Abstract
Purpose
The purpose of the current study was to test the hypothesis that responses of total retinal blood flow (TRBF), inner retinal oxygen delivery (DO2), metabolism (MO2), and extraction fraction (OEF) to hyperoxia are higher after minutes of bilateral common carotid artery occlusion (BCCAO) as compared to days of BCCAO.
Methods
Twenty-eight rats were subjected to BCCAO for 30 minutes (n = 12), 1 day (n = 8), or 3 days (n = 8). Eight of the 12 rats were also evaluated at baseline, prior to BCCAO. During room air breathing (RA) and 100% O2 inspiration (hyperoxia), blood flow and phosphorescence lifetime imaging were performed to measure TRBF and vascular O2 contents, respectively. DO2, MO2, and OEF were calculated from these measurements.
Results
After 30 minutes or 3 days of BCCAO, TRBF did not differ between RA and hyperoxia conditions (P ≥ 0.14) but decreased under hyperoxia after 1 day (P = 0.01). Compared to RA, DO2 and MO2 were increased under hyperoxia after 30 minutes of BCCAO (P ≤ 0.02). Additionally, MO2 was decreased under hyperoxia after 1 day of BCCAO (P = 0.04). OEF was decreased under hyperoxia compared to RA (P < 0.001). Under hyperoxia, TRBF and DO2 were reduced after all BCCAO durations compared to baseline (P ≤ 0.04), whereas MO2 did not differ from baseline after 30 minutes of BCCAO (P = 1.00).
Conclusions
The findings indicate that hyperoxia introduced minutes after ischemia can reduce DO2 impairments and potentially return MO2 to approximately normal values. This information contributes to the knowledge of the effect of supplemental oxygen intervention on TRBF, DO2, MO2, and OEF outcomes after variable durations of ischemia.
Keywords: hyperoxia, BCCAO, retinal ischemia
Inadequate oxygen supply is detrimental to the retinal tissue, which is extremely vulnerable due to its high metabolic activity.1 It is well established that hypoxia plays a central role in the events leading to neuronal cell damage and death due to ischemia.2–4 Retinal hypoxia has been implicated in retinal vascular occlusions and in late stages of diabetic retinopathy.5–10 In central retinal vein occlusion, hypoxia has been confirmed by oxygen tension (PO2) measurements during vitrectomy.11 The severe reduction in blood flow and attempts by the tissue to extract more oxygen has been shown to reduce venous oxygen saturation (SO2).12–14 Moreover, reduction in arterial SO2 was detected in branch and central retinal artery occlusions.15,16 In diabetic subjects, decreased intraocular PO2 was measured during vitrectomy.17 Moreover, in advanced diabetic retinopathy stages, obliterated capillaries reduce oxygen supply to regions of the retina, such that less oxygen is extracted, leading to increased retinal venous SO2, as reported in several studies.18–21 Although there are no available techniques for direct measurement of retinal tissue oxygenation in humans, experimental animal models have shown retinal hypoxia in retinal branch vein occlusion in miniature pigs,22 retinal artery occlusion in cats and rats,23,24 and diabetic cats.25
One treatment approach used to minimize retinal hypoxic damage is to increase oxygen supply by hyperoxia (inspiration of 100% O2). The goal of hyperoxia therapy is to restore cellular oxygenation of damaged cells and stimulate them to function more normally. This method reduced vascular endothelial growth factor expression in mice,26,27 inhibited neovascularization in adult nonhuman primate and mouse retinas,27,28 and increased retinal oxygen consumption in hypoxia induced by retinal detachment in felines.29,30 Additionally, supplemental oxygen via vitreoperfusion in ischemic feline retinas has been shown to be protective of retinal damage when used with ischemia durations of less than 30 minutes.31,32 In patients with central retinal artery occlusion, hyperbaric oxygen treatment improved visual acuity, as long as irreversible infarction had not developed.33–35 Additionally, studies using normobaric hyperoxia treatment in patients reported better potential for improving visual acuity when administered 24 hours after occlusion compared to occlusions over 2 days.36,37 Another study that introduced hyperoxia up to 24 hours after occlusion was unsuccessful in improving visual acuity.38 Moreover, during brain ischemia, animal studies have demonstrated the ability of hyperoxia to provide beneficial effects such as an improvement of blood flow,39,40 an increase in oxygen delivery,39 and a decrease in infarct size39–45 when provided promptly during ischemic injury. Notably, it was found that improvement from hyperoxia in a middle cerebral artery occlusion stroke model was observed in occlusions shorter than 30 to 45 minutes.39,40
We have previously reported reduced total retinal blood flow (TRBF), inner retinal oxygen delivery (DO2) and oxygen metabolism (MO2), and increased oxygen extraction fraction (OEF) both 30 minutes46 and 3 days47,48 after ischemia in rats induced by permanent bilateral common carotid artery occlusion (BCCAO). However, there is limited knowledge of these measurements in response to hyperoxia after the induction of retinal ischemia and specifically how responses to hyperoxia may change dependent on ischemia duration. This may be helpful to assess any potential benefit to damaged tissue. For example, improvements in these metrics in response to hyperoxia may indicate that the tissue has the potential for recovery after different durations of ischemia, whereas a lack of improvement may be suggestive of irreversible damage. The purpose of the current study was to test the hypothesis that the responses of TRBF, DO2, MO2, and OEF to hyperoxia are higher after minutes of BCCAO as compared to days of BCCAO.
Materials and Methods
Animals
All procedures were approved by the University of Southern California Institutional Animal Care and Use Committee and adhered to the articles of the statement of Use of Animals in Ophthalmic and Vision research by the ARVO. The experiments followed the Animal Research: Reporting in Vivo Experiments guidelines. The study was performed in 28 adult (age: 13.5 ± 1.4 weeks) male Long-Evans rats (weight: 360 ± 38 g) (Charles River, San Diego, CA, USA). Before experimental procedures were performed, rats were kept under environmentally controlled conditions with a 12-hour/12-hour light/dark cycle at 20–22°C and had access to food and water. Rats were subjected to permanent ligation of both common carotid arteries (BCCAO) (see below). Under this condition, there was blood flow at a reduced rate to the retinal tissue from the vertebral arteries by way of the circle of Willis and retrograde through the distal internal carotid artery.49 Rats were categorized in three groups according to the duration of BCCAO: 30 minutes (n = 12), 1 day (n = 8), or 3 days (n = 8). These timepoints were chosen based on our previous studies that demonstrated impairments in oxygen metrics after these durations of BCCAO.46–48 After each duration of BCCAO, a randomly selected eye in each rat was imaged during two fractions of the inspired oxygen (FiO2) condition: first under 21% O2 inspiration (spontaneous room air breathing [RA]) and then immediately after 15 minutes of 100% O2 inspiration (hyperoxia). Eight of the rats in the 30-minute BCCAO group were also imaged prior to BCCAO (baseline). Otherwise, rats were imaged only at one duration of ischemia.
Bilateral Common Carotid Artery Occlusion Procedure
During BCCAO procedures, rats were anesthetized by 1.5% to 2.5% isoflurane. The common carotid arteries were accessed via a midline prelaryngeal incision, dissected from the sympathetic and vagus nerves and ligated, as previously described.47,48
Imaging
Prior to imaging, rats were anesthetized by injection of ketamine (90 mg/kg) and xylazine (5 mg/kg). Maintenance doses (45 mg/kg ketamine and 2.5 mg/kg xylazine) were administered during imaging, as needed. As previously described,47,48,50 a catheter was placed in the femoral artery for administration of polystyrene fluorescent microspheres (Life Technologies, Eugene, OR, USA) and Pd-porphine (Frontier Science, Boston, MA, USA). Blood velocity was measured by imaging the movement of the microspheres in the vasculature over time, and PO2 was measured based on the phosphorescence lifetime of Pd-porphine, an oxygen-sensitive molecular probe.47,48,50 The Pd-porphine was administered via the catheter at a dosage of 20 mg/kg. Pupils were dilated with 2.5% phenylephrine (Paragon, Portland, OR, USA) and 1% tropicamide (Bausch and Lomb, Tampa, FL, USA). A glass cover slip with 2.5% hypromellose ophthalmic demulcent solution (HUB Pharmaceuticals, Plymouth, MI, USA) was applied to the eye for hydration and elimination of refractive power. During imaging, anesthesia was sustained and an animal holder with copper tubing that perfused warm water maintained body temperature. Systemic SO2 was measured with a pulse oximeter (SurgiVet V3395 TPR Monitor; Smiths Medical, Waukesha, WI, USA). Saline injections (0.9%) were administered throughout imaging to maintain hydration. Imaging was performed in one eye of each rat.
Retinal venous diameter (D) and blood velocity (V) were measured by our previously described imaging system and used to calculate TRBF.50,51 Measurements of individual vessels on red-free images were averaged to obtain mean arterial and venous D (DA, Dv). Displacement of microspheres over time was determined from analysis of fluorescence images acquired at a high rate to obtain multiple V measurements for each vein and averaged to calculate venous velocity (VV).50,51 Blood flow was calculated in each vein as VVπDV2/4 and then summed over all the veins to calculate TRBF. Retinal vascular oxygen tension (PO2) was measured using our established optical section phosphorescence lifetime imaging system.47,48,50,51 Phosphorescence lifetimes of Pd-porphine in the retinal arteries and veins were converted to PO2 measurements using the Stern–Volmer equation.52,53 Vascular O2 content was calculated as the sum of oxygen bound to hemoglobin and dissolved in blood54: O2 content = SO2 × HgB × C + k × PO2. SO2 is the oxygen saturation calculated from PO2 and the rat hemoglobin dissociation curve,55 HgB is the rat hemoglobin concentration value (13.8 g/dL),56 C is the maximum oxygen-carrying capacity of hemoglobin (1.39 mL O2/g),57 and k is the solubility of oxygen in blood (0.0032 mL O2/dL mm Hg). Arterial (O2A) and venous (O2V) oxygen contents were calculated by averaging values in retinal arteries and veins, respectively. Arteriovenous oxygen content difference (O2AV) was calculated as the difference between O2A and O2V. DO2, MO2, and OEF were calculated as TRBF × O2A, TRBF × O2AV, and MO2/DO2, respectively. Methods of TRBF and PO2 imaging are displayed in the Figure.
Figure.
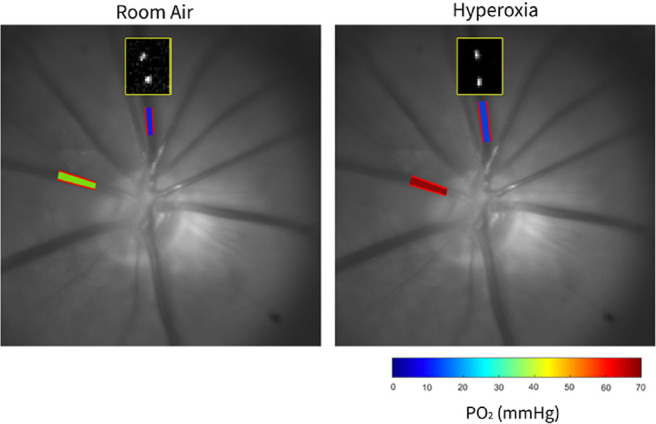
Representation of the method for retinal vascular PO2 and blood flow imaging in the same rat under 30 minutes of BCCAO under RA breathing and under hyperoxia. Yellow-outlined boxes overlaid on the red-free fundus images show the position of intravenous fluorescent microspheres at two time points, depicting similar velocity (distance the microsphere moved during the same time interval). Overlaid on the red-free fundus images are automatically detected retinal vessel boundaries (outlined in red) in a retinal artery and vein, depicting similar arterial and venous diameters between RA and hyperoxia conditions. Retinal arterial and venous PO2 measurements are displayed in pseudo-color. The color bar shows PO2 values in mm Hg. Increased arterial PO2 can be observed under hyperoxia compared to RA.
Data Analysis
Statistical analyses were performed using SPSS Statistics, Version 24 (IBM Armonk, New York, NY, USA). Paired t-tests were used to determine if there was a significant difference in systemic SO2 and O2A between RA and hyperoxia in each BCCAO group. A mixed linear model analysis was performed to determine the effects of BCCAO duration (30 minutes, 1 day, 3 days) and FiO2 condition (RA or hyperoxia) on TRBF, DO2, MO2, and OEF with animal as a random factor to account for multiple measurements in the same animal. If there was a significant interaction effect of BCCAO duration and FiO2 condition, the simple main effects of FiO2 and BCCAO duration were determined using paired t-tests and one-way ANOVAs, respectively. Additionally, TRBF, DO2, MO2, and OEF values in BCCAO groups under hyperoxia were compared to baseline values (0 minutes of BCCAO under RA) using mixed linear model analysis with animal as a random factor and Bonferroni post hoc tests in order to determine whether the metrics recovered to baseline values. P values ≤0.05 were considered significant.
Results
Systemic Condition
Systemic SO2 and O2A measurements are presented in Table 1. Under RA, the systemic SO2 was 75% ± 9%, 74% ± 5%, and 75% ± 4% in the 30 minutes, 1 day, and 3 days BCCAO groups, respectively. Under hyperoxia, the systemic SO2 was 93% ± 1%, 93% ± 4%, and 92% ± 4% in the 30 minutes, 1 day, and 3 days BCCAO groups, respectively. SO2 was significantly higher under hyperoxia than that under RA in all BCCAO groups (P < 0.001). Under RA, O2A was 9.3 ± 2.1 mLO2/dL, 11.7 ± 2.1 mLO2/dL, and 11.5 ± 1.4 mLO2/dL in the 30 minutes, 1 day, and 3 days BCCAO groups, respectively. Under hyperoxia, O2A was 15.8 ± 2.1 mLO2/dL, 17.4 ± 2.7 mLO2/dL, and 17.9 ± 2.2 mLO2/dL in the 30 minutes, 1 day, and 3 days BCCAO groups, respectively. O2A was significantly higher under hyperoxia than that under RA in all BCCAO groups (P < 0.001).
Table 1.
SO2 and O2A Under RA Breathing and Hyperoxia After 30 Minutes, 1 Day, and 3 Days of BCCAO
| BCCAO Duration, Mean ± SD | |||
|---|---|---|---|
| Characteristic | 30 Minutes | 1 Day | 3 Days |
| Systemic SO2 | |||
| RA, % | 75 ± 9 | 74 ± 5 | 75 ± 4 |
| Hyperoxia, % | 93 ± 1 | 93 ± 4 | 92 ± 4 |
| P value | <0.0001 | <0.0001 | <0.0001 |
| O2A (mLO2/dL) | |||
| RA | 9.3 ± 2.1 | 11.7 ± 2.1 | 11.5 ± 1.4 |
| Hyperoxia | 15.8 ± 2.1 | 17.4 ± 2.7 | 17.9 ± 2.2 |
| P value | <0.0001 | <0.0001 | <0.0001 |
Below the SO2 and O2A values are the P values from paired t-tests between RA and hyperoxia in each BCCAO group. Bold indicates significant difference (P ≤ 0.05). SO2 and O2A were significantly higher after hyperoxia compared to RA in the 30 minutes, 1 day, and 3 days BCCAO groups.
Total Retinal Blood Flow
TRBF measurements are presented in Table 2. TRBF at baseline was 6.5 ± 1.1 µL/min. Measurements in the 30 minutes, 1 day, and 3 days BCCAO groups under RA were 1.5 ± 0.8 µL/min, 3.0 ± 1.8 µL/min, and 2.6 ± 0.9 µL/min, respectively. Measurements in the 30 minutes, 1 day, and 3 days BCCAO groups under hyperoxia were 1.9 ± 0.7 µL/min, 1.9 ± 1.2 µL/min, and 2.4 ± 1.4 µL/min, respectively. FiO2 condition and BCCAO duration had a significant interaction effect on TRBF (P = 0.005). TRBF did not differ between RA and hyperoxia after 30 minutes or 3 days of BCCAO (P ≥ 0.18) but was decreased under hyperoxia as compared to RA after 1 day of BCCAO (P = 0.008). Under RA, TRBF was lower after 30 minutes of BCCAO compared to 1 day of BCCAO (P = 0.03), whereas under hyperoxia, there were no differences in TRBF among BCCAO groups (P = 1.00). Compared to baseline, TRBF was reduced in all BCCAO groups under hyperoxia (P < 0.001).
Table 2.
TRBF, DO2, and MO2 at Baseline and Under RA Breathing and Hyperoxia After 30 Minutes, 1 Day, and 3 Days of BCCAO
| BCCAO Duration, Mean ± SD | ||||
|---|---|---|---|---|
| Characteristic | Baseline | 30 Minutes | 1 Day | 3 Days |
| TRBF (µL/min) | ||||
| RA | 6.5 ± 1.1 | 1.5 ± 0.8 | 3.0 ± 1.8 | 2.6 ± 0.9 |
| Hyperoxia | 1.9 ± 0.7 | 1.9 ± 1.2 | 2.4 ± 1.4 | |
| P value | 0.18 | 0.008 | 0.41 | |
| DO2 (nLO2/min) | ||||
| RA | 937 ± 168 | 147 ± 97 | 381 ± 284 | 303 ± 114 |
| Hyperoxia | 311 ± 139 | 352 ± 254 | 450 ± 284 | |
| P value | 0.006 | 0.46 | 0.07 | |
| MO2 (nLO2/min) | ||||
| RA | 267 ± 95 | 143 ± 95 | 182 ± 102 | 214 ± 89 |
| Hyperoxia | 241 ± 75 | 104 ± 75 | 152 ± 44 | |
| P value | 0.02 | 0.04 | 0.06 | |
Below the TRBF, DO2, and MO2 values are the P values from paired t-tests between RA and hyperoxia in each BCCAO group. Bold indicates significant difference (P ≤ 0.05). In response to hyperoxia, only MO2 after 30 minutes of BCCAO approximated baseline values.
Oxygen Delivery
DO2 measurements are presented in Table 2. DO2 at baseline was 937 ± 168 nLO2/min. Measurements in the 30 minutes, 1 day, and 3 days BCCAO groups under RA were 147 ± 97 nLO2/min, 381 ± 284 nLO2/min, and 303 ± 114 nLO2/min, respectively. Measurements in the 30 minutes, 1 day, and 3 days BCCAO groups under hyperoxia were 311 ± 139 nLO2/min, 352 ± 254 nLO2/min, and 450 ± 284 nLO2/min, respectively. FiO2 condition and BCCAO duration had a significant interaction effect on DO2 (P = 0.03). DO2 was increased under hyperoxia as compared to RA after 30 minutes of BCCAO (P = 0.006). There was no statistically significant change in DO2 under hyperoxia after 1 or 3 days of BCCAO (P ≥ 0.07). Under RA, DO2 was lower after 30 minutes of BCCAO compared to 1 day of BCCAO (P = 0.02), while under hyperoxia, DO2 was not significantly different among BCCAO groups (P ≥ 0.54). Compared to baseline, DO2 was reduced in all BCCAO groups under hyperoxia (P ≤ 0.04).
Oxygen Metabolism
MO2 measurements are presented in Table 2. MO2 at baseline was 267 ± 95 nLO2/min. Measurements in the 30 minutes, 1 day, and 3 days BCCAO groups under RA were 143 ± 95 nLO2/min, 182 ± 102 nLO2/min, and 214 ± 89 nLO2/min, respectively. Measurements in the 30 minutes, 1 day, and 3 days BCCAO groups under hyperoxia were 241 ± 75 nLO2/min, 104 ± 75 nLO2/min, and 152 ± 44 nLO2/min, respectively. FiO2 condition and BCCAO duration had a significant interaction effect on MO2 (P < 0.001). MO2 was increased under hyperoxia as compared to RA after 30 minutes of BCCAO (P = 0.02). Additionally, MO2 was decreased under hyperoxia as compared to RA after 1 day of BCCAO (P = 0.04). There were no significant differences in MO2 among BCCAO groups under RA (P ≥ 0.35), while under hyperoxia, MO2 was higher after 1 and 3 days of BCCAO compared to 30 minutes of BCCAO (P ≤ 0.02). MO2 was not significantly different than baseline after 30 minutes of BCCAO under hyperoxia (P = 1.00), but was lower than baseline after 1 and 3 days of BCCAO (P ≤ 0.02).
Oxygen Extraction Fraction
FiO2 condition and BCCAO duration did not have a significant interaction effect on OEF (P = 0.41). However, both BCCAO duration and FiO2 condition had significant main effects on OEF (P < 0.001). OEF was lower under hyperoxia (0.57 ± 0.04) compared to that under RA (0.79 ± 0.04) (P < 0.001). OEF after 30 minutes of BCCAO (0.90 ± 0.05) was higher than that after 1 and 3 days of BCCAO (0.54 ± 0.06 and 0.59 ± 0.06, respectively) (P ≤ 0.003). Under hyperoxia, OEF was higher than baseline after 30 minutes of BCCAO (P < 0.001) but was not different from baseline after 1 and 3 days of BCCAO (P ≥ 0.30).
Discussion
This is the first time, to our knowledge, that the effect of hyperoxia on physiologic retinal parameters after various durations of partial ischemia has been reported. It was also novel that we measured these parameters in the same animals at baseline, which is rarely possible clinically. The results confirmed our hypothesis that responses in oxygen metrics are dependent on BCCAO duration.
Choroidal Oxygen Supply
In numerous studies, the choroid has been shown to contribute an increased amount of oxygen to the retina during hyperoxia.58–61 In a normally perfused retina under RA, an increase in oxygen causes vasoconstriction. In addition, with the administration of hyperoxia, blood flow decreases to autoregulate O2 delivery since there is increased oxygen from the choroid.62,63 Additionally, MO2, which indicates the rate at which O2 is extracted from the retinal circulation, cannot represent some of the O2 consumed by the part of the inner retina close to the choroid. So hyperoxia could cause MO2 to be reduced even if the total amount of oxygen consumed by the inner retina was the same. Therefore, without ischemia, hyperoxia should decrease TRBF, DO2 (the product of blood flow and O2A), and MO2. However, if hyperoxia increases these factors, the increase could be falsely low because of the amount of oxygen being supplied by the choroid to part of the inner retina. Alternatively, if hyperoxia decreases these factors, it may indicate that the results are dominated by choroidal oxygen supply. Additionally, BCCAO causes choroidal blood flow to be decreased. This makes interpretation of our results more difficult.
Total Retinal Blood Flow
TRBF showed no response to hyperoxia after 30 minutes of BCCAO. In a normally perfused rat retina, administration of supplemental O2 causes a decrease in blood flow.62,63 However, in the current study, the lack of TRBF response to hyperoxia suggests a decreased autoregulatory vasoconstriction due to sustained oxygen demand under experimentally induced reduced blood flow for a short duration. In contrast, after 1 day of BCCAO, hyperoxia caused TRBF to decrease, which may be attributed to an increase in choroidal oxygen supply, consistent with a physiologic retinal vascular response.62,63 The reason for the finding of no significant change in TRBF in response to hyperoxia after 3 days of BCCAO is not clear, although we may speculate lower oxygen demand due to cell death after longer durations of BCCAO.
Oxygen Delivery
Since DO2 is the product of TRBF and O2A, both of these factors contribute to its response to hyperoxia. DO2 increased in response to hyperoxia after 30 minutes of BCCAO, primarily due to increased O2A. However, after 1 day of BCCAO, no change to DO2 was observed in response to hyperoxia, which can be attributed to decreased TRBF, as previously discussed, coupled with an increase in O2A. Similarly, hyperoxia caused no change in DO2 after 3 days of BCCAO. At longer durations of BCCAO, lower oxygen demand due to cell death and increased oxygen supply from the choroidal circulation contributed to maintaining DO2.
Oxygen Metabolism
We found that MO2 increased and approximated to normal levels in response to hyperoxia after 30 minutes of BCCAO. With such a short duration of reduced blood flow, very few retinal cells would have become unable to consume oxygen. Thus, with increased oxygen availability, retinal cells were able to extract enough oxygen to essentially normalize MO2. Additionally, because of the possible contribution of O2 from the choroid, the improvement in MO2 may be underestimated. In contrast, after 1 and 3 days of BCCAO, there was a decrease in MO2 in response to hyperoxia. Since it is unlikely that 15 minutes of hyperoxia would result in a significant loss in ability to metabolize oxygen, this decrease in MO2 appears to be attributable to an increase in oxygen availability from the choroid.
Oxygen Extraction Fraction
OEF was lower under hyperoxia compared to that under the RA condition, in which the tissue was metabolizing nearly all of the oxygen being delivered by the vasculature. Hyperoxia significantly increased oxygen availability to the retina, reducing OEF to a level that reflected healthier tissue. Furthermore, regardless of FiO2 condition, OEF was found to be higher after 30 minutes of BCCAO than that after 1 and 3 days. After such a short duration of BCCAO, the retina was extracting an average of 90% of the oxygen being delivered to the retina through the vasculature, which indicates marginally oxygenated tissue. On the other hand, after longer durations, choroidal flow presumably would have improved, causing a decrease in MO2 as discussed, which would have resulted in a lower OEF (0.55–0.60). Moreover, OEF under hyperoxia reverted to approximately normal values after 1 and 3 days of BCCAO.
Hyperoxia Treatment Window
Responses of DO2, MO2, and OEF to hyperoxia demonstrate the higher treatment potential after a short duration of ischemia compared to durations of a day or more. Other studies have similarly found that hyperoxia is most effective as a treatment when administered between 15 and 45 minutes after the onset of ischemia in neural and retinal tissue and that reducing the time between injury and treatment enhances the degree of improvement.34,39,40,64 Additionally, a study found that transient ischemia in neural tissue was more treatable with hyperoxia compared to permanent ischemia.65 Our results imply that decreasing the time interval between ischemic insult and hyperoxia treatment would optimize the potential for tissue to recover.
Limitations
The current study had several limitations. First, we did not measure systemic blood oxygen tension directly, although systemic SO2 was measured. Second, anesthesia used during imaging caused some degree of systemic hypoxia, as was confirmed by SO2 measurements, which may have limited our findings of responses to hyperoxia. Third, the small sample size may have increased the probability of making a type II error, such that some statistically significant differences may not have been found when they actually existed. Fourth, for calculations of O2A and O2V from the hemoglobin dissociation curve, a constant value for blood pH was used and variations due to hyperoxia 66 were not accounted for. Fifth, it is possible that preconditioning by the ischemic insult may trigger endogenous neuroprotection, thus augmenting the effect of hyperoxia. Future studies are needed to investigate and differentiate the benefits of these two factors.
Conclusion
Our findings suggest that administration of supplemental oxygen minutes after ischemia has the potential to mitigate oxygen delivery impairments and revert oxygen metabolism approximately to normal values. Overall, the findings of the current study contribute to the knowledge of the effect of supplemental oxygen intervention on impaired retinal oxygen metrics due to experimental ischemia.
Acknowledgments
The authors thank James Burford for performing animal procedures.
Supported by the National Eye Institute, Bethesda, Maryland (grants EY017918 and EY029220), and an unrestricted departmental award from Research to Prevent Blindness, New York, New York.
Disclosure: S. Leahy, None; N. Matei, None; N.P. Blair, None; M. Shahidi, (P)
References
- 1. Braun RD, Linsenmeier RA, Goldstick TK.. Oxygen consumption in the inner and outer retina of the cat. Invest Ophthalmol Vis Sci. 1995; 36: 542–554. [PubMed] [Google Scholar]
- 2. Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. Br Med Bull. 2009; 92: 7–32. [DOI] [PubMed] [Google Scholar]
- 3. Zauner A, Daugherty WP, Bullock MR, Warner DS.. Brain oxygenation and energy metabolism: part I-biological function and pathophysiology. Neurosurgery. 2002; 51: 289–301; discussion 302. [PubMed] [Google Scholar]
- 4. Stinear C. Prediction of recovery of motor function after stroke. Lancet Neurol. 2010; 9: 1228–1232. [DOI] [PubMed] [Google Scholar]
- 5. Stefansson E, Olafsdottir OB, Eliasdottir TS, et al.. Retinal oximetry: metabolic imaging for diseases of the retina and brain. Prog Retin Eye Res. 2019; 70: 1–22. [DOI] [PubMed] [Google Scholar]
- 6. Bresnick GH, De Venecia G, Myers FL, Harris JA, Davis MD.. Retinal ischemia in diabetic retinopathy. Arch Ophthalmol. 1975; 93: 1300–1310. [DOI] [PubMed] [Google Scholar]
- 7. Hamanaka T, Akabane N, Yajima T, Takahashi T, Tanabe A.. Retinal ischemia and angle neovascularization in proliferative diabetic retinopathy. Am J Ophthalmol. 2001; 132: 648–658. [DOI] [PubMed] [Google Scholar]
- 8. Glacet-Bernard A, Miere A, Houmane B, Tilleul J, Souied E.. Nonperfusion assessment in retinal vein occlusion: comparison between ultra-widefield fluorescein angiography and widefield optical coherence tomography angiography. Retina. 2021; 41: 1202–1209. [DOI] [PubMed] [Google Scholar]
- 9. Rilven S, Torp TL, Grauslund J.. Retinal oximetry in patients with ischaemic retinal diseases. Acta Ophthalmol. 2017; 95: 119–127. [DOI] [PubMed] [Google Scholar]
- 10. Yang S, Liu X, Li H, Xu J, Wang F.. Optical coherence tomography angiography characteristics of acute retinal arterial occlusion. BMC Ophthalmol. 2019; 19: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williamson TH, Grewal J, Gupta B, Mokete B, Lim M, Fry CH.. Measurement of PO2 during vitrectomy for central retinal vein occlusion, a pilot study. Graefes Arch Clin Exp Ophthalmol. 2009; 247: 1019–1023. [DOI] [PubMed] [Google Scholar]
- 12. Eliasdottir TS, Bragason D, Hardarson SH, Kristjansdottir G, Stefansson E.. Venous oxygen saturation is reduced and variable in central retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol. 2015; 253: 1409. [DOI] [PubMed] [Google Scholar]
- 13. Hardarson SH, Stefansson E.. Oxygen saturation in central retinal vein occlusion. Am J Ophthalmol. 2010; 150: 871–875. [DOI] [PubMed] [Google Scholar]
- 14. Traustason S, la Cour M, Larsen M.. Retinal vascular oximetry during ranibizumab treatment of central retinal vein occlusion. Br J Ophthalmol. 2014; 98: 1208–1211. [DOI] [PubMed] [Google Scholar]
- 15. Hardarson SH, Elfarsson A, Agnarsson BA, Stefansson E.. Retinal oximetry in central retinal artery occlusion. Acta Ophthalmol. 2013; 91: 189–190. [DOI] [PubMed] [Google Scholar]
- 16. Gehlert S, Dawczynski J, Hammer M, Strobel J.. Haemoglobin oxygenation of retinal vessels in branch retinal artery occlusions over time and correlation with clinical outcome [in German]. Klin Monbl Augenheilkd. 2010; 227: 976–980. [DOI] [PubMed] [Google Scholar]
- 17. Holekamp NM, Shui YB, Beebe D.. Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am J Ophthalmol. 2006; 141: 1027–1032. [DOI] [PubMed] [Google Scholar]
- 18. Jorgensen CM, Hardarson SH, Bek T.. The oxygen saturation in retinal vessels from diabetic patients depends on the severity and type of vision-threatening retinopathy. Acta Ophthalmol. 2014; 92: 34–39. [DOI] [PubMed] [Google Scholar]
- 19. Hardarson SH, Stefansson E.. Retinal oxygen saturation is altered in diabetic retinopathy. Br J Ophthalmol. 2012; 96: 560–563. [DOI] [PubMed] [Google Scholar]
- 20. Hammer M, Vilser W, Riemer T, et al.. Diabetic patients with retinopathy show increased retinal venous oxygen saturation. Graefes Arch Clin Exp Ophthalmol. 2009; 247: 1025–1030. [DOI] [PubMed] [Google Scholar]
- 21. Blair NP, Wanek J, Felder AE, et al.. Retinal oximetry and vessel diameter measurements with a commercially available scanning laser ophthalmoscope in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2017; 58: 5556–5563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pournaras CJ, Tsacopoulos M, Strommer K, Gilodi N, Leuenberger PM.. Experimental retinal branch vein occlusion in miniature pigs induces local tissue hypoxia and vasoproliferative microangiopathy. Ophthalmology. 1990; 97: 1321–1328. [DOI] [PubMed] [Google Scholar]
- 23. Yu DY, Cringle SJ, Yu PK, Su EN.. Intraretinal oxygen distribution and consumption during retinal artery occlusion and graded hyperoxic ventilation in the rat. Invest Ophthalmol Vis Sci. 2007; 48: 2290–2296. [DOI] [PubMed] [Google Scholar]
- 24. Braun RD, Linsenmeier RA.. Retinal oxygen tension and the electroretinogram during arterial occlusion in the cat. Invest Ophthalmol Vis Sci. 1995; 36: 523–541. [PubMed] [Google Scholar]
- 25. Linsenmeier RA, Braun RD, McRipley MA, et al.. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci. 1998; 39: 1647–1657. [PubMed] [Google Scholar]
- 26. Liu H, Zhang W, Xu Z, Caldwell RW, Caldwell RB, Brooks SE.. Hyperoxia causes regression of vitreous neovascularization by downregulating VEGF/VEGFR2 pathway. Invest Ophthalmol Vis Sci. 2013; 54: 918–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pournaras CJ, Miller JW, Gragoudas ES, et al.. Systemic hyperoxia decreases vascular endothelial growth factor gene expression in ischemic primate retina. Arch Ophthalmol. 1997; 115: 1553–1558. [DOI] [PubMed] [Google Scholar]
- 28. Zhang W, Yokota H, Xu Z, et al.. Hyperoxia therapy of pre-proliferative ischemic retinopathy in a mouse model. Invest Ophthalmol Vis Sci. 2011; 52: 6384–6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang S, Linsenmeier RA.. Hyperoxia improves oxygen consumption in the detached feline retina. Invest Ophthalmol Vis Sci. 2007; 48: 1335–1341. [DOI] [PubMed] [Google Scholar]
- 30. Mervin K, Valter K, Maslim J, Lewis G, Fisher S, Stone J.. Limiting photoreceptor death and deconstruction during experimental retinal detachment: the value of oxygen supplementation. Am J Ophthalmol. 1999; 128: 155–164. [DOI] [PubMed] [Google Scholar]
- 31. Blair NP, Baker DS, Rhode JP, Vitreoperfusion Solomon M.. A new approach to ocular ischemia. Arch Ophthalmol. 1989; 107: 417–423. [DOI] [PubMed] [Google Scholar]
- 32. Blair NP, Shaw WE, Dunn R, Tsukarhara Y, Floro C, Rusin MM.. Limitation of retinal injury by vitreoperfusion initiated after onset of ischemia. Arch Ophthalmol. 1991; 109: 113–118. [DOI] [PubMed] [Google Scholar]
- 33. Hadanny A, Maliar A, Fishlev G, et al.. Reversibility of retinal ischemia due to central retinal artery occlusion by hyperbaric oxygen. Clin Ophthalmol. 2016; 11: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soares A, Gomes NL, Mendonça L, Ferreira C.. The efficacy of hyperbaric oxygen therapy in the treatment of central retinal artery occlusion. BMJ Case Rep. 2017; 2017: bcr2017220113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lopes AS, Basto R, Henriques S, et al.. Hyperbaric oxygen therapy in retinal arterial occlusion: epidemiology, clinical approach, and visual outcomes. Case Rep Ophthalmol Med. 2019; 2019: 9765938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Augsburger JJ, Magargal LE.. Visual prognosis following treatment of acute central retinal artery obstruction. Br J Ophthalmol. 1980; 64: 913–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Linsenmeier RA, Zhang HF.. Retinal oxygen: from animals to humans. Prog Retin Eye Res. 2017; 58: 115–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Atebara NH, Brown GC, Cater J.. Efficacy of anterior chamber paracentesis and Carbogen in treating acute nonarteritic central retinal artery occlusion. Ophthalmology. 1995; 102: 2029–2034; discussion 2034–2035. [DOI] [PubMed] [Google Scholar]
- 39. Shin HK, Dunn AK, Jones PB, et al.. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007; 130: 1631–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singhal AB, Dijkhuizen RM, Rosen BR, Lo EH.. Normobaric hyperoxia reduces MRI diffusion abnormalities and infarct size in experimental stroke. Neurology. 2002; 58: 945–952. [DOI] [PubMed] [Google Scholar]
- 41. Xu J, Zhang Y, Liang Z, et al.. Normobaric hyperoxia retards the evolution of ischemic brain tissue toward infarction in a rat model of transient focal cerebral ischemia. Neurol Res. 2016; 38: 75–79. [DOI] [PubMed] [Google Scholar]
- 42. Esposito E, Mandeville ET, Hayakawa K, Singhal AB, Lo EH.. Effects of normobaric oxygen on the progression of focal cerebral ischemia in rats. Exp Neurol. 2013; 249: 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen C, Cui H, Li Z, Wang R, Zhou C.. Normobaric oxygen for cerebral ischemic injury. Neural Regen Res. 2013;8: 2885–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Henninger N, Bouley J, Nelligan JM, Sicard KM, Fisher M.. Normobaric hyperoxia delays perfusion/diffusion mismatch evolution, reduces infarct volume, and differentially affects neuronal cell death pathways after suture middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2007; 27: 1632–1642. [DOI] [PubMed] [Google Scholar]
- 45. Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ.. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006; 26: 1274–1284. [DOI] [PubMed] [Google Scholar]
- 46. Karamian P, Burford J, Farzad S, Blair NP, Shahidi M.. Alterations in retinal oxygen delivery, metabolism, and extraction fraction during bilateral common carotid artery occlusion in rats. Invest Ophthalmol Vis Sci. 2019; 60: 3247–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matei N, Leahy S, Auvazian S, Thomas B, Blair NP, Shahidi M.. Relation of retinal oxygen measures to electrophysiology and survival indicators after permanent, incomplete ischemia in rats. Transl Stroke Res. 2020; 11: 1273–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leahy S, Farzad S, Blair NP, Shahidi M.. Retinal oxygen delivery, metabolism, and extraction fraction during long-term bilateral common carotid artery occlusion in rats. Sci Rep. 2020; 10: 10371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blair NP, Leahy S, Nathanael M, Shahidi M.. Control of retinal blood flow levels by selected combinations of cervical arterial ligations in rat. Exp Eye Res. 2020; 197: 108088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wanek J, Teng PY, Blair NP, Shahidi M.. Inner retinal oxygen delivery and metabolism under normoxia and hypoxia in rat. Invest Ophthalmol Vis Sci. 2013; 54: 5012–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wanek J, Teng PY, Albers J, Blair NP, Shahidi M.. Inner retinal metabolic rate of oxygen by oxygen tension and blood flow imaging in rat. Biomed Opt Express. 2011; 2: 2562–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shonat RD, Kight AC.. Oxygen tension imaging in the mouse retina. Ann Biomed Eng. 2003; 31: 1084–1096. [DOI] [PubMed] [Google Scholar]
- 53. Lakowicz JR, Szmacinski H, Nowaczyk K, Berndt KW, Johnson M.. Fluorescence lifetime imaging. Anal Biochem. 1992; 202: 316–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shapiro BA, Peruzzi WT, Kozlowski-Templin R.. Clinical Application of Blood Gases . St. Louis, MO: Mosby; 1994: 427. [Google Scholar]
- 55. Cartheuser CF. Standard and pH-affected hemoglobin-O2 binding curves of Sprague-Dawley rats under normal and shifted P50 conditions. Comp Biochem Physiol Comp Physiol. 1993; 106: 775–782. [DOI] [PubMed] [Google Scholar]
- 56. Crystal G. Principles of cardiovascular physiology. In: Estefanous FG, Barash PG, Reves JG, eds. Cardiac Anesthesia: Principles and Clinical Practice. Philadelphia, PA: Lippincott Williams & Wilkins; 2001: 37–57. [Google Scholar]
- 57. West J. Pulmonary Physiology and Pathophysiology: An Integrated, Case-Based Approach. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 58. Yu DY, Cringle SJ.. Retinal degeneration and local oxygen metabolism. Exp Eye Res. 2005; 80: 745–751. [DOI] [PubMed] [Google Scholar]
- 59. Linsenmeier RA, Yancey CM.. Effects of hyperoxia on the oxygen distribution in the intact cat retina. Invest Ophthalmol Vis Sci. 1989; 30: 612–618. [PubMed] [Google Scholar]
- 60. Palkovits S, Lasta M, Told R, et al.. Retinal oxygen metabolism during normoxia and hyperoxia in healthy subjects. Invest Ophthalmol Vis Sci. 2014; 55: 4707–4713. [DOI] [PubMed] [Google Scholar]
- 61. Cringle SJ, Yu DY.. Regulation of oxygen tension in the mammalian retina during systemic hyperoxia is species dependent. Adv Exp Med Biol. 2018; 1072: 241–244. [DOI] [PubMed] [Google Scholar]
- 62. Pechauer AD, Tan O, Liu L, et al.. Retinal blood flow response to hyperoxia measured with en face Doppler optical coherence tomography. Invest Ophthalmol Vis Sci. 2016; 57: OCT141–OCT145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Her C. Hyperoxia-induced decrease in organ blood flow. Anesthesiology. 2008; 108: 168–169; author reply 169–170. [DOI] [PubMed] [Google Scholar]
- 64. Perkins SA, Magargal LE, Augsburger JJ, Sanborn GE.. The idling retina: reversible visual loss in central retinal artery obstruction. Ann Ophthalmol. 1987; 19: 3–6. [PubMed] [Google Scholar]
- 65. Veltkamp R, Sun L, Herrmann O, et al.. Oxygen therapy in permanent brain ischemia: potential and limitations. Brain Res. 2006; 1107: 185–191. [DOI] [PubMed] [Google Scholar]
- 66. Nummela A, Hamalainen I, Rusko H.. Effect of hyperoxia on metabolic responses and recovery in intermittent exercise. Scand J Med Sci Sports. 2002; 12: 309–315. [DOI] [PubMed] [Google Scholar]


