Abstract
Background
The risk of transfusion transmitted dengue (DENV) is increasingly recognized and poses a risk to blood safety as well as spreading into non-immune communities.
Objectives
To determine dengue serological profile, environmental risk, knowledge, and preventive measures among blood donors in a national blood bank in northern Egypt.
Methods
A total of 500 blood donors were enrolled into this study between June and September 2018. Socio-demographic and medical data were collected using a predesigned questionnaire. Blood samples were screened for anti-DENV IgM, anti-DENV IgG and non-structural protein 1 antigen (DENV-NS1 antigen).
Results
History of past dengue exposure was identified in 10.2% of blood donors. No samples (0.0%) tested positive for anti-DENV IgG, IgM or NS1 antigen. At the time of blood donation, no individuals had any symptoms suggestive of a dengue-related illness. Dengue exposure strongly correlated with travel to the Kingdom of Saudi Arabia (KSA), Sudan and the El-Quseir outbreak area in Egypt. Knowledge of dengue and prevention methods was found to be substantially deficient, and the relatively higher level of knowledge among exposed donors did not translate into appropriate preventative measures.
Conclusions
Our risk assessment shows the impact of travel on DENV exposure and highlights its potential threat to disease spread in Egypt. Dengue awareness programs are urgently needed for effective prevention of transmission.
Keywords: Dengue, Screening, Blood donor, Risk, Knowledge, Practices, Egypt
1. Introduction
Dengue virus (DENV) infection is a major public health problem globally and endemic in more than 100 countries with an estimated 100 million infected cases and 25,000 deaths per year.1, 2, 3, 4, 5 Over the past few decades, DENV transmission has increased significantly around the globe with more than 2.5 billion people at risk of infection.3,5, 6, 7 Notable outbreaks of dengue fever (DF) in Egypt were reported in 1799, 1871, 1928, and 1937.8 Recently, the country has experienced an outbreak in 2015 in the Dayrout district of the Assiut Governorate.9 In 2017, a total of 110 people were confirmed to have DF in the Red Sea city of Quseir and the Qena governorate of Upper Egypt.10 Consequently, Egypt was recognized as having an intermediate probability of dengue infection on the global dengue map.3
Most commonly, DENV transmission occurs from the bite of an infected female Aedes aegypti mosquito (sometimes Aedes albopictus) with humans as the major amplifying host for the virus.4,7 During the viremic phase, dengue can become a blood-borne illness in both symptomatic and asymptomatic individuals.11 It is plausible that climatic change, rapid urbanization, population growth, increased international travel and breakdown of vector control measures have all greatly contributed to the recent disease emergence in Egypt.12 The vector has a known distribution in Egypt7 and the potential role of foreign visitors or Egyptians returning from neighboring African and Southeast Asian countries with known dengue endemicity is of great concern. Some of these visitors may arrive while viremic and, thereby facilitate DENV spread.
The prevention of and response to dengue infection and other arboviruses involve developing and implementing preparedness plans. So far, no dengue prevention and control programs have been put into place on a national scale by the Ministry of Health and Population (MoHP) in Egypt which require community participation. There have been no large-scale studies either to assess the current knowledge, attitudes and behaviors (KAB) about DENV transmission and its prevention conducted in Egypt.
Transfusion- and transplantation-associated dengue cases have been reported, and, although rare, still represent risks to the safety of blood products and transfusions. Asymptomatic blood donors in endemic areas may serve as potential vehicles of transmission which could be a serious source of virus dissemination in the wider community.11,13 The presence of anti-DENV antibodies is a further important cause of concern in transfusion medicine due to their immunogenic potential.14 However, mandatory screening of blood donors for dengue would be expensive in a developing country and should only be implemented after an evaluation of the risks posed by otherwise healthy blood donors. Blood banks in disease-endemic countries rely on verbal questioning to rule out the risk of transfusion-transmitted dengue, although this cannot rule out asymptomatic infection.11
Given the absence of an approved blood screening test for DENV in Egypt and in response to new epidemiological data, we would like to determine the seroprevalence of DENV antibodies among blood donors in a selected blood bank to assess the safety of blood donation services in Egypt. Evidence of exposure among blood donors would be useful for future implementation of immunization and blood screening policies. We have also assessed the KAP related to DENV and explored socio-demographic and environmental determinants of its exposure among blood donors.
Together, the present study could help to give an overview of the key parameters needed for preparedness planning, epidemic detection and emergency response for DENV containment and control tailored to the local context.
2. Methods
2.1. Study settings, design, and population
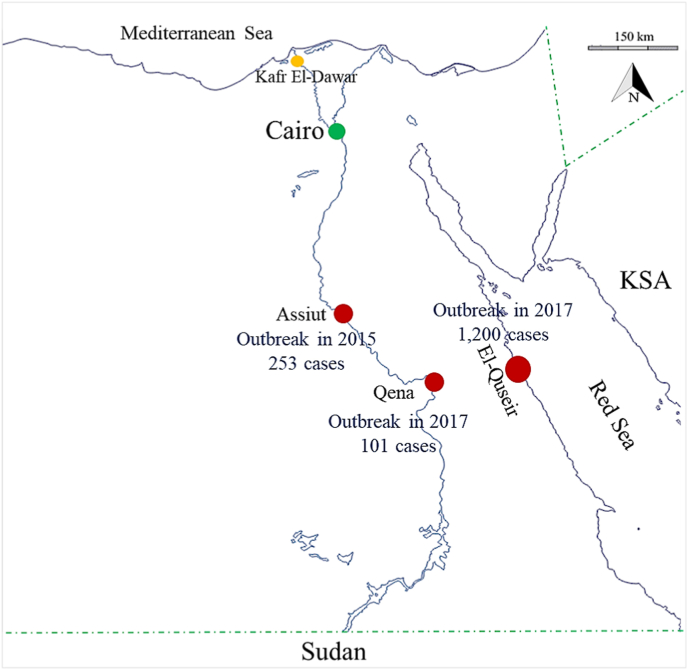
A cross-sectional study was conducted at the blood bank of Kafr El-Dawar General Hospital. Kafr El-Dawar is a rural city in the El-Behira governorate (31.14°N, 30.13°E) (Fig. 1). It has a population density of approximately 1385.8 inhabitants/km.2 Like many agricultural cities in the Nile delta, Kafr El-Dawar has experienced exceedingly rapid urbanization and economic activity.15
Fig. 1.
Map of Egypt showing the city of the study setting (yellow circle), cities that experienced dengue outbreaks in the past few years (red circles), and the capital of Egypt (green circle). Boundaries with dengue endemic neighboring countries are indicated by green dashed lines. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The target population included blood donors attending the selected blood bank for blood donation.
2.2. Sampling
No data regarding the prevalence of DENV virus infection/exposure among blood donors in Egypt is available. However, the prevalence among the general population in some cities in Upper Egypt, where outbreaks of dengue have been previously reported, was found to be 12.09%.16 Using an alpha error = 5% and a precision = 3%, the minimum required sample size was found to be 454, and we eventually enrolled 500 participants. The sample size was calculated using Epi-Info software (version 7). Blood donors who had been accepted for blood donation according to the policy set up by the Egyptian Ministry of Health and Population, and who had agreed to participate in the study were enrolled consecutively until the required sample size was reached.
2.3. Data collection methods and tools
Investigators conducted in-person interviews using a predesigned structured questionnaire form17 (File S1). All serum samples were screened for anti-DENV antibodies [IgM-IgG] and DENV NS1 Ag (non-structural protein 1 antigen), using the Dengue Combo rapid diagnostic test (Biopanda Reagents, UK) to test for dengue viremia, active exposure, or previous exposure. The assay was performed according to the manufacturer's instructions. The status of blood borne viral exposure [hepatitis C (HCV) Ab, hepatitis B (HBV) surface antigen (HBsAg), and HIV Ab] was checked in all tested donors' samples as this is routinely done in all blood banks in Egypt after obtaining participants' consent.
2.4. Case definition
-
-
Dengue exposure is defined by seroreactivity to anti-DENV IgG on a single immunoassay. This can be supported by history and/or a proven medical record of past dengue infection.
-
-
Active dengue infection is defined by seroreactivity to DENV NS1 Ag and/or anti-DENV IgM (with or without anti-DENV IgG seroreactivity).
2.5. Statistical analysis
Collected data was reviewed for accuracy and completeness and analyzed using the Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, Version 20.0, Armonk, NY: IBM Corp. Released 2011).
2.6. Scoring
Environmental risk, any knowledge of dengue and practices for dengue prevention were assessed using open-ended questions. Correct answers were scored 1 point, whereas wrong/“do not know” answers were scored with 0 point. Each outcome was computed as the sum of participant responses and categorized based on the modified Bloom's cut-off point. The total score was qualified as “high” if exceeding 75% of the total score, “average” for scores from 50% to 75% and low for scores below 50%.
3. Results
3.1. Characteristics of the study population
Study subjects were predominantly men (95.4%) with a median age of 32 years (range 18–50), rural residents (79.2%), and of low socioeconomic background (92.2%). Other socio-demographic characteristics are provided in Table 1. Donations were aimed at family members (directed donation).
Table 1.
Sociodemographic characteristics of the study participants.
| Total (n=500) |
History of dengue exposure |
p | ||||||
|---|---|---|---|---|---|---|---|---|
| No (n=449) |
Yes (n=51) |
|||||||
| No. | % | No. | % | No. | % | |||
| Age categories | 18 - <25 | 81 | 16.2 | 79 | 17.6 | 2 | 3.9 | 0.017 |
| 25 - <35 | 274 | 54.8 | 246 | 54.8 | 28 | 54.9 | ||
| 35–50 | 145 | 29.0 | 124 | 27.6 | 21 | 41.2 | ||
| Mean ± SD. | 32.1 ± 7.2 | 31.8 ± 7.3 | 34.3 ± 5.4 | t= −3.0 p= 0.009 | ||||
| Sex | Male | 477 | 95.4 | 426 | 94.9 | 51 | 100.0 | 0.098 |
| Female | 23 | 4.6 | 23 | 5.1 | 0 | 0.0 | ||
| Residence | Urban | 104 | 20.8 | 92 | 20.5 | 12 | 23.5 | 0.612 |
| Rural | 396 | 79.2 | 357 | 79.5 | 39 | 76.5 | ||
| Period of residence | <1 year | 9 | 1.8 | 9 | 2.0 | 0 | 0.0 | 0.593 |
| From 1 to 5 years | 20 | 4.0 | 18 | 4.0 | 2 | 3.9 | ||
| >5 years | 471 | 94.2 | 422 | 94.0 | 49 | 96.1 | ||
| Marital status | Single | 154 | 30.8 | 147 | 32.7 | 7 | 13.7 | 0.037 |
| Married | 342 | 68.4 | 298 | 66.4 | 44 | 86.3 | ||
| Widowed | 3 | 0.6 | 3 | 0.7 | 0 | 0.0 | ||
| Divorced | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | ||
| Education | Illiterate | 27 | 5.4 | 27 | 6.0 | 0 | 0.0 | 0.002 |
| Read and write | 87 | 17.4 | 78 | 17.4 | 9 | 17.6 | ||
| Primary school | 58 | 11.6 | 57 | 12.7 | 1 | 2.0 | ||
| Secondary (high) school | 189 | 37.8 | 158 | 35.2 | 31 | 60.8 | ||
| University education | 139 | 27.8 | 129 | 28.7 | 10 | 19.6 | ||
| Occupation | Unemployed/ not working | 136 | 27.2 | 132 | 29.4 | 4 | 7.8 | <0.001 |
| Farmer/ Agriculture work | 49 | 9.8 | 47 | 10.5 | 2 | 3.9 | ||
| Public sector worker | 39 | 7.8 | 36 | 8.0 | 3 | 5.9 | ||
| Professional job | 59 | 11.8 | 52 | 11.6 | 7 | 13.7 | ||
| Craft work | 109 | 21.8 | 84 | 18.7 | 25 | 49.0 | ||
| Retired | 3 | 0.6 | 3 | 0.7 | 0 | 0.0 | ||
| Student | 62 | 12.4 | 60 | 13.4 | 2 | 3.9 | ||
| Housewife | 3 | 0.6 | 3 | 0.7 | 0 | 0.0 | ||
| Auxiliary worker | 40 | 8.0 | 32 | 7.1 | 8 | 15.7 | ||
| Socioeconomic standarda | Low (<21) | 461 | 92.2 | 414 | 92.2 | 47 | 92.2 | 0.990 |
| Middle (21–31.5) | 39 | 7.8 | 35 | 7.8 | 4 | 7.8 | ||
| Smoking | Never | 96 | 19.2 | 89 | 19.8 | 7 | 13.7 | 0.251 |
| Current smoker | 294 | 58.8 | 263 | 58.6 | 31 | 60.8 | ||
| Ex-smokerb | 16 | 3.2 | 16 | 3.6 | 0 | 0.0 | ||
| Passive smoking | 94 | 18.8 | 81 | 18.0 | 13 | 25.5 | ||
| Smoking other than cigarettes | Never | 448 | 89.6 | 403 | 89.8 | 45 | 88.2 | 0.736 |
| Yes, frequent (water pipe, shisha) | 52 | 10.4 | 46 | 10.2 | 6 | 11.8 | ||
SD: Standard deviation.
No history of alcohol intake or substance abuse was reported.
a: socioeconomic standard was calculated according to a scoring system developed by Fahmy and El-Sherbini.
b: includes smoker if quitted less than 1 year ago.
3.2. Serological profile of DENV infection
History of exposure to dengue was reported by 51 donors (10.2%), whereas anti-DENV IgG, anti-DENV IgM, and DENV NS1 Ag were not detected in any serum samples. At the time of blood donation, none of the donors showed symptoms consistent with the presence of dengue or other viral illness, although 8 (1.6%) and 1 (0.2%) were found seropositive for HCV and HBsAg antibodies, respectively (asymptomatic chronic cases). Likewise, none of the exposed donors had symptoms suggestive of dengue in the 3 months before the current blood donation, although all of them reported having had an episode of DF in the past (2016 to 2018) (Table 2).
Table 2.
Travel and medical history of the study participants.
| Total (n=500) |
History of dengue exposure |
p | ||||||
|---|---|---|---|---|---|---|---|---|
| No (n=449) |
Yes (n=51) |
|||||||
| No. | % | No. | % | No. | % | |||
| Travel | No | 440 | 88.0 | 440 | 98.0 | 0 | 0.0 | <0.001 |
| Yes | 60 | 12.0 | 9 | 2.0 | 51 | 100.0 | ||
| Saudi Arabia | 46 | 9.2 | 9 | 2.0 | 37 | 72.5 | ||
| Sudan | 1 | 0.2 | 0 | 0.0 | 1 | 2.0 | ||
| Red Sea (Egypt)a | 13 | 2.6 | 0 | 0.0 | 13 | 25.5 | ||
| Year of travel | 2016 | 17 | 3.4 | 2 | 22.2 | 15 | 29.4 | |
| 2017 | 29 | 5.8 | 3 | 33.3 | 26 | 51.0 | ||
| 2018 | 14 | 2.8 | 4 | 44.4 | 10 | 19.6 | ||
| History of mosquito bite in last 3 months | No | 216 | 43.2 | 174 | 38.8 | 42 | 82.4 | 0.001 |
| Yes | 284 | 56.8 | 275 | 61.2 | 9 | 17.6 | ||
| History of vaccination in the past year | No | 498 | 99.6 | 447 | 99.6 | 51 | 100.0 | 0.001 |
| Yes | 2 | 0.4 | 2 | 0.4 | 0 | 0.0 | ||
| Clinical symptoms in the past 3 monthsb | Yes | 166 | 33.2 | 160 | 35.6 | 6 | 11.8 | 0.001 |
| Fever | 14 | 2.8 | 13 | 2.9 | 1 | 2.0 | 0.701 | |
| Headache | 150 | 30.0 | 145 | 32.4 | 5 | 9.8 | 0.001 | |
| Skin rash | 3 | 0.6 | 3 | 0.7 | 0 | 0.0 | 0.558 | |
| Joint pain | 5 | 1.0 | 5 | 1.1 | 0 | 0.0 | 0.449 | |
| Fatigue | 20 | 4.0 | 18 | 4.0 | 2 | 3.9 | 0.976 | |
| Dizziness | 2 | 0.4 | 2 | 0.4 | 0 | 0.0 | 0.633 | |
| Myalgia | 19 | 3.8 | 19 | 4.2 | 0 | 0.0 | 0.134 | |
| Vomiting | 7 | 1.4 | 6 | 1.3 | 1 | 2.0 | 0.719 | |
| Nausea | 7 | 1.4 | 6 | 1.3 | 1 | 2.0 | 0.719 | |
| Abd pain | 21 | 4.2 | 20 | 4.5 | 1 | 2.0 | 0.400 | |
| Dark urine | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0.736 | |
| Loss of appetite | 4 | 0.8 | 4 | 0.9 | 0 | 0.0 | 0.499 | |
| Altered consciousness | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0.736 | |
| Serological profile of screened bloodborne viruses | HCV | 8 | 1.6 | 8 | 1.8 | 0 | 0.0 | 0.337 |
| HBV | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0.736 | |
| HIV | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | – | |
a; a city in Egypt lying 500 km far from the city of study setting, and in which outbreaks of dengue fever were reported in 2016 and 2017.
b; No history of bleeding, conjunctival suffusion, chills, rigors, jaundice, eye pain, confusion, photophobia, or blurred vision was reported.
No history of hospitalization, major/minor operations, receiving blood or blood products, injections, Hijama (bloodletting), acupuncture, electrolysis, piercing, tattooing, injury, animal bite, abortion, or contact with blood in past 3 months was reported.
DENV exposure showed no significant association with either sex, residence, or socioeconomic background of donors. However, the increase of DENV seropositivity among potential blood donors, related significantly to travel history, working activity, and level of education (p < 0.05) (Table 1).
3.3. Environmental risk assessment
All exposed donors had a history of travel to dengue-endemic or outbreak areas (p < 0.001). The overall environmental risk score was categorized as average among 64.4% and low among 33.8% of the study participants with no significant difference between exposed and non-exposed donors. Housing conditions that favor insect breeding, particularly house flies and mosquitoes, were reported by most of the study participants (94.4%). The presence of stray animals in residential areas was common as well (94.2%). Conversely, the presence of animals including pets in households was negligible (5.0%).
Latrines predominately of ventilated improved pit (V.I.P) (58.8%) and aqua privy (41.0%) types were available in all households except for two (3.9%) of the exposed donors who reported having a pit latrine.
Wastewater was mainly disposed of through a municipal sewerage system (71.0%) or septic tank (29.0%). Solid waste was disposed of by the municipality (64.2%) and/or burned (45.6%), although most participants (97.0%) reported the presence of litter and garbage heaps in their neighborhood (Table 3) (Fig. 2).
Table 3.
Environmental risk assessment among the study population.
| Total (n=500) |
History of dengue exposure |
p | ||||||
|---|---|---|---|---|---|---|---|---|
| No (n=449) |
Yes (n=51) |
|||||||
| No. | % | No. | % | No. | % | |||
| Travel to dengue endemic area | Yes | 60 | 12.0 | 9 | 2.0 | 51 | 100.0 | <0.001 |
| Shape of the house | Apartment | 484 | 96.8 | 436 | 97.1 | 48 | 94.1 | 0.251 |
| Dwelling | 16 | 3.2 | 13 | 2.9 | 3 | 5.9 | ||
| Type of building material | Red bricks | 500 | 100.0 | 449 | 100.0 | 51 | 100.0 | ND |
| Concrete | 500 | 100.0 | 449 | 100.0 | 51 | 100.0 | ||
| Presence of insects and rodents | No | 3 | 0.6 | 3 | 0.7 | 0 | 0.0 | 0.558 |
| Yes | 497 | 99.4 | 446 | 99.3 | 51 | 100.0 | ||
| House flies | 496 | 99.2 | 445 | 99.1 | 51 | 100.0 | 0.499 | |
| Mosquitos | 495 | 99.0 | 444 | 98.9 | 51 | 100.0 | 0.449 | |
| Creeping insects (ants, cockroaches) | 161 | 32.2 | 153 | 34.1 | 8 | 15.7 | 0.008 | |
| Rodents | 3 | 0.6 | 2 | 0.4 | 1 | 2.0 | 0.184 | |
| Presence of stray animals in residential places | No | 29 | 5.8 | 25 | 5.6 | 4 | 7.8 | 0.510 |
| Yes | 471 | 94.2 | 424 | 94.4 | 47 | 92.2 | ||
| Housing conditions favoring mosquito breeding | No | 3 | 0.6 | 3 | 0.7 | 0 | 0.0 | 0.558 |
| Yes | 497 | 99.4 | 446 | 99.3 | 51 | 100.0 | ||
| Presence of peridomestic water containers | 326 | 65.2 | 286 | 63.7 | 40 | 78.4 | 0.036 | |
| Having plants indoor | 319 | 63.8 | 280 | 62.4 | 39 | 76.5 | 0.047 | |
| Having plants outdoor | 485 | 97.0 | 435 | 96.9 | 50 | 98.0 | 0.646 | |
| Presence of a near-by water canal | 399 | 79.8 | 357 | 79.5 | 42 | 82.4 | 0.632 | |
| Presence of near-by water collection sites | 374 | 74.8 | 331 | 73.7 | 43 | 84.3 | 0.099 | |
| Presence of a near-by chocked sewage/drainage system | 344 | 68.8 | 305 | 67.9 | 39 | 76.5 | 0.211 | |
| Presence of near-by garbage heaps | 483 | 96.6 | 434 | 96.7 | 49 | 96.1 | 0.828 | |
| Availability of latrine in the house | No | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ND |
| Yes | 500 | 100.0 | 449 | 100.0 | 51 | 100.0 | ||
| Pit latrine | 2 | 0.4 | 0 | 0.0 | 2 | 3.9 | <0.001 | |
| V.I.P latrine | 294 | 58.8 | 265 | 59.0 | 29 | 56.9 | 0.767 | |
| Aqua privy | 205 | 41.0 | 184 | 41.0 | 21 | 41.2 | 0.978 | |
| Method of wastewater disposala | Sewerage system | 355 | 71.0 | 310 | 69.0 | 45 | 88.2 | 0.004 |
| Septic tank | 145 | 29.0 | 139 | 31.0 | 6 | 11.8 | 0.004 | |
| Method of solid waste disposal | Scattered | 485 | 97.0 | 434 | 96.7 | 51 | 100.0 | 0.185 |
| Burning | 228 | 45.6 | 214 | 47.7 | 14 | 27.5 | 0.006 | |
| Municipal | 321 | 64.2 | 279 | 62.1 | 42 | 82.4 | 0.004 | |
| Presence of animals in household, including pets | No | 475 | 95.0 | 425 | 94.7 | 50 | 98.0 | 0.134 |
| Yes | 25 | 5.0 | 24 | 5.3 | 1 | 2.0 | ||
| Cat | 3 | 0.6 | 3 | 0.7 | 0 | 0.0 | 0.558 | |
| Dog | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0.736 | |
| Cattle | 23 | 4.6 | 22 | 4.9 | 1 | 2.0 | 0.342 | |
| Donkey | 3 | 0.6 | 3 | 0.7 | 0 | 0.0 | 0.558 | |
| Sheep | 3 | 0.6 | 3 | 0.7 | 0 | 0.0 | 0.558 | |
| Camel | 19 | 3.8 | 19 | 4.2 | 0 | 0.0 | 0.134 | |
| Hoarse | 2 | 0.4 | 2 | 0.4 | 0 | 0.0 | 0.633 | |
| Livestock bransb | None | 478 | 95.6 | 428 | 95.3 | 50 | 98.0 | 0.370 |
| In the backyard | 12 | 2.4 | 11 | 2.4 | 1 | 2.0 | 0.829 | |
| Far from house | 10 | 2.0 | 10 | 2.2 | 0 | 0.0 | 0.282 | |
| Total environmental risk score | 12.5±2.8 | 12.4±2.8 | 13.4±2.3 | t= −1.93 p= 0.057 | ||||
| Low | 169 | 33.8 | 157 | 35.0 | 12 | 23.5 | 0.130 | |
| Average | 322 | 64.4 | 283 | 63.0 | 39 | 76.5 | ||
| High | 9 | 1.8 | 9 | 2.0 | 0 | 0.0 | ||
a; no use of cesspool, trench, or open defecation was reported.
b; brans were not reported to be in the same household, or occupying the ground floor.
Fig. 2.
Photos from the city of the study setting showing local housing and roads, and outdoor environmental conditions favoring mosquito breeding [water canals, flooding, water collection sites, stagnant water ponds, chocked sewage/drainage system, garbage heaps and stray animals in residential areas].
3.4. Knowledge of dengue infection
The level of knowledge among study participants regarding dengue infection and transmission was mainly (94.4%) scored as low, although 52.9% of exposed donors achieved average knowledge scores (p < 0.001). Most exposed respondents were aware of the danger of mosquito bites (62.7%), knew dengue as a disease (90.2%), transmittable by mosquito bites (92.2%), and correctly identified some of the potential indoor and outdoor mosquito breeding sites (p < 0.001). The treating physician was the major source of information on dengue among the exposed participants (96.1%) (p < 0.001), although none of the respondents was aware that dengue could be contracted through blood transfusion (Table 4).
Table 4.
Knowledge of dengue infection among the study participants.
| Total (n=500) |
History of dengue exposure |
p | ||||||
|---|---|---|---|---|---|---|---|---|
| No (n=449) |
Yes (n=51) |
|||||||
| No. | % | No. | d | No. | % | |||
| Awareness of danger of mosquito bite | No | 467 | 93.4 | 448 | 99.8 | 19 | 37.3 | <0.001 |
| Yes | 33 | 6.6 | 1 | 0.2 | 32 | 62.7 | ||
| Knowledge of diseases transmitted through mosquito bit | No | 452 | 90.4 | 448 | 99.8 | 4 | 7.8 | <0.001 |
| Yes | 48 | 9.6 | 1 | 0.2 | 47 | 92.2 | ||
| Malaria | 18 | 3.6 | 0 | 0.0 | 18 | 35.3 | <0.001 | |
| Dengue | 48 | 9.6 | 1 | 0.2 | 47 | 92.2 | <0.001 | |
| Yellow fever | 1 | 0.2 | 0 | 0.0 | 1 | 2.0 | 0.003 | |
| Ever heard about dengue fever | No | 453 | 90.6 | 448 | 99.8 | 5 | 9.8 | <0.001 |
| Yes | 47 | 9.4 | 1 | 0.2 | 46 | 90.2 | ||
| know how dengue is transmitteda | Do not know | 451 | 90.2 | 448 | 99.8 | 3 | 5.9 | <0.001 |
| Mosquito bite | 49 | 9.8 | 1 | 0.2 | 48 | 94.1 | <0.001 | |
| Source of information | NA | 449 | 89.8 | 448 | 99.8 | 1 | 2.0 | <0.001 |
| Neighbors | 5 | 1.0 | 0 | 0.0 | 5 | 9.8 | <0.001 | |
| Treating physician | 50 | 10.0 | 1 | 0.2 | 49 | 96.1 | <0.001 | |
| Friends | 19 | 3.8 | 0 | 0.0 | 19 | 37.3 | <0.001 | |
| Family | 1 | 0.2 | 0 | 0.0 | 1 | 2.0 | 0.003 | |
| Indoor mosquito breeding sites | Do not know | 469 | 93.8 | 447 | 99.6 | 22 | 43.1 | <0.001 |
| Garbage bin | 30 | 6.0 | 2 | 0.4 | 28 | 54.9 | <0.001 | |
| Plant container | 22 | 4.4 | 1 | 0.2 | 21 | 41.2 | <0.001 | |
| Kitchen/bathroom drain free | 3 | 0.6 | 0 | 0.0 | 3 | 5.9 | <0.001 | |
| Un covered water containers | 19 | 3.8 | 0 | 0.0 | 19 | 37.3 | <0.001 | |
| Water in trays under the fridge | 8 | 1.6 | 0 | 0.0 | 8 | 15.7 | <0.001 | |
| Flowerpot trays | 11 | 2.2 | 0 | 0.0 | 11 | 21.6 | <0.001 | |
| Outdoor mosquito breeding sites | Do not know | 470 | 94.0 | 447 | 99.6 | 23 | 45.1 | <0.001 |
| Garbage heaps | 29 | 5.8 | 2 | 0.4 | 27 | 52.9 | <0.001 | |
| Outdoor drains | 2 | 0.4 | 1 | 0.2 | 1 | 2.0 | 0.062 | |
| Floor leaves | 1 | 0.2 | 0 | 0.0 | 1 | 2.0 | 0.003 | |
| Un covered peridomestic water containers | 21 | 4.2 | 1 | 0.2 | 20 | 39.2 | <0.001 | |
| Nearby water canals | 25 | 5.0 | 1 | 0.2 | 24 | 47.1 | <0.001 | |
| Near-by stagnant water ponds | 27 | 5.4 | 0 | 0.0 | 27 | 52.9 | <0.001 | |
| Total knowledge score | 1.0±3.3 | 0.04±0.64 | 9.53±4.82 | t= −14.04 p <0.001 | ||||
| Low | 472 | 94.4 | 448 | 99.8 | 24 | 47.1 | <0.001 | |
| Average | 28 | 5.6 | 1 | 0.2 | 27 | 52.9 | ||
| High | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
NA: not applicable.
ND: not determined.
a; Blood transfusion, unsafe injections, eating unclean vegetables, drinking unclean water, surgery, animal bite, flies, contact with rodents were not selected among responses to possible moods of dengue virus transmission.
3.5. Preventive measures against dengue
All participants showed unsatisfactory practices toward dengue prevention, although exposed donors demonstrated relatively better prevention practices compared to non-exposed ones (p= 0.010). When considering measures taken to prevent mosquito bites during travel, the use of repellents (17.4%) was almost the single action taken. Popular protective methods used in households included window screens (97.8%), repellents and mosquito coils (60.6%), and insecticides (28.6%). Most respondents (97.2%) reported that the authorities did take measures to prevent mosquito breeding, mainly through fogging outside the house, and a few experienced authority inspections of mosquito larvae in their neighborhoods. All participants had access to clean water and most of them stored water at home in refrigerators (99.2%). Responses about preventive practices to reduce indoor/outdoor mosquito breeding and mosquito-human contact included placing all garbage that can accumulate water into closed bins (91.8%), covering water containers in the home (75.4%), avoiding placing any water containers outdoors (75.0%), and removing water from trays under the fridge (15.4%) (Table 5).
Table 5.
Mosquito control measures adopted by the study participants.
| Total (n=500) |
History of engue exposure |
p | ||||||
|---|---|---|---|---|---|---|---|---|
| No (n=449) |
Yes (n=51) |
|||||||
| No. | % | No. | d | No. | % | |||
| Measures taken to prevent mosquito bite while traveling? | No | 412 | 82.4 | 368 | 82.0 | 44 | 86.3 | 0.443 |
| Yes | 88 | 17.6 | 81 | 18.0 | 7 | 13.7 | ||
| Repellents | 87 | 17.4 | 81 | 18.0 | 6 | 11.8 | 0.263 | |
| Mosquito net | 2 | 0.4 | 0 | 0.0 | 2 | 3.9 | <0.001 | |
| Window screens | 1 | 0.2 | 0 | 0.0 | 1 | 2.0 | 0.003 | |
| Stay indoor between dusk and dawn | 1 | 0.2 | 0 | 0.0 | 1 | 2.0 | 0.003 | |
| Wearing long sleeves and pants | 2 | 0.4 | 0 | 0.0 | 2 | 3.9 | <0.001 | |
| Chemoprophylaxis | 1 | 0.2 | 0 | 0.0 | 1 | 2.0 | 0.003 | |
| Measures taken to control mosquito/insects in households | No | 5 | 1.0 | 5 | 1.1 | 0 | 0.0 | 0.449 |
| Yes | 495 | 99.0 | 444 | 98.9 | 51 | 100.0 | ||
| Insecticides | 143 | 28.6 | 127 | 28.3 | 16 | 31.4 | 0.644 | |
| Repellents, coils | 303 | 60.6 | 269 | 59.9 | 34 | 66.7 | 0.349 | |
| Window screens | 489 | 97.8 | 440 | 98.0 | 49 | 96.1 | 0.376 | |
| Sleep under mosquito net | 5 | 1.0 | 1 | 0.2 | 4 | 7.8 | <0.001 | |
| Traps | 2 | 0.4 | 0 | 0.0 | 2 | 3.9 | <0.001 | |
| Measures done by health authority to control mosquitoes | No | 13 | 2.6 | 10 | 2.2 | 3 | 5.9 | 0.120 |
| Yes | 487 | 97.4 | 439 | 97.8 | 48 | 94.1 | ||
| Inspection of mosquito larvae inside the house | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Inspection of mosquito larvae outside the house | 5 | 1.0 | 4 | 0.9 | 1 | 2.0 | 0.467 | |
| Fogging inside the house | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Fogging outside the house | 486 | 97.2 | 438 | 97.6 | 48 | 94.1 | 0.159 | |
| Put larvicidal in potentially breeding sites | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Measures to prevent indoor/outdoor mosquito breeding | No | 39 | 7.8 | 35 | 7.8 | 4 | 7.8 | 0.990 |
| Yes | 461 | 92.2 | 414 | 92.2 | 47 | 92.2 | ||
| Place all garbage that can accumulate water into closed bin | 459 | 91.8 | 413 | 92.0 | 46 | 90.2 | 0.660 | |
| Change water in plant container | 7 | 1.4 | 7 | 1.6 | 0 | 0.0 | 0.369 | |
| Keep drain free from blockage | 7 | 1.4 | 7 | 1.6 | 0 | 0.0 | 0.369 | |
| Cover all water containers | 377 | 75.4 | 333 | 74.2 | 44 | 86.3 | 0.057 | |
| Removing water in trays under the fridge | 77 | 15.4 | 74 | 16.5 | 3 | 5.9 | 0.047 | |
| Remove water from flowerpot trays | 3 | 0.6 | 2 | 0.4 | 1 | 2.0 | 0.184 | |
| Avoid placing any water containers outdoor | 375 | 75.0 | 331 | 73.7 | 44 | 86.3 | 0.050 | |
| Avoid placing any unused tyres, cracked pots outdoor | 27 | 5.4 | 24 | 5.3 | 3 | 5.9 | 0.872 | |
| Eliminating standing water around the house | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Level defective floor surfaces that can collect water, if any | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Adding larvicide in water containers | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| Method of water storage in households | Water tank | 4 | 0.8 | 4 | 0.9 | 0 | 0.0 | 0.499 |
| Zeera | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0.736 | |
| Refrigerator | 496 | 99.2 | 445 | 99.1 | 51 | 100.0 | 0.499 | |
| Keeping water storage containers tightly closed | Yes | 432 | 86.4 | 384 | 85.5 | 48 | 94.1 | 0.090 |
| Total practice score | 9.6±1.8 | 9.6±1.8 | 10.0±1.2 | t= −2.66 p= 0.010 | ||||
| Low | 500 | 100.0 | 449 | 100.0 | 51 | 100.0 | ND | |
| Average | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
| High | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | ||
A: a kind of large water jar made of pottery and used for drinking water storage in rural communities in Egypt.
3.6. Correlation between knowledge, practices, and environmental risk among study participants
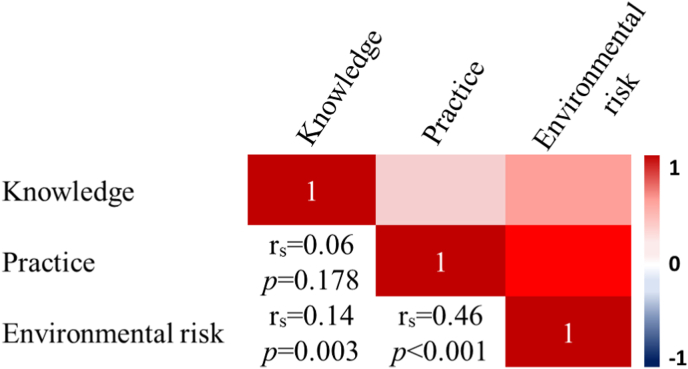
The correlation of knowledge, practices and environmental risk scores overall revealed a significant positive correlation between knowledge and environmental risk (rs = 0.14, p = 0.003) and practice and environmental risk (rs = 0.46, p < 0.001), although the degree of correlation was fair (rs < 0.5). The correlation between knowledge and practice was remarkably low (rs = 0.06, p = 0.178) (Fig. 3).
Fig. 3.
A corrplot visualizing a correlation matrix of participant dengue-related knowledge, practices and environmental risk.
4. Discussion
Blood donors with asymptomatic dengue can contribute to the risk of transfusion-transmitted dengue. The Association for the Advancement of Blood & Biotherapies’ (AABB) Transfusion Transmitted Diseases Committee has categorized dengue as a high priority blood transmissible agent.18 However, transfusion-associated DENV is not recognized as a problem in Egypt given the relative scarcity of the disease. Screening of blood for this pathogen is costly and should be endorsed only after careful disease risk assessment.
Surveillance studies based on blood donors' donations have been used as an alternative strategy to estimate population prevalence by detecting circulating antibodies. Dengue seroprevalence screening has also been widely performed in blood donor samples in several studies worldwide (Table S1). No data on the seroprevalence of DENV among blood donors is available so far in Egypt. In the present study, we have assessed dengue seroprevalence among 500 blood donors in a rural city in northern Egypt. The detection of anti-dengue IgG antibodies in healthy donors is expected since IgG can persist over long periods compared to IgM.19 However, we were not able to detect anti-DENV IgG in the enrolled donors although almost 10% of them reported a history of exposure. This could be attributed to the poor sensitivity of the rapid test used. Indeed, the performance of some commercially available dengue rapid tests regarding real-time quantitative reverse transcription polymerase chain reaction and enzyme-linked immunosorbent assay results is quite low and their sensitivity can range from 40% to 60%.20
Testing of circulating DENV in healthy blood donors is crucial since transmission from blood transfusion is possible. All tested blood samples were seronegative for anti-DENV IgM and DENV-NS1 Ag. This allows to rule out most probably acute dengue infection. Indeed, given the small sample size, it was unlikely to be able to detect an acute/current DENV infection since there was no known dengue outbreak in the region at that time and donors infected in another country would probably have cleared the infection by the time they returned to Egypt and presented to donate blood. This can also reflect the effectiveness of pre-screening questionnaires, physical examination, and body temperature check of blood donors.
Our recorded DENV exposure rate is surprisingly high in a country with only occasional disease occurrence when considering the seroprevalence of dengue antibodies in blood donors of an endemic country/region which can be as high as 26.53%.21 Indeed, 10.2% of our donors had experienced some form of dengue infection several months before the study. Of the exposed donors, 75% had acquired dengue during their stay in KSA and one donor had contracted it in the Sudan, two countries of high disease endemicity. The remaining 25% of the exposed donors reported having had episodes of DF in 2017 during the dengue outbreak in El-Quseir,22 an industrial city in the Red Sea governorate in Egypt. This city has geographical proximity and traffic connections with KSA and the Sudan (Fig. 1), where this group of donors used to relocate for work.
Results from this study may reflect the exposure in adults since the median age of donors was 32. This is, however, expected to rise in numbers along with more adult samples. It is also difficult to correlate this set of data with the overall population because of a lack of samples from younger age groups (children and adolescents).
There might be an epidemiological link between the aforementioned dengue outbreaks in Egypt and endemic DENV infection in KSA. Indeed, Ae. aegypti and Ae. albopictus mosquitoes are both found in Egypt. However, DF is not endemic in the country, and for an outbreak to occur the virus must be introduced by viremic travelers, most probably from neighboring endemic countries. Given the established distribution of vectors in Egypt, there is a potential for dengue onward local transmission that might become established later as an endemic disease.
The lack of serological evidence of active dengue among donors was expected. However, this does not rule out the presence of asymptomatic active carriers who could transmit the virus to prospective recipients. Indeed, DENV-RNA can be detected in asymptomatic blood donors regardless of detectable levels of DENV specific antibodies, particularly in dengue endemic areas.23, 24, 25 Detection of viral RNA may not be achievable for routine large-scale screening in blood banks, particularly in low resource countries. Serological screening for the DENV-NS1 antigen is the alternative test for early diagnosis of asymptomatic DENV viremic donors.26
Detection of anti-DENV IgG in donor blood does not imply virus transmission to the recipients. However, the transmission of this class of antibodies may increase recipient's risk of developing serious forms of the disease, such as dengue hemorrhagic fever (DHF) and/or dengue shock syndrome (DSS)] upon infection with a different viral serotype. These cross-reactive heterotypic non-neutralizing and partially neutralizing antibodies can also enhance viral infectivity through antibody-dependent enhancement, which in turn has a negative impact on host innate immune responses.27, 28, 29 Prospective studies are warranted to assess the significance of this phenomenon in transfused subjects.
Transfusion of exposed blood with dengue specific antibodies may represent an additional threat to high-risk groups. Of concern are infants, young children, pregnant mothers, immunocompromised and chronic disease patients. The Egyptian population is highly burdened by a variety of liver diseases including hepatitis A,B and C as well as bilharziasis and fatty liver.30 Since hepatic dysfunction is a well-recognized feature of dengue,31 the transmission DENV or its cross-reactive antibodies through infected blood may cause serious disease in liver patients.
Dengue is a growing problem worldwide and increasingly reported among international travelers. In the present report, travel or even relocation to another city was strongly correlated with dengue exposure. Travelers returning from dengue endemic countries may offer to donate blood and since DENV can appear in the blood approximately 7 days before the appearance of symptoms,24 such asymptomatic carriers constitute a risk to blood safety. Hence, screening for DENV markers among blood donors with a recent travel history will strengthen blood transfusion safety and control disease dissemination and severity. Alternatives could be the deferral of blood donation for 6 months following return from a dengue endemic region or techniques such as pathogen inactivation.
Implementation of prevention and dengue control strategies require integrated epidemiological information to improve knowledge of factors related to local transmission. We have attempted in this study to address this aspect because the population in Egypt is at equal risk of contracting the infection from mosquito bites. Besides the presence of mosquito vectors, we have traced several environmental factors in the city of our study setting that together with limited vector and disease surveillance could facilitate sustained dengue transmission (Fig. 2). Due to the rapid urbanization of this rural community, there seems to be serious deficiencies in basic infrastructures and municipal services. This has resulted in the accumulation of garbage heaps in residential areas and water stagnation following flooding and stormwater which can turn into breeding ground for mosquitoes.
Despite the occurrence of several dengue outbreaks in Egypt, we have recorded a limited understanding of dengue among blood donors. Inadequate practices in preventing mosquito breeding have reflected this knowledge gap. The relatively higher level of awareness among exposed donors regarding dengue transmission and prevention methods did not, however, translate into effective preventative practices. Indeed, participant practices were not motivated by the awareness of the disease and its prevention but rather by mosquito nuisance. Thus, raising community awareness of dengue should receive more attention and support from health authorities.
Mass media plays a crucial role in conveying health information to the public.32,33 However, we found that the only source of dengue knowledge among study participants came from their health care providers (HCPs). Some studies have identified specific gaps in the knowledge about dengue prevention and management among HCPs that may reflect a lack of training.34,35Research and development of educational strategies to increase knowledge and practices of effective control measures among the general population are highly recommended. Employing mass and social media in this regard might be more effective in disseminating information and stress the community responsibility about its prevention.
In conclusion, potential blood donors can be exposed to DENV, as evidenced by their history of exposure. This warrants the integration of DENV screening into routine blood transfusion testing to ensure blood safety. Large community-based seroprevalence studies are required to estimate the true burden of infection in blood donors and the wider general population. Much remains to be done to fill up the gap in dengue knowledge and prevention practices. The present work constitutes the first step toward a better definition of DENV circulation in the Egyptian community and should help guiding preparedness plans and public health interventions to fight the infection. The present results are also important in terms of the national dengue awareness program in Egypt.
We recognize several limitations to this study including its relatively small sample size from only one blood bank. This might bring into question the inferred prevalence and limit the generalization of our research findings. Most participants were inevitably male because of the low proportion of women who donate blood in Egypt. Our screening approach was also limited by the absence of confirmatory testing. This is crucial particularly when there is a possibility of false-positive/negative results given the poor sensitivity of the rapid diagnostic test used. The use of enzyme-linked immunosorbent assay for antibodies or antigen determination would have been a better choice to test for DENV seroprevalence given its better sensitivity and specificity compared to the rapid test. We should have confirmed the results using real-time polymerase chain reaction as a reference method to eliminate false-negative results.
Ethical consideration
Ethical approval and consent to participate.
The study does not involve any work done on animals.
Conflicts of interest
All authors declare no conflicts of interest.
Consent for publication
All authors approved the manuscript for publication.
Availability of supporting data
All data are fully available without restriction by the corresponding author at ekram.wassim@alexu.edu.eg and through the public data repository http://www.opendatarepository.org/
Funding statement
Author own work. No funding or financial support was received.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We would like to acknowledge the study participants for accepting to participate in the study.
Footnotes
The study was approved by the institutional review board and the ethics committee of the High Institute of Public Health affiliated with Alexandria University, Egypt. We sought the permission and support of the local health authorities to conduct the study in the selected districts in Alexandria. The study was conducted in accordance with the international ethical guidelines and of the Declaration of Helsinki. Informed written consent was obtained from each participant after explaining the aim and concerns of the study. Data sheets were coded by number to ensure anonymity and confidentiality of the participants' data.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jve.2022.100077.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Normile D. Tropical medicine. Surprising new dengue virus throws a spanner in disease control efforts. Science. 2013;342:415. doi: 10.1126/science.342.6157.415. [DOI] [PubMed] [Google Scholar]
- 2.Arima Y., Edelstein Z.R., Han H.K., Matsui T. Epidemiologic update on the dengue situation in the Western Pacific region, 2011. Western Pac Surveill Response J. 2013;4:47–54. doi: 10.5365/WPSAR.2012.3.4.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatt S., Gething P.W., Brady O.J., et al. The global distribution and burden of dengue. Nature. 2013;496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson M.E., Chen L.H. Dengue: update on epidemiology. Curr Infect Dis Rep. 2015;1:457. doi: 10.1007/s11908-014-0457-2. [DOI] [PubMed] [Google Scholar]
- 5.Stanaway J.D., Shepard D.S., Undurraga E.A., et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:712–723. doi: 10.1016/S1473-3099(16)00026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilder-Smith A., Byass P. The elusive global burden of dengue. Lancet Infect Dis. 2016;16:629–631. doi: 10.1016/S1473-3099(16)00076-1. [DOI] [PubMed] [Google Scholar]
- 7.Humphrey J.M., Cleton N.B., Reusken C.B., Glesby M.J., Koopmans M.P., Abu-Raddad L.J. Dengue in the Middle East and north Africa: a systematic review. PLoS Neglected Trop Dis. 2016;10 doi: 10.1371/journal.pntd.0005194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger S. GIDEON: Global Infectious Diseases and Epidemiology Online Network. O'Reilly Media, Inc; 2010. Infectious diseases of Egypt 2010 edition.www.gideononline.com [Google Scholar]
- 9.World Health Organization Dengue fever – Egypt: disease outbreak news. 2015. https://www.who.int/csr/don/12-november-2015-dengue/en/ Available at:
- 10.Abdelkader N.A. Dengue fever. Egypt J Intern Med. 2018;30:47–48. [Google Scholar]
- 11.Tomashek K.M., Margolis H.S. Dengue: a potential transfusion-transmitted disease. Transfus. 2011;51:1654–1660. doi: 10.1111/j.1537-2995.2011.03269.x. [DOI] [PubMed] [Google Scholar]
- 12.Murray N.E., Quam M.B., Wilder-Smith A. Epidemiology of dengue: past, present and future prospects. Clin Epidemiol. 2013;5:299–309. doi: 10.2147/CLEP.S34440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oh H.B., Muthu V., Daruwalla Z.J., Lee S.Y., Koay E.S., Tambyah P.A. Bitten by a bug or a bag? Transfusion-transmitted dengue: a rare complication in the bleeding surgical patient. Transfus. 2015;55:1655–1661. doi: 10.1111/trf.13054. [DOI] [PubMed] [Google Scholar]
- 14.Ribas-Silva R.C., Eid A.A. Dengue antibodies in blood donors. Rev Bras Hematol Hemoter. 2012;34:193–195. doi: 10.5581/1516-8484.20120048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Julia Z., Andrey K. Urbanization dynamics in Egypt: factors, trends, perspectives. Arab Stud Q. 2013;35:20–38. [Google Scholar]
- 16.Hussen M.O., Sayed A.S.M., Abushahba M.F.N. Sero-epidemiological study on Dengue fever virus in humans and camels at Upper Egypt. Vet World. 2020;13:2618–2624. doi: 10.14202/vetworld.2020.2618-2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahmy S.I., Nofal L.M., Shehata S.F., El Kady H.M., Ibrahim H.K. Updating indicators for scaling the socioeconomic level of families for health research. J Egypt Publ Health Assoc. 2015;90:1–7. doi: 10.1097/01.EPX.0000461924.05829.93. [DOI] [PubMed] [Google Scholar]
- 18.Stramer S.L., Hollinger F.B., Katz L.M., et al. Emerging infectious disease agents and their potential threat to transfusion safety. Transfus. 2009;49(Suppl 2):1S–29S. doi: 10.1111/j.1537-2995.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 19.Eick S.M., Dale A.P., McKay B., et al. Seroprevalence of dengue and Zika virus in blood donations: a systematic review. Transfus Med Rev. 2019;33:35–42. doi: 10.1016/j.tmrv.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Jang W.S., Kwak S.Y., May W.L., Yang D.J., Nam J., Lim C.S. Comparative evaluation of three dengue duo rapid test kits to detect NS1, IgM, and IgG associated with acute dengue in children in Myanmar. PLoS One. 2019;14 doi: 10.1371/journal.pone.0213451. e0213451-e0213451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L., Li Y., Lu S., et al. Epidemiological survey and screening strategy for dengue virus in blood donors from Yunnan Province. BMC Infect Dis. 2021;21:104. doi: 10.1186/s12879-021-05810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abozeid S., Elsayed A.K., Schaffner F., Samy A.M. Re-emergence of Aedes aegypti in Egypt. Lancet Infect Dis. 2018;18:142–143. doi: 10.1016/S1473-3099(18)30018-5. [DOI] [PubMed] [Google Scholar]
- 23.Linnen J.M., Vinelli E., Sabino E.C., et al. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfus. 2008;48:1355–1362. doi: 10.1111/j.1537-2995.2008.01772.x. [DOI] [PubMed] [Google Scholar]
- 24.Dias L.L., Amarilla A.A., Poloni T.R., Covas D.T., Aquino V.H., Figueiredo L.T. Detection of dengue virus in sera of Brazilian blood donors. Transfus. 2012;52:1667–1671. doi: 10.1111/j.1537-2995.2012.03729.x. [DOI] [PubMed] [Google Scholar]
- 25.Lanteri M.C., Busch M.P. Dengue in the context of "safe blood" and global epidemiology: to screen or not to screen? Transfus. 2012;52:1634–1639. doi: 10.1111/j.1537-2995.2012.03747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pok K.Y., Lai Y.L., Sng J., Ng L.C. Evaluation of nonstructural 1 antigen assays for the diagnosis and surveillance of dengue in Singapore. Vector Borne Zoonotic Dis. 2010;10:1009–1016. doi: 10.1089/vbz.2008.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dejnirattisai W., Jumnainsong A., Onsirisakul N., et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–748. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modhiran N., Kalayanarooj S., Ubol S. Subversion of innate defenses by the interplay between DENV and pre-existing enhancing antibodies: TLRs signaling collapse. PLoS Neglected Trop Dis. 2010;4:e924. doi: 10.1371/journal.pntd.0000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murphy B.R., Whitehead S.S. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 30.Alboraie M., Youssef N., Sherief A.F., et al. Egyptian liver library: an indexed database for liver disease evidence in Egypt. Arab J Gastroenterol. 2019;20:109–113. doi: 10.1016/j.ajg.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Samanta J., Sharma V. Dengue and its effects on liver. World J Clin Cases. 2015;3:125–131. doi: 10.12998/wjcc.v3.i2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hairi F., Ong C.H., Suhaimi A., et al. A knowledge, attitude and practices (KAP) study on dengue among selected rural communities in the Kuala Kangsar district. Asia Pac J Publ Health. 2003;15:37–43. doi: 10.1177/101053950301500107. [DOI] [PubMed] [Google Scholar]
- 33.Nalongsack S., Yoshida Y., Morita S., Sosouphanh K., Sakamoto J. Knowledge, attitude and practice regarding dengue among people in Pakse, Laos. Nagoya J Med Sci. 2009;71:29–37. [PMC free article] [PubMed] [Google Scholar]
- 34.Ho T.S., Huang M.C., Wang S.M., Hsu H.C., Liu C.C. Knowledge, attitude, and practice of dengue disease among healthcare professionals in southern Taiwan. J Formos Med Assoc. 2013;112:18–23. doi: 10.1016/j.jfma.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Mohammed Yusuf A., Abdurashid Ibrahim N. Knowledge, attitude and practice towards dengue fever prevention and associated factors among public health sector health-care professionals: in Dire Dawa, eastern Ethiopia. Risk Manag Healthc Pol. 2019;12:91–104. doi: 10.2147/RMHP.S195214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are fully available without restriction by the corresponding author at ekram.wassim@alexu.edu.eg and through the public data repository http://www.opendatarepository.org/