio and 95% confidence interval for the composite outcome, effectiveness outcome, and safety outcome, in the early OAC use group and in the delayed OAC use group a .
| Stroke severity | Mild | Moderate | Severe | |||
|---|---|---|---|---|---|---|
| OAC use | Early vs. no use | Delayed vs. no use | Early vs. no use | Delayed vs. no use | Early vs. no use | Delayed vs. no use |
| Composite outcome | ||||||
| 0·75 (0·65, 0·87) | 0·73 (0·62, 0·85) | 0·73 (0·61, 0·88) | 0·82 (0·67, 1·00) | 0·79 (0·68, 0·92) | 0·89 (0·73, 1·08) | |
| Stratified by: | ||||||
| NOAC | 0·64 (0·47, 0·85) | 0·61 (0·49, 0·76) | 0·59 (0·38, 0·93) | 0·73 (0·55, 0·97) | 0·77 (0·51, 1·17) | 1·10 (0·84, 1·45) |
| Warfarin | 0·89 (0·74, 1·06) | 0·88 (0·68, 1·14) | 0·79 (0·63, 1·00) | 0·76 (0·55, 1·06) | 0·86 (0·72, 1·03) | 0·61 (0·45, 0·82) |
| Effectiveness outcome | ||||||
| 0·83 (0·70, 0·98) | 0·69 (0·58, 0·83) | 0·88 (0·72, 1·07) | 0·84 (0·68, 1·03) | 0·82 (0·70, 0·95) | 0·76 (0·63, 0·92) | |
| Stratified by: | ||||||
| NOAC | 0·78 (0·56, 1·07) | 0·52 (0·40, 0·68) | 0·93 (0·59, 1·46) | 0·82 (0·63, 1·08) | 0·58 (0·39, 0·86) | 1·02 (0·78, 1·32) |
| Warfarin | 0·86 (0·69, 1·06) | 0·99 (0·75, 1·30) | 0·95 (0·75, 1·20) | 0·80 (0·56, 1·14) | 0·98 (0·82, 1·18) | 0·52 (0·39, 0·71) |
| Safety outcome | ||||||
| 0·96 (0·76, 1·22) | 0·75 (0·61, 0·93) | 1·08 (0·80, 1·46) | 0·94 (0·69, 1·28) | 1·67 (1·30, 2·13) | 1·16 (0·88, 1·53) | |
| Stratified by: | ||||||
| NOAC | 1·00 (0·58, 1·71) | 0·77 (0·58, 1·03) | 1·13 (0·61, 2·10) | 0·81 (0·51, 1·28) | 2·10 (1·13, 3·92) | 1·18 (0·78, 1·78) |
| Warfarin | 0·95 (0·71, 1·28) | 0·73 (0·52, 1·02) | 1·07 (0·73, 1·57) | 1·11 (0·68, 1·80) | 1·76 (1·29, 2·39) | 1·05 (0·69, 1·59) |
Abbreviations: AF, atrial fibrillation; NOAC, non-vitamin K antagonist oral anticoagulant; OACs, oral anticoagulants.
Note: Statistically significant values are denoted in bold.
A sequence of PS-matched cohorts for each of the three outcomes was constructed on each day from the first to the 30th day of admission. Data of PS-matched cohorts were pooled together for analyses.
AF Patients with Severe Stroke
Early use of OACs, compared with no use, was associated with a 0.79-fold (95% CI: 0.68–0.92) risk of composite outcome, 0.82-fold (95% CI: 0.70–0.95) risk of effectiveness outcome, and 1.67-fold (95% CI: 1.30–2.13) risk of safety outcomes. In NOAC- and warfarin-specific analyses, early use of NOAC was associated with a 0·58-fold (95% CI: 0·39–0·86) risk of effectiveness outcome and a 2.10-fold (95% CI: 1·13–3·92) risk of safety outcome. On the contrary, delayed use of warfarin was associated with a 0·52-fold (95% CI: 0.35–0.71) risk of effectiveness outcomes and was not associated with an increased risk of safety outcomes ( Table 2 ).
Early versus Delayed Use of OACs and the Risk of Outcomes
Across the stroke severity level, the risk of composite or effectiveness outcomes did not significantly differ between the early use and the delayed use groups ( Table 3 ). However, a trend of an increased risk of safety outcomes associated with the early use of OACs was observed, particularly in patients with severe stroke (HR: 1·44, 95% CI: 0·99–2·09, delayed use as reference). In AF patients with severe stroke, the risk of effectiveness outcome was lower in the early use than the delayed use of NOAC (HR: 0·57, 95% CI: 0·35–0·91); the opposite was observed in comparing the early use with the delayed use of warfarin (HR: 1.88, 95% CI: 1.33–2.68).
Table 3. Hazard ratio and 95% confidence intervals for the composite outcome, effectiveness outcome, and safety outcome, comparing the early use with the delayed use of OACs in mixed treatment comparison a .
| Stroke severity | Mild | Moderate | Severe |
|---|---|---|---|
| Composite outcome | 1·03 (0·83, 1·27) | 0·89 (0·68, 1·17) | 0·89 (0·69, 1·14) |
| Stratified by: | |||
| NOAC | 1·05 (0·73, 1·52) | 0·81 (0·48, 1·37) | 0·70 (0·43, 1·15) |
| Warfarin | 1·01 (0·74, 1·39) | 1·04 (0·70, 1·55) | 1·41 (0·99, 2·00) |
| Effectiveness outcome | 1·20 (0·94, 1·54) | 1·05 (0·79, 1·40) | 1·08 (0·85, 1·38) |
| Stratified by: | |||
| NOAC | 1·50 (0·99, 2·28) | 1·13 (0·67, 1·92) | 0·57 (0·35, 0·91) |
| Warfarin | 0·87 (0·61, 1·23) | 1·19 (0·78, 1·82) | 1·88 (1·33, 2·68) |
| Safety outcome | 1·28 (0·93, 1·76) | 1·15 (0·75, 1·77) | 1·44 (0·99, 2·09) |
| Stratified by: | |||
| NOAC | 1·30 (0·70, 2·40) | 1·40 (0·65, 3·01) | 1·78 (0·84, 3·75) |
| Warfarin | 1·30 (0·83, 2·04) | 0·96 (0·52, 1·79) | 1·68 (0·998, 2·82) |
Abbreviations: AF, atrial fibrillation; NOAC, non-vitamin K antagonist oral anticoagulant; OACs, oral anticoagulants.
Note: Statistically significant values are denoted in bold.
Reference group for all mixed treatment comparison was the delayed OAC use.
Net Clinical Benefit for OAC Use
In patients with mild or moderate stroke, early or delayed use of OACs was associated with a statistically significant NCB, as opposed to no OAC use ( Table 4 ). In patients with severe stroke, use of OACs, compared with no use, was also associated with a NCB although the benefit did not reach statistical significance in the delayed use group.
Table 4. Net clinical benefit for the early use and for the delayed use of OACs compared with no use.
| Effectiveness outcome | Safety outcome | Net clinical benefit (95% CI) a | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Person-year (p-yr) | Incidence (1,000 p-yr) | n | Person-year | Incidence (1,000 p-yr) | Weighting factor b | |||
| 1.5 | 2.0 | 3.0 | |||||||
| Mild stroke | |||||||||
| Early use | 570 | 2,009.0 | 283.7 | 349 | 2,493.6 | 140.0 | 0.16 (0.09, 0.23) | 0.17 (0.08, 0.25) | 0.19 (0.08, 0.30) |
| No use | 250 | 600.6 | 416.3 | 119 | 753.3 | 158.0 | Ref | Ref | Ref |
| Delayed use | 447 | 1,813.9 | 246.4 | 330 | 2,245.7 | 146.9 | 0.10 (0.04, 0.15) | 0.11 (0.04, 0.17) | 0.13 (0.04, 0.22) |
| No use | 352 | 1,119.6 | 314.4 | 226 | 1,356.6 | 166.6 | Ref | Ref | Ref |
| Moderate stroke | |||||||||
| Early use | 356 | 908.6 | 391.8 | 190 | 1,152.0 | 164.9 | 0.31 (0.18, 0.45) | 0.34 (0.19, 0.49) | 0.38 (0.19, 0.58) |
| No use | 179 | 280.6 | 638.0 | 68 | 323.8 | 210.0 | Ref | Ref | Ref |
| Delayed use | 269 | 814.2 | 330.4 | 170 | 1,021.3 | 166.4 | 0.20 (0.09, 0.30) | 0.22 (0.10, 0.34) | 0.27 (0.10, 0.44) |
| No use | 203 | 448.1 | 453.0 | 96 | 445.7 | 215.4 | Ref | Ref | Ref |
| Severe stroke | |||||||||
| Early use | 487 | 742.9 | 655.6 | 219 | 1,032.3 | 212.1 | 0.25 (0.11, 0.38) | 0.25 (0.09, 0.40) | 0.25 (0.05, 0.44) |
| No use | 330 | 365.1 | 903.9 | 95 | 450.1 | 211.1 | Ref | Ref | Ref |
| Delayed use | 321 | 597.8 | 537.0 | 141 | 853.6 | 165.2 | 0.12 (−0.01, 0.26) | 0.14 (−0.01, 0.29) | 0.18 (−0.002, 0.37) |
| No use | 249 | 412.7 | 603.3 | 99 | 485.0 | 204.1 | Ref | Ref | Ref |
Abbreviations: CI, confidence interval; OAC, oral anticoagulant.
Net clinical benefit was calculated as: (rate of effectiveness outcome in the no use group – rate of effectiveness outcome in the early [or delayed] use group − weighting factor × (rate of safety outcome in the early [or delayed] use group – rate of safety outcome in the no use group), originally proposed by Singer et al. 36
Discussion
This study provided the first evidence of a large-scale population and evaluated the effects of early or delayed OAC initiation in a population with AF after AIS by PS-matched cohort on each day since admission, stratified by stroke severity, to overcome immortal-time biases by emulating RCTs. Herein, the key finding was that: first, both early and delayed OAC use could reduce the risk of composite and effectiveness outcomes for each stroke severity. Second, compared with delayed use of OAC, early use, based on the recommendations of current clinical guidelines, was not associated with an excessive risk of composite outcomes. Third, in subjects with severe stroke, early treatment may result in a higher bleeding risk when compared with delayed treatment, despite presenting similar risks for the effectiveness and composite outcomes.
Previous observational studies regarding the timing of OAC initiation after AIS have presented conflicting results. For example, Paciaroni et al have reported that the optimal time to initiate OACs was 4 to 14 days from stroke onset. 8 Similar results have been observed in the RAF-NOAC study, with the lowest composite rates of recurrence and major bleeding for those who initiated NOACs between 3 and 14 days. 9 The 2018 American Heart Association/American Stroke Association guidelines recommended that secondary prevention with OACs should be appropriately instituted within the first 2 weeks, 38 whereas United Kingdom guidelines 39 recommended that OAC administration be deferred until at least 14 days from the onset in patients with disabling ischemic stroke.
However, more recent studies have not supported the 14-day recommendation. Yaghi et al have conducted a registry from eight comprehensive stroke centers and found that OACs started in the 0- to 3-day period were not associated with higher recurrent ischemic events or ICH when compared with those initiated at 4 to 14 days. 40 Clinical Relevance of Microbleeds In Stroke-2 (CROMIS-2) also suggested that early OAC (0–4 days) after AF-related AIS or TIA was not associated with a difference in the composite outcome of stroke, TIA, or death at 90 days, when compared with delayed OAC (≥5 days or never started). 41
Given that the delayed initiation of OAC was not associated with obvious clinical benefits, early use of OACs in mild and moderate AIS patients with AF might be a reasonable alternative. Our finding is consistent with the previous two small randomized trials, 42 43 which provided reassurance regarding the safety of early initiation of administration of rivaroxaban or dabigatran in patients with mild-to-moderate ischemic stroke (NIHSS < 9).
Previous recommendations for the delayed initiation of OAC were based on concerns of hemorrhagic transformation after AIS. However, in our analysis, at least in subjects with mild-to-moderate stroke severity, a delayed OAC use for secondary prevention in patients with AF and AIS is not an evidence-based recommendation and should not be employed as routine clinical practice; however, in severe patients with AIS, delaying the use of OACs may reduce the risk of bleeding events. RCTs are needed to define the appropriate timing of OACs initiation.
One of the major advantages of our study is the large study population from a nationwide cohort, providing the opportunity to perform PS matching with sufficient event numbers for statistical inference. Another important strength is the comprehensive analytic framework in our study, especially the approach in dealing with immortal-time bias. For research questions involving strategies with different timings, immortal-time bias and confounding bias are difficult issues to resolve in observational studies. By utilizing the day-by-day PS-matching approach, we reduced the immortal-time bias and confounding bias when comparing different strategies. Furthermore, in the present real-world cohorts, we employed the novel mixed treatment comparison meta-analysis techniques for indirect comparisons to obtain relative effects of early versus delayed OAC use in AF patients with AIS.
Several limitations of the present study need to be acknowledged. First, selection bias is an inherent limitation of observational studies. Second, the disease status and outcomes were identified by validated algorithms, 16 17 18 19 20 21 22 23 28 which might not represent patients' real conditions as the codes were designed to claim health insurance. Third, there may be residual confounding from unmeasured or unknown covariates as NHIRD was unable to provide laboratory data such as international normalized ratio to evaluate the controlled efficacy of VKAs or imaging data, including CT scan, MRI, and echocardiography, to fully evaluate the clinical status. Claims-based databases also lack the information regarding stroke lesion volume; therefore, the current study applied the validated tool, SSI, 25 26 27 for assessing stroke severity rather than NIHSS. The latter, however, is the most widely accepted tool to assess the severity of stroke. Fourth, our study samples were recruited repeatedly in different cohorts to imitate an RCT design. Our estimated results could be confounded by unsatisfied independence. Owing to similar inclusion criteria, methodology, and controlled variables in our study, the consistency assumption of the indirect comparisons is less concerning. 44 Lastly, the delayed or early initiation of OACs was based on current guideline recommendations, and future studies to determine the most appropriate timing to resume OACs are warranted.
Conclusion
In patients with AF admitted for AIS, early initiation of OACs and the routine delayed use appeared to result in a comparable risk of composite clinical outcome across the levels of stroke severity. The risk of bleeding events seemed to be similar for all the OAC use groups in patients with mild-to-moderate AIS. However, such a risk was particularly concerning for patients with severe AIS who resumed OACs early. The current study findings support an early OAC initiation in AF patients with mild-to-moderate AIS and a routine delayed use of OACs in those with severe AIS to avoid a serious bleeding event. The optimal timing of OAC initiation after AIS requires further investigation.
Funding Statement
Funding None.
Conflict of Interest G.Y.H.L.: investigator for the OPTIMAS trial; consultant for Bayer/Janssen, BMS/Pfizer, Boehringer Ingelheim, Verseon, and Daiichi-Sankyo; speaker for BMS/Pfizer, Boehringer Ingelheim, and Daiichi-Sankyo. No fees were directly received in person.
Ethical Approval
The study protocol was approved by ethic committee of Taipei Veterans General Hospital.
Note
All authors confirm that they had full access to all the data in the study and accept responsibility to submit for publication. However, our study does not contain data from any individual. The datasets used and analyzed during the current study are available from the corresponding author on reasonable request. The authors declare that they have no competing interests. This article reflects the views of the authors and does not represent the U.S. Food and Drug Administration's views or policies.
Author Contributions
C.-E.C., H.-M.C., and Y.-W.T. conceived and designed the research. Statistical analysis was performed by W.-L. Wu, P.-Y.C., W.-T.W., and H.-C.C.. W.-T.W., P.-Y.C., Y.-W.T., S.-H.C., and H.-M.C. drafted the article. C.-H.C. and C.-E.C. made critical revision of the article for key intellectual content. Each author contributed important intellectual content during article drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. H.-M.C., C.-H.C., C.-E.C., and Y.-W.T. undertake that this study has been reported honestly, accurately, and transparently, that no important aspects of the study have been omitted, and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
These authors contributed equally to this work. Note: The review process for this paper was fully handled by Christian Weber, Editor-in-Chief.
What is known about this topic?
Evidence has suggested that high-risk patients with atrial fibrillation (AF) should routinely administer lifelong oral anticoagulants (OACs) for secondary stroke prevention.
The 2018 European Heart Rhythm Association (EHRA) practical guide proposed a “1–3–6–12 days rule” to resume OAC after an acute ischemic stroke in patients with AF, which is an expert consensus opinion, lacking supporting evidence from large-scale randomized controlled trials or real-world observational studies.
What does this paper add?
Both early and delayed OAC uses could reduce composite and effectiveness outcomes for each stroke severity.
Compared with delayed use of OAC, early use, based on the recommendations of current clinical guidelines, was not associated with an excessive risk of composite outcomes.
In subjects with severe stroke, early treatment may result in a higher bleeding risk when compared with delayed treatment, despite presenting similar risks for the effectiveness and composite outcomes.
Supplementary Material
References
- 1.GBD 2016 Stroke Collaborators . Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(05):439–458. doi: 10.1016/S1474-4422(19)30034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf P A, Abbott R D, Kannel W B. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22(08):983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.January C T, Wann L S, Calkins H. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140(02):e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 4.ESC Scientific Document Group . Steffel J, Verhamme P, Potpara T S. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330–1393. doi: 10.1093/eurheartj/ehy136. [DOI] [PubMed] [Google Scholar]
- 5.Paciaroni M, Bandini F, Agnelli G. Hemorrhagic transformation in patients with acute ischemic stroke and atrial fibrillation: time to initiation of oral anticoagulant therapy and outcomes. J Am Heart Assoc. 2018;7(22):e010133. doi: 10.1161/JAHA.118.010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ESC Scientific Document Group . Kirchhof P, Benussi S, Kotecha D. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 7.CROMIS-2 collaborators . Wilson D, Ambler G, Shakeshaft C. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): a multicentre observational cohort study. Lancet Neurol. 2018;17(06):539–547. doi: 10.1016/S1474-4422(18)30145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paciaroni M, Agnelli G, Falocci N. Early recurrence and cerebral bleeding in patients with acute ischemic stroke and atrial fibrillation: effect of anticoagulation and its timing: the RAF study. Stroke. 2015;46(08):2175–2182. doi: 10.1161/STROKEAHA.115.008891. [DOI] [PubMed] [Google Scholar]
- 9.Paciaroni M, Agnelli G, Falocci N. Early recurrence and major bleeding in patients with acute ischemic stroke and atrial fibrillation treated with non-vitamin-K oral anticoagulants (RAF-NOACs) study. J Am Heart Assoc. 2017;6(12):e007034. doi: 10.1161/JAHA.117.007034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.SAMURAI Study Investigators . Yoshimura S, Koga M, Sato S. Two-year outcomes of anticoagulation for acute ischemic stroke with nonvalvular atrial fibrillation - SAMURAI-NVAF study. Circ J. 2018;82(07):1935–1942. doi: 10.1253/circj.CJ-18-0067. [DOI] [PubMed] [Google Scholar]
- 11.Seiffge D J, Traenka C, Polymeris A. Early start of DOAC after ischemic stroke: risk of intracranial hemorrhage and recurrent events. Neurology. 2016;87(18):1856–1862. doi: 10.1212/WNL.0000000000003283. [DOI] [PubMed] [Google Scholar]
- 12.Macha K, Volbers B, Bobinger T. Early initiation of anticoagulation with direct oral anticoagulants in patients after transient ischemic attack or ischemic stroke. J Stroke Cerebrovasc Dis. 2016;25(09):2317–2321. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Cappellari M, Carletti M, Danese A, Bovi P. Early introduction of direct oral anticoagulants in cardioembolic stroke patients with non-valvular atrial fibrillation. J Thromb Thrombolysis. 2016;42(03):393–398. doi: 10.1007/s11239-016-1393-9. [DOI] [PubMed] [Google Scholar]
- 14.CROMIS-2, RAF, RAF-DOAC, SAMURAI, NOACISP LONGTERM, Erlangen and Verona registry collaborators . Seiffge D J, Paciaroni M, Wilson D. Direct oral anticoagulants versus vitamin K antagonists after recent ischemic stroke in patients with atrial fibrillation. Ann Neurol. 2019;85(06):823–834. doi: 10.1002/ana.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SAMURAI Study Investigators . Mizoguchi T, Tanaka K, Toyoda K. Early initiation of direct oral anticoagulants after onset of stroke and short- and long-term outcomes of patients with nonvalvular atrial fibrillation. Stroke. 2020;51(03):883–891. doi: 10.1161/STROKEAHA.119.028118. [DOI] [PubMed] [Google Scholar]
- 16.Chan Y-H, Yen K-C, See L-C. Cardiovascular, bleeding, and mortality risks of dabigatran in asians with nonvalvular atrial fibrillation. Stroke. 2016;47(02):441–449. doi: 10.1161/STROKEAHA.115.011476. [DOI] [PubMed] [Google Scholar]
- 17.Chao T F, Liu C J, Wang K L. Using the CHA2DS2-VASc score for refining stroke risk stratification in ‘low-risk’ Asian patients with atrial fibrillation. J Am Coll Cardiol. 2014;64(16):1658–1665. doi: 10.1016/j.jacc.2014.06.1203. [DOI] [PubMed] [Google Scholar]
- 18.Chao T F, Liu C J, Wang K L. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol. 2015;65(07):635–642. doi: 10.1016/j.jacc.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 19.Chao T F, Wang K L, Liu C J. Age threshold for increased stroke risk among patients with atrial fibrillation: a nationwide cohort study from Taiwan. J Am Coll Cardiol. 2015;66(12):1339–1347. doi: 10.1016/j.jacc.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 20.Chao T-F, Liu C-J, Tuan T-C. Rate-control treatment and mortality in atrial fibrillation. Circulation. 2015;132(17):1604–1612. doi: 10.1161/CIRCULATIONAHA.114.013709. [DOI] [PubMed] [Google Scholar]
- 21.Cheng C L, Kao Y H, Lin S J, Lee C H, Lai M L. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20(03):236–242. doi: 10.1002/pds.2087. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh C Y, Chen C H, Li C Y, Lai M L. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc. 2015;114(03):254–259. doi: 10.1016/j.jfma.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Lin L J, Cheng M H, Lee C H, Wung D C, Cheng C L, Kao Yang Y H. Compliance with antithrombotic prescribing guidelines for patients with atrial fibrillation–a nationwide descriptive study in Taiwan. Clin Ther. 2008;30(09):1726–1736. doi: 10.1016/j.clinthera.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Chang C-H, Lee Y-C, Tsai C-T. Continuation of statin therapy and a decreased risk of atrial fibrillation/flutter in patients with and without chronic kidney disease. Atherosclerosis. 2014;232(01):224–230. doi: 10.1016/j.atherosclerosis.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 25.Sung S F, Hsieh C Y, Lin H J. Validity of a stroke severity index for administrative claims data research: a retrospective cohort study. BMC Health Serv Res. 2016;16(01):509. doi: 10.1186/s12913-016-1769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sung S-F, Chen S C-C, Hsieh C-Y, Li C-Y, Lai E C-C, Hu Y-H. A comparison of stroke severity proxy measures for claims data research: a population-based cohort study. Pharmacoepidemiol Drug Saf. 2016;25(04):438–443. doi: 10.1002/pds.3944. [DOI] [PubMed] [Google Scholar]
- 27.Sung S-F, Hsieh C-Y, Kao Yang Y-H. Developing a stroke severity index based on administrative data was feasible using data mining techniques. J Clin Epidemiol. 2015;68(11):1292–1300. doi: 10.1016/j.jclinepi.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Lin C C, Hu H Y, Luo J C. Risk factors of gastrointestinal bleeding in clopidogrel users: a nationwide population-based study. Aliment Pharmacol Ther. 2013;38(09):1119–1128. doi: 10.1111/apt.12483. [DOI] [PubMed] [Google Scholar]
- 29.Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167(04):492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- 30.Hernán M A, Sauer B C, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70–75. doi: 10.1016/j.jclinepi.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenbaum P R, Rubin D B. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(01):33–38. [Google Scholar]
- 32.Hernán M A, Alonso A, Logan R. Observational studies analyzed like randomized experiments: an application to postmenopausal hormone therapy and coronary heart disease. Epidemiology. 2008;19(06):766–779. doi: 10.1097/EDE.0b013e3181875e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fine J P, Gray R J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 34.Austin P C, Lee D S, Fine J P. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(06):601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoyer A. Metaanalysis with R. G.Schwarzer, J. R.Carpenter, G.Rücker (2015). Berlin, DE: Springer. ISBN: 978–3-319–21415–3. Biom J. 2017;59(01):216–217. [Google Scholar]
- 36.Singer D E, Chang Y, Fang M C, Borowsky L H, Pomernacki N K, Udaltsova N. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151:297–305. doi: 10.7326/0003-4819-151-5-200909010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chao T F, Lip G Y, Liu C J. Validation of a modified CHA2DS2-VASc score for stroke risk stratification in asian patients with atrial fibrillation: a nationwide cohort study. Stroke. 2016;47(10):2462–2469. doi: 10.1161/STROKEAHA.116.013880. [DOI] [PubMed] [Google Scholar]
- 38.Powers W J, Rabinstein A A, Ackerson T. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 39.Rudd A G, Bowen A, Young G R, James M A. The latest national clinical guideline for stroke. Clin Med (Lond) 2017;17(02):154–155. doi: 10.7861/clinmedicine.17-2-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yaghi S, Trivedi T, Giles J. Abstract 119: initiating oral anticoagulation 4 to 14 days after a cardioembolic stroke is not associated with a reduction in ischemic or hemorrhagic events: the IAC Multicenter cohort. Stroke. 2020;51 01:A119–A119. [Google Scholar]
- 41.Clinical relevance of Microbleeds in Stroke (CROMIS-2) collaborators . Wilson D, Ambler G, Banerjee G. Early versus late anticoagulation for ischaemic stroke associated with atrial fibrillation: multicentre cohort study. J Neurol Neurosurg Psychiatry. 2019;90(03):320–325. doi: 10.1136/jnnp-2018-318890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phase 2 Exploratory Clinical Study to Assess the Effects of Xarelto (Rivaroxaban) Versus Warfarin on Ischemia, Bleeding, and Hospital Stay in Acute Cerebral Infarction Patients With Non-valvular Atrial Fibrillation (Triple AXEL) Study Group . Hong K-S, Kwon S U, Lee S H. Rivaroxaban vs warfarin sodium in the ultra-early period after atrial fibrillation–related mild ischemic stroke: a randomized clinical trial. JAMA Neurol. 2017;74(10):1206–1215. doi: 10.1001/jamaneurol.2017.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ng K H, Sharma M, Benavente O. Dabigatran following acute transient ischemic attack and minor stroke II (DATAS II) Int J Stroke. 2017;12(18):910–914. doi: 10.1177/1747493017711947. [DOI] [PubMed] [Google Scholar]
- 44.Shim S, Yoon B-H, Shin I-S, Bae J-M. Network meta-analysis: application and practice using Stata. Epidemiol Health. 2017;39:e2017047–e2017047. doi: 10.4178/epih.e2017047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


