Abstract
Background Andexanet alfa (andexanet) is approved for specific anticoagulation reversal in patients with life-threatening or uncontrolled bleeding during treatment with rivaroxaban or apixaban. There is limited experience with andexanet in patients with acute bleeding on edoxaban.
Methods Patients with acute major bleeding within 18 hours of edoxaban intake were prospectively enrolled. Patients received a bolus and 2-hour follow-on infusion of andexanet. The co-primary efficacy outcomes were change in antifactor Xa activity and the percentage of patients achieving excellent or good hemostasis, 12 hours after andexanet treatment. Efficacy was analyzed in patients with confirmed major bleeding and baseline antifactor Xa activity ≥40 ng/mL. Safety was analyzed in all patients.
Results Thirty-six patients (mean age: 82 years, 61.1% male and 91.7% with atrial fibrillation) with acute major bleeding on edoxaban received andexanet. The primary site of bleeding was intracranial in 29 patients (80.6%). In the efficacy population ( n = 28), median antifactor Xa activity decreased from 121.1 (interquartile range [IQR]: 70.3–202.4) ng/mL at baseline to 24.0 (IQR: 77.7–83.7) ng/mL at the end of andexanet bolus (median decrease: 68.9%, 95% confidence interval [CI]: 56.1–77.7%). Excellent or good hemostasis at 12 hours was achieved in 78.6% (95% CI: 59.0–91.7%) of patients. Within 30 days, four patients (11.1%) experienced a thrombotic event and four others (11.1%) died.
Conclusion In patients with acute major bleeding on edoxaban, andexanet significantly decreased antifactor Xa activity. Hemostatic efficacy was similar to that observed in patients with bleeding on rivaroxaban or apixaban. Thrombotic events occurred at a rate expected in such patients.
Keywords: andexanet alfa, reversal, edoxaban, intracranial hemorrhage, bleeding
Introduction
Management of acute major bleeding in patients receiving factor Xa inhibitor therapy can be challenging. Although major extracranial bleeding is more common, intracranial hemorrhage is generally the most feared complication of anticoagulation by both patients and physicians. 1 2 3 Rapid and specific reversal of anticoagulant activity is aimed at restoring hemostasis in patients experiencing significant bleeding during treatment with such agents. Andexanet alfa (andexanet) is a recombinant decoy molecule that specifically binds and sequesters factor Xa inhibitors. 4 5 It is currently approved in the United States, Europe, and selected other locations for patients with life-threatening or uncontrolled bleeding during treatment with rivaroxaban and apixaban, but has so far not been approved for reversal of edoxaban. The oral factor Xa inhibitor edoxaban is currently approved in several countries for the prophylaxis of stroke and systemic embolism in patients with atrial fibrillation and for the prevention and treatment of venous thromboembolism. 6 7 Previous studies in individuals with atrial fibrillation demonstrated associations of edoxaban exposure and corresponding antifactor Xa activity with clinical outcomes. 8 9 10 11 12
The single-arm, international, prospective Andexanet Alfa, a Novel Antidote to the Anticoagulation Effects of Factor Xa Inhibitors (ANNEXA-4) study was designed to assess the efficacy and safety of andexanet in patients experiencing acute major bleeding on an oral factor Xa inhibitor or the low-molecular-weight heparin enoxaparin. 5 13 The study had originally included very few patients receiving edoxaban. After the main results were published, enrollment continued to accumulate additional data for patients on this oral anticoagulant. In this study, we report outcomes of all patients receiving andexanet for anticoagulant reversal of edoxaban.
Methods
Eligibility Criteria
Inclusion and exclusion criteria of the ANNEXA-4 study have been described in detail previously. 5 13 In short, patients aged 18 years or older with acute major bleeding and recent (i.e., within 18 hours) intake of an oral factor Xa inhibitor or enoxaparin were prospectively enrolled. Only patients with acute bleeding and recent intake of edoxaban were included in this report. Major bleeding was defined as bleeding fulfilling one or more of the following criteria: bleeding that was potentially life-threatening; acute, overt bleeding associated with a fall in hemoglobin level by ≥2 g/dL or associated with a hemoglobin level of ≤8 g/dL if no baseline hemoglobin was available; or acute bleeding in a critical area or organ (such as intraspinal, pericardial, or intracranial). For patients entering the study with intraspinal or intracranial hemorrhage, a head computed tomography (CT) or magnetic resonance imaging (MRI) scan demonstrating the bleeding was required. Key exclusion criteria were planned surgery within 12 hours (not including minimally invasive surgery or procedures), presence of intracerebral hemorrhage associated with a Glasgow Coma Scale score <7 or volume >60 mL, primarily visible (i.e., musculoskeletal or intra-articular) bleeding, a recent (i.e., within 2 weeks) thromboembolic event, and an expected survival of <1 month. Participants were required to provide written informed consent; in some cases, proxy or emergency consent was obtained. The study was approved by the local institutional review board at each participating center. The ANNEXA-4 study is registered on ClinicalTrials.gov (unique identifier, NCT02329327).
Study Procedures
Data on patient characteristics, medical history, edoxaban treatment, concomitant medication, and details on the qualifying bleeding event were collected at baseline. Depending on the dosage and time of the last dose of edoxaban, one of two dosing regimens of andexanet was administered ( Supplementary Table S1 [available in the online version]). In short, an intravenous andexanet bolus of 800 mg and a subsequent follow-on infusion of 960 mg over 2 hours were administered to patients who had received >30 mg or an unknown dose of edoxaban within <8 hours or at an unknown time (high dose). Patients who had received up to 30 mg edoxaban within <8 hours or at an unknown time, and those who had received edoxaban ≥8 hours before, received a 400 mg andexanet bolus, followed by a 480 mg follow-on infusion (low dose). Blood samples to determine antifactor Xa activity were obtained before, during (at the end of bolus and after the follow-on infusion), and after andexanet treatment (at 4, 8, and 12 hours). A modified antifactor Xa activity assay with exogenously added bovine factor Xa was used to assess anticoagulant activity before and after andexanet treatment, as described previously. 14 Patients who entered the study with a qualifying intracranial hemorrhage on CT or MRI were required to undergo repeat imaging at 1 hour and 12 hours after administration of andexanet. There was a single follow-up visit at 1 month after enrollment.
Outcomes
The co-primary efficacy outcomes of this study were the median percent change in antifactor Xa activity from baseline to end of andexanet bolus, and the percentage of patients achieving excellent or good hemostasis at 12 hours after andexanet treatment, according to prespecified criteria adapted from Sarode et al ( Supplementary Table S2 [available in the online version]). 15 Safety outcomes included thrombotic events (myocardial infarction, stroke, transient ischemic attack, deep vein thrombosis, pulmonary embolism, and systemic embolism) and death up until 30 days after treatment with andexanet. Each case was reviewed by at least two members of an independent endpoint adjudication committee to confirm the patients met criteria to be included in the efficacy analysis, to determine hemostatic efficacy, and to adjudicate safety outcomes, including the classification of the type of death as cardiovascular, noncardiovascular, or uncertain. An independent data and safety monitoring board reviewed study data for safety.
Statistical Analysis
Efficacy (i.e., change in antifactor Xa activity and the percentage of patients with excellent or good hemostasis at 12 hours) was analyzed in two different patient populations. These were patients with confirmed major bleeding on edoxaban and a baseline antifactor Xa activity ≥40 and ≥75 ng/mL, respectively. The threshold ≥40 ng/mL was introduced through an amendment to the statistical analysis plan because many patients entering ANNEXA-4 on edoxaban had a baseline antifactor Xa activity below the original threshold ≥75 ng/mL for inclusion into the efficacy analysis; a threshold ≥75 ng/mL had been used in the analyses evaluating efficacy of andexanet in patients with bleeding on apixaban or rivaroxaban. 5 The safety population included all patients enrolled in ANNEXA-4 with bleeding on edoxaban and who received andexanet. Baseline characteristics were presented for all patients receiving andexanet (the safety population) and for patients with baseline antifactor Xa activity ≥40 and ≥75 ng/mL, respectively. Continuous variables are summarized as mean ± standard deviation or median (interquartile range [IQR]), as appropriate, and categorical variables are shown as counts and percentages. For patients evaluated for efficacy, the median antifactor Xa activity is presented at baseline, end of andexanet bolus administration, end of andexanet follow-on infusion, as well as at 4, 8, and 12 hours after andexanet infusion, and box plots were used for visualization. The median percent change in antifactor Xa activity from baseline to end of bolus and other time points is shown with corresponding distribution-free 95% confidence intervals (CIs); this was done for patients for whom antifactor Xa activity was available both at baseline and at the respective time point after andexanet administration. Further, the on-treatment nadir, defined as the minimum antifactor Xa activity at the end of andexanet bolus or end of follow-on infusion, and the median percent change from baseline to the on-treatment nadir are presented with 95% CIs; if there were missing data on antifactor Xa activity for either the end of bolus or the end of follow-on infusion, the on-treatment nadir was set to the one that was available. In patients included in the efficacy analyses, the percentage of effective hemostasis at 12 hours after andexanet administration was calculated. The Clopper–Pearson method, based on the exact binomial distribution, was used to calculate corresponding 95% CIs. 16 The rates of a first thrombotic event and mortality within 30 days following andexanet administration were calculated for the safety population. These are presented overall and in three time intervals following administration of andexanet: up until day 5, days 6 to 14, and days 15 to 30. Cases with a first thrombotic event after restarting anticoagulation are highlighted. Events that occurred on the same day as restarting anticoagulation were considered to have occurred before the restart. Several exploratory analyses were conducted, including (1) antifactor Xa activity before and after andexanet treatment according to edoxaban dosage, (2) hemostatic efficacy among patients with atrial fibrillation or flutter according to the HAS-BLED score, (3) safety outcomes among patients with atrial fibrillation or flutter according to the CHA 2 DS 2 -VASc score, (4) a descriptive analysis of the Glasgow Come Scale score of patients before and after andexanet treatment, and (5) summary statistics describing patients who did not achieve excellent or good hemostasis.
Availability of Data and Material
Alexion, AstraZeneca Rare Disease will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development . Link to data request form: https://alexion.com/contact-alexion/medical-information .
Role of the Funding Source
The ANNEXA-4 study was jointly designed and planned by the Population Health Research Institute at McMaster University, Hamilton, Ontario, Canada, and the manufacturer of andexanet, Portola Pharmaceuticals, now Alexion, AstraZeneca Rare Disease, Boston, Massachusetts, United States. The study was funded by Portola Pharmaceuticals. The study was coordinated and all statistical analyses were performed at the Population Health Research Institute. The sponsor was not involved in data collection but did have access to the data.
Results
From December 2016 through March 2020, a total of 36 patients experiencing bleeding on edoxaban received andexanet, including 10 patients who were part of the previously published ANNEXA-4 full cohort. 5 Baseline characteristics of the edoxaban-treated patients receiving andexanet are shown in Table 1 . Overall, the mean age of patients was 81.5 years, 61.1% were male, and 91.7% had atrial fibrillation as the primary indication for oral anticoagulation. Eight patients (22.2%) had suffered a prior stroke. Twenty-eight patients (77.8%) with confirmed major bleeding had a baseline antifactor Xa activity ≥40 ng/mL, and 20 patients (55.6%) had a baseline antifactor Xa activity ≥75 ng/mL. In these groups evaluated for efficacy, the primary site of bleeding was intracranial in 22 (78.6%) and 16 patients (80.0%), respectively.
Table 1. Baseline characteristics of patients.
| Safety population ( N = 36) | Patients with baseline antifactor Xa activity ≥40 ng/mL ( N = 28) | Patients with baseline antifactor Xa activity ≥75 ng/mL ( N = 20) | |
|---|---|---|---|
| Age (y), mean ± SD | 81.5 ± 6.3 | 81.4 ± 6.3 | 81.2 ± 6.9 |
| Male sex, n (%) | 22 (61.1) | 18 (64.3) | 13 (65.0) |
| White race, n (%) | 31 (86.1) | 24 (85.7) | 17 (85.0) |
| Body mass index (kg/m 2 ), mean ± SD | 25.1 ± 3.7 | 24.9 ± 3.3 | 24.1 ± 2.6 |
| Estimated creatinine clearance, a n (%) | |||
| Missing data | 1 (2.8) | 1 (3.6) | 0 (0) |
| <30 mL/min | 5 (13.9) | 3 (10.7) | 2 (10.0) |
| 30–59.9 mL/min | 17 (47.2) | 16 (57.1) | 13 (65.0) |
| ≥60 mL/min | 13 (36.1) | 8 (28.6) | 5 (25.0) |
| Primary indication for anticoagulation, b n (%) | |||
| Atrial fibrillation | 33 (91.7) | 26 (92.9) | 19 (95.0) |
| Atrial flutter | 1 (2.8) | 1 (3.6) | 0 (0) |
| Venous thromboembolism c | 2 (5.6) | 1 (3.6) | 1 (5.0) |
| Medical history, n (%) | |||
| Myocardial infarction | 3 (8.3) | 3 (10.7) | 3 (15.0) |
| Stroke | 8 (22.2) | 8 (28.6) | 7 (35.0) |
| Deep-vein thrombosis | 3 (8.3) | 2 (7.1) | 2 (10.0) |
| Atrial fibrillation | 33 (91.7) | 26 (92.9) | 19 (95.0) |
| Heart failure | 7 (19.4) | 6 (21.4) | 3 (15.0) |
| Diabetes mellitus | 4 (11.1) | 3 (10.7) | 2 (10.0) |
| Hypertension | 33 (91.7) | 26 (92.9) | 18 (90.0) |
| CHA 2 DS 2 -VASc score, median (IQR) d | 4 (3–5) | 4 (3–5) | 4 (3–5) |
| HAS-BLED score, median (IQR) d | 2 (2–3) | 2 (2–3) | 2 (2–3) |
| Hemoglobin (g/L), mean ± SD | 118.2 ± 31.1 | 121.2 ± 31.7 | 121.9 ± 30.8 |
| Platelet count (10 9 /L), mean ± SD | 224.5 ± 80.0 | 226.1 ± 86.0 | 223.6 ± 90.8 |
| Primary site of bleeding, n (%) | |||
| Intracranial, any | 29 (80.6) | 22 (78.6) | 16 (80.0) |
| Intracranial, associated with trauma e | 6 (20.7) | 4 (18.2) | 3 (18.8) |
| Gastrointestinal | 7 (19.4) | 6 (21.4) | 4 (20.0) |
| Edoxaban dosage, n (%) | |||
| 60 mg once daily | 20 (55.6) | 16 (57.1) | 13 (65.0) |
| 30 mg once daily | 15 (41.7) | 11 (39.3) | 6 (30.0) |
| 15 mg once daily | 1 (2.8) | 1 (3.6) | 1 (5.0) |
| Baseline antifactor Xa activity (ng/mL), median (IQR) | 95.1 (57.1–196.8) | 121.1 (70.3–202.4) | 160.5 (106.2–222.2) |
| Time from last dose of edoxaban to andexanet bolus (h), median (IQR) | 9.2 (6.2–13.0) | 9.4 (6.0–12.9) | 8.7 (5.2–11.5) |
| Time from presentation at the emergency department to andexanet bolus (h), median (IQR) | 2.8 (1.8–4.3) | 2.9 (2.3–4.3) | 2.8 (2.0–3.9) |
Abbreviations: INR, international normalized ratio; IQR, interquartile range; SD, standard deviation.
Creatinine clearance estimated according to the Cockcroft–Gault formula.
If >1 primary indication for anticoagulation recorded: if atrial fibrillation was present, this was listed as the primary indication; if present, venous thromboembolism was considered primary in the remaining patients.
Venous thromboembolism refers to prevention or treatment of deep-vein thrombosis and pulmonary embolism.
Reported for patients with atrial fibrillation or atrial flutter. The CHA 2 DS 2 -VASc score ranges from 0 to 9 (congestive heart failure [1], hypertension [1], age ≥75 years [2], diabetes [1], prior stroke or transient ischemic attack [2], vascular disease [1], age 65–74 years [1], female sex [1]). A modified HAS-BLED score is reported, ranging from 0 to 7 (hypertension [systolic blood pressure >160 mm Hg at baseline [1], abnormal kidney function [1], abnormal liver function [1], prior stroke [1], bleeding history [not including the qualifying bleeding event] or anemia [1], age >65 years [1], concomitant use of antiplatelet agents or nonsteroidal anti-inflammatory drugs [1]). As opposed to the original HAS-BLED score, the categories labile INR (not applicable) and alcohol use (data not available) were not considered.
Denominators for percentage with traumatic intracranial hemorrhage are all patients with any intracranial hemorrhage.
Change in Antifactor Xa Activity
In patients with baseline antifactor Xa activity ≥40 ng/mL, the median antifactor Xa activity decreased from 121.1 (IQR: 70.3–202.4) ng/mL at baseline to 24.0 (IQR: 17.7–83.7) ng/mL at the end of andexanet bolus administration, corresponding to a median 68.9% (95% CI: 56.1–77.7%) decrease ( Fig. 1A ). At the end of the andexanet follow-on infusion, the median antifactor Xa activity was 30.2 (IQR: 18.3–68.0) ng/mL, a median 68.6% (95% CI: 57.2–77.6%) decrease. The median antifactor Xa activity at the time of the on-treatment nadir was 24.4 (IQR: 14.1–62.2) ng/mL, corresponding to a median 71.3% (95% CI: 65.2–82.3%) decrease. The median reduction in antifactor Xa activity at 4, 8, and 12 hours after andexanet administration was 34.4, 48.6, and 58.5%, respectively. Supplementary Fig. S1 (available in the online version) shows antifactor Xa activity at baseline and after administration of andexanet according to edoxaban dosage (60 mg once daily vs. 30 mg once daily).
Fig. 1.
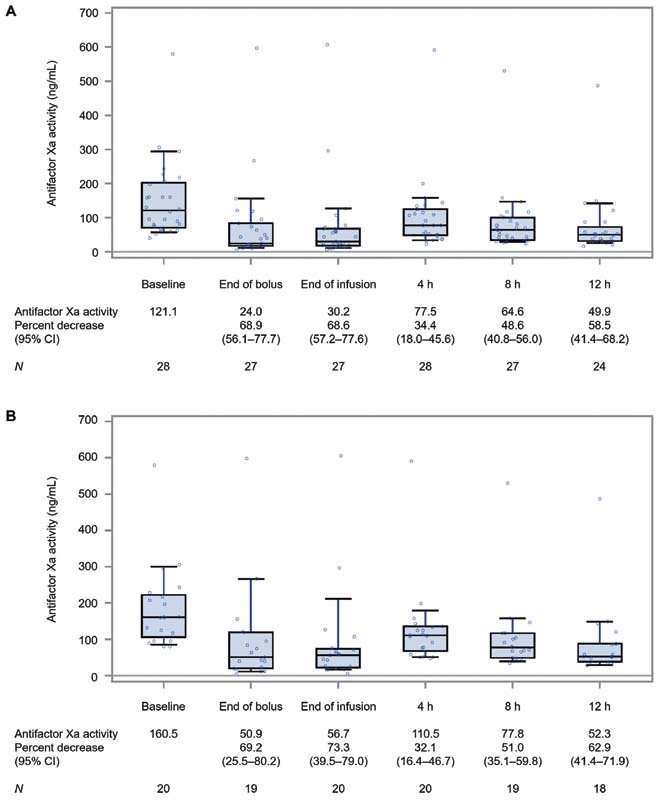
Antifactor Xa activity at baseline and after administration of andexanet. ( A ) Patients on edoxaban with baseline antifactor Xa activity ≥40 ng/mL. ( B ) Patients on edoxaban with baseline antifactor Xa activity ≥75 ng/mL. The median antifactor Xa activity for each time point is marked as a horizontal line within the box. The bottom and top of the box denote the 25th and 75th percentiles, respectively. The lower and upper whiskers indicate the 10th and 90th percentiles, respectively. CI, confidence interval; N , number of patients with data.
In patients with baseline antifactor Xa activity ≥75 ng/mL, the median antifactor Xa activity decreased from 160.5 (IQR: 106.2–222.2) ng/mL at baseline to 50.9 (IQR: 19.9–119.4) ng/mL at the end of andexanet bolus, a median 69.2% (95% CI: 25.5–80.2%) decrease ( Fig. 1B ). At the end of the andexanet follow-on infusion, the median antifactor Xa activity was 56.7 (IQR: 21.8–73.9) ng/mL, a median 73.3% (95% CI: 39.5–79.0%) decrease. The median antifactor Xa activity at the time of the on-treatment nadir was 49.8 (IQR: 18.2–69.4) ng/mL, corresponding to a median 75.6% (95% CI: 57.2–84.6%) decrease. The median reduction in antifactor Xa activity at 4, 8, and 12 hours after andexanet administration was 32.1, 51.0, and 62.9%, respectively.
Hemostatic Efficacy
In patients with baseline antifactor Xa activity ≥40 ng/mL, independently adjudicated hemostasis at 12 hours was rated as excellent in 78.6% (95% CI: 59.0–91.7%) of patients overall and in 81.8% (95% CI: 59.7–94.8%) of patients with intracranial hemorrhage ( Table 2 ). There were no patients with good hemostatic efficacy. Among the 17 patients with intracranial hemorrhage who had intracerebral bleeding, excellent hemostasis was achieved in 76.5% (95% CI: 50.1–93.2%).
Table 2. Effective hemostasis at 12 hours after andexanet administration.
| Patients with baseline antifactor Xa activity ≥40 ng/mL ( N = 28) | Patients with baseline antifactor Xa activity ≥75 ng/mL ( N = 20) | |||||
|---|---|---|---|---|---|---|
| Patients | Excellent or good hemostasis, n (%) | 95% CI (%) | Patients | Excellent or good hemostasis, n (%) | 95% CI (%) | |
| All | 28 | 22 (78.6) | 59.0–91.7 | 20 | 15 (75.0) | 50.9–91.3 |
| Patients with intracranial hemorrhage | 22 | 18 (81.8) | 59.7–94.8 | 16 | 13 (81.3) | 54.4–96.0 |
| Patients with intracerebral bleeding a | 17 | 13 (76.5) | 50.1–93.2 | 12 | 9 (75.0) | 42.8–94.5 |
Abbreviation: CI, confidence interval.
Patients with intracerebral bleeding are a subset of patients with intracranial hemorrhage.
In patients with baseline antifactor Xa activity ≥75 ng/mL, hemostasis at 12 hours was excellent in 75.0% (95% CI: 50.9–91.3%) of patients overall and in 81.3% (95% CI: 54.4–96.0%) of those with intracranial hemorrhage. Among 12 patients with intracerebral bleeding, excellent hemostasis was achieved in 75.0% (95% CI: 42.8–94.5%).
Safety Outcomes
The rates and timing of thrombotic events and mortality after administration of andexanet are shown in Table 3 . Within 30 days, four patients (11.1%) experienced at least one thrombotic event. In total, there were two patients who had an ischemic stroke. One patient suffered a transient ischemic attack, and another patient had both a deep vein thrombosis and a pulmonary embolism. There were no cases of myocardial infarction or non-central nervous system systemic embolism. One of the four patients with a thrombotic event had been restarted on an anticoagulant prior to their first event. A total of four patients, none of whom had experienced a thrombotic event after andexanet treatment, died, corresponding to a 30-day mortality rate of 11.1%. Three of these deaths were classified as cardiovascular.
Table 3. Safety outcomes through 30 days after andexanet administration.
| Safety population ( N = 36) | ||||
|---|---|---|---|---|
| Total | Up to 5 days | Days 6–14 | Days 15–30 | |
| Patients with at least one thrombotic event, n (%) | 4 (11.1) | 2 (5.6) | 1 (2.8) | 1 (2.8) |
| Myocardial infarction, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ischemic stroke, n (%) | 2 (5.6) | 1 (2.8) | 1 (2.8) | 0 (0) |
| Transient ischemic attack, n (%) | 1 (2.8) | 1 (2.8) | 0 (0) | 0 (0) |
| Deep vein thrombosis, a n (%) | 1 (2.8) | 0 (0) | 0 (0) | 1 (2.8) b |
| Pulmonary embolism, a n (%) | 1 (2.8) | 0 (0) | 0 (0) | 1 (2.8) b |
| Systemic embolism, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Death, c n (%) | 4 (11.1) | 2 (5.6) | 1 (2.8) | 1 (2.8) |
| Cardiovascular, n (%) | 3 (8.3) | 1 (2.8) | 1 (2.8) | 1 (2.8) |
| Noncardiovascular, n (%) | 1 (2.8) | 1 (2.8) | 0 (0) | 0 (0) |
Deep vein thrombosis and pulmonary embolism occurred in the same patient.
Patient had been restarted on heparin on day 5, and the deep vein thrombosis/pulmonary embolism occurred on day 15.
None of the patients who died was reported to have experienced a thrombotic event.
Exploratory Analyses
An analysis of hemostatic efficacy according to the HAS-BLED score in patients with atrial fibrillation or flutter with baseline antifactor Xa activity ≥40 ng/mL showed similar results for patients with estimated low and high risk of bleeding ( Supplementary Table S3 [available in the online version]). A safety analysis according to the CHA 2 DS 2 -VASc score in patients with atrial fibrillation or flutter suggested a higher rate of thrombotic events in individuals with higher score categories; all deaths of patients with atrial fibrillation or flutter occurred in those with a CHA 2 DS 2 -VASc score of 5 or greater ( Supplementary Table S4 [available in the online version]). A descriptive analysis of patients with available data showed that the median Glasgow Coma Scale score was 15 before, as well as 1 hour, 12 hours, and 30 days after andexanet treatment ( Supplementary Table S5 [available in the online version]). Finally, baseline characteristics and outcomes of patients who did not achieve excellent or good hemostasis are summarized in Supplementary Table S6 (available in the online version); median baseline antifactor Xa activity was 162.4 (IQR: 80.6–294.4) ng/mL and 208.0 (IQR: 116.8–294.4) ng/mL in patients with baseline antifactor Xa activity of ≥40 ng/mL ( n = 6) and ≥75 ng/mL ( n = 5), respectively. There were no thrombotic events and two deaths in patients who did not achieve effective hemostasis through 30 days after andexanet treatment.
Discussion
Several agents have been developed for rapid and specific reversal of oral anticoagulant therapy in patients experiencing acute bleeding. 5 15 17 Andexanet is the only drug that is approved for specific anticoagulation reversal in patients with uncontrolled bleeding on the direct factor Xa inhibitors rivaroxaban and apixaban. There is, however, limited experience with this agent in patients receiving edoxaban. In this study, we report outcomes of patients receiving andexanet for acute major bleeding on edoxaban, most of whom had intracranial hemorrhage. Andexanet rapidly and significantly reduced antifactor Xa activity, and there was a high percentage of patients with effective hemostasis at 12 hours after andexanet administration. Thirty-day rates of a first thrombotic event and mortality were similar to those previously reported for patients receiving andexanet for bleeding during treatment with other oral factor Xa inhibitors. 5
Data from participants enrolled in the pivotal Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial demonstrate a very strong correlation between trough edoxaban plasma concentration and antifactor Xa activity, which was consistent across different dosing regimens. 10 Further, trough plasma concentrations of edoxaban at 1 month after randomization were associated with the incidence of subsequent major bleeding, with a steep increase in risk for higher edoxaban concentrations. 10 In ENGAGE AF-TIMI 48, rates of intracranial hemorrhage were four and three per 1,000 patient-years during treatment with the high- and low-dose edoxaban regimens, respectively; approximately half (49.2 and 43.9%, respectively) of intracranial hemorrhages occurring on edoxaban were fatal. 6 18 Corresponding rates of major gastrointestinal bleeding were 15 (high-dose regimen) and 9 (low-dose regimen) per 1,000 patient-years, and approximately 10% of these events were considered life-threatening or resulted in death. 6 12 Notably, bleeding rates during treatment with an oral anticoagulant are likely to be higher in an unselected patient population, outside the setting of a randomized clinical trial. If uncontrolled bleeding occurs during treatment with edoxaban or other factor Xa inhibitors, specific reversal of anticoagulant activity is a biologically plausible concept that is aimed at restoring hemostasis. We have previously shown that andexanet led rapidly to a decrease in antifactor Xa activity in patients receiving the drug for acute major bleeding during treatment with rivaroxaban (median: 92%, 95% CI: 88–94% reduction) or apixaban (median: 92%, 95% CI: 91–93% reduction). 5 In this study of patients on edoxaban, there was a median 68.9% (95% CI: 56.1–77.7%) and 69.2% (95% CI: 25.5–80.2%) decrease in antifactor Xa activity at the end of andexanet bolus administration in patients with baseline antifactor Xa activity ≥40 and ≥75 ng/mL, respectively. The reduction in antifactor Xa activity with andexanet appears to be smaller in patients on edoxaban than in patients on rivaroxaban or apixaban. Among the three available oral factor Xa inhibitors, edoxaban has the largest volume of distribution, indicating a drug's tendency to distribute from plasma into other compartments. However, andexanet can only bind, and subsequently sequester, factor Xa inhibitor molecules that are circulating in plasma. One study has shown a lower relative maximum decrease in antifactor Xa activity and a higher molar ratio of andexanet to oral anticoagulant at maximum reversal for individuals treated with edoxaban versus rivaroxaban. 19 This finding has been related to a higher relative redistribution of edoxaban from the extravascular space into plasma and would explain a lower reduction in antifactor Xa activity with andexanet for edoxaban compared with other oral factor Xa inhibitors. 5 Despite the lower median decrease in antifactor Xa activity, the adjudicated percentage of patients with excellent hemostasis at 12 hours after andexanet administration was 78.6% (95% CI: 59.0–91.7%) and 75% (95% CI: 50.9–91.3%), depending on the threshold of baseline antifactor Xa activity for inclusion into the efficacy analysis. For the subset of patients with a qualifying intracranial hemorrhage, the percentage with excellent hemostasis was 81.8% (95% CI: 59.7–94.8%) and 81.3% (95% CI: 54.4–96.0%) for patients with baseline antifactor Xa activity ≥40 and ≥75 ng/mL, respectively. There were no patients with good hemostatic efficacy. These results are consistent with the observed percentage of patients with excellent or good hemostasis receiving rivaroxaban (80%, 95% CI: 72–88%) and apixaban (83%, 95% CI: 77–90%). 5 Similarly, rates of thrombotic events and 30-day mortality after andexanet administration were comparable to those observed in the full ANNEXA-4 cohort (10 and 14%, respectively). 5 Without a control group, it is impossible to know whether the observed thrombotic events were predominantly caused by the underlying disease or associated with andexanet treatment. One out of four patients with a thrombotic event had been restarted on anticoagulation after andexanet treatment. In addition, in two patients, a first event occurred at least 5 days after andexanet treatment, and none of the four patients who died within 30 days was reported to have experienced a thrombotic event after andexanet treatment. Furthermore, there was not a single thrombotic event in any of the preclinical studies of andexanet in healthy volunteers. 4 19
Limitations
Our study has several limitations. First, the number of patients enrolled in this study was small. Thus, the extent of uncertainty around the reported estimates for efficacy and safety of andexanet is greater than what was observed for patients on other oral factor Xa inhibitors, and the results should be interpreted with reasonable caution. Second, we assessed the effect of andexanet on exogenous antifactor Xa activity to evaluate anticoagulation reversal. However, a recent study suggested that endogenous antifactor Xa activity may be a more biologically relevant marker of edoxaban activity. 11 Third, while there was independent adjudication of the type of death (i.e., cardiovascular or noncardiovascular), there was no adjudication of the definite underlying cause. Fourth, our results are based on a prospective, single-arm cohort study without a control group. The ongoing ANNEXA-I trial (ClinicalTrials.gov, unique identifier NCT03661528) is evaluating andexanet against usual care in a randomized clinical trial of patients with intracranial hemorrhage during treatment with an oral factor Xa inhibitor, including edoxaban.
Conclusion
In this prospective, single-arm cohort study in patients with acute bleeding on edoxaban, andexanet significantly decreased antifactor Xa activity. In patients with baseline antifactor Xa activity ≥40 ng/mL, excellent hemostasis at 12 hours was observed in 78.6% of patients overall and in 81.8% of those with intracranial hemorrhage; thrombotic events occurred at a rate expected in such patients. An ongoing randomized clinical trial is evaluating andexanet against usual care in the setting of intracranial hemorrhage during treatment with an oral factor Xa inhibitor, including edoxaban.
Funding Statement
Funding ANNEXA-4 was funded by Portola Pharmaceuticals Inc., South San Francisco, California, United States, now Alexion, AstraZeneca Rare Disease, Boston, Massachusetts, United States, following acquisition by Alexion.
Footnotes
Conflict of Interest A.P.B and L.X. have nothing to report. J.W.E. reports honoraria and/or research grants from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo Inc., Pfizer, and Servier. S.M. reports grant support from Daiichi Sankyo Inc., Bayer, and Aspen Pharma; serving on advisory boards for Bayer, Bristol-Myers Squibb/Pfizer, Boehringer Ingelheim, and Portola; receiving lecture fees from Portola; and serving on an adjudication committee for AbbVie. T.J.M. reports executive/steering committee payments for ANNEXA-I and ENRICH-AF studies from PHRI/McMaster University; consulting income from CSL Behring; and grants from NHLBI and Genentech. M.C. reports serving on a data and safety monitoring board for Bayer; receiving personal funding or serving on advisory boards for Bristol Myers Squibb Canada, CSL Behring, Servier Canada, Asahi Kasei, Precision Biologics, and Hemostasis Reference Laboratory; preparation of educational material and/or presentations and/or moderation of sessions for Pfizer, Alexion, CSL Behring, and Diagnostica Stago; and individual stock ownership for Alnylam. P.Y. reports former employment with Portola Pharmaceuticals Inc., now Alexion, AstraZeneca Rare Disease. P.C. reports former employment with Portola Pharmaceuticals Inc., now Alexion, AstraZeneca Rare Disease. G.L. reports employment with Portola Pharmaceuticals Inc., now Alexion, AstraZeneca Rare Disease. S.J.C. reports institutional research grants and honoraria from Boehringer Ingelheim, Portola/Alexion Pharmaceuticals, Bristol-Myers Squibb/Pfizer, Bayer, and Daiichi Sankyo Inc.
What is known about this topic?
Andexanet alfa (andexanet) is a recombinant decoy molecule approved for specific anticoagulation reversal in patients with life-threatening or uncontrolled bleeding during treatment with the oral factor Xa inhibitors rivaroxaban and apixaban.
There is limited experience with andexanet in patients with acute major bleeding on edoxaban.
What does this paper add?
In this prospective, single-arm cohort study of 36 patients with acute major bleeding on edoxaban, andexanet significantly decreased antifactor Xa activity (median decrease: 68.9% from baseline to the end of andexanet bolus).
Hemostatic efficacy at 12 hours, and 30-day rates of thrombotic events and death in patients treated with edoxaban were similar to the results obtained in patients receiving andexanet for major bleeding on other factor Xa inhibitors.
Supplementary Material
References
- 1.Held C, Hylek E M, Alexander J H. Clinical outcomes and management associated with major bleeding in patients with atrial fibrillation treated with apixaban or warfarin: insights from the ARISTOTLE trial. Eur Heart J. 2015;36(20):1264–1272. doi: 10.1093/eurheartj/ehu463. [DOI] [PubMed] [Google Scholar]
- 2.Ruff C T, Giugliano R P, Braunwald E.Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials Lancet 2014383(9921):955–962. [DOI] [PubMed] [Google Scholar]
- 3.Steiner T, Weitz J I, Veltkamp R. Anticoagulant-associated intracranial hemorrhage in the era of reversal agents. Stroke. 2017;48(05):1432–1437. doi: 10.1161/STROKEAHA.116.013343. [DOI] [PubMed] [Google Scholar]
- 4.Siegal D M, Curnutte J T, Connolly S J. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373(25):2413–2424. doi: 10.1056/NEJMoa1510991. [DOI] [PubMed] [Google Scholar]
- 5.ANNEXA-4 Investigators . Connolly S J, Crowther M, Eikelboom J W. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326–1335. doi: 10.1056/NEJMoa1814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ENGAGE AF-TIMI 48 Investigators . Giugliano R P, Ruff C T, Braunwald E. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 7.Hokusai-VTE Investigators . Büller H R, Décousus H, Grosso M A. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369(15):1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 8.Weitz J I, Connolly S J, Patel I. Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost. 2010;104(03):633–641. doi: 10.1160/TH10-01-0066. [DOI] [PubMed] [Google Scholar]
- 9.Salazar D E, Mendell J, Kastrissios H. Modelling and simulation of edoxaban exposure and response relationships in patients with atrial fibrillation. Thromb Haemost. 2012;107(05):925–936. doi: 10.1160/TH11-08-0566. [DOI] [PubMed] [Google Scholar]
- 10.Ruff C T, Giugliano R P, Braunwald E.Association between edoxaban dose, concentration, anti-Factor Xa activity, and outcomes: an analysis of data from the randomised, double-blind ENGAGE AF-TIMI 48 trial Lancet 2015385(9984):2288–2295. [DOI] [PubMed] [Google Scholar]
- 11.Yin O QP, Antman E M, Braunwald E. Linking endogenous factor Xa activity, a biologically relevant pharmacodynamic marker, to edoxaban plasma concentrations and clinical outcomes in the ENGAGE AF-TIMI 48 trial. Circulation. 2018;138(18):1963–1973. doi: 10.1161/CIRCULATIONAHA.118.033933. [DOI] [PubMed] [Google Scholar]
- 12.Aisenberg J, Chatterjee-Murphy P, Friedman Flack K. Gastrointestinal bleeding with edoxaban versus warfarin: results from the ENGAGE AF-TIMI 48 trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis In Myocardial Infarction) Circ Cardiovasc Qual Outcomes. 2018;11(05):e003998. doi: 10.1161/CIRCOUTCOMES.117.003998. [DOI] [PubMed] [Google Scholar]
- 13.ANNEXA-4 Investigators . Connolly S J, Milling T J, Jr, Eikelboom J W. Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med. 2016;375(12):1131–1141. doi: 10.1056/NEJMoa1607887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milling T J, Jr, Connolly S J, Conley P B. Andexanet alfa for bleeding with factor Xa inhibitors. Reply. N Engl J Med. 2019;381(02):192–193. doi: 10.1056/NEJMc1906058. [DOI] [PubMed] [Google Scholar]
- 15.Sarode R, Milling T J, Jr, Refaai M A. Efficacy and safety of a 4-factor prothrombin complex concentrate in patients on vitamin K antagonists presenting with major bleeding: a randomized, plasma-controlled, phase IIIb study. Circulation. 2013;128(11):1234–1243. doi: 10.1161/CIRCULATIONAHA.113.002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clopper C J, Pearson E S. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26(04):404–413. [Google Scholar]
- 17.Pollack C V, Jr, Reilly P A, van Ryn J. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(05):431–441. doi: 10.1056/NEJMoa1707278. [DOI] [PubMed] [Google Scholar]
- 18.ENGAGE AF-TIMI 48 Steering Committee and Investigators . Nelson S E, Giugliano R P, Antman E M. Intracranial hemorrhage in patients with atrial fibrillation receiving anticoagulation with warfarin or edoxaban: An in-depth analysis from the ENGAGE AF-TIMI 48 randomized trial. J Clin Neurosci. 2021;86:294–300. doi: 10.1016/j.jocn.2020.10.036. [DOI] [PubMed] [Google Scholar]
- 19.Lu G, Conley P B, Leeds J M. A phase 2 PK/PD study of andexanet alfa for reversal of rivaroxaban and edoxaban anticoagulation in healthy volunteers. Blood Adv. 2020;4(04):728–739. doi: 10.1182/bloodadvances.2019000885. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Alexion, AstraZeneca Rare Disease will consider requests for disclosure of clinical study participant-level data provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://alexion.com/our-research/research-and-development . Link to data request form: https://alexion.com/contact-alexion/medical-information .


