Abstract
This experimental study compared leak pressures and completion time of intestinal anastomoses performed by novice veterinarians and a Board-certified surgeon using simple interrupted and simple continuous suture patterns. Grossly normal jejunal segments (n = 108) from 6 fresh canine cadavers were used to harvest 8-cm cooled canine cadaveric jejunal segments that were randomly assigned to a control group (12 segments) and 4 treatment groups (24 segments/group, 12 constructs/group): i) simple interrupted anastomoses performed by a Board-certified surgeon (BSI); ii) simple continuous anastomoses performed by a Board-certified surgeon (BSC); iii) simple interrupted anastomoses performed by novice veterinarians (NSI); and iv) simple continuous anastomoses performed by novice veterinarians (NSC). Median (range) initial leak pressure (ILP) for control was 400.2 mmHg (226.0 to 500.0 mmHg), BSI 37.4 (14.4 to 124.0), BSC 32.5 (13.4 to 91.0), NSI 36.5 (22.9 to 62.0), and NSC 47.5 (8.9 to 120.0). No difference was noted between experience (P = 0.73, P = 0.53), suture technique (P = 0.07, P = 0.38), or across treatment groups (P = 0.17, P = 0.94), for ILP or MIP (maximum intraluminal pressure), respectively. Time to construct completion differed based on suture technique (P < 0.0001) and experience (P < 0.0001). The median and mean ILP of all anastomoses exceeded physiologic intraluminal peristaltic pressures. Simple continuous anastomoses were faster to complete overall. Both handsewn anastomosis techniques are appropriate for intestinal anastomoses.
Résumé
Cette étude expérimentale a comparé les pressions de fuite et le temps de complétion d’anastomoses intestinales réalisées par des vétérinaires novices et un chirurgien certifié comme spécialiste en utilisant des schémas de suture simples interrompus et continus simples. Des segments jéjunaux grossièrement normaux (n = 108) de six cadavres canins frais ont été utilisés pour prélever des segments jéjunaux cadavériques canins refroidis de 8 cm qui ont été assignés au hasard à un groupe témoin (12 segments) et à quatre groupes de traitement (24 segments/groupe, 12 constructions/groupe) : i) anastomoses simples interrompues réalisées par un chirurgien agréé par le Board (BSI); ii) des anastomoses continues simples réalisées par un chirurgien certifié par le Board (BSC); iii) les anastomoses simples interrompues réalisées par des vétérinaires novices (NSI); et iv) des anastomoses continues simples réalisées par des vétérinaires novices (NSC). La pression de fuite initiale médiane (plage) pour le témoin était de 400,2 mmHg (226,0 à 500,0 mmHg), BSI 37,4 (14,4 à 124,0), BSC 32,5 (13,4 à 91,0), NSI 36,5 (22,9 à 62,0) et NSC 47,5 (8,9 à 120,0). Aucune différence n’a été notée entre l’expérience (P = 0,73, P = 0,53), la technique de suture (P = 0,07, P = 0,38) ou entre les groupes de traitement (P = 0,17, P = 0,94), pour l’ILP ou la MIP (pression intraluminale maximale), respectivement. Le temps de complétion de l’assemblage différait en fonction de la technique de suture (P < 0,0001) et de l’expérience (P < 0,0001). L’ILP médian et moyen de toutes les anastomoses dépassait les pressions péristaltiques intraluminales physiologiques. Les anastomoses continues simples étaient globalement plus rapides à réaliser. Les deux techniques d’anastomose cousues à la main conviennent aux anastomoses intestinales.
(Traduit par Docteur Serge Messier)
Introduction
Intestinal resection and anastomoses are commonly performed to remove unhealthy or non-viable intestinal tissue for a multitude of indications including neoplasia, intestinal perforation, and gastrointestinal obstruction (1–6). An array of anastomotic techniques have been described, each of which fall under one of two main categories: handsewn or stapled anastomoses. Although the dehiscence rate for small intestinal anastomoses has been reported to be as high as 28%, the dehiscence rate between handsewn and stapled anastomoses has not been reported to differ in patients without evidence of septic peritonitis (1–8). Despite several benefits of stapled anastomotic techniques (7–9), handsewn techniques remain popular, likely due to ready availability of materials, substantial cost differences, and applicability in patients of varying sizes compared with stapling devices.
Handsewn anastomoses are most often performed using an appositional pattern, including simple interrupted, simple continuous, and modified Gambee techniques, in an effort to minimize mucosal eversion. Mucosal eversion, which occurs more commonly when using simple interrupted than continuous techniques, is associated with increased inflammation, delayed healing, and increased potential for adhesion formation (10,11). Contrarily, a modified Gambee technique minimizes mucosal eversion by excluding the mucosa, resulting in improved approximation of the submucosal layer. Presumptively, the improved apposition may be responsible for the greater leak pressure reported for the modified Gambee pattern when compared to the other appositional suture patterns for small intestinal enterotomies (12,13). However, the clinical significance of the greater leak pressure is assumed to be minimal given that all appositional patterns leaked at pressures that exceeded maximum physiologic peristaltic pressures (15 to 25 mmHg). Since the modified Gambee takes significantly longer to perform, simple interrupted and simple continuous patterns remain popular techniques (9).
To the authors’ knowledge, direct comparisons between experience and two different handsewn anastomotic techniques (interrupted and continuous) using leak pressure testing has not previously been reported. Leak pressures for simple continuous handsewn anastomoses have previously been reported, and this handsewn technique is considered a viable option when performing intestinal anastomoses, with mean leak pressures consistently above maximum physiologic intraluminal pressure (14,15). The objective of this study was to compare leak pressures and completion time of intestinal anastomoses performed by novice veterinarians and a Board-certified surgeon using simple interrupted and simple continuous suture patterns. We hypothesized that simple interrupted anastomoses would have higher leak pressures compared to simple continuous techniques performed by novice veterinarians. Our second hypothesis was that there would be no difference in leak pressures for a Board-certified surgeon regardless of technique. Our third hypothesis was that simple continuous anastomoses would result in a shorter surgical time than simple interrupted anastomoses, regardless of experience. Lastly, we hypothesized that a Board-certified surgeon would have higher leak pressures than a novice veterinarian.
Materials and methods
Specimens
This study was approved by the University of Florida Institutional Animal Care and Use Committee and used methods similar to previous studies (14,15). Grossly normal small intestinal tissue was obtained from 6 middle-aged canine cadavers weighing between 15 and 30 kg. All dogs were euthanized with intravenous pentobarbital-phenytoin sodium for reasons unrelated to this study and subsequently obtained from a local shelter. Within 6 h of euthanasia, the jejunum was harvested from each cadaver, flushed with isotonic saline (0.9% NaCl solution), and stored at 4°C for less than 24 h prior to construct completion and leak pressure testing.
In total, 108, 8-cm long jejunal segments were harvested from the 6 cadavers, with each cadaver contributing 18 segments that were randomly assigned to a control group (n = 2 segments) and 4 treatment groups (n = 4 segments/group):
i) simple interrupted anastomoses performed by a Board-certified surgeon (BSI);
ii) simple continuous anastomoses performed by a Board-certified surgeon (BSC);
iii) simple interrupted anastomoses performed by novice veterinarians (NSI); and
iv) simple continuous anastomoses performed by novice veterinarians (NSC).
For each treatment group, 2 segments were used to assemble a single anastomotic construct. Thus, each treatment group contained 2 anastomotic constructs per cadaver, giving rise to a total of 12 anastomotic constructs per treatment group.
All anastomoses in the BSI and BSC groups were performed by a single Board-certified veterinary surgeon (P.J.R.) with 8 y post-graduate experience. Anastomoses for the NSI and NSC groups were performed by 2 novice veterinarians (C.M.F., K.M.M.). Novice veterinarians were defined as veterinarians who had performed no more than 2 resection and anastomoses in clinical patients. Novice veterinarians included a surgery specialty intern with 1 y post-graduate experience and a small animal surgical resident with 2 y post-graduate experience and < 6 mo of clinical residency training.
Control segments
Control segments were used to assess the integrity of the gastrointestinal segments. No incisions were created, and no suture was placed in control segments prior to leak pressure testing.
Simple continuous handsewn anastomosis
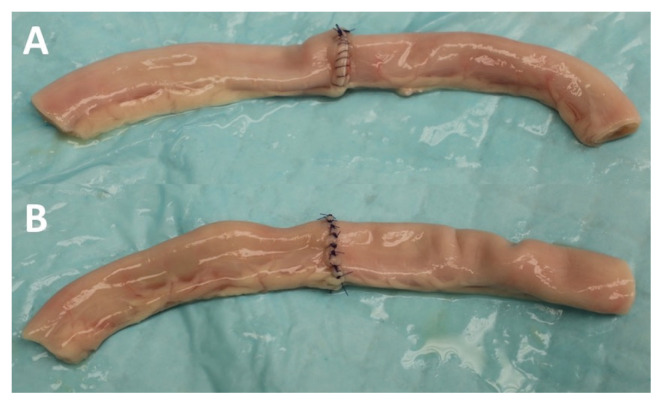
Simple continuous handsewn anastomoses (Figure 1 A) were performed according to a standard surgical technique with 4-0 glycomer 631 (Medtronic, Minneapolis, Minnesota, USA) swaged onto a CV-23 half-circle, 17-mm tapered needle (10). One assistant (M.W.) held the bowel via digital manipulation during construct completion. Two separate simple continuous suture lines were initiated 180° to each other and secured with 4 square throws. One suture line began at the mesenteric border and the second at the antimesenteric border. Full-thickness suture bites were positioned 2 to 3 mm apart and 2 to 3 mm from the anastomotic edge. The body of the needle was used to eliminate eversion of the mucosa. Non-crushing tension was applied on the suture line which was secured with 4 square throws when the last loop was placed.
Figure 1.
Images of the handsewn anastomosis constructs. A — Simple continuous anastomosis. B — Simple interrupted anastomosis.
Simple interrupted handsewn anastomosis
Simple interrupted handsewn anastomoses (Figure 1 B) were performed following the same standard surgical technique stated (10). One simple interrupted suture was placed at the mesenteric border while the second was placed 180° opposite to the first at the antimesenteric border. Full-thickness simple interrupted sutures were placed 2 to 3 mm apart and 2 to 3 mm from the anastomotic edge between the 2 initiating sutures until construct completion. Application of non-crushing tension was applied as each suture was tied with 4 square throws, leaving suture tags approximately 3 mm in length.
Leak pressure testing
Within 2 h of construct completion, controls and anastomotic constructs were leak-pressure tested using a previously described method (14–16). Rochester-Carmalt forceps were used to occlude the jejunal segment ends; adjacent to the forceps, two 18-gauge, 1.25-inch, IV catheters were introduced into the lumen of the jejunum. Constructs were positioned on a platform with a white pad placed underneath to monitor for evidence of gross dye leakage (Figure 2). A pressure transducer, transducer amplifier, and pressure monitoring system (ADInstruments, Sydney, Australia) were connected to one of the catheters. The pressure transducer was calibrated before each use via a sphygmomanometer (Welch Allyn, Skaneateles Falls, New York, USA) and situated level with the construct. A 0.5-L bag of 0.9% NaCl dyed with methylene blue (dilution, 1:500) to enhance visualization of leakage was connected to the second catheter via an IV fluid line. Air was evacuated from the pressure tubing and IV lines with 0.9% NaCl solution prior to attachment to the catheters.
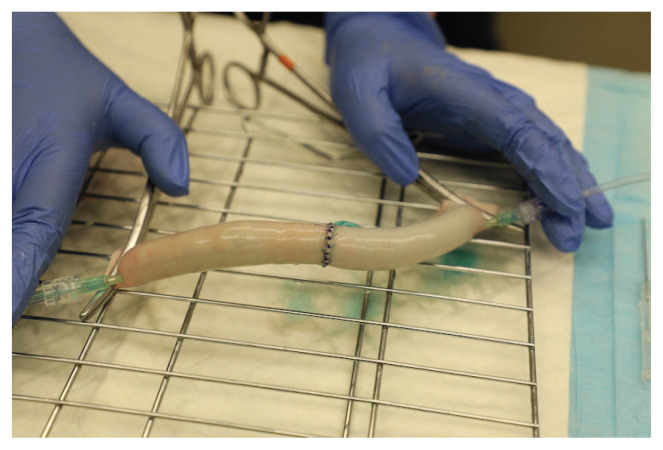
Figure 2.
Image of the leak-testing design with a simple interrupted anastomosis construct. Rochester-Carmalt forceps occlude the segment on both ends and 2 IV catheters are inserted into the intestinal lumen. The catheter on the left is attached to a syringe pump for infusion with 0.9% NaCl solution diluted with methylene blue. The catheter on the right side is attached to the pressure transducer.
The pressure monitoring system recorded intraluminal pressure continuously during infusion of the segments at a rate of 999 mL/h via an IV infusion pump (Heska, Loveland, Colorado, USA). Control segments were tested until maximum sensor pressure was reached (> 500 mmHg), serosal tearing was noted, or gross leakage around the infusion or pressure catheter was observed. For all anastomotic constructs, one investigator (M.W.) reported when gross dye leakage was initially visualized while a second investigator (C.M.F) recorded the pressure and location of leakage. This was defined as the initial leak pressure (ILP). Infusion was continued until the maximum intraluminal pressure (MIP) was reached, defined as the pressure at which the intraluminal pressure plateaued for 5 s, the maximum sensor pressure was reached (> 500 mmHg), or an abrupt decline occurred due to catastrophic failure of the anastomotic construct.
Statistical analysis
Based on a pre-study power analysis, it was concluded that a sample size of ≥ 5 constructs per group was necessary to detect appreciable differences with a power of 90% and α = 0.05. The distribution of ILP, MIP, and time to construct completion of each anastomosis was assessed with descriptive statistics. A linear mixed model was used to test for difference between the handsewn technique used, ILP, MIP, experience, and construct completion time. A mixed model was used to account for correlations in results reported from the same cadaver. Statistical significance was set at P ≤ 0.05. All statistical analyses were performed in SAS JMP (SAS Institute, Cary, North Carolina, USA).
Results
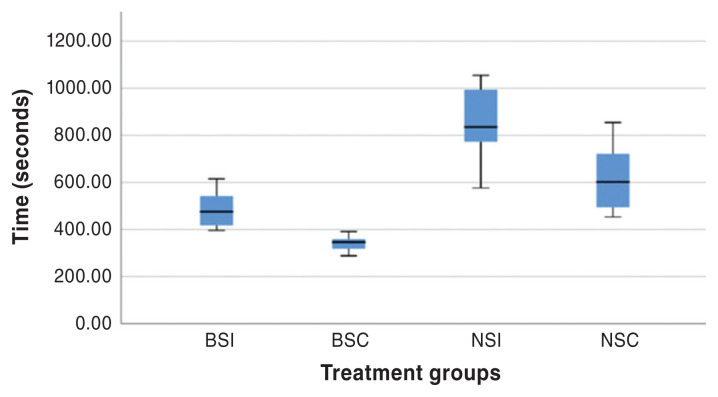
At the time of harvest, all cadaveric intestinal segments were grossly normal. The median (range) and mean ± SD ILP, MIP, and time to construct completion for all controls and anastomosis constructs are presented in Tables 1 and 2, respectively. The distribution of ILP, MIP, and time to construct completion for all treatment groups are presented in Figures 3, 4, and 5, respectively.
Table 1.
All measured variables for controls and 4 treatment groups.
| Construct | Time to construct completion (seconds) | ILP (mmHg) | MIP (mmHg) |
|---|---|---|---|
| Control | … | 400.2 (226.0–500.0) | 417.7 (230.0–500.0) |
| BSI | 475.5 (396.0–615.0) | 37.4 (14.4–124.0) | 98.5 (60.0–313.0) |
| BSC | 346.0 (288.0–391.0) | 32.5 (13.4–91.0) | 94.7 (42.0–184.0) |
| NSI | 835.0 (576.0–1055.0) | 36.5 (22.9–62.0) | 114.0 (57.0–195.0) |
| NSC | 601.5 (453.0–854.0) | 47.5 (8.9–120.0) | 128.0 (20.0–230.0) |
Note: Values are median (range).
… — Not applicable; BSI — Board-certified surgeon simple interrupted anastomoses; BSC — Board-certified surgeon simple continuous anastomoses; NSI — Novice veterinarians simple interrupted anastomoses; NSC — Novice veterinarians simple continuous anastomoses.
Table 2.
All measured variables for controls and 4 treatment groups.
| Construct | Time to construct completion (seconds) | ILP (mmHg) | MIP (mmHg) |
|---|---|---|---|
| Control | … | 388.3 ± 86.0 | 425.8 ± 83.4 |
| BSI | 481.4 ± 73.8 | 52.3 ± 32.2 | 126.4 ± 70.0 |
| BSC | 342.4 ± 30.2 | 42.3 ± 24.0 | 106.1 ± 46.5 |
| NSI | 857.9 ± 142.8 | 37.9 ± 11.4 | 116.7 ± 38.6 |
| NSC | 623.5 ± 135.6 | 57.2 ± 37.4 | 128.5 ± 53.7 |
Note: Values are mean ± SD.
… — Not applicable; BSI — Board-certified surgeon simple interrupted anastomoses; BSC — Board-certified surgeon simple continuous anastomoses; NSI — Novice veterinarians simple interrupted anastomoses; NSC — Novice veterinarians simple continuous anastomoses.
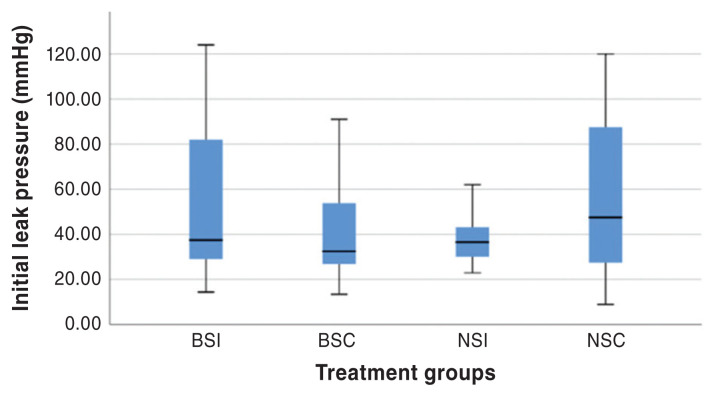
Figure 3.
Boxplot of ILP for all constructs. Each box represents the 25th to 75th percentiles, the horizontal line represents the median, and the whiskers represent the range. BSI — Board-certified surgeon simple interrupted anastomoses; BSC — Board-certified surgeon simple continuous anastomoses; NSI — Novice veterinarians simple interrupted anastomoses; NSC — Novice veterinarians simple continuous anastomoses.
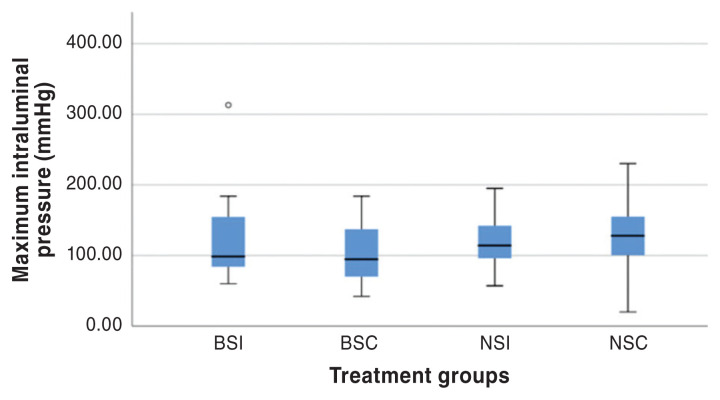
Figure 4.
Boxplot of MIP for all constructs. Each box represents the 25th to 75th percentiles, the horizontal line represents the median, and the whiskers represent the range. The circle identifies a potential outlier. BSI — Board-certified surgeon simple interrupted anastomoses; BSC — Board-certified surgeon simple continuous anastomoses; NSI — Novice veterinarians simple interrupted anastomoses; NSC — Novice veterinarians simple continuous anastomoses.
Figure 5.
Boxplot of time to construction for all constructs. Each box represents the 25th to 75th percentiles, the horizontal line represents the median, and the whiskers represent the range. BSI — Board-certified surgeon simple interrupted anastomoses; BSC — Board-certified surgeon simple continuous anastomoses; NSI — Novice veterinarians simple interrupted anastomoses; NSC — Novice veterinarians simple continuous anastomoses.
When evaluating ILP, there was no difference between experience (P = 0.73), suture technique (P = 0.07), or interactive effects between experience and suture technique (P = 0.17). Similarly, when evaluating MIP, there was no difference between experience (P = 0.53), suture technique (P = 0.38), or interactive effects between experience and suture technique (P = 0.94). The time to completion of constructs differed between treatment groups (P < 0.0001), with simple interrupted anastomoses taking the longest time to perform. Anastomoses performed by a Board-certified surgeon result in faster surgical times (P < 0.0001) than those performed by novice veterinarians, regardless of the suture technique utilized. Simple interrupted anastomoses performed by novice veterinarians took significantly longer.
All anastomotic constructs demonstrated initial leakage at the holes made by the needle during suture bites, regardless of the suture pattern or experience. For control segments, 2 of 12 reached the maximum sensor pressure (> 500 mmHg) without any gross evidence of leakage, and the remaining ten segments exhibited serosal tearing leading to leakage.
Discussion
This study used a previously validated ex-vivo canine model (14–17) to compare leak pressures, construct completion time, and experience when performing 2 handsewn intestinal anastomosis techniques. The first hypothesis was rejected as there was no difference in ILP or MIP between simple interrupted and simple continuous anastomosis techniques for novice veterinarians. In support of our second and third hypotheses, leak pressures did not differ for the Board-certified surgeon, regardless of technique, and simple continuous anastomoses consistently resulted in a faster anastomosis, regardless of experience. The fourth hypothesis was rejected as there was not a statistically significant difference when comparing leak pressures between different experience levels.
In the present study, there was no difference in ILP or MIP when comparing simple interrupted and simple continuous suture patterns, regardless of experience. Median and mean intraluminal leak pressures across all treatment groups in our study exceeded the maximum physiologic intraluminal jejunal pressure induced by peristalsis, which has been reported to be 15 to 25 mmHg in healthy, non-anesthetized dogs (18). Although prior studies have shown that appositional suture patterns, including simple interrupted, simple continuous, and modified Gambee techniques, maintain intraluminal diameter and have comparable resistance to leakage, this is the first study to directly compare 2 appositional techniques with regard to experience (13,14). In the authors’ experience, simple interrupted techniques are oftentimes advocated for novice veterinarians despite an absence of data demonstrating an increased risk of dehiscence with a simple continuous technique (19–22). The results of this study support safe use of a simple continuous anastomosis pattern by novice veterinarians. Although the modified Gambee technique was not evaluated in the present study due to its reported increased operative time (13), similar results may be expected as the technique does not differ substantially from a simple continuous pattern.
In a study performed by Ellison et al (11), intestinal healing of dogs was compared between simple interrupted approximating, simple interrupted crushing, and simple continuous approximating anastomosis techniques. Both non-crushing approximating patterns resulted in less vascular disturbance to the anastomotic edges when compared to the crushing pattern (11). Moreover, the crushing technique induced greater mucosal eversion than both approximating patterns, with the simple continuous pattern resulting in the least eversion and adhesion formation (11). The simple continuous pattern was determined to be preferable due to more mature fibrous tissue formation and superior vascularity at 6 wk (11). Prior studies have reported no difference in dehiscence rates when comparing simple interrupted and simple continuous techniques (2,10). Based on the findings from our study and prior studies (2,10,11,13), both simple interrupted and simple continuous approximating anastomoses are appropriate in a clinical setting.
This study supported our hypothesis that simple continuous techniques resulted in a shorter surgical time regardless of experience. Decreased surgical times are especially advantageous in critical patients, as increasing ASA status and emergent procedures have been associated with an increased likelihood of death in dogs and cats (23,24). Prolongation of the anesthetic period and procedure duration are documented risk factors of death in dogs, horses, and humans (23,25–28). Stapling techniques have been shown to decrease surgical times regardless of experience with a recent study (14) showing a 10-fold increase in time for sutured anastomoses (1–3,8,14,29,30). Studies have shown no statistical significance in dehiscence rates between handsewn and stapled anastomosis techniques in the absence of septic peritonitis (1–3,7,8,30), and prior leak-pressure studies have shown no difference in ILP between handsewn and stapled techniques (14,15). However, surgical staples have inherent disadvantages, including added surgical cost, limited applicability for small dogs and cats, limited use at proximal and distal intestinal anastomosis sites, and reports of secondary foreign body obstructions at previous staple lines (1,7,8). Due to these limitations, handsewn techniques remain commonly used in veterinary medicine due to increased availability, reduced cost, ease of use, and ability to use in numerous intestinal locations (1,2,9,31). This study demonstrated that simple interrupted handsewn techniques resulted in increased surgical time by approximately 1.4 times, which may be disadvantageous in more critical patients. This contrasts with a study by Duell et al (2) that showed no significant difference in mean surgery duration when comparing simple interrupted and simple continuous suture patterns; however, that was a retrospective study that was not able to isolate anastomosis time alone. Simple continuous suture patterns, especially in the hands of an experienced surgeon, should be considered with critical patients or when stapling equipment is not an option.
Although experience did not differ regarding ILP and MIP, anastomoses performed by a Board-certified surgeon resulted in faster surgical times compared to novice veterinarians, regardless of the suture technique used. These findings support a previous study reporting that less experienced veterinarians had significantly longer surgical times when performing handsewn anastomoses (29). In humans, prolonged surgical times exceeding 2 h and inadequate experience, such as surgeons performing a lower volume of intestinal anastomoses per year, have been described as risk factors for dehiscence (32,33). Although studies evaluating such risk factors have yet to be performed in veterinary medicine, Jardel et al (31) reported satisfactory surgical outcomes when novice veterinarians were instructed to perform functional end-to-end stapled intestinal anastomoses after one training session. Although the handsewn anastomoses performed satisfactorily regarding leak pressures for both novice veterinarians and the Board-certified surgeon in the present study, handsewn anastomoses are technically more demanding when compared to the automated nature of staplers and, therefore, the 2 techniques may not perform comparably in-vivo.
Of note, in the current study, the ILP for simple continuous constructs performed by novice veterinarians was highly variable, with the lowest ILP being 8.9 mmHg, which is well below the reported range of physiologic intraluminal pressures (15 to 25 mmHg). This finding may highlight the technically challenging aspect of simple continuous anastomosis closures for novice veterinarians, including variation in tension when tightening and placing subsequent sutures, which could result in uneven distribution of pressure along the suture line. This is in comparison to simple interrupted closures, in which these factors may be less of a concern, and which resulted in less variation in ILP and MIP outcomes in the current study. Of note, placement of suture bites 2 to 3 mm apart was performed via gross examination and was not specifically measured before suture bite placement; therefore, some variability in suture bite placement may also have affected these findings. Although simple continuous anastomosis closures result in decreased surgical time, novice veterinarians may consider the use of simple interrupted suture patterns due to more consistent outcomes, especially in stable patients.
The ILP for simple interrupted constructs performed by the Board-certified surgeon had a more variable range when compared to novice veterinarians, with the ILP ranging from 14.4 to 124 mmHg. Likewise, time to construct completion for simple interrupted constructs by the Board-certified surgeon compared to simple continuous was more variable. We attribute these findings to the Board-certified surgeon more commonly performing simple continuous resection and anastomoses in a clinical setting. This may also have been affected by overall gastrointestinal size and diameter, leading to a variation in the number of sutures placed to complete constructs, although cadaver size was limited to between 15 and 30 kg and the same cadavers were used by the novice veterinarian.
The major limitation of this study is its ex-vivo nature. Intestinal tissue from cadavers likely differs in compliance compared to live tissue. Leak testing of the anastomotic repair primarily challenges the surgical site under tension via intraluminal pressure testing, which assesses the suture pattern and tissue strength alone without accounting for fibrin seal formation and wound healing factors in live animals. Live tissue undergoes multifactorial stressors including cyclic stress, active peristalsis, systemic disease, and the potential for peritonitis, all of which cannot be replicated ex-vivo. Therefore, our ex-vivo results may not correspond to in-vivo anastomoses, especially considering that fibrin seal formation is absent in cadaveric tissue. Another limitation of our study was that a single Board-certified surgeon was compared to multiple novice veterinarians. We consider this a limitation as using multiple novice veterinarians revealed no significant difference between ILP across a varied skill set, whereas a single Board-certified surgeon may have eliminated potential variability associated with overall skill and technique. The Board-certified surgeon more commonly performs simple continuous resection and anastomoses in a clinical setting, which may have attributed to the variability noted with time to construct completion and ILP in the simple interrupted constructs. There was also a wide range of values recorded across treatment groups, which could be due to variable intestinal quality, observer error, pressure measuring device failure, or other unforeseen factors.
In conclusion, this study revealed that the median and mean ILP and MIP of all anastomoses exceeded physiologic intraluminal peristaltic pressures regardless of experience. Simple continuous anastomoses were faster to complete, and an experienced surgeon more rapidly completed both anastomosis techniques when compared to novice veterinarians. Based on the findings of this study, both handsewn anastomosis techniques may be considered for intestinal resection and anastomosis, regardless of experience, with simple continuous anastomoses resulting in shorter surgical time. However, novice veterinarians may consider the use of simple interrupted suture patterns for a more consistent outcome, especially in clinically stable patients.
Acknowledgments
Study design, data collection, manuscript composition (Fruehwald); study design, data collection, composition and review of the manuscript (Regier, Mullen); data collection, composition, and review of the manuscript (Waln, McNamara); study design, data interpretation, manuscript review (Colee).
References
- 1.DePompeo CM, Bond L, George YE, et al. Intra-abdominal complications following intestinal anastomoses by suture and staple techniques in dogs. J Am Vet Med Assoc. 2018;253:437–443. doi: 10.2460/javma.253.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duell JR, Thieman Mankin KM, Rochat MC, et al. Frequency of dehiscence in hand-sutured and stapled intestinal anastomoses in dogs. Vet Surg. 2016;45:100–103. doi: 10.1111/vsu.12428. [DOI] [PubMed] [Google Scholar]
- 3.Davis DJ, Demianiuk RM, Musser J, Podsiedlik M, Hauptman J. Influence of preoperative septic peritonitis and anastomotic technique on the dehiscence of enterectomy sites in dogs: A retrospective review of 210 anastomoses. Vet Surg. 2018;47:125–129. doi: 10.1111/vsu.12704. [DOI] [PubMed] [Google Scholar]
- 4.Allen DA, Smeak DD, Schertel ER. Prevalence of small intestinal dehiscence and associated clinical factors: A retrospective study of 121 dogs. J Am Anim Hosp Assoc. 1992;28:70–76. [Google Scholar]
- 5.Ralphs SC, Jessen CR, Lipowitz AJ. Risk factors for leakage following intestinal anastomosis in dogs and cats. J Am Vet Med Assoc. 2003;223:73–77. doi: 10.2460/javma.2003.223.73. [DOI] [PubMed] [Google Scholar]
- 6.Snowdon KA, Smeak DD, Chiang S. Risk factors for dehiscence of stapled functional end-to-end intestinal anastomoses in dogs: 53 cases (2001–2012) Vet Surg. 2016;45:91–99. doi: 10.1111/vsu.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brundage SI, Jurkovich GJ, Hoyt DB, et al. Stapled versus sutured gastrointestinal anastomoses in the trauma patient: A multicenter trial. J Trauma. 2001;51:1054–1061. doi: 10.1097/00005373-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Farrah JP, Lauer CW, Bray MS, et al. Stapled versus hand-sewn anastomoses in emergency general surgery: A retrospective review of outcomes in a unique patient population. J Trauma Acute Care Surg. 2013;74:1187–1192. doi: 10.1097/TA.0b013e31828cc9c4. [DOI] [PubMed] [Google Scholar]
- 9.Ellison GW, Case JB, Regier PJ. Intestinal surgery in small animals: Historical foundations, current thinking, and future horizons. Vet Surg. 2019;48:1171–1180. doi: 10.1111/vsu.13275. [DOI] [PubMed] [Google Scholar]
- 10.Weisman DL, Smeak DD, Birchard SJ, Zweigart SL. Comparison of a continuous suture pattern with a simple interrupted pattern for enteric closure in dogs and cats: 83 cases (1991–1997) J Am Vet Med Assoc. 1999;214:1507–1510. [PubMed] [Google Scholar]
- 11.Ellison GW, Jokinen MP, Park RD. End-to-end approximating intestinal anastomosis in the dog: A comparative fluorescein dye, angiographic and histopathologic evaluation. J Am Anim Hosp Assoc. 1982;18:729–736. [Google Scholar]
- 12.Giuffrida MA, Brown DC. Small intestine. In: Tobias KM, Johnston SA, editors. Veterinary Surgery: Small Animal. St. Louis, Missouri: Saunders; 2012. pp. 1732–1760. [Google Scholar]
- 13.Kieves NR, Krebs AI, Zellner EM. A comparison of ex vivo leak pressures for four enterotomy closures in a canine model. J Am Anim Hosp Assoc. 2018;54:71–76. doi: 10.5326/JAAHA-MS-6459. [DOI] [PubMed] [Google Scholar]
- 14.Mullen KM, Regier PJ, Waln M, Fox-Alvarez WA, Colee J. Gastrointestinal thickness, duration, and leak pressure of six intestinal anastomoses in dogs. Vet Surg. 2020;49:1315–1325. doi: 10.1111/vsu.13490. [DOI] [PubMed] [Google Scholar]
- 15.Fealey MJ, Regier PJ, Steadman Bs, Case JB, Garcia-Pereira F. Initial leak pressures of four anastomosis techniques in cooled cadaveric canine jejunum. Vet Surg. 2020;49:480–486. doi: 10.1111/vsu.13392. [DOI] [PubMed] [Google Scholar]
- 16.Aeschlimann KA, Mann FA, Middleton JR, Belter RC. Comparison of enterotomy leak pressure among fresh, cooled, and frozen-thawed porcine jejunal segments. Am J Vet Res. 2018;79:576–580. doi: 10.2460/ajvr.79.5.576. [DOI] [PubMed] [Google Scholar]
- 17.Duffy DJ, Chang YJ, Balko JA, Moore GE. Ex vivo comparison of the effect of storage temperature on canine intestinal leakage pressures. Vet Surg. 2020;49:496–501. doi: 10.1111/vsu.13339. [DOI] [PubMed] [Google Scholar]
- 18.Tasaka K, Farrar JT. Intraluminal pressure of the small intestine of the unanesthetized dog. Pflugers Arch. 1976;364:35–44. doi: 10.1007/BF01062909. [DOI] [PubMed] [Google Scholar]
- 19.Dehoff WB. Simple interrupted approximating technique for intestinal anastomosis. J Am Anim Hosp Assoc. 1973;9:483–489. [Google Scholar]
- 20.Hamilton JE. Reappraisal of open intestinal anastomosis. Ann Surg. 1967;168:917–923. doi: 10.1097/00000658-196706000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett RR, Zydeck FA. A comparison of single layer suture patterns for intestinal anastomosis. J Am Vet Med Assoc. 1970;157:1075–1080. [PubMed] [Google Scholar]
- 22.Ott BS, Doyle MD, Greenawald KA. Single layer intestinal anastomosis. J Am Vet Med Assoc. 1968;153:1442–1453. [PubMed] [Google Scholar]
- 23.Brodbelt DC, Pfeiffer DU, Young LE, Wood JLN. Results of the confidential enquiry into perioperative small animal fatalities regarding risk factors for anesthetic-related death in dogs. J Am Vet Med Assoc. 2008;233:1096–1104. doi: 10.2460/javma.233.7.1096. [DOI] [PubMed] [Google Scholar]
- 24.Brodbelt DC, Pfeiffer DU, Young LE, et al. Risk factors for anaesthetic-related death in cats: Results from the confidential enquiry into perioperative small animal fatalities (CEPSAF) Br J Anaesth. 2007;99:617–623. doi: 10.1093/bja/aem229. [DOI] [PubMed] [Google Scholar]
- 25.Tiret L, Desmonts JM, Hatton F, Vourc’h G. Complications associated with anaesthesia — A prospective survey in France. Can Anaesth Soc J. 1986;33:336–344. doi: 10.1007/BF03010747. [DOI] [PubMed] [Google Scholar]
- 26.Fowkes FG, Lunn JN, Farrow SC, Robertson IB, Samuel P. Epidemiology in anaesthesia. III: Mortality risk with coexisting physical disease. Br J Anaesth. 1982;54:819–825. doi: 10.1093/bja/54.8.819. [DOI] [PubMed] [Google Scholar]
- 27.Pottecher T, Tiret L, Desmonts JM, Hatton F, Bilaine J, Otteni JC. Cardiac arrest related to anaesthesia: A prospective survey in France (1978–1982) Eur J Anaesthesiol. 1984;1:305–318. [PubMed] [Google Scholar]
- 28.Johnston GM, Eastment JK, Wood JLN, Taylor PM. Confidential enquiry of perioperative equine fatalities (CEPEF): Mortality results of Phases 1 and 2. Vet Anaesth Analg. 2002;29:159–170. doi: 10.1046/j.1467-2995.2002.00106.x. [DOI] [PubMed] [Google Scholar]
- 29.Vick L, Vick K, Borman K, Salameh JR. Face, content and construct validities of inanimate intestinal anastomoses simulations. J Surg Educ. 2007;64:365–368. doi: 10.1016/j.jsurg.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Coolman BR, Ehrhart N, Pijanowski G, Ehrhart EJ, Coolman SL. Comparison of skin staples with sutures for anastomosis of the small intestine in dogs. Vet Surg. 2000;29:293–302. doi: 10.1053/jvet.2000.7539. [DOI] [PubMed] [Google Scholar]
- 31.Jardel N, Hidalgo A, Leperlier D, et al. One stage functional end-to-end stapled intestinal anastomosis and resection performed by nonexpert surgeons for the treatment of small intestinal obstruction in 30 dogs. Vet Surg. 2011;40:216–222. doi: 10.1111/j.1532-950X.2010.00784.x. [DOI] [PubMed] [Google Scholar]
- 32.Golub R, Golub RW, Cantu R, Stein HD. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg. 1999;184:364–372. [PubMed] [Google Scholar]
- 33.Byrn JC, Schlager A, Divino CM, Weber KJ, Baril DT, Aufses AH., Jr The management of 38 anastomotic leaks after 1,684 intestinal resections. Dis Colon Rectum. 2006;65:1346–1353. doi: 10.1007/s10350-006-0653-8. [DOI] [PubMed] [Google Scholar]