Abstract
Aims
COVID-19 had a devastating impact on patients with severe aortic stenosis (AS). Like many cardiac procedures, transcatheter aortic valve replacement (TAVR) services were suspended during the first wave of COVID-19. We took the opportunity to evaluate the clinical outcomes and identify any delays at different stages of the TAVR pathway during the COVID-19 pandemic.
Methods
Prospectively collected data on 210 consecutive TAVR patients between March 2019 and March 2021 were analysed. We compared the clinical outcomes and 30-day mortality rates of TAVR cases pre-pandemic and during the pandemic. We also looked to identify any time lags from the initial referral to respective stages of the TAVR workup.
Results
A total of 134 patients underwent TAVR prior to the national lockdown (March 2019-March 2020), compared to 76 patients during COVID-19 (April 2020–April 2021). Success rates of TAVR were similar (99% prior to the pandemic and 97.4% during COVID-19). The 30-day survival rates were 98.6% and 94.7%, respectively. Median length of stay post TAVR was 2 days during COVID-19 and 2.5 days prior to the pandemic (p = 0.064). Patients were seen quicker in clinic (median of 33 days) during COVID-19, compared to 51 days before COVID-19 (p = 0.044). No significant difference in times from referral to discussion at TAVR multidisciplinary team (MDT) meetings, CT Aortogram and TAVR implantation, in both groups.
Conclusions
Reconfiguring the patient pathway during COVID-19 allowed TAVR to be performed safely, with a similar success rate and no excess complications or increased 30-day mortality. There proved to be no delay in the respective stages of patient TAVR workup, during the pandemic.
Keywords: Aortic stenosis, TAVR, COVID-19
Graphical abstract
1. Introduction
The prevalence of aortic stenosis (AS) is rising due to increased life expectancy in the elderly population. Untreated, symptomatic, severe AS carries significant mortality and morbidity. With a mortality rate of about 25% per year,1 timely intervention is pivotal. 3.4% of patients above 75 years of age suffer from severe AS, and 40.5% of these patients are deemed too high risk for conventional surgical aortic valve replacement.2 The figures reflect how AS treatment is becoming increasingly reliant on transcatheter aortic valve replacement (TAVR). In the United Kingdom (UK) alone, exponential growth in TAVR has seen 41 centres performing 5197 procedures prior to the COVID-19 pandemic.3
Like most countries worldwide, the COVID-19 pandemic generated unprecedented challenges to the UK's National Health Service (NHS). To protect the public and NHS staffs, hospitals were advised to defer all elective work, including TAVR since March 2020.4 There have been reports on how the COVID-19 pandemic affected cardiac procedures on a whole,3 , 5 , 6 but there has been limited literature on its impact on the TAVR patient pathway.
During this challenging time, we strove to keep our TAVR service aloft to protect the interests of our patients. We adapted using various mechanisms to ensure the safety of the patients and our staffs, while ensuring logistical issues were kept to a minimum. We evaluated the clinical outcomes and time delays in the TAVR patient management pathway during the pandemic compared to an age and risk factor-matched cohort of patients prior to COVID-19.
2. Methods
2.1. Procedural planning outline and data collection
The first lockdown was implemented across the United Kingdom on March 16th 2020. Thus, we categorised our TAVR patients into two groups: those who had TAVR between March 2019 and March 2020, as the ‘pre-COVID’ group, and those who had TAVR after the start of the national lockdown (April 2020–April 2021), as the ‘COVID’ group. Our tertiary centre serves for a population of 2.5 million and provided in-patient treatment for 5590 COVID-positive patients over a 12-month period. We analysed prospectively collected data confirming to the National Institute for Cardiovascular Outcomes Research (NICOR) registry of 210 consecutive patients who underwent TAVR between March 2019 and April 2021.
Information on patient characteristics, procedural details including device success, complications, in-hospital clinical outcomes and 30-day mortality were collated. TAVR device success and complications were based on Valve Academic Research Consortium-2 (VARC-2) criteria.7
All patients referred to the TAVR team were reviewed by a TAVR cardiologist, discussed in TAVR multidisciplinary team (MDT) meetings and underwent TAVR planning computed tomographic (CT) according to the standard practice.
We compared time lags for each stage of the TAVR workup (initial referral to outpatient review, referral to TAVR CT Aortogram, referral to MDT discussions and referral to valve implantation) between patients who underwent TAVR between March 2019 and March 2020 (Control group = 134) and those who underwent TAVR between April 2020 and April 2021 (COVID Group = 76).
A two-stage written consent form was mandated for all patients undergoing TAVR at our centre. To reduce inherent bias, all procedures were performed by the same TAVR operators pre-COVID-19 and during the pandemic.
2.2. Objectives and end points
The study was performed to examine the differences and feasibility of performing TAVR during the COVID-19 pandemic. The primary end point was any significant delay during the pandemic in TAVR workup and valve implantation. Secondary end points included the length of stay in hospital post TAVR, the number of TAVR patients who contracted COVID-19 in hospital and mortality related to COVID-19.
2.3. Reconfiguring the TAVR pathway during COVID-19
The pandemic required us to adapt and change the way we brought patients in for investigations and how we followed them up. We carried out the following:
-
a)
Virtual and phone clinics to evaluate symptomatology in the absence of face-to-face and technician led valve clinics.
-
b)
Encouraging inpatient referrals for TAVR from other specialties, enabling quicker assessment of suitability by TAVR operators.
-
c)
Liaising with the radiology department for dedicated weekly TAVR CT Aortogram slots during COVID-19.
-
d)
Admitting patients from neighbouring referring hospitals for ‘one stop service’-assessment, coronary angiography, CT Aortogram and TAVR implantation.
2.4. Statistical analysis
Data were analysed using GraphPad Prism version 9.0 (GraphPad Software, California, USA). Continuous variables are presented as mean ± standard deviation (SD) and categorical variables as both absolute and percentage numbers. Means were compared using either the unpaired t-test or chi-square test. Statistical significance was defined as a p-value <0.05.
3. Results
3.1. Baseline characteristics
Table 1 summarises the pre-procedural patient characteristics. 134 patients underwent TAVR in the year prior to the national lockdown (March 2019–March 2020), compared to 76 patients during the first year of the pandemic (April 2020–April 2021). The mean age of control cohort was 81.9 SD 6.4 years, compared to 80.9 SD 6.9 years in the COVID-19 group. 59% of the control group and 43% of the COVID-19 group were female. There were no significant differences in the co-morbidities between patients done prior to COVID-19 and those done during the pandemic. 10.4% of patients in the pre-COVID-19 group had previous myocardial infarction compared to 14.5% in the COVID-19 group (p = 0.387). The pre-pandemic group had slightly more history of strokes compared to the COVID-19 group, but this was not statistically significant (23.8% vs 13.2%, p = 0.062). In terms of left ventricular function, 11.9% of the pre-COVID-19 group had an ejection fraction <35%, compared to 13.2% in the COVID-19 group (p = 0.797).
Table 1.
Patient characteristics
| Control (n = 134) | COVID-19 (n = 76) | P-value | |
|---|---|---|---|
| Age (years) | 81.9 ± 6.4 | 80.9 ± 6.9 | 0.296 |
| Female | 79 (59) | 33 (43.4) | 0.03 |
| Body Mass Index (kg/m2) | 27.9 ± 5.9 | 28.1 ± 4.6 | 0.389 |
| Diabetes Mellitus | 37 (27.6) | 17 (22.4) | 0.404 |
| Creatinine (mmol/l) | 106.2 ± 75.2 | 104.9 ± 85.9 | 0.390 |
| Previous MI | 14 (10.4) | 11 (14.5) | 0.387 |
| Previous Cardiac Surgery | 11 (8.2) | 13 (17.1) | 0.051 |
| Prior balloon-aortic valvuloplasty (BAV) | 4 (3) | 1 (1.3) | 0.446 |
| Coronary artery disease in >1 artery∗ | 9 (6.7) | 7 (9.2) | 0.513 |
| Chronic pulmonary disease | 49 (36.6) | 28 (36.8) | 0.968 |
| Severe liver disease | 5 (3.7) | 1 (1.3) | 0.313 |
| Previous cerebrovascular disease | 32 (23.8) | 10 (13.2) | 0.062 |
| Extracardiac arteriopathy | 13 (9.7) | 9 (11.8) | 0.626 |
| Atrial fibrillation | 56 (41.8) | 19 (25) | 0.015 |
| Katz Index | 5.4 ± 0.9 | 5.7 ± 0.5 | 0.228 |
| Left Ventricular Ejection Fraction (%) | |||
| >50 | 92 (68.7) | 51 (67.1) | 0.817 |
| 36-49 | 26 (19.4) | 15 (19.7) | 0.953 |
| <35 | 16 (11.9) | 10 (13.2) | 0.797 |
| EuroSCORE II(%) | 4.51 ± 7.1 | 4.6 ± 4.4 | 0.344 |
| Primary Aortic Valve Pathology | |||
| Aortic Stenosis | 130 (97) | 73 (96) | 0.709 |
| Mixed aortic valve disease | 4 (3) | 3 (3.9) | 0.709 |
| Mean aortic valve gradient (mmHg) | 49.3 ± 15.5 | 45.1 ± 15.4 | 0.133 |
| Aortic valve area (cm2) | 0.8 ± 0.6 | 0.9 ± 0.8 | 0.007 |
| Extensive calcification of ascending aorta | 9 (6.7) | 4 (5.3) | 0.675 |
| Symptoms | |||
| CCS angina III or IV | 1 (0.7) | 2 (2.6) | 0.269 |
| NYHA class III or IV | 120 (89.5) | 64 (84.2) | 0.259 |
Values are mean ± SD or N (%).
EuroSCORE = European System for Cardiac Operative Risk Evaluation; MSCT = multislice computed tomographic; CCS = Canadian Cardiovascular Society; NYHA = New York Heart Association.
Coronary artery disease defined as >70% stenosis.
The majority of our TAVR cases were in the moderate risk category (mean EuroSCORE II of 4.55 SD 5.5). Our cohort of patients had mild to moderate impairment of their baseline functional status. Mean Katz index in the control group was 5.4 SD 0.9, while in the COVID-19 cohort it was 5.7 SD 0.5. Severe AS (96.5%) was the most common indication for TAVR, and the most common symptom was dyspnoea (86.9%).
3.2. Procedural characteristics
Procedural characteristics are detailed in Table 2 . Almost all the cases were done via femoral access, under local anaesthesia and conscious sedation. In the COVID-19 group, 3 patients required alternative access for TAVR implantation as transfemoral approach was not feasible. A transaxillary approach was performed in these patients. General anaesthesia and transesophageal echocardiogram (TOE) were only required in 1 patient from the COVID 19 group, thus protecting our operators from aerosol generating procedures.8 There were less urgent and emergent cases during the COVID-19 pandemic; 5.3% of cases vs 14.9% of cases prior to the lockdown. We postulate this was down to patients being less active and avoiding normal activities due to the strict lockdown measures implemented by the UK health authorities. Therefore, they were less likely to seek emergency services or report deterioration of symptoms. There was also a 37% drop in A&E attendance nationwide17 during the first months of lockdown compared to the previous month, which would explain the significant reduction in cases.
Table 2.
Procedural characteristics
| Procedural characteristics | Control (n = 134) | COVID-19 (n = 76) | P-value |
|---|---|---|---|
| Urgent | 18 (13.4) | 4 (5.3) | 0.063 |
| Emergency | 2 (1.5) | 0 (0) | 0.254 |
| Anaesthesia | |||
| Conscious sedation | 134 (100) | 75 (98.7) | 0.183 |
| General anaesthetic | 0 (0) | 1 (1.3) | |
| Vascular access route | |||
| Transfemoral | 134 (100) | 73 (96.1) | 0.02 |
| Transaxillary | 0 (0) | 3 (3.9) | |
| TOE during procedure | 0 (0) | 1 (1.3) | 0.183 |
| Cerebral protection use | 0 (0) | 0 (0) | |
| Pre-implantation balloon valvuloplasty | 71 (53) | 15 (19.7) | <0.001 |
| Successful valve deployment† | 133 (99.3) | 74 (97.4) | 0.269 |
| Post insertion balloon dilatation | 12 (9.0) | 4 (5.3) | 0.319 |
| End procedural mean aortic gradient (mmHg) | 11.6 ± 5.3 | 12 ± 4.9 | 0.7 |
| End procedural aortic valve area (cm2) | 1.44 ± 0.4 | 1.59 ± 0.5 | 0.381 |
| End procedural paravalvular leak | |||
| None | 69 (51.5) | 42 (55.3) | 0.599 |
| Mild | 60 (44.8) | 33 (43.4) | 0.849 |
| Moderate | 5 (3.7) | 1 (1.3) | 0.313 |
Values are mean ± SD or N (%).
Successful valve deployment defined as per Valve Academic Research Consortium 2 recommendations. TOE-Transesophageal echocardiogram.
Our centre uses a mixture of balloon-expandable transcatheter heart valves, namely SAPIEN 3 Ultra and SAPIEN 3 (Edwards Lifesciences, Irvine, California), and self-expanding valves, such as Evolut R (Medtronic, Minneapolis, Minnesota) and Accurate Neo (Boston Scientific, Massachusetts). Towards the end of 2019, we transitioned to the newer generation SAPIEN 3 Ultra, in place of the SAPIEN 3. .42.4% of balloon-expandable valves used in 2019 were SAPIEN 3 Ultra, compared to 73.6% in 2020. SAPIEN 3 Ultra is associated with less frequent pre-dilatation compared to SAPIEN 3,18 which explains why our COVID-19 cohort had a lower incidence of pre-dilatation prior to valve implantation.
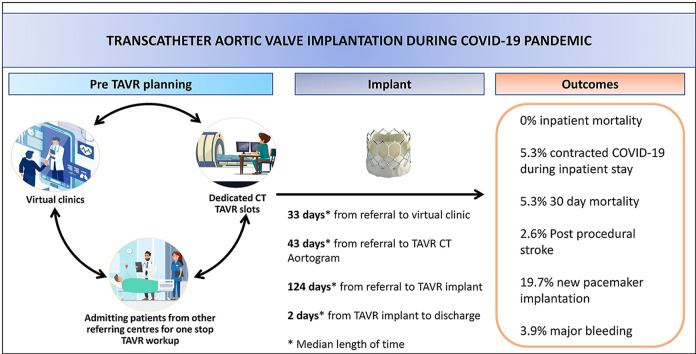
Figure 1.
Summary of outcomes.
Success rates of TAVR were similar in both groups (99% prior to the pandemic and 97.4% during COVID-19, p = 0.269). In addition, more than 96% of patients in both groups had no or only mild paravalvular leak post TAVR.
3.3. Complications and clinical outcomes
No immediate procedural mortality was observed in either group (Table 3 ). At 30 days, survival rate in both groups were 98.6% and 94.7%, respectively (p = 0.142). Median length of stay post TAVR was 2 days during COVID-19, compared to 2.5 days prior to the pandemic (p = 0.064). Despite the relatively brief stay of TAVR patients in hospital, 4 patients (5.3%) developed symptoms of and tested positive for COVID-19 on the ward. Fortunately, none of them required high flow oxygen or prolonged hospital stay. Procedural complications were not significantly different pre-COVID-19 and during COVID-19. A full comparison of complications, including post procedural strokes or MI, acute kidney injury, life threatening bleeding, vascular injury, permanent pacemaker implantation, valve malpositioning, endocarditis, and tamponade, can be found in Table 3.
Table 3.
Complications and clinical outcomes
| Complications | Control (n = 134) | COVID-19 (n = 76) | P-value |
|---|---|---|---|
| Peri procedural MI | 0 (0) | 0 (0) | - |
| Post procedural stroke | 6 (4.5) | 2 (2.6) | 0.439 |
| Life threatening bleeding∗ | 0 (0) | 1 (1.3) | 0.2 |
| Major bleeding∗ | 3 (2.2) | 2 (2.6) | 0.926 |
| AKI Stage 2 and 3† | 1 (0.7) | 1 (1.3) | 0.725 |
| Major vascular complications‡ | 4 (3) | 5 (6.6) | 0.267 |
| Percutaneous device closure failure | 4 (3) | 2 (2.6) | 0.811 |
| New pacemaker implantation | 25 (18.7) | 15 (19.7) | 0.942 |
| Endocarditis | 1 (0.7) | 0 (0) | 0.433 |
| Cardiac tamponade | 0 (0) | 1 (1.3) | 0.2 |
| Valve malpositioning |
1 (0.7) |
0 (0) |
0.433 |
| Clinical outcomes |
Control (n = 134) |
COVID-19 (n = 76) |
P-value |
| Time from referral to clinic (days) | 51 (17-89) | 33 (8-66) | 0.044 |
| Time from referral to TAVR MDT (days) | 82 (42-156) | 73 (30-148) | 0.27 |
| Time from referral to CT Aortogram (days) | 48 (23-109) | 43 (20-125) | 0.66 |
| Time from referral to TAVR (days) | 100 (62-189) | 124 (58-187) | 0.906 |
| Length of stay in hospital (days) | 2.5 (2-9) | 2 (2-4) | 0.064 |
| Immediate procedural mortality | 0 (0) | 0 (0) | - |
| Contracted COVID-19 as inpatient | 0 (0) | 4 (5.3) | 0.124 |
| 30-day mortality | 2 (1.4) | 4 (5.3) | 0.142 |
Values are N (%) or mean ± SD. Times from referral to length of stay are presented as median (interquartile range) ∗Bleeding and † Acute kidney injury as defined by the Acute Kidney Injury Network criteria.
Major vascular complications as defined by the Valve Academic Research Consortium 2 criteria.
Due to the reconfiguration of the TAVR pathway during COVID-19, we found that patients were seen quicker in clinic during the pandemic. Median time after COVID-19 was 33 days, compared to 51 days before COVID-19 (p = 0.044). Furthermore, despite the incredible strain on our CT department during COVID-19, our weekly TAVR CT slot ensured that TAVR patients did not fall in the pecking order in getting their pre-procedure CT Aortogram. Time from online referral to CT Aortogram was 43 days, during the pandemic, compared to 48 days in the control group (p = 0.66).
There was, likewise, no significant difference in times from referral to discussion at the TAVR MDT meeting and TAVR implantation, in both groups (Fig. 1 ).
4. Discussion
To our knowledge, this is the first reported real world data of the impact of COVID-19 on the TAVR service from a UK centre. We describe our experience at the height of the COVID-19 pandemic in the United Kingdom, when deaths were at an all-time high and no vaccines were readily available.
This study has shown that TAVR can be safely performed during the COVID-19 pandemic, with no excess mortality or procedural complications. Our results corroborate with the outcomes of TAVR patients during the COVID-19 pandemic in Israel, as described by Valdebenito et al.9
During the global pandemic, maintaining our TAVR service was essential in ensuring that patients who require aortic valve intervention had access to life saving treatment. The rationale for safeguarding the TAVR service despite the global crisis was also due to the fact that intensive care units were overwhelmed with COVID-19 patients requiring critical care beds. Elective cardiac surgery had to be postponed and in certain patients with poor short-term outcomes, who were initially scheduled for surgical aortic valve replacements (sAVR), our local HEART team advocated referral to TAVR due to the waiting times.
COVID-19 also posed new challenges in the pre-TAVR work-up stages.10 Face-to-face clinics were largely replaced with virtual clinics across the UK, and valvular symptoms surveillance was done remotely.11 The rapid adaptability to telephone clinics was vital in reducing waiting times from referral to patient assessment in the era of COVID-19. Our study showed that patients who were referred for TAVR were seen quicker during the pandemic despite the public health restrictions. Arranging CT Aortogram slots proved particularly challenging at the time, where CT scanners were focused on COVID-19 imaging. Furthermore, we were conscious of the trade-off between exposing patients with aortic valve disease to COVID-19 during visits to hospital and achieving the necessary prerequisite investigations for TAVR implant.
Reconfiguration of our local TAVR pathway was pivotal in delivering a safe and uninterrupted service. Perhaps the most challenging aspect in sustaining the TAVR service was ensuring fine details were taken care of. Examples include effective triage to treat those at highest risk of decompensating first, liaising with the radiology department for urgent scans, mobilising the already diminished, redeployed workforce to adequately cover labs, and managing beds for post TAVR patients. Needless to say, the importance of TAVR coordinators, TAVR specialist nurses, and the wider multidisciplinary team who kept the cogs turning cannot be understated.12
Despite the advancement of TAVR, sAVR remains the gold standard for the treatment of AS in the UK.13 The decision to ‘divert’ a selection of low-intermediate risk patients down the TAVR pathway, although necessitated by circumstances surrounding COVID-19, is in-line with recent recommendations.14 In addition, data from the PARTNER 2 trial demonstrated equivocal outcomes of sAVR and TAVR at 2 years, with regard to intermediate risk patients.15 However, whilst 5-year outcomes of TAVR in general are excellent and comparable to sAVR,16 we have to be mindful that little is known about long-term TAVR valve durability. Long-term monitoring of these subgroup of patients will answer this question and help advocate for patients' choice in the future.
5. Study limitations
Our study inherits limitations common to any registry-based retrospective study. This was an observational study and no randomisation occurred. However, patient selection criteria for TAVR was uniform before and during the pandemic. Secondly, we acknowledge the small sample size in the COVID-19 group. In complying with the UK NHS specialty guidance of deferring all non-emergency interventions for the first month of the national lockdown, no TAVR cases were performed in the initial stages of the lockdown. We were also unable to maintain the number of TAVR referrals from neighbouring hospitals during the pandemic despite deliberately protecting our TAVR services. We postulate that the number of referrals decreased as neighbouring hospitals struggled with the capacity to follow up on their valvular heart patients, due to the redeployment of the cardiology team to help with COVID patient care. Lastly, as this was a single centre study, the results could not be generalised to other TAVR centres.
6. Conclusion
Our local experience of maintaining TAVR services during the COVID-19 pandemic is encouraging. We have shown that TAVR success rate, complication rate and 30-day mortality are similar to the patient group prior to the pandemic. Contrary to our hypothesis, there was no delay at any point of the patient treatment pathway during the COVID-19 pandemic. With the race between COVID-19 vaccines and new variants gripping the globe, what is certain is many hurdles remain. We hope that our experience and perspective will benefit other TAVR centres who are adjusting to the demands of the pandemic.
Contributorship statement
AG, RV, SJ and KB planned the study, performed the procedures and carried out patient follow up. JT, TT and JI carried out the data collection. JT and AG did the statistics. JT wrote the manuscript and submitted to the journal. AG, RV, SJ and KB improved the manuscript. AG supervised the research and guaranteed the research progress.
Funding
No external funding was received for this project.
Conflicts of interest
None declared.
Acknowledgement
We would like to thank the wider TAVR MDT and members of the acute coronary care unit in the Trent Cardiac Centre, Nottingham City Hospital, for their dedication and assistance throughout the COVID-19 pandemic.
Footnotes
Peer review under responsibility of Hellenic Society of Cardiology.
References
- 1.Carabello B.A., Paulus W.J. Aortic stenosis. Lancet [Internet] 2009;373(9667):956–966. doi: 10.1016/S0140-6736(09)60211-7. Available from: [DOI] [PubMed] [Google Scholar]
- 2.Osnabrugge R.L.J., Mylotte D., Head S.J., et al. Aortic stenosis in the elderly: Disease prevalence and number of candidates for transcatheter aortic valve replacement: A meta-analysis and modeling study. J Am Coll Cardiol. 2013;62(11):1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed M.O., Banerjee A., Clarke S., et al. Impact of COVID-19 on cardiac procedure activity in England and associated 30-day mortality. Eur Hear J - Qual Care Clin Outcomes. 2020;44 doi: 10.1093/ehjqcco/qcaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gov.UK . 2020. Government launches campaign urging people to stay at home this Easter.https://www.gov.uk/government/news/coronavirus-government-launches-campaign-urging-people-to-stay-at-home-this-easter Available at: [Google Scholar]
- 5.Leyva F., Zegard A., Okafor O., Stegemann B., Luman P., Qiu T. Cardiac operations and interventions during the COVID-19 pandemic: a nationwide perspective. EP Eur. 2021:1–9. doi: 10.1093/europace/euab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ludman P. Transcatheter Aortic Valve Implantation. UK TAVR Audit Data 2007-2017. Available at: https://www.bcis.org.uk/wp-content/uploads/2018/11/TAVR-slide-deck-to-2017-data-15-11-2018.pdf. Accessed February 26, 2021.
- 7.Kappetein A.P., Head S.J., Généreux P., et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: The valve academic research consortium-2 consensus document (varc-2) Eur J Cardio-Thoracic Surg. 2012;42(5):782–795. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 8.Skulstad H., Cosyns B., Popescu B., et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020:1–7. doi: 10.1093/ehjci/jeaa072. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdebenito M., Massalha E., Barbash I., et al. Transcatheter aortic valve implantation during the COVID-19 pandemic. Am J Cardiol. 2021;145:97–101. doi: 10.1016/j.amjcard.2020.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanguturi V., Lindman B., Pibarot P., et al. Managing aortic stenosis in the COVID 19 era. J Am Coll Cardiol Intv. 2020;13:1937–1944. doi: 10.1016/j.jcin.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah B., Schlosshan D., McConkey H., et al. Outpatient management of heart valve disease following the COVID-19 pandemic: implications for present and future care. Heart. 2020 Oct;106(20):1549–1554. doi: 10.1136/heartjnl-2020-317600. [DOI] [PubMed] [Google Scholar]
- 12.Tchetche D., Biase C., Brochado B., Mastrokostopoulos How to make TAVR more efficient. Interv Cardiol. 2019;14(1):31–33. doi: 10.15420/icr.2018.28.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgartner H., Falk V., Bax J.J., et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2786. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 14.Khialani B., MacCarthy P. Transcatheter management of severe aortic stenosis during the COVID-19 pandemic. Heart. 2020;106:1183–1190. doi: 10.1136/heartjnl-2020-317221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leon M.B., Smith C.R., Mack M.J., et al. Transcatheter or surgical aortic valve replacement in intermediate risk patients. N Engl J Med. 2016;374:1609–1620. doi: 10.1056/NEJMoa1514616. [DOI] [PubMed] [Google Scholar]
- 16.Makkar R.R., Thourani V.H., Mack M.J., et al. Five-Year Outcomes of Transcatheter or Surgical Aortic-Valve Replacement. N Engl J Med. 2020;382(9):799–809. doi: 10.1056/NEJMoa1910555. [DOI] [PubMed] [Google Scholar]
- 17.A&E Attendances and Emergency Admissions 2021-22. Available at: https://www.england.nhs.uk/statistics/statistical-work-areas/ae-waiting-times-and-activity/ae-attendances-and-emergency-admissions-2021-22/. Acessed at 22nd May 2022.
- 18.Rheude T., Pellegrini C., Lutz J., et al. Transcatheter aortic valve replacement with balloon-expandable valves: Comparison of SAPIEN 3 Ultra versus SAPIEN 3. J Am Coll Cardiol Intv. 2020;13(22):2631–2638. doi: 10.1016/j.jcin.2020.07.013. [DOI] [PubMed] [Google Scholar]